Abstract
BACKGROUND
Antibodies that block programmed death 1 (PD-1) protein improve survival in patients with advanced non–small-cell lung cancer (NSCLC) but have not been tested in resectable NSCLC, a condition in which little progress has been made during the past decade.
METHODS
In this pilot study, we administered two preoperative doses of PD-1 inhibitor nivolumab in adults with untreated, surgically resectable early (stage I, II, or IIIA) NSCLC. Nivolumab (at a dose of 3 mg per kilogram of body weight) was administered intravenously every 2 weeks, with surgery planned approximately 4 weeks after the first dose. The primary end points of the study were safety and feasibility. We also evaluated the tumor pathological response, expression of programmed death ligand 1 (PD-L1), mutational burden, and mutation-associated, neoantigen-specific T-cell responses.
RESULTS
Neoadjuvant nivolumab had an acceptable side-effect profile and was not associated with delays in surgery. Of the 21 tumors that were removed, 20 were completely resected. A major pathological response occurred in 9 of 20 resected tumors (45%). Responses occurred in both PD-L1-positive and PD-L1-negative tumors. There was a significant correlation between the pathological response and the pretreatment tumor mutational burden. The number of T-cell clones that were found in both the tumor and peripheral blood increased systemically after PD-1 blockade in eight of nine patients who were evaluated. Mutation-associated, neoantigen-specific T-cell clones from a primary tumor with a complete response on pathological assessment rapidly expanded in peripheral blood at 2 to 4 weeks after treatment; some of these clones were not detected before the administration of nivolumab.
CONCLUSIONS
Neoadjuvant nivolumab was associated with few side effects, did not delay surgery, and induced a major pathological response in 45% of resected tumors. The tumor mutational burden was predictive of the pathological response to PD-1 blockade. Treatment induced expansion of mutation-associated, neoantigen-specific T-cell clones in peripheral blood. (Funded by Cancer Research Institute–Stand Up 2 Cancer and others; ClinicalTrials.gov number, NCT02259621.)
ANTIBODIES THAT BLOCK THE IMMUNE inhibitory pathway of programmed death 1 (PD-1) protein have provided a major treatment advance in patients with cancer.1 In some patients with advanced non–small-cell lung cancer (NSCLC), these drugs unleash antitumor immunity, resulting in tumor regression and improved survival.2-6
Effective therapies are needed for patients with early-stage NSCLC. Five-year survival rates range from 50% for stage IA disease to 20% for stage IIIA disease, with most patients having postsurgical tumor relapse.7 Perioperative platinum-based chemotherapy is associated with a survival rate that is only 5.4 percentage points higher than that with surgery alone, with rates of toxic effects of grade 3 or higher of more than 60%.8,9 PD-1 pathway blockade in patients with early-stage lung cancer may have enhanced antitumor effects owing to greater fitness of host immunity and reduced tumor clonal heterogeneity.10 Neoadjuvant immunotherapy is attractive, since the primary tumor may be leveraged as an antigen source for expansion and activation of tumor-specific T cells and systemic surveillance of micrometastases. In addition, neoadjuvant approaches provide an opportunity to study the in vivo effect of PD-1 blockade on the primary-tumor microenvironment and peripheral blood.
We performed this pilot study to examine the safety and feasibility of the use of neoadjuvant nivolumab in a small group of patients with early NSCLC. We also examined the relationship between the tumor genomic profile and the pathological response, the effects of nivolumab on the primary-tumor microenvironment, and the dynamics of intratumoral and peripheral neoantigen-specific T-cell clonotypes.
METHODS
PATIENTS
Eligible patients were 18 years of age or older and had stage I, II, or IIIA NSCLC that was deemed to be surgically resectable before enrollment. All the patients had an Eastern Cooperative Oncology Group performance-status score of 0 or 1 (on a 5-point scale in which higher numbers reflect greater disability), normal organ function, and adequate pulmonary function.11 Key exclusion criteria were immunodeficiency, ongoing systemic immunosuppressive therapy, active autoimmune or infectious disease, and clinically significant concurrent cancer.
STUDY DESIGN
This single-group study was developed by the authors and conducted at two medical centers in the United States. The patients received two doses of intravenous nivolumab (at a dose of 3 mg per kilogram of body weight) every 2 weeks. It was planned that surgery would be performed approximately 4 weeks after the first dose. All the patients provided written informed consent.
The primary end points were safety and feasibility. The key secondary and exploratory end points were radiologic and pathological responses to treatment and immunologic, genomic, and pathological correlates of response in blood and tumor (Fig. S1 in Supplementary Appendix 1, available with the full text of this article at NEJM.org).
All the patients were monitored for adverse events, according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.12 Feasibility was prospectively defined as any delay in the planned surgery of no more than 37 days (i.e., a surgical delay of >30 days and 7 days for scheduling). In a safety run-in phase, an initial 6 patients were followed for perioperative adverse events of grade 3 or 4 for 90 days after the administration of the last nivolumab dose (or day 30 after surgery). With the goal of exploring the antitumor immune response in depth, the study then expanded to enroll a total of 20 patients who underwent complete tumor resection.
All the patients underwent baseline tumor staging, including pretreatment biopsy, pathological evaluation of mediastinal lymph nodes (if indicated) by means of bronchoscopy or mediastinoscopy, positron-emission tomography–computed tomography (PET–CT), and contrast-enhanced CT or magnetic resonance imaging of brain and chest; chest CT was repeated within 7 days before surgery. Changes in tumor size were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.13 Resection of the primary tumor and lymph nodes was completed according to institutional standards. All the patients were offered conventional adjuvant chemotherapy or radiotherapy, if such therapy was clinically indicated, and were followed for recurrence-free and overall survival.
STUDY OVERSIGHT
This study was approved by the institutional review boards at Johns Hopkins University and Memorial Sloan Kettering Cancer Center. The study was designed and the manuscript was written by the authors, who vouch for the accuracy and completeness of the data reported and adherence to the study protocol. No one who is not an author contributed to the writing of the manuscript. Nivolumab was supplied by Bristol-Myers Squibb; the company played no other role in the study or the report. The study protocol is available at NEJM.org
PATHOLOGICAL ASSESSMENTS
Primary lung tumor and lymph-node surgical specimens were staged according to the criteria of the American Joint Committee on Cancer (seventh edition) for evaluating tumor size, affected lymph nodes, and metastases.7 Primary tumors were assessed for the percentage of residual viable tumor that was identified on routine hematoxylin and eosin staining, and tumors with no more than 10% viable tumor cells were considered to have had a major pathological response.14,15 The results of immunohistochemical and multiplex immunofluorescence analyses of tumors are described in the Methods section in Supplementary Appendix 1.16
EXOME SEQUENCING AND NEOANTIGEN PREDICTION
Details regarding whole-exome sequencing, which was performed on pretreatment tumor samples and matched normal tissue samples, and bioinformatics analyses are provided in Supplementary Appendix 2, available at NEJM.org. We compared tumor and normal sequence data to identify somatic and germline alterations using the VariantDx software pipeline, focusing on single-base substitutions, small insertions and deletions, and copy-number changes.17 We applied exome data combined with each patient’s major histocompatibility complex (MHC) class I haplotype in a neoantigen prediction platform that evaluates binding affinities of somatic peptides to class I MHC, antigen processing, self-similarity, and gene expression.18
IMMUNOLOGIC ANALYSES AND T-CELL RECEPTOR SEQUENCING
We performed assays to evaluate immunologic correlates, as described in the Methods section in Supplementary Appendix 1. Briefly, we used T-cell receptor DNA sequencing to define T-cell clonal distribution and functional specificity for mutant tumor antigens.
STATISTICAL ANALYSIS
Side effects, adverse events, and feasibility were continuously monitored. We made the assumption that treatment would not be feasible if the probability that surgery would be delayed was 90% or more for more than 25% of the patients. We also determined that the treatment was not safe if the probability was 70% or more that the risk of grade 3 or 4 toxic effects was more than 25%. We used the reverse Kaplan-Meier method to calculate the median duration of follow-up. The follow-up range includes all follow-up times. We calculated recurrence-free survival from the date of surgery until disease recurrence or death using the Kaplan-Meier method.
We performed pathological, genomic, and immunologic analyses on available biospecimens, and correlative data were analyzed as described in the Methods section in Supplementary Appendix 1. Reported P values are two-sided, and the significance level was set at 0.05 for all analyses unless otherwise noted.
RESULTS
CHARACTERISTICS OF THE PATIENTS
From August 2015 through October 2016, we enrolled 22 patients, all of whom received at least one dose of nivolumab. Of these patients, 21 were eligible for inclusion in the study (Table 1). Among them, 62% had adenocarcinoma, 81% had stage II or IIIA disease, and 86% were current or former smokers. One patient was discovered to have small-cell lung carcinoma and discontinued the study treatment.
Table 1.
Characteristics of the Patients at Baseline, According to Pathological Response.*
| Characteristic | All Patients (N = 21) |
Patients with Major Pathological Response (N = 9) |
Patients without Major Pathological Response (N = 11)† |
|---|---|---|---|
| Age at enrollment — yr | |||
| Mean ±SD | 66.9±8.3 | 67.7±8.3 | 65.8±8.5 |
| Median (range) | 67 (55–84) | 66 (57–79) | 67 (55–84) |
| Sex — no. (%) | |||
| Female | 11 (52) | 6 (67) | 4 (36) |
| Male | 10 (48) | 3 (33) | 7 (64) |
| Histologic diagnosis — no. (%) | |||
| Adenocarcinoma | 13 (62) | 6 (67) | 6 (55) |
| Squamous-cell carcinoma | 6 (29) | 2 (22) | 4 (36) |
| Other‡ | 2 (10) | 1 (11) | 1 (9) |
| Clinical disease stage — no. (%)§ | |||
| I | 4 (19) | 2 (22) | 2 (18) |
| II | 10 (48) | 5 (56) | 5 (45) |
| IIIA | 7 (33) | 2 (22) | 4 (36) |
| Smoking status — no. (%) | |||
| Never | 3 (14) | 1 (11) | 2 (18) |
| Former or current | 18 (86) | 8 (89) | 9 (82) |
A major pathological response was defined as the identification of 10% or less of residual viable tumor cells in the resected primary tumor. Percentages may not total 100 because of rounding.
One patient’s tumor was unresectable and therefore could not be evaluated for pathological response.
Other histologic diagnoses were pleomorphic carcinoma in a patient with a major pathological response and adenosquamous carcinoma in a patient without a major pathological response.
The clinical disease stage was evaluated according to the criteria of the American Joint Committee on Cancer, seventh edition.
SAFETY AND FEASIBILITY
Neoadjuvant nivolumab was not associated with any previously unreported toxic effects. Treatment-related adverse events of any grade occurred in 5 of 22 patients (23%; 95% confidence interval [CI], 7.8 to 45.4), and only one event was of grade 3 or higher (Table S1 in Supplementary Appendix 1). Of the 22 patients, 20 received the two planned doses of nivolumab; one patient with grade 3 pneumonia underwent an uncomplicated surgical resection after one dose of nivolumab, and another was deemed to be ineligible for the study according to the protocol. There were no treatment-related surgical delays, as defined in the protocol. The median interval between the administration of the second dose of nivolumab and surgery was 18 days (range, 11 to 29), and 20 of the 21 eligible patients (95%) underwent complete tumor resection. During surgery, one patient with stage IIIA NSCLC was found to have tracheal invasion, so complete tumor resection could not be performed.
CLINICAL ACTIVITY
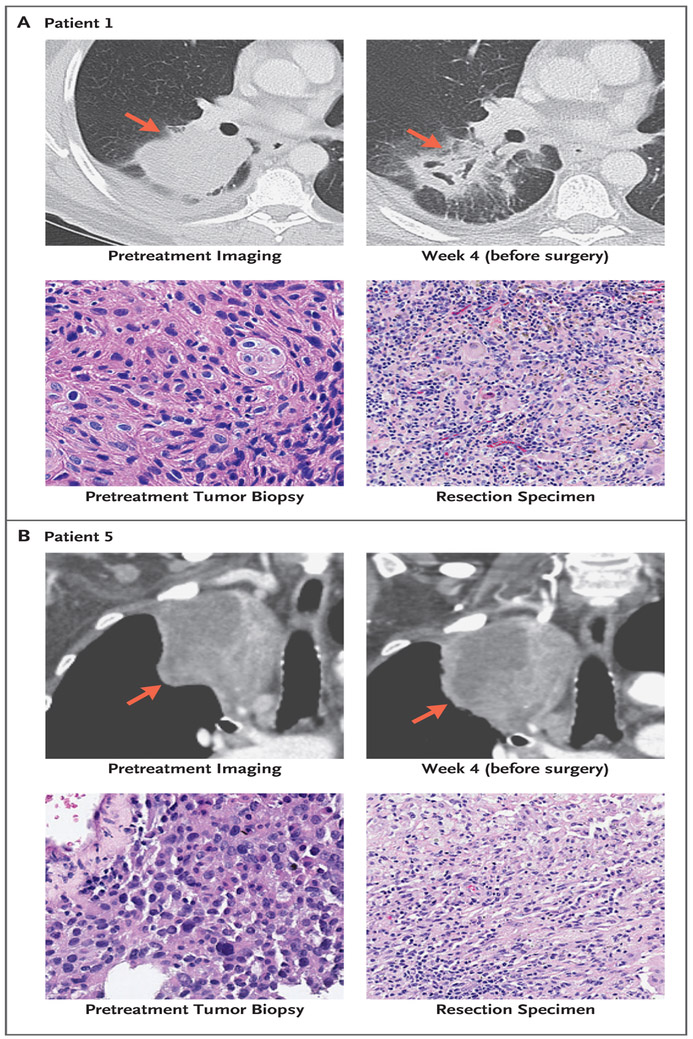
Representative radiologic and pathological responses after two preoperative doses of nivolumab are shown in Figure 1. Of the 21 patients who had radiographic results that could be evaluated, 2 patients (10%) had a partial response, 18 (86%) had stable disease, and 1 (5%) had disease progression. Among the 20 patients who had undergone resection, pathological down-staging from the pretreatment clinical stage occurred in 8 patients (40%) (Tables S2 and S3 in Supplementary Appendix 1).
Figure 1. Patterns of Radiologic and Pathological Response to Neoadjuvant Therapy with Nivolumab.
Panel A shows computed tomographic (CT) imaging of the chest of a 62-year-old male smoker (Patient 1) with stage IIB squamous lung cancer before and after the administration of nivolumab. In the upper row, the pretreatment scan before the infusion of nivolumab shows a primary tumor mass measuring 8 cm in diameter in the lower right lobe of the lung (arrow).A scan performed before surgery shows 35% shrinkage with associated tumor cavitation (arrow). In the lower row, shown are representative sections of tumor specimens obtained from Patient 1 before the administration of nivolumab (left) and after the administration (right) (hematoxylin and eosin staining). This patient had 100% pathological regression of the large primary lung tumor but had residual lymph-node metastases in the resection specimen. Panel B shows CT of the chest of a 78-year-old female former smoker (Patient 5) with stage IIIA lung adenocarcinoma, who received two doses of nivolumab preoperatively. In the upper row, the tumor is larger on imaging performed after the administration of nivolumab than before administration (arrows), possibly because of infiltration of immune cells into the tumor. In the lower row, shown are representative sections of tumor specimens obtained from Patient 5 before the administration of nivolumab (left) and after administration (right) (hematoxylin and eosin staining). Neoplastic cells are present throughout the pretreatment specimen, whereas in the post-treatment specimen, there was 90% tumor regression.
At a median of 12 months of postoperative follow-up (range, 0.8 to 19.7), 16 of 20 patients (80%) who had undergone surgical resection were alive and recurrence-free. One patient without recurrence died from a traumatic head injury that was unrelated to the study treatment. Three patients had disease progression. The first patient, who had 75% residual primary tumor at resection, was found to have a solitary brain lesion 2 months after surgery; after treatment with stereotactic radiation therapy, the patient had no further recurrence at more than 16 months of follow-up. A second patient, who had 5% residual tumor at resection, had a mediastinal lymph-node recurrence that was treated with concurrent chemotherapy and radiation therapy and was free from further progression at more than 12 months of follow-up. A third patient, who had 80% residual tumor, had distant metastatic disease 1 year after surgical resection and died from recurrent lung cancer 4 months later.
The median duration of recurrence-free survival had not been reached at the time of data analysis. The rate of recurrence-free survival at 18 months was 73% (95% CI, 53 to 100) (Fig. S2 in Supplementary Appendix 1).
PATHOLOGICAL FINDINGS AFTER NEOADJUVANT PD-1 BLOCKADE
A major pathological response occurred in 9 of 20 patients (45%; 95% CI, 23 to 68) who had samples that could be evaluated (Fig. 2A, and Table S3 in Supplementary Appendix 1). Three patients had a complete pathological response (i.e., no viable tumor cells) in the primary tumor; however, one of these patients had residual tumor in hilar lymph nodes (Fig. 1A, and Fig. S7 in Supplementary Appendix 1). The median degree of pathological regression in the primary tumor was −65% (range, −100 to 0). Despite apparent tumor enlargement on preoperative CT (possibly because of immune-cell infiltration into the tumor), one patient had a major pathological response (Fig. 1B), and another had a complete pathological response. In primary tumors with a major pathological response, we observed large numbers of infiltrating lymphocytes and macrophages, a finding that was compatible with an immunologic mechanism of response, along with necrotic tumor associated with fibrotic tissue repair. The expression of programmed death ligand 1 (PD-L1) could be evaluated in pretreatment biopsy samples obtained from 15 patients (Table S2 in Supplementary Appendix 1). A major pathological response occurred in both PD-L1-positive and PD-L1-negative tumors (Fig. 2A).
Figure 2. Pathological Assessment of Response to Neoadjuvant Blockade of Programmed Death 1 (PD-1).
Panel A shows pathological regression in the resected primary lung tumor after neoadjuvant administration of nivolumab, according to the percentage of remaining viable tumor cells, for each of the 20 patients who underwent surgical resection. The gray horizontal line indicates the threshold for a major pathological response (90% regression). Clinical and pathological features that include the presence or absence of lymph-node (LN) metastases in the surgical specimen and preoperative radiologic response (according to Response Evaluation Criteria in Solid Tumors [RECIST]) are annotated for each patient. AC denotes adenocarcinoma, SCC squamous-cell carcinoma, PR partial response, SD stable disease, and PD-L1 programmed death ligand 1. Also shown are biopsy specimens obtained before (Panel B) and after (Panel C) neoadjuvant administration of nivolumab in a patient (MD043-008) who had a major pathological response (multiplex immunofluorescence staining). With this staining technique, visible structures include cytokeratin-positive tumor cells (orange), CD68+ macrophages (magenta), FoxP3+ regulatory T cells (yellow), CD8+ T cells (green), PD-1+ cells (red), and PD-L1+ cells (white). In the pretreatment specimen, only a few intratumoral macrophages are seen expressing PD-L1. However, there are multiple foci where PD-L1 and PD-1 are expressed in close proximity to each other (inset with white circle) in the pretreatment specimen. Focal, geographic tumor-cell PD-L1 expression was observed in an adaptive pattern (not shown in this image). After two doses of nivolumab, the tumor is infiltrated by liquid containing CD8+ and PD-1+ immune cells. Some of the infiltrating immune cells express PD-L1, which is consistent with an adaptive immune resistance mechanism.19,20
One patient with a major pathological response had three pretreatment biopsy samples with PD-L1-negative tumor cells but PD-L1-positive infiltrating immune cells. To further explore this case, multiplex immunofluorescence analysis was performed. A pretreatment specimen contained PD-L1-positive, CD68+ macrophages abutting PD-1-positive, CD8+ T cells (Fig. 2B). The surgical specimen contained an influx of CD8+ T cells and had a higher expression of PD-L1 on immune cells than was found in the pretreatment biopsy sample, a finding that was consistent with an adaptive PD-L1 up-regulation mechanism (Fig. 2C).19,20
GENOMIC ANALYSES
To examine the landscape of genomic alterations, predicted neoantigens, and their potential association with pathological response, we performed whole-exome sequencing of pretreatment tumors obtained from 12 patients who had adequate available tissue (Tables S4 through S7 in Supplementary Appendix 2).17 A median of 92 somatic mutations (range, 5 to 366) per tumor were noted and specific driver mutations identified, including in TP53, KRAS, CDKN2A, ARID1A, NOTCH1, and RB1, findings that were consistent with previous observations in patients with NSCLC (Tables S8, S9, and S10 in Supplementary Appendix 2 and Fig. S3 in Supplementary Appendix 1).21,22
Of the 12 patients who provided samples for sequencing, 11 had undergone complete tumor resection and could be evaluated for tumor response. Among these 11 patients, a significantly higher mean (±SE) mutational burden was observed in tumors with a major pathological response than in tumors without a major response (311±55 vs. 74±60, P=0.01 by exact Wilcoxon test) (Fig. 3A). The number of sequence alterations was inversely associated with the percentage of residual tumor (Fig. 3B). No significant correlation was noted between the mutational burden and tumor PD-L1 expression. Candidate mutation-associated neoantigens were computationally predicted from somatic variants in pretreatment tumors and therefore were proportional to the mutational burden. On average, 89 algorithm-predicted, mutation-associated neoantigens per primary tumor were identified (median, 63; range, 1 to 219) (Table S8 in Supplementary Appendix 2). There was a correlation between the candidate mutation-associated neoantigen burden and the pathological response, a finding similar to that for the mutational burden (Fig. S4 in Supplementary Appendix 1).
Figure 3. Association between Mutational Burden and Pathological Response to PD-1 Blockade.
Panel A shows the number of sequence alterations in pretreatment tumor samples obtained from 11 patients who underwent surgical resection and had sufficient pretreatment tissue available for sequencing, with differential responses to PD-1 blockade. Patients who had a major pathological response were found to carry a significantly higher number of somatic sequence alterations than those without a major pathological response, with a mean (±SE) number of 311±55 and 74±60, respectively (P=0.01 by exact Wilcoxon test). Panel B shows the number of sequence alterations per tumor, which was found to be inversely associated with the percentage of residual viable tumor cells after nivolumab treatment (Spearman’s rho, −0.75; P=0.008). The dashed black line indicates the linear regression line, and the dashed gray lines indicate the upper and lower boundaries of the 95% confidence interval. AC denotes adenocarcinoma, AS adenosquamous carcinoma, and SCC squamous-cell carcinoma.
We investigated the potential enrichment of alterations in specific genes or gene pathways, including Ras signaling, DNA repair, and interferon-γ, but did not identify differential enrichment in patients with or without a major pathological response. We found no alterations in immune-related genes of interest, including CD274, PDCD1, CTLA4, B2M, and HLA, or inactivating mutations in JAK1 and JAK2.
DYNAMICS OF ANTITUMOR IMMUNE RESPONSE
To test the hypothesis that nivolumab increased the frequency of tumor-specific T-cell clones in the tumor as well as systemically, we initially performed deep sequencing of T-cell receptor-β chain CDR3 regions (TCRseq) to examine the effects of the drug on the repertoire of T-cell clones found both in tumors and in peripheral blood in 9 patients (3 with a major pathological response and 6 without a major pathological response)23 (Table S2 in Supplementary Appendix 1). At the time of resection, tumors with a major pathological response had a higher frequency of T-cell clones that were shared between intratumoral and peripheral compartments and a higher clonality of the T-cell population (i.e., a higher proportion of total T cells constituted by a restricted number of distinct clones) than did tumors without a major pathological response (Fig. S5A in Supplementary Appendix 1). This finding is in concordance with analyses in patients with advanced melanoma, who had increased clonality of tumor-infiltrating lymphocytes in response to PD-1 blockade.24
After treatment, 8 of 9 patients for whom blood samples obtained before and after treatment were available had peripheral expansion of multiple T-cell clones that were also found in the tumor at the time of resection. Many of these clones were not detected in the peripheral blood before treatment (Fig. S6 in Supplementary Appendix 1).
Longitudinal analysis of Patient 1, in whom the primary tumor had had a complete pathological response (Fig. 1A, and Tables S2 and S3 and Fig. S7 in Supplementary Appendix 1), showed a large but transient expansion of shared intratumoral T-cell clones in the peripheral blood after treatment initiation and before surgical resection. All these clones were more frequent in the resected tumor than in normal lung tissue (Fig. S5B in Supplementary Appendix 1).
To address the hypothesis that a substantial component of antitumor immunity after PD-1 blockade is directed toward mutation-associated neoantigens, we used a recently described assay to identify and functionally evaluate mutation-associated, neoantigen-specific T-cell clones in blood obtained on the day of nivolumab initiation (day −28) and at 44 days after surgery (day 44).25 We used 47 candidate neoantigen peptides to stimulate CD8+ lymphocytes in culture, and T-cell clonal expansion was analyzed by means of TCRseq. A statistical algorithm was used to define mutation-associated neoantigens that induced specific expansion of at least one T-cell clone. In pretreatment blood samples, specific T-cell responses were detected against 19 of the 47 candidate mutation-associated neoantigens, 7 of which were still detectable at day 44. In addition, 7 new mutation-associated, neoantigen-specific T-cell responses had developed by day 44, which supports the notion that neoadjuvant PD-1 blockade induces new systemic tumor-specific reactivity. Three T-cell clones in peripheral blood samples that were obtained on day 44 and that were reactive against mutation-associated neoantigen number 7 collectively represented 1.7% of all lymphocytes infiltrating the pretreatment tumor specimen; these clones were also detected in both a resected lymph node without tumor infiltration and a resected lymph node that was microscopically infiltrated (Fig. 4). These clones, which expanded in an independent replicate stimulation, showed transient expansion in peripheral blood after the initiation of PD-1 blockade, a finding that was reminiscent of antiviral T-cell responses.
Figure 4. Identification of Mutation-Associated, Neoantigen-Specific T Cells after Neoadjuvant Treatment with Nivolumab.
An antigen-recognition assay that evaluates in vitro expansion of T-cell clones after peptide stimulation was performed with the use of 47 algorithm-predicted, candidate mutation-associated neoantigens on peripheral-blood T cells obtained from Patient 1 before the administration of nivolumab (on day −28) and after administration (on day 44 after surgical resection) to determine the repertoire of functional mutation-associated neoantigen recognition. Seven mutation-associated neoantigens were recognized at both time points. The 47 peptides that were chosen for analysis represented the top 10 ImmunoSelect-R pipeline (Personal Genome Diagnostics) peptides that were predicted to bind specifically to each of the patient’s five HLA class I alleles. Three peptides were excluded because they were predicted to bind multiple HLA alleles. Three T-cell clones from a peripheral-blood sample obtained on day 44 that specifically expanded in culture to mutation-associated neoantigen (MANA) number 7 were found in the pretreatment tumor-biopsy specimen and the resection specimen (Panel A). Also shown are the frequencies of the three T-cell clones in longitudinal analysis of peripheral-blood T cells before and after treatment (Panel B) and in a tumor-biopsy sample before treatment, in resected tumor, in normal lung, and in tumor-involved and tumor-uninvolved lymph nodes at resection (Panel C). TCR Vβ CDR3 AA denotes T-cell receptor Vγ complementarity-determining region 3 amino acid.
DISCUSSION
We observed that neoadjuvant administration of two doses of nivolumab in patients with early-stage lung cancer was associated with few immediate adverse events, did not delay planned surgery, and led to a major pathological response in 45% of tumors that could be evaluated. Despite the high rate of major pathological response on histologic examination of the primary lung tumors, only two patients had a radiologic partial response. Two patients in whom tumors had increased in size on presurgical CT scans (although the increase was less than RECIST-defined progression) were found to have minimal or no residual tumor in the surgical specimen. These findings represent pathological evidence supporting the possibility that some patients may derive clinical benefit from immunotherapy without initial radiographic tumor shrinkage and that this process occurs because of immune-cell infiltration into the tumor, rather than true tumor growth.26,27
We also examined the possibility that neoadjuvant PD-1 blockade could enhance the systemic priming of antitumor T cells, thereby potentially eliminating micrometastatic cancer that otherwise might cause postsurgical relapse. Preclinical tumor studies in mice had underpinned this concept.28 Studies in mice had also shown the importance of dendritic cells in the antitumor effects of PD-1 pathway blockade, which suggested that PD-1 blockade not only worked to directly unleash intratumoral T-cell killing but also enhanced tumor antigen–driven priming of T cells.29 Indeed, PD-1 blockade had been shown to enhance the early stages of T-cell activation in lymph nodes.30 We hypothesized that when neoadjuvant PD-1 inhibitors are administered, tumor-specific T cells are expanded in the primary tumor mass (as has been shown in advanced melanoma) and will then travel systemically to other tissues and seek out the micrometastatic tumor deposits that drive postsurgical relapse. This notion is supported by our findings that a substantial number of T-cell clones that had expanded in the peripheral blood after PD-1 blockade were found in the tumor and that the sum proportion of T-cell clones that were shared between tumor and peripheral blood before treatment was significantly higher in patients who went on to have a major pathological response. Using our assay for evaluation of the T-cell repertoire, we showed in a patient with a major pathological response that T-cell clones specific for mutation-associated neoantigens were rapidly expanded in peripheral blood after neoadjuvant PD-1 blockade. Three of these clones were found in the resected tumor and lymph nodes.
The achievement of a major pathological response in patients with early-stage lung cancer was highly associated with an increased tumor mutational burden, a finding that was reminiscent of responses to PD-1 blockade in patients with advanced NSCLC. More important, our findings indicate that the mutational burden is a primary determinant of the depth of pathological response to PD-1 blockade. Furthermore, after PD-1 blockade, multispectral analyses of a tumor with a major pathological response showed new infiltration with PD-1-positive CD8+ T cells. A major pathological response was observed in some tumors lacking tumor-cell PD-L1 expression but in which infiltrating immune cells were highly positive for PD-L1, findings that are reminiscent of PD-L1 expression patterns in colorectal cancers with mismatch-repair deficiency.31
Neoadjuvant treatment is conventionally used to shrink tumors before surgery. The response to neoadjuvant therapy as assessed at the time of surgery may also be prognostic. In a study of neoadjuvant chemotherapy for nonsquamous NSCLC, 22% of tumors had a major pathological response to therapy, and such responses were associated with long-term survival.32 In this context, the rate of major pathological response of 45% that was reported in our pilot study is encouraging, as is the relatively low number of recurrences after a median of 1 year of follow-up. In other studies, retrospective analyses have suggested that the occurrence of a major pathological response after neoadjuvant chemotherapy was associated with improved recurrence-free and overall survival, and this association may be stronger than other potential surrogates.14,15
The limitations of our study include, but are not limited to, the small number of patients who were enrolled and the short postoperative follow-up period. Larger studies are needed to determine the most effective duration of neoadjuvant therapy and the best predictive biomarkers of response and to correlate the pathological response resulting from neoadjuvant immunotherapy with overall survival. Neoadjuvant immune checkpoint blockade is being investigated across a diverse range of tumor types and settings, including phase 3 trials in breast cancer and NSCLC (ClinicalTrials.gov numbers, NCT03036488, NCT02998528, NCT02519322, NCT02296684, NCT02845323, and NCT02575222). Long-term follow-up of these studies will be necessary to define the role of neoadjuvant PD-1 blockade in reducing recurrences and curing early-stage cancers.
Supplementary Material
Acknowledgments
Supported by a Cancer Immunology Translational Cancer Research Grant (SU2C-AACR-DT1012) from Cancer Research Institute-Stand Up 2 Cancer, Johns Hopkins Bloomberg–Kimmel Institute for Cancer Immunotherapy, Bristol-Myers Squibb, International Immuno-Oncology Network, LUNGevity Foundation, International Association for the Study of Lung Cancer, Prevent Cancer Foundation, Lung Cancer Foundation of America, MacMillan Foundation, Eastern Cooperative Oncology Group-American College of Radiology Imaging Network, grants (CA121113, CA006973, CA180950, T32 CA193145, and R01 CA142779) from the National Institutes of Health, Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, Commonwealth Foundation, a grant (P30 CA008748) from Memorial Sloan Kettering Cancer Center, and a grant (P30 CA006973) from Johns Hopkins University Cancer Center. Stand Up 2 Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and their families who participated in this research; and Matthew Lindsley, Barbara Coleman, Heather Schneider, Vilmos Adleff, Carolyn Hruban, Lexa Hartman, and other members of our clinical and laboratory research teams for their assistance in these studies.
Contributor Information
P.M. Forde, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
J.E. Chaft, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine in New York
K.N. Smith, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
V. Anagnostou, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
T.R. Cottrell, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
M.D. Hellmann, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine in New York
M. Zahurak, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
S.C. Yang, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
D.R. Jones, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine in New York
S. Broderick, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
R.J. Battafarano, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
M.J. Velez, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine in New York
N. Rekhtman, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine in New York
Z. Olah, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine in New York
J. Naidoo, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
K.A. Marrone, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
F. Verde, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
H. Guo, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
J. Zhang, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
J.X. Caushi, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
H.Y. Chan, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
J.-W. Sidhom, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
R.B. Scharpf, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
J. White, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
E. Gabrielson, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
H. Wang, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
G.L. Rosner, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
V. Rusch, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine in New York
J.D. Wolchok, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine in New York; the Ludwig Collaborative in New York; the Parker Institute for Cancer Immunotherapy, San Francisco charlotte, NC; Swim Across America Laborarotary, Charlotte, NC
T. Merghoub, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine and the Ludwig Collaborative in New York; the Parker Institute for Cancer Immunotherapy, San Francisco and Swim Across America Laboratory, Charlotte, NC.
J.M. Taube, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
V.E. Velculescu, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
S.L. Topalian, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
J.R. Brahmer, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
D.M. Pardoll, Bloomberg–Kimmel Institute for Cancer Immunotherapy and the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore
REFERENCES
- 1.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced nonsmall-cell lung cancer. J Clin Oncol 2015; 33:2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016;375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 5.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 7.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of Malignant Tumours. J Thorac Oncol 2007; 2:706–14. [DOI] [PubMed] [Google Scholar]
- 8.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552–9. [DOI] [PubMed] [Google Scholar]
- 9.Wakelee HA, Dahlberg SE, Keller SM, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2017;18:1610–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- 12.Revised National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 for adverse event reporting (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40). [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 14.Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014; 15(1):e42–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 2016;374:2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones S, Anagnostou V, Lytle K, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med 2015;7:283ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anagnostou V, Smith KN, Forde PM, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov 2017;7:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraldo NA, Kaunitz GJ, Cottrell TR, et al. The differential association of PD-1, PD-L1, and CD8+ cells with response to pembrolizumab and presence of Merkel cell polyomavirus (MCPyV) in patients with Merkel cell carcinoma (MCC). Cancer Res 2017;77:662. abstract. [Google Scholar]
- 20.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational land scape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava SK, Robins HS. Palindromic nucleotide analysis in human T cell receptor rearrangements. PLoS One 2012; 7(12):e52250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016;34:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res 2009;15:7116–8. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6: 1382–99. [DOI] [PubMed] [Google Scholar]
- 29.Salmon H, Idoyaga J, Rahman A, et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 2016;44:924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood 2007;110:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaft JE, Rusch V, Ginsberg MS, et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous non-small-cell lung cancers. J Thorac Oncol 2013;8:1084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.