Abstract
Introduction
Given the growing burden of venous thromboembolism (VTE) worldwide and the paucity of data from the developing world, the aim of this study was to audit the characteristics, risk factors and length of hospital stay of patients with VTE presenting to a tertiary hospital emergency centre in Johannesburg, South Africa.
Methods
The study was a retrospective record review of all patients who presented with VTE to a tertiary academic emergency centre in Johannesburg, South Africa from 1 April 2012 to 30 March 2013.
Results
Venous thromboembolism was identified in 74 patients; 56 (75.7%) with isolated deep vein thrombosis, 13 (17.6%) with pulmonary embolism and five (6.8%) who had a concurrent deep vein thrombosis with pulmonary embolism. The median age of the patients was 40 years old (range 19–90). The female to male ratio was 2:1. HIV infection, tuberculosis and history of immobilisation were the most common risk factors. The median duration of hospital stay was 14 days (range 4–36). A therapeutic International Normalised Ratio at discharge was only reached in 36.5% of patients.
Conclusion
Venous thromboembolism presentation to the emergency centre is not common, but the risks associated with the morbidity and mortality related to it makes it important despite its relative scarcity. The prevalence of HIV infection amongst patients with VTE is concerning – not only related to the frequency of the pathology but also due to HIV not being factored into the common VTE risk stratification scores.
Keywords: Venous thromboembolism, Pulmonary embolism, Venous thrombosis, Emergency department, Developing countries
African Relevance
-
•
Despite the risks, there is a paucity of venous thromboembolism data in developing countries.
-
•
HIV prevalence is high; HIV infection is not a factor included in the common venous thromboembolism scoring systems.
-
•
Venous thromboembolism guideline changes make outpatient management an option for resource-limited environments.
Introduction
The presentation of patients with venous thromboembolism (VTE), ranging from asymptomatic deep vein thrombosis (DVT) to fatal pulmonary embolism (PE), is commonplace in emergency centres in high-income countries [1], [2]. It is also a common cause of morbidity and mortality [3]. However, little data exist regarding the incidence and management of VTE in low- and middle-income countries (LMICs) [4], [5].
Some of the conventional risk factors for VTE include increasing age, cancer, obesity, smoking, surgery, immobilisation and hormone therapy [3]. Despite initial controversy, Human Immunodeficiency Virus (HIV) infection is now acknowledged as an independent risk factor for VTE [3], [6]. Due to the higher prevalence of HIV infection in LMICs, particularly in sub-Saharan Africa, patients who present with VTE may have very different characteristics than patients who present with VTE in high-income countries [7].
Given the growing burden of VTE worldwide and the paucity of data from LMICs, the aim of this study was to audit the characteristics, risk factors and length of hospital stay of patients with VTE presenting to a tertiary hospital emergency centre.
Methods
This study was a retrospective record review of all patients who presented with VTE to a tertiary academic emergency centre in Johannesburg, South Africa from 1 April 2012 to 30 March 2013. The emergency centre has approximately 60,000 patient visits per annum. This research was approved by the Human Research Ethics Committee of the Faculty of Health Sciences of the University of the Witwatersrand [M130607].
Patients presenting with VTE were identified through the emergency centre registers as well as the radiology department records of DVT ultrasound and computed tomography pulmonary angiograms for PE. The files of these patients were then retrieved from the records department and the data captured by a single abstractor according to a structured data collection sheet. Common VTE risk factors (Table 1) were sought within the patient files. As the study was an audit, the abstractor was not blinded to the outcomes. Any conflicting data were discussed and clarified with an emergency physician.
Table 1.
Venous thromboembolism risk factors, N = 70*, **.
| VTE Risk Factors | Number of patients (%) |
|---|---|
| HIV Positive | 35 (50.0) |
| Tuberculosis | 21 (30.0) |
| Immobilisation | 17 (24.3) |
| Smoking | 11 (15.7) |
| Active Cancer | 7 (10.0) |
| Post-Partum | 6 (8.6) |
| Recent Surgery | 5 (7.1) |
| Trauma | 5 (7.1) |
| Central venous catheter in affected limb | 3 (4.3) |
| Obesity*** | 3 (4.3) |
| Previous history of VTE | 3 (4.3) |
| Systemic Lupus Erythematous | 2 (2.9) |
VTE, venous thromboembolism; HIV, human immunodeficiency virus; *, four patients were excluded due to lack of documentation; **, patients may have had more than one risk factor; ***, not always documented in the file.
Patients were only included if they had presented to the emergency centre with a complaint that lead to a diagnosis of PE or DVT (i.e. hospital-acquired VTE was not included). Patients were included even if the diagnosis was not made or suspected in the emergency centre.
Patients that were admitted with VTE were given subcutaneous low molecular weight heparin (enoxaparin) and then bridged to oral warfarin.
Results
Venous thromboembolism was identified in 74 patients; 56 (75.7%) with isolated DVT and 13 (17.6%) with isolated PE. There were five (6.8%) patients who had a concurrent DVT with PE.
The median age of the patients with VTE was 40 years (range 19–90). Thirty-five patients (47.3%) were below the age of 40 years (Fig. 1). There was no statistically significant difference between the median age of patients with DVT (39 years; range 23–90) compared to the median age of patients with PE (44 years old; range 19–73). 74% of the patients with VTE were Black, 15% Coloured and 11% Caucasian. The female to male ratio was 2:1. Venous thromboembolism risk factors that were identified from the patients’ files are summarised in Table 1.
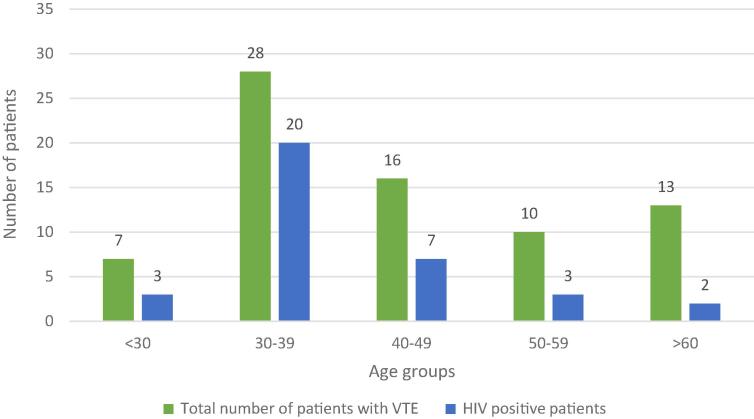
Fig. 1.
HIV prevalence amongst the patients presenting with VTE by age group. VTE, venous thromboembolism; HIV, Human Immunodeficiency Virus.
There were 30 HIV positive patients below the age of 50 years (85.7% of all HIV positive patients). Twenty-four (68.6%) of the HIV positive patients were taking highly active antiretroviral therapy. Fig. 1 shows the distribution of HIV infection per age group. The median duration of hospital stay was 14 days (range 4–36). Hospital length of stay is shown in Fig. 2.
Fig. 2.
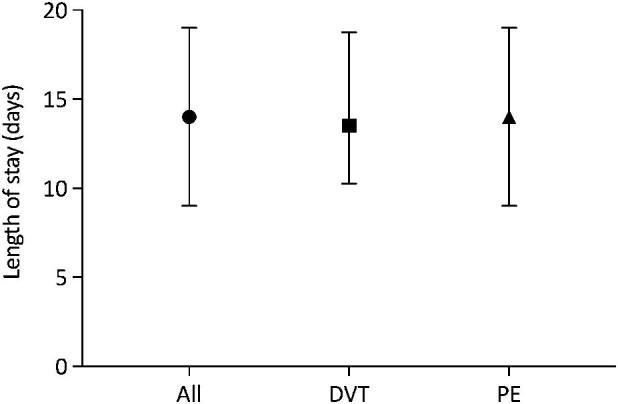
Duration of hospital stay in patients with VTE* (median with inter-quartile ranges). DVT, deep vein thrombosis; PE, pulmonary embolism; *, Data available for 58 patients.
A therapeutic International Normalised Ratio (INR) at discharge was reached in 36.5% (n = 27) of patients, while a therapeutic INR was not reached in 18 (24.3%) patients; follow-up was arranged for a repeat INR in the latter group. The INR at discharge was not documented in 29 (39.2%) patients.
Discussion
Whilst only 1:1000 patients who presented to the emergency centre had VTE, the significance of the potential morbidity and mortality associated with this condition outweighs its relative scarcity. This is marginally higher than rates reported in the literature of 67–80 per 100,000 people [2].
Similar to previous studies, DVT was unsurprisingly more common than PE [1]. Although increasing age is known to be a risk factor for VTE, the median age of our population was 40 years, which is the same as that found by Awolesi et al. at an urban district hospital in KwaZulu-Natal, South Africa [8]. The younger age of these patients is most likely related to the higher rate of HIV infection in this age group (Fig. 1). HIV infection was the most commonly associated risk factor for VTE, followed by tuberculosis. Some of the patients with HIV had co-infection with tuberculosis. This may have contributed to VTE in itself but also may have been potentiated by the immobility secondary to chronic illness, further predisposing them to VTE. Although male gender is known to be a risk factor, as with Awolesi et al.’s cohort, there was also a female preponderance in our study [8], [9].
More than half of the HIV infected patients were taking highly active antiretroviral therapy. The therapy itself is an independent risk factor for VTE [6]. This, as well as the high prevalence of HIV in South Africa, underscores our concerns about the exclusion of these risk factors in the major VTE scoring systems, such as the Modified Wells Scoring System, the Revised Geneva Scoring System and the Pulmonary Embolism Rule Out Criteria score [10], [11], [12]. These scoring systems were not utilised in this study’s emergency centre when the data from this study were collected. However, these scoring systems are frequently used in the South African clinical setting despite lack of validation in our population.
The modifiable risk factors of smoking and obesity could possibly have been underestimated due to lack of documentation in the patient files. Cheng et al., however, emphasised both of their associations with VTE in their 2013 meta-analysis [13].
As was the procedure in our hospital, the traditional management of patients with VTE requires hospital admission in order for patients to be started on parenteral heparin (either low molecular weight or unfractionated) and then be bridged to an oral vitamin K antagonist (e.g. warfarin) [1]. Patients are usually kept in hospital until their INR is within the therapeutic range, which may take several days [14]. This is echoed in the 2013 Southern African Society of Thrombosis and Haemostasis guidelines [15]. This model led to a median length of stay of two weeks for our patients. Even with this protracted admission, only 36.5% of patients were discharged with an INR in the therapeutic range, and even though a therapeutic INR was obtained, the time in the therapeutic range for warfarin is commonly only 60–68% [16], [17]. But there are also costs (monetary and health-related) linked to hospital admission that need to be taken into account. Although data were not collected regarding morbidity related to admission, it is known that hospital admission itself is not without negative sequelae such as nosocomial pneumonia, urinary tract infections and bedsores.
The 2013 Southern African Society of Thrombosis and Haemostasis guidelines were released prior to the registration of the non-vitamin K oral anticoagulants (NOACs) in South Africa [15]. The advent of NOACs, such as dabigatran, rivaroxaban, apixaban and edoxaban has challenged the treatment paradigm. The 2016 American College of Chest Physicians guidelines now recommend the use of NOACs over vitamin K antagonists as primary therapy [18]. There is also increasing evidence to support outpatient management and direct discharge from the emergency centre for low-risk patients with DVT and/or PE [1], [18], [19]. The largest of these trials will be completed in August 2017 in the United States [20].
Kline et al. showed that patients preferred the autonomy associated with outpatient management [21]. This may also have a knock-on effect leading to better patient compliance. The ramifications of VTE admission, continued INR monitoring and higher incidence of severe bleeding complications needs to be balanced against the higher costs of utilising the newer, safer NOACs for outpatient treatment. Perhaps all these factors combined are sufficient to justify outpatient management of VTE in South Africa? If feasible and economically viable, this could potentially be beneficial for the outpatient management of VTE from the emergency centre in LMICs. Further cost-benefit and outcome-related prospective studies would better answer this question.
These data were collected from 2012 to 2013 prior to the implementation of VTE protocols in the emergency centre. The retrospective nature of this audit made missing data a constraint for analysis, e.g. not all patients may have been asked about all possible risk factors. This was a single centre study making wider applicability limited. Without the benefit of electronic health records or a registry to obtain the data, some VTE patients may have been inadvertently excluded. Data regarding morbidity and mortality were not collected.
Venous thromboembolism presentation to the emergency centre is not common, but the risks associated with the morbidity and mortality related to it makes it important despite its relative scarcity. The prevalence of HIV infection amongst patients with VTE is concerning, not only related to the frequency of the pathology but also due to HIV not being factored into the common VTE risk stratification scores.
Conflicts of interest
LG has received honoraria from Bayer Healthcare for speaking on venous thromboembolism. No financial support was given for this study; the authors covered all costs incurred.
Dissemination of results
Results from this study were shared with the management of the Hospital.
Authors’ contributions
LG conceived the original idea. MW collected the data. LG and MW carried out the analysis of the data. LG drafted the manuscript. LG and MW approved the final version that was submitted.
Footnotes
Peer review under responsibility of African Federation for Emergency Medicine.
References
- 1.Singer A.J., Thode H.C., Jr., Peacock W.F. Admission rates for emergency department patients with venous thromboembolism and estimation of the proportion of low risk pulmonary embolism patients: a US perspective. Clin Exp Emerg Med. 2016;3(3):126–131. doi: 10.15441/ceem.15.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf H.R., Tsai J., Siddiqi A., Boulet S.B., Soucie J.M. Emergency Department visits by patients with venous thromboembolism, 1998–2009. J Hospital Admin. 2012 Sep;1(1) [Google Scholar]
- 3.Streiff M.B., Agnelli G., Connors J.M., Crowther M., Eichinger S., Lopes R. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombol. 2016 Jan;41(1):32–67. doi: 10.1007/s11239-015-1317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamerkar D.R., John M.J., Desai S.C., Dsilva L.C., Joglekar S.J. Arrive: a retrospective registry of Indian patients with venous thromboembolism. Indian J Crit Care Med. 2016 Mar;20(3):150–158. doi: 10.4103/0972-5229.178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan A. The case for venous thromboembolism prophylaxis in Africa. East Afr Med J. 2009 Dec;86(12 Suppl):S108–S109. doi: 10.4314/eamj.v86i12.62921. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen L.D., Dybdal M., Gerstoft J., Kronborg G., Larsen C.S., Pedersen C. HIV and risk of venous thromboembolism: a Danish nationwide population-based cohort study. HIV Med. 2011 Apr;12(4):202–210. doi: 10.1111/j.1468-1293.2010.00869.x. [DOI] [PubMed] [Google Scholar]
- 7.The Global HIV/AIDS Epidemic. HIV.gov <https://www.aids.gov/hiv-aids-basics/hiv-aids-101/global-statistics/> [accessed 25.01.2016].
- 8.Awolesi D., Naidoo M., Cassimjee M.H. The profile and frequency of known risk factors or comorbidities for deep vein thrombosis in an urban district hospital in KwaZulu-Natal. S Afr J HIV Med. 2016;17(1):a425. doi: 10.4102/sajhivmed.v17i1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veller M., Pillai J. Lower limb venous thrombosis. Contin Med Educ. 2009;27(7):306. [Google Scholar]
- 10.Douma R.A., Gibson N.S., Gerdes V.E., Büller H.R., Wells P.S., Perrier A. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Thromb Haemost. 2009 Jan;101(1):197–200. [PubMed] [Google Scholar]
- 11.Klok F.A., Mos I.C., Nijkeuter M., Righini M., Perrier A., Le Gal G. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008 Oct 27;168(19):2131–2136. doi: 10.1001/archinte.168.19.2131. [DOI] [PubMed] [Google Scholar]
- 12.Kline J.A., Mitchell A.M., Kabrhel C., Richman P.B., Courtney D.M. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004 Aug;2(8):1247–1255. doi: 10.1111/j.1538-7836.2004.00790.x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y.-J., Liu Z.-H., Yao F.-J., Zeng W.-T., Zheng D.-D. Current and former smoking and risk for venous thromboembolism: a systematic review and meta-analysis. PLoS Med. 2013;10(9):e1001515. doi: 10.1371/journal.pmed.1001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahlon P., Nabi S., Arshad A., Jabbar A., Haythem A. Warfarin dosing and time required to reach therapeutic international normalized ratio in patients with hypercoagulable conditions. Turk J Haematol. 2016 Dec 1;33(4):299–303. doi: 10.4274/tjh.2015.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson B.F., Louw S., Büller H., Mer M., de Jong P.R., Rowji P. Venous thromboembolism: prophylactic and therapeutic practice guideline. S Afr Med J. 2013 Feb 15;103(4):261–267. doi: 10.7196/samj.6706. [DOI] [PubMed] [Google Scholar]
- 16.Caldeira D., Cruz I., Morgado G., Stuart B., Gomes C., Martins C. Evaluation of time in therapeutic range in anticoagulated patients: a single-center, retrospective, observational study. BMC Res Notes. 2014 Dec;9(7):891. doi: 10.1186/1756-0500-7-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pokorney S.D., Simon D.N., Thomas L., Fonarow G.C., Kowey P.R., Chang P. Patients' time in therapeutic range on warfarin among US patients with atrial fibrillation: results from ORBIT-AF registry. Am Heart J. 2015 Jul;170(1):141–148. doi: 10.1016/j.ahj.2015.03.017. 148.e1. [DOI] [PubMed] [Google Scholar]
- 18.Kearon C., Akl E.A., Ornelas J., Blaivas A., Jimenez D., Bounameaux H. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Beam D.M., Kahler Z.P., Kline J.A. Immediate discharge and home treatment with rivaroxaban of low-risk venous thromboembolism diagnosed in two U.S. Emergency Departments: a one-year preplanned analysis. Acad Emerg Med. 2015 Jul;22(7):788–795. doi: 10.1111/acem.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabrhel C. Outpatient treatment of pulmonary embolism and deep vein thrombosis: impact of a new protocol on emergency department efficiency and patient safety. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 – [25 January 2017]. Available from: <https://clinicaltrials.gov/ct2/show/record/NCT02532387> Identifier: NCT02532387.
- 21.Kline J.A., Kahler Z.P., Beam D.M. Outpatient treatment of low-risk venous thromboembolism with monotherapy oral anticoagulation: patient quality of life outcomes and clinician acceptance. Patient Prefer Adherence. 2016 Apr;15(10):561–569. doi: 10.2147/PPA.S104446. [DOI] [PMC free article] [PubMed] [Google Scholar]