Abstract
Objective
Sex differences in the manifestation of psychiatric disorders, including anxiety disorders, are among the most prominent findings in psychiatry. The study of Turner syndrome (TS), caused by X‐monosomy, has the potential to reveal mechanisms that underline male/female differences in neuropsychiatric disorders. The amygdala has been implicated in numerous neuropsychiatric disorders. Previous studies suggest an effect of TS on amygdala volume as well as on amygdala‐related behaviors such as anxiety. Our objective is to investigate the amygdala shape in TS. Specifically, we tested whether amygdala enlargements in TS are localized to specific nuclei implicated in anxiety, such as the basomedial nucleus.
Experimental design: We use a surface‐based analytical modeling approach to contrast 41 pre‐estrogen treatment girls with TS (mean age 8.6 ± 2.4) with 34 age‐and sex‐matched typically developing (TD) controls (mean age 8.0 ± 2.8). Anxiety symptoms were assessed using the Revised Children's Manifest Anxiety Scale ‐ 2 (RCMAS‐2) in both groups.
Principal observations: TS was associated with anomalous enlargement of the amygdala. Surface‐based modeling revealed shape differences (increased radial‐distances) in bilateral basal and basomedial nuclei within the basolateral complex. RCMAS‐2 Total Anxiety t‐score was significantly higher in participants with TS compared with TD controls (P = 0.012).
Conclusions
Group differences in global amygdala volumes were driven by local morphological increases in areas that are critically involved in face emotion processing and anxiety. In the context of increased amygdala volumes in TS, our results also showed increased worry and social anxiety in young girls with TS compared with TD. Hum Brain Mapp 37:1593‐1601, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: Turner syndrome, X‐monosomy, amygdala, anxiety, shape analysis and structural MRI
INTRODUCTION
Turner syndrome (TS) arises from the complete or partial absence of an X chromosome in females. In girls that have a complete absence of the X chromosome gonadal (ovarian) dysgenesis occurs early in development, resulting in a lack of exposure to sex hormones compared to typically developing (TD) girls. Neuroimaging studies of developing girls with TS have consistently demonstrated an association between TS and the structure of specific cortical brain regions [Brown et al., 2004; Green et al., 2014; Lepage et al., 2013]. Alterations in subcortical brain structure, in regions such as the amygdala, are also described in children, adolescents, and adults with TS, with many of these studies finding anomalous increases in amygdala volume [Good et al., 2003; Kesler et al., 2004; Lepage et al., 2013]. Such findings are intriguing given prior work documenting a high prevalence of anxiety behaviors [McCauley et al., 1986; Schmidt et al., 2006] and deficits in social cognition [Hong et al., 2011; Lepage et al., 2013a; McCauley et al., 1987; Romans et al., 1998] in individuals with TS, as such behaviors are themselves linked with increased amygdala volume in children without TS [Qin et al., 2014]. More specifically, girls with TS are thought to have impaired recognition of emotional facial expressions compared to TD females, with specific deficits in the recognition of fear [Lawrence et al., 2003; Mazzola et al., 2006]. Functional neuroimaging studies have additionally demonstrated altered activation in the amygdala [Skuse et al., 2005] and cognitive control regions [Hong et al., 2014] in girls with TS as they perform face emotion recognition tasks. Taken together, these findings, in context with prior work emphasizing the amygdala's role in fear [Dejean et al., 2015] and social perception [Rutishauser et al., 2015] provide persuasive evidence for a role of altered amygdala function in the behavioral phenotype associated with TS.
The amygdala is not a homogenous subcortical structure, but rather is a complex of nuclei. Advances in brain image acquisition and analysis enable evaluation of the amygdala complex at a higher level of granularity than before. This level of granularity allows for more sensitive localization of differences in amygdala structure. For example analysis of three‐dimensional amygdala structure is used in different neuropsychiatric conditions such as psychosis [Mahon et al., 2015], Alzheimer disease [Cavedo et al., 2011] and genetic conditions [Haas and Reiss, 2012]. Further, this analysis is used to explore brain and behavior correlations in clinical populations [Peng et al., 2014].
Although TS appears to affect amygdala structure and function, knowledge of amygdala morphology in TS is currently constrained to measurements of volume [Good et al., 2003; Kesler et al., 2004; Lepage et al., 2013]. These measurements are global and lack sensitivity to local changes that might underlie overall between‐group volume differences. Accordingly, in this study, we sought to identify amygdala subregions that contribute to these overall volume differences between TS and TD. In particular, we tested the hypothesis that local alterations in amygdala subregions that support face recognition functions—the basal and basomedial nuclei [Janak and Tye, 2015]—would disproportionately contribute to overall volume differences. Further, given the frequent occurrence of anxiety symptoms in individuals with TS [McCauley et al., 1986; Schmidt et al., 2006], as well as associations reported between anxiety symptoms and amygdala morphology [Etkin et al., 2009; Qin et al., 2014] in non‐TS populations, we tested whether associations between amygdala structure and anxiety symptoms were significantly different between TS and TD.
METHODS
Participants
The sample comprised 41 girls with TS and 34 TD girls. Exclusion criteria for both groups included premature birth (gestational age < 34 weeks), low birth weight (<2000 g) and known diagnosis of a major psychiatric condition such as psychotic or mood disorder or current neurological disorder including seizures. All participants in the TS group had X‐monosomy, as confirmed by standard karyotype analysis of at least 20 cells, which allows exclusion of 11% mosaicism or greater with a 0.90 confidence [Hook, 1977]. Girls with TS exhibiting mosaic or uncommon structural karyotypes were excluded. Estrogen replacement therapy is provided to induce puberty and the development of secondary sexual characteristics in individuals with X‐monosomy, typically at age 14 or after [Bondy and Turner Syndrome Study, 2007]. In this study, we included only girls with TS due to X‐monosomy before estrogen administration. Thus, in this sample, we are able to examine the effects of absence of one complete X chromosome on amygdala structure before the administration of exogenous estrogen. The sample used in this study overlaps with previously published work [Hong et al., 2014; Lepage et al., 2013a, 2013b]. The local Institutional Review Board of the Stanford University School of Medicine approved this study and informed written consent was obtained from a legal guardian for all participants. Written assent was obtained from participants over 7 years of age.
Cognitive and Behavioral Assessments
General intellectual functioning of all participants was assessed using the Wechsler Preschool and Primary Scale of Intelligence – III (WPPSI‐III; [Wechsler, 2002]) or the Wechsler Intelligence Scale for Children – IV (WISC‐IV; [Wechsler, 2003]). Anxiety symptoms were assessed using the Revised Children's Manifest Anxiety Scale ‐ 2 (RCMAS‐2; [Reynolds and Richmond, 2008]). The RCMAS‐2 consists of a total of 49 yes/no items that together contribute to a total anxiety score and to scores on three subscales: Physiological Anxiety, Worry, Social Anxiety. Scaled scores from the RCMAS‐2 were used in this study. Scaled scores were available for children between the age 6 and 12 years; T‐scores >60 indicate significant anxiety.
MRI
Participants underwent behavioral training in a mock MRI scanner before their actual scan to desensitize them to the appearance and sounds of an MRI environment and to help participants learn to limit motion. Imaging data were collected between 2006 and 2011 on a Signa HDxt 3.0 T whole‐body MR system (GE Medical Systems, Milwaukee, WI) using a standard birdcage headcoil. Anatomic images were acquired using a fast spoiled gradient‐recalled echo sequence with the following parameters: 124 coronal slices, repetition time [TR]/echo time [TE] = 6.4/2 ms, inversion time [TI] = 300 ms, flip angle = 15°, number of excitations = 3, field of view = 22 × 22 cm, matrix = 256 × 256, 1.5 mm thickness, acquisition time = 14 min 43 s.
Amygdala Segmentations
The amygdalae were segmented on each participant's scan using a combination of automated and manual approaches. First, automatic segmentation of the amygdalae was performed using a well‐validated probabilistic region‐of‐interest labeling technique within the FreeSurfer software package (ver 5.0 http://surfer.nmr.mgh.harvard.edu/). The technical details of the FreeSurfer procedures used are extensively described in prior publications [Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2002, 2004].
Following Freesurfer processing, amygdalae were visually inspected by a trained image analyst (K.C.F.) who was blind to diagnostic, demographic, and clinical variables. This analyst had excellent inter‐ and intra‐rater reliability (intraclass correlation coefficients > 0.95) and made minor corrections, as necessary, according to previously established protocols [Haas et al., 2014]. Manual editing of the amygdala was required for 8 subjects (7 in the TS group, 1 in the TD group; Fisher's Exact Test, P = 0.13).
Statistical Analyses of Demographic and Questionnaire Measures
We performed statistical analyses using The R Project for Statistical Computing (R) (http://www.r-project.org). Unpaired t‐tests were used to compare age, IQ, and RCMAS‐2 scores between TS and TD.
Statistical Analyses of Amygdala Volume
Prior to shape analysis, the main effect of group on total amygdala volume was assessed. Analysis was performed using analysis of covariance (ANCOVA) with group (TS, TD) as a between‐group factor, left or right volume as the dependent variable, and age, verbal IQ (VIQ) and total brain volume as covariates. In this model, VIQ did not contribute a significant proportion of variance to the model for either the left or right amygdala. Thus, final analyses used only age and total brain volume as covariates. There was no significant difference in age (P = 0.33) and total brain volume (P = 0.60) between groups.
Amygdala Volume‐Behavior Correlations
Correlations between amygdala volume and RCMAS‐2 total and subscale scores within groups were examined using partial correlation controlling for age and total brain volume.
Statistical Analyses of Three‐Dimensional Amygdala Structure
We performed statistical analysis of amygdala shape using Shape Tools (http://www.loni. ucla.edu/Software/ShapeTools), a software package that is effective in evaluating condition‐specific alterations in subcortical morphometry [Foland‐Ross et al., 2013; Haas et al., 2014; Peng et al., 2013]. The amygdalae were initially oriented to standard space based on each subject's whole brain transformation (9‐parameter) to the ICBM‐152 brain template using FMRIB's Linear Image Registration Tool [Jenkinson et al., 2002; Jenkinson and Smith, 2001]. The whole brain transformation (9‐parameter) removes gross size differences between the amygdala. Amygdalae surface points across subjects were subsequently matched by equalizing the points’ spatial frequency within brain slices across participants. An amygdala centroid, or the mean position of all the points in all coordinate directions, was then computed, through which a medial line was drawn for each amygdala. The distance from this medial line to each of the uniformly distributed surface points of the mesh, or the radial distance, served as the main dependent variable in all shape analyses [Thompson et al., 2004].
At each surface point along the surface mesh, an ANCOVA model was run with group (TS vs. TD) as a between‐group factor, radial distances for each surface point as the dependent variable and age and VIQ as covariates. Again, as this model indicated that VIQ did not significantly contribute to radial distances, this variable was removed from final analyses. Total brain volume was not included as a covariate in our analyses of three dimensional shape differences since the whole brain registration performed during shape preprocessing adjusted for the overall variation in brain size.
As in previous studies [Foland‐Ross et al., 2013; Haas et al., 2014; Peng et al., 2013], correction for multiple comparisons was performed using permutation testing (10,000 iterations) for superior and inferior halves of the left and right amygdalae [Thompson et al., 2003, 2004]. This method compares the statistical features observed in the true experiment to those occurring with randomly assigned groups or covariate values. An inherent limitation of the method used to analyze amygdala shape [Thompson et al., 2003, 2004] is that the software dictates that analyses are conducted separately for superior and inferior halves of the left and right amygdalae. Therefore, to correct for the resulting multiple comparisons of the four amygdala halves, a further correction was conducted using false discovery rate (FDR) [Benjamini and Hochberg, 1995]. P‐value of 0.05 was considered as the significance threshold after permutation testing and FDR corrections.
For interpretation of the results, the locations of significant between‐group differences and within‐group correlations were examined visually with reference to amygdala subregional anatomy. Amygdala subregional anatomy was based on the Atlas of the Human Brain [Mai et al., 2007], that provided an operationalized approach for partitioning this structure into subregions (http://caportal.cis.jhu.edu/wiki/tutorials/amygdala/amygdala.html ). A widely used subdivision into the basolateral complex and the centromedial amygdala is based on specific functional characteristics, connections to other brain regions and cytoarchitectonics [LeDoux, 2000; Pitkanen et al., 2000]. The basolateral complex is further divided to the lateral nucleus, basal nucleus (also referred to as the basolateral nucleus) and basomedial nucleus [Mahon et al., 2015; Mai et al., 2007].
Three‐Dimensional Amygdala Structure‐Behavior Correlations
Post hoc Pearson's correlations were conducted at each surface point within and across groups to investigate associations between localized variation of radial distance and RCMAS‐2 total and subscale scores. After correcting for multiple comparisons (as described above), each P‐value was plotted onto an amygdala surface mesh model (as these analyses were exploratory in nature, no further correction with FDR was used.).
RESULTS
Cognitive and Behavioral Measures Analyses
There was no significant difference in age between groups (P = 0.33). As expected, the control group scored significantly higher for all IQ measures (all P’s ≤ 0.001). Although the group means for both TD and TS girls for Total Anxiety score and subscale scores were within the normal range (T scores ranging between 40 and 60 [Reynolds and Richmond, 2008]), RCMAS‐2 Total Anxiety t‐score was significantly higher in participants with TS compared with TD controls (P = 0.012; Table 1). Moreover, four (12.5%) girls with TS scored at or above at‐risk level for anxiety compared to none in the TD group. Scores on the Worry and Social Anxiety subscales of the RCMAS‐2 were significantly higher in TS compared with TD (P's = 0.003 and 0.033, respectively). Figure 1 shows the anxiety score distributions across TS and TD.
Table 1.
Population Characteristics
| TS | TD | P value | |
|---|---|---|---|
| Number of participants | 41 | 34 | – |
| GH | 27, U = 1 | – | – |
| Estrogen | None | – | |
| Mean (SD) | Mean (SD) | ||
| Age | 8.6 (2.4) | 8.0 (2.8) | NS |
| Range | 4.0–12.9 | 3.6–12.6 | – |
| FSIQ | 92.8 (13.5) | 117.8 (12.1) | <0.001 |
| VIQ | 102.5 (13.1) | 117.0 (15.1) | <0.001 |
| PIQ | 90.8 (15.8) | 115.7 (12.8) | <0.001 |
| Total Anxietya | 46.6 (9.9) | 40.2 (7.8) | =0.012 |
| Physiological Anxietya | 46.3 (8.2) | 44.0 (8.7) | NS |
| Worrya | 48.3 (9.6) | 39.9 (9.3) | =0.003 |
| Social Anxietya | 44.9 (9.9) | 39.7 (7.4) | =0.033 |
Note: GH: growth hormone; U: unknown; FSIQ: full‐scale intelligence quotient; VIQ: verbal intelligence quotient; PIQ: performance intelligence quotient; NS: not significant.
RCMAS‐2, Revised Children's Manifest Anxiety Scale; TS (n = 32); TD (n = 21).
Figure 1.
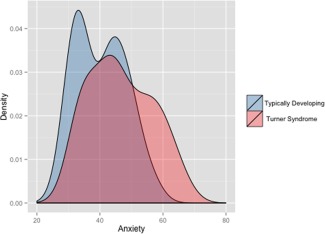
Distribution plot of Total Anxiety Scores on the Revised Children's Manifast Anxiety Scale‐2 (RCMAS‐2). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Amygdala Volume Analyses
Bilateral amygdala volumes were greater in the TS group compared with TD. There was a significant main effect of group (TS vs. TD) on bilateral amygdala volume after controlling for age and total brain volume (left: F(1,71) = 27.96, right F(1,71) = 20.50, all P’s < 0.0001); the TS group showed increased volumes in left (mean difference = 182.87 mm3, t = 5.29, P < 0.0001) and right amygdala (mean difference =166.28 mm3, t = 4.53, P < 0.0001 Age was positively correlated with left amygdala volume (F(1,71) = 4.64, P < 0.05) and approached significance (positive correlation) for the right amygdala (F(1,71) = 3.52, P = 0.06) across groups. Within TS and control groups, age was positively correlated with left and right amygdala volumes (all P's < 0.05). No significant correlations were found between VIQ and amygdala volume bilaterally for either the TS or TD groups.
Amygdala Shape Analyses
We observed overall bilateral increases of radial distances in the TS group compared with the TD group (p < 0.05 for all comparisons, FDR corrected) after controlling for age. Changes were observed in areas that visually corresponded to the basomedial and basal nuclei, expanding to the lateral nucleus in the left amygdala of the TS group (Fig. 2). Age was positively correlated with radial distances of the left amygdala across groups (P < 0.05). Within groups, a positive correlation between age and radial distance of the left amygdala was present only in the TS group (P < 0.05). No significant correlations were found between age and right amygdala radial distance.
Figure 2.

Surface based comparison of amygdala shape between TS and TD controls. Top row, left, and right amygdala from superior and inferior view. Results show bilateral increased radial distances in areas corresponding to the basomedial and basal nuclei bilaterally, and extending to the lateral nucleus in the left amygdala in TS compared to TD (P‐values were corrected for multiple comparisons). Legend; segmentation of the amygdala to four subregions, left and right amygdala are presented using the superior view. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Amygdala Volume‐Behavior Correlations
Within groups, no correlations were observed between total volumes of the left or right amygdala and RCMAS‐2 total and subscale scores (all P’s ≥ 0.095, not FDR corrected).
Exploratory Amygdala shape‐Behavior Correlations
No significant between‐group differences were observed for associations between brain and behavior. However, for the TS group only, higher Total Anxiety and Social Anxiety scores were associated with reduced radial distance in the left amygdala (P = 0.029 and P = 0.037, respectively, not FDR corrected). Visual inspection of the results indicated that these associations extended along the left basal nucleus.
DISCUSSION
In this study, we sought to investigate tTS‐related anomalies in amygdala volume and shape in a large cohort of prepubertal girls. Overall, we found X monosomy to be associated with anomalous enlargement of the amygdala, and further, that group differences in global volume were driven by local morphological increases in the basal and basomedial nuclei, areas that are critically involved in face emotion processing. Our analyses of the association between three‐dimensional amygdala structure and anxiety symptoms did not reveal significant differences between the groups.
Our findings of amygdala enlargement in girls with TS are in line with previous results from studies of girls, as well as adolescents and adults with TS [Good et al., 2003; Kesler et al., 2004; Lepage et al., 2013]. The effects of X monosomy on amygdala shape and volume are particularly intriguing in light of sexual dimorphism of amygdala volume and shape in healthy controls. Greater amygdala volumes have been previously observed in males compared with females [Giedd et al., 2012; Goddings et al., 2014], with these difference driven by increases in radial distances of the “superficial” amygdala [Kim et al., 2012]. It is tempting, therefore, to speculate that the differences in amygdala shape, seen here between TS and TD girls, is the result of an X‐dosage effect that may also underlie differences were seen between TD boys and girls. However, as we did not collect data from TD boys in our study, we cannot attribute, at this time, the anatomical differences observed in TS to naturally occurring sex‐differences. It is noteworthy that both the previous study of healthy adults [Kim et al., 2012] and our present study results indicate that the effect of sex chromosomes on amygdala structure appear localized to anatomic subregions of this structure.
Our study of girls with complete absence of one X‐chromosome allows for initial modeling of the effects of a single X chromosome in humans. The influence of TS on amygdala morphology can theoretically be related to the direct effects of having a single X chromosome (i.e., haploinsufficiency of either PAR genes or genes that escape X inactivation) and/or indirect hormonal effects (i.e., lack of normative levels of circulating sex hormones). With respect to the latter point, it is particularly noteworthy that our study included young girls with TS who were sampled before the start of estrogen replacement therapy. Moreover, androgen levels are positively correlated with amygdala volumes in both males and females [Neufang et al., 2009]. Importantly, androgen (testosterone and 5‐alphe dihydrotestosterone) and estrogen levels have not been found to be different between girls with TS and TD girls under the age of 10 years, but have been found to be reduced in girls with TS compared with TD above the age of 10 years [Apter et al., 1982]. Thus, if amygdala volumes were affected only by sex hormones, we should have found reduced amygdala volumes in TS. In support of the interpretation that the influence of TS on amygdala morphology relates to the direct effects of having a single X chromosome, Raznahan et al. found an effect of sex chromosome number on amygdala structure in X monosomic (XO), XX, and XY mice. Contrary to ovarian dysgenesis that develops in females with X‐monosomy, XO mice are free from this reproductive anomaly and putatively have approximately the same hormonal profile as XX mice. Thus in mice, sex chromosomes appear to affect amygdala structure independently from sex hormones. Further, these observations of X monosomy effects on amygdala structure in mice, independent from sex hormones [Raznahan et al., 2013], is in agreement with the hypotheses that X monosomy has a direct effect on amygdala structure in humans as observed in our study.
We observed localized effects of X‐monosomy on amygdala structure. Specifically, we identified increases in radial distance within amygdala subregions that are critically involved in the processing of fearful faces. In general, inputs from sensory cortices and the thalamus project information to the lateral nuclei of the amygdala, from which information is sent to the basal and basomedial nuclei. The basal and the basolateral nuclei, in turn, are highly connected with other brain regions involved in the mediation of a behavioral response to threatening stimuli as evoked in humans by recognition of fearful faces, including the dorsal anterior cingulate and orbital preforontal cortices, the hippocampus and sensory association areas [Janak and Tye, 2015; Sah et al., 2003]. As we note above, several studies of individuals with TS demonstrate the disorder to be associated with deficits in the recognition of fearful (but not happy or sad) faces [Lawrence et al., 2003; Mazzola et al., 2006]. Findings from functional imaging studies of TS using face recognition tasks additionally find that affected individuals show a reduction in BOLD response to fearful faces compared with controls in both the amygdala [Skuse et al., 2005] and the dorsolateral prefrontal, anterior, and posterior cingulate cortices [Hong et al., 2014] suggesting that anomalies in emotion processing in TS extend beyond the amygdala. Our results corroborate these functional studies by providing evidence of specific structural aberrations in amygdala nuclei, specifically the basal and basomedial nuclei, that support processing of fearful faces stimuli.
Contrasting TS and TD on scores of the RCMAS‐2, a behavioral measure of anxiety, yielded significant differences between the groups. Girls with TS girls endorsed significantly more anxiety symptoms than TD girls. Although the group means for both the TD and TS groups for Total Anxiety score and subscale scores were within the normal range (T scores ranging between 40 and 60 [Reynolds and Richmond, 2008]), girls with TS showed broader distribution of RCMAS‐2 scores, with 12% of participants scoring above the “at risk” level (T score ≥ 60; Fig. 1). These results are in line with prior reports of anxiety symptoms in adults with TS [McCauley et al., 1986; Schmidt et al., 2006].
With respect to brain and behavior correlations, while we found an inverse correlation between amygdala structure and anxiety symptoms within the TS group; a similar association was not detected in the TD group. We speculate that the lack of findings for the TD group might stem from the restricted variance of the anxiety scores in these participants. Moreover, no significant between‐group differences were observed for this association. Thus, our hypothesis predicting that amygdala structure and anxiety symptoms would be differentially correlated in TS and TD was not supported.
With respect to the direction of brain‐behavior correlations within the TS group, we found higher anxiety scores in girls with TS to be associated with reduced radial distances the left amygdala. Thus, the direction of the brain‐behavior correlation was not thematically consistent with observed differences in radial distances between groups in our study. One possible explanation for this finding relates to deficits in recognition of emotions (specifically fear) in this population. For example, Skuse et al. have demonstrated that high skin conduction recordings in response to anxiety‐inducing stimulus were negatively associated with fear recognition scores in TS [Skuse et al., 2005 ]. In contrast, high skin conduction recordings in response to anxiety‐inducing stimuli were positively associated with fear recognition scores in TD controls [Skuse et al., 2005 ]. Thus, neurobiological mechanisms underlying the negative association between physiological measures of anxiety and fear recognition in TS might also contribute to the negative correlation between amygdala structure and anxiety symptoms observed here.
Limitations and Conclusions
One limitation of out study is the lack of physiological measures of anxiety in TS. Physiological measures of arousal (such as skin conduction recordings) in response to an anxiety‐inducing stimulus, in addition to self‐rating assessments for anxiety, might have provided a better estimate of anxiety levels in the TS group [Skuse et al., 2005 ]. Another limitation of this study is that the slice thickness (1.5 mm) and in‐plane resolution (∼1 mm) used for image acquisition did not permit finer delineation of distinct nuclei contained within the amygdala. Such finer delineation can be obtained using higher magnetic field (7 Tesla – 7T) compared with 3T currently used in brain imaging studies of children. Thus, our reference to anatomic subregions within the amygdala was strictly inferred from visual landmarks.
In conclusion, we used a novel, mesh‐mapping method to localize TS‐related alterations in amygdala structure in a carefully recruited sample of prepubertal girls. We found that TS‐related increases in amygdala volumes appear to be driven by local expansion in areas corresponding to the basal and basomedial nuclei, areas that are implicated in fearful face processing and anxiety. In the context of increased amygdala volumes in TS, our results also showed increased worry and social anxiety in young girls with TS compared with TD. Although we did not find between group differences in anxiety‐amygdala radial distance associations, further studies comparing groups of girls with TS to non‐TS girls with similar levels of anxiety symptoms might provide insights into the specific brain‐based mechanisms that lead to anxiety in the context of a single X chromosome.
ACKNOWLEDGMENTS
The authors would like to thank the Turner Syndrome Society and the Turner Syndrome Foundation made this work possible. The authors would like to sincerely thank all of the families who kindly volunteered to participate. Dr. Reiss is an unpaid medical advisor for the Turner Syndrome Society and Turner Syndrome Foundation. The funding sources mentioned above had no role in the study design; in the collection, analysis and interpretation of the data.
REFERENCES
- Apter D, Lenko HL, Perheentupa J, Soderholm A, Vihko R (1982): Subnormal pubertal increases of serum androgens in Turner's syndrome. Horm Res 16:164–173. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the False Discovery Rate ‐ a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met 57:289–300. [Google Scholar]
- Bondy CA, Turner Syndrome Study G (2007): Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab 92:10–25. [DOI] [PubMed] [Google Scholar]
- Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht M, Reiss AL (2004): A volumetric study of parietal lobe subregions in Turner syndrome. Dev Med Child Neurol 46:607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavedo E, Boccardi M, Ganzola R, Canu E, Beltramello A, Caltagirone C, Thompson PM, Frisoni GB (2011): Local amygdala structural differences with 3T MRI in patients with Alzheimer disease. Neurology 76:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Dejean, C , Courtin, J , Rozeske, RR , Bonnet, MC , Dousset, V , Michelet, T , Herry, C : Neuronal Circuits for Fear Expression and Recovery: Recent Advances and Potential Therapeutic Strategies. Biol Psychiatry 78:298–306. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD (2009): Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 66:1361–1372. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Foland‐Ross LC, Thompson PM, Sugar CA, Narr KL, Penfold C, Vasquez RE, Townsend J, Fischer J, Saharan P, Bearden CE, Altshuler LL (2013): Three‐dimensional mapping of hippocampal and amygdalar structure in euthymic adults with bipolar disorder not treated with lithium. Psychiatry Res 211:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK (2012): Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ 3:19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ (2014): The influence of puberty on subcortical brain development. Neuroimage 88:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Lawrence K, Thomas NS, Price CJ, Ashburner J, Friston KJ, Frackowiak RS, Oreland L, Skuse DH (2003): Dosage‐sensitive X‐linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain 126:2431–2446. [DOI] [PubMed] [Google Scholar]
- Green T, Chromik LC, Mazaika PK, Fierro K, Raman MM, Lazzeroni LC, Hong DS, Reiss AL (2014): Aberrant parietal cortex developmental trajectories in girls with Turner syndrome and related visual‐spatial cognitive development: A preliminary study. Am J Med Genet B Neuropsychiatr Genet 165B:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Reiss AL (2012): Social brain development in williams syndrome: The current status and directions for future research. Front Psychol 3:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Sheau K, Kelley RG, Thompson PM, Reiss AL (2014): Regionally specific increased volume of the amygdala in Williams syndrome: Evidence from surface‐based modeling. Hum Brain Mapp 35:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, Dunkin B, Reiss AL (2011): Psychosocial functioning and social cognitive processing in girls with Turner syndrome. J Dev Behav Pediatr 32:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, Bray S, Haas BW, Hoeft F, Reiss AL (2014): Aberrant neurocognitive processing of fear in young girls with Turner syndrome. Soc Cogn Affect Neurosci 9:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook EB (1977): Exclusion of chromosomal mosaicism: tables of 90%, 95% and 99% confidence limits and comments on use. Am J Hum Genet 29:94–97. [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM (2015): From circuits to behaviour in the amygdala. Nature 517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Garrett A, Bender B, Yankowitz J, Zeng SM, Reiss AL (2004): Amygdala and hippocampal volumes in Turner syndrome: A high‐resolution MRI study of X‐monosomy. Neuropsychologia 42:1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim N, Kim S, Hong S, Park K, Lim S, Park JM, Na B, Chae Y, Lee J, Yeo S, Choe IH, Cho SY, Cho G (2012): Sex differences in amygdala subregions: Evidence from subregional shape analysis. Neuroimage 60:2054–2061. [DOI] [PubMed] [Google Scholar]
- Lawrence K, Kuntsi J, Coleman M, Campbell R, Skuse D (2003): Face and emotion recognition deficits in Turner syndrome: A possible role for X‐linked genes in amygdala development. Neuropsychology 17:39–49. [PubMed] [Google Scholar]
- LeDoux JE (2000): Emotion circuits in the brain. Ann Rev Neurosci 23:155–184. [DOI] [PubMed] [Google Scholar]
- Lepage, JF , Mazaika, PK , Hong, DS , Raman, M , Reiss, AL (2013): Cortical Brain Morphology in Young, Estrogen‐Naive, and Adolescent, Estrogen‐Treated Girls with Turner Syndrome. Cereb Cortex 23:2159–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage JF, Dunkin B, Hong DS, Reiss AL (2013a): Impact of cognitive profile on social functioning in prepubescent females with Turner syndrome. Child Neuropsychol 19:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage JF, Hong DS, Mazaika PK, Raman M, Sheau K, Marzelli MJ, Hallmayer J, Reiss AL (2013b): Genomic imprinting effects of the X chromosome on brain morphology. J Neurosci 33:8567–8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon PB, Lee DS, Trinh H, Tward D, Miller MI, Younes L, Barta PE, Ratnanather JT (2015): Morphometry of the amygdala in schizophrenia and psychotic bipolar disorder. Schizophr Res 164:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Paxinos G, Voss T (2007): Atlas of Human Brain, 3rd ed. Academic Press. 525 B St., San Diego, California, USA. [Google Scholar]
- Mazzola F, Seigal A, MacAskill A, Corden B, Lawrence K, Skuse DH (2006): Eye tracking and fear recognition deficits in Turner syndrome. Soc Neurosci 1:259–269. [DOI] [PubMed] [Google Scholar]
- McCauley E, Sybert VP, Ehrhardt AA (1986): Psychosocial adjustment of adult women with Turner syndrome. Clin Genet 29:284–290. [DOI] [PubMed] [Google Scholar]
- McCauley E, Kay T, Ito J, Treder R (1987): The Turner syndrome: cognitive deficits, affective discrimination, and behavior problems. Child Dev 58:464–473. [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz‐Dahlmann B, Fink GR, Konrad K (2009): Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex 19:464–473. [DOI] [PubMed] [Google Scholar]
- Peng, DX , Kelley, RG , Quintin, EM , Raman, M , Thompson, PM , Reiss, AL (2013): Cognitive and behavioral correlates of caudate subregion shape variation in fragile X syndrome. Hum Brain Mapp 35:2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng DX, Kelley RG, Quintin EM, Raman M, Thompson PM, Reiss AL (2014): Cognitive and behavioral correlates of caudate subregion shape variation in fragile X syndrome. Hum Brain Mapp 35:2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A (2000): Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci 911:369–391. [DOI] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V (2014): Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry 75:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Probst F, Palmert MR, Giedd JN, Lerch JP (2013): High resolution whole brain imaging of anatomical variation in XO, XX, and XY mice. Neuroimage 83:962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RC, Richmond OB (2008): RCMAS‐2, Revised Childen's Manifest Anxiety Scale. Los Angeles, CA: Western Psychological Services. p 83.
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL (1998): Transition to young adulthood in Ullrich‐Turner syndrome: Neurodevelopmental changes. Am J Med Genet 79:140–147. [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Adolphs R: The primate amygdala in social perception ‐ insights from electrophysiological recordings and stimulation. Trends Neurosci 38:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J (2003): The amygdaloid complex: Anatomy and physiology. Physiol Rev 83:803–834. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Cardoso GM, Ross JL, Haq N, Rubinow DR, Bondy CA (2006): Shyness, social anxiety, and impaired self‐esteem in Turner syndrome and premature ovarian failure. JAMA 295:1374–1376. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Morris JS, Dolan RJ (2005): Functional dissociation of amygdala‐modulated arousal and cognitive appraisal, in Turner syndrome. Brain 128:2084–2096. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW (2003): Dynamics of gray matter loss in Alzheimer's disease. J Neurosci 23:994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW (2004): Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage 22:1754–1766. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2002): Wechsler Prescholl and Primary Scale of Intelligence, 3th ed. (WIPPSI‐III): Technical and interactive manual. San Antonio, TX: The Psychological Corporation.
- Wechsler D (2003): Wechsler Intelligence Scale for Children, 4th ed (WISC‐IV). Technical and interactive manual. San Antonio, TX: The Psychological Corporation.


