Abstract
Oral delivery of vaccines is highly desirable, yet it has met with limited success. Previously we developed allergen-free pollen grains as a novel approach for oral vaccination. We showed that spores of Lycopodium clavatum can be used for oral vaccination. However, it is unknown if pollens of other species can be similarly used as an oral vaccine carrier. Therefore, in this study, we evaluated common ragweed (RW) pollen (Ambrosia elatior) for its oral vaccination potential. Allergen-free RW pollens were prepared from natural pollens through chemical treatment. Eight weekly oral doses of ovalbumin (OVA) formulated with treated RW generated strong systemic (anti-OVA IgG, IgG1, IgG2a, and IgA) and mucosal (anti-OVA IgA) immune responses that sustained for at least three months after vaccination. Mucosal IgA against OVA was found in the lung lavage, feces, saliva, and vaginal secretion. Moreover, three and half months after the last immunization OVA-specific plasma cells were found in the bone marrow that actively secreted IgG and IgG1 antibodies. No IgE against RW-specific proteins was detected in the serum. Overall, RW pollen demonstrated potential for oral vaccine delivery.
Keywords: Microencapsulation, Mucosal vaccination, Oral vaccine delivery, Pollen grain oral vaccination, Ragweed vaccine delivery
Graphical abstract
1. Introduction
The adult human digestive tract is approximately 32 m2 in area (Helander and Fandriks, 2014). In addition to helping in absorption of nutrients, this large surface also forms a gateway for a plethora of pathogens. Colonization by invading pathogens at the gastrointestinal and other mucosal entry points can be prevented by secretory immunoglobulin A (sIgA), which is found on the mucosal surfaces (Holmgren and Czerkinsky, 2005; Mantis et al., 2011). In addition to eliciting systemic immunity, vaccination through the oral route has the advantage of inducing sIgA and other facets of mucosal immunity (Alignani et al., 2005; Sarti et al., 2011). Oral vaccination has other multifarious advantages over traditional parenteral vaccines including ease of administration, lower cost, needle-free painless delivery, and better patient compliance (Renukuntla et al., 2013; Vela Ramirez et al., 2017). Despite these advantages, subunit-based oral vaccination is difficult to implement. This is because the harsh acidic and proteolytic environment in the stomach can cause the vaccine subunit proteins to degrade. Further, the thick mucus layer on the intestine, the tight junctions between the intestinal epithelial cells that limit permeability of large molecules, and oral tolerance, together pose major challenges for oral vaccine delivery (Lycke, 2012; Vela Ramirez et al., 2017).
Several strategies have been developed to overcome these barriers (Shakya et al., 2016). These include use of live attenuated bacterial and viral vectors (Kotton and Hohmann, 2004), plant-based edible vaccines (Tacket, 2007), particulate delivery systems based on liposomes (Schwendener, 2014), immune stimulating complexes (ISCOMS) (Sjolander and Cox, 1998), and micro- and nano-encapsulation systems (Igartua et al., 1998; Sarti et al., 2011). Subunit protein-based vaccines provide a safer alternative to live attenuated microbe-based vaccines; however, they are poorly immunogenic and thus require a strong adjuvant to increase vaccine efficacy. Orally delivered bacterial enterotoxins such as cholera toxin (CT) from Vibrio cholerae or heat-labile enterotoxin (LT) from Escherichia coli can fulfill the role of mucosal adjuvants (Clements et al., 1988; Holmgren et al., 1993), however, their toxicity prohibits their use in humans (Shakya et al., 2016). Particulate delivery systems are another option to increase the potency of oral vaccines because they can enhance protein stability, increase bioavailability, and can enhance the immune response. In this regard, several polymers such as poly (lactic-co-glycolic acid) (PLGA) and chitosan have been tested (Borges et al., 2005; De Smet et al., 2013; Eldridge et al., 1990; Petersen et al., 2011; van der Lubben et al., 2003). However, low encapsulation efficiency, the potential of vaccine degradation from organic solvents that are used during particle formation, and the short-lived immune responses are the major barriers to successful implementation of particulate oral delivery systems (Woodrow et al., 2012).
Pollen grains (PGs) have emerged as a novel microparticulate carrier for oral therapeutics (Atwe et al., 2014; Diego-Taboada et al., 2013; Potroz et al., 2017). PGs are nature’s tough microcapsules that transport the precious plant male gametophyte for plant reproduction (Krichevsky et al., 2007). The shell of a PG is resistant, and it protects the fragile gametophyte and biomolecules residing in its interior from harsh environmental conditions. The pollen shell is bilayered. The outer wall is called the exine. It primarily consists of sporopollenin, a highly chemical resistant biopolymer (Mackenzie et al., 2015). The exine is covered with sticky lipid-rich pollen coat (pollenkitt, tryphine) (Pacini and Hesse, 2005). The inner wall is called the intine, and it is made of cellulose (Jiang et al., 2013). PGs also contain numerous proteins and enzymes that are essential for pollen development and fertilization (McCormick). Some of these pollen proteins can cause allergic reactions (Bordas-Le Floch et al., 2015). Therefore, it is critical to remove them from PG shells, for which several chemical treatment methods have been developed (Atwe et al., 2014; Mundargi et al., 2016; Prabhakar et al., 2017).
Previously, we have shown that oral delivery of ovalbumin (OVA) formulated with Lycopodium clavatum spore can elicit both systemic and mucosal antibody responses in mice (Atwe et al., 2014). Besides Lycopodium, PGs of other plant species have not been investigated for oral vaccination. There is a great degree of variation in size, shape, and surface morphology amongst pollens of different plant species (Atwe et al., 2014). These intrinsic variations can have an impact on immune responses. Therefore, the purpose of this study was to determine whether the immunoenhancing property of Lycopodium spore is shared by other PGs or not. To do so we have selected the common ragweed (RW) pollen for oral vaccination. RW pollens differ in size, shape, and surface architecture from Lycopodium spores. The focus of this study was to characterize the systemic and mucosal immune responses in mice by using OVA as a model antigen and RW pollen as the oral vaccine delivery system.
2. Materials and methods
2.1. Pollen, chemicals, proteins, and antibodies
Common RW (Ambrosia elatior) pollen was obtained from Pharmallerga (Lisov, Czech Republic). All chemicals and materials were purchased from Fisher Scientific (PA, USA) unless otherwise stated. Tween 20 and phosphate-citrate buffer tablets were purchased from Sigma-Aldrich. Phosphate-buffered saline (PBS) was bought from Mediatech, Inc. (Manassas, VA, USA). All cell culture reagents, lane marker sample reducing buffer, Coomassie brilliant blue G-250 dye, fluorescein isothiocyanate (FITC)-conjugated OVA, and O-phenylenediamine (OPD) tablets were purchased from Thermo Fisher Scientific (Waltham, MA, USA). OVA was purchased from MP Biomedicals (Solon, OH, USA). Non-fat dry milk, 4–20% mini-PROTEAN TGX precast gels for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Tris/Glycine/SDS electrophoresis buffer, and protein standards were obtained from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Carbamoylcholine chloride was obtained from Tocris Bioscience (Bio-Techne, Minneapolis, MN, USA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, IgG1, IgG2a, IgA, and IgE antibodies were bought from Southern Biotech (Birmingham, AL, USA). Common RW extract was obtained from Greer® (Lenoir, NC, USA). Red blood cell lysis buffer was purchased from eBioscience, Inc. (San Diego, CA, USA).
2.2. Animals
Female BALB/c mice (age 6–8 weeks) were purchased from Charles River Laboratories (Wilmington, MA, USA) and maintained at Texas Tech University (TTU) Animal Care Service facility (TX, USA), which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experiments were performed according to TTU Institutional Animal Care and Use Committee (IACUC) approved protocols.
2.3. Chemical treatment to prepare empty RW pollen microcapsules
To prepare protein-free RW pollen shell, 30 g of raw RW was first refluxed in 350 ml of acetone at 70 °C for 18 h. Solutions were cooled and filtered using a acuum iltration unit illipore igma Billerica, MA, USA). Pollens were then stirred in 400 ml of 85% orthophosphoric acid at 60 °C for seven days. After acidolysis, pollen solutions were cooled, diluted with ultrapure Milli-Q water, filtered, and washed sequentially with hot Milli-Q water, acetone, 2 M HCl, 2 M NaOH, Milli-Q water, acetone, and 70% ethanol. After overnight air-drying, pollens were refluxed in 350 ml of 1 M KOH for 12 h at 80 °C (renewed after 6 h with fresh solution). Pollen solutions were then diluted with Milli-Q water, filtered, and washed with hot Milli-Q water, acetone, and 70% ethanol. Finally, pollens were dried at 60 °C for 24 h and stored at room temperature until use.
2.4. RW shell characterization
To examine the interior of the RW shell, samples of RW pollen were manually cracked by grinding with dry ice, mounting on an aluminum stub, and coating with gold-palladium in a sputter coater (Technics Hummer V sputter coater, Anatech USA, CA, USA). The structure of RW was visualized with a scanning electron microscope (Hitachi S-4300 E/N FESEM, NY, USA). Carbon, hydrogen, and nitrogen content in the pollen samples were analyzed using PerkinElmer 2400 Series II CHNS/O Analyzer (PerkinElmer, Inc., Waltham, MA, USA). Protein percentage was calculated from total nitrogen content by multiplying it with a nitrogen-to-protein conversion factor of 6.25 (Atwe et al., 2014). All measurements were performed in triplicate.
2.5. Confocal laser scanning microscopy
Chemically-treated RW pollens (5 mg) were incubated with FITC-OVA solution (300 µg FITC-OVA in 0.3 ml PBS) overnight at room temperature under vacuum (20 in. of Hg). Pollens were then carefully removed from the solution, air-dried, and imaged with a confocal microscope (Nikon Eclipse Ti-E, Nikon Instruments Inc., Melville, NY, USA). RW pollens were excited by 488 and 561 nm lasers and images were captured with Nikon NIS-Elements AR software (version 4.13.01).
2.6. SDS-PAGE
SDS-PAGE was used to compare protein content in natural and chemically-treated RW pollen (Uddin and Gill, 2017). Dry RW pollens (natural or after chemical treatment) were first broken using a magnetic stir bar at 1000 rpm for 6 h at room temperature. PBS was then added (1 ml per 20 mg RW) and stirred for another 24 h at 4 °C. Solutions were filtered with Vivaspin™ centrifugal concentrators (1000 kD MWCO, Sartorius AG, Goettingen, Germany) at 10,000 × g for 5 min at 4 °C. These solutions were analyzed for protein content.
SDS-PAGE was also used to determine if OVA adsorbs onto RW surface. Chemically-treated RW pollens were incubated overnight with an OVA solution (5 mg RW per 100 µg OVA in 0.3 ml PBS) under vacuum and filtered. The filtered pollens were either dried at 4 °C (before wash group), or washed three times with PBS and then dried (after wash group). Dried RW from both groups were heated with sample loading buffer (60 mM Tris-HCl, 1% SDS, 10% glycerol, 20 mM dithiothreitol in PBS) at a concentration of 20 mg/ml for 10 min at 95 °C to extract OVA adsorbed on the RW walls. Pollens were separated from the liquid by centrifugal filtration in Vivaspin™ centrifugal concentrators as described above. The different samples along with OVA solution (333 µg/ml) as a control were analyzed.
2.7. Vaccine formulations and immunizations
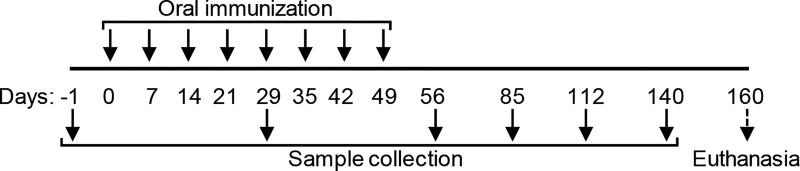
OVA was used as a model vaccine antigen. Each oral dose comprised of 5 mg treated RW formulated with 100 µg OVA in 0.3 ml PBS. Mice (n = 10 per group) were immunized via oral gavage with a 20G curved animal feeding needle (Cadence, Inc. Staunton, VA, USA). OVA alone (without RW) and RW alone (without OVA) were used as control formulation. A total of eight weekly doses were administered (Fig.1).
Fig. 1.
Immunization and sample collection schedule.
2.8. Sample collection
Blood was collected from mouse submandibular (facial) vein and kept at room temperature for 2 h to clot. 10 fecal droppings were collected from each mouse and incubated with 500 µl o PB at room temperature or 1 h. Both blood and fecal samples were centrifuged at 15,000 × g for 10 min at 4 °C to collect supernatants. Vaginal washes were collected by pipetting 40 µl o sterile PB into the aginal tract. Salivary flow was collected from mouse oral cavity by intraperitoneal injection of 50 µl of PBS containing carbamoylcholine chloride (40 µg/ml) as saliva stimulant. All samples were collected every 28 days for up to three months after the last dose (Fig. 1). Baseline serum and fecal samples were collected before vaccination (day -1). Three and half months after the last dose, mice were euthanized and tracheal region of each mouse was exposed for bronchoalveolar lavage (BAL) fluid collection. An 18G 1.25-inch catheter was used to collect BAL fluid by injecting and aspirating out 0.5 ml PBS. Vaginal wash, saliva, and BAL fluids were centrifuged at 500 × g for 5 min at 4 °C to collect supernatants. All samples were stored at −80 °C until analysis.
2.9. Bone marrow and splenocyte cell culture
Three and half months after the last booster dose, vaccinated mice (n = 7 mice) were euthanized and bone marrow cells were harvested from both hind legs of each mouse according to a published protocol (Uddin and Gill, 2017). Bone marrow cells were then cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/ml of penicillin, 100 µg/ml of streptomycin, and 0.25 µg/ml of amphotericin B. Splenocytes were prepared and also cultured in the above-mentioned medium. Bone marrow cells and splenocytes were plated at a concentration of 1 × 107 cells/ml per well of a 12 well plate, and cultured at 37 °C in a 5% CO2 incubator for four days. Supernatants were collected every 24 h, centrifuged at 200 × g for 5 min at 4 °C and stored at −80 °C until analysis.
2.10. Enzyme-linked immunosorbent assay (ELISA)
OVA-specific antibodies were measured with ELISA as described previously (Atwe et al., 2014). Immune response was characterized by measuring serum anti-OVA IgG, IgG1, IgG2a, IgE, and IgA; fecal anti-OVA IgA; salivary anti-OVA IgA; vaginal anti-OVA IgA; BAL fluid anti-OVA IgA; and anti-OVA IgG, IgG1, and IgG2a antibodies secreted by bone marrow cells and splenocytes. Serum samples were first analyzed at a dilution of 1:25. Serum samples that showed reading at optical density (OD) ≥ 3 were analyzed further using serial dilution (two-fold dilution from 1:25 to 1:102400). Serum OVA-specific IgG and IgG1 responses were then expressed as endpoint titer, which was defined as the reciprocal of the highest dilution above cut-off value (mean OD + 3 × standard deviation (SD) of non-immunized baseline sera collected at day -1) (Park et al., 2009). Mice that did not show any response or showed a response less than the cut-off point were assigned the lowest titer i.e. 25. Fecal samples, vaginal washes, and saliva samples from individual animals were analyzed at a dilution of 1:5. BAL fluid, splenocytes, and bone marrow cell culture supernatants were analyzed at a dilution of 1:2. All samples were diluted in PBST (0.05% Tween 20 in PBS) and analyzed in triplicate. Internal antibody controls were used in all ELISA plates to confirm consistency in the ELISA procedure. These serum and fecal antibody controls were generated in-house by three monthly intranasal vaccinations of mice (n = 10) with 5 µg CT and 25 µg OVA.
To measure IgE specific for RW pollen protein, lyophilized RW allergen crude extract purchased from Greer® (Lenoir, NC) was dissolved in PBS at a concentration of 20.2 µg/ml to obtain protein concentration of 5 µg/ml. This solution was used to coat ELISA plates instead of OVA.
2.11. Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). A two-tailed t-test was used for analysis of pollen protein content data, bone marrow plasma cell response, and BAL fluid mucosal response. Inter-group comparisons for ELISA and other tests were done with one-way ANOVA and post hoc Tukey test. Significance was considered for p < 0.05.
3. Results
3.1. Hollow RW shell for oral vaccination
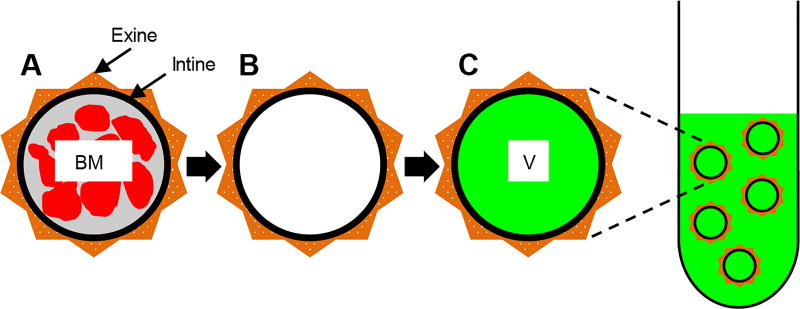
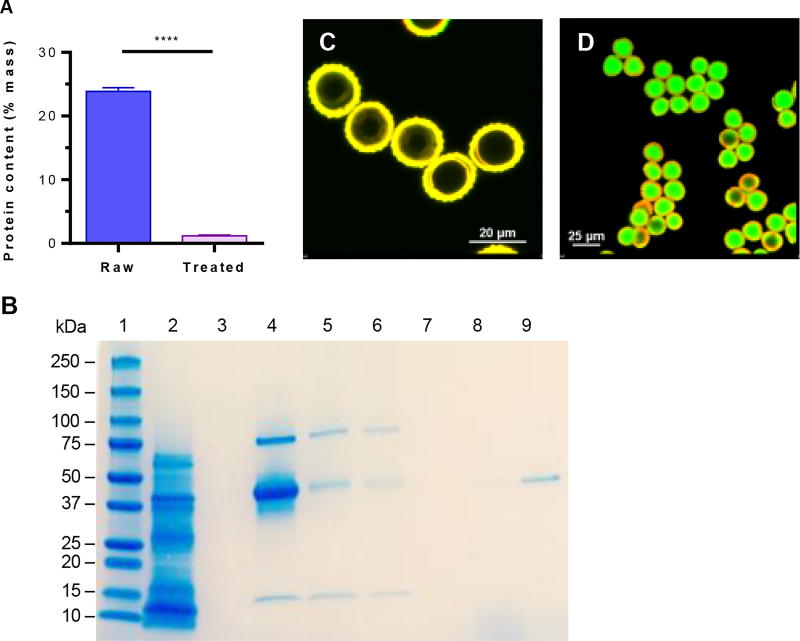
The concept of using PGs for oral vaccination is shown in Fig. 2. Natural raw pollens (Fig. 2A) are chemically treated to obtain clean, protein-free, and hollow pollen shells (Fig. 2B), which are then formulated in a vaccine solution (Fig. 2C). The vaccine-filled pollens stay suspended in the vaccine solution at all times until oral gavage. This allows shells to remain filled with the vaccine. Electron microscopy showed that the chemically treated RW pollens were morphologically intact, and had no visible residue on the outer surface nor the inner cavity (Fig. S1). Approximately 71.9 ± 1.1% of the initial mass was removed from RW pollen during chemical treatment, and the protein content significantly decreased from 23.9 ± 0.6% to 1.2 ± 0.1% (Fig. 3A). This reduction is similar to our previously published result (Uddin and Gill, 2017). In the determination of protein content, we have assumed that 100% of the measured nitrogen is emanating from residual proteins. However, sporopollenin, the material that makes up the exine of pollens also contains nitrogen in its chemical structure (Descolas-Gros and Schölzel, 2007). Consequently, this calculated residual protein value is an overestimate that serves to provide the upper limit rather than the actual residual protein content.
Fig. 2.
Schematic of pollen grains (PGs) as an oral vaccine delivery system. (A) A natural raw PG shell with plant-derived biomolecules (BM) surrounded by a wall. (B) A chemically treated PG with an intact shell and a clean core. (C) A chemically treated PG shell filled with vaccine (V) solution. PGs are suspended in a vaccine solution, and as a result, the vaccine is in the PG core and also surrounds it.
Fig. 3.
Characterization of ragweed (RW) pollen. (A) Elemental analysis of RW showing a reduction in protein content after chemical treatment. Values shown are mean ± SD for three independent experiments. p ≤ 0.0001 [****]. (B) SDS-PAGE analysis of RW. Lane 1: protein ladder; lane 2: extract from raw RW pollen; lane 3: extract from treated RW pollen; lane 4: OVA; lane 5: chemically treated RW incubated with OVA, filtered, and dried (before washing group); lane 6: chemically treated RW incubated with OVA, filtered, washed three times, and dried (after washing group); lane 7: supernatant from third wash; lane 8: supernatant from second wash; lane 9: supernatant from first wash. The gel image is a representative of three independent experiments with similar results. Confocal micrographs of dry treated RW pollens (C) before FITC-OVA encapsulation, (D) after FITC-OVA encapsulation. All confocal micrographs are merged images obtained after excitation with 488 and 561 nm lasers.
The absence of proteins in chemically-treated RW pollen was additionally confirmed using SDS-PAGE. RW pollens were broken using a stir bar to help extract any residual proteins. Extracts from RW pollen before chemical treatment contained the expected proteins (Fig. 3B: lane 2) (Bordas-Le Floch et al., 2015), but after chemical treatment, no proteins were detected (Fig. 3B: lane 3). This indicates that a robust cleaning was achieved with the chemical treatment, and protein-free pollen shells were obtained.
3.2. Adsorption of OVA on RW surface
Treated RW pollen surface have the potential to adsorb proteins (Uddin and Gill, 2017). Therefore, using SDS-PAGE analysis we studied OVA adsorption on to RW surfaces. The OVA solution showed three protein bands (Fig. 3B: lane 4) instead of a single band. We believe this is due to impurities such as ovotransferrin (76 kD) and lysozyme (14.4 kD), which are typically found in commercially available OVA (45 kD) (Uddin and Gill, 2017). Similar bands were seen when RW were analyzed after they were incubated with the OVA solution and then either filtered and dried but not washed (Fig. 3B: lane 5, before washing group) or were washed three times (Fig. 3B: lane 6, after washing group). This shows that small quantities of OVA get’s adsorbed on the RW pollen surfaces, and stays bound despite thorough washing. The thoroughness of the washing was confirmed by the lack of protein bands in the last two washes (Fig. 3B: lane 7,8). In contrast, the first wash did remove some of the loosely attached OVA from RW (Fig. 3B: lane 9)
3.3. FITC-OVA loading into RW pollen shell
Once the native proteins and other biomolecules were removed from the RW pollens, the empty space inside the RW shell became available for vaccine loading. To facilitate loading of the vaccine solution into RW, vacuum was used. Confocal micrographs showed hollow RW pollen shells (Fig. 3C) get filled with FITC-OVA solution (Fig. 3D).
3.4. RW + OVA induce strong systemic immune response
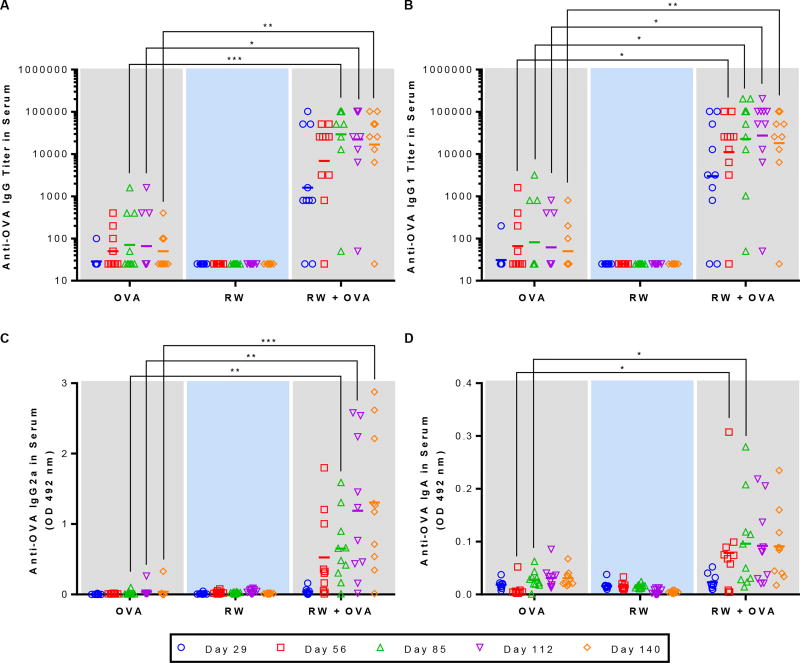
We evaluated the efficacy of RW-based oral vaccination by measuring antibody responses in serum using ELISA. Mice that were fed OVA alone (no RW) or RW alone (no OVA) were used as negative controls in this study. As expected, OVA-specific antibody responses were not detected in mice before immunization (day -1) (data not shown). When mice were gavaged with OVA alone, no significant increase in anti-OVA IgG was seen (Fig. 4A). However, when RW pollens were included in the formulation, titers of OVA-specific IgG increased significantly as compared to the OVA alone group (Fig. 4A). In the RW + OVA group, the titers increased as the number of doses administered increased. The geometric mean titer was two orders of magnitude higher for the RW + OVA group as compared to the OVA alone group on day 85. These high titers were sustained for up to three months after the last oral dose. Individual mouse serum analysis shows that at day 85, 90% of the mice vaccinated with RW formulation showed a high response (titer > 12,800). In contrast, majority of the OVA alone control mice did not show any response, and only 30% of these control mice showed a low response (titer < 1600). These results demonstrate that addition of RW in the OVA formulation not only increased the titers but also increased the number of high responders. As expected, RW alone group did not generate any OVA-specific IgG (Fig. 4A).
Fig. 4.
Serum antibody response against OVA. Mice were orally immunized with 100 µg OVA alone, 5 mg treated ragweed (RW) pollen alone, or 100 µg OVA and 5 mg RW every week for eight weeks. (A) Anti-OVA IgG titer. (B) Anti-OVA IgG1 titer. (C) Anti-OVA IgG2a absorbance value. (D) Anti-OVA IgA absorbance value. Each symbol represents the endpoint titer or the mean absorbance of an individual mouse n = 10). p ≤ 0.05 [*] p ≤ 0.01 [**] p ≤ 0.001 [***]. short horizontal bar represents the geometric mean titer or the mean absorbance of the group).
We further analyzed anti-OVA IgG subtypes (IgG1 and IgG2a) and IgA in serum samples. Serum IgG1 response followed a similar course as IgG, with the RW formulation showing significantly higher (p < 0.01) titers (Fig 4B). IgG2a and IgA samples were analyzed at 1:25 dilution. Mice gavaged with OVA alone did not show any IgG2a response (Fig. 4C). On the other hand, in the RW + OVA group, the IgG2a response continuously increased from day 29 to day 112 (two months after the last dose), and it remained high up to day 140 (three months after the last dose). At day 140 IgG2a in RW + OVA group was significantly higher than that of OVA alone (p < 0.01). Serum analysis was also positive for OVA-specific IgA. It was significantly higher (p < 0.05) on day 56 and day 85 in the RW + OVA group (Fig. 4D), and remained at those elevated levels till day 140. This shows that RW can induce both IgG1 and IgG2a subtypes, indicating stimulation of both TH1 and TH2 pathways. As expected, no antibody responses were observed at any time points in mice that were vaccinated with only RW without OVA.
3.5. RW + OVA induce mucosal immune response
We next determined IgA response in various mucosal compartments. Due to the limited volume of the mucosal samples, we chose to measure OD at a fixed dilution of 1:5 rather than measuring antibody titer. This dilution was determined from a pilot vaccination study (Fig. S2).
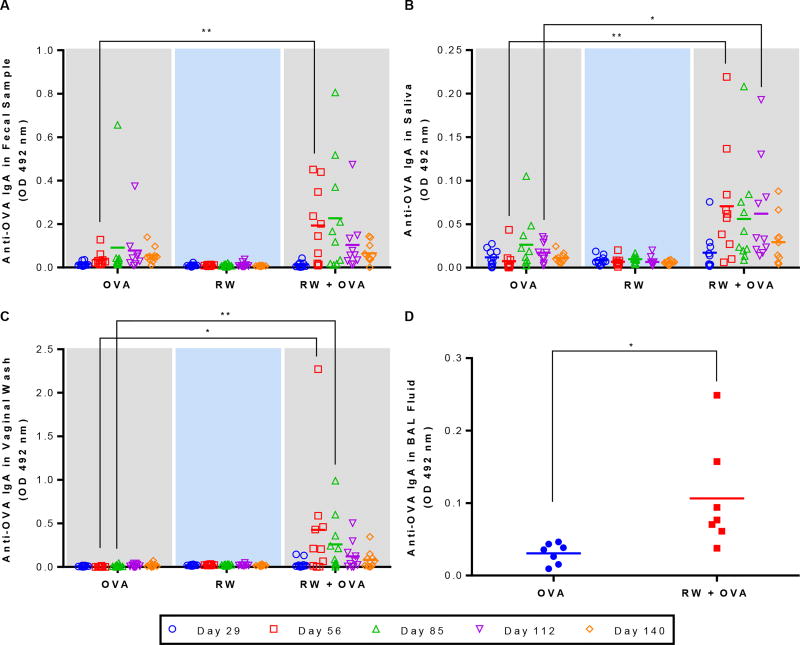
IgA response in fecal samples was low on day 29 for all groups (Fig. 5A). Fecal IgA increased significantly (p < 0.01) for the RW + OVA formulation at day 56 and reached a maximum at day 85. Two months after the last booster dose, IgA response started to decrease for all groups. IgA response in saliva was less strong. Nonetheless, the RW + OVA group had a significantly (p < 0.01) higher salivary IgA at days 56 through 112 (Fig. 5B). On day 112, anti-OVA IgA in saliva of RW + OVA mice was significantly higher than mice of OVA alone group. Surprisingly, IgA in vaginal secretion at day 56 and 85 was also more pronounced and significantly higher (p < 0.05) in the RW + OVA group (Fig. 5C). In contrast, control mice did not show any vaginal IgA response. Lung lavages of RW + OVA gavaged mice also had significantly higher (p < 0.05) IgA response on day 160 (Fig. 5D).
Fig. 5.
Mucosal antibody response against OVA at different mucosal sites. Mice were orally immunized with 100 µg OVA alone, 5 mg treated ragweed (RW) pollen alone, or 100 µg OVA and 5 mg RW every week for eight weeks. Anti-OVA IgA response in (A) fecal samples, (B) saliva, (C) vaginal wash, and (D) BAL fluid (day 160). Each symbol represents the response from an individual mouse (n = 10 for fecal, saliva, and vaginal wash; n = 7 for BAL fluid). Feces, saliva, and vaginal secretions were diluted in PBST at 1:5 dilution while BAL fluids were diluted at 1:2 dilution in PB T. p ≤ 0.05 [*] p ≤ 0.01 [**] (horizontal bar represents the group mean)
3.6. RW + OVA promote OVA-specific plasma cells in bone marrow
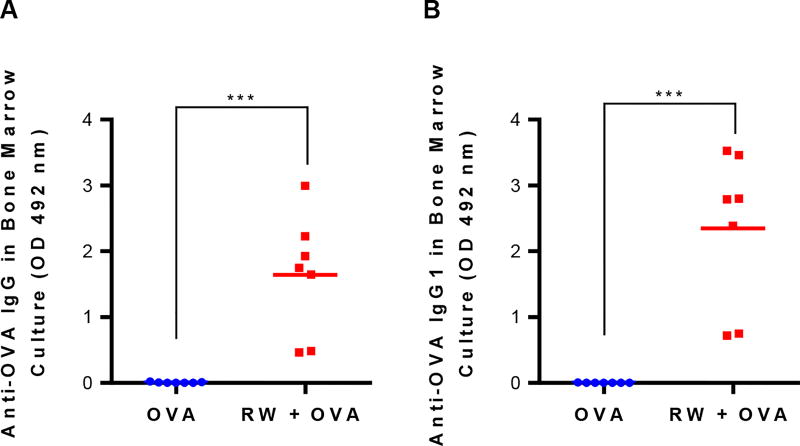
Since we observed sustained antibody levels in the RW + OVA gavaged mice, we wanted to study if vaccinated mice had developed OVA-specific plasma cells. Therefore, three and half months after the last booster dose, splenocytes and bone marrow cells were harvested and cultured for four days without any stimulation. Fig. 6 shows that bone marrow cells from mice vaccinated with RW + OVA secreted significantly higher (p < 0.001) anti-OVA IgG (Fig. 6A) and IgG1 (Fig. 6B) antibodies than the OVA alone control formulation. However, IgG2a was not detected in the bone marrow cell culture medium (data not shown). This is consistent with serum antibody levels wherein high IgG1 but moderate IgG2a levels were seen. Splenocytes did not show any antibody secretion (data not shown). Anti-OVA IgG secretion increased with longer culture times, and it reached a plateau on the fourth day (Fig. S3).
Fig. 6.
Antibody-secreting plasma cell response in mouse bone marrow culture at day 160. Mice were orally immunized with 100 µg OVA alone or 100 µg OVA and 5 mg treated ragweed (RW) pollen every week for eight weeks. Bone marrow cells from the vaccinated mice were cultured for four days without any stimulation and secreted antibodies were measured with ELISA: (A) anti-OVA IgG, (B) anti-OVA IgG1. Cell culture supernatants were diluted in PBST at 1:2 dilution. Each symbol represents the response from an individual mouse (n = 7). p ≤ 0.001 [***] (horizontal bar represents the group mean)
3.7. RW formulation did not cause allergic response
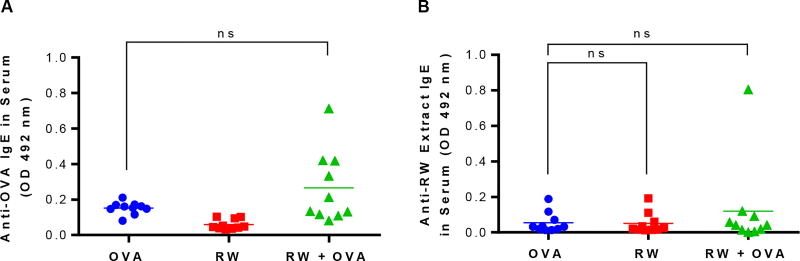
To determine if the RW formulation can cause an allergic response, serum IgE antibodies were analyzed against OVA and RW extract. The RW extract contains allergenic proteins. Serum samples from individual mice were analyzed with ELISA at 1:25 dilution. Serum analysis showed low anti-OVA and anti-RW extract IgE for all groups and these responses were not significantly different from each other (p > 0.05) (Fig. 7). The absence of high IgE responses further supports our results that treated RW do not contain any allergenic proteins, and that immunization with RW does not cause allergic response to the antigen (OVA).
Fig. 7.
Serum IgE antibody response. Mice were orally immunized with 100 µg OVA alone, 5 mg treated ragweed (RW) pollen alone, or 100 µg OVA and 5 mg RW every week for eight weeks. IgE response against (A) OVA at day 85, and (B) RW pollen extract at day 140 were measured with ELISA. Serum samples were diluted in PBST at 1:25 dilution. Each symbol represents the response from an individual mouse (n = 10). ns = not significant (horizontal bar represents the group mean)
4. Discussion
While our previous study showed a prolonged antigen-specific immune response when mice were orally vaccinated with a Lycopodium spore-based formulation (Atwe et al., 2014), similar studies evaluating the immunostimulating properties of other pollens are lacking. Therefore, to determine whether pollens of other species also possess adjuvant properties, we characterized the immune responses produced by RW pollen as an oral vaccine carrier. RW pollens are spherically shaped with a diameter measuring 15–18 µm. Their surface is covered with short spines. In contrast, Lycopodium spores are much larger with a diameter of 25–30 µm. They have an asymmetric architecture with a bulbous face and an opposite face containing a trilete joint. Lycopodium spores are not covered with spines. Instead, they are covered with architectures resembling a crown. This dramatic difference between RW pollens and Lycopodium spores prompted us to select RW for this investigation.
PGs in their native state contain a large number of proteins, glycoproteins, lipids, and enzymes, which have potential to cause allergies (Bordas-Le Floch et al., 2015). Several methods have been developed to remove these materials from PGs without damaging the pollen shells (Atwe et al., 2014; Mundargi et al., 2016). To clean RW pollens, we have developed a novel treatment process. This is because the conventional treatment protocol that is widely used for cleaning Lycopodium spores does not work for most other pollens including RW pollen. Instead of producing clean and discrete RW pollens, the use of Lycopodium treatment protocol produces RW pollens that are clumped and entrapped in an insoluble matter. Using the new treatment protocol, we were able to generate clean, intact, and non-aggregated RW pollens.
We prepared protein-free intact RW pollens and confirmed removal of allergens with several complementary techniques including, scanning electron microscopy examination, elemental analysis, and gel electrophoresis. We have further confirmed removal of lipids, proteins, and nucleic acids in RW after chemical treatment using Fourier transform infrared (FTIR) spectroscopy. These data are included in a separate publication.
Chemical treatment did not alter the physical structure of RW pollens (Fig S1). We utilized the open aperture of the treated RW pollen (Fig. S1 F,G) to fill it with OVA solution. Entry of OVA solution into RW pollens was facilitated by application of low vacuum, which removed air from the RW pollens and generated a negative pressure in the pollens compared to the surrounding liquid. This pressure gradient allowed entry of surrounding liquid into the RW pollens.
Loading of OVA inside the RW core was confirmed using confocal microscopy. OVA can also get adsorbed on the surfaces of chemically treated pollen, especially since they possess micron- and nano-sized decorative features and pockets. In line with this postulate, we have recently shown that OVA does adsorb on to RW pollens when OVA at 1.67 mg/ml concentration was used (Uddin and Gill). However, for oral gavage, the formulation contains OVA in about fivefold lower amount. Therefore, we wanted to assess if adsorption can still occur when OVA is present at this low concentration of 0.33 mg/ml. Using SDS-PAGE analysis we found that not only is OVA adsorbed on RW pollen surface, but it stays attached despite thorough washing. This result is significant because it shows that RW can transport OVA in its core (in soluble form), and on its surfaces (in adsorbed form).
A strong systemic IgG antibody response was generated after oral vaccination with OVA and RW pollen. Both IgG1 and IgG2a antibodies were observed, although the IgG1 subtype was higher than the IgG2a subtype. This is consistent with our previous study where Lycopodium showed an IgG1-biased immune response (Atwe et al., 2014). In addition to the systemic immune response, oral vaccination is also expected to produce a mucosal immune response. Similar to our previous study with Lycopodium (Atwe et al., 2014), in this study too, oral vaccination with RW produced anti-OVA IgA in intestinal secretions. In addition, we also observed IgA antibody response in saliva, vaginal washes, and lung lavages. Thus, oral immunization with RW formulations provides a simple way to induce mucosal responses in both local and distant mucosal sites.
To confirm that RW alone cannot induce an antigen-specific immune response, we immunized mice with RW pollen but without OVA. No OVA-specific antibody response was detected in serum or mucosal samples of these mice. Therefore, bone marrow and BAL samples from these mice were not analyzed further.
It is desirable to have a vaccine formulation that can generate long-lasting immunity. Therefore, we measured antibody levels in serum and mucosal samples three months after the last vaccine dose. We saw that while systemic antibody levels remained high, the mucosal antibody levels decreased slightly. It is thought that sustained antibody levels are maintained by long-lived plasma cells that reside mainly in the bone marrow, and a small proportion in the spleen (Manz et al., 1997; Radbruch et al., 2006). Lemke and colleagues found long-lived OVA-specific plasma cells in the bone marrow even nine months after oral immunization with CT (Lemke et al., 2016). Therefore, in this study, we also examined if the persistent humoral response seen after RW vaccination is due to bone marrow- or spleen-resident long-lived plasma cells. Based on continuous antibody secretion in ex vivo cultures, we conclude that RW-based oral vaccination does generate plasma cells, and they exclusively reside in the bone marrow.
RW pollen is known to cause allergies. Although chemical treatment allowed us to remove the allergy-causing native proteins from RW, we still wanted to determine if any allergic response was being generated. Since IgE plays a critical role in the pathogenesis of allergy (Holgate and Polosa, 2008), we measured serum IgE responses against both OVA protein and RW allergen extract (similar to that used in skin allergy tests). Our results showed no significant IgE response against OVA or RW proteins in the vaccinated mice.
Pollen-based formulation departs from the current practice of using micro and nanoparticles for oral vaccine delivery. These particles are often less than 5 µm in diameter. Particles similar in size to RW (15 µm in diameter) or larger have only been rarely tested. It is also common to administer a high vaccine quantity (up to 100-fold higher than injection) with multiple booster immunizations to achieve a good immune response (Pavot et al., 2012). For example, six oral doses (200 µg BmpB antigen/dose) of M cell targeting PLGA particles (3.7 µm) were used to develop a vaccine against swine dysentery (Jiang et al., 2014). Lower antigen concentration and fewer doses often provide only partial vaccine responders. For example, three doses (5 µg OVA/dose) of PLGA nanoparticles (211 nm) incorporated with a M cell targeting moiety successfully immunized only 63% of the mice in a cohort (Garinot et al., 2007). Moreover, in a separate study, six doses (500 µg bovine serum albumin/dose) of PLGA particles (1.5 µm) were needed to generate a prolonged immune response (Igartua et al., 1998). Intraperitoneal priming along with four oral booster doses (1 mg OVA/dose) using polyacrylamide microparticles (2.55 µm) was only able to enhance salivary IgA while serum IgG response did not change after vaccination (O'Hagan et al., 1989). Nine or more doses (100–468 µg antigen/dose) of starch microparticle-based (2–3 µm) oral vaccine formulations were used in other studies (Moreno-Mendieta et al., 2014; Rydell and Sjöholm, 2005; Wikingsson and Sjöholm, 2002). In contrast to these studies, in our current study, eight doses of RW formulation not only had a high percentage of responding mice (90%), but it also generated higher serum antibody titer, and mucosal antibodies in the gut and other mucosal sites. Furthermore, both mucosal and systemic antibodies persisted for an extended period of time. This data demonstrates the potential of using RW for oral vaccine delivery.
Generally, oral vaccine formulations incorporate adjuvants such as flagellin, CT, LT, monophosphoryl lipid A (MPLA), CpG oligodeoxynucleotides to strengthen the immune responses (Shakya et al., 2016; Vela Ramirez et al., 2017). Although we did not use adjuvants, it may be worthwhile to evaluate safe adjuvants such as MPLA and CpG to determine if their addition can help to reduce the number of oral vaccine doses.
RW pollen grains have pores in their wall that link the inside of pollens to their outside. Since these pores always remain open, any OVA that enters the pollen grains can easily come out. As a result, to keep pollens filled at all times, they are kept submerged in the OVA solution. This formulation approach makes it tough to determine release kinetics because to perform these studies the outer solution must be removed, such as through filtration. In doing so, the solution inside the pollens also comes out. In our recently published study we have postulated a mechanism of how despite a leaky architecture, RW pollens can stimulate an immune response (Uddin and Gill, 2017). Using Caco-2 cells to model the human intestinal epithelial cells, we showed that RW pollens are not cytotoxic nor do they increase the permeability of the Caco-2 monolayers. This suggests that RW pollens do not facilitate antigen uptake by merely increasing intestinal permeability. We, however, observed internalization of RW pollen across the mouse intestinal barrier in vivo, which shows that RW carries the antigen directly across the intestinal barrier where it can stimulate the resident immune cells to generate an adaptive immune response. We also found that RW stimulate Caco-2 cells to secrete inflammatory cytokines that can recruit innate immune cells including macrophages and dendritic cells, both of which are antigen presenting cells. We have also observed that RW pollens activate primary mouse macrophages and primary dendritic cells ex vivo. This implies a dual role of RW both as an immunostimulant and a delivery system (Uddin and Gill). While the RW-mediated innate activation pathway of macrophages and dendritic cells remains elusive, we postulate that pattern-recognition receptors (PRRs) such as toll-like receptors (TLRs), NOD-like receptors (NLRs) or C-type lectin receptors (CLRs) present in the antigen presenting cells (Kawai and Akira, 2011) may uniquely interact with the sporopollenin wall chemical moiety, thereby activating the innate immune cells. More studies are needed to fully investigate this mechanism.
Although the humoral responses of RW formulation were discussed in this study, the cellular responses were not characterized. Additional studies are needed to characterize the cellular immunity of RW-based oral vaccines. Considering our current study, other pollen species also need to be investigate to identify pollen species that might be better as oral vaccine carriers.
RW pollens are relatively inexpensive and the chemical treatment process used to clean them is also simple. Additionally, the pollen-vaccine formulation is simple to prepare because it involves a convenient single step process of mixing the subunit protein and pollens under mild vacuum. To study the physical stability of the RW pollen after encapsulation, OVA encapsulated pollens were separated from the vaccine solution, air-dried, and imaged. RW pollen retained their physical integrity and morphological homogeneity after OVA encapsulation (Fig. S1I–J). There was no detrimental effect of vacuum on RW exine wall. Salt from PBS along with possible OVA residue was seen on the exine surface (Fig. S1K) and on the intine surface (Fig. S1L). RW pollens do not form clumps in the liquid formulation and are thus amenable to a relatively simple mixing and vacuum-loading step as part of large scale manufacturing.
In contrast, many polymeric particles previously used for oral vaccination require the use of harsh solvents to encapsulate the vaccine, and this can adversely affect vaccine efficacy. Pollens are currently collected on a commercial scale to cater to the allergy pharmaceutical industry to extract allergens. This large-scale availability of pollens in high purity and meeting the pharmaceutical industry standards, implies that scale up of pollens for oral vaccination is feasible. We obtain pollens for research from a vendor who supplies pollens to the pharmaceutical industry. Purity of the RW pollens used in our studies is reflected in the electron micrograph image of the ensemble of natural RW pollens where no other pollen species can be observed (Fig. S1A). Thus, the RW-based oral vaccine delivery system can offer a simple and economically viable alternative to polymeric particles and needle-based vaccination.
5. Conclusion
Our current study demonstrates that RW pollen can be made allergen-free and engineered to carry vaccine proteins. An oral vaccine formulation can be prepared by simple mixing of vaccine antigens with chemically-treated RW pollen. In addition to encapsulating antigens inside the pollen core, a small amount of antigen can also get adsorbed on the pollen surfaces. Induction of both mucosal and systemic immune responses can be attained with this RW formulation. A key aspect of the mucosal response was that antigen-specific IgA was induced in local (i.e. intestinal), and distal (i.e. vaginal, oral cavity, and respiratory tract) mucosal sites. The humoral antibody response remained elevated for at least three months after the last booster dose. Significant levels of OVA-specific antibody-secreting plasma cells were found in the bone marrow of the vaccinated mice. Overall this study lays the groundwork to further explore the RW pollen and other pollen species for oral vaccine delivery.
Supplementary Material
Acknowledgments
Acknowledgments and conflict of interest disclosure
This research was supported by the National Institutes of Health (NIH) [grant number DP2HD075691] and the Defense Advanced Research Projects Agency (DARPA) [grant number N66001-12-1-4251]. HSG is a co-inventor on a patent related to the development of pollen grains for oral vaccines. This potential conflict of interest has been disclosed and is managed by Texas Tech University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
References
- Alignani D, Maletto B, Liscovsky M, Ropolo A, Moron G, Pistoresi-Palencia MC. Orally administered OVA/CpG-ODN induces specific mucosal and systemic immune response in young and aged mice. J. Leukoc. Biol. 2005;77:898–905. doi: 10.1189/jlb.0604330. [DOI] [PubMed] [Google Scholar]
- Atwe SU, Ma Y, Gill HS. Pollen grains for oral vaccination. J. Control. Release. 2014;194:45–52. doi: 10.1016/j.jconrel.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordas-Le Floch V, Groeme R, Chabre H, Baron-Bodo V, Nony E, Mascarell L, Moingeon P. New insights into ragweed pollen allergens. Curr. Allergy Asthm. R. 2015;15:63. doi: 10.1007/s11882-015-0565-6. [DOI] [PubMed] [Google Scholar]
- Borges O, Borchard G, Verhoef JC, de Sousa A, Junginger HE. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int. J. Pharm. 2005;299:155–166. doi: 10.1016/j.ijpharm.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Clements JD, Hartzog NM, Lyon FL. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- De Smet R, Demoor T, Verschuere S, Dullaers M, Ostroff GR, Leclercq G, Allais L, Pilette C, Dierendonck M, De Geest BG, Cuelier CA. β-Glucan microparticles are good candidates for mucosal antigen delivery in oral vaccination. J. Control. Release. 2013;172:671–678. doi: 10.1016/j.jconrel.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Descolas-Gros C, Schölzel C. Stable isotope ratios of carbon and nitrogen in pollen grains in order to characterize plant functional groups and photosynthetic pathway types. New Phytol. 2007;176:390–401. doi: 10.1111/j.1469-8137.2007.02176.x. [DOI] [PubMed] [Google Scholar]
- Diego-Taboada A, Maillet L, Banoub JH, Lorch M, Rigby AS, Boa AN, Atkin SL, Mackenzie G. Protein free microcapsules obtained from plant spores as a model for drug delivery: ibuprofen encapsulation, release and taste masking. J. Mater. Chem. B. 2013;1:707–713. doi: 10.1039/c2tb00228k. [DOI] [PubMed] [Google Scholar]
- Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tice TR. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the peyer's patches. J. Control. Release. 1990;11:205–214. [Google Scholar]
- Garinot M, Fiévez V, Pourcelle V, Stoffelbach F, des Rieux A, Plapied L, Theate I, Freichels H, Jérôme C, Marchand-Brynaert J, Schneider Y-J, Préat V. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J. Control. Release. 2007;120:195–204. doi: 10.1016/j.jconrel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Helander HF, Fandriks L. Surface area of the digestive tract – revisited. Scand. J. Gastroenterol. 2014;49:681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- Holmgren J, Lycke N, Czerkinsky C. Cholera toxin and cholera B subunit as oral— mucosal adjuvant and antigen vector systems. Vaccine. 1993;11:1179–1184. doi: 10.1016/0264-410x(93)90039-z. [DOI] [PubMed] [Google Scholar]
- Igartua M, Hernandez RM, Esquisabel A, Gascon AR, Calvo MB, Pedraz JL. Enhanced immune response after subcutaneous and oral immunization with biodegradable PLGA microspheres. J. Control. Release. 1998;56:63–73. doi: 10.1016/s0168-3659(98)00077-7. [DOI] [PubMed] [Google Scholar]
- Jiang J, Zhang Z, Cao J. Pollen wall development: the associated enzymes and metabolic pathways. Plant Biol. 2013;15:249–263. doi: 10.1111/j.1438-8677.2012.00706.x. [DOI] [PubMed] [Google Scholar]
- Jiang T, Singh B, Li H-S, Kim Y-K, Kang S-K, Nah J-W, Choi Y-J, Cho C-S. Targeted oral delivery of BmpB vaccine using porous PLGA microparticles coated with M cell homing peptide-coupled chitosan. Biomaterials. 2014;35:2365–2373. doi: 10.1016/j.biomaterials.2013.11.073. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kotton CN, Hohmann EL. Enteric pathogens as vaccine vectors for foreign antigen delivery. Infect. Immun. 2004;72:5535–5547. doi: 10.1128/IAI.72.10.5535-5547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky A, Kozlovsky SV, Tian G-W, Chen M-H, Zaltsman A, Citovsky V. How pollen tubes grow. Dev. Biol. 2007;303:405–420. doi: 10.1016/j.ydbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Lemke A, Kraft M, Roth K, Riedel R, Lammerding D, Hauser AE. Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice. Mucosal Immunol. 2016;9:83–97. doi: 10.1038/mi.2015.38. [DOI] [PubMed] [Google Scholar]
- Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- Mackenzie G, Boa AN, Diego-Taboada A, Atkin SL, Sathyapalan T. Sporopollenin, the least known yet toughest natural biopolymer. Front. Mater. 2015;2:66. [Google Scholar]
- Mantis NJ, Rol N, Corthesy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- McCormick S. Pollen. Curr. Biol. 23:R988–R990. doi: 10.1016/j.cub.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Moreno-Mendieta SA, Guillén D, Espitia C, Hernández-Pando R, Sanchez S, Rodríguez-Sanoja R. A novel antigen-carrier system: The Mycobacterium tuberculosis Acr protein carried by raw starch microparticles. Int. J. Pharm. 2014;474:241–248. doi: 10.1016/j.ijpharm.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Mundargi RC, Potroz MG, Park JH, Seo J, Lee JH, Cho N-J. Extraction of sporopollenin exine capsules from sunflower pollen grains. RSC Adv. 2016;6:16533–16539. [Google Scholar]
- O'Hagan DT, Palin K, Davis SS, Artursson P, Sjöholm I. Microparticles as potentially orally active immunological adjuvants. Vaccine. 1989;7:421–424. doi: 10.1016/0264-410x(89)90156-4. [DOI] [PubMed] [Google Scholar]
- Pacini E, Hesse M. Pollenkitt – its composition, forms and functions. Flora. 2005;200:399–415. [Google Scholar]
- Park KS, Lee J, Ahn SS, Byun Y-H, Seong BL, Baek YH, Song M-S, Choi YK, Na YJ, Hwang I, Sung YC, Lee CG. Mucosal immunity induced by adenovirus-based H5N1 HPAI vaccine confers protection against a lethal H5N2 avian influenza virus challenge. Virology. 2009;395:182–189. doi: 10.1016/j.virol.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Pavot V, Rochereau N, Genin C, Verrier B, Paul S. New insights in mucosal vaccine development. Vaccine. 2012;30:142–154. doi: 10.1016/j.vaccine.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Petersen LK, Ramer-Tait AE, Broderick SR, Kong C-S, Ulery BD, Rajan K, Wannemuehler MJ, Narasimhan B. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials. 2011;32:6815–6822. doi: 10.1016/j.biomaterials.2011.05.063. [DOI] [PubMed] [Google Scholar]
- Potroz MG, Mundargi RC, Gillissen JJ, Tan E-L, Meker S, Park JH, Jung H, Park S, Cho D, Bang S-I, Cho N-J. Plant-based hollow microcapsules for oral delivery applications: toward optimized loading and controlled release. Adv. Funct. Mater. 2017;27:1700270. [Google Scholar]
- Prabhakar AK, Lai HY, Potroz MG, Corliss MK, Park JH, Mundargi RC, Cho D, Bang S-I, Cho N-J. Chemical processing strategies to obtain sporopollenin exine capsules from multi-compartmental pine pollen. J. Ind. Eng. Chem. 2017;53:375–385. [Google Scholar]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGC, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013;447:75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydell N, Sjöholm I. Mucosal vaccination against diphtheria using starch microparticles as adjuvant for cross-reacting material (CRM197) of diphtheria toxin. Vaccine. 2005;23:2775–2783. doi: 10.1016/j.vaccine.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Sarti F, Perera G, Hintzen F, Kotti K, Karageorgiou V, Kammona O, Kiparissides C, Bernkop-Schnurch A. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A. Biomaterials. 2011;32:4052–4057. doi: 10.1016/j.biomaterials.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther. Adv. Vaccines. 2014;2:159–182. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya AK, Chowdhury MYE, Tao W, Gill HS. Mucosal vaccine delivery: current state and a pediatric perspective. J. Control. Release. 2016;240:394–413. doi: 10.1016/j.jconrel.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolander A, Cox JC. Uptake and adjuvant activity of orally delivered saponin and ISCOM™ accines. Adv. Drug Deliv. Rev. 1998;34:321–338. doi: 10.1016/s0169-409x(98)00046-5. [DOI] [PubMed] [Google Scholar]
- Tacket CO. Plant-based vaccines against diarrheal diseases. Trans. Am. Clin. Climatol. Assoc. 2007;118:79–87. [PMC free article] [PubMed] [Google Scholar]
- Uddin MJ, Gill HS. Ragweed pollen as an oral vaccine delivery system: mechanistic insights. J. Control. Release. 2017;268:416–426. doi: 10.1016/j.jconrel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lubben IM, Kersten G, Fretz MM, Beuvery C, Coos Verhoef J, Junginger HE. Chitosan microparticles for mucosal vaccination against diphtheria: oral and nasal efficacy studies in mice. Vaccine. 2003;21:1400–1408. doi: 10.1016/s0264-410x(02)00686-2. [DOI] [PubMed] [Google Scholar]
- Vela Ramirez JE, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017;114:116–131. doi: 10.1016/j.addr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikingsson LD, Sjöholm I. Polyacryl starch microparticles as adjuvant in oral immunisation, inducing mucosal and systemic immune responses in mice. Vaccine. 2002;20:3355–3363. doi: 10.1016/s0264-410x(02)00288-8. [DOI] [PubMed] [Google Scholar]
- Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu. Rev. Biomed. Eng. 2012;14:17–46. doi: 10.1146/annurev-bioeng-071811-150054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.