Summary
The intestinal microbiota provides colonization resistance against invading pathogens, limiting pathogen expansion, transmission into the environment, and infection of naïve hosts. Previously described mechanisms of microbiota-mediated colonization resistance have been identified by observing loss of colonization resistance after community perturbations such as antibiotic treatment or diet changes. Here, we identify a microbiota-mediated mechanism of colonization resistance against the intestinal bacterial pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium) by comparing high-complexity communities with different levels of colonization resistance. Using two inbred mouse strains with significantly different infection dynamics and S. Typhimurium intestinal burdens, we demonstrate that Bacteroides species mediate colonization resistance against S. Typhimurium in vivo through the production of the short-chain fatty acid propionate. We show that propionate directly inhibits S. Typhimurium growth in vitro by disrupting intracellular pH homeostasis. This work provides the first mechanistic understanding of the role of individualized microbial communities in host-to-host variability of pathogen transmission.
Keywords: Colonization resistance, Salmonella, propionate, Bacteroides, metabolism, intestine, bacterial pathogenesis, intracellular pH, microbiota
Introduction
Colonization resistance is crucial in preventing pathogen invasion in several niches within animal hosts. Microbiota-mediated mechanisms of colonization resistance inhibit infection at a variety of host sites including the skin, vagina, lungs, urinary tract and intestine (Bäumler and Sperandio, 2016). Resistance mechanisms have been best characterized in the intestine, where trillions of commensal microbes occupy the intestinal lumen and interact intimately with the host as well as each other in complex microbial networks (Bäumler and Sperandio, 2016).
Intestinal commensals mediate colonization resistance against bacterial pathogens through indirect and direct mechanisms (Sassone-Corsi and Raffatellu, 2015). Indirect mechanisms of colonization resistance occur primarily through engagement and activation of antibacterial host immune pathways, altering epithelial and immune cell function to promote anti-pathogen immunity (Bevins and Salzman, 2011; Sassone-Corsi and Raffatellu, 2015). Commensal microbes can also limit enteric pathogen colonization and expansion through direct microbe-microbe interactions. These mechanisms are less well characterized, and include competition for niches and nutrients, contact-dependent killing, and production of antagonistic molecules (Buffie et al., 2014; Deriu et al., 2013; Hecht et al., 2016; Maltby et al., 2013; Sassone-Corsi et al., 2016). Together, these colonization resistance mechanisms provide host protection against enteric bacterial pathogens, which rely on robust colonization and expansion in the intestines for efficient transmission into the environment.
Salmonella enterica serovars are important human pathogens responsible for millions of infections worldwide that range from self-limiting gastroenteritis to invasive typhoid fever (Parry et al., 2002). In oral infection models in mice, S. enterica serovar Typhimurium (S. Typhimurium) robustly colonizes sites in the distal intestine such as the cecum and colon (Lam and Monack, 2014). From these sites, S. Typhimurium is shed via the feces into the environment, where it can infect naïve hosts (Lam and Monack, 2014). In order to expand to high levels in the distal intestine, S. Typhimurium utilizes a number of key virulence factors such as Salmonella Pathogenicity Islands SPI-1, SPI-2, and SPI-6, which encode secretion systems that deliver effector proteins into host cells and competing Enterobacteriaceae (Lam and Monack, 2014; Sana et al., 2016).
We and others have demonstrated that the endogenous intestinal commensal microbiota provides colonization resistance against Salmonella infection, as oral antibiotic treatment enhances pathogen expansion and fecal shedding (Barthel et al., 2003; Lawley et al., 2007). Commensal-mediated colonization resistance mechanisms against intestinal Salmonella infection have so far only been studied in colitis models of infection in which mice are pre-treated with antibiotics prior to infection (Barthel et al., 2003). Disruption of the resident commensal community in this model limits the generalizability of mechanistic findings to clinical infections in humans, in which a subset of humans become chronic asymptomatic carriers of intestinal infection while others control intestinal pathogen expansion (Parry et al., 2002). Indeed, while prior antibiotic treatment has been linked to Salmonella infection in various human studies, the prevalence of this association accounts for only a subset of infections (<25% of cases) (Malik et al., 2017). In this study, we utilize a mouse model of oral S. Typhimurium infection in which mice are not pretreated with an antibiotic to uncover a novel mechanism of colonization resistance. We demonstrate that commensal Bacteroides species limit S. Typhimurium infection in the distal gut. We find that Bacteroides spp. production of the short-chain fatty acid metabolic byproduct propionate directly inhibits S. Typhimurium growth in vitro by disrupting intracellular pH homeostasis and limits distal intestinal expansion and fecal shedding in vivo.
RESULTS
The intestinal microbiota controls distal intestinal Salmonella expansion and fecal shedding
We and others have demonstrated that Salmonella distal intestinal colonization and shedding in the feces vary between inbred mouse strains (Lawley et al., 2007; Rivera-Chávez et al., 2016). The mechanisms that control intestinal levels of Salmonella in different strains of mice are currently unknown. To gain insight into mechanisms that influence the ability of S. Typhimurium to colonize these sites, we evaluated the kinetics of infection in two inbred strains of mice: 129X1/SvJ (129) mice and C57BL/6 nramp-1+/+ (B6N) mice. These well-established animal models of S. Typhimurium infection permit the examination of both acute and chronic stages of infection (Brown et al., 2013; Monack et al., 2004). Both mouse strains harbor functional alleles of nramp-1, which encodes a multipass transmembrane protein and functions as a divalent metal transporter (Brown et al., 2013; Gruenheid et al., 1997). Mice with a functional nramp-1 allele control the levels of systemic Salmonella and survive longer than mice harboring a nonfunctional allele (Arpaia et al., 2011).
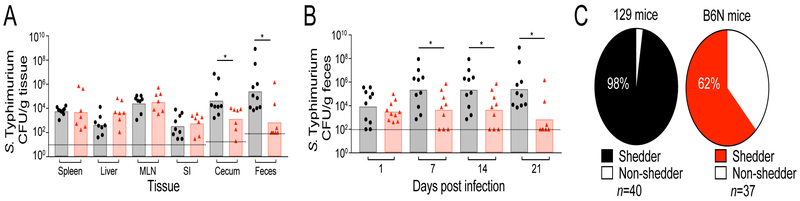
We orally inoculated 129 and B6N mice with S. Typhimurium and examined pathogen burdens in systemic and intestinal tissues. After 21 days post-infection (p.i.), 129 and B6N mice were colonized to similar levels in systemic and proximal intestinal tissues such as the spleen, liver, mesenteric lymph nodes, and small intestine (Figure 1A). However, 129 mice surprisingly harbored significantly more S. Typhimurium compared to B6N mice in the cecum and colon (Figure 1A). Consistent with this observation, 129 mice shed significantly more S. Typhimurium in the feces throughout infection (7, 14, and 21 days p.i.) (Figure 1B). Additionally, the proportion of mice shedding S. Typhimurium during infection was significantly different between these strains; while 98% of infected 129 mice shed S. Typhimurium in the feces, only 62% of infected B6N mice shed detectable levels of pathogen (Figure 1C). This observation suggests that a distal intestine-specific factor may control intestinal Salmonella expansion and fecal shedding in inbred strains of mice.
Figure 1. S. Typhimurium distal gut colonization and fecal shedding varies between inbred mouse strains.
129 (black) and B6N (red) mice were infected with S. Typhimurium. A) Tissue burdens at 21 days p.i. MLN=mesenteric lymph nodes, SI=small intestine. B) Fecal shedding. C) The proportion of shedders in infected mice. Shedder/non-shedder proportions were quantified by combining scores of mice shedding at each time point. Dotted lines represent the limit of detection. Data represent two independent experiments with 5–10 animals per group. Error bars represent the standard error of the mean. For shedding and tissue data, significance was calculated using a two-tailed Mann-Whitney test, *: p<0.05, **: p<0.01. For shedding status analysis, proportion distributions were analyzed by a two-tailed Fisher’s exact test (p<0.0001). Unless noted, conditions are not significant.
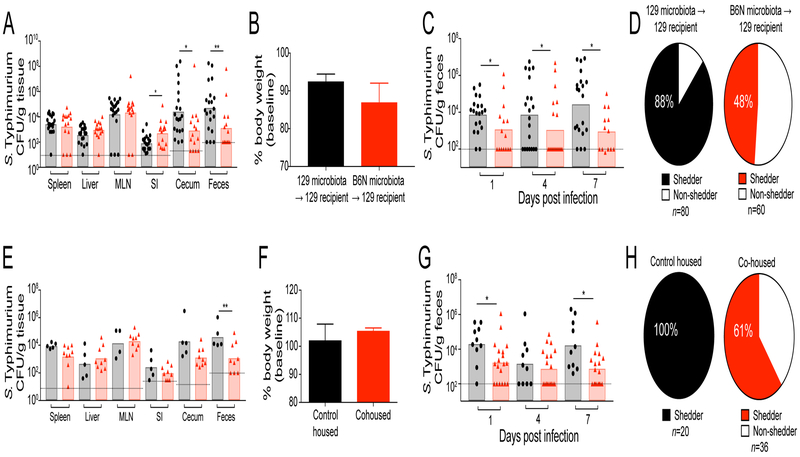
S. Typhimurium interacts with the vast community of commensal microbes in the distal intestine (Sassone-Corsi and Raffatellu, 2015). The composition of these microbial communities is diverse among inbred mouse strains, similar to the diversity that has been observed in human populations (Carmody et al., 2015; Friswell et al., 2010). Indeed, the microbiotas of 129 and C57BL/6 mice are known to have unique community compositions (Gulati et al., 2012). Considering this, we hypothesized that the composition of the intestinal microbiota in 129 and B6N mice controls intestinal Salmonella expansion and fecal shedding. To determine the relative effects of host genetics and the intestinal microbiota on S. Typhimurium infection, we transplanted the fecal microbiotas from B6N mice (referred to as a fecal microbiota transplant, FMT) into 129 mice (Figure S1A). The FMT did not affect S. Typhimurium colonization of systemic tissues or overall host health (Figure 2A–B). However, the FMT of the intestinal microbiota from B6N mice was sufficient to limit S. Typhimurium intestinal expansion and fecal shedding in 129 mice and significantly reduced the proportion of hosts that shed pathogen (Figure 2A,C-D). The commensal bacteria responsible for this phenotype are naturally transmissible and exert a dominant effect on intestinal Salmonella expansion, as cohousing 129 mice with B6N mice produced results similar to FMT (Figure 2E–H, S1B).
Figure 2. The intestinal microbiota controls intestinal Salmonella expansion in the distal gut and fecal shedding.
A-D) 129 mice received fecal microbiota transplant (FMT) with 129 (black) or B6N (red) microbiotas prior to infection with S. Typhimurium. A) Tissue burdens at 21 days p.i. MLN=mesenteric lymph nodes, SI=small intestine. B) Host body weight at 21 days p.i. C) Fecal shedding. D) The proportion of shedders in infected mice. E-H) 129 mice were housed with their own genotype (control housed, black) or with B6N mice (cohoused, red) prior to S. Typhimurium infection. E) Tissue burdens at 21 days p.i. F) Host body weight at 21 days p.i. G) Fecal shedding. H) The proportion of shedders in infected mice. Dotted lines represent the limit of detection. Bar graph data represent two independent experiments with 5–10 animals per group. Error bars represent the standard error of the mean. For shedding and tissue data, significance was calculated using a two-tailed Mann-Whitney test, *: p<0.05, **: p<0.01. For shedding status analysis, proportion distributions were analyzed by a two-tailed Fisher’s exact test (p<0.0001 for FMT, p<0.0009 for cohousing). Unless noted, conditions are not significant.
Bacteroidales spp. are more abundant in B6N microbiota recipients
Two features of microbial communities have been postulated to mediate colonization resistance to pathogens: community species richness and community composition. Highly diverse microbial communities are associated with protection against invading pathogens; perturbations that reduce species richness predispose hosts to enteric infections (Desai et al., 2016; Lawley et al., 2007). In addition to overall species richness, community composition can drive microbiota-mediated colonization resistance. Recent studies have illustrated that the presence or absence of specific commensals controls infection by enteric pathogens (Brugiroux et al., 2016; Buffie et al., 2014; Miki et al., 2017).
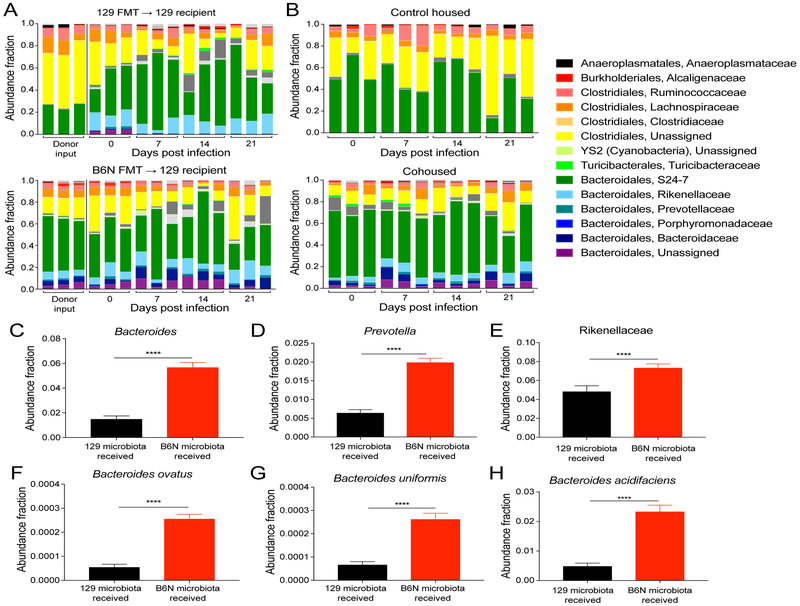
To gain mechanistic insight into the enhanced colonization resistance of B6N mice, we analyzed changes to the intestinal bacterial communities caused by transplantation and cohousing using 16S rRNA sequencing. To test whether community species richness was associated with B6N microbiota-mediated colonization resistance, we first measured α-diversity in animals receiving B6N and 129 microbiotas. Phylogenetic and nonphylogenetic α-diversity measurements did not differentiate animals receiving B6N and 129 microbiotas, indicating that the disparity in S. Typhimurium expansion was not due to a reduction in species richness in the community (Figure S2A). To further characterize B6N and 129 microbiotas, we performed β-diversity analyses on these communities. Principal coordinate analysis (PCoA) of unweighted UniFrac β-diversity showed clear sample clustering of mice from the transplantation and cohousing experiments. Sample clustering was associated with the microbiota each mouse received, and not with other variables in our experimental design (Figure S2B–I). Taken together, these results suggest that microbiota community composition, rather than species richness, drive microbiota-mediated colonization resistance against intestinal S. Typhimurium infection.
Based on these findings, we hypothesized that commensal bacteria mediating colonization resistance would be either exclusive to or more abundant in mice receiving B6N microbiotas. Analysis of community composition revealed Bacteroidales taxonomic groups that were present in mice that had received B6N microbiotas and absent in mice that had received 129 microbiotas (Figure 3A–B). Indeed, random forest supervised learning analyses identified several Bacteroidales taxonomic groups that were predictive features of B6N microbiotas (Table S1) (Knights et al., 2011). Three of these groups (genera Bacteroides and Prevotella, and family Rikenellaceae) were significantly more abundant in mice that had received B6N microbiotas by transplantation or cohousing (Figure 3C–H, S3). Previous studies demonstrated that closely related Enterobacteriaceae spp. mediate colonization resistance against Salmonella infection through competition for similar niche requirements (Brugiroux et al., 2016; Sassone-Corsi et al., 2016). Importantly, we found that Enterobacteriaceae spp. were not predictive of B6N microbiotas or more abundant in animals receiving B6N microbiotas (Figure S3, Table S1).
Figure 3. Bacteroidales spp. are more abundant in animals receiving B6N microbiotas.
16S rRNA gene sequencing abundances from FMT and cohousing experiments on days 0, 7, 14, and 21 p.i. A-B) Abundance of bacterial families in A) FMT and B) cohousing experiments. Each mouse is represented by an individual bar. Three representative animals are displayed per group (total n=5–10 mice per group). C-H) Abundance of Bacteroidales spp. identified by supervised learning. C) Bacteroides, D) Prevotella, E) Rikenellaceae, F) B. ovatus, G) B. uniformis, H) B. acidifaciens. Abundances represent data combined from FMT and cohousing experiments at all time points (D0, 1, 7, 14, 21 p.i., n=25–50 mice per group). Significance was calculated using the Kruskall-Wallis test with Bonferroni correction for multiple hypotheses with the p-value indicated (****: p<0.0001). Error bars represent standard error of the mean.
Bacteroides-derived products directly limit S. Typhimurium growth in vitro
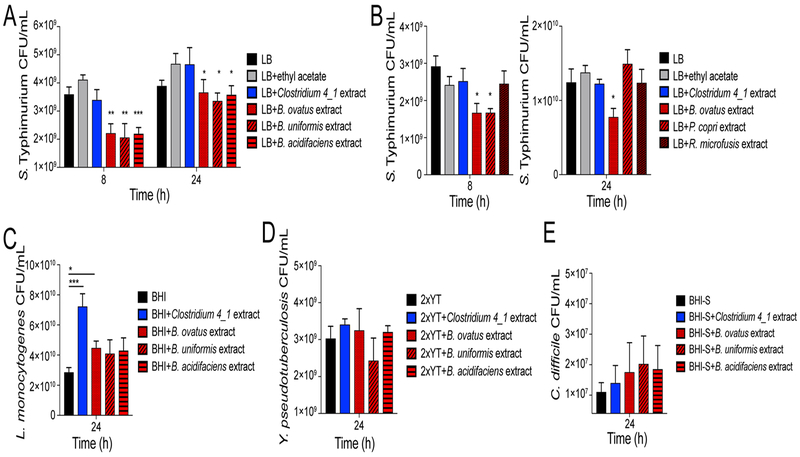
Commensal bacteria mediate colonization resistance against pathogens through direct antibacterial mechanisms such as production of antagonistic molecules or direct niche competition (Maltby et al., 2013; Sassone-Corsi et al., 2016), as well as via indirect mechanisms such as enhancing host immunity (Abt et al., 2016). We first investigated whether Bacteroidales spp. directly limit pathogen growth using an in vitro assay. In this assay, organic extracts of spent medium from anaerobically-grown commensals were resuspended in rich medium. S. Typhimurium was subsequently grown anaerobically in these microbe-free extracts (Figure S4A). Products produced by Bacteroides spp. limited anaerobic S. Typhimurium growth at 8 and 24 h post-inoculation as measured by colony forming units (CFU) (Figure 4A, S4B).
Figure 4. Bacteroides spp.-derived products limit S. Typhimurium growth in vitro.
Medium was supplemented with organic extracts of supernatants isolated from anaerobically grown commensal bacteria. A-B) S. Typhimurium CFU/mL. C) L. monocytogenes CFU/mL. D) Y. pseudotuberculosis CFU/mL. E) C. difficile CFU/mL. All extracts were resuspended at 1X concentration. Data represent 3 independent experiments with 3–5 replicates. Error bars represent the standard error of the mean. Significance was calculated using a two-tailed Mann-Whitney test comparing conditions to LB control; NS: not significant, *: p<0.05, **: p<0.01, ***: p<0.001. Unless noted, conditions are not significant.
Human Bacteroides isolates as well as mouse isolates from B6N mice limited S. Typhimurium growth (data not shown), indicating that limitation of growth is likely conserved among Bacteroides spp. Interestingly, limitation of growth was specific to Bacteroides spp., as extracted supernatants from Clostridiaceae, Prevotella, and Rikenellaceae species did not limit S. Typhimurium growth (Figure 4B, S4C). Furthermore, Bacteroides spp. products did not limit growth of other enteric pathogens such as Listeria monocytogenes, Yersinia pseudotuberculosis, or Clostridioidies difficile, suggesting that growth limitation is specific to S. Typhimurium (Figure 4C–E). Taken together, these results demonstrate that products released from Bacteroides spp. limit S. Typhimurium in vitro growth in a specific and direct manner.
Propionate production by Bacteroides spp. limits Salmonella growth in vitro
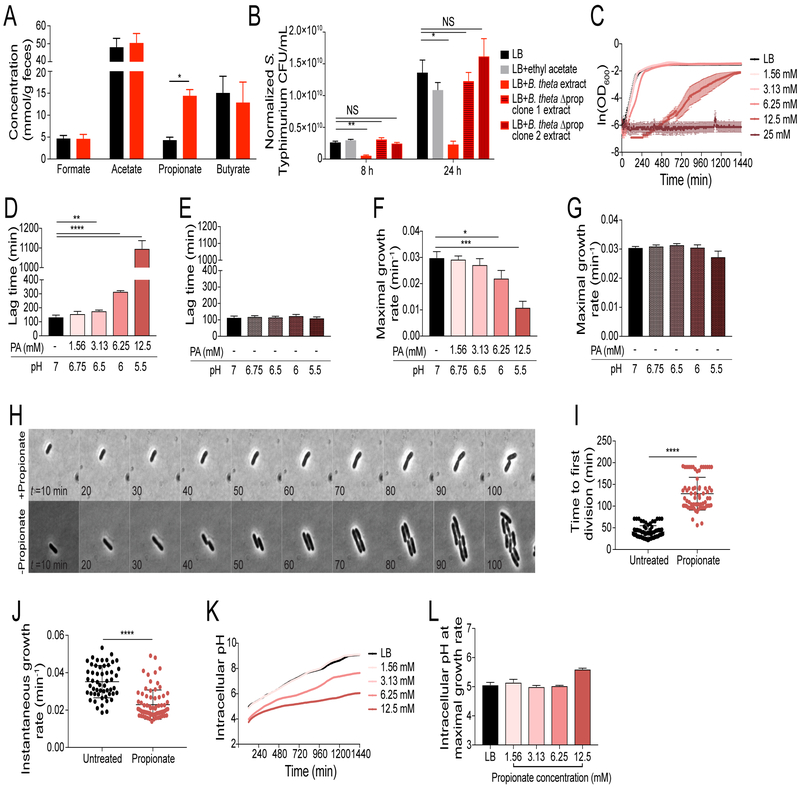
In the intestine, Bacteroides spp. ferment complex sugars into a number of byproducts, including short-chain fatty acids (SCFAs) such as formate, acetate, propionate, and butyrate (Besten et al., 2013). These molecules are extremely abundant in the distal intestine, reaching millimolar concentrations in the lumen (Besten et al., 2013). We measured fecal SCFA levels in uninfected 129 and B6N mice to determine whether the abundance of these molecules predisposes hosts to different levels of S. Typhimurium fecal shedding. We found that while concentrations of formate, acetate, and butyrate were similar between 129 and B6N mice, levels of propionate were three-fold higher in B6N mice than in 129 mice (Figure 5A).
Figure 5. Propionate produced by Bacteroides spp. limits S. Typhimurium growth.
A) Fecal short chain fatty acid (SCFA) concentrations in uninfected B6N (red) and 129 (black) mice measured by gas chromatography-mass spectrometry (GC-MS). Data represent 2 independent experiments with 4–5 mice per group. Error bars represent the standard error of the mean. B) S. Typhimurium CFU/mL grown in medium supplemented with organic extracts of supernatants isolated from anaerobically grown Bt isolates. Extract concentration was normalized to Bt CFU/mL. Data represent 3 independent experiments with 4 replicates. Error bars represent the standard error of the mean. C-D, F) Anaerobic growth curves of S. Typhimurium in LB supplemented with propionic acid. C) OD600. D) Lag time. F) Maximal growth rate. Data represent 2 independent experiments with 3 replicates. Error bars represent the standard deviation. OD600 was measured every 5 min for 24 h. E, G) Anaerobic growth curves of S. Typhimurium in LB broth supplemented with propionic acid or pH-matched LB broths. E) Lag time. G) Maximal growth rate. Data represent 2 independent experiments with 3 replicates. Error bars represent standard deviation. H-J) Time lapse imaging of anaerobic Salmonella grown on LB with and without propionate. H) Kymograph of representative single cells during growth. I) Time to the first cellular division. J) Maximal growth rate. Data represent at least 60 cells per condition. Error bars represent standard deviation. K-L) Anaerobic growth curves of S. Typhimurium in LB broth supplemented with propionic acid. K) Intracellular pH measurements over time. L) Intracellular pH measurement at maximal growth rate. Data represent 2 independent experiments with 3 replicates. Error bars represent standard deviation. For SCFA and commensal extract growth curves, significance was calculated using a two-tailed Mann-Whitney test; NS: not significant, *: p<0.05, **: p<0.01. For lag time, maximal growth rate, time to first division, and instantaneous elongation rate measurements, significance was calculated using an unpaired two-tailed t-test; *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001. Unless noted, conditions are not significant.
Propionate production is a well-characterized feature of Bacteroides spp., resulting from catabolism of succinate (Reichardt et al., 2014). This pathway is highly conserved within the Bacteroides genus, with many species producing propionate during in vitro growth (Figure S4D). To determine whether Bacteroides production of propionate is necessary to limit S. Typhimurium growth in vitro, we utilized previously published mutant strains of B. thetaiotaomicron (Bt) that are unable to produce propionate (Figure S4E) (Kovatcheva- Datchary et al., 2015). As these mutants have a minor growth defect, we performed organic extractions on supernatants from these cultures and normalized the extract concentration based on input Bt CFUs (Figure S4F). We found that while S. Typhimurium growth was limited by wild type Bt products, growth was not limited by products from propionate-production mutants (Figure 5B). Importantly, products from complemented propionate-production mutant strains of Bt limited S. Typhimurium growth (Figure S4E,G). Taken together, these data suggest that propionate production by Bacteroides spp. is necessary to limit S. Typhimurium growth.
To test whether propionate is sufficient to limit S. Typhimurium growth in vitro, we compared anaerobic growth with and without exogenous propionate. At the population level, propionate inhibited growth of S. Typhimurium in a dose-dependent manner by extending lag phase by several hours and reducing maximal growth rate by two- to threefold (Figure 5C-D,F). To gain more insight into how propionate limits S. Typhimurium growth, we performed microscopy in an anaerobic chamber on S. Typhimurium cells grown on LB agar pads with and without propionate. At the single-cell level, propionate extended the time to the first cellular division as well as reduced the instantaneous elongation rate during exponential growth (Figure 5H–J).
Since propionate significantly acidifies bacterial growth media (Ricke, 2003), we first hypothesized that propionate extends bacterial lag phase by acidifying the extracellular space. To test this hypothesis, we measured the pH of LB broth with known concentrations of propionate and made pH-matched LB broth with HCl. The lag times and maximal growth rates of S. Typhimurium in pH-matched LB broth were similar to those in LB alone (Figure 5E,G, S4H), indicating that propionate acidification of the extracellular medium is not sufficient to inhibit growth. When propionate is protonated it can diffuse across Gram-negative bacterial membranes into the cytoplasm, where it dissociates into a proton and propionate anion (Ricke, 2003). This process has been proposed to acidify the intracellular cytoplasm, which has negative consequences for bacterial growth and division (Ricke, 2003). To test whether propionate-mediated acidification of the bacterial cytoplasm is associated with extended lag phase, we measured intracellular pH during growth using a cell-permeable pH-sensitive dye. We found that S. Typhimurium cells grown in LB were able to rapidly buffer their cytoplasm up to a more neutral pH during growth (Figure 5K), consistent with previously published measurements of intracellular pH during aerobic growth (Choi and Groisman, 2016). In contrast, cells grown in propionate buffered their cytoplasm at a significantly slower rate (Figure 5K). This cytoplasmic acidification is reminiscent of an acid-stress response in which Salmonella is unable to buffer its cytoplasm effectively (Choi and Groisman, 2016). Remarkably, S. Typhimurium grown in all conditions reached maximal growth rate at a similar, slightly acidic pH (Figure 5L). These data indicate that the significantly slower buffering of intracellular pH likely underlies the prolonged lag phase and reduced growth rate observed during propionate treatment.
Taken together, these data demonstrate that Bacteroides spp. production of propionate is necessary and sufficient to limit S. Typhimurium growth in vitro. Propionate inhibition of in vitro growth is a result of extended lag time and slower maximal growth rate, which is associated with disruption of intracellular pH homeostasis. Our study provides the first mechanistic insight into the mode of action of SCFAs as bacterial growth inhibitors.
Salmonella intestinal expansion in 129 mice does not depend on Type 3 Secretion System 1-mediated tissue invasion
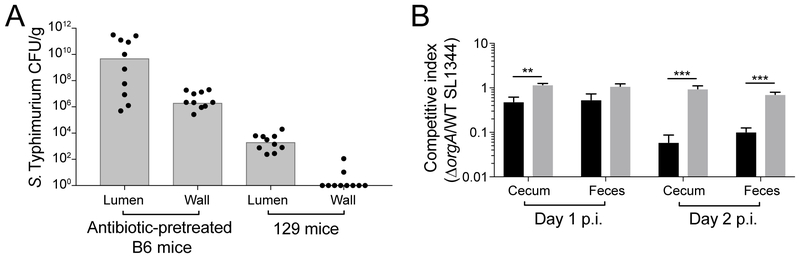
We next investigated whether propionate modulates Salmonella virulence in vivo as a mechanism of colonization resistance. Previous studies have shown that propionate can decrease Salmonella virulence by inhibiting expression of the Type 3 Secretion System 1 (T3SS1) (Hung et al., 2013). This macromolecular needle encoded on Salmonella pathogenicity island 1 (SPI-1) facilitates invasion into the distal gut epithelium by injecting effectors into host cells to mediate pathogen uptake (Hapfelmeier et al., 2005). In antibiotic-pretreated C57BL/6 mice, T3SS1-dependent invasion into host intestinal tissues such as the cecum is required for sustained intestinal S. Typhimurium expansion during early stages of infection (within the first 2 days) (Hapfelmeier et al., 2005). We have previously shown that the T3SS1 is required for high levels of S. Typhimurium colonization of the distal gut at 30 days p.i. of 129 mice (Lawley et al., 2007). However, the contribution of the T3SS1 during early stages of infection has not been investigated in our mouse model.
To compare the contribution of T3SS1-mediated tissue invasion to intestinal S. Typhimurium expansion during early stages of infection in antibiotic-pretreated C57BL/6 (B6) mice and in our 129 mouse model, we determined pathogen levels in luminal and gentamicin-treated tissue compartments of the cecum. Consistent with previously published data, we recovered high levels of extracellular S. Typhimurium in intestinal contents as well as high levels of intracellular pathogen in gentamicin-protected intestinal tissue in antibiotic-pretreated B6 mice (Figure 6A). In contrast, we did not recover any intracellular S. Typhimurium from within the cecal tissue of 129 animals despite significant pathogen burdens recovered in luminal contents (Figure 6A). These results suggest that contrary to its importance in antibiotic-pretreated mouse models, T3SS1-mediated invasion does not contribute to intestinal S. Typhimurium expansion at early stages of infection in 129 mice.
Figure 6. S. Typhimurium intestinal luminal expansion does not depend on Type 3 Secretion System 1-mediated gut tissue invasion.
A) 129 mice and antibiotic-pretreated B6 mice were infected with S. Typhimurium. CFU/g cecal contents and tissue at 1 day p.i. Bars represent the median. B) Antibiotic pre-treated B6 (black) and 129 (gray) mice were infected with a 1:1 mixture of wild type (WT) and ΔorgA SL1344. Data represent 2 independent experiments with 5 animals in each group. Error bars represent the standard error of the mean. Significance was calculated using an unpaired two-tailed t-test; **: p<0.01, ***: p<0.001. Unless noted, conditions are not significant.
We further supported this conclusion by infecting 129 mice and antibiotic-pretreated B6 mice with a 1:1 mixture of wild type and a T3SS1 mutant (ΔorgA) of S. Typhimurium. While the T3SS1 mutant was significantly outcompeted by wild type in antibiotic-pretreated B6 mice by 1 day p.i., the T3SS1 mutant was not significantly outcompeted by wild type in our 129 mouse model (competitive index ~1, Figure 6B). Taken together, these data demonstrate that the T3SS1 does not contribute to colonization of the distal gut during early stages of infection in 129 mice. Thus, the increased levels of propionate in the intestines of the B6N mice is not likely to mediate colonization resistance against S. Typhimurium through inhibition of T3SS1-mediated tissue invasion.
Bacteroides spp. production of propionate mediates colonization resistance against S. Typhimurium
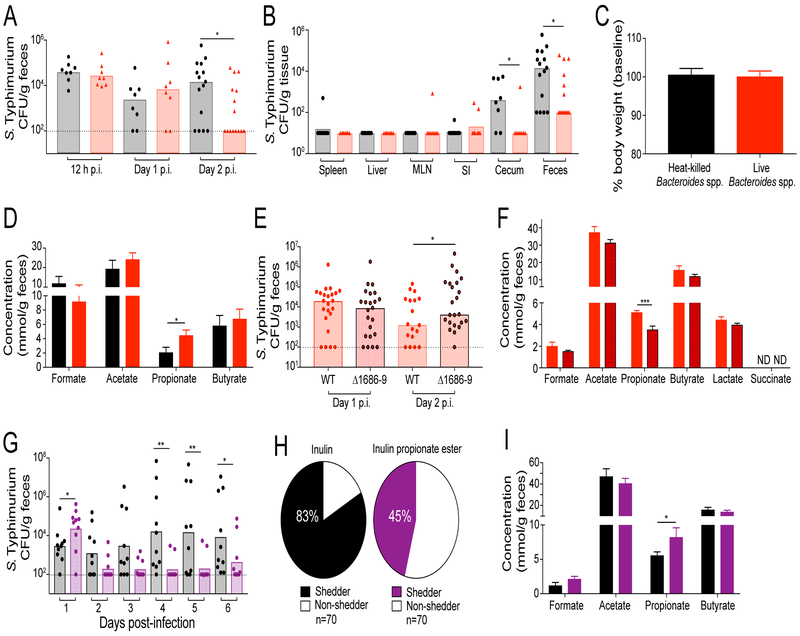
To determine whether Bacteroides spp. limit intestinal S. Typhimurium infection in vivo, we gavaged 129 mice with a cocktail of live or heat-killed Bacteroides spp. prior to infection with S. Typhimurium (Figure S5A). The Bacteroides spp. included human strains (B. ovatus, B. uniformis, B. acidifaciens) as well as mouse strains isolated from B6N mice (B. acidifaciens). By monitoring Bacteroides spp. abundance by plating, we found that live Bacteroides spp. stably colonized the 129 distal intestine of 129 mice (Figure S5B). Mice that received a live cocktail of Bacteroides spp. contained significantly less S. Typhimurium in the distal gut at 2 days p.i. compared to mice that received heat-killed Bacteroides spp. (Figure 7A–B).
Figure 7. Bacteroides spp. mediate colonization resistance against S. Typhimurium.
A-D) 129 mice were administered a cocktail of either heat-killed (black) or live (red) Bacteroides spp. prior to infection with S. Typhimurium. A) Fecal shedding. B) Tissue burdens at 2 days p.i. C) Host weight at 2 days p.i. D) Cecal content SCFA concentrations at 2 days p.i. Dotted lines represent the limit of detection. Data represent 2 independent experiments with 4–8 animals in each group. Error bars represent the standard error of the mean. E-F) 129 mice were administered live wild-type (red outline) or a cocktail of propionate-production mutants (black outline) of Bt prior to infection with S. Typhimurium. E) Fecal shedding at 2 days p.i. F) Cecal SCFA concentrations 2 days p.i. Data represent 2 independent experiments with 8–15 animals per group. G-I) 129 mice were treated daily with either 1% inulin (black) or 1% inulin propionate ester solution (purple) and infected with S. Typhimurium. G) Fecal shedding. H) The proportion of shedders in infected mice. Shedder/non-shedder proportions were quantified by combining scores of mice shedding at each time point. I) Cecal content SCFA concentrations at 7 days p.i. Data represent 2 independent experiments with 4–6 animals per group. For tissue burden, fecal shedding, and SCFA concentration measurements, significance was calculated using the two-tailed Mann-Whitney test, *: p<0.05, **: p<0.01. Unless noted, conditions are not significant.
One possible explanation for reduced levels of intestinal S. Typhimurium in mice that received live Bacteroides spp. is that these commensal bacteria enhance the host immune response to infection. To test this possibility, we characterized several immune metrics to assess overall changes in the host response to infection, immune pathways that are altered by Bacteroides spp. (Hooper et al., 2003), and immune responses crucial for control of S. Typhimurium infection (Dolowschiak et al., 2016; Godinez et al., 2008; Kulagina et al., 2006; Maier et al., 2014). These immunological analyses indicated that host weight, histology, antimicrobial peptide expression, immune cell population size, and pro-inflammatory cytokine production were similar in S. Typhimurium-infected mice that received heat-killed or live Bacteroides spp. (Figure 7C, S6). In contrast, the levels of propionate in 129 mice colonized with live Bacteroides spp. were significantly higher compared to mice gavaged with heat-killed Bacteroides spp. (Figure 7D). Taken together, these findings suggest that rather than indirect colonization resistance via engagement of host immune pathways, Bacteroides-derived propionate mediates colonization resistance through direct limitation of Salmonella growth.
To test whether Bacteroides spp. production of propionate is necessary to mediate colonization resistance against S. Typhimurium, 129 mice were gavaged with cocktails of either wild type or propionate-production mutant Bt (Figure S5C). Both wild type and propionate-production mutants stably colonized the distal intestine of 129 mice (Figure S5D). We found that S. Typhimurium fecal shedding was significantly lower in animals colonized with wild type Bt compared to the propionate-production mutants, which correlated with significantly higher levels of intestinal propionate (Figure 7E–F). To test whether propionate is sufficient to mediate colonization resistance against Salmonella, 129 mice were administered either an inulin propionate ester solution that has been shown to significantly increase levels of intestinal propionate or an inulin solution as a control (Figure S5E) (Chambers et al., 2015). Treatment with the inulin propionate ester significantly reduced S. Typhimurium fecal shedding compared to treatment with inulin alone (Figure 7G). Inulin propionate ester treatment also significantly reduced the proportion of hosts that shed pathogen (Figure 7H). Importantly, this protection was associated with a significant increase in intestinal levels of propionate, but no changes were observed in the levels of other SCFAs (Figure 7I). Taken together, these results demonstrate that Bacteroides spp. production of propionate mediates colonization resistance against intestinal Salmonella infection.
Discussion
Collectively, our results demonstrate that Bacteroides spp. limit intestinal S. Typhimurium expansion and fecal shedding without affecting systemic pathogen colonization or overall host health. Our data indicate that this colonization resistance is not mediated by enhancing the host antibacterial immune response or through inhibition of pathogen virulence. Rather, colonization resistance is due to the production of a commensal metabolite that directly limits pathogen growth. Overall, this study provides the first example of a colonization resistance mechanism identified from the unperturbed microbiota that highlights host protection from enteric infection by commensal metabolism.
Virtually all characterized mechanisms of colonization resistance to date have been described by observing the loss of colonization resistance in antibiotic-treated animal models in which the intestinal microbiota has been perturbed (Bäumler and Sperandio, 2016). We have identified a highly distinct mechanism explaining different levels of colonization resistance in unperturbed microbiotas, demonstrating the impact that a single metabolite can have on infection. The mechanism we identify has important implications for understanding the role of individualized microbial communities in person-to-person variability in pathogen transmission.
In this study, we leveraged the observation that intestinal infection varied in inbred mouse strains (Figure 1). Decades of scientific research have leveraged the genetic diversity of inbred mouse strains to identify host genes that regulate S. Typhimurium infection (Boehme, 1970; Hormaeche, 1979). In contrast, the role of the commensal microbiota during infection of these strains had not been characterized; our findings highlight microbiota composition as an important factor mediating divergent host outcome and infection dynamics in inbred mouse strains. We imagine that probing microbiota function using available ‘omics’ technologies may uncover additional mechanisms underlying infection heterogeneity. The general strategy of comparing host-microbe interactions across inbred mouse strains may prove effective for uncovering other microbiota-mediated mechanisms in the future. Additionally, our findings may offer insight into why and how certain human populations control Salmonella infections, while others do not.
While Bacteroides spp. and propionate production have been correlated with protection against Salmonella infection in the literature, the functional importance of these associations have not been deeply investigated. For example, one study comparing colonization resistance of conventionalized mice with a low-complexity microbiota (LCM) indicated that Bacteroides spp. were strongly correlated with colonization resistance, although this trend was not explored (Stecher et al., 2010). More recently, it was shown that a 12-member defined community of commensals provided some colonization resistance against Salmonella infection compared to germ-free mice (Brugiroux et al., 2016). Interestingly, this community included a Bacteroides species but its contribution to colonization resistance was not addressed (Brugiroux et al., 2016). Finally, Bacteroides spp. have been shown to speed intestinal clearance of a S. Typhimurium SPI-2 mutant after antibiotic treatment; fecal metabolomics identified a role for vitamin B6 in intestinal clearance through an unknown mechanism (Miki et al., 2017). Careful examination of the reported metabolomics results revealed that propionate levels were also statistically significantly associated with host colonization resistance, suggesting that the propionate-mediated colonization resistance mechanism we describe here could have contributed to this previously described phenotype.
It is increasingly clear that SCFAs have anti-pathogenic properties. However, the effects that have been described within a mammalian host are largely a result of indirect mechanisms of immune activation rather than direct limitation of growth (Jung et al., 2015; Peng et al., 2009). In addition, SCFAs are known to regulate Salmonella virulence programs (El-Gedaily et al., 1997, Huang et al., 2008, Van Immerseel, 2003). In this study, we demonstrated that Bacteroides spp. limitation of intestinal Salmonella expansion through production of propionate is mediated by direct limitation of pathogen growth (Figure 5–7). We were surprised to find that the magnitude of in vitro propionate-mediated growth inhibition we observed is quite large. If we imagine directly competing S. Typhimurium grown with and without the presence of propionate, the growth inhibition provided by propionate could compound to a 10 to 50-fold reduction in bacterial fitness over time. This difference is recapitulated in our in vivo experiments, where we observed a 10 to 100-fold decrease in S. Typhimurium levels in animals colonized with Bacteroides spp. or treated with an inulin propionate ester.
It has long been understood that SCFAs can directly inhibit bacterial growth in vitro, but the underlying mechanism(s) of this inhibition have been largely mysterious (McHan and Shotts, 1993). SCFAs freely diffuse across bacterial membranes; once inside the cytosol, they dissociate into anions and protons, leading to inhibition of cellular growth (Ricke, 2003). Here, we show that propionate inhibits S. Typhimurium growth by significantly extending lag time and growth rate at the population and single-cell levels. This phenotype is distinct from other described increases in lag time that are associated with an increased fraction of dormant cells (Peters et al., 2016). Our findings suggest that when intracellular concentrations of propionate are high, cells are unable to raise their internal pH to facilitate cellular functions required for growth, leading to an extended lag time (Figure 5).
Bacteroides spp. metabolize succinate through enzymatic steps culminating in the production of propionate as well as ATP and CO2, which are crucial for Bacteroides to survive in carbon-limiting conditions like the intestinal lumen (Dimroth and Schink, 1998; Reichardt et al., 2014). Bacteroides spp. release propionate into the environment rather than utilize the metabolite for growth; the genomes of sequenced Bacteroides spp. do not contain genes homologous to known propionate utilization loci (Hammelman et al., 1996). Furthermore, human studies show direct correlations between Bacteroides spp. abundance and propionate levels (Salonen et al., 2014). Taken together, these observations are consistent with propionate as a byproduct of Bacteroides metabolism. Viewed in this light, our work presents a novel example in which a byproduct of normal commensal metabolism provides colonization resistance against invading enteric pathogens.
Propionate and other SCFAs have been utilized to control enteric pathogen infection in agricultural animals since the 1960s, and have even been proposed as a dietary supplement alternative to antibiotic treatment (Ricke, 2003). We demonstrated that the addition of Bacteroides spp. to mice leads to propionate production that is sufficient to limit pathogen expansion (Figure 7). Moreover, we showed that the prebiotic inulin propionate ester is sufficient to limit Salmonella fecal shedding, establishing that propionate mediates colonization resistance (Figure 7). Overall, these findings suggest that the addition of live Bacteroides spp., other SCFA-producing commensals, or even SCFA-related prebiotics to agricultural animals or humans may alter the intestinal environment to help control spread of Salmonella and perhaps other related enteric Enterobacteriaceae pathogens.
STAR Methods
Ethics Statement
Experiments involving animals were performed in accordance with NIH guidelines, the Animal Welfare Act, and US federal law. All animal experiments were approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC) and overseen by the Institutional Animal Care and Use Committee (IACUC) under Protocol ID 12826. Animals were housed in a centralized research animal facility accredited by the Association of Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
Mouse Strains and Husbandry
C56BL/6 nramp-1+/+ male mice were a generous donation from Dr. Greg Barton at the University of California Berkeley (Arpaia et al., 2011). C57BL/6 nramp-1+/+ male mice were crossed with wild-type C57BL/6 nramp-1−/− female mice from Jackson Laboratories. Embryos were transferred into specific pathogen-free CD1 fosters. C57BL/6 nramp-1+/− heterozygous pups were crossed to generate a colony of homozygous C57BL/6 nramp-1 +/+ animals that were verified by genotyping. 129X1/SvJ mice were obtained from Jackson Laboratories or an in-house 129X1/SvJ colony. Male and female mice of both gentoypes (6–14 weeks old) were housed under specific pathogen-free conditions in filter-top cages that were changed bi-monthly by veterinary or research personnel. Sterile water and food were provided ad libitum. Mice were given at least one week to acclimate to the Stanford Animal Biohazard Research Facility prior to experimentation.
Mouse age, sex, lineage, and source facility were tracked for all experiments and did not track with infection outcomes.
Genotyping
A 514-base pair fragment of the nramp-1 gene was amplified by PCR from tail snips and amplified by PCR, purified, and sequenced using primers listed in Table S2. nramp-1 status was determined based on the G169D amino acid substitution (Brown et al., 2013).
Bacterial Strains and Growth Conditions
For all mouse infections, S. Typhimurium strains were maintained aerobically on LB agar supplemented with 200 μg/mL streptomycin (LB-strep) or 200 μg/mL streptomycin + 15 μg/mL tetracycline (LB-strep-tet) and grown aerobically overnight at 37 °C with aeration in LB-strep broth. Bacterial cultures were spun down and washed with sterile phosphate-buffered saline (PBS) before resuspension for infection. Prior to in vitro growth curve experiments, S. Typhimurium was maintained anaerobically on LB-strep plates and grown anaerobically for 24 h at 37 °C in LB broth without shaking.
Listeria monocytogenes was maintained anaerobically on Brain Heart Infusion (BHI) plates and grown anaerobically for 24 h at 37 °C BHI broth without shaking.
Clostridioides difficile was maintained anaerobically on BHI-S (BHI+ 0.5% yeast extract + 0.1% cysteine) plates and grown anaerobically for 24 h at 37 °C in BHI-S broth without shaking.
Yersinia pseudotuberculosis was maintained anaerobically on 2xYT plates and grown anaerobically for 24 h at 37 °C in 2xYT broth without shaking.
We isolated two Bacteroides acidifaciens strains from the feces of C57BL/6 nramp-1+/+ mice used in this study. Two fresh fecal pellets from mice were collected directly into 500 μL PBS. Pellets were homogenized, and serial dilutions were plated in an anerobic chamber onto pre-reduced BHI-BA plates supplemented with 100 μg/mL gentamicin (BHI-BA-gent). Plates were incubated anaerobically at 37 °C for 72 h and individual colonies were streaked onto BHI-BA plates. Plates were incubated anaerobically 37 °C for 48 h and individual colonies were grown for 24 h in TYG broth supplemented with 500 μg/mL cysteine. Genomic DNA was isolated from cultures, and a 16S rRNA gene segment was amplified using the primers listed in Table S2 and sent for DNA sequencing to identify the taxonomy of each strain.
Commensals were maintained and grown as follows: Bacteroides spp. on BHI-BA plates and in TYG broth supplemented with 500 μg/mL cysteine; Prevotella copri on BHI-BA plates and PYG broth; Rikenella microfusis on RCM plates and RCM broth supplemented with 0.05% agar; Clostridiaceae sp. DT4 on RCM plates and RCM broth. Commensals were grown anaerobically at 37 °C standing for 24–72 h without shaking to stationary phase before being utilized for in vitro experiments. For animal experiments, Bacteroides spp. were grown anaerobically at 37 °C standing for 24 h without shaking, spun down and resuspended in 1 mL pre-reduced PBS. Heat-killed Bacteroides spp. were incubated in PBS for 20 min at 95 °C. Heat-killed and live commensal cocktails were pooled and resuspended in PBS for infection.
Published Bt strains were generated in a thymidine kinase mutant (Δtdk) background (Kovatcheva-Datchary et al., 2015). Expression of thymidine kinase was restored by chromosomally integrating a pNBU2 plasmid containing the tdk gene. Chromosomal insertion. Bt strains were incubated aerobically with an S17λ helper E. coli strain carrying pNBU2-tdk for 24 h prior to anaerobic incubation for 48 h on BHI-BA plates supplemented with gentamicin and erythromycin. Bt propionate production was restored by chromosomally integrating a pNBU2 plasmid containing BT1684–1689 as well as 200bp up- and down-stream of the operon containing predicted promoter region. Bt mutant strains were incubated aerobically with an S17λ helper E. coli strain carrying pNBU2-1684–89 for 24 h prior to anaerobic incubation for 48 h on BHI-BA plates supplemented with gentamicin and tetracycline. All Bt clones were screened by PCR for chromosomal insertion using primers described in Table S2. Complemented strains were functionally assessed by measuring SCFA levels in culture supernatants.
Fecal Microbiota Transplantation Experiments
6–10 week-old male and female 129X1SvJ mice were housed in cage setups with autoclaved bedding, food, and water. Mice were administered an ANVM (1 g/L ampicillin, 0.5 g/L vancomycin, 1 g/L neomycin, 1 g/L metronidazole in MilliQ water) antibiotic cocktail for 2–4 weeks. Ablation of the resident microbiota was assessed by aerobic plating on BHI plates supplemented with 10% horse blood (BHI-BA plates). The antibiotic cocktail was removed 12 h prior to transplantation. 6–14 week-old male and female 129X1/SvJ and C57BL/6 nramp1+/+ mice were used as fecal microbiota transplant donors. Fresh fecal pellets were collected from donor mice and homogenized in sterile PBS (2 pellets/mL). Fecal suspensions were immediately gavaged into recipient mice (100 μL/recipient). Animals were transplanted for three consecutive days followed by a 4-week equilibration period prior to infection.
Cohousing Experiments
6–10 week-old female 129X1/SvJ mice were housed together (four mice per cage) or cohoused with 6–10 week-old female C57BL/6 nramp1+/+ mice (two of each genotype per cage). Animals were housed for 4 weeks prior to infection.
Bacteroides spp. Gavage Experiments
Prior to gavage with commensals, 6–14 week-old male and female 129X1/SvJ mice were sampled for baseline Bacteroides abundance in the feces. 1–2 fresh fecal pellets were collected into tubes and resuspended in 500 μL PBS. Fecal contents were weighed and serial dilutions were plated on BHI-BA-gent plates. CFU/g feces were determined by counting gentamicin-resistant colonies >1mm in size after 48 h of anaerobic growth at 37 °C. For Bacteroides cocktail experiments, mice were gavaged with a cocktail of heat-killed or live Bacteroides spp. including human and mouse isolates of B. ovatus, B. uniformis, and B. acidifaciens (100 μL/mouse containing 107-108 CFU of each commensal). For Bt experiments, mice were gavaged with 108 CFU wild type or a cocktail of propionate-production mutant Bt. Bacteroides spp. abundance was tracked in the feces during a 7-day equilibration period to assess colonization prior to infection as well as throughout the course of infection.
Mouse Infections
Mice were infected with 107-108 CFU S. Typhimurium by oral gavage. For competitive index experiments, mice were infected with a 1:1 mixture of 108 CFU of each S. Typhimurium strain by oral gavage. Individual mice were distinguished by tail markings, and weight was tracked throughout the duration of infection. 1–2 fresh fecal pellets were collected into tubes and resuspended in 500 μL PBS. Fecal contents were weighed and CFU/g feces were determined by plating serial dilutions on LB plates supplemented with 200 μg/mL streptomycin.
For terminal experiments, mice were sacrificed at the indicated time points. Mice were euthanized with carbon dioxide and blood was collected in heparin solution by cardiac puncture. Sterile dissection tools were utilized to collect spleen, liver, mesenteric lymph nodes (MLN), small intestine, cecum, and colon. For tissue invasion assays, luminal cecal contents were removed and cecal tissue was washed twice with PBS. Cecal tissue was incubated in RPMI+100 μg/mL gentamicin for 45 min at room temperature and washed twice in PBS. Tissues were collected in 1–3 mL PBS and homogenized. Homogenates were plated as serial dilutions on LB agar supplemented with 200 μg/mL streptomycin to enumerate CFU/g tissue. Infections were conducted with 5–10 animals per group as indicated. Each experiment was conducted twice to ensure reproducibility.
16S rRNA Gene Sequencing
Fecal samples were collected from fecal microbiota transplant and cohousing experiment mice at baseline prior to infection (day 0) as well as weekly throughout infection (day 1, 7, 14, 21). Samples were maintained at −80°C prior to fecal DNA extraction using MoBio PowerSoil DNA Isolation Kits. The V4 region of the 16S rRNA gene was amplified using Illumina 515F and 806R barcoded primers (5PRIME HotMasterMix). PCR products were cleaned (MoBio UltraClean PCR Clean-Up Kit) and quantified in duplicate (Quant-iT High-Sensitivity dsDNA Assay Kit). Amplicons were pooled, ethanol precipitated, and submitted for sequencing. Paired-end sequencing was performed on the Illumina MiSeq platform at the Mayo Clinic Medical Genome Facility.
Analysis of 16S Gene Sequencing
Sequence quality was assessed using QIIME v. 1.9.1 (Caporaso et al., 2010). 12,951,465 reads were produced by the MiSeq run, of which 9,284,642 passed default quality filtering in split_ libraries.py. FastQC v0.11.4 (http://www.bioinformatics.babraham.ac.uk/ projects/fastqc/) was also used to inspect per tile and per base quality. Sequences that passed quality filtering were clustered using an open-reference OTU-picking approach implemented in pick_open_reference_otus.py. Taxonomy was assigned using UCLUST (Edgar, 2010) against the GreenGenes training set v. 13.8 (McDonald et al., 2012; Werner et al., 2012). A phylogenetic tree was constructed from a PyNAST alignment using FastTree (Price et al., 2010). After OTU picking, the sequence table contained 1,015 distinct OTUs. OTUs present in <2 samples were removed such that the final filtered table contained 334 samples and 8,927,541 total reads (68.9% of input sequences). Prior to analyses, the OTU table was rarefied to 10,000 sequences/sample. α–diversity was calculated in QIIME with alpha_diversity.py and unweighted uniFrac β–diversity was calculated with beta_diversity_through_plots.py. Principal coordinate analysis (PCoA) plots were rendered with Emperor (Vázquez-Baeza et al., 2013). Differences in taxonomic abundance were determined with group_significance.py and significance was determined with the Kruskall-Wallis test using Bonferroni-corrected p-values. Commensals of interests were identified using supervised_learning.py, which implements a random forest classifier. Analysis metrics are indicated at the L6 and L7 taxonomic level for Bacteroidales spp. Metrics for fecal microbiota transplant and cohousing experiments include mean decrease in accuracy for each taxonomic group when removed from the analysis and the rank of predictive power.
Organic Extractions of Commensal Supernatants
Stationary phase commensal cultures were frozen at −20 °C degrees prior to use. For each growth curve experiment, one biological replicate of a commensal culture was thawed and centrifuged at 6,000 rpm for 5 min. Culture supernatant was mixed 1:1 with ethyl acetate. Organic extraction was vortexed and centrifuged at 1,000 rpm for 10 min. The organic phase was removed and evaporated using a SpeedVac. Organic extract pellets were resuspended in culture medium (LB, BHI, or BHI-S), filter-sterilized, and pre-reduced prior to use. One biological replicate culture was prepared for an entire growth curve culture such that the same extract was sampled for S. Typhimurium growth at 2, 4, 8, and 24 h post inoculation. For Bt extract experiments (Figure 5B, S4E-G), organic extract pellets were resuspended in LB at a concentration normalized to Bt CFU/mL values of extracted cultures (the wild-type organic extract was resuspended at 1X concentration, while the mutant extract was resuspended at 2.4X concentration to normalize for the 2.4X growth defect).
SCFA Concentration Measurements
For fecal SCFA measurements, 75–150 mg frozen cecal contents were thawed, homogenized in water, and centrifuged at 2350 rpm at 4 °C for 30 s. For bacterial cultures, cells were grown anaerobically in media for 24 h, removed from the anaerobic chamber, and centrifuged at 800 rpm for 5 min. Standard curves of SCFAs and other relevant acids (formate, acetate, propionate, butyrate, lactate and succinate) were prepared by dissolving sodium salts of acids at concentrations of 20, 2, 0.2 and 0.02 mM in water. All samples were kept on ice during sample preparation. Samples were acidified with HPLC-grade 37% HCl and vortexed to mix. An organic extraction was performed twice on acidified samples with diethyl ether. Samples were derivatized with MTBSFTA (Sigma) and incubated for 30 min at 60 °C prior to quantification using gas chromatography-mass spectroscopy (GC-MS). Samples were run on an Agilent (HP) 7890/5975 single quadrupole GC-MS. Using a 7683B autosampler, 1 μL split injections (1:100) were made onto a DB-5MSUI capillary column (30 m length, 0.25 mm ID, 0.25 μm film thickness; Agilent) using helium as the carrier gas (1 mL/min, constant flow mode). The inlet temperature was 200 °C and the transfer line temperature was 300 °C. GC temperature was held at 60 °C for 2 min, ramped at 40° C/min to 160 °C, then ramped at 80 °C/min to 320 °C and held for 2 min; total run time was 8.5 min. The mass spectrometer used electron ionization (70 eV) and the scan range was m/z 50–400, with a 3.75 min solvent delay. Retention times were as follows: formate, 3.92 min; acetate, 4.22 min; propionate, 4.69 min; butyrate, 5.08 min; lactate, 6.34 min; succinate, 6.98 min. Peak area integration was determined for each major peak in sample spectra. Standard curves were generated for each molecule of interest by linear regression and unknown concentrations in samples were determined by interpolating the peak area integration of each molecule of interest based on standard curves for that molecule.
In Vitro Growth Curve Experiments
Bacteria were grown for 24 h under anaerobic conditions and diluted 1:100 to begin growth curve experiments. Samples were taken at 2, 4, 8, and 24 h of growth and plated to determine CFU/mL. Each experiment was conducted with 3–5 replicates per condition, and experiments were conducted a total of 3 times.
For propionate growth curve experiments, strains were grown on LB-strep plates. Single colonies were inoculated into 3mL LB broth anaerobically and grown at 37 °C without shaking overnight. To measure growth dynamics from stationary phase, a 1:200 dilution of overnight culture was inoculated into 200 uL of medium. Medium was supplemented with varying concentrations of propionic acid (1.56–100mM). The plate was covered with an optical film, with small holes poked at the side of each well to allow aeration. Incubation and OD measurements were performed with an Epoch 2 plate reader (BioTek) at 37 °C with continuous shaking and OD600 was measured at 5-min intervals for 24 h. The plate reader was contained within an anerobic chamber. Blank values were subtracted from OD measurements and the derivative of ln(OD) with respect to time was computed. The maximal growth rate was calculated by finding the peak of the smoothed derivative of ln(OD) with respect to time. The duration of lag phase was defined as the time at which a culture first reached maximal growth rate.
For extracellular pH experiments, pH was determined for LB broth supplemented with varying concentrations of propionate. pH-matched LB solutions were made by mixing different amounts of HCl, and pH was measured with pH strips. For intracellular pH experiments, the ratiometric dye BCECF-AM (1 μM, Life Technologies) was added to culture conditions. BCECF-AM is cell-permeable, and only fluorescent when modified in the cytoplasm. Intracellular pH was determined by the ratio of emission from 485 and 440 spectra. To generate a standard curve for intracellular pH measurements, LB broth solutions were made at fixed pH (pH=2–13) by mixing different amounts of HCl and pH was measured with pH strips. S. Typhimurium was grown anaerobically overnight as described above and diluted 1:10 into conditions with 1 μM BCECF-AM and 50 μM CCCP. CCCP depolarizes the membrane, allowing for equilibration of extracellular and intracellular pH, rendering measurements suitable for the generation of a calibration curve. The emission ratio of BCECF-AM was measured over the course of 5 hours to allow the dye to equilibrate. The ratio of λem485 to λem440 was measured and used to generate a standard curve. The ratiometric measurements of BCECF-AM under different concentrations of propionate and extracellular pH were converted to absolute intracellular pH values by performing linear interpolation to the standard curve measurements obtained previously.
Single Cell Imaging
S. Typhimurium was grown anaerobically overnight prior to spotting on either an LB agar pad as a control or a pad supplemented with 10 mM propionic acid. Cells were imaged every minute for 4–6 h at 37 °C. Phase-contrast images were acquired with a Nikon Ti-E inverted microscope (Nikon Instruments) using a 100X (NA 1.40) oil-immersion objective and a Neo 5.5 sCMOS camera (Andor Technology). The microscope was outfitted with an active-control environmental chamber for temperature regulation (HaisonTech, Taipei, Taiwan), and was housed in the same anaerobic chamber used to grow cultures. Images were acquired using μManager v.1.4 (www.micro-manager.org) (Edelstein et al., 2001).
The MATLAB (Mathworks, Natick, MA, USA) image processing code Morphometrics (Ursell et al., 2017) was used to segment cells and to identify cell outlines from phase-contrast microscopy images. A local coordinate system was generated for each cell outline using a method adapted from MicrobeTracker (Sliusarenko et al., 2011). Local cell widths were calculated as the distances between contour points perpendicular to the cell midline. The cell volume is defined as the sum of the volumes of cylinders defined by these meshlines, assuming cylindrical symmetry about the long axis of the cell. Instantaneous elongation rate was calculated as the rate of change of area normalized to initial cell area (dA/Adt). The lag time was computed as the time for each cell to perform its first division (τd).
Generation of Inulin Propionate Ester
Inulin propionate ester was synthesized following a previously published procedure for inulin esterification (Hartzell et al., 2013). Inulin (20 g, 0.123 mol with MW 162.15/fructose unit, degree of polymerization=2–60), propionic anhydride (18.3 g, .014 mol), 1-methyl imidazole (10.1 g, 0.123 mol) was combined in 60mL DMSO and stirred at room temperature for 4 h. The mixture was diluted with 120mL water. The reaction mixture was dialyzed against water for 2 days at 4°C. Following dialysis, samples were freeze-dried. The degree of ester formation was calculated through comparison of the proton NMR of inulin and the esterified product. Inulin has no alkyl groups in the 1–2ppm range where the methyl group of propionic esters are found. Although the NMR peaks of carbohydrate oligomers vary, examination of the 1–2ppm region and calculation of a ratio of the remaining protons in the 3.5–5.5ppm range determined that the esterified inulin contained an average of 0.7 propionate groups per fructose unit. Bruker 500-MHz spectra in d6DMSO: inulin 1H NMR (500 MHz, DMSO-d6) δ 5.29 – 5.22 (m, 1H), 4.80 (d, J = 6.5 Hz, 1H), 4.70 (q, J = 8.1, 5.9 Hz, 1H), 4.08 (ddt, J = 11.7, 7.7, 4.5 Hz, 1H), 3.89 (q, J = 7.2 Hz, 1H), 3.75 – 3.65 (m, 2H), 3.57 (dd, J = 21.2, 8.7 Hz, 3H), 2.59 (q, J = 1.8 Hz, 1H); propionylated inulin 1H NMR (500 MHz, DMSO-d6) 3.7–5.6 (m, 8H), 2.2 – 2.5 (m, 3H), 1.0 – 1.1 (m, 3H).
For animal experiments, mice were orally gavaged with 200 μL 1% inulin or 1% inulin propionate ester daily starting the day prior to infection. On the day of infection, animals were administered solutions 4 h prior to infection. Starting at 1 dpi, animals were administered solutions 4 h prior to fecal sampling.
Histological Analysis
Portions of the spleen, liver, small intestine, cecum and colon were harvested for histology. Tissues were fixed in 10% buffered neutral formalin, routinely processed, embedded in paraffin, sectioned and stained with hematoxylin and eosin. Blinded slides were scored by a veterinary pathologist and scores represent a combination of scores of inflammation severity and distribution for each tissue.
Flow Cytometry
Spleens from mice were perfused with digestion buffer (HBSS+Ca2++Mg2+ + 40 μg/mL DNase + 50 μg/mL Liberase TL), dissociated with forceps, and incubated at 37 °C for 15 min. EDTA was added at a final concentration of 0.5 M and incubated at 37 °C for 5 min. to halt digestion. Single cell suspensions were passed through a 70- μm filter and washed with FACS buffer (PBS + 2% fetal bovine serum (FBS) + 2 mM EDTA). Red blood cells were lysed with ACK Lysis Buffer for 2 min at room temperature and washed once with FACS buffer before staining. All single-cell suspensions were centrifuged at 4 °C at 400g for 5 min.
Colons were harvested from mice and feces and mesenteric fat were removed. Colons were opened and washed in PBS, and then minced into 0.5-cm pieces and washed with HBSS + 1 mM DTT at 37 °C with shaking at 200 rpm for 15 min. Wash buffer was decanted and colons were washed with HBSS + 5% FBS + 25 mM HEPES at 37 °C at 200 rpm for 30 min. Wash buffer was decanted, and colon pieces were further minced into 1 mm pieces and incubated in RPMI + 5% FBS + 0.167 mg/mL Liberase TL + 0.25 mg/mL DNase at 37 °C at 200 rpm for 30 min. Colon pieces were mashed through a 100- μm filter and passed through a 70- μm filter. Single-cell suspensions were washed in FACS buffer before staining. All single cell suspensions were centrifuged at 4 °C at 400g for 5 min.
For surface cell marker staining, single-cell suspensions were incubated in Fc Block (TruStain fcx anti-mouse CD16/32) at room temperature for at least 10 min and washed with PBS before staining. Cells were stained on ice for 30 min in PBS with a surface marker antibody cocktail of Live/Dead Fixable Blue Viability Dye, CD45-Brilliant Violet 510, CD19-Alexa Fluor 780, CD11b-Brilliant Violet 785, CD11c-PE-Cy7, Ly6G-BUV 395, Ly6C-APC, CD3e-Pac Blue, NK1.1-e650 NC, and CD64-PE, or with CD4 antibodies for splenocyte compensation controls. Cells were washed with FACS buffer and fixed at room temperature for 10 min with a Cytofix/Cytoperm Kit. Cells were washed with Cytofix/Cytoperm buffer and FACS buffer before being run on an LSR Fortessa cytometer. Data were acquired with DIVA software and analyzed using FlowJo v. 10.0.8 software using the gating strategy shown in Figure S7. The percentage of intact, live CD45+ immune cells in each sample was utilized for downstream analyses. Cell populations were gated as described in Figure S7.
RNA Isolation and Gene Expression
Small intestine and cecal tissue were isolated from mice and stored at −80 °C until use. Tissue was homogenized by bead beating and RNA was isolated using an RNeasy Mini Kit. cDNA was synthesized from RNA samples (SuperScript III First-Strand Synthesis Kit) and gene-specific primers (Table S2) were used to amplify transcripts (FastStart Universal SYBR Green Master Mix).
Statistics
Prism was used to create all figures and perform statistical analyses for S. Typhimurium infections. Unpaired Mann-Whitney tests were used to compare CFU burdens in tissues and feces in all infection experiments. Significance was defined by as indicated in figure legends.
All statistical analyses of microbiota composition were performed using QIIME. Group significance analyses were performed using default parameters. Supervised learning analyses were conducted using the random forest classifier with 10-fold cross-validation and default parameters.
Competitive Index (CI) values were calculated as previously described (Beuzón and Holden, 2001).
Supplementary Material
Related to Figure 2. Experimental design of fecal microbiota transplant and cohousing experiments. A) 129 mice were administered a cocktail of antibiotics in the drinking water prior to fecal microbiota transplant (FMT) with either 129 or B6N microbiotas. Mice were given FMTs for three consecutive days after antibiotic treatment. FMTs were allowed to stabilize for 4 weeks prior to S. Typhimurium infection. B) 129 mice were housed with their own genotype (control housed) or with B6N mice (cohoused) for 4 weeks prior to S. Typhimurium infection.
Related to Figure 3. 16S rRNA gene sequencing diversity analyses on fecal genomic DNA isolated from FMT and cohousing experiments on days 0, 7, 14, and 21 postinfection. A-B) α- diversity is the same between 129 mice receiving 129 (red) and B6N (blue) microbiotas across rarefaction depths, for both observed OTUs (top) and Faith’s Phylogenetic Diversity (bottom, PD_whole_tree). B-F) β-diversity PCoA plots of 129 mice receiving 129 or B6N FMTs. B) Samples separate well by microbiota received. Black=129 microbiota received, red=B6N microbiota. C) Samples do not separate by infection status. White=uninfected, black=infected. D) Samples do not separate by shedding status. White=low shedder (<106 CFU/g feces), gray=moderate shedder (106-108 CFU/g feces), black=supershedder (>108 CFU/g feces). E) Samples do not separate by day postinfection. White=day 0, light gray=day 7, dark gray=day 14, black=day 21. F-I) PCoA plots of control or cohoused 129 mice. F) Samples separate well by microbiota received. Black=129 microbiota received, red=B6N microbiota. G) Samples do not separate by infection status. White=uninfected, black=infected. H) Samples do not separate by shedding status. White=low shedder (<106 CFU/g feces), gray=moderate shedder (106-108 CFU/g feces), black=supershedder (>108 CFU/g feces). I) Samples do not separate by day post-infection. White=day 0, light gray=day 7, dark gray=day 14, black=day 21.
Related to Figure 3. 16s rRNA gene sequencing abundance analysis of fecal genomic DNA isolated from FMT and cohousing experiments on days 0, 7, 14, and 21 postinfection. A) There is not a significant difference in the abundance of Enterobacteriaceae spp. prior to infection between mice receiving 129 vs. B6N microbiotas in FMT experiment. Significance was calculated using the Kruskall-Wallis test; NS: not significant. B-C) The abundance of Bacteroidales spp. identified by supervised learning as predictive features of B6N microbiotas: family S24–7, genus Odoribacter. D-I) Abundance dynamics of Bacteroidales spp. identified by supervised learning as predictive features of B6N microbiotas in 129 animals receiving either a 129 or B6N FMT: D) Bacteroides, E) Prevotella, F) Rikenellaceae, G) B. ovatus, H) B. uniformis, I) B. acidifaciens. Black=129 microbiota received, red=B6N microbiota received. J-O) Abundance dynamics of Bacteroidales spp. identified by supervised learning as predictive features of B6N microbiotas in control or cohoused 129 mice: J) Bacteroides, K) Prevotella, L) Rikenellaceae, M) B. ovatus, N) B. uniformis, O) B. acidifaciens. Black=control housed, red=cohoused. Error bars represent standard error of the mean. Dotted lines represent the limit of detection.
Related to Figures 4 and 5. Anaerobic growth curves of bacterial growth in rich medium supplemented with organic extracts of supernatants isolated from anaerobically-grown commensal bacteria. A) Schematic of experimental approach for production of organic extracts of supernatants isolated from anaerobically grown commensal bacteria. B-C) S. Typhimurium was grown in LB broth without and with organic extracts. CFU/mL were measured by plating at 2, 4, 8, and 24 h. B. ovatus=Bacteroides ovatus, B. uniformis=Bacteroides uniformis, B. acidifaciens=Bacteroides acidifaciens, P. copri = Prevotella copri, R. microfusis=Rikenella microfusis. All extracts were resuspended at 1× concentration. D) Propionate concentration in supernatants from Bacteroides spp. cultures grown for 24 h. Data represent three independent cultures. E) SCFA concentrations in supernatants from wild-type, propionate-production mutant, and complemented Bt cultures. Propionate is the only SCFA with differential production between WT, mutants, and complemented strains. Significance was calculated using a two-tailed Mann-Whitney test; NS: not significant, ****: p<0.0001. Unless noted, conditions are not significant. F) Bt CFU/mL after 24 h of growth, utilized for normalization of S. Typhimurium growth in Figure 5B, S4. Data represent 3 independent experiments with 3–5 replicates. Error bars represent the standard error of the mean. G) S. Typhimurium CFU/mL are significantly decreased in LB supplemented with organic extracts from Bt producing propionate, but not in propionate-production mutants or in the ethyl acetate negative control. Extract concentration was normalized to Bt CFU/mL. Data represent 3 independent experiments with 4 replicates. Error bars represent the standard error of the mean. H) S. Typhimurium anaerobic growth curves in which LB broth was pHmatched to pH of propionic acid at conditions indicated in Figure 5C-E. S. Typhimurium displays similar growth in pH-matched LB solutions and LB control. For commensal extract growth curve experiments and SCFA concentrations, significance was calculated using a two-tailed Mann-Whitney test. For CFU/mL calculations, significance was calculated using an unpaired two-tailed t test. NS: not significant, *: p<0.05, **: p<0.01, ***: p<0.001. Unless noted, conditions are not significant.
Related to Figure 7. A-B) 129 mice were administered a cocktail of either heat-killed or live Bacteroides spp. (B. ovatus, B. uniformis, B. acidifaciens) 7 days prior to infection with S. Typhimurium. A) Experimental design. B) Bacteroides spp. fecal shedding is increased in mice administered a live cocktail of Bacteroides spp. (red) but not in mice administered a heat-killed cocktail (black). C-D) 129 mice were administered live wild type or a cocktail of propionate-production (Δ1686–89) Bt 7 days prior to infection with S. Typhimurium. C) Experimental design. D) Bt fecal shedding was similar between mice administered wild-type Bt (red) and propionate production mutants (black). E) Experimental design in which 129 mice were treated daily with either 1% inulin or 1% inulin propionate ester solution and infected with S. Typhimurium. For fecal shedding experiments, significance was calculated using a two-tailed Mann-Whitney test; ***: p<0.001, ****: p<0.0001. Unless noted, conditions are not significant.
Related to Figure 7. 129 mice were administered heat-killed (black) or live (red) Bacteroides spp. 7 days prior to infection with S. Typhimurium. Mice were sacrificed at 2 days post-infection for histological, expression, and flow cytometry analysis. A) Histopathology of spleen, liver, small intestine, cecum, and colon was scored. B-C) RNA from B) small intestine and C) cecal tissue was isolated and antimicrobial peptide gene expression was measured relative to GAPDH expression. mBD3=mouse β-defensin 3, Ang4=angiogenin 4. D-G) Flow cytometric analysis from D-E) spleen and F-G) colon tissue. D, F) CD19+ B cells, CD3ε+ T cells, and NK1.1+ NK cells were quantified as a percentage of total CD45+ immune cells. E,G) CD11chi dendritic cells, CD11c- CD11b+ mononuclear phagocytes, and CD11c- CD11b+ Ly6G+ neutrophils were quantified as a percentage of total CD45+ immune cells. H) RNA from cecal tissue was isolated and cytokine gene expression was measured relative to GAPDH expression. Data represent two independent experiments with 7–8 animals in each group. Error bars represent standard error of the mean. Significance was calculated using the two-tailed Mann- Whitney test; no comparisons were significant.
Related to Figure 7. 129 mice were administered heat-killed or live Bacteroides spp. 7 days prior to infection with S. Typhimurium. Mice were sacrificed at 2 days post-infection for flow cytometry analysis. Single-cell suspensions were stained with antibodies. Cells were gated on forward and side scatter to exclude debris and select for single cells. A hierarchical gating strategy was used on live cells to identify B cells, T cells, NK cells, dendritic cells, mononucleocytes, and neutrophils as depicted.
Supplementary Tables
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| S. Typhimurium SL1344, StrepR | Monack Lab strain collection | N/A |
| S. Typhimurium SL1344 ΔorgA, StrepR/TetR | Monack Lab strain collection | N/A |
| Listeria monocytoqenes 10403S | Monack Lab strain collection | N/A |
| Clostridioides difficile 630 | Sonnenburg Lab strain collection | N/A |
| Yersinia pseudotuberculosis IP2666 | Monack Lab strain collection | N/A |
| Bacteroides ovatus | ATCC | 8483 |
| Bacteroides uniformis | ATCC | 8492 |
| Bacteroides acidifaciens | DSMZ | 15896 |
| Bacteroides acidifaciens, mouse isolate 1 | This study, C57BL/6 Nramp+/+ feces | N/A |
| Bacteroides acidifaciens, mouse isolate 2 | This study, C57BL/6 Nramp+/+ feces | N/A |
| Bacteroides thetaiotaomicron VPI Δtdk | Sonnenburg Lab strain collection | N/A |
| Bacteroides thetaiotaomicron VPI Δ1686–1689 Δtdk, clone 1.2 | Kovatcheva-Datchary et al., 2015 | N/A |
| Bacteroides thetaiotaomicron VPI A1686–1689Δtdk, clone 1.8 | Kovatcheva-Datchary et al., 2015 | N/A |
| Prevotella copri | DSMZ | 18025 |
| Rikenella microfusis | ATCC | 29728 |
| Clostridiaceae sp. 4 1 37FAA | ATCC | 35704 |
| E. coli S17λ pir | Sonnenburg Lab strain collection | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| LB Broth, Miller | Fisher Scientific | BP1426 |
| LB Agar, Miller | Fisher Scientific | BP1425 |
| Brain Heart Infusion | BD | 237500 |
| Sheep Blood | Hemostat Laboratories | DSB500 |
| PowerSoil DNA Isolation Kit | MoBIO | 12955 |
| 5PRIME HotMasterMix | QuantaBio | 2200400 |
| UltraClean PCR Cleanup Kit | MoBIO | 12596 |
| Quant-iT High-Sensitivity dsDNA Assay Kit | ThermoFisher Scientific | Q33120 |
| Sodium formate | Sigma | 71539 |
| Sodium acetate | Sigma | S2889 |
| Sodium propionate | Sigma | P1880 |
| Sodium butyrate | Sigma | 303410 |
| Sodium L-lactate | Sigma | L7022 |
| Succinic Acid, disodium salt | Sigma | S2378 |
| MTBSFTA | Sigma | 394882 |
| 37% HCI, HPLC-grade | Sigma | 339253 |
| DNase | Roche | 10104159001 |
| Liberase TL | Roche | 05401020001 |
| ACK Lysis Buffer | Lonza | 10–548E |
| BCECF-AM | ThermoFisher Scientific | B1170 |
| Propionic acid (<99.7%) | Sigma | 402907 |
| Inulin | Orafti | N/A |
| Inulin propionate ester | This study | N/A |
| Dialysis tubing, MWCO 500 | Spectrum Laboratories | 131060 |
| Critical Commercial Assays | ||
| Cytofix/Cytoperm Kit | BD Biociences | 554714 |
| RNeasy Mini Kit | QIAGEN | 74106 |
| Superscript III First-Strand Synthesis Kit | Invitrogen | 18080 |
| FastStart Universal SYBR Green Master Mix | Roche | 04913914001 |
| Deposited Data | ||
| 16s Sequencing Data | Mendeley | http://dx.doi.orq/10.1 7632/79h97qpxz5.1 |
| Antibodies | ||
| TruStain fcx anti-mouse CD16/32(Fc Block) | Biolegend | 101320 |
| Live/Dead Fixable Blue Viability Dye | Invitrogen | L34962 |
| CD45-Brilliant Violet 510 | Biolegend | 103138 |
| CD19-Alexa Fluor 780 | eBioscience | 47–0193-82 |
| CD11 b-Brilliant Violet 785 | Biolegend | 101243 |
| CD11c-PE-Cy7 | BD Biosciences | 558079 |
| Ly6G-BUV395 | BD | 563978 |
| Ly6C-APC | eBioscience | 17–5932-82 |
| CD3e-Pac Blue | eBioscience | 48–0032-82 |
| NK1.1-e650NC | eBioscience | 95–5941-42 |
| CD64-PE | BioLegend | 139304 |
| Experimental Models: Organisms/Strains | ||
| 129SvJ/X1 | Jackson Laboratory | Cat# 000691 |
| C57BL/6 Nramp+/+ | In-house colony | N/A |
| Oligonucleotides | ||
| For primers used in this study, see Table S2. | ||
| Recombinant DNA | ||
| Plasmid: pHBU2-tdk, CarbR/ErmR | Sonnenburg Lab | N/A |
| Plasmid: pNBU2–7686–1689, CarbR/TetR | This study | N/A |
| Software and Algorithms | ||
| QIIMEv. 1.9.1 | Caporasoetal., 2010 | www.qiime.orq |
| FastQCv. 0.11.4 | http://www.bioinformatics.bab raham.ac.uk/proiects/fastqc/ | N/A |
| MATLAB | Mathworks | https://www.mathwor ks.com/products/mat lab.html |
| FACSDIVA | BD Biosciences | http://www.bdbioscie nces.com/us/instrum ents/clinical/software/flow-cvtometrv-acquisition/bd-facsdiva-software/m/333333/o verview |
| FlowJo v. 10.0.8 | FlowJo, LLC | https://www.flowio.co m/ |
| Prism | GraphPad | https://www.qraphpa d.com/scientific-software/prism/ |
Acknowledgements
The authors thank Dr. Eric Martens for sharing bacterial strains, protocols, and counsel on Bacteroides genetics, and Wes Whitaker and Elizabeth Stanley Shepherd for assistance with Bacteroides complementation. The authors are grateful to Juliana Idoyaga, Katharine Ng, and Andrew Hryckowian, Phoom Chairatana, Andres Aranda-Diaz, David Schneider and the members of the Monack Lab for assistance with various aspects of this manuscript. The authors acknowledge the Stanford Veterinary Service Center for expertise on animal husbandry and experiments, and the Mass Spectrometry Laboratory for GCMS expertise. Research reported in this publication was supported by grants R01-AI116059 from NIAID (DM) and R01-DK085025 from NIDDK (JLS), the Paul Allen Stanford Discovery Center on Systems Modeling of Infection (DM and KCH), a National Science Foundation Graduate Research Fellowship and award T32GM007276 from NIGMS (AJ), award F32AI133917 from NIAID (MR), and NSF CAREER Award MCB-1149328 (KCH). JLS and KCH are Chan Zuckerberg Biohub Investigators. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- Abt MC, Buffie CG, Su ac B, Becattini S, Carter RA, Leiner I, Keith JW, Artis D, Osborne LC, and Pamer EG (2016). TLR-7 activation enhances IL-22-mediated colonization resistance against vancomycin-resistant enterococcus. Science Translational Medicine 8, 327ra25–327ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Godec J, Lau L, Sivick KE, McLaughlin LM, Jones MB, Dracheva T, Peterson SN, Monack DM, and Barton GM (2011). TLR Signaling Is Required for Salmonella typhimurium Virulence. Cell 144, 675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, and Hardt WD (2003). Pretreatment of Mice with Streptomycin Provides a Salmonella enterica Serovar Typhimurium Colitis Model That Allows Analysis of Both Pathogen and Host. Infection and Immunity 71, 2839–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler AJ, and Sperandio V (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besten, den G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, and Bakker BM (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzón CR, and Holden DW (2001). Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes and Infection 3, 1345– 1352. [DOI] [PubMed] [Google Scholar]
- Bevins CL, and Salzman NH (2011). Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Micro 9, 356–368. [DOI] [PubMed] [Google Scholar]
- Boehme DH (1970). Resistance to salmonella infections in inbred mouse strains. Bull N Y Acad Med 46, 499–508. [PMC free article] [PubMed] [Google Scholar]
- Brown DE, Libby SJ, Moreland SM, McCoy MW, Brabb T, Stepanek A, Fang FC, and Detweiler CS (2013). Salmonella entericaCauses More Severe Inflammatory Disease in C57/BL6 Nramp1 G169Mice Than Sv129S6 Mice. Vet Pathol 50, 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugiroux S, Beutler M, Pfann C, Garzetti D, Ruscheweyh H-J, Ring D, Diehl M, Herp S, Lötscher Y, Hussain S, et al. (2016). Genome-guided design of a defined mouse microbiota that confers colonization resistance against <italic>Salmonella enterica</italic> serovar Typhimurium. Nature Microbiology 1–12. [DOI] [PubMed] [Google Scholar]
- Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, et al. (2014). Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517, 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody RN, Gerber GK, Luevano JM Jr, Gatti DM, Somes L, Svenson KL, and Turnbaugh PJ (2015). Diet Dominates Host Genotype in Shaping the Murine Gut Microbiota. Cell Host & Microbe 17, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SEK, MacDougall K, Preston T, Tedford C, Finlayson GS, et al. (2015). Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, and Groisman EA (2016). Acidic pH sensing in the bacterial cytoplasm is required for Salmonellavirulence. Molecular Microbiology 101, 1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, and Raffatellu M (2013). Probiotic Bacteria Reduce Salmonella Typhimurium Intestinal Colonization by Competing for Iron. Cell Host & Microbe 14, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, et al. (2016). A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P, and Schink B (1998). Energy conservation in the decarboxylation of dicarboxylic acids by fermenting bacteria. Arch. Microbiol 170, 69–77. [DOI] [PubMed] [Google Scholar]
- Dolowschiak T, Mueller AA, Pisan LJ, Feigelman R, Felmy B, Sellin ME, Namineni S, Nguyen BD, Wotzka SY, Heikenwalder M, et al. (2016). IFN-γ Hinders Recovery from Mucosal Inflammation during Antibiotic Therapy for Salmonella Gut Infection. Cell Host & Microbe 20, 238–249. [DOI] [PubMed] [Google Scholar]
- Edelstein A, Amodaj N, Hoover K, Vale R, and Stuurman N (2001). Computer Control of Microscopes Using μManager (Hoboken, NJ, USA: John Wiley & Sons, Inc.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. [DOI] [PubMed] [Google Scholar]
- El-Gedaily A, Paesold G, Chen CY, Guiney DG, and Krause M (1997). Plasmid virulence gene expression induced by short-chain fatty acids in Salmonella dublin: identification of rpoS-dependent and rpo-S-independent mechanisms. Journal of Bacteriology 179, 1409–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber F, Thiennimitr P, Spiga L, Byndloss MX, Litvak Y, Lawhon S, Andrews-Polymenis HL, Winter SE, and Bäumler AJ (2017). Respiration of Microbiota-Derived 1,2-propanediol Drives Salmonella Expansion during Colitis. PLoS Pathog 13, e1006129–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friswell MK, Gika H, Stratford IJ, Theodoridis G, Telfer B, Wilson ID, and McBain AJ (2010). Site and Strain-Specific Variation in Gut Microbiota Profiles and Metabolism in Experimental Mice. PLoS ONE 5, e8584–e8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, Santos RL, Dandekar S, Tsolis RM, and Baumler AJ (2008). T Cells Help To Amplify Inflammatory Responses Induced by Salmonella enterica Serotype Typhimurium in the Intestinal Mucosa. Infection and Immunity 76, 2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Pinner E, Desjardins M, and Gros P (1997). Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J. Exp. Med 185, 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati AS, Shanahan MT, Arthur JC, Grossniklaus E, Furstenberg, von RJ, Kreuk L, Henning SJ, Jobin C, and Sartor RB (2012). Mouse Background Strain Profoundly Influences Paneth Cell Function and Intestinal Microbial Composition. PLoS ONE 7, e32403–e32411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammelman TA, O’Toole GA, Trzebiatowski JR, Tsang AW, Rank D, and Escalante-Semerena JC (1996). Identification of a new prp locus required for propionate catabolism in Salmonella typhimurium LT2. FEMS Microbiology Letters 137, 233–239. [DOI] [PubMed] [Google Scholar]
- Hapfelmeier S, Stecher B, Barthel M, Kremer M, Muller AJ, Heikenwalder M, Stallmach T, Hensel M, Pfeffer K, Akira S, et al. (2005). The Salmonella Pathogenicity Island (SPI)-2 and SPI-1 Type III Secretion Systems Allow Salmonella Serovar typhimurium to Trigger Colitis via MyD88-Dependent and MyD88-Independent Mechanisms. J.I 174, 1675–1685. [DOI] [PubMed] [Google Scholar]
- Hartzell AL, mez MXAXM-GX, Hutkins RW, and Rose DJ (2013). Synthesis and in vitro digestion and fermentation of acylated inulin. Bioactive Carbohydrates and Dietary Fibre 1, 81–88. [Google Scholar]
- Hooper LV, Stappenbeck TS, Hong CV, and Gordon JI (2003). Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol 4, 269–273. [DOI] [PubMed] [Google Scholar]
- Hormaeche CE (1979). Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology 37, 311–318. [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Suyemoto M, Garner CD, Cicconi KM, and Altier C (2008). Formate Acts as a Diffusible Signal To Induce Salmonella Invasion. Journal of Bacteriology 190, 4233–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C-C, Garner CD, Slauch JM, Dwyer ZW, Lawhon SD, Frye JG, McClelland M, Ahmer BMM, and Altier C (2013). The intestinal fatty acid propionate inhibits Salmonella invasion through the post-translational control of HilD. Molecular Microbiology 87, 1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T-H, Park JH, Jeon W-M, and Han K-S (2015). Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract 9, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knights D, Costello EK, and Knight R (2011). Supervised classification of human microbiota. FEMS Microbiol Rev 35, 343–359. [DOI] [PubMed] [Google Scholar]
- Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, and Bäckhed F (2015). Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metabolism 22, 971–982. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Shaffer KM, Anderson GP, Ligler FS, and Taitt CR (2006). Antimicrobial peptide-based array for Escherichia coli and Salmonella screening. Analytica Chimica Acta 575, 9–15. [DOI] [PubMed] [Google Scholar]
- Lam LH, and Monack DM (2014). Intraspecies Competition for Niches in the Distal Gut Dictate Transmission during Persistent Salmonella Infection. PLoS Pathog 10, e1004527–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, and Monack DM (2007). Host Transmission of Salmonella enterica Serovar Typhimurium Is Controlled by Virulence Factors and Indigenous Intestinal Microbiota. Infection and Immunity 76, 403– 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier L, Diard M, Sellin ME, Chouffane E-S, Trautwein-Weidner K, Periaswamy B, Slack E, Dolowschiak T, Stecher B, Loverdo C, et al. (2014). Granulocytes Impose a Tight Bottleneck upon the Gut Luminal Pathogen Population during Salmonella Typhimurium Colitis. PLoS Pathog 10, e1004557–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik U, Armstrong D, Ashworth M, Dregan A, L’Esperance V, McDonnell L, Molokhia M, and White P (2017). Association between prior antibiotic therapy and subsequent risk of community-acquired infections: a systematic review. J. Antimicrob. Chemother [DOI] [PubMed]
- Maltby R, Leatham-Jensen MP, Gibson T, Cohen PS, and Conway T (2013). Nutritional Basis for Colonization Resistance by Human Commensal Escherichia coli Strains HS and Nissle 1917 against E. coli O157:H7 in the Mouse Intestine. PLoS ONE 8, e53957–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, and Hugenholtz P (2012). An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME Journal 6, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHan F, and Shotts EB (1993). Effect of short-chain fatty acids on the growth of Salmonella typhimurium in an in vitro system. Avian Dis. 37, 396–398. [PubMed] [Google Scholar]
- Miki T, Goto R, Fujimoto M, Okada N, and Hardt W-D (2017). The Bactericidal Lectin RegIIIβ Prolongs Gut Colonization and Enteropathy in the Streptomycin Mouse Model for Salmonella Diarrhea. Cell Host & Microbe 1–14. [DOI] [PubMed] [Google Scholar]
- Monack DM, Bouley DM, and Falkow S (2004). Salmonella typhimuriumPersists within Macrophages in the Mesenteric Lymph Nodes of Chronically Infected Nramp1+/+Mice and Can Be Reactivated by IFNγ Neutralization. J. Exp. Med 199, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry CM, Hien TT, Dougan G, White NJ, and Farrar JJ (2002). Typhoid fever. N. Engl. J. Med 347, 1770–1782. [DOI] [PubMed] [Google Scholar]
- Peng L, Li ZR, Green RS, Holzman IR, and Lin J (2009). Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. Journal of Nutrition 139, 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Colavin A, Shi H, Czarny TL, Larson MH, Wong S, Hawkins JS, Lu CHS, Koo B-M, Marta E, et al. (2016). A Comprehensive, CRISPR-based Functional Analysis of Essential Genes in Bacteria. Cell 165, 1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MN, Dehal PS, and Arkin AP (2010). FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt N, Duncan SH, Young P, Belenguer A, Leitch CM, Scott KP, Flint HJ, and Louis P (2014). Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. The ISME Journal 8, 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke SC (2003). Perspectives on the Use of Organic Acids and Short Chain Fatty Acids as Antimicrobials. 1–8. [DOI] [PubMed] [Google Scholar]
- Rivera-Chávez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE, Xu G, Velazquez EM, Lebrilla CB, Winter SE, et al. (2016). Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host & Microbe 19, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen A, Lahti L, rvi JSA, Holtrop G, Korpela K, Duncan SH, Date P, Farquharson F, Johnstone AM, Lobley GE, et al. (2014). Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. 8, 2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana TG, Flaugnatti N, Lugo KA, Lam LH, Jacobson A, Baylot V, Durand E, Journet L, Cascales E, and Monack DM (2016). SalmonellaTyphimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc. Natl. Acad. Sci. U.S.a. 113, E5044–E5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi M, and Raffatellu M (2015). No Vacancy: How Beneficial Microbes Cooperate with Immunity To Provide Colonization Resistance to Pathogens. J.I 194, 4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi M, Nuccio S-P, Liu H, Hernandez D, Vu CT, Takahashi AA, Edwards RA, and Raffatellu M (2016). Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 540, 280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliusarenko O, Heinritz J, Emonet T, and Jacobs-Wagner C (2011). High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Molecular Microbiology 80, 612–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga L, Winter MG, de Carvalho TF, Zhu W, Hughes ER, Gillis CC, Behrendt CL, Kim J, Chessa D, Andrews-Polymenis HL, et al. (2017). An Oxidative Central Metabolism Enables Salmonella to Utilize Microbiota-Derived Succinate. Cell Host & Microbe 22, 291–301. e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Chaffron S, Käppeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, Mering, von, C., et al. (2010). Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog 6, e1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell T, Lee TK, Shiomi D, Shi H, Tropini C, Monds RD, Colavin A, Billings G, Bhaya-Grossman I, Broxton M, et al. (2017). Rapid, precise quantification of bacterial cellular dimensions across a genomic-scale knockout library. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F (2003). Invasion of Salmonella enteritidis in avian intestinal epithelial cells in vitro is influenced by short-chain fatty acids. International Journal of Food Microbiology 85, 237–248. [DOI] [PubMed] [Google Scholar]
- Vázquez-Baeza Y, Pirrung M, Gonzalez A, and Knight R (2013). EMPeror: a tool for visualizing high-throughput microbial community data. Gigascience 2, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JJ, Koren O, Hugenholtz P, DeSantis TZ, Walters WA, Caporaso JG, Angenent LT, Knight R, and Ley RE (2012). Impact of training sets on classification of high-throughput bacterial 16s rRNA gene surveys. The ISME Journal 6, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Related to Figure 2. Experimental design of fecal microbiota transplant and cohousing experiments. A) 129 mice were administered a cocktail of antibiotics in the drinking water prior to fecal microbiota transplant (FMT) with either 129 or B6N microbiotas. Mice were given FMTs for three consecutive days after antibiotic treatment. FMTs were allowed to stabilize for 4 weeks prior to S. Typhimurium infection. B) 129 mice were housed with their own genotype (control housed) or with B6N mice (cohoused) for 4 weeks prior to S. Typhimurium infection.
Related to Figure 3. 16S rRNA gene sequencing diversity analyses on fecal genomic DNA isolated from FMT and cohousing experiments on days 0, 7, 14, and 21 postinfection. A-B) α- diversity is the same between 129 mice receiving 129 (red) and B6N (blue) microbiotas across rarefaction depths, for both observed OTUs (top) and Faith’s Phylogenetic Diversity (bottom, PD_whole_tree). B-F) β-diversity PCoA plots of 129 mice receiving 129 or B6N FMTs. B) Samples separate well by microbiota received. Black=129 microbiota received, red=B6N microbiota. C) Samples do not separate by infection status. White=uninfected, black=infected. D) Samples do not separate by shedding status. White=low shedder (<106 CFU/g feces), gray=moderate shedder (106-108 CFU/g feces), black=supershedder (>108 CFU/g feces). E) Samples do not separate by day postinfection. White=day 0, light gray=day 7, dark gray=day 14, black=day 21. F-I) PCoA plots of control or cohoused 129 mice. F) Samples separate well by microbiota received. Black=129 microbiota received, red=B6N microbiota. G) Samples do not separate by infection status. White=uninfected, black=infected. H) Samples do not separate by shedding status. White=low shedder (<106 CFU/g feces), gray=moderate shedder (106-108 CFU/g feces), black=supershedder (>108 CFU/g feces). I) Samples do not separate by day post-infection. White=day 0, light gray=day 7, dark gray=day 14, black=day 21.
Related to Figure 3. 16s rRNA gene sequencing abundance analysis of fecal genomic DNA isolated from FMT and cohousing experiments on days 0, 7, 14, and 21 postinfection. A) There is not a significant difference in the abundance of Enterobacteriaceae spp. prior to infection between mice receiving 129 vs. B6N microbiotas in FMT experiment. Significance was calculated using the Kruskall-Wallis test; NS: not significant. B-C) The abundance of Bacteroidales spp. identified by supervised learning as predictive features of B6N microbiotas: family S24–7, genus Odoribacter. D-I) Abundance dynamics of Bacteroidales spp. identified by supervised learning as predictive features of B6N microbiotas in 129 animals receiving either a 129 or B6N FMT: D) Bacteroides, E) Prevotella, F) Rikenellaceae, G) B. ovatus, H) B. uniformis, I) B. acidifaciens. Black=129 microbiota received, red=B6N microbiota received. J-O) Abundance dynamics of Bacteroidales spp. identified by supervised learning as predictive features of B6N microbiotas in control or cohoused 129 mice: J) Bacteroides, K) Prevotella, L) Rikenellaceae, M) B. ovatus, N) B. uniformis, O) B. acidifaciens. Black=control housed, red=cohoused. Error bars represent standard error of the mean. Dotted lines represent the limit of detection.
Related to Figures 4 and 5. Anaerobic growth curves of bacterial growth in rich medium supplemented with organic extracts of supernatants isolated from anaerobically-grown commensal bacteria. A) Schematic of experimental approach for production of organic extracts of supernatants isolated from anaerobically grown commensal bacteria. B-C) S. Typhimurium was grown in LB broth without and with organic extracts. CFU/mL were measured by plating at 2, 4, 8, and 24 h. B. ovatus=Bacteroides ovatus, B. uniformis=Bacteroides uniformis, B. acidifaciens=Bacteroides acidifaciens, P. copri = Prevotella copri, R. microfusis=Rikenella microfusis. All extracts were resuspended at 1× concentration. D) Propionate concentration in supernatants from Bacteroides spp. cultures grown for 24 h. Data represent three independent cultures. E) SCFA concentrations in supernatants from wild-type, propionate-production mutant, and complemented Bt cultures. Propionate is the only SCFA with differential production between WT, mutants, and complemented strains. Significance was calculated using a two-tailed Mann-Whitney test; NS: not significant, ****: p<0.0001. Unless noted, conditions are not significant. F) Bt CFU/mL after 24 h of growth, utilized for normalization of S. Typhimurium growth in Figure 5B, S4. Data represent 3 independent experiments with 3–5 replicates. Error bars represent the standard error of the mean. G) S. Typhimurium CFU/mL are significantly decreased in LB supplemented with organic extracts from Bt producing propionate, but not in propionate-production mutants or in the ethyl acetate negative control. Extract concentration was normalized to Bt CFU/mL. Data represent 3 independent experiments with 4 replicates. Error bars represent the standard error of the mean. H) S. Typhimurium anaerobic growth curves in which LB broth was pHmatched to pH of propionic acid at conditions indicated in Figure 5C-E. S. Typhimurium displays similar growth in pH-matched LB solutions and LB control. For commensal extract growth curve experiments and SCFA concentrations, significance was calculated using a two-tailed Mann-Whitney test. For CFU/mL calculations, significance was calculated using an unpaired two-tailed t test. NS: not significant, *: p<0.05, **: p<0.01, ***: p<0.001. Unless noted, conditions are not significant.
Related to Figure 7. A-B) 129 mice were administered a cocktail of either heat-killed or live Bacteroides spp. (B. ovatus, B. uniformis, B. acidifaciens) 7 days prior to infection with S. Typhimurium. A) Experimental design. B) Bacteroides spp. fecal shedding is increased in mice administered a live cocktail of Bacteroides spp. (red) but not in mice administered a heat-killed cocktail (black). C-D) 129 mice were administered live wild type or a cocktail of propionate-production (Δ1686–89) Bt 7 days prior to infection with S. Typhimurium. C) Experimental design. D) Bt fecal shedding was similar between mice administered wild-type Bt (red) and propionate production mutants (black). E) Experimental design in which 129 mice were treated daily with either 1% inulin or 1% inulin propionate ester solution and infected with S. Typhimurium. For fecal shedding experiments, significance was calculated using a two-tailed Mann-Whitney test; ***: p<0.001, ****: p<0.0001. Unless noted, conditions are not significant.
Related to Figure 7. 129 mice were administered heat-killed (black) or live (red) Bacteroides spp. 7 days prior to infection with S. Typhimurium. Mice were sacrificed at 2 days post-infection for histological, expression, and flow cytometry analysis. A) Histopathology of spleen, liver, small intestine, cecum, and colon was scored. B-C) RNA from B) small intestine and C) cecal tissue was isolated and antimicrobial peptide gene expression was measured relative to GAPDH expression. mBD3=mouse β-defensin 3, Ang4=angiogenin 4. D-G) Flow cytometric analysis from D-E) spleen and F-G) colon tissue. D, F) CD19+ B cells, CD3ε+ T cells, and NK1.1+ NK cells were quantified as a percentage of total CD45+ immune cells. E,G) CD11chi dendritic cells, CD11c- CD11b+ mononuclear phagocytes, and CD11c- CD11b+ Ly6G+ neutrophils were quantified as a percentage of total CD45+ immune cells. H) RNA from cecal tissue was isolated and cytokine gene expression was measured relative to GAPDH expression. Data represent two independent experiments with 7–8 animals in each group. Error bars represent standard error of the mean. Significance was calculated using the two-tailed Mann- Whitney test; no comparisons were significant.
Related to Figure 7. 129 mice were administered heat-killed or live Bacteroides spp. 7 days prior to infection with S. Typhimurium. Mice were sacrificed at 2 days post-infection for flow cytometry analysis. Single-cell suspensions were stained with antibodies. Cells were gated on forward and side scatter to exclude debris and select for single cells. A hierarchical gating strategy was used on live cells to identify B cells, T cells, NK cells, dendritic cells, mononucleocytes, and neutrophils as depicted.