Abstract
Introduction and Objective
Social media has been proposed as a possibly useful data source for pharmacovigilance signal detection. This study primarily aimed to evaluate the performance of established statistical signal detection algorithms in Twitter/Facebook for a broad range of drugs and adverse events.
Methods
Performance was assessed using a reference set by Harpaz et al., consisting of 62 US Food and Drug Administration labelling changes, and an internal WEB-RADR reference set consisting of 200 validated safety signals. In total, 75 drugs were studied. Twitter/Facebook posts were retrieved for the period March 2012 to March 2015, and drugs/events were extracted from the posts. We retrieved 4.3 million and 2.0 million posts for the WEB-RADR and Harpaz drugs, respectively. Individual case reports were extracted from VigiBase for the same period. Disproportionality algorithms based on the Information Component or the Proportional Reporting Ratio and crude post/report counting were applied in Twitter/Facebook and VigiBase. Receiver operating characteristic curves were generated, and the relative timing of alerting was analysed.
Results
Across all algorithms, the area under the receiver operating characteristic curve for Twitter/Facebook varied between 0.47 and 0.53 for the WEB-RADR reference set and between 0.48 and 0.53 for the Harpaz reference set. For VigiBase, the ranges were 0.64–0.69 and 0.55–0.67, respectively. In Twitter/Facebook, at best, 31 (16%) and four (6%) positive controls were detected prior to their index dates in the WEB-RADR and Harpaz references, respectively. In VigiBase, the corresponding numbers were 66 (33%) and 17 (27%).
Conclusions
Our results clearly suggest that broad-ranging statistical signal detection in Twitter and Facebook, using currently available methods for adverse event recognition, performs poorly and cannot be recommended at the expense of other pharmacovigilance activities.
Electronic supplementary material
The online version of this article (10.1007/s40264-018-0699-2) contains supplementary material, which is available to authorized users.
Key Points
| Social media has been suggested as a possibly valuable data source for signal detection in pharmacovigilance. This study focused on the evaluation of disproportionality analysis in combined Twitter and Facebook data. |
| A large number of drugs and a breadth of adverse events were considered. Two different reference sets were used to benchmark predictive performance, one based on labelling changes and one based on validated safety signals. |
| Twitter/Facebook data displayed no predictive value for either of the reference sets, which was contrasted by considerably better performance for the conventional pharmacovigilance data source VigiBase. Therefore, broad-ranging statistical safety signal detection in Twitter and Facebook cannot be recommended. |
Introduction
During the period 2014–17, the Innovative Medicines Initiative WEB-RADR (WEB-Recognizing Adverse Drug Reactions) project addressed key research questions relevant to the potential use of social media for pharmacovigilance.
The advent and massive uptake of social media as a communication tool provides opportunities and challenges in many fields, including pharmacovigilance [1, 2]. One relevant question is whether social media may have value as an independent hypothesis-generating tool in pharmacovigilance, to be used in addition to other data sources such as spontaneous reports of adverse events or electronic health records. If indeed valuable, the vast amount of information generated through social media would require a well-defined approach with regard to monitoring, reporting, analysing and evaluating potential adverse reactions, signals and other medical insights related to medicines. The underlying assumption in the utilisation of social media for signal detection is that the type of discussions in social media could either be of a different nature (i.e. different experiences with medications) or take place at a different time than spontaneous reports. If either one of these assumptions holds, social media could indeed be used as a general tool for the detection of either different adverse drug reactions (ADRs) or earlier detection of ADRs relative to other data sources, specifically spontaneous individual case safety reports (ICSRs).
The research presented in this article focuses on the use of social media for aggregate statistical signal detection using spontaneous data as a comparator, specifically VigiBase.
Past investigations into the utility of social media for signal detection have been somewhat limited in the scope of methods, products and events (e.g. abuse or misuse) [3–6], or focused on the recognition of adverse events in single posts [7, 8]. In contrast, this work aims to present a comprehensive analysis of the use of social media for the detection of safety signals for a wide range of products using statistical methods. Specifically, our primary aim was to evaluate the predictive ability and timeliness of statistical signal detection using disproportionality analysis in broad-coverage social media such as Twitter and Facebook. To this end, both validated safety signals and label changes were used for benchmarking. Additional aims were to investigate the potential utility of statistical signal detection in patient fora, and to assess the clinical relevance of Twitter and Facebook posts for signal detection purposes.
Data and Methods
Data Extraction and Aggregation
Social Media Data Extraction
The raw social media data were provided by Epidemico, one of the WEB-RADR partners. All source data were in the form of free text posts originating from publicly available content from Twitter, Facebook and various patient fora. These data were acquired either directly from the respective social media platforms or through a third-party vendor. To maximise the signal-to-noise ratio and to increase relevance to drug safety, posts were retrieved using a list of search terms referring to medical product names, including brand names, active ingredients, generic terms and common misspellings.
After acquisition, the data underwent classification, mapping to medical products, de-duplication and mapping to MedDRA®, using the Epidemico algorithm described previously [5, 7, 9]. In this process, each post was assigned an indicator score between 0 and 1: a score close to 0 means the post contains language that does not resemble an adverse-event discussion (usually spam), whereas a score close to 1 indicates that the post more closely resembles language describing an adverse event. The symptom taxonomy contains a list of MedDRA® Preferred Terms (PTs, 2167 for the analyses in this work), with a set of colloquial phrases (synonyms) that social media users have used to describe each PT.
The medical product taxonomy contains information for drugs, medical devices, vaccines, cosmetics and dietary supplements. For each of these products, the taxonomy includes both a canonical name, search terms, synonyms (including misspellings and slang terms) and active ingredients to enable downstream grouping at the substance level.
Selection of Drugs
Data collection from social media was performed for a pre-specified set of drugs, matching the reference sets used for the performance analysis (see Sect. 2.2). In total, 75 substances (or substance combinations) were included, originating either from the publicly available reference set by Harpaz et al. [10] or from the internally developed WEB-RADR reference set. Products contributing to the latter are presented in the Electronic Supplementary Material (ESM).
Social Media Foreground Data from Facebook and Twitter
Facebook and Twitter data were acquired and the resulting posts were processed as described in Sect. 2.1.1. For the Harpaz substances, 2,024,528 posts were collected with a post date between 1 March 2012 and 31 March 2015 (23% Facebook; 77% Twitter). The total number of posts for the WEB-RADR substances was 4,254,896 (35% Facebook; 65% Twitter), collected over the same period.
The number of Twitter/Facebook posts available for analysis of the Harpaz substances with at least one adverse event, and an indicator score of 0.4 or higher was 224,884, whereas there were 465,608 such posts available for the WEB-RADR substances, using the same indicator score threshold. Subsets of data were constructed by applying indicator score thresholds, as shown in Table 1.
Table 1.
Number of Twitter/Facebook (FB) posts for different indicator score thresholds
| Indicator score threshold | Posts (Twitter/FB) on Harpaz substances | Posts (Twitter/FB) on WEB-RADR substances |
|---|---|---|
| 0.4 | 224,884 | 465,608 |
| 0.5 | 128,199 | 274,554 |
| 0.6 | 39,461 | 98,677 |
| 0.7 | 19,120 | 46,121 |
| 0.8 | 10,028 | 22,785 |
| 0.9 | 5232 | 10,757 |
| 0.99 | 2130 | 3606 |
Social Media Foreground Data from Patient Fora
In addition to Twitter/Facebook, there are more focused social media channels of potential interest for pharmacovigilance. These patient fora are online communities where patients, family members and providers come together to discuss diseases and treatments, often limited to a very narrow disease area. For this study, patient fora were selected relevant to the WEB-RADR drugs (and associated indications) by the respective marketing authorisation holders participating in WEB-RADR. Patient forum data were investigated separately from Twitter/Facebook to assess its value for pharmacovigilance signal detection. The complete list of included patient fora is available in the ESM.
The procedure of data provision was the same as for Twitter/Facebook (see Sect. 2.1.3), with the difference that a single indicator score threshold of 0.7 was used. In addition, patient forum posts were only obtained for the WEB-RADR substances, and not for the Harpaz drugs. A total number of 42,721 posts on the 38 WEB-RADR substances from 407 patient fora covering the period 1 March 2012 to 31 March 2015 were collected.
Social Media Background Data
In addition to social media foreground posts, additional posts were collected to provide a broader dataset and more robust estimates of background posting rates. These background posts were collected using the same classifier as the foreground posts, without limitation to the product name, provided the post contained at least one product.
A total of 4,294,658 posts with indicator scores of 0.4 or above were collected, primarily from Twitter (3,056,043 posts, 64%) and Facebook (1,718,892, 36%), with a very small percentage of posts coming from patient fora and discussion groups (310 total). A total of about 1150 different drugs were included in the background data.
As with the foreground data, multiple sets of background posts were created using indicator score thresholds between 0.4 and 0.99.
For each analysis, the applicable foreground data were merged with the background data of the same indicator score threshold.
VigiBase Data
VigiBase, the World Health Organization (WHO) global database of ICSRs [11], was used as a comparator data source against which social media statistical signal detection performance was contrasted. VigiBase is an established repository of adverse event and suspected ADR reports with data from 135 countries. As of 11 March 2018, VigiBase contained 16,870,313 reports in total.
A core extraction of reports from the inception of VigiBase up to March 2015 was performed, although no reports from before March 2012 were used in comparative analyses with social media. Reports were taken from a frozen VigiBase version as of 16 October, 2015 containing 14,897,935 reports in total. All active reports were included except those where the submitting country was different from the country in which the event occurred, and each report was assigned a receipt date as the date of the most recent follow-up. No exclusion of reports was performed on the basis of type of report, type of reporter or other related criteria. Only suspect/interacting drugs were considered.
Aggregated Datasets
From the core datasets of social media posts and VigiBase reports described above, corresponding aggregated datasets were generated at the product-event combination (PEC) level. These aggregated data were subsequently used to compute disproportionality metrics from the different data sources. In the first instance, all combinations of the Harpaz drugs and the various medical concepts defined in the Harpaz reference set [10] were considered, as well as all combinations of the WEB-RADR drugs presented in the ESM and individual MedDRA® PTs. For each PEC in each considered data source, monthly cumulative counts were generated for the following: (1) number of posts/reports on the combination; (2) number of posts/reports on the drug; (3) number of posts/reports on the event; and (4) total number of posts/reports.
For social media, foreground and background posts were put together to form the equivalent of a traditional database of ICSRs, such as VigiBase. As mentioned above, for patient forum posts, a single indicator score threshold of 0.7 was used. For Twitter/Facebook posts, seven different indicator score thresholds between 0.4 and 0.99 were considered (see Table 1), each generating a different aggregate dataset. For brevity, these will be referred to as ‘Social 0.4’, ‘Social 0.5’, and so on.
For the PECs included in the Harpaz reference set (see Sect. 2.2.1), monthly cumulative counts were generated for the period March 2012 to March 2015, using February 2012 as the baseline. For the PECs derived from the WEB-RADR drugs in the ESM, cumulative counts were available from April 2012 to March 2015. For the latter set of PECs, one version of cumulative VigiBase counts used the start of VigiBase as the baseline, and another version used March 2012 as the baseline. Only the latter version was used when comparing social media and VigiBase; this was also the version used to determine which PECs qualified for inclusion into the WEB-RADR reference set according to the definitions of positive and negative controls in Sect. 2.2.2.
Reference Sets
Harpaz Reference Set
The publicly available reference set by Harpaz et al. is based on US Food and Drug Administration labelling changes performed during the year 2013 [10], which coincide temporally with the collected social media data.
The Harpaz reference contains 62 positive controls, i.e. labelling changes, on 55 drugs and 38 events. Each event is defined by a set of MedDRA® PTs, of which some are considered narrow and some broad with respect to the corresponding event. In this study, only narrow terms were included. Each positive control has an index date corresponding to the date of the labelling revision; for the purposes of this study, the month in which that date fell is used as the index date. The reference set also contains 75 negative controls generated by randomly pairing drugs and events occurring among the positive controls, and manually excluding those with a known, i.e. labelled, association between the drug and event.
WEB-RADR Reference Set
For various reasons, the Harpaz reference in isolation was deemed insufficient to reliably assess the value of signal detection in social media. First, the Harpaz reference set is limited in size. Second, its included label changes are severely restricted in geography and time. Finally, and most importantly, whereas labelling changes occur very late in the pharmacovigilance process, safety signals usually occur significantly earlier and are more relevant for protecting patient safety, regardless whether they will appear on a product label. The construction of a more relevant reference set therefore focused on the concept of the “validated safety signal”, i.e. a safety signal with some evidence suggestive of a causal drug/event relationship beyond statistical disproportionality. Additionally, there is intrinsic scientific value in using two different and independent reference sets. Therefore, a larger reference set was generated based on proprietary information on the products listed in the ESM. This WEB-RADR reference set contains 200 positive controls defined thus:
A PEC (on MedDRA® PT level) identified by the manufacturer as a validated signal for the first time in the period between 1 May 2012 and 31 March 2015, that had either (i) at least two posts in the Social 0.7 dataset, or (ii) at least two reports in the aggregated VigiBase dataset, by 31 March 2015, and whose adverse event term belonged to the set of 2,167 PTs included in the symptom taxonomy.
Each positive control was assigned an index date, defined as the month in which it reached the status of a validated signal. The specific indicator score threshold of 0.7 was chosen on account of being considered a default quality threshold [9].
Negative controls were defined correspondingly in the following manner:
A PEC (on MedDRA® PT level) not contained in any HLT linked to any positive control or any listed/labelled PT for the product, and that had either (i) at least two posts in the Social 0.7 dataset, or (ii) at least two reports in the aggregated VigiBase dataset, by 31 March 2015, and whose adverse event term belonged to the set of 2,167 PTs included in the symptom taxonomy.
Each participating manufacturer generated its own set of positive and negative controls for its included products, and each control was anonymised. All data extraction for this reference set was performed in a decentralised manner at the respective manufacturers, and forwarded in an anonymised form for aggregate central analysis. A flowchart describing in detail the construction of the WEB-RADR reference set is provided in the ESM.
Statistical Signal Detection in Social Media Data
Disproportionality analysis is the state-of-the-art statistical approach to support the detection of drug safety signals in spontaneous reports [12, 13]. It was therefore selected as the investigational method for evaluating the potential of statistical signal detection in social media data. Disproportionality analysis highlights pairs of drugs and adverse event terms (or groups of terms) with higher-than-expected reporting. Such reporting associations do not in themselves qualify as drug safety signals [13, 14], and are referred to here as signals of disproportionate reporting (SDRs).
Disproportionality Analysis Measures and Algorithms
Two common measures of disproportionality were considered in this study: the Proportional Reporting Ratio (PRR) [15], and the Information Component (IC) [16, 17]. Each measure can be applied as part of different signal detection algorithms, whose performance may vary [18]. This study considers four commonly used algorithms, one based on the IC and three based on the PRR:
IC025 > 0,
PRR > 2 and N ≥ 3,
PRR > 2 and N ≥ 3 and χ2 ≥ 4,
PRR025 > 1 and N ≥ 3,
where IC025 is the lower endpoint of a 95% credibility interval for the IC, χ2 is the (uncorrected) statistic of a χ2-test and PRR025 is the lower endpoint of a 95% confidence interval for PRR. These algorithms were applied to VigiBase and the various social media data sources retrospectively in monthly intervals. For social media data, the computations refer to numbers of posts rather than reports.
Performance Evaluations
Analyses at the Product-Event Combination Level
Statistical signal detection performance was evaluated in social media and VigiBase data by two methods: receiver operating characteristics (ROC) at fixed time points, and the time required to detect positive controls as SDRs. Additionally, the time to the first social media post was measured.
Receiver Operating Characteristics
Receiver operating characteristic curves display sensitivity and specificity at all possible thresholds of a classifier algorithm. In this study, sensitivity and specificity were computed for the four disproportionality algorithms in Twitter/Facebook data, forum post data and VigiBase data, using the Harpaz and WEB-RADR reference sets as benchmarks. In addition, the performance of the raw post/report count (denoted N) was tested. This is a useful reference point for disproportionality analysis, and may capture potential issues with the reference set [19].
For the Harpaz reference, data from 1 March 2012 and onwards were used. Positive controls were evaluated in the month prior to their respective index dates, i.e. just before they were labelled. Negative controls were evaluated in December 2013, which is the point in time when their lack of association was established.
For the WEB-RADR reference set, two main analyses were performed. The first included VigiBase data only, and served as a validation of the reference itself. Data were collected from the start of VigiBase to the month prior to the respective index dates of the positive controls, and to March 2015 for negative controls. The second analysis, in which social media data were compared to VigiBase data, was intended to be similar in design to the Harpaz analysis. However, this resulted in limited data collection periods for the positive controls, and consequently unreliable results (see the ESM for details). Instead, the full data collection period between April 2012 and March 2015 was used for all controls, which means that positive controls were evaluated after their index dates.
For the PRR algorithms presented in Sect. 2.3.1, ROC curves were generated on the basis of the PRR or PRR025 value, and any PEC not meeting the auxiliary conditions on N or χ2 was classified as negative. For some PECs, the PRR was mathematically undefined, and for some PECs with zero posts or reports, data were missing to compute both the PRR and the IC. All such cases were considered negative classifications.
The area under the ROC curve (AUC) is a common measure of overall predictive performance, which is also used in this study. An AUC of 0.5 corresponds to random classification.
Timeliness Analyses
All timeliness analyses concerned positive controls only. The time required to first detection as a SDR (if ever) was analysed for all data sources, using both reference sets. Data were collected for Twitter/Facebook (Social 0.4/0.7), forum posts and VigiBase from March 2012 for the Harpaz reference, and April 2012 for the WEB-RADR reference set. For each disproportionality algorithm, the month of discovery of each positive control was compared to its index date.
Further analyses were performed for the social media datasets using the WEB-RADR reference set only. First, the month of the first post (if any) of each signal was compared to its index date. Given the available study period (April 2012 to March 2015), this provides a conservative bound on the timing of the first potential warning in social media. Finally, it was investigated whether there were any signals whose first Twitter/Facebook post (within the study period) preceded the first spontaneous report in the corresponding manufacturer’s internal database, unrestricted in time.
Post-Level Content Analysis
In addition to aggregate analyses, an assessment of “posts-of-interest” was undertaken to further evaluate the potential value of social media for the identification of drug safety issues.
The primary aim was to quantify the strength of evidence in social media for positive controls actually detected using aggregate methods in social media. A secondary aim was to measure the quality of the information present. The assessors were selected from the respective marketing authorisation holders, and were pharmacovigilance personnel experienced in the assessment of ICSRs.
For 25 positive controls (i.e. signals) from the WEB-RADR reference set detected in Social 0.4 before their index date (using the IC025 > 0 algorithm), the full texts of the corresponding Twitter/Facebook posts were inspected by an expert from the company manufacturing that product.
- Each post was assessed using survey questions covering four areas:
- Does the post contain the correct drug and event?
- Is the event an actual adverse experience?
- Is there supporting information in the post?
- Does cumulative evidence exist across multiple posts?
In addition, a comparative analysis was performed by contrasting the results for low indicator score posts (0.4 ≤ indicator score < 0.7) to high indicator score posts (indicator score ≥ 0.7).
This analysis could be considered an assessment of precision and recall of the Epidemico algorithm on a small but independent test set.
Results
Overview of Reference Sets and Aggregated Datasets
Table 2 provides an overview of the various reference sets and their respective coverage in the considered datasets. Generally, the coverage in social media is low.
Table 2.
Overview information on the considered combinations of reference sets and datasets
| Reference set | Positive controls | Negative controls | Dataseta | Positive controls N ≥ 1b n (%) |
Positive controls N ≥ 3b n (%) |
Negative controls N ≥ 1b n (%) |
Negative controls N ≥ 3b n (%) |
|---|---|---|---|---|---|---|---|
| Harpaz | 62 | 75 | VigiBase | 41 (66) | 29 (47) | 36 (48) | 24 (32) |
| Social 0.4 | 13 (21) | 5 (8) | 17 (23) | 8 (11) | |||
| Social 0.5 | 8 (13) | 5 (8) | 8 (11) | 2 (3) | |||
| Social 0.6 | 3 (5) | 2 (3) | 2 (3) | 2 (3) | |||
| Social 0.7 | 3 (5) | 1 (2) | 2 (3) | 2 (3) | |||
| Social 0.8 | 3 (5) | 1 (2) | 2 (3) | 2 (3) | |||
| Social 0.9 | 3 (5) | 1 (2) | 2 (3) | 2 (3) | |||
| Social 0.99 | 3 (5) | 1 (2) | 2 (3) | 2 (3) | |||
| WEB-RADR | 200 | 5332 | VigiBase | 197 (98) | 180 (90) | 5072 (95) | 3853 (72) |
| Social 0.4 | 98 (49) | 75 (38) | 2527 (47) | 1879 (35) | |||
| Social 0.5 | 85 (42) | 56 (28) | 2294 (43) | 1653 (31) | |||
| Social 0.6 | 46 (23) | 26 (13) | 1461 (27) | 879 (16) | |||
| Social 0.7 | 42 (21) | 20 (10) | 1345 (25) | 772 (14) | |||
| Social 0.8 | 37 (18) | 19 (10) | 1267 (24) | 679 (13) | |||
| Social 0.9 | 35 (18) | 17 (8) | 1216 (23) | 624 (12) | |||
| Social 0.99 | 34 (17) | 14 (7) | 1176 (22) | 585 (11) | |||
| Forum posts | 61 (30) | 28 (14) | 1657 (31) | 886 (17) |
a‘Social 0.X’ means social media data from Twitter and Facebook, with a post-level threshold on the indicator score of 0.X. For forum posts, an indicator score threshold of 0.7 was used
bThese figures refer to the specific time points at which data were extracted for positive and negative controls for the purposes of receiver operating characteristic analysis
Table 3 shows the amount of data for each WEB-RADR substance in each of the different datasets. The variability both within and across datasets is considerable; in particular, there are many drugs with very few social media posts.
Table 3.
Number of WEB-RADR substance mentionings in Twitter/Facebook (FB) and patient fora; and the number of reports in VigiBase
| WEB-RADR substance(s) | No. of Twitter/FB postsa | % | No. of patient forum posts | % | No. of VigiBase reports | % |
|---|---|---|---|---|---|---|
| Methylphenidate | 13,248 | 28.0 | 11,178 | 19.8 | 24,042 | 3.6 |
| Topiramate | 5190 | 11.0 | 4036 | 7.2 | 15,889 | 2.4 |
| Diclofenac | 4310 | 9.1 | 1081 | 1.9 | 66,782 | 10.0 |
| Terbinafine | 3706 | 7.8 | 1152 | 2.0 | 19,983 | 3.0 |
| Levetiracetam | 2927 | 6.2 | 1372 | 2.4 | 14,597 | 2.2 |
| Vardenafil hydrochloride | 2753 | 5.8 | 6023 | 10.7 | 5692 | 0.85 |
| Propofol | 2268 | 4.8 | 435 | 0.77 | 14,694 | 2.2 |
| Carbamazepine | 1671 | 3.5 | 1191 | 2.1 | 47,209 | 7.1 |
| Insulin glargine | 1619 | 3.4 | 2752 | 4.9 | 26,830 | 4.0 |
| Baclofen | 1187 | 2.5 | 2740 | 4.9 | 15,667 | 2.4 |
| Zolpidem | 1152 | 2.4 | 2417 | 4.3 | 21,593 | 3.2 |
| Clomipramine | 950 | 2.0 | 844 | 1.5 | 8423 | 1.3 |
| Propranolol | 830 | 1.8 | 2184 | 3.9 | 13,987 | 2.1 |
| Zolmitriptan | 651 | 1.4 | 207 | 0.37 | 2581 | 0.39 |
| Tamoxifen | 597 | 1.3 | 3821 | 6.8 | 14,373 | 2.2 |
| Estradiol | 578 | 1.2 | 2084 | 3.7 | 25,924 | 3.9 |
| Clozapine | 450 | 0.95 | 485 | 0.86 | 91,511 | 13.7 |
| Ethinylestradiol, gestodene | 432 | 0.91 | 16 | 0.03 | 4300 | 0.65 |
| Filgrastim | 427 | 0.90 | 1366 | 2.4 | 7732 | 1.2 |
| Oxcarbazepine | 306 | 0.65 | 758 | 1.3 | 9412 | 1.4 |
| Fingolimod | 291 | 0.62 | 63 | 0.11 | 17,806 | 2.7 |
| Pegfilgrastim | 246 | 0.52 | 1063 | 1.9 | 7190 | 1.1 |
| Metoprolol tartrate | 236 | 0.50 | 574 | 1.0 | 26,900 | 4.0 |
| Clopidogrel | 178 | 0.38 | 838 | 1.5 | 36,138 | 5.4 |
| Atenolol | 168 | 0.36 | 936 | 1.7 | 23,272 | 3.5 |
| Budesonide | 154 | 0.33 | 756 | 1.3 | 13,245 | 2.0 |
| Interferon-β-1b | 151 | 0.32 | 26 | 0.05 | 16,139 | 2.4 |
| Letrozole | 140 | 0.30 | 4786 | 8.47 | 7891 | 1.2 |
| Dienogest | 126 | 0.27 | 73 | 0.13 | 279 | 0.04 |
| Omalizumab | 116 | 0.25 | 126 | 0.22 | 8469 | 1.3 |
| Denosumab | 63 | 0.13 | 829 | 1.5 | 16,954 | 2.5 |
| Teriflunomide | 63 | 0.13 | 20 | 0.04 | 2965 | 0.4 |
| Artemether, lumefantrine | 27 | 0.06 | 4 | 0.01 | 667 | 0.10 |
| Alemtuzumab | 23 | 0.05 | 23 | 0.04 | 3255 | 0.49 |
| Sorafenib | 23 | 0.05 | 35 | 0.06 | 13,703 | 2.1 |
| Romplostim | 20 | 0.04 | 51 | 0.09 | 5658 | 0.85 |
| Dronedarone | 16 | 0.03 | 36 | 0.06 | 4344 | 0.65 |
| Ranibizumab | 5 | 0.01 | 100 | 0.18 | 10,301 | 1.6 |
aAt an indicator score threshold of 0.7
Receiver Operating Characteristic Analyses
Harpaz Reference Set
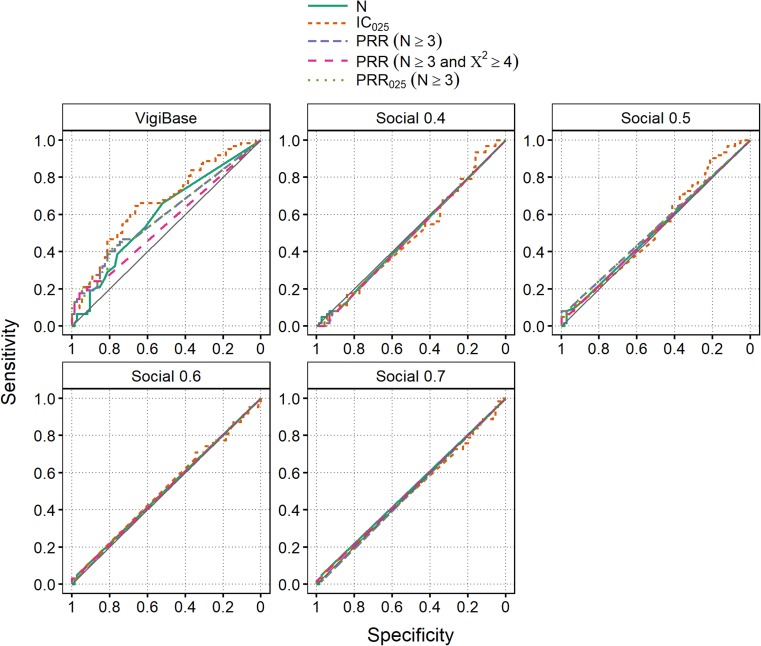
ROC curves for Twitter/Facebook and VigiBase for the Harpaz reference are shown in Fig. 1. The overall performance in Twitter/Facebook is poor, with all ROC curves close to the diagonal, i.e. near random classification. The AUC ranges between 0.48 and 0.53 across all algorithms and all indicator score thresholds. This is in concordance with the low figures displayed in Table 2. Performance in VigiBase is better: AUC ranges between 0.55 and 0.67, with IC025 attaining the maximum.
Fig. 1.
Receiver operating characteristic curves for the Harpaz reference set, using data from March 2012 up to the month prior to the index dates for positive controls, and up to December 2013 for negative controls. ‘Social 0.X’ means Twitter/Facebook data with a post-level indicator score threshold of 0.X. The area under the curve ranges between 0.55 and 0.67 in VigiBase, and is 0.53 or lower in Twitter/Facebook. The diagonal represents a random classifier. IC Information Component, PRR Proportional Reporting Ratio
WEB-RADR Reference Set
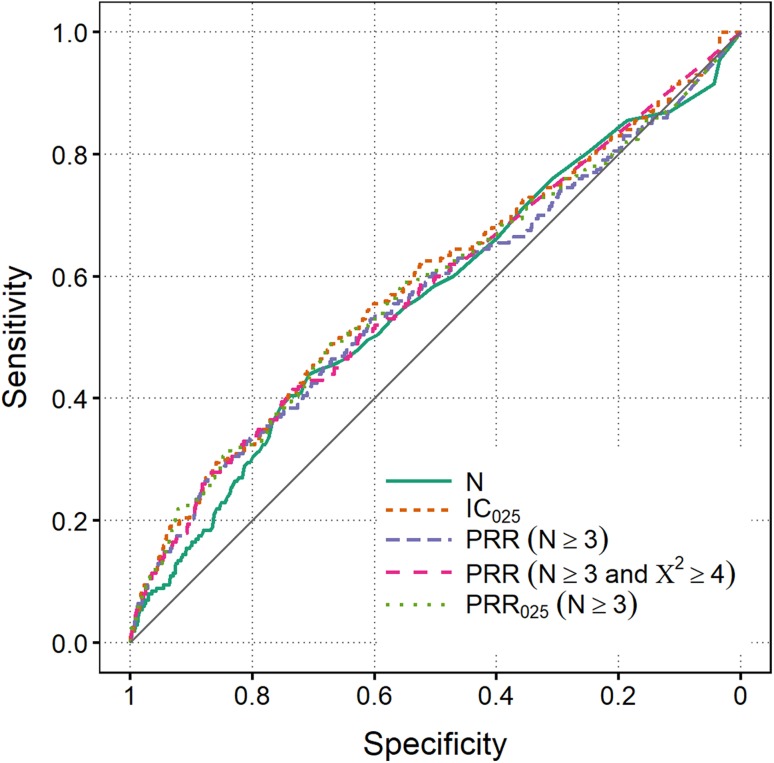
The predictive performance of disproportionality analysis for the WEB-RADR reference set in VigiBase, using all historically available data, is depicted in Fig. 2. Although performance is not very good, there is above-random discrimination between positive and negative controls. In a sensitivity analysis where only those positive controls later confirmed as ADRs were used (n = 70), the AUC for IC025 increased from 0.56 in Fig. 2 to 0.62. Here, a confirmed ADR was defined as “a safety signal where sufficient evidence exists to suspect a causal relationship between the signal and the drug and that may require a mitigation action”. This means that a positive control may only be classified as a confirmed ADR if the validated signal underwent a full evaluation of all available data by the company. In comparable settings, values as high as 0.74 have previously been observed [19], which suggests that the WEB-RADR reference is challenging. Nonetheless, because this reference yields above-random predictive ability in VigiBase even when evaluating positive controls prior to their index dates, it is considered a valid reference set for the purposes of this study.
Fig. 2.
Receiver operating characteristic curves for VigiBase based on the WEB-RADR reference set. All historical data are used, up to the month prior to the index dates for positive controls, and up to March 2015 for negative controls. Area under the curve values range between 0.56 and 0.59. The diagonal represents a random classifier. IC Information Component, PRR Proportional Reporting Ratio
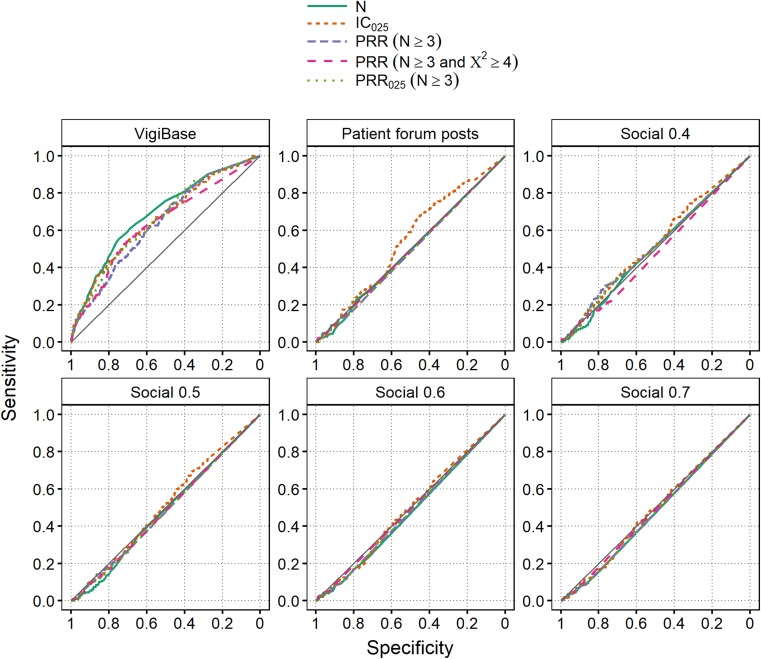
The results for the social media datasets and VigiBase when restricted to the period between April 2012 and March 2015, and evaluating all controls at the end of this period, are provided in Fig. 3. Although data are collected beyond the signalling dates of the positive controls, social media displays no predictive ability. Indeed, results for Twitter/Facebook are very similar to those observed for the Harpaz reference (see Fig. 1), with AUCs ranging between 0.47 and 0.53 across all algorithms and indicator score thresholds. For patient forum posts, there is a peculiar pattern for IC025 in the right side of the curve. However, this part of the curve corresponds to an algorithm of about IC025 > − 10 with a majority of true positives having zero posts; hence, there is no practical value in this finding. Results for VigiBase are generally good, and in particular better than in Fig. 2: AUC ranges between 0.64 and 0.69. This is expected based on the more extended data collection period, and emphasises the relative underperformance of social media data. Restricting the positive controls to confirmed ADRs only (see above) did not change the results.
Fig. 3.
Receiver operating characteristic curves for the WEB-RADR reference set, using data from April 2012 up to March 2015 for both positive and negative controls. ‘Social 0.X’ means Twitter/Facebook data with a post-level indicator score threshold of 0.X. The area under the curve ranges between 0.64 and 0.69 in VigiBase, and is 0.55 or lower in all social media datasets. The diagonal represents a random classifier. For the common algorithm IC025 > 0, sensitivity in VigiBase is 0.38 (at specificity 0.83). For patient forum posts, sensitivity is 0.14 (at specificity 0.88); and for Twitter/Facebook, sensitivity is 0.08 or lower. IC Information Component, PRR Proportional Reporting Ratio
Timeliness Analyses
Harpaz Reference Set
The time to SDR detection for positive controls of the Harpaz reference is summarised in Fig. 4, for Twitter/Facebook and VigiBase data. As expected from the ROC analysis, the results for social media are rather poor.
Fig. 4.
Time to signal of disproportionate reporting detection for the positive controls of the Harpaz reference set, relative to their respective index dates. Data were collected from March 2012 and onwards. ‘Social 0.X’ means Twitter/Facebook data with a post-level indicator score threshold of 0.X. IC Information Component, PRR Proportional Reporting Ratio
A single PEC, guanfacine/hallucinations, was detected in Social 0.7 prior to its index date. It was captured by all disproportionality algorithms in March 2013, 5 months prior to its labelling change (for reference, detection in VigiBase occurred in May 2012). In Social 0.4, only four (6%) of all positive controls were detected with any of the disproportionality algorithms prior to their index dates. The corresponding number in VigiBase was 17 (27%).
Comparing Twitter/Facebook and VigiBase head to head, no PEC was detected earlier in Social 0.7 than in VigiBase, with any disproportionality algorithm. In 31 cases, the opposite occurred. For Social 0.4, the corresponding numbers were 4 and 29 PECs, respectively.
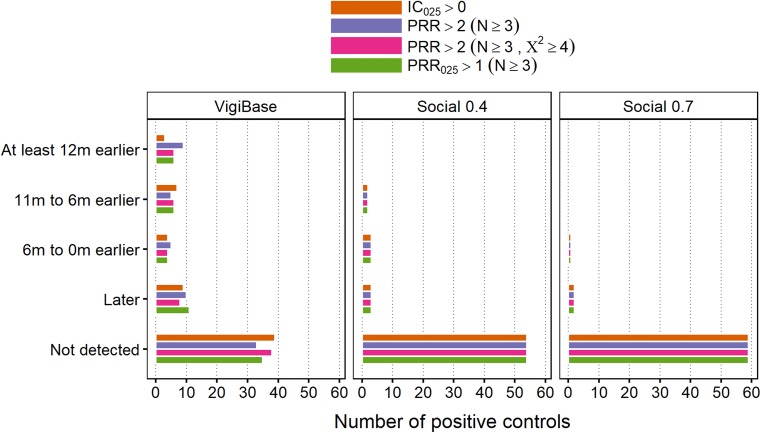
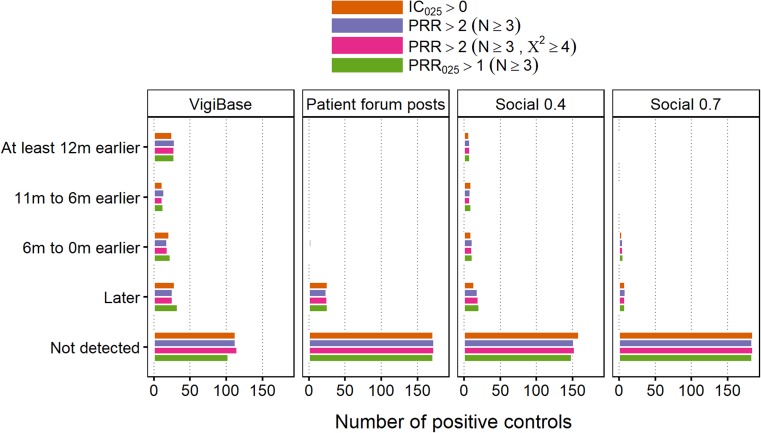
WEB-RADR Reference Set
Timeliness of SDR detection in Twitter/Facebook, patient fora and VigiBase are shown for the positive controls of the WEB-RADR reference set in Fig. 5. Performance in Twitter/Facebook relative to VigiBase is similar to that seen for the Harpaz reference. In patient forum posts, there are more PECs detected in total than in Social 0.7; however, detection appears to be more delayed.
Fig. 5.
Time to signal of disproportionate reporting detection for the positive controls in the WEB-RADR reference set, relative to their respective index dates. ‘Social 0.X’ means Twitter/Facebook data with a post-level indicator score threshold of 0.X. Forum posts were extracted with an indicator score threshold of 0.7. Data were collected from April 2012 and onwards. IC Information Component, PRR Proportional Reporting Ratio
In Social 0.7, there were in total five (3%) PECs detected strictly before their index dates, with any disproportionality algorithm. The corresponding numbers were 31 (16%) and 1 (0.5%) for Social 0.4 and patient forum posts, respectively, while in VigiBase there were 66 (33%) such PECs.
It should be noted that this analysis is biased against VigiBase because the definition of positive controls excludes PTs that were not considered in the social media extraction pipeline. Any such control might however appear in other data sources, including VigiBase.
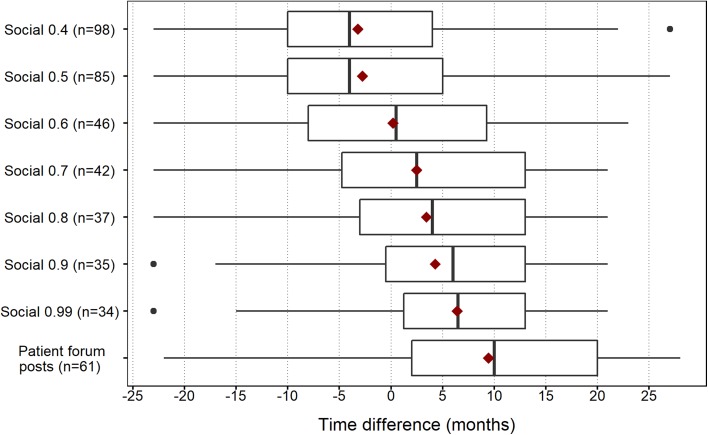
For the same positive controls, the distribution of time to occurrence of the first social media post is shown in Fig. 6. For Twitter/Facebook, the results clearly show that requiring higher quality posts (i.e. higher indicator score) implies later occurrence of the first post. This is expected, as the set of posts with a lower indicator score threshold also includes all posts with higher scores. Posting in the considered patient forums occurs generally later than in Twitter/Facebook, which agrees with the SDR timeliness analysis.
Fig. 6.
Distribution of time differences between the occurrence of the first post and the index date, for positive controls in the WEB-RADR reference set. Vertical bars indicate medians and diamonds indicate means. Note that only positive controls with at least one post have been included; the sample sizes are given for each dataset separately (with the total number of positive controls being 200). ‘Social 0.X’ means Twitter/Facebook data with a post-level indicator score threshold of 0.X. Forum posts were extracted with an indicator score threshold of 0.7. Data were collected from April 2012 and onwards
Comparing the occurrence of the first social media post (within the study period) to the manufacturers’ internal databases of spontaneous reports (unrestricted in time), two positive controls appeared earlier in social media. Both had indicator scores between 0.4 and 0.5, and are presumably of low quality. The time differences were small: 1.1 and 0.5 months, respectively. It is important to stress that this number is a lower limit because there might have been posts on other PECs prior to the start of our study period.
Post-Level Content Analysis
A total of 631 social media posts were inspected, corresponding to 25 positive controls from the WEB-RADR reference set detected as SDRs prior to their signalling date.
Individual Posts
The results of the content analysis of individual posts are presented in Table 4. These results demonstrate that, at least when using a low indicator score threshold, little information can be gleaned from the posts themselves that would aid in the medical triage process, i.e. to determine whether the disproportionality alert should be further considered as a potential safety issue.
Table 4.
Results of the content analysis of individual posts
| Question | Yes n (%) |
Strengthen n (%) |
Neutral n (%) |
Weaken n (%) |
|---|---|---|---|---|
| Does the post contain the correct drug? | 594 (94.1) | |||
| Does the post contain the correct medical adverse event? | 462 (73.2) | |||
| If the post contains the correct drug and medical event, is the medical event an actual adverse experience? | 250 (39.6) | |||
| Does the post relate the medical event to the drug of interest? | 199 (79.6)a | |||
| Is there evidence that the patient really took the drug? | 109 (43.6)a | |||
| Is there information on latency? | 24 (9.6)a | 8 (33.3) | 16 (66.7) | 0 (0) |
| Is there a description on the course of the adverse event? | 49 (19.6)a | 11 (22.4) | 36 (73.5) | 2 (4.1) |
| Is there any mention/discussion in the post on risk factors (including lifestyle, medical history, comorbidity, indication) and/or co-medication? | 33 (13.2)a | 3 (9.1) | 22 (66.7) | 8 (24.2) |
| Does the post contain patient characteristics: age, sex, weight, height? | 7 (2.8)a | 1 (14.3) | 6 (85.7) | 0 (0) |
| Is there any description as to whether/how the event affected the quality of life of the patient? | 29 (11.6)a |
aThe denominator for this question is the 250 posts containing the correct drug and medical event, and where the medical event was an actual adverse experience
Inspection of the posts revealed duplication among the retrieved Twitter/Facebook posts. For example, one PEC had five posts available that corresponded to only two unique Tweets. For another PEC, the same Tweet was duplicated five times. This reduces the information available for triage and highlights the question whether these PECs should have been identified as SDRs at all. The issue of duplication was not further investigated here, but merits more attention.
Post-Series Assessment (Cumulative Strength of Evidence)
For each of the 25 PECs, the entire series of posts was assessed for strength of evidence, and the results are presented in Table 5. For three positive controls, the inspected posts would have strengthened the signal: in two of the signals, some of the retrieved posts contained enough information for causality assessment (time to onset and outcome were present and associated the event with the drug); in the third signal, the large amount of identified posts (70) in itself was considered evidence strengthening. Of note, the posts with evidence for causality both had indicator scores > 0.7.
Table 5.
Results of the questions on cumulative strength of evidence in the assessment of individual posts
| Yes n (%) |
Strengthen n (%) |
Neutral n (%) |
Weaken n (%) |
|
|---|---|---|---|---|
| Consistency of pattern of symptoms | 4 (16) | 0 (0) | 24 (96) | 1 (4) |
| Consistency of time to onset | 2 (8) | 0 (0) | 25 (100) | 0 (0) |
| Identifiable subgroup at risk | 0 (0) | 0 (0) | 25 (100) | 0 (0) |
| Conclusion: would the series of posts (i.e. cumulative evidence) strengthen/neutralise/weaken the suspicion of a causal association? | 3 (12) | 21 (84) | 1 (4) |
Sub-Analysis by Indicator Score
The results of the post-level assessment stratified by indicator score are given in Table 6. Adverse events were detected with approximately the same accuracy (~ 70%) in low- and high-quality posts. However, posts with a low indicator score only contained an actual adverse experience 34% (178/524) of the time, whereas higher quality posts, while fewer in number, were much more trustworthy in that respect (72/107, i.e. 67% of these posts contained an actual adverse experience).
Table 6.
Difference in quality and content between posts with an indicator score < 0.7 (LT07) and those with an indicator score ≥ 0.7 (GE07)
| LT07 subset n/N (%) |
GE07 subset n/N (%) |
|
|---|---|---|
| Does the post contain the correct drug? | 488/524 (93.1) | 106/107 (99.1) |
| Does the post contain the correct medical adverse event? | 387/524 (73.9) | 75/107 (70.1) |
| If the post contains the correct drug and medical event, is the medical event an actual adverse experience? | 178/524 (34.0) | 72/107 (67.3) |
The proportion of true positive posts retrieved in high-quality posts compared to that retrieved in low-quality posts is 72/178 = 40%. These 178 true positive posts are a subset of all true positive posts (for the 25 PECs identified as signals), and this proportion of 40% may be considered an upper bound on recall (sensitivity) of the algorithm with an indicator score threshold of ≥ 0.7. These results highlight the trade-off between quality and sensitivity: there are many more posts with a lower indicator score than a high indicator score (5:1 ratio), but the average information content in the low-quality posts is much less than those of the higher quality posts. The recall is 2.5 times higher in the low-quality posts but the precision is half of the high-quality posts.
Discussion
This study investigated the potential usefulness of social media as a broad-based stand-alone data source for statistical signal detection in pharmacovigilance. Our results provide very little evidence in favour of social media in this respect: in neither of the two complementary reference sets, containing validated safety signals and label changes, respectively, did standard disproportionality analysis yield any predictive ability in a large dataset of combined Facebook and Twitter posts. In contrast, ICSR data from VigiBase collected during matching time periods performed well. Likewise, very rarely did the first post or the first occurrence of disproportionality precede the actual time point of signalling, whereas in VigiBase this was much more frequent. The same lack of predictive performance was seen in a non-exhaustive sample of posts from patient fora. Finally, manual assessment of Facebook and Twitter posts underlying 25 early signals of disproportionality showed that only 40% of posts contained the correct drug and the correct event as an adverse experience, and for only three of those 25 signals did the posts strengthen the belief in a causal association.
We have identified four main possible explanations for these results. First, for the majority of our included drugs, there seems to be low activity in the social media platforms we have studied. Indeed, the high number of drugs with very low post counts retrieved with the standard data collection pipeline we have employed is remarkable and suggests that there is limited value of social media as a general pharmacovigilance data source.
Second, automatic adverse event recognition in individual posts is difficult, and affects any downstream analysis. In our study, over 600 posts were assessed manually, with precision estimated at 40% for a post-level indicator score threshold of 0.4. One potential explanation for this low performance may be that the underlying classification algorithm is not optimised for the rare types of events that are of interest in signal detection. In addition, adverse-event recognition relies on symptom taxonomies that contain colloquial phrases used in social media posts. The quality of adverse event recognition relies partly on these taxonomies, and there is likely room for improvement. We acknowledge that the poor signal detection performance observed in social media may be owing partially to shortcomings of the underlying adverse event recognition algorithms. However, these algorithms are considered standard and are already in use within the pharmaceutical industry.
Third, the selection and design of reference sets has an obvious influence on the results. We used reference sets that matched our aim, which was to investigate general statistical signal detection. The positive results observed for VigiBase clearly suggest that these references were capable of identifying predictive performance. In fact, the WEB-RADR reference set was restricted to events that the underlying data extraction pipeline was able to identify, which would, if anything, introduce bias in favour of social media. At the same time, both our references contain positive controls populated within the existing pharmacovigilance system, which is largely driven by spontaneous reporting. Therefore, any truly novel signal present in social media would incorrectly appear as a false positive in our study. This issue could only be circumvented by conducting a prospective surveillance study in both data sources, which is laborious and difficult to scale, and was beyond our scope and resources.
Finally, our study was restricted to aggregate measures (i.e. disproportionality analysis and plain counting of reports or posts) developed for the purpose of analysing spontaneous reporting data. It is conceivable that other methods tailored to the analysis of social media data, or even other disproportionality algorithms, would have performed better. However, an argument against this possibility is the low amount of data found in social media in the first place.
The major strength of our study is the breadth and size of the two complementary reference sets employed, which also yielded very consistent results. In fact, the number and types of drugs covered in the two references is very broad and allows for generalisability of the conclusions. In addition, a major aspect of the work is the fact that we did not only use labelled events as positive controls, but also safety signals. As discussed earlier, the concept of a safety signal is more encompassing and relevant to pharmacovigilance than a labelling change. The labelling events of the Harpaz reference constitute an interesting case study, but are not truly representative of the actual day-to-day workings of continual detection and assessment of safety signals, many of which do not eventually appear on product labels, but are subject to further monitoring, e.g. in risk management plans. Last, the fact that statistical SDRs were complemented by an inspection of individual posts also solidifies the conclusions. In fact, manual inspection and assessment of the underlying content of an SDR should always be performed, if possible, when ascertaining the value of a new pharmacovigilance data source such as social media.
There are several limitations in the current study that need to be acknowledged. Most importantly, the period covered by the study is quite limited, with only 3 years’ worth of posts being analysed. For the WEB-RADR reference, this precluded our intended ROC analysis at the point of signalling, and forced us to use all available data beyond the signalling dates. Ideally, any follow-on work would use a longer data collection period generally, and particularly prior to the index dates of the positive controls. Another potential improvement would be to link together different posts from the same user, to capture adverse events that are not mentioned in the same post as the drug. Furthermore, we covered relatively few patient fora, and the number of posts retrieved was very small compared with Facebook and Twitter. Well-known patient discussion sites such as Patients Like Me and other subscription sites were not covered in this study. We did not make any distinction between different types of patient fora and placed all of them into one category, which may have resulted in dilution.
Most other work in this area has focused on identifying and optimising the recognition of single adverse events from social media [5, 7, 8, 20, 21], while relatively few papers focused on the actual assessment of utility of social media in providing evidence for ADRs relative to traditional data sources [6, 22]. Other studies do focus on the possible uses of social media above and beyond spontaneous reporting systems, but do not provide specific comparisons in performance [9]. The conclusions in this article point at the limited utility of social media (at least Twitter and Facebook) even as an additive source for strengthening an initial hypothesis, as the quality of most underlying posts is severely lacking. Other studies [4, 23] did establish that in areas of abuse, large volumes of discussion and new information are readily available in social media and provide a depth and richness of content usually not seen in spontaneous reporting systems. This is not inconsistent with the findings in our study, which did not attempt to single out specific areas of interest.
Our findings of low post counts and high proportions of falsely included posts suggest that improved adverse event recognition from social media posts is a priority area for future research, especially if improved algorithms are able to find and correctly identify adverse experiences across the MedDRA® spectrum. Further, although we used traditional methods for finding SDRs, there may be methods more suitable for social media, for example, methods that take into account the likelihood that a social media post does indeed contain an actual adverse medical event (as opposed to, for example, an indication). Clearly, however, this remains to be demonstrated, and is less of a priority until relevant posts can be retrieved with satisfying sensitivity and specificity. As highlighted above, there may be signals specific to social media that were not part of the Harpaz and WEB-RADR reference sets. This could be investigated through prospective monitoring of social media alongside traditional spontaneous data sources.
Nevertheless, it is important to point out that for a majority of drugs, there simply does not seem to be much activity in social media. Any future work should therefore focus on either specific drugs and/or specific areas of interest. Finally, whereas the goal of our work was to assess the utility of social media as a first-line signal detection tool across drugs and events, there are other potential applications of social media in pharmacovigilance that have not been explored here. Some examples include signal strengthening, signal validation and patients’ overall perception of benefit-risk balance. Closed patient fora designed around the views and experiences of individual patients might be especially suitable for such in-depth analyses. Even for signal strengthening or signal confirmation of signals detected in other sources, however, it is debatable whether social media (at least Twitter and Facebook) would add value based on our experience from inspecting 600 posts, which yielded very little confirmatory information. A potential issue in using individual posts is that companies, under current regulations, would need to report these to regulatory authorities, increasing the burden on the pharmacovigilance personnel.
Conclusions
Our study clearly suggests that general social media such as Facebook and Twitter are currently not worthwhile to employ for the purpose of broad-ranging statistical signal detection at the expense of other pharmacovigilance activities. Although future improvements to adverse event recognition in social media posts in terms of performance and coverage of events may revise this recommendation, social media is not expected to become a first-line signal detection system. It may, however, serve as a useful complement in specific niche areas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are indebted to the national centres who make up the World Health Organization Programme for International Drug Monitoring and contribute reports to VigiBase. However, the opinions and conclusions of this study are not necessarily those of the various centres nor of the World Health Organization. Further, the authors are indebted to the following colleagues, past or present, within the WEB-RADR consortium who provided technical support that enabled the research presented herein: Beatrice Bourdin, Michael Goodman, Rajesh Gosh, Zeshan Iqbal, Kristina Juhlin, Julia Lien, Carrie Pierce, Amy Purrington, Sue Rees and Harold Rodriguez.
Funding
The research leading to these results was conducted as part of the WEB-RADR consortium (http://webradr.eu), which is a public–private partnership coordinated by the Medicines and Healthcare products Regulatory Agency. The WEB-RADR project has received support from the Innovative Medicine Initiative Joint Undertaking (http://www.imi.europa.eu) under Grant Agreement No. 115632, resources of which are composed of financial contributions from the European Union’s Seventh Framework Programme (FP7/2007-2013) and the European Federation of Pharmaceutical Industries and Associations companies’ in-kind contribution.
Conflict of interest
Magnus Lerch provided scientific advice and support to Bayer Pharma AG within the WEB-RADR project and has received compensation for his work from Bayer AG. Antoni Wisniewski is a full-time paid employee of AstraZeneca and holds shares in AstraZeneca. Ola Caster, Juergen Dietrich, Marie-Laure Kürzinger, Simon Maskell, G. Niklas Norén, Stéphanie Tcherny-Lessenot, Benoit Vroman and John van Stekelenborg have no conflicts of interest that are directly relevant to the content of this study.
References
- 1.Zeng D, Chen H, Lusch R, Li SH. Social media analytics and intelligence. IEEE Intell Syst. 2010;25:13–16. doi: 10.1109/MIS.2010.151. [DOI] [Google Scholar]
- 2.Edwards IR, Lindquist M. Social media and networks in pharmacovigilance. Drug Saf. 2011;34:267–271. doi: 10.2165/11590720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Yang CC, Yang H, Jiang L, Zhang M. Social media mining for drug safety signal detection. Proceedings of the 2012 international workshop on smart health and wellbeing (SHB ‘12), Maui, HI, United States, 29 Oct-2 Nov 2012, p. 33–40. 10.1145/2389707.2389714.
- 4.Sarker A, O’Connor K, Ginn R, Scotch M, Smith K, Malone D, et al. Social media mining for toxicovigilance: automatic monitoring of prescription medication abuse from Twitter. Drug Saf. 2016;39:231–240. doi: 10.1007/s40264-015-0379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierce CE, Bouri K, Pamer C, Proestel S, Rodriguez HW, Van Le H, et al. Evaluation of Facebook and Twitter monitoring to detect safety signals for medical products: an analysis of recent FDA safety alerts. Drug Saf. 2017;40:317–331. doi: 10.1007/s40264-016-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya M, Snyder S, Malin M, Truffa MM, Marinic S, Engelmann R, et al. Using social media data in routine pharmacovigilance: a pilot study to identify safety signals and patient perspectives. Pharmaceut Med. 2017;31:167–174. [Google Scholar]
- 7.Freifeld CC, Brownstein JS, Menone CM, Bao W, Filice R, Kass-Hout T, et al. Digital drug safety surveillance: monitoring pharmaceutical products in Twitter. Drug Saf. 2014;37:343–350. doi: 10.1007/s40264-014-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocos A, Fiks AG, Masino AJ. Deep learning for pharmacovigilance: recurrent neural network architectures for labeling adverse drug reactions in Twitter posts. J Am Med Inform Assoc. 2017;24:813–821. doi: 10.1093/jamia/ocw180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell GE, Seifert HA, Reblin T, Burstein PJ, Blowers J, Menius JA, et al. Social media listening for routine post-marketing safety surveillance. Drug Saf. 2016;39:443–454. doi: 10.1007/s40264-015-0385-6. [DOI] [PubMed] [Google Scholar]
- 10.Harpaz R, Odgers D, Gaskin G, DuMouchel W, Winnenburg R, Bodenreider O, et al. A time-indexed reference standard of adverse drug reactions. Sci Data. 2014;1:140043. doi: 10.1038/sdata.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inform J. 2008;42:409–419. doi: 10.1177/009286150804200501. [DOI] [Google Scholar]
- 12.Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–436. doi: 10.1002/pds.1742. [DOI] [PubMed] [Google Scholar]
- 13.CIOMS Working Group XIII . Practical aspects of signal detection in pharmacovigilance. Geneva: CIOMS; 2010. [Google Scholar]
- 14.Delamothe T. Reporting adverse drug reactions. BMJ. 1992;304:465. [Google Scholar]
- 15.Evans SJW, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10:483–486. doi: 10.1002/pds.677. [DOI] [PubMed] [Google Scholar]
- 16.Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54:315–321. doi: 10.1007/s002280050466. [DOI] [PubMed] [Google Scholar]
- 17.Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22:57–69. doi: 10.1177/0962280211403604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candore G, Juhlin K, Manlik K, Thakrar B, Quarcoo N, Seabroke S, et al. Comparison of statistical signal detection methods within and across spontaneous reporting databases. Drug Saf. 2015;38:577–587. doi: 10.1007/s40264-015-0289-5. [DOI] [PubMed] [Google Scholar]
- 19.Norén GN, Caster O, Juhlin K, Lindquist M. Zoo or savannah? Choice of training ground for evidence-based pharmacovigilance. Drug Saf. 2014;37:655–659. doi: 10.1007/s40264-014-0198-z. [DOI] [PubMed] [Google Scholar]
- 20.Leaman R, Wojtulewicz L, Sullivan R, Skariah A, Yang J, Gonzalez G. Towards internet-age pharmacovigilance: extracting adverse drug reactions from user posts to health-related social networks. Proceedings of the 2010 workshop on biomedical natural language processing (BioNLP ‘10), Uppsala, Sweden, 15 July 2010, p. 117–25.
- 21.Bian J, Topaloglu U, Yu F. Towards large-scale twitter mining for drug-related adverse events. Proceedings of the 2012 international workshop on smart health and wellbeing (SHB ‘12), Maui, HI, United States, 29 Oct–2 Nov 2012, p. 25–32. 10.1145/2389707.2389713. [DOI] [PMC free article] [PubMed]
- 22.Kürzinger ML, Schuck S, Texier N, Adbellaoui R, Faviez C, Pouget J, et al. Web-based signal detection using medical forums data in France. J Med Internet Res. 2018 doi: 10.2196/10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson SL, Bell GH, Gilbert M, Davidson EJ, Winter C, Barratt JM, et al. Using social listening data to monitor misuse and nonmedical use of Bbpropion: a content analysis. JMIR Public Health Surveill. 2017;3:e6. doi: 10.2196/publichealth.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.