Abstract
Oral fostamatinib is an orally administered small molecule spleen tyrosine kinase (SYK) inhibitor approved for the treatment of adults with chronic immune thrombocytopenia (ITP) who have an inadequate response to a previous treatment. Fostamatinib has a unique mechanism of action, whereby its active metabolite targets the SYK-mediated pathway of platelet destruction. In clinical trials, fostamatinib provided durable responses in adults with chronic ITP who had not responded or had relapsed following treatment with one or more prior ITP therapies, including corticosteroids, thrombopoietin receptor agonists, rituximab, and/or splenectomy. Most patients who respond to fostamatinib maintain platelet counts of > 50 × 109/L for periods of ≥ 12 months. The most common adverse events reported with fostamatinib in clinical trials were diarrhea, hypertension, nausea, and increased transaminase levels.
Adis evaluation of fostamatinib in the treatment of adults with chronic immune thrombocytopenia (ITP)
| A small molecule spleen tyrosine kinase (SYK) inhibitor that inhibits platelet destruction via a distinct mode of action |
| Achieves durable responses in patients with inadequate responses to previous treatments for chronic ITP |
| Common adverse events include diarrhea, hypertension, nausea and increased transaminase levels |
| Patients should be monitored regularly throughout treatment for hypertension, hepatotoxicity, and neutropenia |
What is the rationale for developing fostamatinib to treat chronic ITP?
Immune thrombocytopenia (ITP) is a heterogeneous autoimmune disorder with a variable clinical course and treatment response [1]. The pathogenesis is not fully clarified, but both antibody-mediated and/or T cell-mediated platelet destruction are involved [2, 3]. Primary ITP is defined as thrombocytopenia (platelet count < 100 × 109/L) without any known cause (accounts for ≈ 80% of ITP cases), whereas secondary ITP can be attributed to a coexisting condition (e.g. systemic lupus erythematosus, antiphospholipid syndrome, Evan’s syndrome, chronic lymphocytic leukemia, autoimmune lymphoproliferative syndrome following bone-marrow or solid organ transplantation, hepatitis C, HIV infection, Helicobacter pylori infection, other miscellaneous systemic infections, common variable immune deficiency, post-transfusion purpura, myelodysplasia and other conditions, as well as being induced by drugs that lead to the production of autoantibodies or occurring post-vaccination) [3, 4]. When the disease has been present for > 1 year, it is termed chronic ITP [4]. The most common clinical manifestations of ITP are bleeding, purpura, and fatigue. Adult patients have an increased risk of morbidity and mortality, particularly when therapy does not control platelet counts. The aim of therapy is to maintain a hemostatic platelet count (generally considered to be at least 20–30 × 109/L) and minimize treatment toxicity [4].
Estimates of the incidence and prevalence of ITP in adults vary [4, 5]. Studies indicate an annual incidence of about 1.6–3.3 per 100,000 adults and a prevalence of 4.0–23.6 per 100,000 patient-years [4, 5].
Because of the multifactorial pathogenesis of ITP, current treatment options are diverse [1, 4]. First-line treatments are aimed at suppressing auto-antibody production and/or impeding platelet destruction, and include corticosteroids, intravenous immunoglobulin, and anti-D immunoglobulin [1, 4]. However, first-line treatments do not often lead to durable remissions, with subsequent treatments frequently being required. Although treating corticosteroid-refractory ITP with a thrombopoietin receptor agonist (TRA; e.g. romiplostim or eltrombopag) has shown promising results [1, 4], a substantial proportion of ITP patients remain refractory to treatment. As a result, additional therapeutic approaches are needed.
One such novel approach is fostamatinib (Tavalisse™), which is an orally bioavailable, small molecule spleen tyrosine kinase (SYK) inhibitor that is rapidly converted to the major active metabolite R406 in the gut [6, 7]. R406 potently inhibits signal transduction of Fc-activating receptors and B cell receptors, leading to reduced antibody-mediated destruction of platelets [6].
For whom is fostamatinib indicated?
In the USA, fostamantinib is approved to treat thrombocytopenia in adults with chronic ITP who have had an insufficient response to a previous treatment [8] (Table 1). Dosage adjustments are not needed in patients with renal or hepatic impairment, or those who are elderly (Table 1) [8]. According to population analyses, the pharmacokinetics of recommended dosages of fostamatinib are not altered to a clinically relevant extent by age, sex or race/ethnicity, nor are they altered to a clinically relevant extent in patients with renal or hepatic impairment [8, 9] (Table 1).
Table 1.
Summary of the US prescribing information of fostamatinib (Tavalisse™) in the treatment of thrombocytopenia in adults with chronic immune thrombocytopenia who have had an insufficient response to a previous treatment [8]
| How is fostamatinib available? | |
| 100 and 150 mg film-coated tablets to be taken with or without food | |
| How should fostamatinib be administered? | |
| Initial dosage | 100 mg twice daily for 4 weeks |
| Dosage adjustment | ↑ to 150 mg twice daily after 4 weeks if platelet count has not ↑ to ≥ 50 × 109/L |
| Maintenance dosage | Use the lowest dosage to achieve and maintain a platelet count of ≥ 50 × 109/L |
| Missed dose | Take the next dose at its scheduled time |
| Discontinuation after 12 weeks | Discontinue if platelet count does not ↑ to a level sufficient to avoid clinically important bleeding |
| Management of toxicity | Modify dosage (including ↓, interruption or discontinuation) based on tolerability |
| ↓ from 150 mg twice daily to 100 mg twice daily, then to 150 mg in the morning, then to 100 mg in the morning as needed; if further ↓ to < 100 mg/day are required, discontinue fostamatinib | |
| How should platelet counts be monitored? | |
| Prior to treatment | Obtain baseline assessments |
| During treatment | Perform monthly platelet count until a stable count of ≥ 50 × 109/L is achieved |
| Continue regular monitoring | |
| How should fostamatinib be used in special populations? | |
| Patients with renal or hepatic impairment, or who are elderly | No specific dosage recommendations (no alterations in fostamatinib pharmacokinetics) |
| Pregnant women | Should not be used due to potential for fetal harm (animal data) |
| Breastfeeding women | Breastfeeding is not advised during, and for ≥ 1 month after, treatment (potential for serious adverse reactions in the infant) |
| Women of childbearing potential | Advise the use of effective contraception during, and for ≥ 1 month after, treatment |
| What are the pharmacokinetic properties of R406 (the major metabolite of fostamatinib)? | |
| Absolute bioavailablity | 55% |
| Median time to Cmax | ≈ 1.5 h (range 1–4 h) |
| Plasma protein binding | 98.3% in vitro |
| Mean volume of distribution | 256 L at steady state |
| Terminal elimination half-life | ≈ 15 h |
| Excretion | 80% in the feces; ≈ 20% in the urine |
| What clinically relevant drug interactions may potentially occur with fostamatinib? | |
| Strong CYP3A4 inhibitor (e.g. ketoconazole) | Monitor for fostamatinib-related toxicities (exposure to R406 may ↑) |
| Strong CYP3A4 inducers (e.g. rifampicin) | Concomitant use not recommended (exposure to R406 may ↓) |
| Substrates of CYP3A4 (e.g. simvastatin), BCRP (e.g. rosuvastatin) and P-gp (e.g. digoxin) | Monitor for toxicities of the substrate (concentrations of the substrate may ↑) |
Cmax maximum plasma concentration, CYP cytochrome P450, P-gp P-glycoprotein, ↑ increase(d), ↓ decrease/reduction
What potential drug interactions are associated with fostamatinib?
R406 is metabolized primarily via cytochrome P450 (CYP) 3A4 oxidization and UDP glucuronosyltransferase (UGT) 1A9 glucuronidation. Therefore, concomitant use of fostamatinib with strong CYP3A4 inhibitors may increase exposure to R406 (monitoring for fostamatinib-related toxicities is recommended) and concomitant use with strong CYP3A4 inducers may decrease exposure to R406 (concomitant use is not recommended) [Table 1] [8].
When fostamatinib is co-administered with CYP3A4, BCRP, or P-glycoprotein substrates, exposure to the agent acting as a substrate may increase, and patients should be monitored for substrate-related toxicities (Table 1).
What is the efficacy of fostamatinib in chronic ITP?
Phase 3 trials
The efficacy of oral fostamatinib in the treatment of adults with chronic ITP was demonstrated in two randomized, double-blind, phase 3 trials (pooled results are reported) [10]. Patients were considered refractory to ITP treatment having received a median of three unique prior therapies (range 1–13), including corticosteroids, splenectomy, TRAs, and/or rituximab. At study entry, the median duration of disease was 8.7 years (range 0.3–53) in fostamatinib recipients and 7.8 years (range 0.4–45) in placebo recipients, and mean platelet counts in the respective groups were 16.1 × 109/L (range 1–51) and 19.8 × 109/L (range 1–156) [10]. Patients were randomized to fostamatinib 100 mg twice daily or placebo for 24 weeks, with an option to increase the fostamatinib dosage from 100 mg twice daily to 150 mg twice daily after 4 weeks depending on platelet counts (88% of patients increased their fostamatinib dosage at or after week 4) [10].
Response rates and platelet counts
A stable response by week 24 (primary endpoint; see Table 2 for definition) was achieved in significantly more patients receiving fostamatinib than receiving placebo [10]. Similarly, the proportion of patients achieving an overall response was significantly higher with fostamatinib than placebo, according to a post hoc analysis (Table 2). Among patients achieving a stable response, 15 of 18 (83%) demonstrated a response at 5 of 6 clinic visits, and 14 of 18 (77%) at all 6 clinic visits between weeks 14 and 24. The median time to achieve a platelet count of ≥ 50 × 109/L among stable and overall responders was 15.5 and 15 days, respectively [10].
Table 2.
Efficacy of oral fostamatinib in patients with chronic immune thrombocytopenia in the pooled analysis of two randomized, double-blind, 24-week phase 3 trials [10]
| Outcome | Total study population | FOS subgroups based on overall response to FOS | ||
|---|---|---|---|---|
| FOS (n = 101) | PL (n = 49) | With an overall response (n = 43) | Without an overall response (n = 58) | |
| Stable responsea (% of pts) | 18* | 2 | ||
| Overall responseb (% of pts) | 43* | 14 | ||
| Bleeding-related serious AE (% of pts) | 10 | 0 | 7 | |
| Bleeding-related moderate-to-severe AE (% of pts) | 16 | 9 | 10 | |
| Use of rescue medication (% of pts) | 45 | 16 | 34 | |
AE adverse event, FOS fostamatinib, PL placebo, pts patients
*p < 0.01 vs PL
aPrimary endpoint; defined as platelet counts ≥ 50 × 109/L on at least 4 of 6 clinic visits that occurred every 2 weeks during weeks 14–24, without requiring rescue therapy after week 10
bDefined as ≥ 1 platelet count ≥ 50 × 109/L during weeks 0–12; determined in a post hoc analysis
Over the 24 weeks of the trial, median platelet counts in stable and overall responders to fostamatinib were 95 and 52 × 109/L, respectively [10]. In both the stable and overall fostamatinib responders (evaluable n = 15–18 and 20–41 at each time point, respectively), median platelet counts increased to > 50 × 109/L by week 2, and remained > 50 × 109/L for all subsequent 2-week timepoints up to week 24, with the exception of week 4, where there was a slight decrease. In contrast, in the placebo group, median platelet counts were < 50 × 109/L from week 2 to 12 (n = 32–43 at each timepoint) [10], after which most placebo non-responders enrolled in the extension study [11].
Among patients with severe thrombocytopenia at baseline (i.e. platelet counts < 15 × 109/L), an increase in platelet counts of ≥ 20 × 109/L to ≥ 30 × 109/L at weeks 12 and 24 was achieved by 21 and 15% of 47 fostamatinib recipients, respectively, compared with 5 and 0% of 21 placebo recipients [10].
A response to fostamatinib therapy occurred in all subgroups regardless of age, sex, prior therapy (splenectomy, rituximab, or TRA), baseline platelet count (≤ 15 × vs > 15 × 109/L), or duration of ITP at study entry [10]. A numerically higher response to fostamatinib was achieved in younger patients (aged < 65 years) than in older patients, and in those with platelet counts of 15–30 × 109/L than in those with counts of < 15 × 109/L.
Bleeding-related events and rescue medication use
None of the patients with an overall response to fostamatinib experienced a bleeding-related serious adverse event compared with 10% of placebo recipients, and 7% of patients without an overall response to fostamatinib (Table 2) [10]. Relative to both the placebo group and the subgroup without an overall response to fostamantinib, numerically fewer fostamatinib recipients with an overall response experienced a moderate or severe bleeding-related adverse event or received rescue therapy (e.g. corticosteroids, platelet transfusion) [Table 2] [10].
Long-term extension study
One hundred and twenty-three patients in the phase 3 trials received fostamatinib in an open-label, long-term extension [11]. Most patients who did not respond to fostamatinib, and most placebo recipients, entered the extension at week 12 of the phase 3 trials. A stable response to fostamatinib was defined as a platelet count ≥ 50 × 109/L at 4 of 6 evaluations during weeks 14–24 of the phase 3 trials, or ≥ 50 × 109/L within 12 weeks of starting therapy without the need for rescue therapy and ≥ 50 × 109/L at two of three subsequent monthly evaluations during the extension study [11].
Most patients who achieved a stable response to fostamatinib were able to maintain a stable response for ≥ 12 months, and up to > 24 months in some individuals [11]. Among 117 patients who received fostamatinib for ≥ 12 months, 17 of 25 (68%) maintained a stable response, with a median platelet count of 111 and 115 × 109/L after 12 and 52 weeks of treatment, respectively (median platelet count prior to fostamatinib was 23 × 109/L) [11]. Although the Kaplan–Meier median duration of a stable response for the 17 patients who maintained a response has not yet been reached, it is estimated to be > 28 months (range 12 to > 28 months) [11]. Bleeding-related serious adverse events occurred in 9% of patients, with all but one of these events occurring in patients without a stable response.
Among patients who received placebo during the phase 3 studies and then switched to fostamatinib, 10 of 44 (23%) achieved a stable response compared with one (2%) patient during the placebo phase (p < 0.01) [11].
What is the tolerability profile of fostamatinib?
In the 24-week phase 3 trials in adults with chronic ITP, treatment-emergent adverse events (TEAEs) were reported in 83 and 75% of patients in the fostamatinib (100 or 150 mg twice daily) and placebo groups, respectively (pooled data), with the majority of these being mild (39 and 56% of TEAEs in the fostamatinib and placebo groups, respectively) or moderate (42 vs 25%) in severity [10]. Figure 1 shows the most commonly reported categories of TEAEs and their severity [10].
Fig. 1.
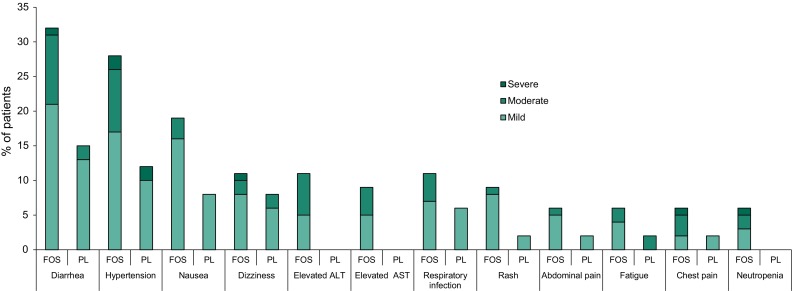
Treatment-emergent adverse events reported in > 5% of 102 fostamatinib (FOS) recipients and at a numerically higher incidence than in 48 placebo (PL) recipients in two pooled phase 3 trials in patients with chronic immune thrombocytopenia [10]. Diarrhea includes diarrhea and frequent bowel movement; hypertension includes hypertension, blood pressure (BP) increased, diastolic BP (DPB) abnormal, and DBP increased; respiratory infection includes respiratory tract infection (RTI), upper RTI, lower RTI, and viral upper RTI; rash includes rash, rash erythematous and rash macular; abdominal pain includes abdominal pain, and abdominal pain upper; neutropenia includes neutropenia and neutrophil count decreased
In the pooled fostamatinib and placebo groups, TEAEs led to discontinuation in generally similar proportions of patients (10 vs 8%), although the proportions requiring dose reductions (9 vs 2%) or interruptions (18 vs 10%) resulting from an adverse event were numerically higher in the fostamatinib group [10]. The most common TEAEs (incidence of ≥ 2%) that led to fostamatinib dose reductions or interruptions were hypertension, diarrhea, increased ALT levels, and influenza-like illness. Three severe TEAEs led to treatment withdrawal in fostamatinib recipients, including one case each of non-serious chest pain and syncope, pneumonia, and thrombocytopenia. No patients who achieved a stable response, and three patients who achieved an overall response, discontinued fostamatinib therapy due to a TEAE [10].
Serious TEAEs were reported in 13 and 21% of patients in the fostamatinib and placebo groups, respectively; however, the serious events were consider to be related to treatment in only 4 and 2% of patients in the respective groups [10].
In the long-term extension study, 75% of 123 patients experienced at least one TEAE, and 52% of patients had at least one treatment-related adverse event [11]. The most frequent TEAEs were diarrhea (28% of patients), hypertension (15%), petechia (15%) and epistaxis (14%). Most TEAEs were mild to moderate in severity, with 22% of patients experiencing a serious TEAE event. A TEAE led to treatment withdrawal in 15 (12%) patients, including diarrhea in five patients, liver enzyme elevations in three, and neutropenia in two (all other events were reported in a single patient).
Adverse effects of special interest
Fostamatinib is associated with increases in blood pressure (BP; Fig. 1) [8]; therefore, BP should be regularly monitored in all patients, and hypertension managed based on the PI recommendations (Table 3) [8]. Increases in BP are thought to result from the inhibitory effect of fostamatinib on vascular endothelial growth factor receptor signaling leading to reduced nitric oxide release from the endothelium [12]. After administration of fostamatinib 100 mg twice daily for 28 days, mean placebo-adjusted increases in systolic and diastolic BP were 2.93 and 3.53 mmHg, respectively [8]. Blood pressures ≥ 140/90 mmHg were recorded in 31 and 15% of patients in the fostamatinib and placebo groups, respectively. Among 19 fostamatinib recipients with BP ≥ 140/90 mmHg, BP returned to baseline levels within one week of discontinuing fostamatinib in 11 (58%) patients.
Table 3.
Monitoring and management of hypertension, hepatotoxicity, neutropenia, and diarrhea associated with fostamatinib [8]
| How should BP be monitored and managed? | ||
| Monitoring | Assess at baseline; monitor every 2 weeks until a stable dosage is established, then monthly | |
| Stage 1 hypertension (SBP 130–139 or DBP 80–89 mmHg) | Initiate or ↑ dosage of antihypertensive in patients with ↑cardiovascular risk; adjust as needed until BP is controlled | |
| BP target is not met after 8 weeks: ↓ fostamatinib dosage | ||
| Stage 2 hypertension (SBP ≥ 140 or DBP ≥ 90 mmHg) | Initiate or ↑ dosage of antihypertensive; adjust as needed until BP is controlled | |
| BP remains ≥ 140/90 mmHg for > 8 weeks: ↓ fostamatinib dosage | ||
| BP remains ≥ 160/100 mmHg for > 4 weeks despite aggressive antihypertensive therapy: interrupt or discontinue fostamatinib | ||
| Hypertensive crisis (SBP > 180 and/or DBP > 120 mmHg) | ||
| How should hepatotoxicity be monitored and managed? | ||
| Monitoring | Perform LFTs, including ALT, AST and BL,at baseline, then monthly during treatment | |
| AST/ALT ≥ 3 × ULN and < 5 × ULN | Symptomatic patients (e.g. nausea, vomiting, abdominal pain): interrupt fostamatinib; check LFTs every 72 h until ALT/AST < 1.5 × ULN and total BL < 2 × ULN; resume fostamatinib at next lower daily dose | |
| Asymptomatic patients: check LFTs every 72 h until ALT/AST < 1.5 × ULN and total BL < 2 × ULN; consider dose interruption (or ↓) if ALT/AST remains 3–5 × ULN and total BL remains < 2 × ULN; resume fostamatinib at next lower daily dose when ALT/AST no longer ↑ (< 1.5 × ULN) and total BL remains < 2 × ULN | ||
| AST/ALT ≥ 5 × ULN and total BL < 2 × ULN | Interrupt fostamatinib; check LFTs every 72 h until AST/ALT no longer ↑ (< 1.5 × ULN) and total BL remains < 2 × ULN; resume fostamatinib at next lower daily dose | |
| Discontinue fostamatinib if AST/ALT remain ≥ 5 × ULN for ≥ 2 weeks | ||
| AST/ALT ≥ 3 × ULN and total BL > 2 × ULN | Discontinue fostamatinib | |
| ↑ Unconjugated (indirect) BL in absence of other LFT abnormalities | Continue fostamatinib with frequent monitoring (isolated ↑in unconjugated BL may be due to UGT1A1 inhibition) | |
| How should neutropenia be monitored and managed? | ||
| Monitoring | Perform complete blood counts, including neutrophils, at baseline, and regularly during treatment | |
| ANC < 1.0 × 109/L and remains low after 72 h | Interrupt fostamatinib | |
| ANC > 1.5 × 109/L | Resume fostamatinib at next lower daily dosage | |
| How should diarrhea be managed? | ||
| Onset of symptoms | Provide supportive measures (e.g. dietary changes, hydration and/or antidiarrheal medication) until symptoms resolve | |
| Severe (≥ grade 3) | Interrupt fostamatinib; resume treatment at next lower daily dosage if diarrhea improves to mild (grade 1) | |
ANC absolute neutrophil count, BP blood pressure, BL bilirubin, DBP diastolic BP, LFT liver function test, SBP systolic BP, UGT glucuronosyltransferase, ULN upper limit of normal, ↑ increase(d)/elevated, ↓ decrease
Fostamatinib is also associated with an increased risk of hepatotoxicity (Fig. 1). Liver function tests should be performed regularly, and PI recommendations for the management of hepatotoxicity followed (Table 3). As R406 acts as an inhibitor of UGT1A1, increases in unconjugated bilirubin may occur in the absence of other liver function test abnormalities [8].
Neutropenia and diarrhea have also been associated with fostamatinib (Fig. 1). Complete blood counts, including neutrophil counts, should be performed regularly, and PI recommendations for the management of neutropenia and diarrhea should be followed (Table 3) [8].
What conclusions can be made regarding the use of fostamatinib in chronic ITP?
Oral fostamatinib is an option for the treatment of chronic ITP in adults who have had an insufficient response to a previous treatment. In clinical trials, fostamatinib provided durable responses in adults with chronic ITP who had not responded or relapsed following treatment with one or more prior ITP therapies, including corticosteroids, TRAs, rituximab, and/or splenectomy [10, 11]. Unlike other ITP therapies, fostamatinib has a unique mechanism of action, targeting the SYK-mediated pathway of platelet destruction [6]. Most patients who respond to fostamatinib maintain platelet counts of > 50 × 109/L for periods of ≥ 12 months [11]. The most common adverse events reported with fostamatinib in clinical trials were diarrhea, hypertension, nausea, and increased transaminase levels [10]; monitoring is required throughout treatment because of the risk of hypertension, hepatotoxicity, and neutropenia (Table 3) [8]. Further clinical trial data should help establish if administering fostamatinib earlier in the course of the disease could improve response rates, as well as the comparative efficacy of fostamatinib versus other ITP therapies [10]. Identifying the patient populations who are most likely to respond to fostamatinib may also potentially improve treatment outcomes [10].
Acknowledgements
The manuscript was reviewed by: W. Homenda, Wojewódzki Szpital Specjalistyczny im. J. Korczaka i Akademia Pomorska w Słupsku, Slupsk, Poland; A. A. Khalafallah, Menzies Institute for Medical Research and Faculty of Health Sciences, University of Tasmania, Launceston, TAS, Australia. During the peer review process, Rigel Pharmaceuticals Inc., the marketing-authorization holder of fostamatinib, was also offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
K. McKeage and K.A. Lyseng-Williamson are employees of Adis/Springer, are responsible for the article content and declare no conflicts of interest.
Footnotes
The original version of this article was revised due to a retrospective Open Access request.
Change history
11/16/2018
The article Fostamatinib in chronic immune thrombocytopenia: a profile of its use in the USA, was originally published Online First without open access. After publication in volume 34, issue 10, pages 451–456, Rigel Pharmaceuticals, Inc. requested that the article be Open Choice to make the article an open access publication. Post-publication open access was funded by Rigel Pharmaceuticals, Inc. The article is forthwith distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License.
References
- 1.Niscola P, Scaramucci L, Giovannini M. Spleen tyrosine kinase inhibition: a new promising approach to chronic and refractory immune thrombocytopenia. Immunotherapy. 2018;10(1):5–7. doi: 10.2217/imt-2017-0141. [DOI] [PubMed] [Google Scholar]
- 2.Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP) J Clin Med. 2017;6(2):16. doi: 10.3390/jcm6020016. [DOI] [Google Scholar]
- 3.Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin N Am. 2013;27(3):495–520. doi: 10.1016/j.hoc.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terrell DR, Beebe LA, Vesely SK, et al. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174–180. doi: 10.1002/ajh.21616. [DOI] [PubMed] [Google Scholar]
- 6.Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319(3):998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 7.Baluom M, Grossbard EB, Mant T, et al. Pharmacokinetics of fostamatinib, a spleen tyrosine kinase (SYK) inhibitor, in healthy human subjects following single and multiple oral dosing in three phase I studies. Br J Clin Pharmacol. 2013;76(1):78–88. doi: 10.1111/bcp.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tavalisse™ (fostamatinib disodium hexahydrate): US prescribing information. South San Francisco: Rigel Pharmaceuticals Inc. 2018.
- 9.Martin P, Oliver S, Gillen M, et al. Pharmacokinetic properties of fostamatinib in patients with renal or hepatic impairment: results from 2 phase I clinical studies. Clin Ther. 2015;37(12):2823–2836. doi: 10.1016/j.clinthera.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921–930. doi: 10.1002/ajh.25125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bussel JB, Arnold DM, Cooper N, et al. Long-term maintenance of platelet responses in adult patients with persistent/chronic immune thrombocytopenia treated with fostamatinib: 1-year efficacy and safety results [abstract] Blood. 2017;130(Suppl 1):16. [Google Scholar]
- 12.Skinner M, Philp K, Lengel D, et al. The contribution of VEGF signalling to fostamatinib-induced blood pressure elevation. Br J Pharmacol. 2014;171(9):2308–2320. doi: 10.1111/bph.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]


