Abstract
Introduction
Understanding the occurrence of antiretroviral (ARV)-related adverse events (AEs) among patients receiving second-line antiretroviral therapy (ART) is important in preventing switches to more limited and expensive third-line regimens.
Objective
This study aimed to estimate the rates and examine predictors of AEs among adult HIV-1-infected patients receiving second-line ART in the Right to Care (RTC) clinical cohort in South Africa.
Methods
This was a cohort study of HIV-1-infected adult patients (≥ 18 years of age) initiating standard second-line ART in South Africa from 1 April 2004 to 10 January 2016. Our primary outcome was the development of an AE within 24 months of initiating second-line therapy. We used Kaplan–Meier survival analysis to determine AE incidence in the first 24 months of second-line ART. Predictors of AEs were modelled using a Cox proportional hazards model.
Results
A total of 7708 patients initiated second-line ART, with 44.5% developing at least one AE over the first 24 months of second-line treatment. The highest AE incidence was observed among patients receiving abacavir (ABC) + lamivudine (3TC) + ritonavir-boosted lopinavir/atazanavir (LPVr/ATVr) (52.7/100 person-years (PYs), 95% confidence interval (CI): 42.9–64.8), while patients initiated on a tenofovir (TDF) + emtricitabine (FTC)/3TC + LPVr regimen had the lowest rate of AEs (26.4/100 PYs, 95% CI: 24.9–28.3). Clinical predictors of AEs included experiencing AEs when receiving first-line ART (adjusted hazard ratio (aHR) 2.3, 95% CI: 1.9–2.8), lower CD4 cell count (0–199 vs. ≥ 350 cells/mm3; aHR 1.4, 95% CI: 1.4–1.8), and switching to second-line therapy from an ABC-base first-line regimen (ABC + 3TC + efavirenz/nevirapine [EFV/NVP] vs. TDF + 3TC/FTC + EFV/NVP; aHR 3.4, 95% CI: 1.1–11.1).
Conclusions
The rates of AEs were lowest among patients receiving a TDF-based second-line regimen. Patients with poorer health at the time of switch were at higher risk of AEs when receiving second-line ART and may require closer monitoring to improve the durability of second-line therapy.
Key Points
| Within 24 months of starting second-line therapy at a large HIV clinical cohort in South Africa, close to half of the patients (3429/7708, 44.5%) had experienced at least one second-line antiretroviral therapy-related adverse event (AE). |
| Rates of AEs among patients receiving second-line regimens such as TDF + FTC/3TC + LPVr are lower than that of patients receiving other regimens (3TC + ABC + LPVr/ATVr; AZT + ddI + LPVr; AZT + 3TC + LPVr/ATVr). |
| In resource-limited settings such as South Africa, where access to third-line therapy is not guaranteed, the early detection and effective management of AEs, including low-grade AEs, could improve patient outcomes in second-line therapy. |
Introduction
Over the past 2 decades, expanded access to antiretroviral therapy (ART), in combination with more tolerable regimens, has shifted the impact of human immunodeficiency virus (HIV) infection from causing a fatal disease to a treatable chronic condition. South Africa is currently home to an estimated 7.2 million HIV-1-infected persons and has responded to the epidemic by enrolling 56% (approximately 3,831,730) of HIV-1-infected patients receiving ART, by 2017 [1]. Furthermore, in 2017, approximately 38–45% of all HIV-1-infected persons receiving HIV treatment were virally suppressed [2, 3]. This wide gap between the number of HIV-1-infected patients and those who are suppressed when receiving ART, as well as the growing number of persons entering the HIV treatment cascade, will eventually increase the demand for second-line therapy [1, 3, 4]. Currently, an estimated 17–25% of first-line ART patients in South Africa are expected to experience virologic failure (as defined by the World Health Organization [WHO]) within 5 years [5–8]. Moreover, data from the Right to Care (RTC) clinical cohort, one of the largest ART cohorts in the country, indicate that > 60% of patients not responding to first-line ART are switched to second-line therapy [9–11]. Therefore, it is essential to continuously monitor and appropriately respond to treatment failure risks to keep the ART programme effective and sustainable.
While various studies have quantified and examined the impact of adverse drug reactions (ADRs) among patients receiving first-line therapy, very few have looked at patients receiving second-line ART. In a systematic review examining the efficacy of treatment among ART-naive HIV-infected patients in Rome (Italy), early cessation of first-line ART (within 48 weeks of treatment initiation) was estimated at 25%, with the most common reasons being by patients’ decision (11%), 8% due to ADRs (8%), and 4% due to virologic failure (4%) [12]. We recently demonstrated that South African patients with possible ADRs receiving second-line ART were more likely (approximately 30%) to experience treatment interruption within 2 years of second-line initiation [13]. While early treatment cessation has declined over the years, the removal of CD4 count eligibility criteria for starting ART will likely increase the number of asymptomatic individuals initiated on ART. These relatively healthy patients may be less tolerant of ART-associated ADRs, which may reverse the recent gains made in retention in HIV treatment. Although access to ART has significantly improved, patients in resource-limited settings (including South Africa) still have few HIV treatment options beyond second-line ART, hence the need to closely monitor ART-related ADRs among patients receiving second-line ART [13, 14]. However, the growth of the South African ART programmes was not followed by an equivalent growth in adverse event (AE) monitoring systems, and routine clinical data on ART AEs offers, at best, indicators of ART-associated ADRs.
This study aimed to use routine data to estimate the rate of, and examine predictors of, second-line ART-associated AEs (as a proxy for ADRs) in the first 24 months of second-line ART among adult HIV-1-infected patients in the RTC clinical cohort in South Africa.
Methods
Study Design, Site and Population
Using prospectively collected routine clinical data, we conducted a cohort study of adult (≥ 18 years of age at second-line ART initiation) HIV-1-infected patients who initiated standard first-line ART (Table 1) and subsequently permanently switched to standard second-line ART (triple combination ART, including a standard protease inhibitor [PI]) between 1 April 2004 and 10 January 2016 within the RTC clinical cohort in South Africa [9, 15–20]. The analytic dataset included data from the date of second-line ART initiation (baseline) to 24 months post second-line ART initiation.
Table 1.
Standard first- and second-line antiretroviral therapy regimens in South Africa
| Standard first-line regimens | Standard second-line regimens |
|---|---|
| TDF + 3TC/EFV + EFV/NVP | AZT/ABC-3TC-LPVr |
| AZT + 3TC + EFV/NVP | TDF-3TC/FTC-LPVr |
| d4T + 3TC + EFV/NVP | AZT/ABC-3TC-ATVr |
| ABC + 3TC + EFV/NVP | TDF-3TC/FTC-ATVr |
TDF tenofovir, 3TC lamivudine, EFV efavirenz, NVP nevirapine, AZT zidovudine, d4T stavudine, ABC abacavir, LPVr lopinavir/ritonavir, FTC emtricitabine, ATVr atazanavir/ritonavir
The RTC clinical cohort consists of 10 clinics (three non-governmental organisation [NGO]-run clinics, four public Community Health Centres (CHCs) and three ART clinics embedded in public hospitals) located in the Gauteng and Mpumalanga provinces in South Africa [9]. The clinics were established or expanded as part of the public sector scale-up of ART in the country and receive technical support from RTC, an NGO that is funded by the United States Agency for International Development (USAID). HIV care and treatment within the RTC clinical cohort follows South African National ART guidelines [15–20]. During the study period, CD4 threshold for ART eligibility criteria was revised from ≤ 200 cells/µL in the 2004 guidelines to ≤ 350 cells/µL in the 2013 guideline update, and ≤ 500 cells/µL in 2015. CD4 thresholds were removed from the 2016 HIV treatment policy [15–20]. Moreover, patients not responding to first-line ART (two consecutive viral loads ≥ 1000 copies/mL within a 3-month period, with intensified adherence counselling administered between viral loads) are eligible to switch to second-line therapy [19]. Additionally, in the absence of virologic failure, the decision to switch a patient to second-line therapy may also be made based on diagnosed AEs/resistance to first-line drugs.
Clinical data, including laboratory data from the National Health Laboratory Services (NHLS), from the ART clinics were captured on site and stored in an electronic patient management system, TherapyEdge-HIV™ [9].
Analytic Variables
Adverse Events (AEs) in the Initial 24 Months of Second-Line Antiretroviral Therapy (ART)
In the absence of reliable pharmacovigilance ADR data, we use second-line ART-related AEs as proxy measures. Therefore, the primary outcome was the first occurrence of any one of the following ART-related conditions during medical visits, up to 24 months after second-line ART initiation: dyslipidaemia, dermatitis/skin conditions neuropathy, diarrhoea/nausea/vomiting, gynaecomastia/breast conditions, hepatitis, lactic acidosis, decreased kidney function, anaemia, depression, and sleep disorders/insomnia. These conditions have previously been identified as possible drug reactions to both first- and second-line ARVs [21].
These conditions were ascertained by abstraction from clinical visit notes. However, in addition to clinical notes, anaemia episodes were also identified using haemoglobin (Hb) measurements defined as Hb < 13.0 g/dL in men and < 12.0 g/dL in women. Similarly, episodes of renal insufficiencies while receiving second-line ART were also identified using estimated glomerular filtration rate (eGFR) measures. Renal problems were defined as eGFR ≤ 59 mL/min/1.73 m2 for both males and females.
AEs were also identified up to 6 months before the switch to second-line ART to allow adjustments for unresolved AEs that occurred while receiving first-line ART. AEs occurring while receiving second-line ART were distinguished from those occurring while receiving first-line therapy by the respective start dates of each AE. In addition to the first occurrence of an AE, a measure of the frequency of AEs up to 24 months after the switch to second-line ART was determined.
Baseline Explanatory Variables
We defined baseline as the time of initiating second-line ART, and indicators were considered baseline if they were taken up to 3 months before the initiation of second-line ART. Baseline data included (1) demographic variables; (2) clinical and laboratory variables (e.g. WHO stage, body mass index [BMI], CD4 count and viral load); and (3) treatment variables (e.g. ART regimen, and treatment start and stop date). WHO staging information is generally interpreted as asymptomatic, mildly symptomatic, moderately symptomatic, and severely symptomatic. BMI (measured in kg/m2) was categorised as being underweight (BMI < 18.5), normal (18.5 ≤ BMI < 25), overweight (25 ≤ BMI < 30), and obese (BMI ≥ 30). Additionally, we included a variable summarising the CD4 response when receiving first-line ART, defined as the change in CD4 count from first-line ART initiation to the date of switching to second-line ART.
Follow-Up Time
Person-time accrued from the date of second-line ART initiation until the outcome of interest, completion of 24 months of second-line ART, or the last date seen at the clinic during the first year of second-line ART (for those who died, were lost to follow-up [LTFU], or transferred out).
Statistical Analysis
Data analysis was conducted using STATA version 14 (StataCorp LLC, College Station, TX, USA). Baseline demographic and clinical characteristics were presented using medians and interquartile ranges (IQRs) for continuous variables, while categorical variables were described using percentages. Kaplan–Meier survival analysis was used to determine the incidence of AEs in the first 24 months of second-line ART. Predictors of experiencing an AE (first reported AE within 24 months of second-line initiation) were modelled using complete case analysis with the use of a Cox proportional hazards model. Models were adjusted for baseline demographic and clinical characteristics. Additionally, we looked at the frequency of AEs by the initial second-line ART regimens.
Variables with a p value < 0.05 in crude analyses were entered in the multivariate model. Schoenfeld residuals were used to test the assumption of proportional hazards. Interaction terms with time-varying covariates were created for variables that violated the proportional hazards assumption. Variables were excluded from the multivariate model when the inclusion of the interaction term did not resolve the proportional hazards assumption violation, except for the initiating second-line regimen, in which case the model was stratified.
Results
Cohort Description and Baseline Characteristics
Table 2 presents the demographic and clinical characteristics of the study cohort at second-line ART initiation (N = 7708). Nearly two-thirds of the cohort were female (65.0%), and the median age at second-line initiation was 37.4 years (IQR 32.3–43.9). The median CD4 cell count at second-line ART initiation was 212 cells/µL (IQR 101–344), with 47.2% of patients switching to second-line therapy at a CD4 cell count of < 200 cells/µL. The majority of patients were switched to second-line ART with viral loads ≥ 1000 copies/mL (82.9%). Nearly half of all patients had a normal BMI (47.8%), while close to two-thirds of patients were at WHO stage I (62.8%) at the time of switching to second-line ART.
Table 2.
Demographic and clinical characteristics of patients initiating second-line antiretroviral therapy from 1 April 2004 to 10 January 2016
| Variables | Total [N = 7708] |
|---|---|
| Sex at second-line initiation | |
| Female | 5010 (65.0) |
| Male | 2698 (35.0) |
| Age at second-line initiation, years | |
| Median (IQR) | 37.4 (32.3–43.9) |
| 18–29 | 1220 (15.8) |
| 30–39 | 3489 (45.3) |
| ≥ 40 | 2999 (38.9) |
| CD4 cell count at second-line initiation, cells/mm3 | |
| Median (IQR) | 212 (101–344) |
| 0–199 | 1436 (47.2) |
| 200–349 | 864 (28.4) |
| ≥ 350 | 742 (24.4) |
| Viral load at second-line initiation, copies/mL | |
| Median (IQR) | 11,841 (2100–60,784) |
| < 1000 | 941 (17.0) |
| 1000–9999 | 1686 (30.5) |
| ≥ 10,000 | 2896 (52.4) |
| BMI at second-line initiation | |
| Underweight | 440 (7.8) |
| Normal | 2681 (47.8) |
| Overweight | 1530 (27.3) |
| Obese | 959 (17.1) |
| WHO stage at second-line initiation | |
| I | 2580 (62.8) |
| II | 582 (14.2) |
| III/IV | 944 (23.0) |
| Time on first-line ART before the switch, months | |
| Median (IQR) | 25.5 (13.8–44.8) |
| 0–12 | 1564 (20.3) |
| 12–24 | 2086 (27.1) |
| ≥ 24 | 4058 (52.7) |
| Regimen at second-line initiation | |
| 3TC + ABC + LPVr | 238 (3.1) |
| AZT + 3TC + LPVr/ATVr | 2686 (34.9) |
| AZT + ddI + LPVr | 1501 (19.5) |
| TDF + FTC/3TC + LPVr | 3283 (42.6) |
| Year of second-line initiation | |
| 2004–2009 | 1895 (24.6) |
| 2010–2012 | 3618 (46.9) |
| 2013–2016 | 2195 (28.5) |
| Non-ARV comedication at second-line initiation | |
| No | 4752 (61.7) |
| Yes | 2956 (38.4) |
| *Time (days) to first AE in the first 24 months of second-line ART | |
| Median (IQR) | 84 (28–237) |
| 0–90 | 1810 (52.8) |
| 91–180 | 547 (16.0) |
| ≥ 181 | 1072 (31.3) |
| Time (days) to first AE in the first 24 months of second-line ART by initial second-line regimen [medium (IQR)]* | |
| ABC + 3TC + LPVr/ATVr | 57 (5–155) |
| AZT + 3TC + LPVr/ATVr | 56 (26–181) |
| AZT + ddI + LPVr | 85 (28–224) |
| TDF + FTC/3TC + LPVr | 134 (28–308) |
Data are expressed as n (%) unless otherwise specified
IQR interquartile range, BMI body mass index, WHO World Health Organization, ART antiretroviral therapy, ARV antiretroviral, 3TC lamivudine, ABC abacavir, LPVr lopinavir/ritonavir, ATVr atazanavir/ritonavir, AZT zidovudine, ddI didanosine, TDF tenofovir, FTC emtricitabine, AE adverse event
At the time of second-line ART initiation, 52.7% of patients had been receiving first-line ART for 2 or more years. Overall, 42.6% of patients initiated second-line ART on a regimen of tenofovir (TDF) + emtricitabine (FTC)/lamivudine (3TC) + ritonavir-boosted lopinavir (LPVr), 34.9% were prescribed zidovudine (AZT) + 3TC + LPVr/ritonavir-boosted atazanavir (ATVr), and 19.5% were prescribed AZT + didanosine (ddI) + LPVr. Only 3.1% were switched to 3TC + abacavir (ABC) + LPVr.
AEs in the First 24 Months of Second-Line ART
Overall, 3429 (44.5%) patients experienced an AE in the first 24 months of second-line treatment (Table 3). The overall rate of AEs while receiving second-line ART was 36.7/100 person-years (PYs; 95% confidence interval [CI] 35.3–38.1). This was highest among patients receiving a regimen including ABC (52.7/100 PYs, 95% CI 42.9–64.8) or ddI (51.2/100 PYs, 95% CI 47.5–55.2), while patients switched to a TDF- (26.5/100 PYs, 95% CI 24.9–28.3) or AZT-based second-line regimen had the lowest AE rates (42.8/100 PYs, 95% CI 40.2–45.5). Table 3 presents the first AE experienced by the initial second-line ART regimen, up to 24 months of second-line ART.
Table 3.
Details of AEs, drug substitutions and treatment interruptions among patients with incident AEs up to 24 months of second-line initiation
| Initial second-line ART regimen | Total | ||||
|---|---|---|---|---|---|
| 3TC + ABC + LPVr/ATVr | AZT + 3TC + LPVr/ATVr | AZT + ddI + LPVr | TDF + FTC/3TC + LPVr | ||
| n (col %) | n (col %) | n (col %) | n (col %) | n (col %) | |
| [N = 117] | [N = 1298] | [N = 822] | [N = 1192] | [N = 3429] | |
| First AE in the observation period | |||||
| Anaemia | 80 (68.4) | 1015 (78.2) | 524 (63.6) | 711 (59.7) | 2330 (68.0) |
| Decreased kidney function | 28 (23.9) | 77 (5.9) | 8 (1.0) | 151 (12.7) | 264 (7.7) |
| Dyslipidaemia | 2 (1.7) | 68 (5.2) | 60 (7.3) | 115 (9.7) | 245 (7.1) |
| Gastrointestinal conditions | 2 (1.7) | 52 (4.0) | 83 (10.1) | 82 (6.9) | 219 (6.4) |
| Neuropathy | 2 (1.7) | 28 (2.2) | 44 (5.4) | 52 (4.4) | 126 (3.7) |
| Skin conditions | 3 (2.6) | 29 (2.2) | 49 (6.0) | 40 (3.4) | 121 (3.5) |
| Others | – | 29 (2.2) | 54 (6.6) | 41 (3.4) | 124 (3.6) |
| Frequency of AE episodes in the initial 24 months of second-line ART | |||||
| One | 39 (33.3) | 594 (45.8) | 342 (41.6) | 681 (57.1) | 1656 (48.3) |
| Two | 20 (17.1) | 335 (25.8) | 209 (25.4) | 264 (22.2) | 828 (24.2) |
| Three or more | 58 (49.6) | 369 (28.4) | 271 (33.0) | 247 (20.7) | 945 (27.6) |
| Drug substitutions on second-line ART (24 months) | |||||
| None | 177 (74.4) | 2097 (78.1) | 885 (59.0) | 2608 (79.4) | 5767 (74.8) |
| One | 38 (16.0) | 345 (12.8) | 446 (29.7) | 471 (14.4) | 1300 (16.9) |
| Two | 12 (5.0) | 171 (6.4) | 134 (8.9) | 154 (4.7) | 471 (6.1) |
| Three or more | 11 (4.6) | 70 (2.6) | 36 (2.4) | 50 (1.5) | 167 (2.2) |
| Treatment interruptions on second-line ART (24 months) | |||||
| None | 216 (90.8) | 2491 (92.7) | 1273 (84.8) | 3019 (92.0) | 6999 (90.8) |
| One | 19 (8.0) | 180 (6.7) | 205 (13.7) | 243 (7.4) | 647 (8.4) |
| Two | 2 (0.8) | 13 (0.5) | 22 (1.5) | 17 (0.5) | 54 (0.7) |
| Three or more | 1 (0.4) | 2 (0.1) | 1 (0.1) | 4 (0.1) | 8 (0.1) |
AEs adverse events, ART antiretroviral therapy, 3TC lamivudine, ABC abacavir, LPVr lopinavir/ritonavir, ATVr atazanavir/ritonavir, AZT zidovudine, ddl didanosine, TDF tenofovir, FTC emtricitabine
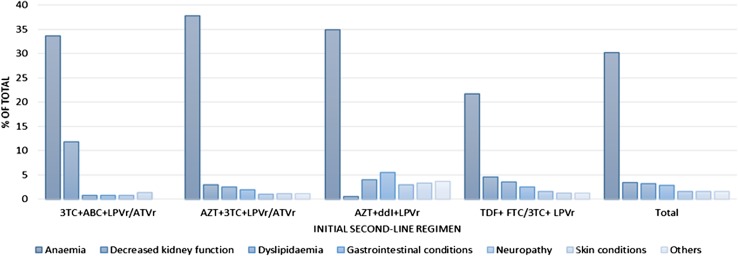
Anaemia was the most common first AE experienced, with 2389 first AE cases (31.0% prevalence and 68.0% of all AEs); 78.5% of these anaemia episodes were mild (24.0% overall), 16.9% were moderate (5% overall), and 4.6% were severe (1.0% overall) (Fig. 1). Nearly half (43.6%) of all first anaemia cases were among patients receiving AZT + 3TC + LPVr/ATVr, while the next most common AE was decreased kidney function (264 cases, or 3% overall), among which 90.9% were mild, 6.3% were moderate and 2.8% were severe cases. Kidney problems were less likely among patients receiving TDF + FTC/3TC + LPVr (5.0% overall) compared with patients receiving ABC + 3TC + LPVr/ATVr (12.0%). After anaemia (35.0% overall), patients receiving AZT + ddI + LPVr also experienced gastrointestinal conditions (6.0%) and dyslipidaemia (4.0%).
Fig. 1.
Proportion of incident adverse events in initial 24 months of second-line ART among HIV-1 infected patients in South Africa
Predictors of AEs in the First 24 Months of Second-Line ART
Table 4 presents the crude (hazard ratio [HR]) and adjusted HR (aHR) estimates of experiencing an AE in the first 24 months of second-line ART. In adjusted analyses, when compared with patients initiating second-line ART of TDF + FTC/3TC + LPVr, patients receiving AZT + ddI + LPVr (aHR 1.5, 95% CI 1.2–1.9) and AZT + 3TC + LPVr/ATVr (aHR 1.3, 95% CI 1.1–1.1) were more likely to experience AEs while receiving second-line ART.
Table 4.
Unadjusted and adjusted estimates of the relationship between demographic and clinical characteristics at second-line ART initiation with AEs by 24 months after second-line ART initiation
| Variable | Cox hazard regression modelling with total sample | Cox regression stratified by first second-line ART regimen | Logistic regression modelling risk of having two or more AEs in the first 24 months (patients with one or more AEs) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n (row%) | Rate/100PY (95% CI) | Crude HR | Adjusted HR | Adjusted HR TDF + FTC/3TC + LPVr |
Adjusted HR AZT + + 3TC + LPVr/ATVr |
Adjusted HR AZT + ddI + LPVr |
Crude odds ratio | Adjusted odds ratio |
|
| ADRs 6 months prior to second-line ART initiation | |||||||||
| No | 1989 (36.9) | 27.3 (26.0–28.7) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 1440 (62.1) | 71.2 (67.2–75.5) | 2.4 (2.2–2.6) | 2.3 (1.9–2.8) | 2.3 (1.7–3.2) | 2.7 (1. 9–3.9) | 2.3 (1.7–3.0) | 2.5 (2.1–2.8) | 2.3 (1.8–2.9) |
| Sex at second-line initiation | |||||||||
| Female | 2372 (47.4) | 41.3 (39.4–43.2) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Male | 1057 (39.2) | 29.4 (27.5–31.4) | 0.8 (0.7–0.8) | 0.6 (0.5–0.7) | 0.4 (0.3–0.7) | 0.7 (0.5–1.1) | 0.5 (0.4–0.7) | 0.8 (0.7–0.9) | 0.7 (0.5–0.9) |
| Age at second-line initiation, years | |||||||||
| 18–30 | 522 (42.8) | 37.9 (34.5–41.7) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 30–40 | 1517 (43.5) | 35.2 (33.3–37.3) | 1.0 (0.9–1.1) | 1.3 (1.0–1.6) | 1.0 (0.7–1.6) | 1.3 (0.7–2.2) | 1.4 (1.0–2.1) | 1.0 (0.8–1.2) | 1.1 (0.8–1.6) |
| ≥40 | 1390 (46.4) | 37.9 (35.7–40.2) | 1.0 (0.9–1.1) | 1.4 (1.0–1.8) | 1.1 (0.7–1.8) | 1.2 (0.7–2.1) | 1.5 (1.0–2.2) | 1.0 (0.9–1.3) | 1.1 (0.7–1.6) |
| CD4 cell count at second-line initiation, cells/mm3 | |||||||||
| 0–199 | 768 (53.5) | 55.7 (51.6–60.2) | 1.8 (1.5–2.1) | 1.4 (1.0–1.8) | 0.7 (0.4–1.3) | 1.8 (1.0–3.2) | 1.8 (1.1–3.0) | 2.1 (1.6–2.8) | 1.6 (1.0–2.4) |
| 200–349 | 403 (46.6) | 38.8 (34.9–43.2) | 1.3 (1.1–1.5) | 1.2 (0.9–1.5) | 0.8 (0.5–1.1) | 1.1 (0.7–1.8) | 1.6 (1.0–2.6) | 1.7 (1.3–2.3) | 1.6 (1.1–2.4) |
| ≥ 350 | 311 (41.9) | 29.5 (26.0–33.5) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| CD4 cell count change between first- and second-line ART initiation, cells/mm3 | |||||||||
| No change (± 50) | 395 (52.0) | 54.0 (48.5–60.1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Increase (50–100) | 157 (50.8) | 48.6 (41.0–57.5) | 0.9 (0.7–1.1) | 1.0 (0.7–1.3) | 1.6 (1.0–2.7) | 1.5 (0.8–2.7) | 0.6 (0.4–0.9) | 1.0 (0.7–1.4) | 1.1 (0.8–1.7) |
| Increase (≥ 100) | 519 (46.5) | 36.5 (33.2–40.2) | 0.7 (0.6–0.8) | 1.0 (0.8–1.2) | 0.8 (0.5–1.2) | 1.5 (0.8–2.6) | 0.9 (0.6–1.3) | 0.7 (0.5–0.9) | 0.9 (0.6–1.4) |
| Decrease (≥ − 50) | 90 (54.2) | 64.9 (51.9–81.1) | 1.2 (0.9–1.5) | 1.1 (0.8–1.7) | 2.5 (1.3–5.0) | 1.8 (0.9–3.9) | 0.6 (0.3–1.2) | 1.4 (0.9–2.2) | 1.5 (0.9–2.5) |
| BMI at second-line initiation, kg/m2 | |||||||||
| Underweight | 257 (58.4) | 62.1 (53.9–71.6) | 1.4 (1.2–1.7) | 1.1 (0.8–1.5) | 0.9 (0.5–1.6) | 1.4 (0.8–2.6) | 1.2 (0.7–2.1) | 1.2 (0.9–1.6) | – |
| Normal | 1265 (47.2) | 41.1 (38.7–43.7) | 1 | 1 | 1 | 1 | 1 | 1 | – |
| Overweight | 651 (42.6) | 33.9 (31.1–36.9) | 0.8 (0.8–0.9) | 0.8 (0.6–1.0) | 0.7 (0.4–1.0) | 0.9 (0.6–1.3) | 0.7 (0.5–1.0) | 1.0 (0.8–1.2) | – |
| Obese | 408 (42.5) | 33.3 (30.0–37.1) | 0.8 (0.7–0.9) | 0.8 (0.6–1.0) | 0.7 (0.5–1.2) | 0.6 (0.3–1.0) | 0.9 (0.6–1.3) | 0.9 (0.7–1.1) | – |
| WHO stage at second-line initiation | |||||||||
| I/II | 1455 (46.0) | 37.2 (35.1–39.4) | 1 | 1 | 1 | 1 | 1 | 1 | – |
| III/IV | 456 (48.3) | 42.4 (38.3–47.0) | 1.1 (1.0–1.3) | 0.9 (0.7–1.2) | 1.1 (0.7–1.6) | 0.9 (0.6–1.4) | 1.1 (0.9–1.3) | – | |
| Regimen at second-line initiation | |||||||||
| 3TC + ABC + LPVr/ATVr | 117 (49.2) | 52.7 (42.9–64.8) | 1.8 (1.5–2.3) | 1.0 (0.5–1.9) | – | – | – | 2.7 (1.8–4.0) | 1.4 (0.5–3.9) |
| AZT + 3TC + LPVr/ATVr | 1298 (48.3) | 42.8 (40.2–45.5) | 1.6 (1.4–1.7) | 1.3 (1.1–1.7) | – | – | – | 1.6 (1.4–1.9) | 1.7 (1.2–2.3) |
| AZT + ddI + LPVr | 822 (54.8) | 51.2 (47.5–55.2) | 1.9 (1.7–2.1) | 1.5 (1.2–1.9) | – | – | – | 1.9 (1.6–2.2) | 2.0 (1.4–2.8) |
| TDF + FTC/3TC + LPVr | 1192 (36.3) | 26.5 (24.9–28.3) | 1 | 1 | – | – | – | 1 | 1 |
| First-line regimen prior to switch | |||||||||
| ABC + 3TC + EFV/NVP | 34 (61.8) | 68.5 (46.6–100.6) | 1.6 (1.1–2.3) | 3.4 (1.1–11.1) | – | – | – | 2.5 (1.2–5.5) | 10.8 (1.2–94.0) |
| TDF + 3TC/FTC + EFV/NVP | 1358 (45.1) | 40.8 (38.5–43.2) | 1 | 1 | – | – | – | 1 | 1 |
| AZT + 3TC + EFV/NVP | 453 (41.6) | 31.2 (28.2–34.6) | 0.8 (0.7–0.9) | 0.9 (0.7–1.2) | – | – | – | 0.8 (0.6–1.0) | 1.0 (0.7–1.5) |
| d4T + 3TC + EFV/NVP | 1584 (44.6) | 34.9 (33.0–36.9) | 0.9 (0.8–1.0) | 1.0 (0.8–1.3) | – | – | – | 1.0 (0.9–1.2) | 1.1 (0.8–1.5) |
| Non-ARV comedication at second-line initiation | |||||||||
| No | 2009 (42.3%) | 33.6 (32.0–35.3) | 1 | 1 | 1 | 1 | 1 | 1 | – |
| Yes | 1420 (48.0%) | 41.8 (39.5–44.3) | 1.2 (1.1–1.3) | 1.0 (0.8–1.2) | 1.4 (1.0–2.0) | 0.9 (0.6–1.3) | 0.9 (0.7–1.2) | 1.1 (1.0–1.3) | – |
Global test before including interaction with time (0.00); cd4 cell count change (0.05); regimen at second-line initiation (0.00); first-line regimen prior to switch (0.01)
Global test after including interaction with time (0.25); cd4 cell count change (0.44); regimen at second-line initiation (0.03); first-line regimen prior to switch (0.05)
ART antiretroviral therapy, ADRs adverse drug reactions, AEs adverse events, HR hazard ratio, PYs person-years, CI confidence interval, BMI body mass index, WHO World Health Organization, 3TC lamivudine, ABC abacavir, LPVr lopinavir/ritonavir, ATVr atazanavir/ritonavir, AZT zidovudine, ddl didanosine, TDF tenofovir, FTC emtricitabine, EFV efavirenz, NVP nevirapine, TDF tenofovir, FTC emtricitabine, d4T stavudine, ARV antiretroviral
In the adjusted model including the total cohort, patients who had experienced an AE in the last 6 months of first-line ART were twice more likely to experience an AE in the first 24 months of second-line ART (aHR 2.3, 95% CI 1.9–2.8). Male patients had a 43% reduced risk compared with females (aHR 0.6, 95% CI 0.5–0.7), and patients who switched to second-line ART at 40 years of age or older (HR1.4, 95% CI 1.0–1.8) were more likely to experience AEs compared with those who were younger (18–30 years) at the switch.
Patients with a lower CD4 cell count at baseline were 38.0% more likely to experience AEs (0–199 vs. ≥ 350 cells/µL: aHR 1.4, 95% CI 1.1–1.8). Patients who were underweight at second-line ART initiation had a higher risk of AEs (underweight vs. normal-weight patients: aHR 1.4, 95% CI 1.2–1.7), whereas obese and overweight patients had a reduced likelihood of AEs (overweight vs. normal BMI: aHR 0.8, 95% CI 0.6–1.0; and obese vs. normal BMI: aHR 0.8, 95% CI 0.6–1.0).
In stratified models, similar results were observed for having a history of AEs before switching to second-line ART, sex, age (in patients receiving a ddI-based regimen), WHO stage, baseline CD4 count (in patients receiving a ddI-based regimen) and BMI. Among patients who were initially switched to TDF + FTC/3TC + LPVr, a CD4 decline after first-line ART initiation was predictive of AEs while receiving second-line ART (CD4 decrease ≥ 50 cells/µL vs. no CD4 change: aHR 2.5, 95% CI 1.3–5.0).
Predictors of Experiencing Two or More AEs Among Patients Who Had Incident AEs in the First 24 Months of Second-Line ART
In logistic regression analyses, predictors of having two or more AEs among patients who had incident AEs (Table 4) were having a history of AEs while receiving first-line ART (adjusted odds ratio [aOR] 2.3, 95% CI 1.8–2.9), having a baseline CD4 count < 199 cells/µL compared with those with CD4 ≥ 350 cells/µL (aOR 1.6, 95% CI 1.0–2.4), starting second-line ART with AZT + 3TC + LPVr/ATV (aOR 1.7, 95% CI 1.2–2.3) or AZT + ddI + LPVr (aOR 2.0, 95% CI 1.4–2.8) compared with those who started on TDF + FTC/3TC + LPVr. Additionally, although imprecise, the risk of multiple AE episodes was higher among patients who had switched from ABC + 3TC + efavirenz/nevirapine (EFV/NVP) [aOR 10.8, 95% CI 1.2–94.0] compared with patients who switched from TDF + 3TC/FTC + EFV/NVP. Compared with women, male patients were less likely to have had multiple AEs in the first 2 years of second-line ART (aOR 0.7, 95% CI 0.5–0.9).
Discussion
We aimed to determine the rates and examine clinical predictors of developing an ART-related AE in the first 24 months of second-line ART among HIV-1-infected adult patients within the RTC clinical cohort. Overall, 44.5% of patients experienced an AE in the 24 months of observation, across second-line ART regimens. This proportion is higher than the 19% observed in a Malawi cohort (12 months follow-up) [22] and 22.3% from a 2011 cross-sectional study (including children) in Uganda [23]. The variations of AE rates for patients receiving second-line ART highlight the inconsistent AE reporting practices and follow-up times across settings and studies [24].
Similar to other sub-Saharan African populations, we found that the rates of AEs were higher among patients receiving a second-line regimen that included AZT and ddI compared with a TDF-based regimen [25, 26]. The first AE was mainly anaemia, more so among patients who started second-line ART with an AZT-containing regimen. Despite the differing study methods, a cross-sectional study among patients receiving second-line ART in Malawi found similar anaemia levels, i.e. 33.2 versus 31.0% in our study [27].
The second most common first AEs were kidney problems, affecting 3% of the sample. While kidney problems were expected to occur mainly among patients receiving TDF, they were more frequent among patients who were switched to an ABC-based second-line regimen [21, 28]. This difference in the occurrence of kidney problems among patients switched to ABC for second-line ART possibly stems from TDF contraindications or pre-existing kidney problems at the time of the switch [29]. Patients who started second-line ART on an ABC-based regimen were also at considerably higher risk of frequent AEs in the first 2 years of second-line ART. Furthermore, patients who experienced AEs in the last 6 months of first-line ART were more susceptible to multiple AEs after the switch to second-line ART. Additional predictors of AEs among patients receiving second-line ART include low baseline BMI and CD4 count. The relationship between CD4 cell count and the development of AEs is unclear in recent literature, with both higher and lower CD4 cell counts having been associated with incident AEs [30, 31]. However, the results suggest that patients with poor health who switch to second-line ART may struggle to clear pre-existing conditions, are less able to cope with the challenge with new drugs, and require closer monitoring.
Demographic factors associated with an increased risk of AEs while receiving second-line ART were older age at second-line ART initiation (≥ 40 vs. 18–30 years) and being female. Similar to our findings, a gender difference in ART discontinuation and AE pattern has been previously observed [32], with women being more likely to develop peripheral neuropathy, particularly at low Hb levels [33–35].
Limitations
The interpretation of these results is limited to the context from which participants were drawn. The RTC cohort consisted of patients from health facilities in Gauteng and Mpumalanga province and may not be representative of patients across South Africa. Due to the lack of formal AE reporting systems in South Africa, AE rates may be underestimated [36–38], and more systematic and structured pharmacovigilance systems are needed to improve the accuracy of reported AEs. While rates of AEs were lower among patients receiving a TDF-based regimen, 36.3% over 2 years is not negligible. Further studies with more extended follow-up periods are needed to assess the longer-term implication of AEs and the potential fluidity in predictors of such events.
Conclusions
TDF in second-line ART is least associated with incident and frequent AEs in the first 24 months of second-line ART. However, patients with poor health indicators at switch, those with a history of AEs while receiving first-line ART, and those with TDF contraindications are at a considerably higher risk of incident AEs when receiving second-line ART, and need closer monitoring and support in order to thrive. Furthermore, the size of the ART programme in South Africa, coupled with high rates of comorbidities, such as tuberculosis, warrants active pharmacovigilance to obtain data on actual ADRs to better monitor the second-line ART programme.
Acknowledgements
The authors gratefully acknowledge the directors and staff of the study sites, as well as Right to Care, the Non-Governmental Organization supporting the study sites through a partnership of the South African National and Gauteng provincial Department of Health with the USAID. Most of all, the authors thank the patients attending the clinics for their continued trust in the treatment provided at the clinic.
Funding
This work was supported by the generous American people through the USAID under the terms of Cooperative Agreement 674-A-12-00020 to RTC; INROADS USAID-674-A-12-00029 to the Health Economics and Epidemiology Research Unit and to Boston University. The contents of this paper are the responsibility of the authors and do not necessarily reflect the views of the funding agencies or participating clinics or patients.
Author contributions
DO, KH and MPF conceptualised the analysis, analysed the data and drafted the manuscript. LCL, JM and LvdB assisted in the analysis and contributed to the interpretation of the results, as well as manuscript preparation.
Conflict of interest
Dorina Onoya, Kamban Hirasen, Liudmyla van den Berg, Jacqui Miot, Lawrence C. Long and Matthew P. Fox have no conflicts of interest that are directly relevant to the content of this study.
Ethical Approval
All data were fully anonymised for analyses. Ethics approval for the retrospective data review was obtained from the Human Research Ethics Committee of the University of Witwatersrand (M140201) and the Boston University Institutional Review Board (H-29768).
Patient Consent
Following Sect. 3 of the recommendations regarding the provisions for waiver or alteration of the informed consent requirements under the South African Department of Health and Human Services (HHS) Regulations at 45 CFR 46.116(d) and the Declaration of Helsinki, a waiver for individual patient consent was obtained.
References
- 1.National Department of Health (South Africa) National Department of Health annual report 2016/2017. Pretoria: National Department of Health; 2017. [Google Scholar]
- 2.UNAIDS. Global AIDS update 2017. UNAIDS; 2017.
- 3.Johnson LF, Dorrington RE, Moolla H. Progress towards the 2020 targets for HIV diagnosis and antiretroviral treatment in South Africa. S Afr J HIV Med. 2017;18(1):a694. doi: 10.4102/sajhivmed.v18i1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estill J, et al. The need for second-line antiretroviral therapy in adults in sub-Saharan Africa up to 2030: a mathematical modelling study. Lancet HIV. 2016;3(3):e132–e139. doi: 10.1016/S2352-3018(16)00016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. Aids. 2010;24(4):563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 6.Fox MP, Van Cutsem G, Giddy J, Maskew M, Keiser O, Prozesky H, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr. 2012;60(4):428. doi: 10.1097/QAI.0b013e3182557785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nglazi MD, Lawn SD, Kaplan R, Kranzer K, Orrell C, Wood R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr. 2011;56(1):e1. doi: 10.1097/QAI.0b013e3181ff0bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2. Geneva: WHO Press; 2016. [PubMed] [Google Scholar]
- 9.Fox MP, Maskew M, Brennan AT, Evans D, Onoya D, Malete G, et al. Cohort profile: the right to care clinical HIV cohort, South Africa. BMJ Open. 2017;7(6):e015620. doi: 10.1136/bmjopen-2016-015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox MP, Maskew M, MacPhail AP, Long L, Brennan AT, Westreich D, et al. Cohort profile: the Themba lethu clinical cohort, Johannesburg, South Africa. Int J Epidemiol. 2012;42(2):430–439. doi: 10.1093/ije/dys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanne IM, Westreich D, Macphail AP, Rubel D, Majuba P, Rie A. Long-term outcomes of antiretroviral therapy in a large HIV/AIDS care clinic in urban South Africa: a prospective cohort study. J Int AIDS Soc. 2009;12(1):38. doi: 10.1186/1758-2652-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prosperi MC, Fabbiani M, Fanti I, Zaccarelli M, Colafigli M, Mondi A, et al. Predictors of first-line antiretroviral therapy discontinuation due to drug-related adverse events in HIV-infected patients: a retrospective cohort study. BMC Infect Dis. 2012;12(1):296. doi: 10.1186/1471-2334-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onoya D, Brennan AT, Berhanu R, Berg L, Buthelezi T, Fox MP. Changes in second-line regimen durability and continuity of care in relation to national ART guideline changes in South Africa. J Int AIDS Soc. 2016;19(1):20675. doi: 10.7448/IAS.19.1.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onoya D, Nattey C, Budgell E, van den Berg L, Maskew M, Evans D, et al. Predicting the need for third-line antiretroviral therapy by identifying patients at high risk for failing second-line antiretroviral therapy in South Africa. AIDS Patient Care STDs. 2017;31(5):205–212. doi: 10.1089/apc.2016.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Department of Health (South Africa) National antiretroviral treatment guidelines 2010. Pretoria: National Department of Health; 2010. [Google Scholar]
- 16.Department of Health (South Africa) National antiretroviral treatment guidelines 2004. Pretoria: National Department of Health; 2004. [Google Scholar]
- 17.Department of Health (South Africa) Circular on new criteria for initiating adults on ART at CD4 count of 350 cells/ml and below 2011. Pretoria: National Department of Health; 2011. [Google Scholar]
- 18.Department of Health (South Africa) National antiretroviral treatment guidelines 2013. Pretoria: National Department of Health; 2013. [Google Scholar]
- 19.Department of Health (South Africa) National consolidated guidelines—for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults 2015. Pretoria: National Department of Health; 2015. [Google Scholar]
- 20.Department of Health (South Africa) Implementation of the universal test and treat strategy for HIV positive patients and differentiated care for stable patients. Pretoria: National Department of Health; 2016. [Google Scholar]
- 21.Orrell C. Antiretroviral adverse drug reactions and their management: how to recognise, manage and avoid adverse effects of antiretrovirals. Continuing Medical Education. 2011;29(6):234–237. [Google Scholar]
- 22.Hosseinipour M, Kumwenda J, Weigel R, Brown L, Mzinganjira D, Mhango B, et al. Second-line treatment in the Malawi antiretroviral programme: high early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med. 2010;11(8):510–518. doi: 10.1111/j.1468-1293.2010.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namukanja PMM. Adverse effects on second-line highly active antiretroviral therapy (HAART) among HIV infected adults and children treated at Mildmay Uganda. Pretoria: University of Limpopo (Medunsa Campus); 2011. http://ulspace.ul.ac.za/handle/10386/532.
- 24.Miller V (ed). ARV drugs, adverse events, case definition, grading, laboratory diagnosis and treatment monitoring. Presentations at the 2nd interest meeting, Feb 28–29 in Geneva, Switzerland; 2010. http://www.hivforum.org/tox-a-aes/59-arv-drugs-adverse-events-case-definition-grading-laboratory-diagnosis-and-treatment-monitoring.
- 25.Ciaffi L, Koulla-Shiro S, Sawadogo A, le Moing V, Eymard-Duvernay S, Izard S, et al. Efficacy and safety of three second-line antiretroviral regimens in HIV-infected patients in Africa. AIDS. 2015;29(12):1473. doi: 10.1097/QAD.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaulding A, Rutherford GW, Siegfried N. Tenofovir or zidovudine in three-drug combination therapy with one nucleoside reverse transcriptase inhibitor and one non-nucleoside reverse transcriptase inhibitor for initial treatment of HIV infection in antiretroviral-naïve individuals. Cochrane Database Syst Rev. 2010;10:008740. doi: 10.1002/14651858.CD008740. [DOI] [PubMed] [Google Scholar]
- 27.Ngongondo M, Rosenberg NE, Stanley CC, et al. Anemia in people on second line antiretroviral treatment in Lilongwe, Malawi: a cross-sectional study. BMC Infect Dis. 2018;18:39. doi: 10.1186/s12879-018-2952-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2006;43(3):278–283. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 29.Evans D, Maskew M, Heneger C, Sanne I. Estimated use of abacavir among adults and children enrolled in public sector antiretroviral therapy programmes in Gauteng Province, South Africa. South Afr J HIV Med. 2012;13(3):134–137. doi: 10.4102/sajhivmed.v13i3.126. [DOI] [Google Scholar]
- 30.Kesselring AM, Wit FW, Sabin CA, Lundgren JD, Gill MJ, Gatell JM, et al. Risk factors for treatment-limiting toxicities in patients starting nevirapine-containing antiretroviral therapy. Aids. 2009;23(13):1689–1699. doi: 10.1097/QAD.0b013e32832d3b54. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Moorman AC, Wood KC, et al. Initiation of antiretroviral therapy at CD4 cell counts ≥ 350 cells/mm3 does not increase incidence or risk of peripheral neuropathy, anemia, or renal insufficiency. J Acquir Immune Defic Syndr. 2008;47(1):27–35. doi: 10.1097/QAI.0b013e31815acacc. [DOI] [PubMed] [Google Scholar]
- 32.Kempf M-C, Pisu M, Dumcheva A, Westfall AO, Kilby JM, Saag MS. Gender differences in discontinuation of antiretroviral treatment regimens. J Acquir Immune Defic Syndr. 2009;52(3):336–341. doi: 10.1097/QAI.0b013e3181b628be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta SA, Ahmed A, Laverty M, Holzman RS, Valentine F, Sivapalasingam S. Sex differences in the incidence of peripheral neuropathy among kenyans initiating antiretroviral therapy. Clin Infect Dis. 2011;53(5):490–496. doi: 10.1093/cid/cir432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arenas-Pinto A, Thompson J, Musoro G, Musana H, Lugemwa A, Kambugu A, et al. Peripheral neuropathy in HIV patients in sub-Saharan Africa failing first-line therapy and the response to second-line ART in the EARNEST trial. J NeuroVirol. 2016;22(1):104–113. doi: 10.1007/s13365-015-0374-7. [DOI] [PubMed] [Google Scholar]
- 35.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38(10):1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 36.Agu KA, Isah MA, Oqua D, Habeeb MA, Agada PO, Ohiaeri SI, et al. Incidence of adverse drug reactions in patients on antiretroviral therapy: a study of pharmaceutical care in HIV interventions in Nigeria. West Afr J Pharm. 2013;24(1):30–42. [Google Scholar]
- 37.Mehta UC. Pharmacovigilance: the devastating consequences of not thinking about adverse drug reactions. CME. 2011;29(6):247–251. [Google Scholar]
- 38.Kiguba R, Karamagi C, Waako P, Ndagije HB, Bird SM. Recognition and reporting of suspected adverse drug reactions by surveyed healthcare professionals in Uganda: key determinants. BMJ Open. 2014;4(11):e005869. doi: 10.1136/bmjopen-2014-005869. [DOI] [PMC free article] [PubMed] [Google Scholar]