Abstract
Intestinal bacteria contribute to the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Recently developed microbial profiling techniques are beginning to shed light on the nature of the changes in the gut microbiota that accompany NAFLD and non-alcoholic steatohepatitis (NASH). In this review, we summarize the role of gut microbiota in the development of NAFLD, NASH, and hepatocellular carcinoma (HCC). We highlight the mechanisms by which gut microbiota contribute to NAFLD/NASH, including through alterations in gut epithelial permeability, choline metabolism, endogenous alcohol production, release of inflammatory cytokines, regulation of hepatic Toll-like receptor (TLR), and bile acid metabolism. In addition, we analyze possible mechanisms for enhanced hepatic carcinogenesis, including alterations in bile acid metabolism, release of inflammatory cytokines, and expression of TLR-4. Finally, we describe therapeutic approaches for NAFLD/NASH and preventive strategies for HCC involving modulation of the intestinal microbiota or affected host pathways. Although recent studies have provided useful information, large-scale prospective studies are required to better characterize the intestinal microbiota and metabolome, in order to demonstrate a causative role for changes in the gut microbiota in the etiology of NAFLD/NASH, to identify new therapeutic strategies for NAFLD/NASH, and to develop more effective methods of preventing HCC.
Keywords: Gut microbiota, Intestinal microbiome, Metabolome, Metagenome, Fatty liver disease, Non-alcoholic fatty liver disease (NAFLD), Non-alcoholic steatohepatitis (NASH), Hepatocellular carcinoma (HCC)
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is currently the most common liver disease worldwide in both adults and children.1–3 NAFLD is defined as the presence of hepatic steatosis in the absence of any secondary causes of hepatic fat accumulation, including excessive alcohol consumption, hepatitis C (genotype 3), Wilson's disease, lipodystrophy, starvation, parenteral nutrition, abetalipoproteinemia, medication (e.g., amiodarone, methotrexate, tamoxifen, corticosteroids, valproate, or antiretroviral medications), Reye’s syndrome, acute fatty liver of pregnancy, HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome, and inborn errors of metabolism (e.g., lecithin cholesterol acyltransferase deficiency, cholesterol ester storage disease, or Wolman’s disease).2
NAFLD includes a spectrum of pathologies, from simple steatosis, defined as the presence of steatosis without any histologic or biochemical injury, to non-alcoholic steatohepatitis (NASH), characterized by steatosis, inflammation, and hepatocyte injury (ballooning), with or without cirrhosis. Patients with NASH have a high likelihood of developing advanced fibrosis and cirrhosis. It is estimated that about one third of the cases of early-stage NASH will progress to stage 3 or 4 fibrosis (cirrhosis) over 5–10 years.4 Additionally, patients with NAFLD are at a higher risk of developing hepatocellular carcinoma (HCC), even in the absence of cirrhosis. In Western countries, 4%–22% of HCC cases are now attributed to NAFLD and the risk of developing HCC for patients with NASH and cryptogenic cirrhosis is between 2.4% over 7 years and 12.8% over 3 years.5–7 NAFLD pathophysiology is multifactorial, involving ecological, genetic, and metabolic factors, such as high energy intake, limited physical activity, and a poorly balanced diet.8,9 Together with epigenetic factors, these promote insulin resistance and hepatic fat accumulation.8,10 The development and progression of inflammation in the steatotic liver was initially proposed to be due to endotoxemia resulting from greater gut permeability.11,12 Recently, however, studies have reported that the intestinal microbiota plays an important role in the pathogenesis of NAFLD.13–15 In addition, obesity induces changes in the composition of the gut microbiota and its metabolites that promote the development of HCC.16 The development of microbial profiling techniques has shed new light on such changes in the gut microbiota and the role of gut bacteria in the development of NAFLD.17
In this review, we focus on the mechanisms by which gut microbiota affect the development of NAFLD/NASH and their progression to HCC. Finally, we discuss potentially effective methods to treat NAFLD/NASH and prevent the development of HCC by modulating the intestinal microbiome.
2. Gut dysbiosis is associated with NAFLD/NASH and HCC
Intestinal dysbiosis is associated with a variety of liver diseases, including NAFLD and NASH.18 However, there have been few studies to date on the relationship between the gut microbiome and NAFLD in humans, and these have yielded inconsistent results (Table 1). For example, patients with NASH had significantly lower levels of Bacteroidetes compared to subjects with simple steatosis and healthy individuals,13 and another study generated similar results in children,19 whereas a third study reported larger numbers of Prevotella, one of the most important genera within the phylum Bacteroidetes, in NASH patients compared to healthy subjects.15 Selected members of the phylum Firmicutes were also more frequent in NAFLD than in non-obese controls (healthy people),14 but lower numbers of Firmicutes were present in NASH patients in another study.15 Finally, while one study reported a larger numbers of the genus Escherichia from the Enterobacteriaceae family in subjects with NASH,15 there was no significant difference in Escherichia coli numbers between NASH patients and subjects with simple steatosis in another paper.13 Taken together, these studies suggest that the composition of the gut microbiota does differ in patients with NAFLD/NASH and controls, but the specific data obtained to date have been inconsistent.
Table 1.
Changes in the intestinal microbiota associated with NAFLD/NASH.
| References | Diseases and groups |
Comparison | Implicated Microbiota Phylum |
Class | Order | Family | Genus | Methodology |
|---|---|---|---|---|---|---|---|---|
| Mouzaki et al.13 | Healthy (n=17), Steatosis (n=11), NASH (n=22) | Healthy vs NASH Steatosis vs NASH |
Bacteroidetes ↓ | Quantitative real-time PCR (Stool sample) | ||||
| Bacteroidetes ↓ | ||||||||
| Firmicutes | Lachnospiraceae | Clostridium coccoides ↑ | ||||||
|
| ||||||||
| Del Chierico et al.19 | Children: Healthy (n=54), NAFLD (n=27), NASH (n=26), Obese (n=8) | Healthy vs all patients | Bacteroidetes ↓ | Rikenellaceae ↓ | ||||
| Porphyromonadaceae | Parabacteroides ↓ | |||||||
| Bacteroidaceae | Bacteroides fragilis ↓ | |||||||
| Firmicutes ↑ | Ruminococcaceae | Oscillospira ↓ | ||||||
| Lachnospiraceae | Dorea ↑, Ruminococcus ↓ | |||||||
| NAFLD vs NASH | Firmicutes ↑ | |||||||
| Bacteroidetes ↓ | ||||||||
| Proteobacteria ↓ | ||||||||
| Actinobacteria ↓ | ||||||||
| NAFLD vs obese | Proteobacteria ↓ | |||||||
| Actinobacteria ↓ | ||||||||
| Healthy vs NASH | Bacteroidetes | Bacteroidaceae ↓ | Bacteroides ↓ | |||||
|
| ||||||||
| Zhu et al.15 | Children: Healthy (n=16), Obese (n=25), NASH (n=22) | Healthy vs Obese | Bacteroidetes ↑ | Prevotellaceae ↑ | Prevotella ↑ | 16S rRNA gene pyrosequencing (Stool sample) | ||
| Rikenellaceae ↓ | Alistipes ↓ | |||||||
| Firmicutes ↓ | Lachnospiraceae ↓ | Blautia ↓ ↓, Coprococcus ↓ , Roseburia ↓ | ||||||
| Eubacteriaceae | Eubacterium ↓ | |||||||
| Ruminococcaceae ↓ | ||||||||
| Healthy vs NASH | Bacteroidetes ↑ | Prevotellaceae ↑ | Prevotella ↑ | |||||
| Rikenellaceae ↓ | Alistipes ↓ | |||||||
| Firmicutes ↓ | Lachnospiraceae ↓ | Blautia ↓, Coprococcus ↓ | ||||||
| Eubacteriaceae | Eubacterium ↓ | |||||||
| Ruminococcaceae ↓ | Oscillospira ↓ | |||||||
| Actinobacteria ↓ | Bifidobacteriaceae ↓ | Bifidobacterium ↓ | ||||||
| Proteobacteria ↑ | Gammaproteobacteri | Enterobacterial | Enterobacteriaceae ↑ | Escherichia ↑ | ||||
| Obese vs NASH | Proteobacteria ↑ | Gammaproteobacteri | Enterobacterial | Enterobacteriaceae ↑ | Escherichia ↑ | |||
|
| ||||||||
| Raman et al.14 | Healthy (n=30), NAFLD (n=30) | Healthy vs NAFLD | Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae ↑ | Lactobacillus ↑ | 16S rRNA gene pyrosequencing (Stool sample) |
| Clostridia | Clostridiales | Lachnospiraceae ↑ | Robinsoniella ↑ , Roseburia ↑, Dorea ↑ | |||||
| Clostridia | Clostridiales | Ruminococcaceae ↓ | Oscillibacter ↓ | |||||
| Clostridia | Clostridiales | Veillonellaceae ↑ | ||||||
| Proteobacteria | Alphaproteobacteria | Kiloniellales | Kiloniellaceae ↑ | |||||
| Gammaproteobacteri | Pasteurellales | Pasteurellaceae ↑ | ||||||
| Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromon adaceae ↓ | |||||
Comparison of condition A vs condition B: ↑ signifies an increase in condition B relative to condition A. ↓ signifies a decrease in condition B relative to condition A. Abbreviations: NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Further studies have also investigated the association between gut dysbiosis, severe NAFLD, and HCC (Table 2). Numbers of the genera Bacteroides, Prevotella, and Ruminococcus significantly differ between patients with F0 or F1 fibrosis and those with significant (F≥2) fibrosis (according to the NASH Clinical Research Network scoring system, F0 = no fibrosis, F1 = perisinusoidal or portal/periportal fibrosis, F2 = perisinusoidal and portal/periportal fibrosis, F3 = bridging fibrosis, and F4 = cirrhosis). The abundance of Bacteroides and Ruminococcus is significantly greater in F≥2 patients, whereas Prevotella is less abundant.20 Firmicutes are more abundant in mild/moderate NAFLD, while Proteobacteria were commoner in advanced fibrosis. At the species level, Eubacterium rectale and Bacteroides vulgatus are the most abundant organisms in mild/moderate NAFLD, while B. vulgatus and E. coli are the most abundant in advanced fibrosis. Finally, Ruminococcus obeum CAG: 39, R. obeum, and E. rectale are significantly less abundant in advanced fibrosis than in mild/moderate NAFLD.21 Thus, NAFLD severity is associated with gut dysbiosis. In addition, alterations in the gut microbiota induced by obesity could promote the development of HCC.16
Table 2.
Changes in the intestinal microbiota associated with the severity of NAFLD.
| References | Disease and groups | Comparison | Implicated Microbiota Phylum | Class | Order | Family | Genus/species | Methodology |
|---|---|---|---|---|---|---|---|---|
| Boursier et al.20 | F0/1 fibrosis without NASH (n=20), F0/1 fibrosis with NASH (n=10), fibrosis F≥2 (n=27) | No NASH vs NASH | Bacteroidetes | Bacteroidaceae ↑ | Bacteroides ↑ | 16S ribosomal RNA gene sequencing (Stool sample) | ||
| Prevotellaceae ↓ | Prevotella ↓ | |||||||
| F0/1 fibrosis vs F=2 fibrosis | Bacteroidetes | Bacteroidaceae ↑ | Bacteroides ↑ | |||||
| Prevotellaceae ↓ | Prevotella ↓ | |||||||
| Firmicutes | Ruminococcaceae | Ruminococcus ↑ | ||||||
| Erysipelotrichaceae ↓ | ||||||||
|
| ||||||||
| Loomba et al.21 | Mild/moderate (stage 0–2 fibrosis) NAFLD (n=72), Advanced fibrosis (stage 3 or 4 fibrosis) (n=14) | stage 0–2 fibrosis vs stage 3 or 4 fibrosis | Proteobacteria ↑ | Whole genome shotgun sequencing of DNA (Stool sample) | ||||
| Firmicutes ↓ | Eubacteriaceae | Eubacterium rectale ↓ | ||||||
| Ruminococcaceae | Ruminococcus obeum CAG:39 ↓, Ruminococcus obeum ↓ | |||||||
Comparison of condition A vs condition B: ↑ signifies an increase in condition B relative to condition A. ↓ signifies a decrease in condition B relative to condition A. Abbreviations: NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
3. NAFLD/NASH is associated with bacterial overgrowth in the small intestine
Patients with NAFLD/NASH not only have compositional changes in the gut microbiota; they are also likely to have small intestinal bacterial overgrowth (SIBO), with the estimated prevalence ranging from 50% to 77.78% .12,22,23 SIBO is defined as a total bacteria growth of more than 105 colony-forming units per milliliter (CFU/mL) of intestinal fluid. Various breath tests, including the glucose-H2 breath test have been used to quantify SIBO, given the difficulty in obtaining cultures.24 Patients with SIBO have a greater risk of hepatic steatosis,25 and SIBO and the presence of metabolic syndrome were independently associated with more severe hepatic steatosis.24 SIBO has an important role in NASH development and progression, because it increases intestinal permeability by disrupting intercellular tight junctions in the gut, enhances hepatic expression of Toll-like receptor (TLR)-4, and thus leads to release of interleukin (IL)-8.22, 23, 26
4. Mechanisms by which the intestinal microbiome impacts NAFLD/NASH
4.1. Gut barrier dysfunction
The intestinal microbiota plays a critical role in the maintenance of the integrity of the intestinal barrier,27 and changes in intestinal epithelial permeability are associated with the development of NAFLD/NASH.28 Mice with defects in intestinal barrier function develop more severe steatohepatitis than control mice fed a diet high in saturated fat, fructose, and cholesterol (HFCD). The colon of patients with NAFLD has lower levels of junctional adhesion molecule A (JAM-A) and higher levels of inflammation than subjects without NAFLD.28 The gut immune system is altered during high fat diet (HFD) feeding.29 Greater intestinal permeability was found in patients with NAFLD and correlated with the severity of steatosis.22 In these studies, intestinal permeability was determined by measuring urinary excretion of 51Cr-ethylenediaminetetra-acetate and evaluating tight junctions in duodenal biopsies by immunohistochemical analysis of the zona occludens.
Studies of animal models have shown correlations between hepatic inflammation and dysfunction of the intestinal mucosal barrier, which suggests that intestinal mucosal barrier malfunction influences the pathogenesis of NAFLD and possibly NASH.30,31 In addition, patients with NAFLD had a high prevalence of SIBO, indicating that alterations in the microbiome may have contributed to the disruption of gut barrier integrity.22 Epithelial microvillus atrophy, disrupted tight junctions, and SIBO were present in patients eating a HFD, and these changes were more severe in those with NASH than in those with NAFLD.32 Administration of oral antibiotics or sequestration of bacterial endotoxins with sevelamer hydrochloride reduced mucosal inflammation and restored normal liver histology in F11r−/ − (the F11r gene encodes JAM-A) mice fed a HFCD,28 while treatment of HFD-fed mice with the local gut anti-inflammatory drug, 5-aminosalicylic acid (5-ASA), reduces gut inflammation, improves intestinal barrier function, improves metabolic parameters, and reduces hepatic steatosis.29
Altered intestinal barrier function may be involved in the pathogenesis of NAFLD/NASH by exposing the liver to higher levels of bacterial inflammatory products, such as lipopolysaccharide (LPS), and to the toxic bacterial metabolome, which includes short-chain fatty acids (SCFAs), ethanol, and other volatile organic compounds (VOCs), such as acetone and butanoic acid.
4.2. Choline metabolism
Choline is an important constituent of cell and mitochondrial membranes. Acetylcholine plays critical roles in diverse physiological processes in the liver, including lipid metabolism, signaling through lipid second messengers, enterohepatic circulation of bile acids, and cholesterol metabolism.33 Choline is an essential nutrient and low-choline diets can cause health problems in humans, including fatty liver disease.34,35 Choline levels are not only influenced by dietary and genetic factors,36 but are also modulated by the gut microbiota.37,38 Subjects consuming a low choline diet had variable populations of Gammaproteobacteria, but they vanished from most of the subjects when high dietary choline levels were restored, suggesting that the level of dietary choline influences the composition of the gut microbiome.39
Conversely, the gut microbiome can also affect choline levels. Enzymes produced by the gut microbiota convert dietary choline to dimethylamine (DMA) and trimethylamine (TMA),40,41 which are absorbed through the microvilli and transported via the portal vein to the liver, where they may promote liver inflammation. Furthermore, low choline levels have been associated with fatty liver.39,42–44 Germ-free mice do not excrete TMA, providing solid evidence for the critical role of gut microbiota in the conversion of choline to TMA.45 Exposure to TMA increases the pH of the growth medium of exposed bacteria, resulting in modifications to antibiotic uptake and transient alterations in antibiotic resistance, providing an alternative means whereby choline and its metabolites can affect community behavior and structure in physically separated bacteria.46 Thus, the gut microbiome may affect the pathogenesis of fatty liver disease by modifying choline metabolism. However, future studies should assess to what extent the changes in the composition of the microbiota described in NAFLD affect choline metabolism and investigate novel therapeutic strategies to regulate choline metabolism.
4.3. Endogenous ethanol production
Ethanol is an important microbial metabolite, and it is principally metabolized to acetate and acetaldehyde. Ethanol can inhibit the tricarboxylic acid cycle and increase production of acetate, which is a substrate for fatty acid synthesis,47 while acetaldehyde has distinct oxidation-dependent metabolic and cytotoxic effects that can result in liver injury.48 Serum alcohol concentration is high in adult NAFLD patients and pediatric NASH patients exhibit higher serum alcohol concentrations than those of healthy controls and non-NASH obese patients.15,49,50. Breath and plasma ethanol levels were also significantly higher in ob/ob mice than in lean control mice.51
In one study there was a significant increase in the abundance of Escherichia in NASH patients.15 Escherichia is a genus of the Enterobacteriaceae family, which may increase the production of ethanol using a mixed-acid fermentation pathway.52–55 In addition, insulin resistance followed by a decrease in alcohol dehydrogenase activity in the liver alters ethanol metabolism.50 Interestingly, ethanol is also produced by intestinal microbiota cultured in the presence of specific concentrations of nutrients.56 The endogenous production of ethanol might, in turn, contribute to the formation of free fatty acids and oxidative stress, further underscoring the potential role of ethanol-producing bacteria in the pathogenesis of NAFLD. Further studies are required to determine the effects of endogenous production of ethanol on the progression of NAFLD and NASH.
4.4. Dysregulation of inflammatory cytokine release
Inflammatory cytokines are thought to play a key role in NAFLD/NASH. Serum tumor necrosis factor alpha (TNF-α) levels are high in patients with NASH,57 while expression of TNF-α and its p55 receptor in the liver of patients with NASH is higher than in patients with simple steatosis.58 Furthermore, a TNF-α gene promoter polymorphism at position 238 may be associated with an increased risk of NAFLD,59 and SIBO in NASH patients is associated with high circulating TNF-α levels.12 Inflammasome deficiency-associated changes in the Firmicute and Bacteroidetes populations are associated with worsening of hepatic steatosis and inflammation via TLR-4 and TLR-9 activation, which leads to higher hepatic TNF-α expression and thus progression of NASH.60 Conversely, probiotic therapy significantly reduces TNF-α, steatosis, and the NASH activity index.61,62 Thus, the gut microbiota may play a role in the pathogenesis of NASH by regulating TNF-α levels.
Another inflammatory cytokine, IL-8, is also involved in NASH.57 The A/A genotype of the IL-8 gene is associated with the progression of NASH,63 while the high prevalence of SIBO in NASH patients is associated with greater expression of TLR-4 and release of IL-8.23 IL-18 also has an effect, slowing the progression of NAFLD/NASH by regulating the gut microbiota.60 Further studies are required to explore potential novel therapeutic strategies targeting inflammatory cytokines for patients with NAFLD/NASH.
4.5. Regulation of hepatic TLRs
TLRs are pattern recognition receptors that play an important role in host defense by recognizing pathogen-associated molecular patterns (PAMPs). TLR-4 is a receptor for LPS, which is a component of gram-negative bacteria, TLR-9 recognizes DNA containing unmethylated CpG motifs, which are common in bacterial DNA,64,65 TLR-2 binds components of gram-positive bacterial cell walls, such as peptidoglycan and lipoteichoic acid,64,65 and TLR-5 recognizes flagellin, the major protein constituent of bacterial flagella.66,67 Recent studies indicate that TLR signaling plays an important role in the progression of NAFLD and NASH,68–70 and conversely the gut microbiota regulates the expression of TLR in patients with NAFLD/NASH. NASH patients also have a higher prevalence of SIBO, which is associated with enhanced expression of TLR-4 in the liver.23 Inflammasome deficiency-associated changes in the gut microbiota lead to worsening of hepatic steatosis and inflammation through activation of TLR-4 and TLR-9, and thus greater hepatic TNF-α expression and NASH progression.60
The TLR-9 signaling pathway induces production of IL-1β by Kupffer cells, leading to steatosis, inflammation, and fibrosis.71 Mice genetically deficient in TLR-5 exhibit hyperphagia and develop features of the metabolic syndrome, which correlate with changes in the composition of the gut microbiota. In addition, transfer of microbiota from TLR-5 knockout mice to wild-type germ-free mice confers many features of the metabolic syndrome on the recipients.72 These findings suggest that interactions between TLR-5 and specific gut microbiota contribute to the development of the metabolic syndrome.
Interestingly, probiotic treatment increased anti-inflammatory cytokine secretion in a TLR-2-dependent manner,73 while Clostridium butyricum induced IL-10 production from intestinal macrophages in acute experimental colitis through TLR-2,74 which suggests that TLR-2 has a dual function. TLR-2 ligands from probiotic bacteria are anti-inflammatory, whereas TLR-2 ligands from the bacteria that are present during obesity induce inflammation. In summary, the gut microbiota may be involved in the pathogenesis of NAFLD and NASH by regulating TLRs, and further studies are needed to identify potential novel therapeutic strategies targeting TLRs for patients with NAFLD/NASH.
4.6. Alterations in bile acid metabolism
Bile acids and their metabolites play important roles in maintaining hepatic glucose, cholesterol, and triglyceride homeostasis, and both bile acid homeostasis and the associated signaling pathways are dysregulated in NAFLD.75 The concentrations of serum bile acids, especially the taurine-conjugated and glycine-conjugated primary and secondary bile acids, are higher in patients with NASH than in healthy volunteers.76 In addition, the gut microbiota has a significant impact on bile acid metabolism,77 and bile acids can have direct effects on the intestinal microbiota by disrupting membranes.78 Thus, there appears to be an association between the gut microbiota and bile acid metabolism. HFD-induced changes in the bile acid pool are mediated through altered gut microbiota,79,80 which in turn contributes to the occurrence of NAFLD by influencing lipid and energy metabolism.
Bile acids may also influence NAFLD progression through regulation of the farnesoid X nuclear receptor (FXR) and the G-protein-coupled receptor (GPCR) TGR5.75 Bile acids may have a role in the regulation of insulin sensitivity by activating TGR5,81 and indeed the treatment of diet-induced obese mice with the TGR5 agonist INT-777 leads to reduced body weight gain and increased energy expenditure in brown adipose tissue.82 The gut microbiota may also affect bile acid metabolism by activating FXR during the onset and progression of hepatic steatosis.83–86 Evidence for this comes from observation of the effects of the gut-restricted FXR agonist fexaramine (Fex), which can reduce diet-induced weight gain, systemic inflammation, and hepatic glucose production, while enhancing thermogenesis and browning of white adipose tissue (WAT), thereby reducing obesity and insulin resistance.87 Furthermore, 6-ethylchenodeoxycholic acid (obeticholic acid) is a potent activator of FXR that ameliorates histological defects in NASH patients.88 However, FXR is able to serve either as an activator or as a direct repressor when it forms a heterodimer with retinoid X receptor a (RXRα/NR2B1) or as a monomer.75 Animals with intestine-specific Fxr disruption had lower hepatic triglyceride accumulation in response to a HFD,84 but both bile acid receptors (FXR and TGR5) may represent important targets for the treatment of the metabolic syndrome,89,90 because administration of TGR5/FXR agonists in these mice improved NAFLD histology and reduced hepatic inflammation by inhibiting the production of inflammatory cytokines by macrophages.91 Indeed, FXR/TGR5 activation regulates the immune phenotype of monocytes and macrophages, both in vitro and in vivo, identifying them as potential targets for the treatment of NAFLD.
Taken together, the role of the complex interplay between the microbiome and the human bile acid pool requires further elucidation with respect to the pathogenesis of NAFLD and NASH.
4.7. Other factors that contribute to the pathogenesis of NAFLD/NASH
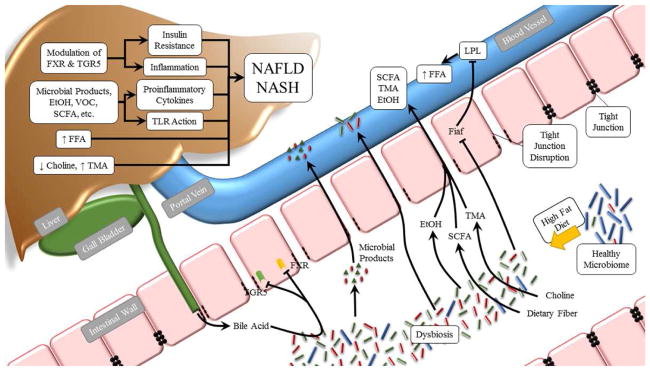
Enteric bacteria decrease the expression of fasting-induced adipocyte factor (Fiaf) in the gut, thereby inducing lipoprotein lipase (LPL) activity and thus greater hepatic accumulation of triglycerides,92 implying a direct link between the intestinal microbiome and fat deposition in the liver. In addition, gut microbiota uses the host’s diet to generate energy, a process which involves the production of SCFAs, including acetate, propionate, and butyrate. The presence of additional SCFAs can increase the quantity of energy-producing substrates absorbed from the intestinal tract, thus contributing to the development of obesity and obesity-related metabolic diseases.93,94 The dysbiosis-associated factors contributing to NAFLD and NASH are illustrated in Fig. 1.
Fig. 1. Effects of the intestinal microbiota on NAFLD/NASH.
HFD feeding results in dysbiosis and intestinal bacterial overgrowth. Dysbiosis leads to greater production of ethanol (EtOH) and SCFA, which are metabolized in the liver. Dietary choline is metabolized by the intestinal microbiota to form TMA, resulting in choline deficiency and hepatic steatosis. The intestinal microbiota suppresses expression of the Fiaf gene by intestinal epithelial cells, resulting in enhanced activity of LPL and greater liberation of free fatty acids (FFA). Greater intestinal permeability leads to translocation of microbial products to the liver and causes inflammation through activation of TLRs and production of inflammatory cytokines, which can drive NAFLD/NASH progression. Dysbiosis also affects bile acid metabolism, which influences the progression of NAFLD through modulation of FXR and TGR5. Abbreviations: HFD, high fat diet; SCFA, short-chain fatty acid; TMA, trimethylamine; Fiaf, fasting-induced adipocyte factor; LPL, lipoprotein lipase; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; FXR, farnesoid X nuclear receptor; TGR5, G-protein-coupled receptor.
5. How does the intestinal microbiota contribute to the development of cirrhosis and HCC in NAFLD?
NAFLD has the potential to evolve into cirrhosis and HCC and the gut microbiota plays an important role in the pathogenesis of cirrhosis and HCC.95 Mice kept under germ-free conditions develop fewer and smaller HCCs than mice housed conventionally, while chronic treatment with a low, non-toxic dose of LPS leads to a significant increase in the number and size of HCCs.96 Furthermore, Yoshimoto et al.16 showed that the administration of antibiotics and gut sterilization could reduce the prevalence of HCCs in obese mice.
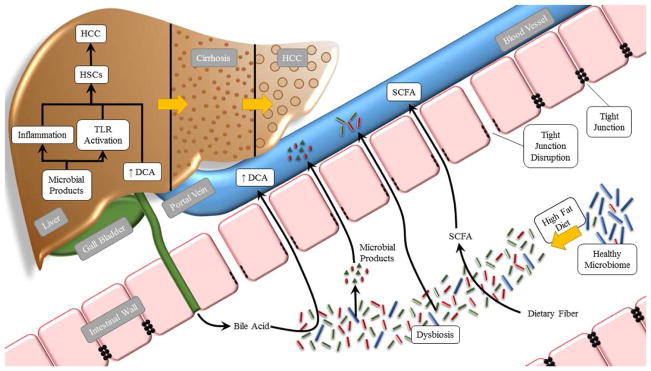
What is the mechanism by which the gut microbiota contributes to NAFLD-associated cirrhosis and HCC? Firstly, dysbiosis of the gut microbiota leads to an increase in secretion of inflammatory cytokines, such as TNF-α and IL-8, which play a key role in the induction and progression of NAFLD to NASH and cirrhosis.97 Consistent with this, serum TNF-α and IL-8 levels were significantly higher in patients with NASH and cirrhosis.57 Secondly, intestinal dysbiosis leads to activation of TLR-4 and TLR-9, resulting in production of IL-1β by Kupffer cells. IL-1β promotes lipid accumulation and cell death in hepatocytes, causing steatosis and inflammation, and stimulates hepatic stellate cells (HSCs) to produce fibrogenic mediators, which promote fibrosis.68,71 In addition, TLR-4 might be directly involved in the pathogenesis of HCC.96 Thirdly, dysbiosis could promote the development of NAFLD-associated HCC by modifying bile acid metabolism. Alterations in the composition of the gut microbiota can result in higher levels of deoxycholic acid (DCA), which provokes a senescence-associated secretory phenotype (SASP) in HSCs, which in turn secrete various inflammatory and tumor-promoting factors in the liver, thus promoting the development of HCCs.16 In summary, the intestinal microbiota may promote the development of NAFLD-associated cirrhosis and HCC by increasing inflammatory cytokine secretion, activating TLR-4 and TLR-9, and modifying bile acid metabolism (Fig. 2). However, further studies are required into the complex interplay between the microbiome and NAFLD-associated cirrhosis and HCC.
Fig. 2. Effects of the intestinal microbiota on HCC tumorigenesis.
Long term ingestion of a HFD results in dysbiosis and intestinal bacterial overgrowth. Greater intestinal permeability leads to translocation of microbial products to the liver and causes inflammation and TLR activation, which can stimulate HSCs to produce pro-fibrotic factors, In addition, changes in the gut microbiota can result in higher levels of DCA, which provoke a senescence-associated secretory phenotype (SASP) in HSCs. All of these factors promote the development of HCCs. Abbreviations: HCC, hepatocellular carcinoma; HFD, high fat diet; TLR, Toll-like recepter; HSCs, hepatic stellate cells; DCA, deoxycholic acid; SCFA, short-chain fatty acid.
6. Modulation of the intestinal microbiome and treatment of NAFLD/NASH
Recent studies of the role of the gut microbiota in the development of NAFLD have provided important evidence to guide the development of a gut microbiota-targeted strategy to prevent or treat NAFLD/NASH and HCC. The most commonly used means of manipulating gut microbiota include the use of probiotic, prebiotic, or synbiotic supplements, or antibiotic treatment. In particular, Lactobacillus and Bifidobacterium are frequently used for their beneficial effects on NAFLD. Probiotic therapies may ameliorate insulin resistance by reducing serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), total cholesterol, and TNF-α in NAFLD patients.98,99 Probiotic treatment has been shown to reduce intrahepatic triglyceride (IHTG) content, AST, TNF-α, C-reactive protein (CRP), homeostasis model assessment of insulin resistance (HOMA-IR), serum endotoxin, steatosis, and the NASH activity index in patients with NASH.61,100 Probiotics can also reduce the levels of ALT, AST, cholesterol, low-density lipoprotein-C (LDL-C), triglycerides, and waist circumference in children with NAFLD.101 In animal models, probiotics protect against the onset of fructose-induced NAFLD by reducing expression of the tight junction protein occludin in the duodenum, attenuating activation of the TLR-4 signaling cascade, and increasing peroxisome proliferator-activated receptor gamma (PPAR-γ) activity.102 Probiotics also limit oxidative and inflammatory liver damage in patients with NAFLD by increasing PPAR-a activity and reducing TNF-α levels, metalloproteinase 2 and metalloproteinase 9 activities, and expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2.103 In particular, Lactobacillus casei strain Shirota (LcS) administration markedly suppressed the development of methionine and choline-deficient diet-induced NASH, as well as lowering serum LPS concentrations, suppressing inflammation and fibrosis in the liver, and reducing inflammation of the colon,104 while administration of Bifidobacteria attenuated NAFLD by modulating the SIRT1-mediated signaling pathway.105 In contrast, Lactobacillus rhamnosus GG (LGG) reduced duodenal nuclear factor-κB inhibitor (IκB) protein expression and restored portal LPS and duodenal tight junction protein concentrations, while also tending to attenuate TNF-α, IL-8R, and IL-1β mRNA expression in the liver.106 In addition, LGG decreased cholesterol levels, mediated by suppression of FXR and FGF-15 signaling.107
Serum AST was significantly lower in NASH patients treated with a combination of Bifidobacterium (Bifidobacterium longum) and fructooligosaccharide than in the control group,61 which implies a potential benefit of the use of synbiotics for the treatment of NASH. Furthermore, Buss et al.108 performed a systematic review of three randomized controlled trials (RCTs) that had tested the efficacy of probiotics, prebiotics or both (synbiotics) for the treatment of NAFLD in adult patients, and showed reductions in aminotransferases in the probiotic- and symbiotic-treated groups. Prebiotic fiber supplements have a beneficial effect in NAFLD because they modify the composition of the gut microbiota, and hence reduce body fat and improve glucose metabolism.109 Therefore, prebiotics, probiotics or both (synbiotics) may hold promise for the treatment of patients with NAFLD in clinical practice. Transplantation with better defined microbial communities or engineered bacterial strains, and substances targeting inflammatory cytokines, choline metabolism, hepatic TLR regulation, and bile acid metabolism might provide novel therapeutic strategies.
7. Conclusions
The pathophysiology of NAFLD involves many factors, and the intestinal microbiota plays an important role in the pathogenesis of both NAFLD and HCC. NAFLD and NASH are associated with alterations in the gut microbial population through various routes, such as alterations in gut epithelial permeability, choline metabolism, endogenous alcohol production, release of inflammatory cytokines, regulation of hepatic TLR, and bile acid metabolism. In addition, altered bile acid metabolism, release of inflammatory cytokines, and TLR-4 expression may promote NAFLD/NASH-associated HCC. Potential targets for NAFLD treatment and for the prevention of HCC include composition of the microbiota, metabolites, and pathways. However, better characterization of the intestinal microbiota and metabolome must be achieved through larger scale clinical research. Microbiome therapy could include the use of probiotic, prebiotic, and synbiotic supplements, or antibiotics.
Acknowledgments
This study was supported by the USA NIH grant R01 AA020703, and by Award Number I01BX002213 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development to B.S.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Browning JD, Cohen JC, Hobbs HH. Patatin-like phospholipase domain-containing 3 and the pathogenesis and progression of pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52:1189–1192. doi: 10.1002/hep.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty fiver disease: practice guideline by the American Gastroenterological Association, American Association for the study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis. 2010;28:162–168. doi: 10.1159/000282081. [DOI] [PubMed] [Google Scholar]
- 5.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 6.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 8.Day CP. Genetic and environmental susceptibility to non-alcoholic fatty liver disease. Dig Dis. 2010;28:255–260. doi: 10.1159/000282098. [DOI] [PubMed] [Google Scholar]
- 9.Finelli C, Tarantino G. Non-alcoholic fatty liver disease, diet and gut microbiota. EXCLI J. 2014;13:461–490. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 12.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouzaki M, Comelli EM, Arendt BM, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 14.Raman M, Ahmed I, Gillevet PM, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868–875. e1–3. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 17.Weinstock GM. Genomic approaches to studying the human microbiota. Nature. 2012;489:250–256. doi: 10.1038/nature11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Chierico F, Nobili V, Vernocchi P, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65:451–464. doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 20.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomba R, Seguritan V, Li W, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25:1054–1062. e5. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 23.Shanab AA, Scully P, Crosbie O, et al. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56(5):1524–34. doi: 10.1007/s10620-010-1447-3. [DOI] [PubMed] [Google Scholar]
- 24.Sabate JM, Jouet P, Harnois F, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18(4):371–7. doi: 10.1007/s11695-007-9398-2. [DOI] [PubMed] [Google Scholar]
- 25.Fialho A, Thota P, McCullough AJ, Shen B. Small Intestinal Bacterial Overgrowth Is Associated with Non-Alcoholic Fatty Liver Disease. J Gastrointestin Liver Dis. 2016;25(2):159–65. doi: 10.15403/jgld.2014.1121.252.iwg. [DOI] [PubMed] [Google Scholar]
- 26.Fu XS, Jiang F. Cisapride decreasing orocecal transit time in patients with nonalcoholic steatohepatitis. Hepatobiliary Pancreat Dis Int. 2006;5(4):534–7. [PubMed] [Google Scholar]
- 27.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8(6):411–20. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 28.Rahman K, Desai C, Iyer SS, et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology. 2016;151(4):733–746. e12. doi: 10.1053/j.gastro.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luck H, Tsai S, Chung J, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21(4):527–42. doi: 10.1016/j.cmet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Gabele E, Dostert K, Hofmann C, et al. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol. 2011;55(6):1391–9. doi: 10.1016/j.jhep.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Wu WC, He CY, Han Z, Jin DY, Wang L. Change of intestinal mucosa barrier function in the progress of non-alcoholic steatohepatitis in rats. World J Gastroenterol. 2008;14(20):3254–8. doi: 10.3748/wjg.14.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao JW, Tang HY, Zhao T, et al. Intestinal mucosal barrier dysfunction participates in the progress of nonalcoholic fatty liver disease. Int J Clin Exp Pathol. 2015;8(4):3648–58. [PMC free article] [PubMed] [Google Scholar]
- 33.Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012;28(2):159–65. doi: 10.1097/MOG.0b013e32834e7b4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchman AL, Dubin MD, Moukarzel AA, et al. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22(5):1399–403. [PubMed] [Google Scholar]
- 35.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8(1):35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 36.Waite KA, Cabilio NR, Vance DE. Choline deficiency-induced liver damage is reversible in Pemt(−/−) mice. J Nutr. 2002;132(1):68–71. doi: 10.1093/jn/132.1.68. [DOI] [PubMed] [Google Scholar]
- 37.al-Waiz M, Mikov M, Mitchell SC, Smith RL. The exogenous origin of trimethylamine in the mouse. Metabolism. 1992;41(2):135–6. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 38.Zeisel SH, Wishnok JS, Blusztajn JK. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther. 1983;225(2):320–4. [PubMed] [Google Scholar]
- 39.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140(3):976–86. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109(52):21307–12. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. 2015;6(2):e02481. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehedint MG, Zeisel SH. Choline's role in maintaining liver function: new evidence for epigenetic mechanisms. Curr Opin Clin Nutr Metab Care. 2013;16(3):339–45. doi: 10.1097/MCO.0b013e3283600d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezzi S, Ramadan Z, Fay LB, Kochhar S. Nutritional metabonomics: applications and perspectives. J Proteome Res. 2007;6(2):513–25. doi: 10.1021/pr060522z. [DOI] [PubMed] [Google Scholar]
- 44.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bain MA, Fornasini G, Evans AM. Trimethylamine: metabolic, pharmacokinetic and safety aspects. Curr Drug Metab. 2005;6(3):227–40. doi: 10.2174/1389200054021807. [DOI] [PubMed] [Google Scholar]
- 46.Letoffe S, Audrain B, Bernier SP, Delepierre M, Ghigo JM. Aerial exposure to the bacterial volatile compound trimethylamine modifies antibiotic resistance of physically separated bacteria by raising culture medium pH. MBio. 2014;5(1):e00944–13. doi: 10.1128/mBio.00944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato T, Morita A, Mori N, Miura S. The role of glycerol-3-phosphate dehydrogenase 1 in the progression of fatty liver after acute ethanol administration in mice. Biochem Biophys Res Commun. 2014;444(4):525–30. doi: 10.1016/j.bbrc.2014.01.096. [DOI] [PubMed] [Google Scholar]
- 48.Elamin E, Masclee A, Troost F, Dekker J, Jonkers D. Cytotoxicity and metabolic stress induced by acetaldehyde in human intestinal LS174T goblet-like cells. Am J Physiol Gastrointest Liver Physiol. 2014;307(3):G286–94. doi: 10.1152/ajpgi.00103.2014. [DOI] [PubMed] [Google Scholar]
- 49.Volynets V, Kuper MA, Strahl S, et al. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD) Dig Dis Sci. 2012;57(7):1932–41. doi: 10.1007/s10620-012-2112-9. [DOI] [PubMed] [Google Scholar]
- 50.Engstler AJ, Aumiller T, Degen C, et al. Insulin resistance alters hepatic ethanol metabolism: studies in mice and children with non-alcoholic fatty liver disease. Gut. 2016;65(9):1564–71. doi: 10.1136/gutjnl-2014-308379. [DOI] [PubMed] [Google Scholar]
- 51.Nair S, Cope K, Risby TH, Diehl AM. Obesity and female gender increase breath ethanol concentration: potential implications for the pathogenesis of nonalcoholic steatohepatitis. American Journal of Gastroenterology. 2001;96(4):1200–4. doi: 10.1111/j.1572-0241.2001.03702.x. [DOI] [PubMed] [Google Scholar]
- 52.Brooks JB, Basta MT, el Kholy AM. Studies of metabolites in diarrheal stool specimens containing Shigella species by frequency-pulsed electron capture gas-liquid chromatography. J Clin Microbiol. 1985;21(4):599–606. doi: 10.1128/jcm.21.4.599-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark DP. The fermentation pathways of Escherichia coli. FEMS Microbiol Rev. 1989;5(3):223–34. doi: 10.1016/0168-6445(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 54.Dawes EA, Foster SM. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956;22(2):253–65. doi: 10.1016/0006-3002(56)90148-2. [DOI] [PubMed] [Google Scholar]
- 55.Paege LM, Gibbs M. Anaerobic dissimilation of glucose-C14 by Escherichia coli. J Bacteriol. 1961;81:107–10. doi: 10.1128/jb.81.1.107-110.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elshaghabee FM, Bockelmann W, Meske D, et al. Ethanol Production by Selected Intestinal Microorganisms and Lactic Acid Bacteria Growing under Different Nutritional Conditions. Front Microbiol. 2016;7:47. doi: 10.3389/fmicb.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bahcecioglu IH, Yalniz M, Ataseven H, et al. Levels of serum hyaluronic acid, TNF-alpha and IL-8 in patients with nonalcoholic steatohepatitis. Hepatogastroenterology. 2005;52(65):1549–53. [PubMed] [Google Scholar]
- 58.Crespo J, Cayon A, Fernandez-Gil P, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34(6):1158–63. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 59.Wang JK, Feng ZW, Li YC, Li QY, Tao XY. Association of tumor necrosis factor-alpha gene promoter polymorphism at sites -308 and -238 with non-alcoholic fatty liver disease: a meta-analysis. J Gastroenterol Hepatol. 2012;27(4):670–6. doi: 10.1111/j.1440-1746.2011.06978.x. [DOI] [PubMed] [Google Scholar]
- 60.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–85. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57(2):545–53. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 62.Sohn W, Jun DW, Lee KN, et al. Lactobacillus paracasei Induces M2-Dominant Kupffer Cell Polarization in a Mouse Model of Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60(11):3340–50. doi: 10.1007/s10620-015-3770-1. [DOI] [PubMed] [Google Scholar]
- 63.Cengiz M, Yasar DG, Ergun MA, Akyol G, Ozenirler S. The role of interleukin-6 and interleukin-8 gene polymorphisms in non-alcoholic steatohepatitis. Hepat Mon. 2014;14(12):e24635. doi: 10.5812/hepatmon.24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 65.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21(4):317–37. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29(3):275–88. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 67.Salazar-Gonzalez RM, McSorley SJ. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Lett. 2005;101(2):117–22. doi: 10.1016/j.imlet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):571–9. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rivera CA, Gaskin L, Allman M, et al. Toll-like receptor-2 deficiency enhances non-alcoholic steatohepatitis. BMC Gastroenterol. 2010;10:52. doi: 10.1186/1471-230X-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wagnerberger S, Spruss A, Kanuri G, et al. Toll-like receptors 1–9 are elevated in livers with fructose-induced hepatic steatosis. Br J Nutr. 2012;107(12):1727–38. doi: 10.1017/S0007114511004983. [DOI] [PubMed] [Google Scholar]
- 71.Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139(1):323–34. e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeon SG, Kayama H, Ueda Y, et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012;8(5):e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayashi A, Sato T, Kamada N, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13(6):711–22. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Fuchs C, Claudel T, Trauner M. Bile acid-mediated control of liver triglycerides. Semin Liver Dis. 2013;33(4):330–42. doi: 10.1055/s-0033-1358520. [DOI] [PubMed] [Google Scholar]
- 76.Ferslew BC, Xie G, Johnston CK, et al. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60(11):3318–28. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–59. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 78.Stacey M, Webb M. Studies on the antibacterial properties of the bile acids and some compounds derived from cholanic acid. Proc R Soc Med. 1947;134(877):523–37. doi: 10.1098/rspb.1947.0029. [DOI] [PubMed] [Google Scholar]
- 79.Serino M, Luche E, Gres S, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61(4):543–53. doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yokota A, Fukiya S, Islam KB, et al. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes. 2012;3(5):455–9. doi: 10.4161/gmic.21216. [DOI] [PubMed] [Google Scholar]
- 81.Mobraten K, Haugbro T, Karlstrom E, Kleiveland CR, Lea T. Activation of the bile acid receptor TGR5 enhances LPS-induced inflammatory responses in a human monocytic cell line. J Recept Signal Transduct Res. 2015;35(5):402–9. doi: 10.3109/10799893.2014.986744. [DOI] [PubMed] [Google Scholar]
- 82.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19(4):338–48. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 84.Jiang C, Xie C, Li F, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125(1):386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li F, Jiang C, Krausz KW, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–35. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159–65. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baars A, Oosting A, Knol J, Garssen J, van Bergenhenegouwen J. The Gut Microbiota as a Therapeutic Target in IBD and Metabolic Disease: A Role for the Bile Acid Receptors FXR and TGR5. Microorganisms. 2015;3(4):641–66. doi: 10.3390/microorganisms3040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31(2):159–65. doi: 10.1097/MOG.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McMahan RH, Wang XX, Cheng LL, et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J Biol Chem. 2013;288(17):11761–70. doi: 10.1074/jbc.M112.446575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767–72. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–43. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 95.De Minicis S, Day C, Svegliati-Baroni G. From NAFLD to NASH and HCC: pathogenetic mechanisms and therapeutic insights. Curr Pharm Des. 2013;19(29):5239–49. [PubMed] [Google Scholar]
- 96.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504–16. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brandi G, De Lorenzo S, Candela M, et al. Microbiota, NASH, HCC and the potential role of probiotics. Carcinogenesis. 2017;38(3):231–240. doi: 10.1093/carcin/bgx007. [DOI] [PubMed] [Google Scholar]
- 98.Aller R, De Luis DA, Izaola O, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15(9):1090–5. [PubMed] [Google Scholar]
- 99.Ferolla SM, Armiliato GN, Couto CA, Ferrari TC. Probiotics as a complementary therapeutic approach in nonalcoholic fatty liver disease. World J Hepatol. 2015;7(3):559–65. doi: 10.4254/wjh.v7.i3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong VW, Won GL, Chim AM, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study Ann Hepatol. 2013;12(2):256–62. [PubMed] [Google Scholar]
- 101.Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J Pediatr Gastroenterol Nutr. 2017;64(3):413–417. doi: 10.1097/MPG.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 102.Wagnerberger S, Spruss A, Kanuri G, et al. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: a mouse model. J Nutr Biochem. 2013;24(3):531–8. doi: 10.1016/j.jnutbio.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 103.Esposito E, Iacono A, Bianco G, et al. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr. 2009;139(5):905–11. doi: 10.3945/jn.108.101808. [DOI] [PubMed] [Google Scholar]
- 104.Okubo H, Sakoda H, Kushiyama A, et al. Lactobacillus casei strain Shirota protects against nonalcoholic steatohepatitis development in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G911–8. doi: 10.1152/ajpgi.00225.2013. [DOI] [PubMed] [Google Scholar]
- 105.Ren T, Huang C, Cheng M. Dietary blueberry and bifidobacteria attenuate nonalcoholic fatty liver disease in rats by affecting SIRT1-mediated signaling pathway. Oxid Med Cell Longev. 2014;2014:469059. doi: 10.1155/2014/469059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ritze Y, Bardos G, Claus A, et al. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9(1):e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim B, Park KY, Ji Y, Park S, Holzapfel W, Hyun CK. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem Biophys Res Commun. 2016;473(2):530–6. doi: 10.1016/j.bbrc.2016.03.107. [DOI] [PubMed] [Google Scholar]
- 108.Buss C, Valle-Tovo C, Miozzo S, Alves de Mattos A. Probiotics and synbiotics may improve liver aminotransferases levels in non-alcoholic fatty liver disease patients. Ann Hepatol. 2014;13(5):482–8. [PubMed] [Google Scholar]
- 109.Parnell JA, Raman M, Rioux KP, Reimer RA. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012;32(5):701–11. doi: 10.1111/j.1478-3231.2011.02730.x. [DOI] [PubMed] [Google Scholar]