Abstract
Graphene-based gas/vapor sensors have attracted much attention in recent years due to their variety of structures, unique sensing performances, room-temperature working conditions, and tremendous application prospects, etc. Herein, we summarize recent advantages in graphene preparation, sensor construction, and sensing properties of various graphene-based gas/vapor sensors, such as NH3, NO2, H2, CO, SO2, H2S, as well as vapor of volatile organic compounds. The detection mechanisms pertaining to various gases are also discussed. In conclusion part, some existing problems which may hinder the sensor applications are presented. Several possible methods to solve these problems are proposed, for example, conceived solutions, hybrid nanostructures, multiple sensor arrays, and new recognition algorithm.
Keywords: Graphene, Gas/Vapor sensor, Chemiresistor, Detection mechanism
Introduction
The past several decades have witnessed a tremendous development of chemical sensors in many fields [1–4]. Gases detecting and harmful vapors with early warning feature are playing increasingly important roles in many fields, including environmental protection, industrial manufacture, medical diagnosis, and national defense. Meanwhile, sensing materials are of intense significance in promoting the combination properties of gas/vapor sensors, such as sensitivity, selectivity, and stability. Thus, various materials [5–13], covering from inorganic semiconductors, metal oxides, and solid electrolytes, to conducting polymers, have been exploited to assemble sensing devices with small sizes, low power consumption, high sensitivity, and long reliability. Among them, nanomaterials, such as carbon nanotubes (CNTs), metal-oxide nanoparticles, and graphenes, are widely used in gas sensing for their excellent responsive characteristics, mature preparation technology, and low cost of mass production, since the traditional silicon-based semiconducting metal-oxide technologies will have reached their limits [14]. Figure 1 shows a module of MQ-9, a SnO2-based gas sensor for CO detection, which can be easily obtained in the market.
Fig. 1.
SnO2-based gas sensor for CO detection, product model: MQ-9
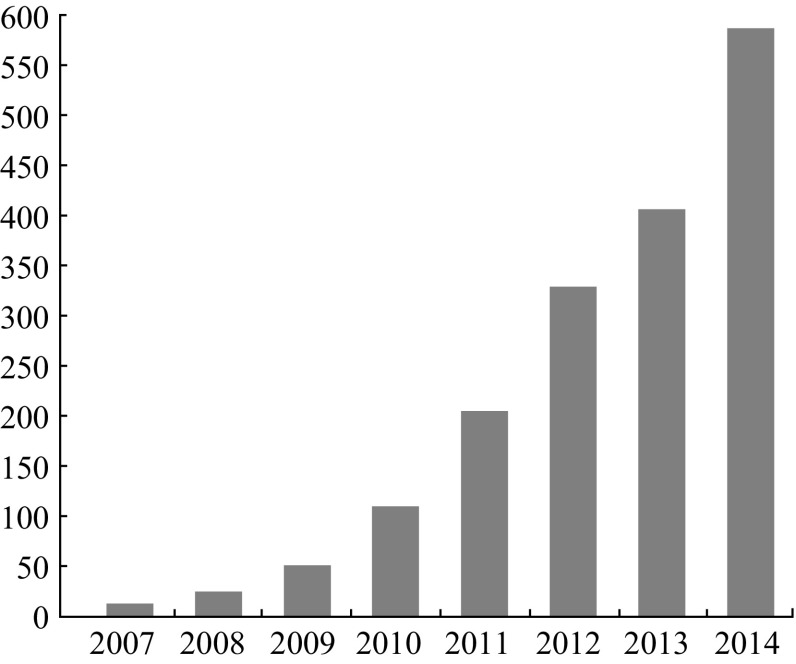
As one of the most fascinating materials, graphene has aroused scientists’ great enthusiasms in its synthesis, modification, and applications in many fields since 2004 [15], due to its remarkable overall properties, for instance, single-atom-thick two-dimensional conjugated structures, room-temperature stability, ballistic transport, and large available specific surface areas [16–39]. Graphene can be served as an ideal platform to carry other components for specific roles, because of its special structure. High conductivity and ballistic transport ensure that graphene exhibits very little signal disturbance when it works as a chemical sensor [40], which do not require auxiliary electric heating devices due to its excellent chemical stability at ambient temperature [16, 27]. All of these features for graphene are beneficial for its sensing properties, making it an ideal candidate for gas/vapor detecting. Therefore, great efforts have been put into the research of graphene-based gas/vapor sensors, leading to a giant leap in the development of graphene-based gas-sensing devices [24, 41–57]. We can clearly see that the number of published papers on graphene-based gas sensors has sharply increased over the period from 2007, as shown in Fig. 2. The first experiment focusing on the detection of gas molecules based on graphene was carried out in 2007. Schedin et al. reported that micrometer-size sensors made from graphene were capable of detecting single gas molecules attached to or detached from graphene’s surface, as depicted in Fig. 3 [24]. Their discovery indicated that graphene had a great potential for detecting and sensing.
Fig. 2.
Histogram detailing the number of graphene-based gas/vapor sensors publications per year for the period from 2007 to 2014 (data obtained from ISI Web of Knowledge, January 28, 2015)
Fig. 3.
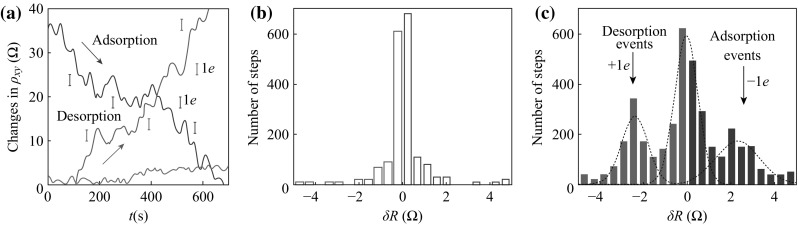
Single-molecule detection. a Examples of changes in Hall resistivity observed near the neutrality point (|n| < 1011 cm−2) during adsorption of strongly diluted NO2 (blue curve) and its desorption in vacuum at 50 °C (red curve). The green curve is a reference—the same device thoroughly annealed and then exposed to pure He. The curves are for a three-layered device in B = 10 T. The grid lines correspond to changes in ρ xy caused by adding one electron charge, e (δR ≈ 2.5 Ω), as calibrated in independent measurements by varying V g. For the blue curve, the device was exposed to 1 ppm of NO2 leaking at a rate of ≈10−3 Ω mbar L s−1. Statistical distribution of step heights, R, in this device without its exposure to NO2 (in helium) (b) and during a slow desorption of NO2 (c). For this analysis, all changes in ρ xy larger than 0.5 Ω and quicker than 10 s (lock-in time constant was 1 s making the response time of ≈6 s) were recorded as individual steps. The dotted curves in textbfc are automated Gaussian fits. Adapted from reference [24]. (Color figure online)
In principle, a sensor is a device, purpose of which is to sense (i.e., to detect) some characteristics of its environs. It detects events or changes in quantities and provides a corresponding output, generally as an electrical or optical signal. According to different forms of reaction with external atmospheres, gas/vapor sensors can be classified into chemiresistor, silicon-based field-effect transistor (FET), capacitance sensor (CS), surface work function (SWF) change transistor, surface acoustic wave (SAW) change transistor, optical fiber sensor (OFS), and so on [58]. Among them, chemiresistor is the most widely used in the construction of gas/vapor sensors and also the most popular product for practical applications, because of its long-history research, simple structure, convenience to implement, room-temperature operation, and relatively low cost [59, 60]. Actually, we usually apply voltage on both electrodes of the device, and detect the current fluctuating over time when gas composition changes. Figure 4 distinctly shows the typical structure of chemiresistors and silicon-based FET devices. An ordinary testing system for the research of gas sensors with chemiresistor structure is also displayed.
Fig. 4.
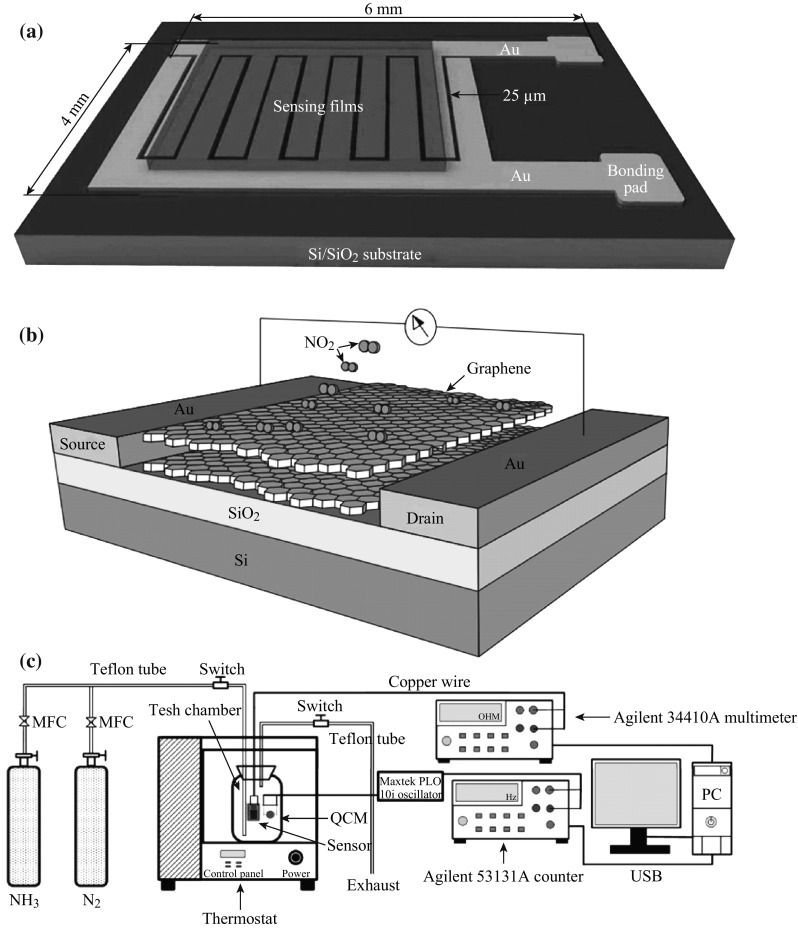
Typical schematic diagram of a chemiresistor, b FET, and c testing system of gas sensors with chemiresistor structure. Adapted from reference [61, 62]
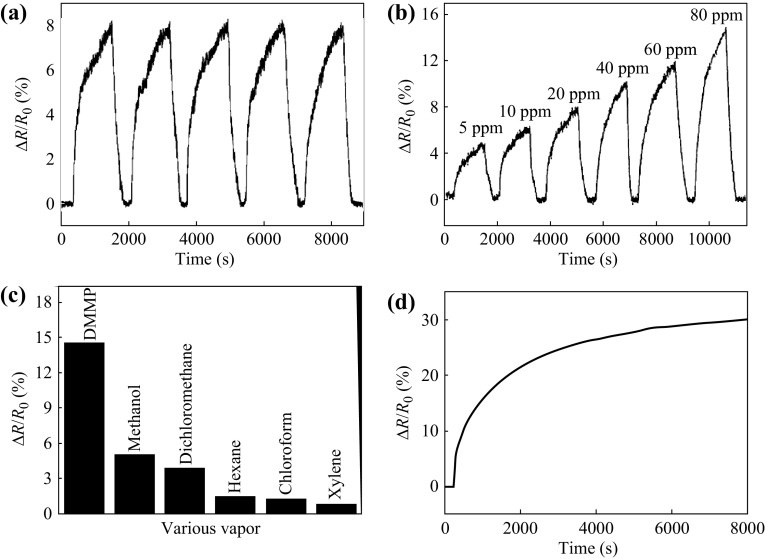
Through real-time monitoring and analyzing the response curves of sensing devices, the realistic realization of vapor detection can be achieved. Figure 5 is an example of real-time response of dimethyl methylphosphonate (DMMP) vapor monitored by para-phenylene diamine-reduced graphene oxide (PPD-RGO)-based vapor sensor. In Fig. 5, the excellent repeatability, low limit of detection, and superior selectivity of the vapor sensor have been distinctly displayed.
Fig. 5.
a Reproducibility of response of the RGO sensor to 20 ppm DMMP vapor. b Response curve of the RGO sensor to DMMP vapor under the concentrations of 5–80 ppm. (c) Response of RGO sensor to DMMP compared with other analytes diluted to 5 % of saturated vapor concentrations. d Response curve of the RGO sensor to DMMP vapor under the concentration of 80 ppm. Adapted from reference [63]
For evaluating the performance of gas/vapor sensors, there are a few critical parameters including component resistance, measure resistance, sensitivity, limit of detection, response time, recovery time, and selectivity. The definitions and formulas of these parameters are summarized in Table 1.
Table 1.
Summary of the definition and formula of sensor parameters
| Parameter | Definition | Formula |
|---|---|---|
| R a | Resistance value of the device, when put into the dry, clean atmosphere | |
| R g | Resistance value of the device, when put into gas to be detected | |
| S | Ratio of variation of resistance () to initial resistance (R a) | |
| LOD | The lowest concentration of target gas that can be distinguished from the common atmosphere, which produces a signal greater than three times the standard deviation of the noise level | |
| T res | Period of time from gas sensor contact with gas to be detected to variation of resistance reach to 90 % of | |
| T rec | Period of time from gas sensor away from gas to be detected to variation of resistance reach to 90 % of | |
| D | Ratio of response of target gas (S c) to response of disturbed gas (S i). |
Synthesis and Properties of Graphene
There are mainly four approaches to synthesize single-layered or few-layered graphene: micromechanical exfoliation, epitaxial growth, vapor deposition, and chemical reduction [64–67]. Novoselov et al. used scotch tapes to repeatedly peel flakes of graphite off the mesas which were fixed onto a SiO2/Si substrate, and the high-purity, single-layered graphene was obtained [15]. By micromechanical exfoliation of highly ordered pyrolytic graphite, crystalline graphene nanosheets with large surface areas and a small number of layers could be obtained [65]. This method is very simple and does not need any special facilities. However, it is limited to laboratory research because of the small size and inefficiency of the production. Berger and his co-workers got graphene thin films which exhibited remarkable two-dimensional (2D) electron gas behaviors through thermal decomposition on the (0001) surface of 6H-SiC [68]. Epitaxial growth, compared with mechanical exfoliation, can realize the preparation of graphene with larger sizes and higher qualities. Hence, this approach is of significant importance for graphene semiconductor devices. Although a great breakthrough has been made for this technique, there is still a long way to go toward mass production of the graphene with uniform thickness and acceptable cost. Chemical vapor deposition (CVD) is the most extensively used method in industrial manufacture considering the merits of controllable sizes and structures. By pyrolysis of carbon-containing compounds, graphene was grown on the surfaces of transition metals, such as Cu [36], Pt [69], Ni [37], Ru [70], and Ir [71]. Copper foil is the most common substrate material to build single-layered graphene. Li and his group have successfully synthesized large-area and uniform graphene films on copper foils with a high quality by CVD techniques using methane as carbon source [36].
In 2006, Stankovich et al. created a bottom-up approach when they incorporated graphene sheets in a composite material and the far-reaching method, which called chemical reduction of graphene oxide, pave the way for graphene’s large-scale production, modification, and application [21]. Figure 6 displays the fabrication process flow of graphene–polymer composite. In 2009, Tung et al. reported a versatile solution-based process for the large-scale production of single-layered chemically converted graphene over the entire area of a silicon/SiO2 wafer [72]. In general, there are three steps to obtain graphene-based composites: (1) strong oxidant, like H2SO4, HNO3, or HClO4, is used to transform graphite to graphite oxide. (2) complete exfoliation of graphite will take place, and molecular-level dispersion of individual graphene oxide (GO) in water or other polar solvent via ultrasonication will be achieved. (3) through the reduction of GO suspended in water or organic solvents, reduced graphene oxide (RGO) can be prepared without changing its morphology. Conductivity of RGO would be partly recovered too. The RGO sheets have quite high specific surface areas, which can be considered as a promising candidate for gas detection.
Fig. 6.

a Suspensions of phenyl isocyanate-treated graphite oxide (1 mg mL−1) and dissolved polystyrene in DMF before (left) and after (right) reduction by N, N-dimethylhydrazine. b Composite powders as obtained after coagulation in methanol. c Hot-pressed composite (0.12 vol% of graphene) and pure polystyrene of the same 0.4-mm thickness and processed in the same way. Adapted from reference [21]
Brodie method [73], Staudenmaier method [74] and Hummers method [75] are three main ways to form GO. Hummers method is becoming the most popular approach to synthesize GO by virtue of its merits, including rapid, easy and relatively safe properties. Various modified Hummers methods have been reported to promote the progress of GO preparation [76–79].
In a sense, the development of chemical reduction can provide equivalent routes for production and modification of graphene materials via wet chemical techniques. As such, the reductant is so important since it can affect the properties of vapor detecting devices to a large degree [80–111]. Fan and co-workers observed that a stable graphene suspension could be quickly prepared by simply heating an exfoliated-GO suspension under strongly alkaline conditions at moderate temperatures (50–90 °C). This interesting reaction provides a green route to the synthesis of graphene with excellent dispersibility in water [81]. Zhu and his team developed a green and facile approach to synthesize chemically converted graphene nanosheets (GNS) through reducing exfoliated GO precursors by reducing sugars, such as glucose, fructose, and sucrose. Their unremitting efforts pave a new way to enlarge the production of widely used GNS with a high quality [95]. Recently, Liu et al. had demonstrated a green and facile approach to synthesize RGO through reduction of GO by Zn powder under acidic condition at room temperature. This approach offers a possibility for the production of RGO with cost-effective, environment-friendly and large-scale characteristics [106].
Recently, we found that PPD-reduced RGO exposed to DMMP exhibited much better response than that of the RGO reduced from hydrazine [63]. At the same time, we confirmed that RGO reduced from aniline exhibited a better response to ammonia, compared with the RGO reduced from hydrazine [107]. The sensing properties of aniline-reduced graphene attached with different states of polyaniline (PANI) had also been studied. The results suggested that free RGO exhibited better response to NH3 and showed higher sensitivity with concentrations at ppm levels compared to those of the RGO attached with acid-doped PANI and de-doped PANI [108].
Properties of Gas/Vapor Sensors
Graphene has shown excellent sensing properties toward NH3, NO2, H2, CO, SO2, H2S, and volatile organic compounds (VOCs). Subsequently, some information from related works was summarized and discussed. Efforts have been made to exploit these sensitivities in the development of new sensor technologies.
Ammonia Detection
Ammonia (NH3) is a compound of nitrogen and hydrogen with the formula NH3, which is a colorless gas with a characteristic pungent smell. Ammonia not only contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers, but also is a building-block for the synthesis of many pharmaceuticals, and is used in many commercial products. Although widely used, this gas is both caustic and hazardous, and thus it is harmful to human and would pollute environment. Therefore, the detection of NH3 is a pressing requirement for the modern society.
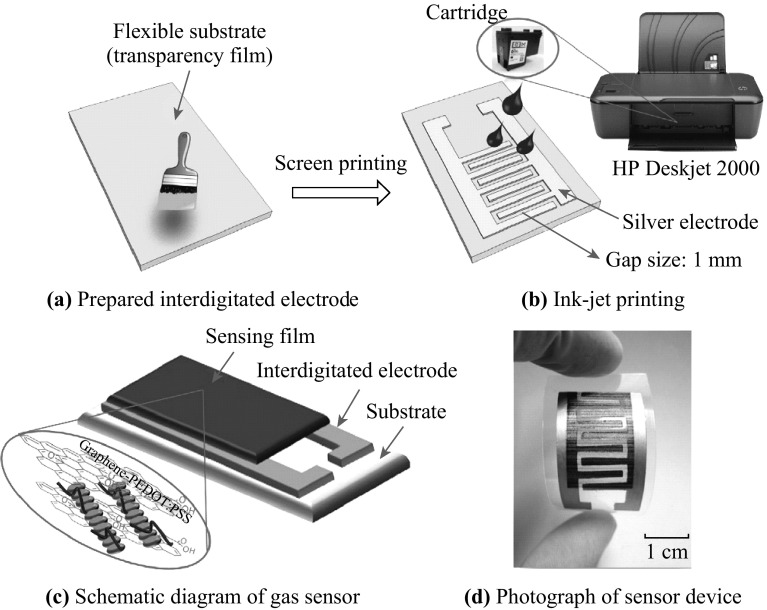
Recently, a great deal of efforts had presented a great leap forward in the development of graphene gas sensors for ammonia detection. Gautam and his team investigated ammonia gas-sensing behaviors of graphene synthesized by CVD, of which the sensitivity and the recovery time were enhanced by the deposition of gold nanoparticles on the surface of graphene films [112]. Yavari et al. manufactured a device which was distinctly superior to commercially available NO2 and NH3 detectors [113]. They found graphene films synthesized by CVD (as displayed in Fig. 7) had an outstanding property of detection of NO2 and NH3 at room temperature. The detection limits of both NO2 and NH3 reached to ppb level. Wu and his co-workers reported a contrast experiment between graphene/PANI nanocomposites, and PANI to explore their sensing properties [61]. The results indicated that the NH3 detection limit of graphene/PANI sensors (ca. 1 ppm) was lower than that of PANI (ca. 10 ppm). This indicated that the sensitivity of graphene/PANI sensors for NH3 detection was enhanced by introduction of graphene into PANI. A simple, low-cost, and practical inkjet-printing technique for fabricating an innovative flexible gas sensor based on graphene–poly (3, 4-ethylenedioxythiophene):poly (styrene sulfonate) (PEDOT:PSS) composite films with high uniformity over a large area was created by Seekaew et al. [114]. Figure 8 clearly depicts a schematic diagram of this brand new gas sensor fabrication process. The ink-jet printed graphene-PEDOT: PSS gas sensor exhibited high response and high selectivity to NH3 in a low concentration ranging from 25 to 1000 ppm at room temperature. This novel and convenient method would provide a new thought for the controllable and mass manufacture of gas detectors. Table 2 summarized recent researches about NH3 detection based on graphene.
Fig. 7.
Optical micrographs of graphene film grown by CVD on Cu and then transferred onto a Si/SiO2 substrate. Gold contact pads in the Van Der Pauw configuration were deposited on the film. Adapted from reference [113]
Fig. 8.
Schematic diagram of gas-sensor fabrication process. Adapted from reference [114]
Table 2.
A summary of recent researches about graphene-based gas sensors for NH3 detection at room temperature
| Sensing material | Structure of sensor | Target gas | T res(s) | LOD | T rec(s) | Ref. |
|---|---|---|---|---|---|---|
| RGO/MnO2 + PANI | Chemiresistor | NH3 | 1080 | 25 %/5 ppm | 240 | [115] |
| RGO/ANI | Chemiresistor | NH3 | 1080 | 10.7 %/5 ppm | 170 | [107] |
| RGO/ANI + PANI | Chemiresistor | NH3 | 1080 | 20 %/20 ppm | 120 | [108] |
| RGO/Py | Chemiresistor | NH3 | 1.4 | 2.4 %/1 ppb | 76 | [116] |
| RGO/Py | Chemiresistor | NH3 | 720 | 4.2 %/50 ppb | 375 | [117] |
| GR + Au | Chemiresistor | NH3 | 1200 | 1 %/6 ppm | 3800 | [112] |
| GR | FET | NH3 | – | 0.49 V/ppm | – | [118] |
| GR | Chemiresistor | NH3 | 21,600 | 3 %/500 ppb | 21,600 | [113] |
| GR + PANI | Chemiresistor | NH3 | 50 | 0.7 %/1 ppm | 23 | [61] |
| GR supported by mica substrate | FET | NH3 | 60 | 4 %/50 ppm | – | [119] |
| GR gated by ionic liquid | FET | NH3 | 33 | 130 ppb | – | [120] |
| Printed GR + PEDOT:PSS | Chemiresistor | NH3 | 180 | 25 ppm | 300 | [114] |
| RGO + P3HT | Chemiresistor | NH3 | 141 | 7.15 %/10 ppm | 488 | [121] |
| RGO/Tannic acid | Chemiresistor | NH3 | 40 | 9.3 %/1310 ppm | 170 | [122] |
| RGO/Cu(OH)2−4+Cu2O | Chemiresistor | NH3 | 28 | 80 %/100 ppm | 206 | [123] |
RGO reduced graphene oxide, GR Graphene, PPD p-phenyldiamine, DMMP dimethyl methyl phosphonate, PANI polyaniline, ANI aniline, Py pyrrole, COP Chemical oxidative polymerization
Nitrogen Dioxide Detection
Nitrogen dioxide is one of several nitrogen oxides with the formula NO2. On one hand, this reddish-brown gas, as one kind of the important chemical feedstocks, is an intermediate in the industrial synthesis of nitric acid. On the other hand, the toxic gas has characteristic sharp, biting odor, and is a prominent air pollutant. The whole society has a strong demand for NO2 detection, in order to curb environmental pollution and keep the safety and health of human beings.
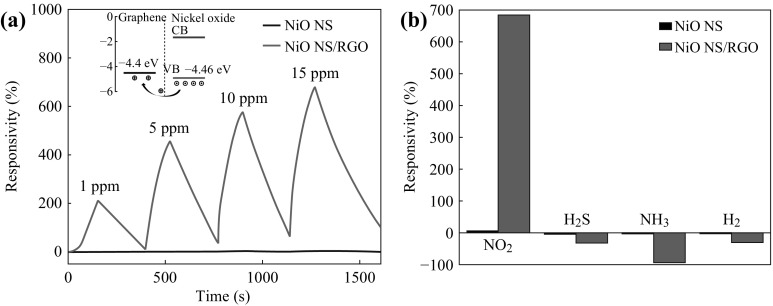
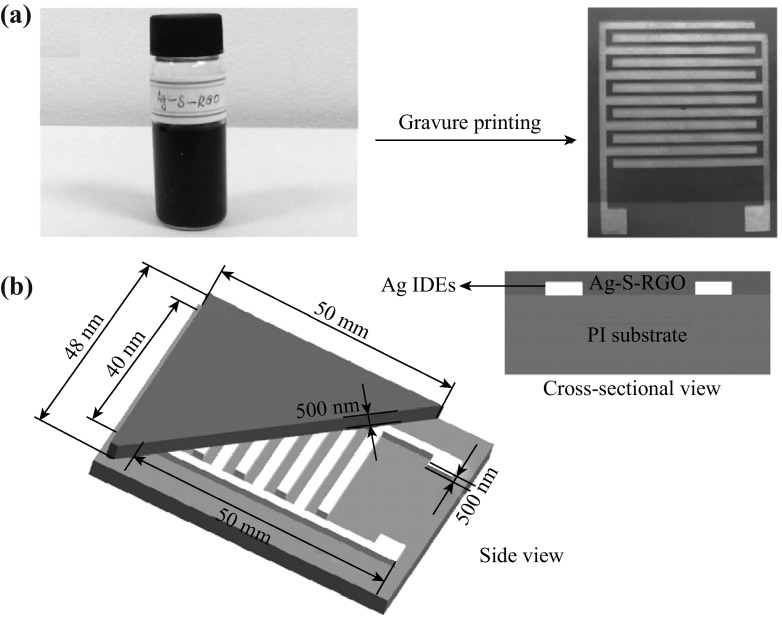
Compared to the development of ammonia detection, there are several reports about NO2 sensing showing the lower detection limit, higher response, and more practical manufacturing techniques. Choi and his co-workers reported a highly sensing NO2 gas sensor based on multilayered graphene films synthesized by a CVD method on a microheater-embedded flexible substrate [124]. The multilayered graphene had a very low detection limit of NO2 at sub-ppm (<200 ppb) levels. It also presented high responses and a short response time, when it was exposed to 1 ppm NO2 at room temperature. Hoa et al. reported that they built a gas sensor with hybrid structures of 2D graphene and 2D NiO nanosheets, sensitivity of which was two orders higher than those of devices based on NiO nanosheets alone toward NO2 even at 1 ppm level [125]. As shown in Fig. 9, the detector had excellent sensing properties, such as high sensitivity and superior selectivity. Nanosphere-like α-Fe2O3-modified RGO nanosheets were prepared by Dong’s team [109]. The 3D-structured nanocomposites exhibited a very high response of 150.63 % to 90 ppm NO2 at room temperature, which was 65.5 times higher than that of pure graphene, and the detection limit could be decreased down to 0.18 ppm. Huang et al. fabricated a gravure-printed chemiresistor-type NO2 sensor based on sulfonated RGO decorated with Ag nanoparticles (RGO/S + Ag) (as depicted in Fig. 10) [126]. Compared with other graphene-based sensors, this device showed more rapid response to NO2. When exposed to 50 ppm NO2, the sensor exhibited a sensitivity of 74.6 %, a response time of 12 s, and a recovery time of 20 s. Recently, Ju et al. reported a bendable and washable electronic textile (e-textile) gas sensors composed of reduced graphene oxides using commercially available yarns and molecular glues through an electrostatic self-assembly method [127]. The resultant e-textile gas sensor possessed the following features: (1) chemical durability to several detergents washing treatments, (2) mechanical stability under 1000 bending tests at an extreme bending radius of 1 mm, and (3) a high response to NO2 gas at room temperature with selectivity to other gases such as acetone, ethanol, ethylene, and CO2. Herein, we summarized recent researches about graphene-based gas sensors for NO2 detection, as shown in Table 3.
Fig. 9.
a Response of NiO nanosheet-based and NiO nanosheet/RGO-based gas sensors in various NO2 concentrations at 200 °C. b Response of NiO nanosheet-based and NiO nanosheet/RGO-based gas sensors in various gases, where the concentrations of NO2, H2S, and NH3 were 100 ppm, and H2 was 4 %. Adapted from reference [125]
Fig. 10.
a Photographs of RGO/S + Ag ink and sensing layer printed onto the PI substrate with Ag-IDEs, respectively. b Schematic of the printed RGO/S + Ag sensor. Adapted from reference [126]
Table 3.
A summary of recent researches about graphene-based gas sensors for NO2 detection at room temperature
| Sensing material | Structure of sensor | Target gas | T res(s) | LOD | T rec(s) | Ref. |
|---|---|---|---|---|---|---|
| GR | Chemiresistor | NO2 | 3000 | 4 %/100 ppb | 3000 | [113] |
| Single-layered GR | FET | NO2 | 3600 | 2.5 ppm | – | [128] |
| Ozone-treated GR | Chemiresistor | NO2 | 900 | 1.3 ppb | 1800 | [129] |
| GR/PMMA on a flexible PET substrate | Chemiresistor | NO2 | 170 | 25 %/200 ppm | – | [130] |
| RGO/hydrazine + WO3 | Chemiresistor | NO2 | – | 5 ppm | – | [131] |
| Multilayered GR | Chemiresistor | NO2 | 1800 | 6 %/1 ppm | – | [124] |
| RGO + NiO | Chemiresistor | NO2 | 125 | 200 %/1 ppm (200 °C) | 250 | [125] |
| Bilayer GR | FET | NO2 | – | Establish a theoretical model | – | [62] |
| RGO/FeCl3 + α-Fe2O3 | Chemiresistor | NO2 | 80 | 180 ppb | 44 | [109] |
| RGO + PVP | QCM | NO2 | – | 20 ppm | – | [132] |
| Printed RGO/S + Ag | Chemiresistor | NO2 | 12 | 74.6 %/50 ppm | 20 | [126] |
| RGO/hydrazine + ZnO | Chemiresistor | NO2 | 165 | 25.6 %/5 ppm | 499 | [133] |
| RGO + SnO2 aerogel | Chemiresistor | NO2 | 190 | 50 ppm | 224 | [134] |
| GO + Cs | Chemiresistor | NO2 | 240 | 90 ppb | 540 | [135] |
| RGO/NaBH4 | Chemiresistor | NO2 | 420 | 11.5 %/5 ppm | 1680 | [136] |
| RGO + SnO2 | Chemiresistor | NO2 | 75 | 3.31 %/5 ppm (50 °C) | 300 | [137] |
| RGO/WO3 | Chemiresistor | NO2 | 540 | 769 %/5 ppm | 1080 | [138] |
| RGO/In2O3 | Chemiresistor | NO2 | 240 | 8.25/30 ppm | 1440 | [139] |
RGO reduced graphene oxide, GO Graphene oxide, GR Graphene, PVP Polyvinylpyrrolidone, QCM quartz crystal microbalance
Hydrogen Detection
While hydrogen (H2) is not very reactive under standard conditions, it does form compounds with most elements. As one of the most important industrial chemicals and potential clean energy facing the future, hydrogen has aroused a great attention. Large-scale preparation, transportation, and application of this material have a strong demand for rapid detection and accurate analysis, which makes H2 detection become a research hotspot recent years.
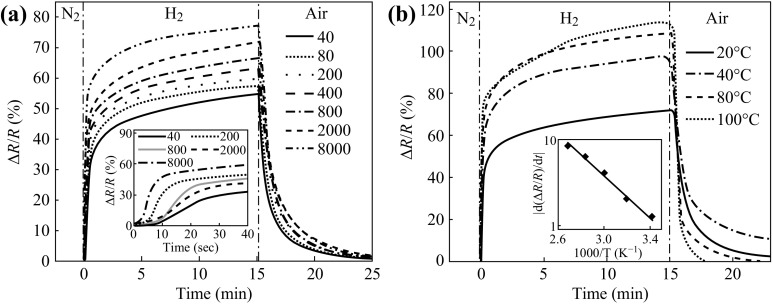
Johnson and his co-workers reported a novel Pd-functionalized multilayered graphene nanoribbon networks with excellent sensitivity to H2 at ppm levels. The fluffy porous material structure and noble metal modification accounted for their fast response and recovery time at room temperature [140]. The relationship between the sensor performance and work temperature was studied as well. Their work offers the possibility of using functionalized graphene-based nanoribbon networks in a wide range of gas/vapor-sensing applications. Figure 11 shows the response of the device varying with the concentration of H2 and work temperature. The real-time response curves of the detector as well as the activation energy of hydrogen detection at background temperatures varied from room temperature to 175 °C were measured by Chu et al. [141]. Three E a (activation energy) were observed dependent on the background temperature: 0.832 eV for 30–60 °C, 0.396 eV for 60–100 °C, and 0.057 eV for 100–170 °C. Their results contribute to the theoretical research of gas/vapor detection. Meanwhile, Chu and his team studied the effect of thickness of the Pt metal layer on hydrogen-sensing sensitivity of Pt-coated and multilayered graphene, and they concluded that the Pt coating improved the response time of the graphene sensor, but decreased the sensitivity [142]. When the thickness of the Pt metal layer was about 1 nm, the sensor presented the highest sensitivity. Mehta and co-workers had successfully fabricated a device with ultrafast response and recovery of hydrogen sensing based on graphene composite layers with Pd and Pt nanoparticles dispersed on graphene layers [143]. Jiang et al. considered the dissociative adsorption of H2 molecules on graphene with mono-atom-vacancies by using density functional theory (DFT) calculations [144]. They demonstrated that this defected graphene was promising for ultrasensitive room-temperature hydrogen sensing and the LOD could even reach to 10−35 mol L−1 theoretically. The reaction pathway of H2 molecule dissociative adsorption on pristine graphene and treated graphene with a monoatom-vacancy was displayed in Fig. 12. Table 4 summarized recent researches about H2 detection based on graphene.
Fig. 11.
a The responses of the Pd-functionalized MLGN network sensor as a function of time when it is exposed to different concentrations of H2 in N2 ranging from 40 to 8000 ppm. b The response as a function of operating temperature in the range 20–100 °C for the MLGN network sensor when exposed to 2000 ppm H2. Adapted from reference [140]
Fig. 12.
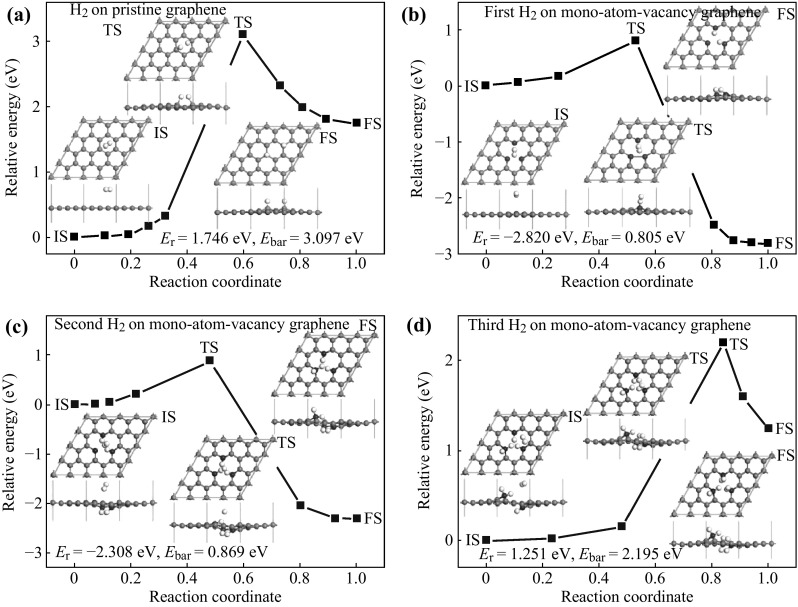
The reaction pathways of H2 molecule dissociative adsorption on pristine graphene (a), and on graphene with a mono-atom-vacancy for the first H2 molecule (b), the second H2 molecule (c), and the third H2 molecule (d). IS, TS, and FS represent initial structure, transition structure, and final structure, respectively. Their atomic structures are shown in the inserts. The energy of the IS is taken to be zero. The units of E bar and E r are eV, where E bar is the energy barrier, and E r is the reaction energy. The gray, black, and white atoms are saturated C, unsaturated C, and H, respectively. Adapted from reference [144]
Table 4.
A summary of recent researches about graphene-based gas sensors for H2 detection at room temperature
| Sensing material | Structure of sensor | Target gas | T res(s) | LOD | T rec(s) | Ref. |
|---|---|---|---|---|---|---|
| Pt/RGO/SiC | FET | H2 | 300 | Voltage shift of ≈100 mV for 1 % H2 (100 °C) | – | [145] |
| GR/Pt | Chemiresistor | H2 | 540 | 16 %/4 vol% | – | [140] |
| Multilayered GR/Pd nanoribbon | Chemiresistor | H2 | 21 | 55 %/40 ppm | 23 | [146] |
| GR/Pt | Chemiresistor | H2 | 700 | 1 % concentration (175 °C) | 700 | [141] |
| GR/Pt | Chemiresistor | H2 | 120 | 80 %/1 % concentration | 1200 | [142] |
| GR/(Pt + Pd) | Chemiresistor | H2 | <2 | 2 % concentration (40 °C) | 18 | [143] |
| GR/Pd | Chemiresistor | H2 | – | 1 % concentration | – | [147] |
| GR/Pd | Chemiresistor | H2 | 900 | 20 ppm | 1800 | [148] |
| GR | First-principle calculation | H2 | – | – | – | [149] |
| RGO/TiO2/(Pd + Pt) | Chemiresistor | H2 | 18 | 92 %/500 ppm (180 °C) | 29 | [150] |
| RGO/SnO2 + Pt | Chemiresistor | H2 | 5 | 1 % concentration | 4 | [151] |
| RGO/Pd | Chemiresistor | H2 | – | 0.20 % | – | [152] |
| GR with mono-atom-vacancy | First-principle calculation | H2 | – | 10−35 mol L−1 | – | [144] |
| RGO/Pd | Chemiresistor | H2 | 1200 | 0.4 %/0.2 ppm | 900 | [153] |
| GO | Chemiresistor | H2 | 270 | 6 %/800 ppm | 306 | [154] |
| PMMA/Pd NPs + SL GR | Chemiresistor | H2 | 108 | 66 %/2 % | 330 | [155] |
| GR/SnO2 NPs | FET | H2 | 1.2 | 3/100 ppm | 1.6 | [156] |
| GO/PEDOT:PSS | Chemiresistor | H2 | 30 | 4.2 %/100 ppm | 25 | [157] |
RGO reduced graphene oxide, GR Graphene, PMMA Polymethylmethacrylate, NPs Nanoparticles, SL Single layer
Carbon Dioxide, Carbon Monoxide, and Methane Detection
Carbon dioxide (CO2), carbon monoxide (CO), and methane (CH4) are very familiar to our daily life, industrial manufacture, and environmental protection. CO2 is not only the primary source of carbon in life, but also of significant impact in air pollution, which can cause global warming. CO is toxic to humans when encountered in concentrations above about 35 ppm. Besides, this colorless, odorless, and tasteless gas is one kind of gaseous fuels, which is widely used as reducing agent in industry. CH4 is the simplest alkane and the main component of natural gases. On one hand, the relative abundance of methane makes it an attractive fuel. On the other hand, it is the chief culprit of a gas explosion. In a word, detection and early warning of these gases is a pressing need for modern society.
Nemade et al. have carried out a lot of work focusing on graphene-based carbon dioxide sensor over the recent years [158]. They fabricated a device with excellent stability, short response and recovery times, and low detection limit based on few-layered graphene synthesized by an electrochemical exfoliation method. It is worth mentioning that this few-layered graphene also showed remarkable sensing features to liquid petroleum gases, which endowed it with a giant potential application. In addition, they investigated the sensing characteristic to CO2 of graphene/Y2O3 quantum dots (QDs) [159], graphene/Sb2O3 QDs [160], and graphene/Al2O3 QDs [161], respectively. The experimental results showed that gas-sensing properties could be changed by different combination of materials. Liu and his team investigated the adsorption of several common gas molecules (CO, SO2, NH3, CO2, N2, H2O, and H2) on Li-decorated T graphene, using DFT [162]. They found that Li-decorated T graphene exhibited a higher sensitivity to CO. Their work provided an insight to build promising gas detectors based on graphene. Wu et al. reported that graphene nanosheets/PANI nanocomposite with a different mass ratio was synthesized and investigated [163]. This hybrid was able to fabricate methane sensor, the detection limit of which decreased with the increasing mass ratio of graphene to PANI. Herein, we summarized recent researches about graphene-based gas sensors for CO2, CO and CH4 detection, as shown in Table 5.
Table 5.
A summary of recent researches about graphene-based gas sensors for CO2, CO, and CH4 detection at room temperature
| Sensing material | Structure of sensor | Target gas | T res(s) | LOD | T rec(s) | Ref. |
|---|---|---|---|---|---|---|
| GR/PANI | Chemiresistor | CH4 | 85 | 10 ppm | 45 | [163] |
| GR/Li | First-principle calculation | CO | – | – | – | [162] |
| GR prepared by mechanical cleavage | Chemiresistor | CO2 | 8 | 9 %/10 ppm | – | [164] |
| GR/Y2O3 QDs | Chemiresistor | CO2 | – | 1.08 %/35 ppm | – | [159] |
| Few-layered GR | Chemiresistor | CO2 | 11 | 3 ppm | 14 | [158] |
| GR reduced by hydrogen plasma | Chemiresistor | CO2 | 240 | 2 %/300 ppm | 240 | [165] |
| GR/Sb2O3 QDs | Chemiresistor | CO2 | 16 | 50 ppm | 22 | [160] |
| GR/Al2O3 QDs | Chemiresistor | CO2 | 14 | 100 ppm (125 °C) | 22 | [161] |
GR Graphene, QDs quantum dots
Sulfur Dioxide and Hydrogen Sulfide Detection
As main atmospheric pollutants, sulfur dioxide (SO2) and hydrogen sulfide (H2S) are very harmful to mankind and animals. In recent years, some researchers reported some novel gas sensors for the detection of SO2 and H2S based on graphene composites. Shen and his team demonstrated that GO nanosheets derived from chemically tailoring acted as a promising material for SO2 gas sensing [166]. The edge-tailored GO nanosheet-based chemiresistive sensor had a wide range of sensitivity as well as a quick response and short recovery time at room temperature. First principle calculations based on DFT were often used to predict the physical properties of specific materials. Through DFT calculation, Liu et al. drew a conclusion that Al-doped defective graphene owned a high reactivity toward SO2, indicating its potential application in SO2 detection [167]. Similarly, Shao et al. found that Cr-doped zigzag graphene nanoribbons were also considered as the potential candidates for SO2 molecular sensors [168]. Tensile strain effects on enhanced adsorption of H2S molecules on Ag-decorated defective graphene composite were investigated using first principles calculations based on DFT by Xian’s team [169]. Their calculations illustrated that a relatively modest tensile strain around 8 % in defective graphene can greatly increase the binding energy of Ag adatom by 44 %, indicating enhanced stabilization of Ag adatom on defective graphene, while the tensile strain had little effects on the sensitivity of Ag-decorated defective graphene composite to H2S molecule. Zhou et al. fabricated a RGO/Cu2O nanocomposite-based sensor with a very low detection limit of 5 ppb at room temperature, which might be on account of high surface activity adsorption of H2S gas molecules due to the absence of any surfactant capping [170]. So far, it is the lowest LOD in the similar types of sensors. Jiang and co-workers had also carried out a fantastic work to realize ultrafast response to H2S within 500 μs, as well as a fast recovery time of less than 30 s [171]. They used magnetic fields with different orientations to control fabrication progress of the Fe2O3/graphene nanosheets. The experimental results illustrated that structural orientation of nanosheets played an essential role in maximizing efficiency of the device. In a word, their remarkable jobs and significant results have greatly promoted the development of graphene-based gas sensors. Table 6 summarized recent researches about SO2 and H2S detection based on graphene.
Table 6.
A summary of recent researches about graphene-based gas sensors for SO2 and H2S detection at room temperature
| Sensing material | Structure of sensor | Target gas | T res(s) | LOD | T rec(s) | Ref. |
|---|---|---|---|---|---|---|
| GR | FET | SO2 | 120 | 100 %/50 ppm | 120 | [172] |
| Edge-tailored GO | FET | SO2 | – | 5 ppm | – | [166] |
| Al-dropped defective GR | First-principle calculation | SO2 | – | – | – | [167] |
| Cr-doped zigzag GR nanoribbons | First-principle calculation | SO2 | – | – | – | [168] |
| Ag-decorated defective GR | First-principle calculation | H2S | – | – | – | [169] |
| Ag-supported Si-doped GR | First-principle calculation | H2S | – | – | – | [173] |
| Fe-dropped defective GR | First-principle calculation | H2S | – | – | – | [174] |
| RGO + Cu2O nanocrystal | Chemiresistor | H2S | 120 | 11 %/5 ppb | 120 | [170] |
| PSS-doped RGO/PANI | Chemiresistor | H2S | <90 | 1 ppm | 150 | [175] |
| RGO/SnO2 NFs | Chemiresistor | H2S | <198 | 1 ppm (200 °C) | <114 | [176] |
| RGO/Fe2O3 | Chemiresistor | H2S | 500 μs | 15 ppm (190 °C) | <30 | [171] |
| GR/porous WO3 NFs | Chemiresistor | H2S | – | 3.9 %/100 ppb (300 °C) | 600 | [177] |
| Zigzag Gr/Cu | First-principle calculation | H2S | [178] | |||
| GR/Ti or GR/Sn | First-principle calculation | SO2/H2S | [179] |
RGO reduced graphene oxide, GO Graphene oxide, GR Graphene, PSS poly 4-styrenesulfonic acid, NFs Nanofibers
Volatile Organic Compounds, Explosives, and Chemical Warfare Agents Detection
Volatile organic compounds (VOCs) are organic chemicals that have a high vapor pressure at room temperature. VOCs are numerous, varied, and ubiquitous. They refer to gases which containing organic compounds, including aromatic hydrocarbon, nitro hydrocarbon, halogenated hydrocarbon, long chain alkane, alcohol, ether, acetone, grease, hydrazine, and so on. Most of them are toxic, flammable, and explosive gases. At present, as the terrible activities are of high frequency, the detection of explosives and chemical warfare agents (CWAs) attracts an increasing attention in many fields and is becoming a hot topic for research.
In general, the study of graphene-based vapor sensors for detection of VOCs, explosives, and CWAs is relatively immature. As such, many novel approaches have been developed to explore the terra incognita.
Dua and co-works developed a rapid and one-step method for the conversion of exfoliated GO into RGO using aqueous vitamin C as a mild and green reducing agent [180]. The RGO-based gas sensor fabricated by inkjet printing techniques was able to detect VOCs at ppb level at room temperature. In 2011, Jiang et al. developed a facile and novel route to synthesize Al2O3/graphene nanocomposites with the aid of supercritical CO2 derived from graphene oxide [181]. The ethanol-sensing features of as-synthesized Al2O3/graphene nanocomposites were firstly reported on the basis of catalytic chemiluminescence mechanisms. They boldly broke through the limitation of the traditional preparation and measurement methods, leading a new way to tackle relevant problems. In the same year, Zhang et al. reported an intrinsic polymer optical fiber (POF) sensor based on graphene, which was described for the purpose of acetone vapor sensing for the first time [182]. Gautam’s team had systematically studied the key parameters (response, recovery, repeatability and reliability) of the sensor based on gold and platinum nanoparticles functionalized graphene for the detection of different organic vapors (acetic acid, ethanol, and acetone) at ppm levels [183].
Tang et al. established a prominent analytical platform for electrochemical sensing determination of nitroaromatic explosive compounds, such as 2,4,6-trinitrotoluene (TNT), which was superior to other TNT-sensing platforms, using uniform and rich-wrinkled graphene films prepared by electrophoretic deposition techniques [184]. The detection of TNT with the concentration of 0.2 ppb in a phosphate buffered saline by differential pulse voltammetry was realized. Fan’s team utilized water-soluble and surface-unmodified graphene quantum dots, which were prepared by a chemical approach from GO, as a novel, effective, and simple fluorescent-sensing platform for ultrasensitive detection of TNT in solution by fluorescence resonance energy transfer quenching for the first time [185]. The detection limit was about 0.495 ppm. Liu et al. used surface enhanced Raman scattering to realize ultratrace detection of TNT (5 × 10−16 M), which was based on p-aminothiophenol functionalized graphene nanosheets decorated with silver nanoparticles [186]. GO modified Au electrode was used as a carbon electrode catalyst for the electrochemical oxidation of chemical warfare agent simulant thiodiglycol (TDG) at room temperature by Singh and his team [187]. Their experiments indicated that GO would be a better alternative material for transition metals in the degradation of chemical warfare agents as well as environmental pollutants. Ganji et al. drew a conclusion that aluminum nitride graphene had stronger interaction with the DMMP molecule and could provide more sensitive signal for a single DMMP molecule, compared with pristine graphene, boron nitride graphene, using ab initio van der Waals density functional calculations [188]. Though some detection process of their experiments could only take place in solution, their excellent work is a useful reference for graphene-based gas detection and has contributed a lot to practical applications in national defense and daily life. Herein, we summarized recent researches about graphene-based vapor sensors for VOCs, explosives, and CWAs detection, as shown in Table 7.
Table 7.
A summary of recent researches about graphene-based vapor sensors for VOCs, explosives, and CWAs detection at room temperature
| Sensing material | Structure of sensor | Target gas | T res(s) | LOD | T rec(s) | Ref. |
|---|---|---|---|---|---|---|
| RGO/PPD | Chemiresistor | DMMP | 1080 | 5 %/5 ppm | 360 | [63] |
| Few-layered GR | Chemiresistor | LPG | 5 | 4 ppm | 18 | [158] |
| RGO/SnO2 NFs | Chemiresistor | Acetone | <198 | 100 ppb (350 °C) | <114 | [176] |
| GO/Au electrode | TDG | [187] | ||||
| Al nitride GR | First-principle calculation | DMMP | [188] | |||
| Uniform and rich-wrinkled GR | TNT | 0.2 ppb | [184] | |||
| GQDs | FRET quenching | TNT | 0.495 ppm | [185] | ||
| GR/Ag + PATP | SERS | TNT | 5 × 10−16 M | [186] | ||
| Printed RGO | Chemiresistor | VOCs | ppb level | [180] | ||
| RGO/Al2O3 | CL | Ethanol | 10 | 1.5 mg/mL−1 (200 °C) | <100 | [181] |
| GR on POF | OFS | Acetone | 44 ppm | [182] | ||
| RGO | FET array | Ethanol | 300 | 17 % | [189] | |
| GR/(Au + Pt) | Chemiresistor | VOCs | 30 %/100 ppm | [183] | ||
| GO/PPr | Chemiresistor | Toluene | 24 ppm | [190] | ||
| Ni NPs/Nafion/GR | CV & EIS | Ethanol | 0.12 mM | [191] | ||
| RGO/ZnFe2O4 | Chemiresistor | Acetone | 4 | 10 ppm (275 °C) | 18 | [192] |
| Si dropped BC3 GR | First-principle calculation | Acetone | [193] | |||
| Self-Assembled GR/PDA | Colorimetric sensor | VOCs | 0.01 % | [194] | ||
| Co3O4 NFs + Ir NPs + GO | Chemiresistor | Acetone | 1.18 %/120 ppb (300 °C) | [195] | ||
| RGO coated optical fiber | OFS | Methanol & Ethanol | 100 ppm | [196] | ||
| RGO/Ag | OFS | Ethanol | 11 | 1 % | 6 | [197] |
| RGO/ZnO + Ag NPs | Chemiresistor | Acetylene | 21.2 | 21.2/100 ppm (150 °C) | 80 | [198] |
| RGO/ZnO + Ag NPs | Chemiresistor | Acetylene | 57 | 12.3/100 ppm (200 °C) | 90 | [199] |
RGO reduced graphene oxide, GO graphene oxide, GR graphene, TDG thiodiglycol, GQDs graphene quantum dots, FRET fluorescence resonance energy transfer quenching, PATP p-aminothiophenol, SERS surface enhanced Raman scattering, CL catalytic chemiluminescence, POF polymer optical fiber, PPr polypyrene, NPs nanoparticles, CV cyclic voltammetry, CIS electrochemical impedance spectroscopy, PDA polydiacetylene, NFs Nanofibers, OFS optical fiber sensor
Response Mechanisms
We have given a brief introduction to the classification of gas/vapor sensors. Considering that the gas-sensing mechanisms of graphene is uncertain and related research is rare, herein, we just give a recognized point of view as a general introduction of the reference of other related literatures [200–203].
Graphene is intrinsically inert and nonselective. Its great efficiency to conduct electricity and distinguishing features of ballistic transport of charges decide that this two-dimensional material is an ideal candidate to serve as a platform or a supporter, in which we can realize many specific functions by doping or compositing with other materials. Once combined with other materials physically or chemically, graphene can show the characteristics of the semiconductor in normal circumstances, of which conductivity is determined by carriers’ concentration. For chemiresistor-type sensors, sensing materials show response to externalities by the change of conductivity, that is the variation of concentration of hole or electron carriers. Bulk porous materials usually have a large specific surface area, hence gas molecules can be easily adsorbed, following by the interaction between gas molecules and specific groups in the graphene surface, and then the gas molecules capture or donate electrons from the sensing material, which changes concentration of the semiconductor’s carriers.
Different doping and reaction conditions may lead to different types of graphene-based semiconductors (p-type or n-type). As we all know, p-type semiconductors refer to those who have a larger hole concentration than electron concentration. In p-type semiconductors, holes are the majority carriers and electrons are the minority carriers. As opposed to p-type semiconductors, n-type semiconductors have a larger electron concentration than hole concentration. In n-type semiconductors, electrons are the majority carriers and holes are the minority carriers. For example, one doped graphene shows characteristic of n-type semiconductors: when it is exposed to a reducing atmosphere, such as NH3, it would get electrons from the gas molecules, leading to an increase of the electron concentration, i.e., a decrease of graphene’s resistance occurs. Likewise, when it is exposed to an oxidation atmosphere, such as NO2, it will deliver electrons to the gas molecules, leading an increase of hole concentration, leading to an increase of graphene’s resistance. Figure 13 demonstrates a general progress of gas sensing, which has been described above. This is the old and universal theory called “Oxygen anion barrier model,” which used to illustrate the mechanism of gas-sensing progress based on metal-oxide semiconductors [204–207].
Fig. 13.
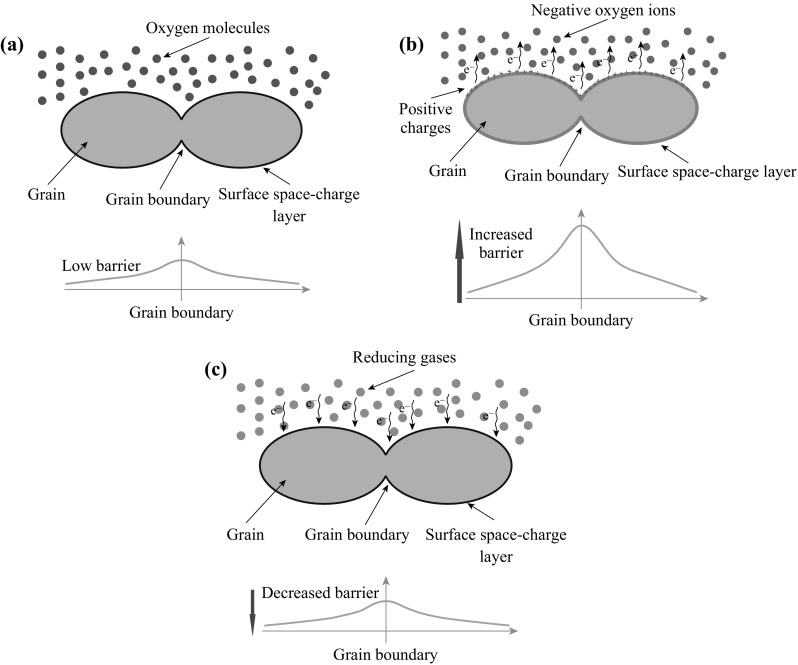
A general progress of gas sensing based on chemiresistor
Zhou et al. have found that the total flow rate had a significant effect on the initial electric resistance of the sensors and their sensing properties to target gases. In addition, an appropriate quantity of deposited RGO solution was critical for sensors’ sensing response and sensitivity. Finally, they raise a novel sensing mechanism for chemiresistors based on RGO at room temperature [208]. Zhu and his team had done an important job to prove that the oxygen functional groups presiding on the surface of reduced graphene oxide could play a vital role in the response for one specific gas. Two types of unprecedented effects could be attributed to the presence of oxygen functional groups, i.e., the selective binding interactions (strong or weak) to different gas molecules, and the impendence to charge interaction between gas molecules and sp2-hybridized carbon areas in RGO [209]. Sometimes, p-type graphene and n-type graphene can transform from one to another by changing the annealing temperature. Wang et al. explored this interesting phenomenon that the slightly reduced p-type graphene showed ultrasensitive gas sensing at room temperature, with a response of 58 % to 1 ppm ethanol, while the graphene could become n-type and insensitive to gas sensing, with a low response of 0.5 % to 50 ppm ethanol, by simply increasing the annealing temperature to about 300 °C [210].
Conclusions
Existing Problems
The interests in the study of nanomaterials have escalated in the recent decades, while the application is still in its infancy. This so-called “game changing” technology has met, one after another, many impediments on its way to large-scale industrialization [40]. Can graphene and graphene-based devices get through the close siege?
Theory can indicate a direction for practice. However, till now, the mechanism of gas sensing based on nanomaterials is not very clear, and quantitative calculation is almost impossible. There is little doubt that graphene thin film has great sensitivity; however, this may lead to another result that it is sensitive to many kinds of gases. Cross-sensitivity means sensor shows similar responses to the different types of gases, and this character may result in false detecting. For example, cross-sensitivity can be a problem in the detection of ethylene oxide, as ethylene oxide requires a very active working electrode catalyst and high operating potential for its oxidation. Therefore, gases which are more easily oxidized like alcohols and carbon monoxide will also give a response. Once a technique reaches the stage of mass production, it will be a completely different compared with the laboratory. One of dire challenges we confronted with is the nonrepeatability of device fabrication. From preparation of sensing materials to construction of gas/vapor sensors, from building of experimental platforms to characterization parameters, none of the uniform criteria is listed, and neither specification of laboratory equipment nor the unified presentation of technological process and synthesis method was reported.
Solutions and Prospects
In order to overcome the problems mentioned above, we put forward several worthwhile schemes and directions. Sensing materials are the core of gas detection in a real-world application. The development of synthesizing novel materials with high sensitivity and selectivity is one of the mainstream trends of gas sensors. Multicomponent classification and hybrid nanostructures which have multifunctions and outstanding performances in practical tasks are at the forefront of current research. By the improved preparation techniques such as modification of graphene, 3D structure tailoring, and thermal treatments, we may make sensors to suit the ideal state.
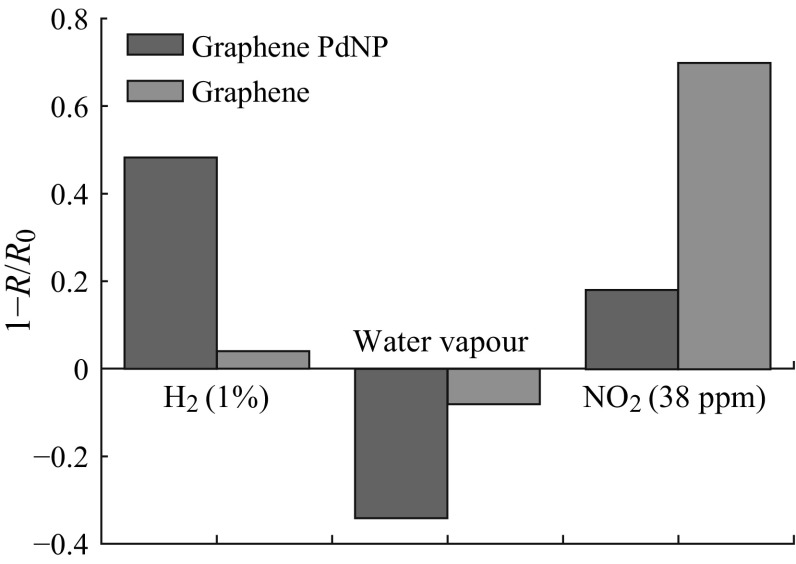
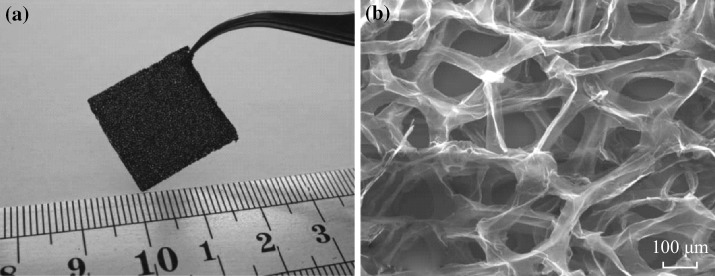
Figure 14 shows a stark contrast between the responses of the graphene/palladium nanoparticle composites to H2, NO2, and humidity and those of pristine graphene. The comparison explicitly instructs that modification can change the sensing properties to a large extent. Yavari and his team manufactured a macro-graphene foam-like 3D network which had both the advantages of the nanostructured and conventional solid-state and conducting-polymer sensors. A surprising sensing property and ppb level detection of NH3 and NO2 in air at room temperature had been demonstrated for this robust, flexible, and novel material [211]. The microporous structure of this graphene foam is demonstrated in Fig. 15.
Fig. 14.
Comparison of the responses to hydrogen, NO2, and humidity of the graphene/Pd NPs composite and of graphene. Adapted from reference [147]
Fig. 15.
a Photograph and b scanning electron micrograph of the microporous graphene foam structure showing a continuous network of 3D interconnected graphene sheets that comprise the walls of the foam-like structure. The robust and flexible graphene foam strips can be easily handled and manipulated. Adapted from reference [211]
Based on the technology of microelectromechanical systems, multiple sensor arrays, in which every unit has different heterostructure and shows different sensing characteristics, can be assembled and expected to have higher sensitivity and improved selectivity. Yi et al. presented a novel materials—sensor integration fabrication strategy, which involved the introduction of micro-injection to fabricate sensing devices. The In2O3 nanowire-like network directly on the surface of coplanar sensors array by structure replication from sacrificial CNTs was obtained on the basis of screen-printing technology and calcination. The device showed that excellent gas-sensing properties benefited from fabrication of coplanar gas sensors arrays and materials, which had special porous nanowire-like network micromorphology. Figure 16 shows a schematic diagram depicting the procedure to prepare the porous In2O3 nanowire-like network and the related devices.
Fig. 16.
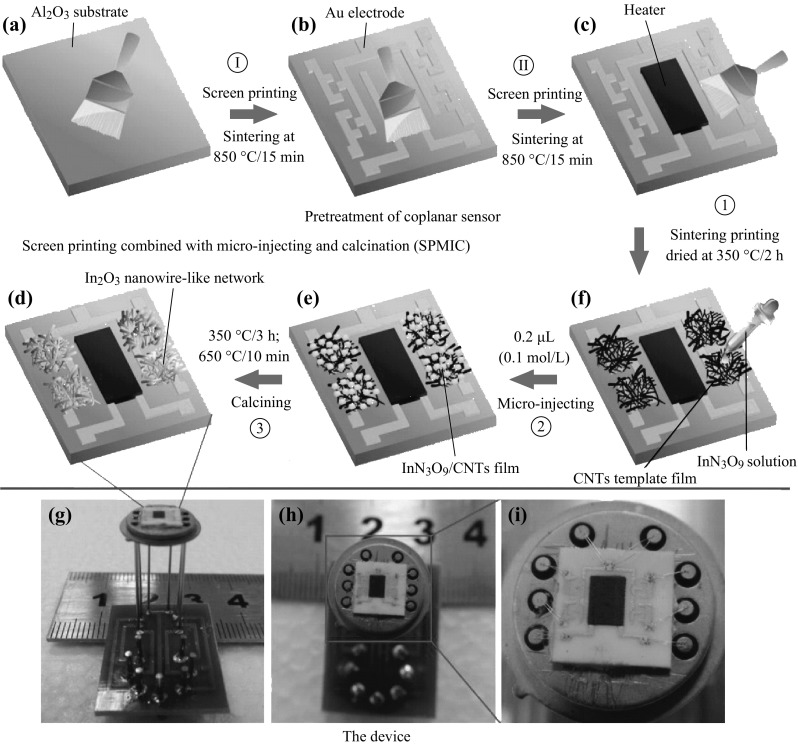
Schematic diagram of the preparation of sensing material and the construction of the device. Adapted from reference [212]
The employment of the new signal-processing technology and recognition algorithm based on single-chip system is an important direction for the development of gas-sensing devices. By the application of dynamic detection, signal processing, and recognition algorithm, gas/vapor sensors with low power consumption, portable volume, and intelligent operation could be achieved [213–215]. Huang and his team had successfully achieved qualitative and quantitative analysis of organophosphorus pesticide residues using temperature-modulated SnO2-based gas sensor, and the quantitative analyses of the pure pesticide vapor and their mixture were performed by fast Fourier transformation [213]. The results showed that the amplitudes of the higher harmonics exhibited characteristic changes depending on the vapor concentration ratio and the kinetics on the sensor surface, as shown in Fig. 17. They made a significant exploratory development in the rapid detection of pesticide residue vapors.
Fig. 17.
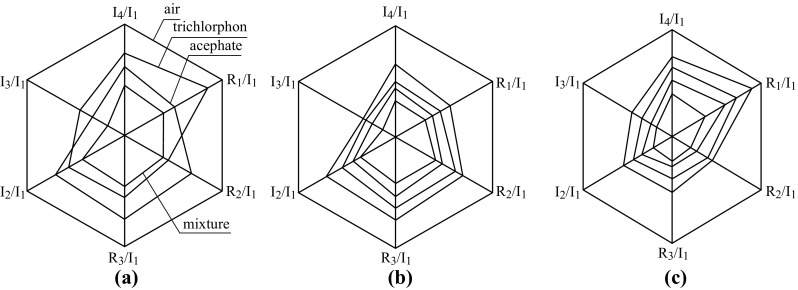
a Characteristic responses of the relative intensities of the higher harmonics. R i and L i are the real and imaginary components of the ith higher harmonic. The analyzed data correspond to the resistance values in the concentrations of 1.053 μg L−1 trichlorphon, 0.75 μg L−1 acephate, and their mixture. b, c Effects of concentrations of pesticide vapor on the relative intensities of the higher harmonic. The concentrations from inside to outside are 0.75, 2.25, 3.75, and 5.25 μg L−1; and 1.053, 3.159, 5.265, and 7.371 μg L−1, respectively. b acephate, c trichlorphon. Adapted from reference [213]
The future of graphene-based gas/vapor sensors looks bright. Continued progress in this field will overcome the current challenges, get through the close siege, and lead to a class of gas sensors with superior sensitivity, excellent selectivity, reduced size, and extended lifetimes for a wide range of environments and applications.
Acknowledgments
The authors gratefully acknowledge the financial supports provided by the National Basic Research Program of China (2013CB932500), the National Natural Science Foundation of China (21171117 and 61574091), the Program for New Century Excellent Talents in University (NCET-12-0356), the Program of Shanghai Academic/Technology Research Leader (15XD1525200), Shanghai Jiao Tong University Agri-X Funding (Agri-X2015007), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning. The authors also acknowledge the analysis support received from the Instrumental Analysis Center of Shanghai Jiao Tong University and the Center for Advanced Electronic Materials and Devices of Shanghai Jiao Tong University.
Contributor Information
Zhi Yang, Phone: +86-21-34206398, Email: zhiyang@sjtu.edu.cn.
Liying Zhang, Phone: +86-21-34206398, Email: liyingzhang@sjtu.edu.cn.
References
- 1.Stevens JG, Bowen LH, Whatley KM. Moessbauer spectroscopy. Anal. Chem. 1990;62(12):125R–139R. doi: 10.1021/ac00211a003. [DOI] [PubMed] [Google Scholar]
- 2.Janata J. Chemical sensors. Anal. Chem. 1992;64(12):196–219. doi: 10.1021/ac00036a012. [DOI] [Google Scholar]
- 3.Janata J, Josowicz M, DeVaney DM. Chemical sensors. Anal. Chem. 1994;66(12):207R–228R. doi: 10.1021/ac00084a010. [DOI] [PubMed] [Google Scholar]
- 4.Janata J, Josowicz M, Vanýsek P, DeVaney DM. Chemical sensors. Anal. Chem. 1998;70(12):179–208. doi: 10.1021/a1980010w. [DOI] [PubMed] [Google Scholar]
- 5.Zee F, Judy JW. Micromachined polymer-based chemical gas sensor array. Sens. Actuators B. 2001;72(2):120–128. doi: 10.1016/S0925-4005(00)00638-9. [DOI] [Google Scholar]
- 6.Itagaki Y, Deki K, Nakashima S, Sadaoka Y. Toxic gas detection using porphyrin dispersed polymer composites. Sens. Actuators B. 2005;108(1–2):393–397. doi: 10.1016/j.snb.2004.10.055. [DOI] [Google Scholar]
- 7.Imad HK, Hassan HA, Abdullah QN. Hydrogen gas sensor based on nanocrystalline SnO2 thin film grown on bare Si substrates. Nano-Micro Lett. 2015;7(2):97–120. doi: 10.1007/s40820-014-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai H, Zhao L, Lu CH, Li C, Shi GQ. Composite nanofibers of conducting polymers and hydrophobic insulating polymers: preparation and sensing applications. Polymer. 2009;50(14):3292–3301. doi: 10.1016/j.polymer.2009.04.066. [DOI] [Google Scholar]
- 9.Mohammadi MR, Fray DJ. Development of nanocrystalline TiO2-Er2O3 and TiO2-Ta2O5 thin film gas sensors: controlling the physical and sensing properties. Sens. Actuators B. 2009;141(1):76–84. doi: 10.1016/j.snb.2009.05.026. [DOI] [Google Scholar]
- 10.Lee JS, Kwon OS, Park SJ, Park EY, You SA, Yoon H, Jang J. Fabrication of ultrafine metal-oxide-decorated carbon nanofibers for DMMP sensor application. ACS Nano. 2011;5(10):7992–8001. doi: 10.1021/nn202471f. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Shi G. Three-dimensional graphene architectures. Nanoscale. 2012;4(18):5549–5563. doi: 10.1039/c2nr31467c. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Chen A, Jen AK. Reducing cross-sensitivity of TiO2-(B) nanowires to humidity using ultraviolet illumination for trace explosive detection. Phys. Chem. Chem. Phys. 2013;15(14):5017–5021. doi: 10.1039/c3cp43454k. [DOI] [PubMed] [Google Scholar]
- 13.Yang G, Lee C, Kim J, Ren F, Pearton SJ. Flexible graphene-based chemical sensors on paper substrates. Phys. Chem. Chem. Phys. 2013;15(6):1798–1801. doi: 10.1039/C2CP43717A. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Appenzeller J, Knoch J, Lin YM, Avouris P. The role of metal-nanotube contact in the performance of carbon nanotube field-effect transistors. Nano Lett. 2005;5(7):1497–1502. doi: 10.1021/nl0508624. [DOI] [PubMed] [Google Scholar]
- 15.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 16.Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA. Two-dimensional gas of massless Dirac fermions in graphene. Nature. 2005;438(7065):197–200. doi: 10.1038/nature04233. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Tan YW, Stormer HL, Kim P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature. 2005;438(7065):201–204. doi: 10.1038/nature04235. [DOI] [PubMed] [Google Scholar]
- 18.Berger C, Song Z, Li X, Wu X, Brown N, et al. Electronic confinement and coherence in patterned epitaxial graphene. Science. 2006;312(5777):1191–1196. doi: 10.1126/science.1125925. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006;97(18):187401. doi: 10.1103/PhysRevLett.97.187401. [DOI] [PubMed] [Google Scholar]
- 20.Son YW, Cohen ML, Louie SG. Energy gaps in graphene nanoribbons. Phys. Rev. Lett. 2006;97(21):216803. doi: 10.1103/PhysRevLett.97.216803. [DOI] [PubMed] [Google Scholar]
- 21.Stankovich S, Dikin DA, Dommett GH, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS. Graphene-based composite materials. Nature. 2006;442(7100):282–286. doi: 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- 22.Geim AK, Novoselov KS. The rise of graphene. Nat. Mater. 2007;6(3):183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 23.Han MY, Ozyilmaz B, Zhang Y, Kim P. Energy band-gap engineering of graphene nanoribbons. Phys. Rev. Lett. 2007;98(20):206805. doi: 10.1103/PhysRevLett.98.206805. [DOI] [PubMed] [Google Scholar]
- 24.Schedin F, Geim AK, Morozov SV, Hill EW, Blake P, Katsnelson MI, Novoselov KS. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007;6(9):652–655. doi: 10.1038/nmat1967. [DOI] [PubMed] [Google Scholar]
- 25.Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 2007;45(7):1558–1565. doi: 10.1016/j.carbon.2007.02.034. [DOI] [Google Scholar]
- 26.Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau CN. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008;8(3):902–907. doi: 10.1021/nl0731872. [DOI] [PubMed] [Google Scholar]
- 27.Bolotin KI, Sikes KJ, Jiang Z, Klima M, Fudenberg G, Hone J, Kim P, Stormer HL. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008;146(9–10):351–355. doi: 10.1016/j.ssc.2008.02.024. [DOI] [Google Scholar]
- 28.Li D, Muller MB, Gilje S, Kaner RB, Wallace GG. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008;3(2):101–105. doi: 10.1038/nnano.2007.451. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Wang X, Zhang L, Lee S, Dai H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science. 2008;319(5867):1229–1232. doi: 10.1126/science.1150878. [DOI] [PubMed] [Google Scholar]
- 30.Nair RR, Blake P, Grigorenko AN, Novoselov KS, Booth TJ, Stauber T, Peres NM, Geim AK. Fine structure constant defines visual transparency of graphene. Science. 2008;320(5881):1308. doi: 10.1126/science.1156965. [DOI] [PubMed] [Google Scholar]
- 31.Stoller MD, Park S, Zhu Y, An J, Ruoff RS. Graphene-based ultracapacitors. Nano Lett. 2008;8(10):3498–3502. doi: 10.1021/nl802558y. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Zhi L, Mullen K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008;8(1):323–327. doi: 10.1021/nl072838r. [DOI] [PubMed] [Google Scholar]
- 33.Castro Neto AH, Peres NMR, Novoselov KS, Geim AK. The electronic properties of graphene. Rev. Mod. Phys. 2009;81(1):109–162. doi: 10.1103/RevModPhys.81.109. [DOI] [Google Scholar]
- 34.Geim AK. Graphene: status and prospects. Science. 2009;324(5934):1530–1534. doi: 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- 35.Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, et al. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature. 2009;457(7230):706–710. doi: 10.1038/nature07719. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Cai W, An J, Kim S, Nah J, et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science. 2009;324(5932):1312–1314. doi: 10.1126/science.1171245. [DOI] [PubMed] [Google Scholar]
- 37.Reina A, Jia X, Ho J, Nezich D, Son H, Bulovic V, Dresselhaus MS, Kong J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009;9(1):30–35. doi: 10.1021/nl801827v. [DOI] [PubMed] [Google Scholar]
- 38.Bae S, Kim H, Lee Y, Xu X, Park JS, et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010;5(8):574–578. doi: 10.1038/nnano.2010.132. [DOI] [PubMed] [Google Scholar]
- 39.Dreyer DR, Park S, Bielawski CW, Ruoff RS. The chemistry of graphene oxide. Chem. Soc. Rev. 2010;39(1):228–240. doi: 10.1039/B917103G. [DOI] [PubMed] [Google Scholar]
- 40.Bogue R. Nanomaterials for gas sensing: a review of recent research. Sensor Rev. 2014;34(1):1–8. doi: 10.1108/SR-03-2013-637. [DOI] [Google Scholar]
- 41.Al-Mashat L, Shin K, Kalantar-Zadeh K, Plessis JD, Han SH, et al. Graphene/polyaniline nanocomposite for hydrogen sensing. J. Phys. Chem. C. 2010;114(39):16168–16173. doi: 10.1021/jp103134u. [DOI] [Google Scholar]
- 42.An XQ, Yu JC, Wang Y, Hu YM, Yu XL, Zhang GJ. WO3 nanorods/graphene nanocomposites for high-efficiency visible-light-driven photocatalysis and NO2 gas sensing. J. Mater. Chem. 2012;22(17):8525–8531. doi: 10.1039/c2jm16709c. [DOI] [Google Scholar]
- 43.Dan Y, Lu Y, Kybert NJ, Luo Z, Johnson AT. Intrinsic response of graphene vapor sensors. Nano Lett. 2009;9(4):1472–1475. doi: 10.1021/nl8033637. [DOI] [PubMed] [Google Scholar]
- 44.Malekalaie M, Jahangiri M, Rashidi AM, Haghighiasl A, Izadi N. Selective hydrogen sulfide (H2S) sensors based on molybdenum trioxide (MoO3) nanoparticle decorated reduced graphene oxide. Mater. Sci. Semicon. Process. 2015;38:93–100. doi: 10.1016/j.mssp.2015.03.034. [DOI] [Google Scholar]
- 45.Fowler JD, Allen MJ, Tung VC, Yang Y, Kaner RB, Weiller BH. Practical chemical sensors from chemically derived graphene. ACS Nano. 2009;3(2):301–306. doi: 10.1021/nn800593m. [DOI] [PubMed] [Google Scholar]
- 46.Ji Q, Honma I, Paek SM, Akada M, Hill JP, Vinu A, Ariga K. Layer-by-layer films of graphene and ionic liquids for highly selective gas sensing. Angew. Chem. Int. Ed. 2010;49(50):9737–9979. doi: 10.1002/anie.201004929. [DOI] [PubMed] [Google Scholar]
- 47.Alaie MM, Jahangiri M, Rashidi AM, Asl AH, Izadi N. A novel selective H2S sensor using dodecylamine and ethylenediamine functionalized graphene oxide. J. Ind. Eng. Chem. 2015;29:97–103. doi: 10.1016/j.jiec.2015.03.021. [DOI] [Google Scholar]
- 48.Khadem SMJ, Abdi Y, Darbari S, Ostovari F. Investigating the effect of gas absorption on the electromechanical and electrochemical behavior of graphene/ZnO structure, suitable for highly selective and sensitive gas sensors. Curr. Appl. Phys. 2014;14(11):1498–1503. doi: 10.1016/j.cap.2014.07.020. [DOI] [Google Scholar]
- 49.Lu G, Ocola LE, Chen J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology. 2009;20(44):445502. doi: 10.1088/0957-4484/20/44/445502. [DOI] [PubMed] [Google Scholar]
- 50.Lu G, Park S, Yu K, Ruoff RS, Ocola LE, Rosenmann D, Chen J. Toward practical gas sensing with highly reduced graphene oxide: a new signal processing method to circumvent run-to-run and device-to-device variations. ACS Nano. 2011;5(2):1154–1164. doi: 10.1021/nn102803q. [DOI] [PubMed] [Google Scholar]
- 51.Rumyantsev S, Liu G, Shur MS, Potyrailo RA, Balandin AA. Selective gas sensing with a single pristine graphene transistor. Nano Lett. 2012;12(5):2294–2298. doi: 10.1021/nl3001293. [DOI] [PubMed] [Google Scholar]
- 52.Song HJ, Zhang LC, He CL, Qu Y, Tian YF, Lv Y. Graphene sheets decorated with SnO2 nanoparticles: in situ synthesis and highly efficient materials for cataluminescence gas sensors. J. Mater. Chem. 2011;21(16):5972–5977. doi: 10.1039/c0jm04331a. [DOI] [Google Scholar]
- 53.Yi J, Park W., II Vertically aligned ZnO nanorods and graphene hybrid architectures for high-sensitive flexible gas sensors. Sens. Actuators B. 2011;155(1):264–269. doi: 10.1016/j.snb.2010.12.033. [DOI] [Google Scholar]
- 54.Akbari E, Arora VK, Enzevaee A, Khaledian M, Yusof R. Gas concentration effects on the sensing properties of bilayer graphene. Plasmonics. 2014;9(4):987–992. doi: 10.1007/s11468-014-9705-4. [DOI] [Google Scholar]
- 55.Omidvar A, Mohajeri A. Edge-functionalized graphene nanoflakes as selective gas sensors. Sens. Actuators B. 2014;202:622–630. doi: 10.1016/j.snb.2014.05.136. [DOI] [Google Scholar]
- 56.Akbari E, Arora VK, Enzevaee A, Ahmadi MT, Saeidmanesh M, Khaledian M, Karimi H, Yusof R. An analytical approach to evaluate the performance of graphene and carbon nanotubes for NH3 gas sensor applications. Plasmonics. 2014;5:726–734. doi: 10.3762/bjnano.5.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bogue R. Graphene sensors: a review of recent developments. Sensor Rev. 2014;34(3):233–238. doi: 10.1108/SR-03-2014-631. [DOI] [Google Scholar]
- 58.Yuan WJ, Shi GQ. Graphene-based gas sensors. J. Mater. Chem. A. 2013;1(35):10078–10091. doi: 10.1039/c3ta11774j. [DOI] [Google Scholar]
- 59.Seiyama T, Kato A, Fujiishi K, Nagatani M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962;34(11):1502–1503. doi: 10.1021/ac60191a001. [DOI] [Google Scholar]
- 60.Shaver PJ. Activated tungsten oxide gas detectors. Appl. Phys. Lett. 1967;11(8):255. doi: 10.1063/1.1755123. [DOI] [Google Scholar]
- 61.Wu ZQ, Chen XD, Zhu SB, Zhou ZW, Yao Y, Quan W, Liu B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B. 2013;178:485–493. doi: 10.1016/j.snb.2013.01.014. [DOI] [Google Scholar]
- 62.Akbari E, Yusof R, Ahmadi MT, Enzevaee A, Kiani MJ, Karimi H, Rahmani M. Bilayer graphene application on NO2 sensor modelling. J. Nanomater. 2014;2014:1–7. doi: 10.1155/2014/534105. [DOI] [Google Scholar]
- 63.Hu NT, Wang YY, Chai J, Gao RG, Yang Z, Kong ESW, Zhang YF. Gas sensor based on p-phenylenediamine reduced graphene oxide. Sens. Actuators B. 2012;163(1):107–114. doi: 10.1016/j.snb.2012.01.016. [DOI] [Google Scholar]
- 64.Subrahmanyam KS, Panchakarla LS, Govindaraj A, Rao CNR. Simple method of preparing graphene flakes by an arc-discharge method. J. Phys. Chem. C. 2009;113(11):4257–4259. doi: 10.1021/jp900791y. [DOI] [Google Scholar]
- 65.Subrahmanyam KS, Vivekchand SRC, Govindaraj A, Rao CNR. A study of graphenes prepared by different methods: characterization, properties and solubilization. J. Mater. Chem. 2008;18(13):1517–1523. doi: 10.1039/b716536f. [DOI] [Google Scholar]
- 66.Paola R, Anming H, Giuseppe C. Synthesis, properties and potential applications of porous graphene: a review. Nano-Micro Lett. 2013;5(4):260–273. doi: 10.1007/BF03353757. [DOI] [Google Scholar]
- 67.Yang Z, Gao RG, Hu NT, Chai J, Cheng YW, Zhang LY, Wei H, Kong ESW, Zhang YF. The prospective two-dimensional graphene nanosheets: preparation, functionalization, and applications. Nano-Micro Lett. 2012;4(1):1–9. doi: 10.1007/BF03353684. [DOI] [Google Scholar]
- 68.Berger C, Song ZM, Li TB, Li XB, Ogbazghi AY, et al. Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J. Phys. Chem. B. 2004;108(52):19912–19916. doi: 10.1021/jp040650f. [DOI] [Google Scholar]
- 69.Land TA, Michely T, Behm RJ, Hemminger JC, Comsa G. STM investigation of single layer graphite structures produced on Pt(111) by hydrocarbon decomposition. Surf. Sci. 1992;264(3):261–270. doi: 10.1016/0039-6028(92)90183-7. [DOI] [Google Scholar]
- 70.Marchini S, Gunther S, Wintterlin J. Scanning tunneling microscopy of graphene on Ru(0001) Phys. Rev. B. 2007;76:075429. doi: 10.1103/PhysRevB.76.075429. [DOI] [Google Scholar]
- 71.Coraux J, N’Diaye AT, Busse C, Michely T. Structural coherency of graphene on Ir(111) Nano Lett. 2008;8(2):565–570. doi: 10.1021/nl0728874. [DOI] [PubMed] [Google Scholar]
- 72.Tung VC, Allen MJ, Yang Y, Kaner RB. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009;4(1):25–29. doi: 10.1038/nnano.2008.329. [DOI] [PubMed] [Google Scholar]
- 73.Brodie BC. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859;149:249–259. doi: 10.1098/rstl.1859.0013. [DOI] [Google Scholar]
- 74.Staudenmaier L. Verfahren zur darstellung der graphitsäure. Ber. Dtsch. Chem. Ges. 1898;31(2):1481–1487. doi: 10.1002/cber.18980310237. [DOI] [Google Scholar]
- 75.Hummers WS, Offeman RE. Preparation of graphitic oxide. JACS. 1958;80(6):1339–1339. doi: 10.1021/ja01539a017. [DOI] [Google Scholar]
- 76.Cao JY, Song LZ, Tang JL, Xu J, Wang WC, Chen ZD. Enhanced activity of Pd nanoparticles supported on Vulcan XC72R carbon pretreated via a modified Hummers method for formic acid electrooxidation. Appl. Surf. Sci. 2013;274:138–143. doi: 10.1016/j.apsusc.2013.02.133. [DOI] [Google Scholar]
- 77.Botas C, Alvarez P, Blanco P, Granda M, Blanco C, et al. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon. 2013;65:156–164. doi: 10.1016/j.carbon.2013.08.009. [DOI] [Google Scholar]
- 78.Chen T, Zeng B, Liu JL, Dong JH, Liu XQ, Wu Z, Yang XZ, Li ZM. High throughput exfoliation of graphene oxide from expanded graphite with assistance of strong oxidant in modified hummers method. J. Phys. Conf. Ser. 2009;188:012051. doi: 10.1088/1742-6596/188/1/012051. [DOI] [Google Scholar]
- 79.Chang CI, Chang KH, Shen HH, Hu CC. A unique two-step Hummers method for fabricating low-defect graphene oxide nanoribbons through exfoliating multiwalled carbon nanotubes. J. Taiwan. Inst. Chem. E. 2014;45(5):2762–2769. doi: 10.1016/j.jtice.2014.05.030. [DOI] [Google Scholar]
- 80.Schniepp HC, Li JL, McAllister MJ, Sai H, Herrera-Alonso M, et al. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B. 2006;110(17):8535–8539. doi: 10.1021/jp060936f. [DOI] [PubMed] [Google Scholar]
- 81.Fan X, Peng W, Li Y, Li X, Wang S, Zhang G, Zhang F. Deoxygenation of exfoliated graphite oxide under alkaline conditions: a green route to graphene preparation. Adv. Mater. 2008;20(23):4490–4493. doi: 10.1002/adma.200801306. [DOI] [Google Scholar]
- 82.Wang G, Yang J, Park J, Gou X, Wang B, Liu H, Yao J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C. 2008;112(22):8192–8195. doi: 10.1021/jp710931h. [DOI] [Google Scholar]
- 83.Shin H-J, Kim KK, Benayad A, Yoon S-M, Park HK, et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 2009;19(12):1987–1992. doi: 10.1002/adfm.200900167. [DOI] [Google Scholar]
- 84.Zhou M, Wang Y, Zhai Y, Zhai J, Ren W, Wang F, Dong S. Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chemistry. 2009;15(25):6116–6120. doi: 10.1002/chem.200900596. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Y, Bao Q, Tang LAL, Zhong Y, Loh KP. Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 2009;21(13):2950–2956. doi: 10.1021/cm9006603. [DOI] [Google Scholar]
- 86.Chen W, Yan L, Bangal PR. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon. 2010;48(4):1146–1152. doi: 10.1016/j.carbon.2009.11.037. [DOI] [Google Scholar]
- 87.Fan Z, Wang K, Wei T, Yan J, Song L, Shao B. An environmentally friendly and efficient route for the reduction of graphene oxide by aluminum powder. Carbon. 2010;48(5):1686–1689. doi: 10.1016/j.carbon.2009.12.063. [DOI] [Google Scholar]
- 88.Fernandez-Merino MJ, Guardia L, Paredes JI, Villar-Rodil S, Solis-Fernandez P, Martinez-Alonso A, Tascon JMD. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C. 2010;114(14):6426–6432. doi: 10.1021/jp100603h. [DOI] [Google Scholar]
- 89.Gao X, Jang J, Nagase S. Hydrazine and thermal reduction of graphene oxide: reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C. 2010;114(2):832–842. doi: 10.1021/jp909284g. [DOI] [Google Scholar]
- 90.Mao S, Lu G, Yu K, Bo Z, Chen J. Specific protein detection using thermally reduced graphene oxide sheet decorated with gold nanoparticle-antibody conjugates. Adv. Mater. 2010;22(32):3521–3526. doi: 10.1002/adma.201000520. [DOI] [PubMed] [Google Scholar]
- 91.Pei S, Zhao J, Du J, Ren W, Cheng H-M. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon. 2010;48(15):4466–4474. doi: 10.1016/j.carbon.2010.08.006. [DOI] [Google Scholar]
- 92.Pham VH, Cuong TV, Nguyen-Phan T-D, Pham HD, Kim EJ, Hur SH, Shin EW, Kim S, Chung JS. One-step synthesis of superior dispersion of chemically converted graphene in organic solvents. Chem. Commun. 2010;46(24):4375–4377. doi: 10.1039/c0cc00363h. [DOI] [PubMed] [Google Scholar]
- 93.Zhang J, Yang H, Shen G, Cheng P, Zhang J, Guo S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010;46(7):1112–1114. doi: 10.1039/B917705A. [DOI] [PubMed] [Google Scholar]
- 94.Zhao J, Pei S, Ren W, Gao L, Cheng H-M. Efficient preparation of large-area graphene oxide sheets for transparent conductive films. ACS Nano. 2010;4(9):5245–5252. doi: 10.1021/nn1015506. [DOI] [PubMed] [Google Scholar]
- 95.Zhu C, Guo S, Fang Y, Dong S. Reducing Sugar: New functional molecules for the green synthesis of graphene nanosheets. ACS Nano. 2010;4(4):2429–2437. doi: 10.1021/nn1002387. [DOI] [PubMed] [Google Scholar]
- 96.Fan Z-J, Kai W, Yan J, Wei T, Zhi L-J, Feng J, Ren Y-M, Song L-P, Wei F. Facile synthesis of graphene nanosheets via Fe reduction of exfoliated graphite oxide. ACS Nano. 2011;5(1):191–198. doi: 10.1021/nn102339t. [DOI] [PubMed] [Google Scholar]
- 97.Guo Y, Wu B, Liu H, Ma Y, Yang Y, Zheng J, Yu G, Liu Y. Electrical assembly and reduction of graphene oxide in a single solution step for use in flexible sensors. Adv. Mater. 2011;23(40):4626–4630. doi: 10.1002/adma.201103120. [DOI] [PubMed] [Google Scholar]
- 98.Luo D, Zhang G, Liu J, Sun X. Evaluation criteria for reduced graphene oxide. J. Phys. Chem. C. 2011;115(23):11327–11335. doi: 10.1021/jp110001y. [DOI] [Google Scholar]
- 99.Sungjin P, Jinho A, Potts JR, Velamakanni A, Murali S, Ruoff RS. Hydrazine-reduction of graphite- and graphene oxide. Carbon. 2011;49(9):3019–3023. doi: 10.1016/j.carbon.2011.02.071. [DOI] [Google Scholar]
- 100.Akhavan O, Kalaee M, Alavi ZS, Ghiasi SMA, Esfandiar A. Increasing the antioxidant activity of green tea polyphenols in the presence of iron for the reduction of graphene oxide. Carbon. 2012;50(8):3015–3025. doi: 10.1016/j.carbon.2012.02.087. [DOI] [Google Scholar]
- 101.Ambrosi A, Chua CK, Bonanni A, Pumera M. Lithium aluminum hydride as reducing agent for chemically reduced graphene oxides. Chem. Mater. 2012;24(12):2292–2298. doi: 10.1021/cm300382b. [DOI] [Google Scholar]
- 102.Shen Y, Jing T, Ren W, Zhang J, Jiang Z-G, Yu Z-Z, Dasari A. Chemical and thermal reduction of graphene oxide and its electrically conductive polylactic acid nanocomposites. Compos. Sci. Technol. 2012;72(12):1430–1435. doi: 10.1016/j.compscitech.2012.05.018. [DOI] [Google Scholar]
- 103.Solis-Fernandez P, Rozada R, Paredes JI, Villar-Rodil S, Fernandez-Merino MJ, Guardia L, Martinez-Alonso A, Tascon JMD. Chemical and microscopic analysis of graphene prepared by different reduction degrees of graphene oxide. J. Alloys Compd. 2012;536:S532–S537. doi: 10.1016/j.jallcom.2012.01.102. [DOI] [Google Scholar]
- 104.Thanh Truong D, Viet Hung P, Bao Khanh V, Hur SH, Shin EW, Kim EJ, Chung JS, Tascon JMD. Clean and effective catalytic reduction of graphene oxide using atomic hydrogen spillover on Pt/gamma-Al2O3 catalyst. Mater. Lett. 2012;86:161–164. doi: 10.1016/j.matlet.2012.07.063. [DOI] [Google Scholar]
- 105.Chua CK, Pumera M. Reduction of graphene oxide with substituted borohydrides. J. Mater. Chem. A. 2013;1(5):1892–1898. doi: 10.1039/C2TA00665K. [DOI] [Google Scholar]
- 106.Liu P, Huang Y, Wang L. A facile synthesis of reduced graphene oxide with Zn powder under acidic condition. Mater. Lett. 2013;91:125–128. doi: 10.1016/j.matlet.2012.09.085. [DOI] [Google Scholar]
- 107.Huang XL, Hu NT, Wang YY, Zhang YF. Ammonia gas sensor based on aniline reduced graphene oxide. Adv. Mater. Res. 2013;669:79–84. doi: 10.4028/www.scientific.net/AMR.669.79. [DOI] [Google Scholar]
- 108.Huang XL, Hu NT, Zhang LL, Wei LM, Wei H, Zhang YF. The NH3 sensing properties of gas sensors based on aniline reduced graphene oxide. Synth. Met. 2013;185:25–30. doi: 10.1016/j.synthmet.2013.09.034. [DOI] [Google Scholar]
- 109.Dong YL, Zhang XF, Cheng XL, Xu YM, Gao S, Zhao H, Huo LH. Highly selective NO2 sensor at room temperature based on nanocomposites of hierarchical nanosphere-like alpha-Fe2O3 and reduced graphene oxide. RSC Adv. 2014;4(101):57493–57500. doi: 10.1039/C4RA10136G. [DOI] [Google Scholar]
- 110.Xiangfeng C, Tao H, Feng G, Yongping D, Wenqi S, Linshan B. Gas sensing properties of graphene-WO3 composites prepared by hydrothermal method. Mat. Sci. Eng. B. 2015;193:97–104. doi: 10.1016/j.mseb.2014.11.011. [DOI] [Google Scholar]
- 111.Deng S, Tjoa V, Fan HM, Tan HR, Sayle DC, Olivo M, Mhaisalkar S, Wei J, Sow CH. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. JACS. 2012;134(10):4905–4917. doi: 10.1021/ja211683m. [DOI] [PubMed] [Google Scholar]
- 112.Gautam M, Jayatissa AH. Ammonia gas sensing behavior of graphene surface decorated with gold nanoparticles. Solid-State Electron. 2012;78:159–165. doi: 10.1016/j.sse.2012.05.059. [DOI] [Google Scholar]
- 113.Yavari F, Castillo E, Gullapalli H, Ajayan PM, Koratkar N. High sensitivity detection of NO2 and NH3 in air using chemical vapor deposition grown graphene. Appl. Phys. Lett. 2012;100:203120. doi: 10.1063/1.4720074. [DOI] [Google Scholar]
- 114.Seekaew Y, Lokavee S, Phokharatkul D, Wisitsoraat A, Kerdcharoen T, Wongchoosuk C. Low-cost and flexible printed graphene-PEDOT:PSS gas sensor for ammonia detection. Org. Electron. 2014;15(11):2971–2981. doi: 10.1016/j.orgel.2014.08.044. [DOI] [Google Scholar]
- 115.Huang X, Hu N, Gao R, Yu Y, Wang Y, Yang Z, Siu-Wai Kong E, Wei H, Zhang Y. Reduced graphene oxide-polyaniline hybrid: Preparation, characterization and its applications for ammonia gas sensing. J. Mater. Chem. 2012;22(42):22488. doi: 10.1039/c2jm34340a. [DOI] [Google Scholar]
- 116.Hu N, Yang Z, Wang Y, Zhang L, Wang Y, Huang X, Wei H, Wei L, Zhang Y. Ultrafast and sensitive room temperature NH3 gas sensors based on chemically reduced graphene oxide. Nanotechnology. 2014;25(2):025502. doi: 10.1088/0957-4484/25/2/025502. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Zhang L, Hu N, Wang Y, Zhang Y, Zhou Z, Liu Y, Shen S, Peng C. Ammonia gas sensors based on chemically reduced graphene oxide sheets self-assembled on Au electrodes. Nanoscale Res. Lett. 2014;9(1):251. doi: 10.1186/1556-276X-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gautam M, Jayatissa AH. Graphene based field effect transistor for the detection of ammonia. J. Appl. Phys. 2012;112:064304. doi: 10.1063/1.4752272. [DOI] [Google Scholar]
- 119.Ben Aziza Z, Zhang Q, Baillargeat D. Graphene/mica based ammonia gas sensors. Appl. Phys. Lett. 2014;105:254102. doi: 10.1063/1.4905039. [DOI] [Google Scholar]
- 120.Inaba A, Yoo K, Takei Y, Matsumoto K, Shimoyama I. Ammonia gas sensing using a graphene field-effect transistor gated by ionic liquid. Sens. Actuators B. 2014;195:15–21. doi: 10.1016/j.snb.2013.12.118. [DOI] [Google Scholar]
- 121.Ye ZB, Jiang YD, Tai HL, Yuan Z. The investigation of reduced graphene oxide/P3HT composite films for ammonia detection. Integr. Ferroelectr. 2014;154(1):73–81. doi: 10.1080/10584587.2014.904148. [DOI] [Google Scholar]
- 122.Yoo S, Li X, Wu Y, Liu WH, Wang XL, Yi WH. Ammonia gas detection by tannic acid functionalized and reduced graphene oxide at room temperature. J. Nanomater. 2014;2014:1–6. doi: 10.1155/2014/497384. [DOI] [Google Scholar]
- 123.Meng H, Yang W, Ding K, Feng L, Guan YF. Cu2O nanorods modified by reduced graphene oxide for NH3 sensing at room temperature. J. Mater. Chem. A. 2015;3(3):1174–1181. doi: 10.1039/C4TA06024E. [DOI] [Google Scholar]
- 124.Choi H, Jeong HY, Lee DS, Choi CG, Choi SY. Flexible NO2 gas sensor using multilayer graphene films by chemical vapor deposition. Carbon Lett. 2013;14(3):186–189. doi: 10.5714/CL.2013.14.3.186. [DOI] [Google Scholar]
- 125.Hoa LT, Tien HN, Luan VH, Chung JS, Hur SH. Fabrication of a novel 2D-graphene/2D-NiO nanosheet-based hybrid nanostructure and its use in highly sensitive NO2 sensors. Sens. Actuators B. 2013;185:701–705. doi: 10.1016/j.snb.2013.05.050. [DOI] [Google Scholar]
- 126.Huang L, Wang Z, Zhang J, Pu J, Lin Y, Xu S, Shen L, Chen Q, Shi W. Fully printed, rapid-response sensors based on chemically modified graphene for detecting NO2 at room temperature. ACS Appl. Mater. Interface. 2014;6(10):7426–7433. doi: 10.1021/am500843p. [DOI] [PubMed] [Google Scholar]
- 127.Ju Yun Y, Hong WG, Choi N-J, Hoon Kim B, Jun Y, Lee H-K. Ultrasensitive and highly selective graphene-based single yarn for use in wearable gas sensor. Sci. Rep. 2015;5:10904–10904. doi: 10.1038/srep10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pearce R, Iakimov T, Andersson M, Hultman L, Spetz AL, Yakimova R. Epitaxially grown graphene based gas sensors for ultra sensitive NO2 detection. Sens. Actuators B. 2011;155(2):451–455. doi: 10.1016/j.snb.2010.12.046. [DOI] [Google Scholar]
- 129.Chung MG, Kim DH, Lee HM, Kim T, Choi JH, Seo DK, Yoo JB, Hong SH, Kang TJ, Kim YH. Highly sensitive NO2 gas sensor based on ozone treated graphene. Sens. Actuators B. 2012;166:172–176. doi: 10.1016/j.snb.2012.02.036. [DOI] [Google Scholar]
- 130.Lee C, Ahn J, Lee KB, Kim D, Kim J. Graphene-based flexible NO2 chemical sensors. Thin Solid Films. 2012;520(16):5459–5462. doi: 10.1016/j.tsf.2012.03.095. [DOI] [Google Scholar]
- 131.Srivastava S, Jain K, Singh VN, Singh S, Vijayan N, Dilawar N, Gupta G, Senguttuvan TD. Faster response of NO(2) sensing in graphene-WO(3) nanocomposites. Nanotechnology. 2012;23(20):205501. doi: 10.1088/0957-4484/23/20/205501. [DOI] [PubMed] [Google Scholar]
- 132.Huang JL, Xie GZ, Zhou Y, Xie T, Tai HL, Yang GJ. Polyvinylpyrrolidone/reduced graphene oxide nanocomposites thin films coated on quartz crystal microbalance for NO2 detection at room temperature. Proc. SPIE. 2014;9285:92850B–92851B. doi: 10.1117/12.2069492. [DOI] [Google Scholar]
- 133.Liu S, Yu B, Zhang H, Fei T, Zhang T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B. 2014;202:272–278. doi: 10.1016/j.snb.2014.05.086. [DOI] [Google Scholar]
- 134.Liu X, Cui JS, Sun JB, Zhang XT. 3D graphene aerogel-supported SnO2 nanoparticles for efficient detection of NO2. RSC Adv. 2014;4(43):22601–22605. doi: 10.1039/c4ra02453b. [DOI] [Google Scholar]
- 135.Piloto C, Notarianni M, Shafiei M, Taran E, Galpaya D, Yan C, Motta N. Highly NO2 sensitive caesium doped graphene oxide conductometric sensors. Beilstein. J. Nanotechnol. 2014;5:1073–1081. doi: 10.3762/bjnano.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Su PG, Shieh HC. Flexible NO2 sensors fabricated by layer-by-layer covalent anchoring and in situ reduction of graphene oxide. Sens. Actuators B. 2014;190:865–872. doi: 10.1016/j.snb.2013.09.078. [DOI] [Google Scholar]
- 137.Zhang H, Feng JC, Fei T, Liu S, Zhang T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators B. 2014;190:472–478. doi: 10.1016/j.snb.2013.08.067. [DOI] [Google Scholar]
- 138.Su P-G, Peng S-L. Fabrication and NO2 gas-sensing properties of reduced graphene oxide/WO3 nanocomposite films. Talanta. 2015;132:398–405. doi: 10.1016/j.talanta.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 139.Gu F, Nie R, Han D, Wang Z. In2O3-graphene nanocomposite based gas sensor for selective detection of NO2 at room temperature. Sens. Actuators B. 2015;219:94–99. doi: 10.1016/j.snb.2015.04.119. [DOI] [Google Scholar]
- 140.Johnson JL, Behnam A, Pearton SJ, Ural A. Hydrogen sensing using pd-functionalized multi-layer graphene nanoribbon networks. Adv. Mater. 2010;22(43):4877–4880. doi: 10.1002/adma.201001798. [DOI] [PubMed] [Google Scholar]
- 141.Chu BH, Lo CF, Nicolosi J, Chang CY, Chen V, Strupinski W, Pearton SJ, Ren F. Hydrogen detection using platinum coated graphene grown on SiC. Sens. Actuators B. 2011;157(2):500–503. doi: 10.1016/j.snb.2011.05.007. [DOI] [Google Scholar]
- 142.Chu BH, Nicolosi J, Lo CF, Strupinski W, Pearton SJ, Ren F. Effect of coated platinum thickness on hydrogen detection sensitivity of graphene-based sensors. Electrochem. Solid-State Lett. 2011;14(7):K43–K45. doi: 10.1149/1.3589250. [DOI] [Google Scholar]
- 143.Kumar R, Varandani D, Mehta BR, Singh VN, Wen Z, Feng X, Muellen K. Fast response and recovery of hydrogen sensing in Pd-Pt nanoparticle-graphene composite layers. Nanotechnology. 2011;22(27):275719. doi: 10.1088/0957-4484/22/27/275719. [DOI] [PubMed] [Google Scholar]
- 144.Jiang QG, Ao ZM, Zheng WT, Li S, Jiang Q. Enhanced hydrogen sensing properties of graphene by introducing a mono-atom-vacancy. Phys. Chem. Chem. Phys. 2013;15(48):21016–21022. doi: 10.1039/c3cp52976b. [DOI] [PubMed] [Google Scholar]
- 145.Kaniyoor A, Jafri RI, Arockiadoss T, Ramaprabhu S. Nanostructured Pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor. Nanoscale. 2009;1(3):382–386. doi: 10.1039/b9nr00015a. [DOI] [PubMed] [Google Scholar]
- 146.Shafiei M, Spizzirri PG, Arsat R, Yu J, du Plessis J, Dubin S, Kaner RB, Kalantar-Zadeh K, Wlodarski W. Platinum/graphene nanosheet/SiC contacts and their application for hydrogen gas sensing. J. Phys. Chem. C. 2010;114(32):13796–13801. doi: 10.1021/jp104459s. [DOI] [Google Scholar]
- 147.Lange U, Hirsch T, Mirsky VM, Wolfbeis OS. Hydrogen sensor based on a graphene: palladium nanocomposite. Electrochimi. Acta. 2011;56(10):3707–3712. doi: 10.1016/j.electacta.2010.10.078. [DOI] [Google Scholar]
- 148.Chung MG, Kim D-H, Seo DK, Kim T, Im HU, Lee HM, Yoo J-B, Hong S-H, Kang TJ, Kim YH. Flexible hydrogen sensors using graphene with palladium nanoparticle decoration. Sens. Actuators B. 2012;169:387–392. doi: 10.1016/j.snb.2012.05.031. [DOI] [Google Scholar]
- 149.Ehemann RC, Krstic PS, Dadras J, Kent PRC, Jakowski J. Detection of hydrogen using graphene. Nanoscale Res. Lett. 2012;7:1–14. doi: 10.1186/1556-276X-7-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Esfandiar A, Ghasemi S, Irajizad A, Akhavan O, Gholami MR. The decoration of TiO2/reduced graphene oxide by Pd and Pt nanoparticles for hydrogen gas sensing. Int. J. Hydrogen Energ. 2012;37(20):15423–15432. doi: 10.1016/j.ijhydene.2012.08.011. [DOI] [Google Scholar]
- 151.Russo PA, Donato N, Leonardi SG, Baek S, Conte DE, Neri G, Pinna N. Room-temperature hydrogen sensing with heteronanostructures based on reduced graphene oxide and tin oxide. Angew. Chem. Int. Ed. 2012;51(44):11053–11057. doi: 10.1002/anie.201204373. [DOI] [PubMed] [Google Scholar]
- 152.Chen X, Yasin FM, Eggers PK, Boulos RA, Duan X, Lamb RN, Iyer KS, Raston CL. Non-covalently modified graphene supported ultrafine nanoparticles of palladium for hydrogen gas sensing. RSC Adv. 2013;3(10):3213–3217. doi: 10.1039/c3ra22986f. [DOI] [Google Scholar]
- 153.Phan D-T, Chung G-S. Characteristics of resistivity-type hydrogen sensing based on palladium-graphene nanocomposites. Int. J. Hydrogen Energ. 2014;39(1):620–629. doi: 10.1016/j.ijhydene.2013.08.107. [DOI] [Google Scholar]
- 154.Singh B, Wang J, Rathi S, Kim G-H. Alignment of graphene oxide nanostructures between microgap electrodes via dielectrophoresis for hydrogen gas sensing applications. Appl. Phys. Lett. 2015;106:203106. doi: 10.1063/1.4921524. [DOI] [Google Scholar]
- 155.Hong J, Lee S, Seo J, Pyo S, Kim J, Lee T. A highly sensitive hydrogen sensor with gas selectivity using a PMMA membrane-coated Pd nanoparticle/single-layer graphene hybrid. ACS Appl. Mater. Interface. 2015;7(6):3554–3561. doi: 10.1021/am5073645. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Z, Zou X, Xu L, Liao L, Liu W, Ho J, Xiao X, Jiang C, Li J. Hydrogen gas sensor based on metal oxide nanoparticles decorated graphene transistor. Nanoscale. 2015;7(22):10078–10084. doi: 10.1039/C5NR01924A. [DOI] [PubMed] [Google Scholar]
- 157.Zheng Y, Lee D, Koo HY, Maeng S. Chemically modified graphene/PEDOT:PSS nanocomposite films for hydrogen gas sensing. Carbon. 2015;81:54–62. doi: 10.1016/j.carbon.2014.09.023. [DOI] [Google Scholar]
- 158.Nemade KR, Waghuley SA. Chemiresistive gas sensing by few-layered graphene. J. Electron. Mater. 2013;42(10):2857–2866. doi: 10.1007/s11664-013-2699-4. [DOI] [Google Scholar]
- 159.Nemade KR, Waghuley A. Carbon dioxide gas sensing application of graphene/Y2O3 quantum dots composite. Int. J. Mod. Phys. 2013;22:380–384. [Google Scholar]
- 160.Nemade KR, Waghuley SA. Role of defects concentration on optical and carbon dioxide gas sensing properties of Sb2O3/graphene composites. Opt. Mater. 2014;36(3):712–716. doi: 10.1016/j.optmat.2013.11.024. [DOI] [Google Scholar]
- 161.Nemade KR, Waghuley SA. Highly responsive carbon dioxide sensing by graphene/Al2O3 quantum dots composites at low operable temperature. Indian J. Phys. 2014;88(6):577–583. doi: 10.1007/s12648-014-0454-1. [DOI] [Google Scholar]
- 162.Liu C-S, Jia R, Ye X-J, Zeng Z. Non-hexagonal symmetry-induced functional T graphene for the detection of carbon monoxide. J. Chem. Phys. 2013;139(3):034704. doi: 10.1063/1.4813528. [DOI] [PubMed] [Google Scholar]
- 163.Wu Z, Chen X, Zhu S, Zhou Z, Yao Y, Quan W, Liu B. Room temperature methane sensor based on graphene nanosheets/polyaniline nanocomposite thin film. IEEE Sens. J. 2013;13(2):777–782. doi: 10.1109/JSEN.2012.2227597. [DOI] [Google Scholar]
- 164.Yoon HJ, Jun DH, Yang JH, Zhou ZX, Yang SS, Cheng MMC. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B. 2011;157(1):310–313. doi: 10.1016/j.snb.2011.03.035. [DOI] [Google Scholar]
- 165.Hafiz SM, Ritikos R, Whitcher TJ, Razib NM, Bien DCS, et al. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuators B. 2014;193:692–700. doi: 10.1016/j.snb.2013.12.017. [DOI] [Google Scholar]
- 166.Shen F, Wang D, Liu R, Pei X, Zhang T, Jin J. Edge-tailored graphene oxide nanosheet-based field effect transistors for fast and reversible electronic detection of sulfur dioxide. Nanoscale. 2013;5(2):537–540. doi: 10.1039/C2NR32752J. [DOI] [PubMed] [Google Scholar]
- 167.Liu X-Y, Zhang J-M, Xu K-W, Ji V. Improving SO2 gas sensing properties of graphene by introducing dopant and defect: a first-principles study. Appl. Surf. Sci. 2014;313:405–410. doi: 10.1016/j.apsusc.2014.05.223. [DOI] [Google Scholar]
- 168.Shao L, Chen G, Ye H, Niu H, Wu Y, Zhu Y, Ding B. Sulfur dioxide molecule sensors based on zigzag graphene nanoribbons with and without Cr dopant. Phys. Lett. A. 2014;378(7–8):667–671. doi: 10.1016/j.physleta.2013.12.042. [DOI] [Google Scholar]
- 169.Xian Q, Qingyuan M, Yuan F. Ping, Strain effects on enhanced hydrogen sulphide detection capability of Ag-decorated defective graphene. Mod. Phys. Lett. B. 2012;26(25):1250166. doi: 10.1142/S0217984912501667. [DOI] [Google Scholar]
- 170.Zhou L, Shen F, Tian X, Wang D, Zhang T, Chen W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale. 2013;5(4):1564–1569. doi: 10.1039/c2nr33164k. [DOI] [PubMed] [Google Scholar]
- 171.Jiang Z, Li J, Aslan H, Li Q, Li Y, et al. A high efficiency H2S gas sensor material: paper like Fe2O3/graphene nanosheets and structural alignment dependency of device efficiency. J. Mater. Chem. A. 2014;2(19):6714–6717. doi: 10.1039/c3ta15180h. [DOI] [Google Scholar]
- 172.Ren Y, Zhu C, Cai W, Li H, Ji H, Kholmanov I, Wu Y, Piner RD, Ruoff RS. Detection of sulfur dioxide gas with graphene field effect transistor. Appl. Phys. Lett. 2012;100:163114. doi: 10.1063/1.4704803. [DOI] [Google Scholar]
- 173.Xian Q, Qing M. Yuan, G. Yu Fei, Ag supported Si-doped graphene for hydrogen sulphide detection: a first-principles investigation. Adv. Mater. Res. 2013;602–604:37–40. [Google Scholar]
- 174.Zhang Y-H, Han L-F, Xiao Y-H, Jia D-Z, Guo Z-H, Li F. Understanding dopant and defect effect on H2S sensing performances of graphene: a first-principles study. Comp. Mater. Sci. 2013;69:222–228. doi: 10.1016/j.commatsci.2012.11.048. [DOI] [Google Scholar]
- 175.Cho S, Lee JS, Jun J, Kim SG, Jang J. Fabrication of water-dispersible and highly conductive PSS-doped PANI/graphene nanocomposites using a high-molecular weight PSS dopant and their application in H2S detection. Nanoscale. 2014;6(24):15181–15195. doi: 10.1039/C4NR04413D. [DOI] [PubMed] [Google Scholar]
- 176.Choi S-J, Jang B-H, Lee S-J, Min BK, Rothschild A, Kim I-D. Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl. Mater. Interface. 2014;6(4):2588–2597. doi: 10.1021/am405088q. [DOI] [PubMed] [Google Scholar]
- 177.Choi S-J, Choi C, Kim S-J, Cho H-J, Hakim M, Jeon S, Kim I-D. Highly efficient electronic sensitization of non-oxidized graphene flakes on controlled pore-loaded WO3 nanofibers for selective detection of H2S molecules. Sci. Rep. 2015;5:8067. doi: 10.1038/srep08067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Berahman M, Sheikhi MH. Hydrogen sulfide gas sensor based on decorated zigzag graphene nanoribbon with copper. Sens. Actuators B. 2015;219:338–345. doi: 10.1016/j.snb.2015.04.114. [DOI] [Google Scholar]
- 179.Tawfik SA, Cui XY, Carter DJ, Ringer SP, Stampfl C. Sensing sulfur-containing gases using titanium and tin decorated zigzag graphene nanoribbons from first-principles. Phys. Chem. Chem. Phys. 2015;17(10):6925–6932. doi: 10.1039/C4CP05919K. [DOI] [PubMed] [Google Scholar]
- 180.Dua V, Surwade SP, Ammu S, Agnihotra SR, Jain S, Roberts KE, Park S, Ruoff RS, Manohar SK. All-organic vapor sensor using inkjet-printed reduced graphene oxide. Angew. Chem. Int. Ed. 2010;49(12):2154–2157. doi: 10.1002/anie.200905089. [DOI] [PubMed] [Google Scholar]
- 181.Jiang Z, Wang J, Meng L, Huang Y, Liu L. A highly efficient chemical sensor material for ethanol: Al2O3/Graphene nanocomposites fabricated from graphene oxide. Chem. Commun. 2011;47(22):6350–6352. doi: 10.1039/c1cc11711d. [DOI] [PubMed] [Google Scholar]
- 182.Zhang H, Kulkarni A, Kim H, Woo D, Kim Y-J, Hong BH, Choi J-B, Kim T. Detection of acetone vapor using graphene on polymer optical fiber. J. Nanosci. Nanotechno. 2011;11(7):5939–5943. doi: 10.1166/jnn.2011.4408. [DOI] [PubMed] [Google Scholar]
- 183.Gautam M, Jayatissa AH. Detection of organic vapors by graphene films functionalized with metallic nanoparticles. J. Appl. Phys. 2012;112:114326. doi: 10.1063/1.4768724. [DOI] [Google Scholar]
- 184.Tang L, Feng H, Cheng J, Li J. Uniform and rich-wrinkled electrophoretic deposited graphene film: a robust electrochemical platform for TNT sensing. Chem. Commun. 2010;46(32):5882–5884. doi: 10.1039/c0cc01212b. [DOI] [PubMed] [Google Scholar]
- 185.Fan L, Hu Y, Wang X, Zhang L, Li F, Han D, Li Z, Zhang Q, Wang Z, Niu L. Fluorescence resonance energy transfer quenching at the surface of graphene quantum dots for ultrasensitive detection of TNT. Talanta. 2012;101:192–197. doi: 10.1016/j.talanta.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 186.Liu M, Chen W. Graphene nanosheets-supported Ag nanoparticles for ultrasensitive detection of TNT by surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2013;46:68–73. doi: 10.1016/j.bios.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 187.Singh VV, Nigam AK, Yadav SS, Tripathi BK, Srivastava A, Boopathi M, Singh B. Graphene oxide as carboelectrocatalyst for in situ electrochemical oxidation and sensing of chemical warfare agent simulant. Sens. Actuators B. 2013;188:1218–1224. doi: 10.1016/j.snb.2013.08.013. [DOI] [Google Scholar]
- 188.Ganji MD, Dalirandeh Z, Khosravi A, Fereidoon A. Aluminum nitride graphene for DMMP nerve agent adsorption and detection. Mater. Chem. Phys. 2014;145(1–2):260–267. doi: 10.1016/j.matchemphys.2014.02.021. [DOI] [Google Scholar]
- 189.Chen B, Liu H, Li X, Lu C, Ding Y, Lu B. Fabrication of a graphene field effect transistor array on microchannels for ethanol sensing. Appl. Surf. Sci. 2012;258(6):1971–1975. doi: 10.1016/j.apsusc.2011.05.101. [DOI] [Google Scholar]
- 190.Zhang L, Li C, Liu A, Shi G. Electrosynthesis of graphene oxide/polypyrene composite films and their applications for sensing organic vapors. J. Mater. Chem. 2012;22(17):8438–8443. doi: 10.1039/c2jm16552j. [DOI] [Google Scholar]
- 191.Sun P, Cai Y, Du S, Xu X, You L, Ma J, Liu F, Liang X, Sun Y, Lu G. Hierarchical alpha-Fe2O3/SnO2 semiconductor composites: hydrothermal synthesis and gas sensing properties. Sens. Actuators B. 2013;182:336–343. doi: 10.1016/j.snb.2013.03.019. [DOI] [Google Scholar]
- 192.Liu F, Chu X, Dong Y, Zhang W, Sun W, Shen L. Acetone gas sensors based on graphene-ZnFe2O4 composite prepared by solvothermal method. Sens. Actuators B. 2013;188:469–474. doi: 10.1016/j.snb.2013.06.065. [DOI] [Google Scholar]
- 193.Moradi M, Noei M, Peyghan AA. DFT studies of Si- and Al-doping effects on the acetone sensing properties of BC3 graphene. Mol. Phys. 2013;111(21):3320–3326. doi: 10.1080/00268976.2013.783720. [DOI] [Google Scholar]
- 194.Wang X, Sun X, Hu PA, Zhang J, Wang L, Feng W, Lei S, Yang B, Cao W. Colorimetric sensor based on self-assembled polydiacetylene/graphene-stacked composite film for vapor-phase volatile organic compounds. Adv. Funct. Mater. 2013;23(48):6044–6050. doi: 10.1002/adfm.201301044. [DOI] [Google Scholar]
- 195.Choi S-J, Ryu W-H, Kim S-J, Cho H-J, Kim I-D. Bi-functional co-sensitization of graphene oxide sheets and Ir nanoparticles on p-type Co3O4 nanofibers for selective acetone detection. J. Mater. Chem. B. 2014;2(41):7160–7167. doi: 10.1039/C4TB00767K. [DOI] [PubMed] [Google Scholar]
- 196.T. Kavinkumar, D. Sastikumar, S. Manivannan, Reduced graphene oxide coated optical fiber for methanol and ethanol vapor detection at room temperature. Proceedings of SPIE 9270, Optoel. Dev. Integr. V, 92700U (2014). doi:10.1117/12.2071841
- 197.Aziz A, Lim HN, Girei SH, Yaacob MH, Mandi MA, Huang NM, Pandikumar A. Silver/graphene nanocomposite-modified optical fiber sensor platform for ethanol detection in water medium. Sens. Actuators B. 2015;206:119–125. doi: 10.1016/j.snb.2014.09.035. [DOI] [Google Scholar]
- 198.Uddin ASMI, Duy-Thach P, Chung G-S. Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sens. Actuators B. 2015;207:362–369. doi: 10.1016/j.snb.2014.10.091. [DOI] [Google Scholar]
- 199.Uddin ASMI, Lee K-W, Chung G-S. Acetylene gas sensing properties of an Ag-loaded hierarchical ZnO nanostructure-decorated reduced graphene oxide hybrid. Sens. Actuators B. 2015;216:33–40. doi: 10.1016/j.snb.2015.04.028. [DOI] [Google Scholar]
- 200.Chaonan X, Tamaki J, Miura N, Yamazoe N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B. 1991;3(2):147–155. doi: 10.1016/0925-4005(91)80207-Z. [DOI] [Google Scholar]
- 201.Rothschild A, Komem Y. The effect of grain size on the sensitivity of nanocrystalline metal-oxide gas sensors. J. Appl. Phys. 2004;95(11):6374–6380. doi: 10.1063/1.1728314. [DOI] [Google Scholar]
- 202.Lee J-H. Gas sensors using hierarchical and hollow oxide nanostructures: overview. Sens. Actuators B. 2009;140(1):319–336. doi: 10.1016/j.snb.2009.04.026. [DOI] [Google Scholar]
- 203.Sang-Zi L, Gugang C, Harutyunyan AR, Sofo JO. Screening of charged impurities as a possible mechanism for conductance change in graphene gas sensing. Phys. Rev. B: Condens. Matter. 2014;90(11):115410. doi: 10.1103/PhysRevB.90.115410. [DOI] [Google Scholar]
- 204.Dayan NJ, Sainkar SR, Karekar RN, Aiyer RC. Formulation and characterization of ZnO: Sb thick-film gas sensors. Thin Solid Films. 1998;325(1–2):254–258. doi: 10.1016/S0040-6090(98)00501-X. [DOI] [Google Scholar]
- 205.Yamazoe N, Sakai G, Shimanoe K. Oxide semiconductor gas sensors. Catal. Surv. Asia. 2003;7(1):63–75. doi: 10.1023/A:1023436725457. [DOI] [Google Scholar]
- 206.Yamazoe N. New approaches for improving semiconductor gas sensors. Sens. Actuators B. 1991;5(1–4):7–19. doi: 10.1016/0925-4005(91)80213-4. [DOI] [Google Scholar]
- 207.Egashira M, Shimizu Y, Takao Y, Sako S. Variations in I–V characteristics of oxide semiconductors induced by oxidizing gases. Sens. Actuators B. 1996;35(1–3):62–67. doi: 10.1016/S0925-4005(96)02015-1. [DOI] [Google Scholar]
- 208.Zhou Y, Jiang YD, Xie T, Tai HL, Xie GZ. A novel sensing mechanism for resistive gas sensors based on layered reduced graphene oxide thin films at room temperature. Sens. Actuators B. 2014;203:135–142. doi: 10.1016/j.snb.2014.06.105. [DOI] [Google Scholar]
- 209.Zhu M, Li X, Chung S, Zhao L, Li X, Zang X, Wang K, Wei J, Zhong M, Zhou K, Xie D, Zhu H. Photo-induced selective gas detection based on reduced graphene oxide/Si Schottky diode. Carbon. 2015;84:138–145. doi: 10.1016/j.carbon.2014.12.008. [DOI] [Google Scholar]
- 210.Wang R-C, Chang Y-M. Switch of p-n electricity of reduced-graphene-oxide-flake stacked films enabling room-temperature gas sensing from ultrasensitive to insensitive. Carbon. 2015;91:416–422. doi: 10.1016/j.carbon.2015.05.012. [DOI] [Google Scholar]
- 211.Yavari F, Chen ZP, Thomas AV, Ren WC, Cheng HM, Koratkar N. High sensitivity gas detection using a macroscopic three-dimensional graphene foam network. Sci. Rep. 2011;1:166. doi: 10.1038/srep00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yi S, Tian SQ, Zeng DW, Xu K, Zhang SP, Xie CS. An In2O3 nanowire-like network fabricated on coplanar sensor surface by sacrificial CNTs for enhanced gas sensing performance. Sens. Actuators B. 2013;185:345–353. doi: 10.1016/j.snb.2013.05.007. [DOI] [Google Scholar]
- 213.Huang X, Lin J, Pi Z, Yu Z. Qualitative and quantitative analysis of organophosphorus pesticide residues using temperature modulated SnO(2) gas sensor. Talanta. 2004;64:538–545. doi: 10.1016/j.talanta.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 214.Huang XJ, Wang LC, Sun YF, Meng FL, Liu JH. Quantitative analysis of pesticide residue based on the dynamic response of a single SnO2 gas sensor. Sens. Actuators B. 2004;99(2–3):330–335. doi: 10.1016/j.snb.2003.11.032. [DOI] [Google Scholar]
- 215.Huang XJ, Liu JH, Shao DL, Pi ZX, Yu ZL. Rectangular mode of operation for detecting pesticide residue by using a single SnO2-based gas sensor. Sens. Actuators B. 2003;96(3):630–635. doi: 10.1016/j.snb.2003.07.006. [DOI] [Google Scholar]