Abstract
Objectives:
The high mortality among HIV/tuberculosis (TB) coinfected patients in Eastern Europe is partly explained by the high prevalence of drug-resistant TB. It remains unclear whether outcomes of HIV/TB patients with rifampicin/isoniazid-susceptible TB in Eastern Europe differ from those in Western Europe or Latin America.
Methods:
One-year mortality of HIV-positive patients with rifampicin/isoniazid-susceptible TB in Eastern Europe, Western Europe, and Latin America was analysed and compared in a prospective observational cohort study. Factors associated with death were analysed using Cox regression models
Results:
Three hundred and forty-one patients were included (Eastern Europe 127, Western Europe 165, Latin America 49). Proportions of patients with disseminated TB (50, 58, 59%) and initiating rifampicin + isoniazid + pyrazinamide-based treatment (93, 94, 94%) were similar in Eastern Europe, Western Europe, and Latin America respectively, whereas receipt of antiretroviral therapy at baseline and after 12 months was lower in Eastern Europe (17, 39, 39%, and 69, 94, 89%). The 1-year probability of death was 16% (95% confidence interval 11–24%) in Eastern Europe, vs. 4% (2–9%) in Western Europe and 9% (3–21%) in Latin America; P <0.0001. After adjustment for IDU, CD4+ cell count and receipt of antiretroviral therapy, those residing in Eastern Europe were at nearly 3-fold increased risk of death compared with those in Western Europe/Latin America (aHR 2.79 (1.15–6.76); P = 0.023).
Conclusions:
Despite comparable use of recommended anti-TB treatment, mortality of patients with rifampicin/isoniazid-susceptible TB remained higher in Eastern Europe when compared with Western Europe/Latin America. The high mortality in Eastern Europe was only partially explained by IDU, use of ART and CD4+ cell count. These results call for improvement of care for TB/HIV patients in Eastern Europe.
Keywords: death, drug susceptibility testing, Eastern Europe, HIV, Latin America, TB:HIV study, treatment, tuberculosis, Western Europe
Introduction
Currently, tuberculosis (TB) is a public health emergency in Eastern Europe [1]. The incidence of TB among HIV-infected populations is rapidly increasing in this region, and the situation is complicated by an increase in the prevalence of multidrug resistant (MDR) TB [2–4]. We have previously documented an excess mortality rate among TB/HIV patients in Eastern Europe, which can only partially be explained by the high prevalence of MDR TB, suboptimal access to drug susceptibility testing (DST), and as a consequence, use of inadequate anti-TB treatment regimens [5].
One of the risk factors for development of MDR TB is previous (inadequate) TB treatment which permitted drug resistance to be selected or amplified [6,7]. By contrast, high rates of MDR TB among new TB cases without a history of prior TB, as well data from modelling studies, suggest that transmission of drug resistant TB plays an important role in the ongoing epidemic of MDR TB in Eastern Europe [8,9]. From a patient as well as from a public health perspective it is therefore important to appropriately treat drug sensitive TB, ensuring that patients are cured and their infection is eliminated. This strategy would help prevent the development and potential further spread of MDR TB. Treatment of MDR TB is complex because of the drug burden, prolonged treatment duration, adverse effects, high cost, and the limited availability of many drugs in low and middle-income regions [10–12]. Besides, the overall success rate of treating MDR TB is only around 50% [13]. By contrast, treatment of drug susceptible-TB is inexpensive, requires fewer clinical (nurse and doctor led) resources, and first-line drugs are widely available in resource-limited settings [14,15]. Provided that recommendations for treatment of drug sensitive-TB are used and adhered to both by clinicians and patients, successful TB outcome can be achieved in a majority of patients, which is according to WHO 2015 standards [16].
In the presence of HIV coinfection, it is also of paramount importance to adequately treat the underlying HIV infection [17]. Timely initiation of antiretroviral therapy (ART) improves immune function and hence prevents of active TB disease, or in the settings of already developed TB, significantly improves outcome [18,19].
We hypothesised that mortality rates among TB/HIV patients with drug sensitive-TB in Eastern Europe would be comparable with those in Western Europe and Latin America. The present analysis aimed to assess and compare mortality rates of HIV patients with drug susceptible TB across regions and identify risk factors associated with mortality. Further, we aimed to describe management of HIV coinfection in patients with drug sensitive-TB.
Methods
The current analyses were conducted within the prospective TB: HIV cohort study. Details of the study design and methodology have been published elsewhere [20]. Briefly, HIV-positive persons aged 16 years or older who were diagnosed with TB between January 2011 and December 2013 in 62 participating HIVand TB clinics in 19 countries from Eastern Europe, Western Europe or Latin America [Eastern Europe (EE) (21 clinics in Belarus, Estonia, Georgia, Latvia, Lithuania, Poland, Romania, Ukraine, Russia), Western Europe (WE) (28 clinics in Belgium, Denmark, France, Italy, Spain, Switzerland, UK), and Latin America (LA) (13 clinics in Argentina, Chile, and Mexico] were enrolled and followed up for 24 months. Demographic, clinical, and laboratory information was prospectively collected on case report forms at TB diagnosis, 6, 12, and 24 months thereafter. The study received approval from Ethics Committees in all countries as per local regulations.
The current analysis is limited to 12 months of follow-up and the database was closed for analysis in May 2015, when all 12 months follow-up data were collected. Baseline was defined as the date when anti-TB treatment was initiated. DSTs, obtained within 4 weeks of baseline were used to confirm mycobacterial susceptibility to rifampicin and isoniazid. Patients without a DST and those with rifampicin and/or isoniazid-resistant TB were excluded from the current analyses. Patients were stratified by their region of residence. Descriptive statistics were used for baseline characteristics. Study definitions in terms of certainty of TB diagnosis, clinical presentation of TB disease, TB treatment regimens, ART regimens, and region of residence have previously been published and reused in the current analysis for consistency [5].
Treatment of underlying HIV infection was analysed by calculating proportion of patients receiving ART at baseline, 3, 6, 9, and 12 months, and by assessing immuno-virological status of the cohort at the same time points. The proportion on ART was calculated as proportion of TB/HIV patients being on ART at a given time point among those alive and not lost to follow-up. The proportion with HIV-RNA < 500 copies/ml and proportion with CD4+ cell count > 200 cells/µl was calculated as the proportion of those alive, not lost to follow-up and being on ART at a given time point. Response to ART was assessed among those who started ART at or within 6 months after their TB diagnosis. It was done by calculating proportion of patients with HIV RNA less than 500 copies/ml in the time period 2–7 months after starting ART out of those alive and still under follow-up. ART was defined as a combination of at least three antiretroviral drugs of any class.
Mortality was compared using Kaplan–Meier survival analysis in which patients were censored at their last clinic visit, date of death, or after 12 months of follow-up, whichever occurred first. Risk factors for death were identified by Cox proportional hazard models that included region of residence, where Western Europe and Latin America were combined because of the small numbers of deaths in these regions. Following ‘a priori’ defined variables were tested in the uni and multivariate models: sex, age (per 10 years increase), history of injection drug use (IDU: yes or no), extent of TB disease (pulmonary or disseminated), use of rifampicin, isoniazid and pyrazinamide in the initial anti-TB treatment regimen, baseline CD4+ cell count (as a categorical variable), and use of ART (yes/no) as a time-updated variable.
Results
Of the 1406 TB/HIV patients in the TB: HIV study, 644 (46%) had culture confirmed TB (360 of 834 enrolled participants in Eastern Europe, 205 of 317 in Western Europe, and 79 of 255 in Latin America, respectively). Of the 644 patients, 495 (77%) had baseline DST results for both rifampicin and isoniazid available, and finally 341/495 (69%) were infected with Mycobacterium tuberculosis susceptible to both rifampicin and isoniazid. The regional distribution was Eastern Europe 127, Western Europe 165, and Latin America 49. These 341 patients were included in the present analyses (Fig. 1).
Fig. 1. Flow chart of TB:HIV Study patients with rifampicin and isoniazid-susceptible tuberculosis included in the analyses.
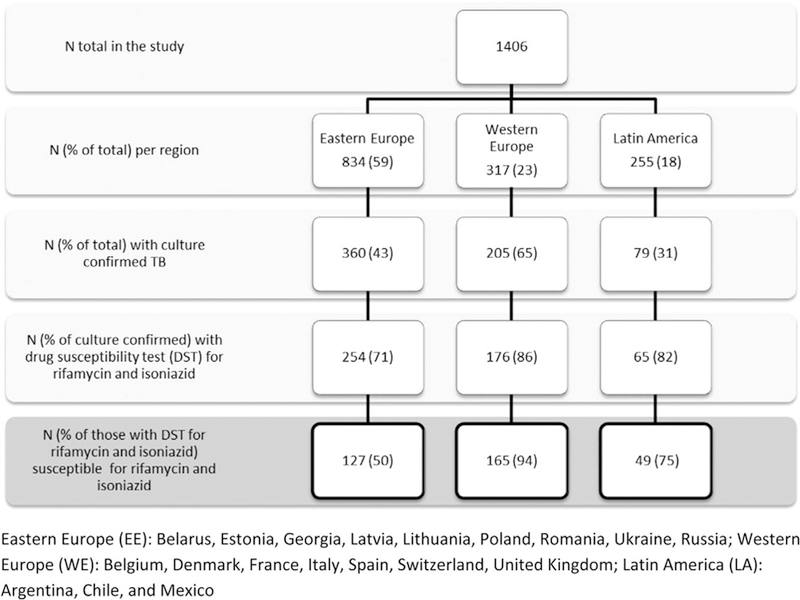
TB, tuberculosis.
The baseline clinical and demographic characteristics of these patients are described in Table 1. Compared with Western Europe and Latin America, patients in Eastern Europe were slightly younger and a greater proportion in this region was coinfected with hepatitis C virus (HCV). The most common risk factors for TB/HIVacquisition in Eastern Europe were: history of IDU, excess alcohol consumption, and/or imprisonment. The proportion of patients with excess alcohol consumption was comparably high in Latin America, but not in Western Europe. More than 90% of patients in all three regions commenced anti-TB treatment that included rifampicin, isoniazid, and pyrazinamide. At the time of TB diagnosis, patients from all regions had advanced immunodeficiency and over half presented with disseminated TB. Despite similar degrees of immunodeficiency and HIV RNA levels, a smaller proportion of those in Eastern Europe were receiving ART at baseline.
Table 1.
Baseline demographic and clinical characteristics of 341 HIV patients with rifampicin and isoniazid-susceptible tuberculosis.
| Total | Eastern Europe N (%) 127 |
Western Europe N (%) 165 |
Latin America N (%) 49 |
P | |
|---|---|---|---|---|---|
| Sex | Male | 98(77.2) | 109 (66.1) | 36 (73.5) | 0.108 |
| Age | Years, median (IQR) | 36.5 (30.9–42.6) | 38.8 (33.0–46.2) | 38.2 (29.2–44.8) | 0.037 |
| Weight | Kg, median (IQR) | 58.0 (50.0–66.0) | 60.0 (54.0–68.0) | 55.0 (45.5–65.5) | 0.225 |
| TB/HIV risk factors | Ever injecting drug use, N (%) | 76 (60.3) | 31 (18.8) | 8 (16.3) | <0.001 |
| History of imprisonment, N (%) | 16 (12.6) | 5 (3.0) | 4 (8.2) | 0.008 | |
| History of excess alcohol consumption, N (%) |
27 (21.3) | 20 (12.1) | 13 (26.5) | 0.026 | |
| Laboratory markers | Haemoglobin (g/dl), median (IQR)a |
11 (9–13) | 11 (9–12) | 11 (10–13) | 0.158 |
| Albumin (g/dl), median (IQR)b | 29 (24–33) | 30 (25–34) | 26 (22–33) | 0.484 | |
| TB disease | Disseminated, N (%) | 64 (50.4) | 95 (57.6) | 29 (59.2) | 0.39 |
| TB treatment | RHZ-based, N (%) | 118 (92.9) | 155 (93.9) | 46 (93.9) | 0.935 |
| RHZ + E, N (%) | 98 (83.1) | 128 (82.6) | 43 (93.5) | 0.181 | |
| RHZ + S, N (%) | 1 (0.8) | 0.426 | |||
| RHZ + ES, N (%) | 10 (8.5) | 3 (1.9) | 1 (2.2) | 0.024 | |
| Hepatitis Cc | Ever, N (%) | 61 (48.0) | 27 (16.4) | 6 (12.2) | <0.001 |
| HIV status | |||||
| Antiretroviral therapy | Yes. N (%) | 21 (16.5) | 64 (38.8) | 19 (38.8) | <0.001 |
| CD4+ cell count, cells/μld | Median (IQR) | 103.5 (35.0–258.0) | 123.0 (35.0–280.0) | 78.0 (28.0–218.0) | 0.31 |
| HIV RNA, log10 copies/mle | Median (IQR) | 5.2 (4.3–5.7) | 5.0 (2.9–5.7) | 4.7 (2.5–5.6) | 0.289 |
Eastern Europe (Belarus, Estonia, Georgia, Latvia, Lithuania, Poland, Romania, Ukraine, Russia); Western Europe (Belgium, Denmark, France, Italy, Spain, Switzerland, United Kingdom); Latin America (Argentina, Chile, and Mexico).
Data for haemoglobin available for 98 (77) patients in EE, 146 (88) in WE, and 41 (84) in LA.
Data for albumin available for 38 (30) patients in EE, 113 (68) in WE, and 26 (53) in LA.
Ever tested positive for hepatitis C antibodies or hepatitis C RNA. Data for hepatitis C available for 91 (72) patients in EE, 121 (73) in WE, and 36 (73) in LA. Missing data are included in the denominator.
Baseline CD4+ cell count was available for 102 (80), 162 (98) and 43 (88) patients in EE, WE, and LA.
Baseline HIV RNA data were available for 68 (54), 157 (95) and 40 (82) patients in EE, WE, and LA.
IQR, interquartile range; R, rifampicin; H, isoniazid; Z, pyrazinamide; E, ethambutol; S, streptomycin.
In Kaplan–Meier analyses of the duration of TB therapy, the time by which 50% of patients had stopped therapy was longer than the guideline-recommended 6 months in all three regions: 8.7 months (interquartile range 7.1–9.7) in Eastern Europe, 9.0 (8.3–9.1) in Western Europe, and 9.8 (9.0–11.5) in Latin America. There was no evidence that the time to stopping TB treatment differed between regions (P = 0.28).
Management of HIV infection after initiation of anti-TB treatment is presented in Fig. 2. The use of ART increased over time in all three regions, but remained significantly lower in Eastern Europe. The proportion of patients receiving ART in Eastern Europe increased from 17 to 54% during the first 3 months after start of anti-TB treatment. Proportion of patients on ART in the two other regions was significantly higher at any time point and at 12 months 94 and 89% of patients in Western Europe and Latin America, respectively, were on ART compared with 69% in Eastern Europe, P < 0.001 (Fig. 2). Despite the increase in ART uptake, the proportion of patients with suppressed HIV RNA (<500 copies/ml), among those who were on ART, was lower in Eastern Europe compared with Western Europe and Latin America. At 12 months after TB diagnosis, 48% of patients on ART in Eastern Europe had HIV RNA less than 500 compared with 86 and 71% in Western Europe and Latin America, respectively, P < 0.001 (Fig. 2).
Fig. 2. Use of ART, HIV RNA and CD4+ cell count status in 341 HIV patients with drug-susceptible tuberculosis during 12 months after initiation of anti-TB therapy.
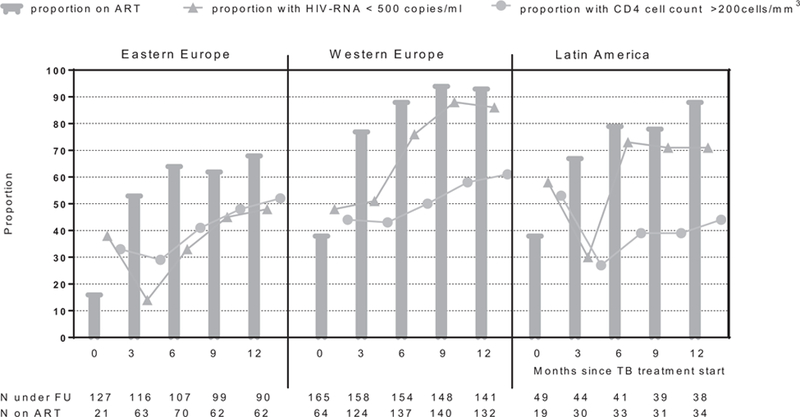
Eastern Europe (Belarus, Estonia, Georgia, Latvia, Lithuania, Poland, Romania, Ukraine, Russia); Western Europe (Belgium, Denmark, France, Italy, Spain, Switzerland, United Kingdom); Latin America (Argentina, Chile, and Mexico).
In addition to describing ARTuse, HIV RNA and CD4+ cell status cross-sectionally at time points after TB diagnosis (Fig. 2), we also looked at the response to ART following the date of ART initiation among those who started ARTat or after TB diagnosis. This analysis showed that in the time period 3–6 months after starting ART, 36% of individuals still alive and under follow-up in Eastern Europe compared with 85% in Western Europe and 80% in Latin America had HIV RNA less than 500 copies/ml (P < 0.001).
At 1 year from baseline, 68 patients (54%) in Eastern Europe, 117 (71%) in Western Europe, and 30 (61%) in Latin America had completed at least six consecutive months of anti-TB treatment, P = 0.009. In total, 17 patients (13%) in Eastern Europe, 17 (10%) in Western Europe, and 7 (14%) in Latin America were lost to follow-up, P = 0.63. In total, 20 (16%) deaths had occurred in Eastern Europe, 7 (4%) in Western Europe, and 4 (8%) in Latin America, P = 0.0031. In Eastern Europe, TB was indicated as the underlying cause of death in 13 (70%) cases, compared with 2 (29%) and 1 (25%) in Western Europe and Latin America, respectively. A majority (9 out of 13) of TB-related death in Eastern Europe occurred within the first 4 months after baseline. The cumulative probability of all cause death after 1 year was 16% (95% confidence interval 11–24%) in Eastern Europe, 4% (2–9%) in Western Europe, and 9% (3–21%) in Latin America (Fig. 3), and numbers did not allow for more detailed analysis on intraregional variability. In the adjusted model, the only significant risk factor for death was region of residence. Thus, treatment of rifampicin and isoniazid-susceptible TB in Eastern Europe was associated with an almost 3-fold increased risk of death compared with Western Europe and Latin America combined (adjusted hazard ratio 2.79; 95% confidence interval 1.15–6.76) (Fig. 4). Association of ART initiation with patients’ prognosis was marginally significant in the unadjusted model only. This association disappeared after adjustment likely because of the small numbers and limited statistical power. IDU was also a significant predictor of death before adjustment, however this also became nonsignificant after adjustment because of the high correlation with the region of residence (Fig. 4)
Fig. 3. Probability of death among 341 HIV patients with rifampicin and isoniazid-susceptible tuberculosis according to their region of residence.

Number of deaths: 20 in EE, 7 in WE and 4 in LA. EE, Eastern Europe (Belarus, Estonia, Georgia, Latvia, Lithuania, Poland, Romania, Ukraine, Russia); LA, Latin America (Argentina, Chile, and Mexico); TB, tuberculosis; WE, Western Europe (Belgium, Denmark, France, Italy, Spain, Switzerland, United Kingdom).
Fig. 4. Cox proportional hazard model of factors associated with 31 deaths in 341 HIV patients with rifampicin- and isoniazid-susceptible tuberculosis.
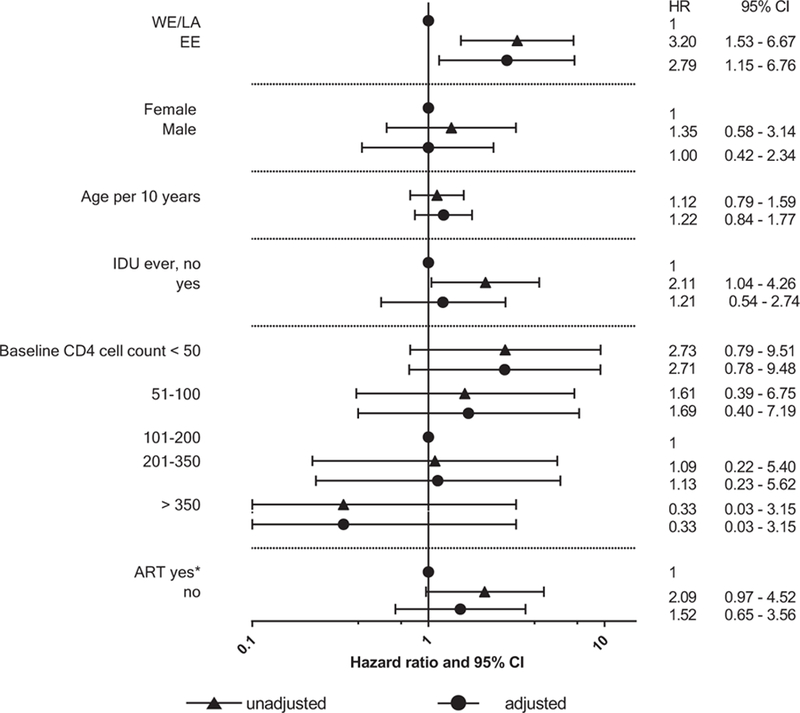
ART, antiretroviral therapy; EE, Eastern Europe (Belarus, Estonia, Georgia, Latvia, Lithuania, Poland, Romania, Ukraine, Russia); HR, hazard ratio; CI, confidence interval; IDU, injecting drug use; LA, Latin America (Argentina, Chile, and Mexico); WE, Western Europe (Belgium, Denmark, France, Italy, Spain, Switzerland, United Kingdom); CD4+ cell count measurement (cells/mm3)
Discussion
The study demonstrates greater mortality rate among HIV patients infected with rifampicin and isoniazid-susceptible TB in Eastern Europe compared with Western Europe and Latin America. This difference persisted after adjustment for clinical characteristics such as IDU, low immune status at the time of TB diagnosis, and receipt of ART during follow-up. Although mortality was similar across the regions within the first 1–2 months, patients in Eastern Europe continued to experience high death rates throughout the first year post-TB diagnosis. By contrast with Eastern Europe, TB/HIV coinfected patients in Latin America, another middle-income region, and in Western Europe, a high-income region, experienced low mortality rates, reflecting high standards of TB and HIV care in the latter mentioned regions [17,21,22]. The differences in TB outcome were only partially explained by factors, related to HIV management, in particular use of ART and CD4+ cell levels, or by other factors included in the model. This underscores the fact that some other factors, probably on a higher level than individual clinics and that we were not able to test, play an important role in patients’ survival, as for example: national and regional healthcare and social infrastructure.
A vast majority of patients from all regions initiated their anti-TB treatment with the standard recommended regimen, that is, rifampicin, isoniazid, and pyrazinamide based indicating that these drugs are widely available and clinicians follow standard recommendations for TB treatment [14]. This is however not enough to ensure a good outcome, particularly in the settings of HIV coinfection. ART should be initiated as soon as possible after TB diagnosis, if a patient was not already receiving it [23–25]. In our study, management of HIV infection in Eastern Europe was suboptimal. Even though ART coverage had increased with time, both usage of ART and response to ART was still much lower than in the other regions, inferring that ART was not properly prescribed and/or taken. By contrast, HIV management in Western Europe was according to the standards; ART coverage was over 90% and nearly 90% of patients on ARTachieved viral suppression [26]. Latin America showed results very close to those in Western Europe, demonstrating that appropriate HIV care is also possible in resource-constrained settings. In our study, initiation of ART did not play a significant role in patients’ survival after adjustment for other risk factors for death. However, binary adjustment for ART initiation (yes vs. no) may not fully reflect the impact of ARTon mortality, and there are other aspects of HIV treatment that we could not adjust for because of the lack of data, and therefore not fully capture all aspects of HIV care. Scale up of ART still remains paramount in the management of HIV infection, although it is also important to ensure that effective drugs are used and that adherence is maximized. In the present study, the most common reason for not starting ART by 2 months after start of anti-TB treatment was patients’ refusal (data not shown), suggesting perhaps that patients may not fully appreciate or are not aware of the life-saving effects of ART. Other factors, as for example, concerns of toxicity, lack of food and/or accommodation might also play a role and need to be overcome. It is therefore a major task not only for clinicians, but also for community and social services to educate, engage and retain patients in care.
One of the drivers of the TB/HIV epidemic in Eastern Europe is IDU, a marginalised population with poor access to and uptake of health services, resulting in late presentation of both HIV and TB, poor rates of TB treatment completion, and frequent ART interruptions [27]. Healthcare systems in Eastern Europe need to better account the special needs of this large patient population, considering its lifestyle and ability to accept continuous treatment [27]. Measures should be taken to help IDU patients to remain in care and overcome structural barriers, improve health literacy, and minimize stigma. There is a need for a patient-centred integrated multidisciplinary approach, involving both HIV and TB clinicians, as well as social support and provision of opiate substitution therapy, which is currently poorly available [28–31]. Integration of HIV and TB services, although improving, is still suboptimal in Eastern Europe and close collaboration of physicians from both specialities is limited. Healthcare infrastructure continues to be driven by a strong vertical system, not allowing for interdisciplinary collaboration [12]. Community involvement and patients’ education and support services need to be improved as well. Of note, we were not able to document any significant differences in mortality within Eastern Europe in explorative analyses stratified by IDU status (yes vs. no), perhaps because of limited numbers, but it may also be because of high prevalence of risk factors such as alcohol abuse and imprisonment among non-IDU individuals. This highlights the need for an approach that supports vulnerable patient groups in addition to individuals who are IDU. Prevalence of HCV coinfection is rather high among HIV/TB patients in Eastern Europe (app. 50% in our study), thus management of this disease should also be integrated.
The study has several strengths and limitations. As a prospective cohort study, our data reflect the ‘real life’ situation in the participating clinics/countries in terms of data availability, such as DST, HIV RNA, CD4+ cell count, at the same time limiting interventions and more careful study of the risk factors, associated with death. Many clinics in Eastern Europe do not have the infrastructure to support patients’ retention in care. Moreover, patients may have to attend different facilities for inpatient and outpatient treatment. Thus the loss to follow-up rate might be high, although major attempts have been taken to minimize it. All missing data were queried, all death, MDR TB cases and at least 10% of random patients were monitored and guidelines on how to minimize loss to follow-up was developed (www.cphiv.dk). The study did not collect detailed data on issues of special relevance for IDU individuals, such as adherence and whether there is an ongoing IDU and how that influences the patients’ life. Because of the limited statistical power, risk factors of death could not be studied in more detail. The fact that the multivariate model did not remove the difference between regions does not exclude the possibility that the variables adjusted for in this model were on the causal pathway. There might be an issue of residual confounding, where the quality and availability of the variables (e.g. HIV related) is insufficient to capture the biological changes that truly occurred. End-stage liver disease because of HCV coinfection might have contributed to the excess mortality in Eastern Europe. However, we were not able to explore this further because of the statistical power limitations. Detailed analysis of causes of death and contributing factors is currently under development. Finally, there may be a general lower life expectancy in Eastern Europe because of differences in socio-economic status, lifestyle, diets, and so on. This is, however, difficult to address in a clinical study and further research is needed.
In conclusion, this study documents an unacceptable high mortality among HIV-positive patients with drug-susceptible TB in Eastern Europe despite widespread use of WHO recommended anti-TB treatment regimens as initial therapy. Management of HIV infection was suboptimal in this region, but did not fully explain the high mortality. Healthcare provision for the TB/HIV population in Eastern Europe needs to be urgently reviewed and improved. This requires a high level of political commitment both locally and globally, and may be achieved by improving collaboration between TB and HIV clinicians, as well as strengthening the existing healthcare infrastructure.
Acknowledgements
Role of each of the contributing authors: D.N.P., A.S., A.M., J.D.L. and O.K. designed the study and analysis plan and wrote the first draft of the report. A.S. performed the statistical analyses under supervision of A.M. and with support for data interpretation by D.N.P., O.K., J.D.L. A.M.W.E., O.K. and D.N.P. coordinated the study. A.P., A.M.S., J.M.M., A.R., H.F., R.F.M., M.H.L., J.T., A.V., and E.G. collected data. All authors interpreted data and critically reviewed and commented on the manuscript. All authors have approved the final version of the manuscript.
Funding: The study has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under EuroCoord grant agreement n° 260694, The Danish Council for Independent Research, the Danish National Research Foundation (grant 126) and the Research Council, Rigshospitalet. TB:HIV study data were pooled with the EuroCoord network (www.EuroCoord.net). We thank the patients who participated in the study and the staff involved at the participating hospitals.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Conference presentation: Results from this study were presented at the 15th European AIDS Conference (EACS), October 21–24, 2015, Barcelona, Spain. Abstract PS2/2.
References
- 1.World Health Organisation. Global tuberculosis report 2015. http://www.who.int/tb/publications/global_report/en/ [Accessed 23 October 2016].
- 2.Acosta CD, Dadu A, Ramsay A, Dara M. Drug-resistant tuberculosis in Eastern Europe: challenges and ways forward. Public Health Action 2014; 4:S3–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeHovitz J, Uuskula A, El-Bassel N. The HIV epidemic in Eastern Europe and Central Asia. Curr HIV/AIDS Rep 2014; 11:168–176. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Drug-resistant TB Surveillance and Response. Supplement to Global Tuberculosis Report 2014. http://www.who.int/tb/publications/global_report/gtbr14_supplement_web_v3.pdf [Accessed 23 October 2016].
- 5.Podlekareva DN, Efsen AM, Schultze A, Post FA, Skrahina AM, Panteleev A, et al. , TB:HIV study group in EuroCoord. Tuberculosis- related mortality in people living with HIV in Europe and Latin America: an international cohort study. Lancet HIV 2016; 3:e120–e131. [DOI] [PubMed] [Google Scholar]
- 6.Skrahina A, Hurevich H, Zalutskaya A, Sahalchyk E, Astrauko A, Hoffner S, et al. Multidrug-resistant tuberculosis in Belarus: the size of the problem and associated risk factors. Bull World Health Organ 2013; 91:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skrahina A, Hurevich H, Zalutskaya A, Sahalchyk E, Astrauko A, van Gemert W, et al. Alarming levels of drug-resistant tuberculosis in Belarus: results of a survey in Minsk. Eur Respir J 2012; 39:1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasipanodya JG, Gumbo T. A meta-analysis of self-administered vs directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis 2013; 57:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen T, Murray M. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat Med 2004; 10:1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange C, Abubakar I, Alffenaar JW, Bothamley G, Caminero JA, Carvalho AC, et al. Management of patients with multidrugresistant/ extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J 2014; 44:23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floyd K, Hutubessy R, Kliiman K, Centis R, Khurieva N, Jakobowiak W, et al. Cost and cost-effectiveness of multidrugresistant tuberculosis treatment in Estonia and Russia. Eur Respir J 2012; 40:133–142. [DOI] [PubMed] [Google Scholar]
- 12.Mansfeld M, Skrahina A, Shepherd L, Schultze A, Panteleev AM, Miller RF, et al. Major differences in organization and availability of healthcare and medicines for HIV/TB coinfected patients across Europe. HIV Med 2015; 16:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Global tuberculosis report 2016. http://www.who.int/tb/publications/global_report/en/ [Accessed 23 October 2016].
- 14.World Health Organization. Treatment of tuberculosis Guidelines Fourth edition. 2009. http://www.who.int/tb/publications/2010/9789241547833/en/ [Accessed 23 October 2016]. [Google Scholar]
- 15.National Institute for Health and Care Excellence (NICE). Tuberculosis NICE guideline. Published: 13 January 2016. www.nice.org.uk/guidance/ng33 [Accessed 23 October 2016]. [Google Scholar]
- 16.Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. , WHO’s Global TB Programme. WHO’s new end TB strategy. Lancet 2015; 385:1799–1801. [DOI] [PubMed] [Google Scholar]
- 17.Pozniak AL, Coyne KM, Miller RF, Lipman MC, Freedman AR, Ormerod LP, et al. , BHIVA Guidelines Subcommittee. British HIV Association guidelines for the treatment of TB/HIV coinfection 2011. HIV Med 2011; 12:517–524. [DOI] [PubMed] [Google Scholar]
- 18.INSIGHT START Study Group. Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365:1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efsen AM, Schultze A, Post FA, Panteleev A, Furrer H, Miller RF, et al. , TB: HIV study group in EuroCoord. Major challenges in clinical management of TB/HIV coinfected patients in Eastern Europe compared with Western Europe and Latin America. PLoS One 2015; 10:e0145380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podlekareva DN, Reekie J, Mocroft A, Losso M, Rakhmanova AG, Bakowska E, et al. , EuroSIDA study in EuroCoord. Benchmarking HIV healthcare: from individual patient care to healthcare evaluation. An example from the EuroSIDA study. BMC Infect Dis 2012; 12:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podlekareva DN, Grint D, Post FA, Mocroft A, Panteleev AM, Miller RF, et al. , HIV-TB Study Group. Healthcare index score and risk of death following tuberculosis diagnosis in HIVpositive patients. Int J Tuberc Lung Dis 2013; 17:198–206. [DOI] [PubMed] [Google Scholar]
- 23.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. , CAMELIA (ANRS 1295–CIPRA KH001) Study Team. Earlier versus later start of antiretroviral therapy in HIVinfected adults with tuberculosis. N Engl J Med 2011; 365:1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. , AIDS Clinical Trials Group Study A5221. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Recommendations for a public health approach; - Second edition. 2016. http://www.who.int/hiv/pub/arv/arv-2016/en/ [Accessed 23 October 2016]. [PubMed] [Google Scholar]
- 26.UNAIDS. 90–90-90 An ambitious treatment target to help end the AIDS epidemic 2014. www.unaids.org/en/resources/documents/ 2014/90-90-90 [Accessed 23 October 2016].
- 27.Wolfe D Paradoxes in antiretroviral treatment for injecting drug users: access, adherence and structural barriers in Asia and the former Soviet Union. Int J Drug Policy 2007; 18: 246–254. [DOI] [PubMed] [Google Scholar]
- 28.Morozova O, Dvoryak S, Altice FL. Methadone treatment improves tuberculosis treatment among hospitalized opioid dependent patients in Ukraine. Int J Drug Policy 2013; 24:e91–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend 2014; 134:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew TA, Yanov SA, Mazitov R, Mishustin SP, Strelis AK, Yanova GV, et al. , Tomsk Tuberculosis Alcohol Working Group. Integration of alcohol use disorders identification and management in the tuberculosis programme in Tomsk Oblast, Russia. Eur J Public Health 2009; 19:16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Integrating collaborative TB and HIV services within a comprehensive package of care for people who inject drugs: consolidated guidelines 2016. www.who.int/tb/publications/integrating-collaborative-tb-and-hiv_services_for_pwid/en/ [Accessed 23 October 2016].


