Abstract
Burosumab (Crysvita®), a fully human IgG1 monoclonal antibody directed at fibroblast growth factor 23 (FGF23), is indicated for the treatment of X-linked hypophosphatemia (XLH), a condition associated with excessive FGF23 production. It directly addresses the excessive FGF23 activity in patients with XLH by binding to FGF23, and inhibiting its signaling. This leads to increased gastrointestinal phosphate absorption and renal phosphate reabsorption, thereby improving serum phosphate levels, and, ultimately, bone mineralization and the risk of bone disease. In clinical trials, subcutaneous burosumab increased serum phosphorus levels in pediatric and adult patients with XLH, as well as significantly improving the severity of rickets in children, and improving pain, stiffness, physical functioning, and fracture/pseudofracture healing in adults. Burosumab is well tolerated by children and adults with XLH, with most treatment-emergent adverse events being of mild to moderate severity.
Adis evaluation of burosumab in the treatment of X-linked hypophosphatemia (XLH)
| Novel monoclonal antibody that directly addresses the excess FGF23 activity in patients with XLH |
| Increases serum phosphate levels to within the normal range in children and adults |
| Improves the severity of rickets and other XLH-related outcomes in children with XLH |
| Improves XLH-related symptoms in adults with XLH |
| Well tolerated, with most adverse events being manageable without intervention |
What is the rationale for using burosumab in X-linked hypophosphatemia (XLH)?
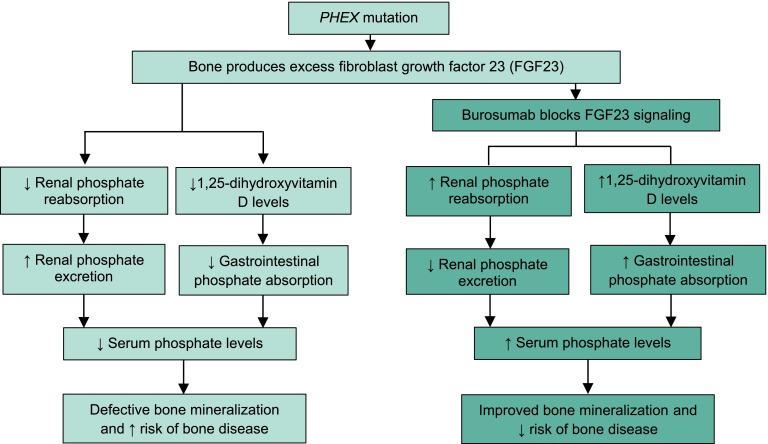
X-linked hypophosphatemia (XLH), which results from mutations in the phosphate-regulating endopeptidase homolog (PHEX) gene, is the most common form of hereditary rickets [1–3]. These mutations cause excessive circulating levels of fibroblast growth factor 23 (FGF23) and, thereby, excessive FGF23 activity. This, in turn, reduces renal tubular reabsorption and increases renal phosphate excretion, resulting in low serum phosphate levels and, ultimately, impaired bone mineralization and bone disease (Fig. 1). In addition, FGF23 reduces renal 1α-hydroxylase activity, thereby lowering serum 1,25-dihydroxyvitamin D [1,25(OH)2D] levels, reducing the gastrointestinal (GI) absorption of phosphate, which also reduces serum phosphate levels and impairs bone mineralization (Fig. 1) [1–3]. Poor bone mineralization may lead to limb deformity, short stature, osteoarthritis, insufficiency fractures, and other poor outcomes [4].
Fig. 1.
Simplified pathophysiology of X-linked hypophosphatemia (lighter colored boxes) and mechanism of action of burosumab in its treatment (darker colored boxes) [3, 5]
Although conventional treatment with calcitriol and phosphate supplements can improve bone mineralization in patients with XLH, their use does not address the underlying deficiencies in renal phosphate reabsorption and 1,25(OH)2D production caused by excessive FGF23 activity [1–4].
Burosumab-twza (Crysvita®; hereafter burosumab), a fully human recombinant IgG1 monoclonal antibody, has been developed to address these defects by binding directly to FGF23. By binding to FGF23, burosumab inhibits FGF23 signaling, thereby increasing tubular phosphate reabsorption and decreasing renal phosphate excretion, as well as increasing serum levels of 1,25(OH)2D and increasing GI absorption of phosphate (Fig. 1) [3, 5]. As a result, serum phosphate levels increase, and, ultimately, bone mineralization is improved and the risk of done disease is decreased (Fig. 1) [3, 5]. This article provides an overview of the current evidence related to the approved use of burosumab in the clinical-practice setting.
For whom is burosumab indicated?
Burosumab, administered subcutaneously, is indicated for the treatment of XLH in many countries worldwide including the USA and those in the EU. In the USA, it is approved to treat XLH in adults and children aged ≥ 1 years [5]; in the EU, burosumab is indicated for the treatment of XLH with radiographic evidence of bone disease in XLH in children 1 year of age and older and adolescents with growing skeletons [6].
Table 1 provides a summary of the prescribing information for burosumab in the USA [5]. Fasting serum phosphorus levels should be assessed prior to treatment initiation and monitored regularly during treatment. The dosage of burosumab may require adjustment based on these levels (i.e., an increase in dosage if fasting serum phosphorus levels are less than the reference range for age, and dose interruption and/or dose reduction if levels are above the normal range; Table 1) [5]. Consult local information for further details.
Table 1.
Summary of the use of burosumab solution for injection (Crysvita®) in the treatment of X-linked hypophosphatemia in adults and pediatric patients aged ≥ 1 year in the USA [5]
| How is burosumab available, what is its route of administration, how should it be stored? | |
| Availability | Single-dose vials containing 10, 20 or 30 mg of burosumab in 1 mL of solution for injection |
| Administration route | Subcutaneous injection into upper arms or thighs, buttocks, or any quadrant of abdomen; rotate injection site with each injection |
| Storage | Refrigerate at 2–8 °C (36–46 °F) in the original package (to protect from light) |
| What steps need to be taken prior to initiating treatment with burosumab? | |
| 1 week prior to initiation: discontinue the use of oral phosphate and active vitamin D analogs (concomitant use of such agents with burosumab is contraindicated due to the ↑ risk of hyperphosphatemia and hypercalcemia) | |
| Measure fasting serum phosphorus: should be < RRA prior to initiation (the use of burosumab is contraindicated if the level is ≥ RRA) | |
| How should the dosage of burosumab be determined in pediatric patients (aged ≥ 1 to <18 years)? | |
| Starting dosage | 0.8 mg/kg rounded to the nearest 10 mg (minimum 10 mg; maximum 90 mg) every 2 weeks |
| Monitor fasting serum phosphorus levels | Measure every 4 weeks during the first 3 months of treatment, and thereafter as appropriate (including 4 weeks after any dosage adjustment) |
| Dosage adjustment based on fasting serum phosphorus levels | Serum phosphorus > the lower limit of RRA and < 5 mg/dL: no dosage change needed |
| Serum phosphorus < RRA: ↑ dosage stepwise to ≈ 2 mg/kg (maximum 90 mg) every 2 weeks | |
| Serum phosphorus > 5 mg/dL: withhold the next dose and assess serum phosphorus in 4 weeks; once serum phosphorus is < RRA, restart burosumab at a ↓ dosage than previously received; reassess in 4 weeks and adjust as required | |
| Do not adjust dosage more frequently than every 4 weeks | |
| How should the dosage of burosumab be determined in adult patients (aged ≥ 18 years)? | |
| Starting dosage | 1 mg/kg rounded to the nearest 10 mg (maximum dose 90 mg) administered every 4 weeks |
| Monitor fasting serum phosphorus levels | Measure 2 weeks post-dose on a monthly basis for the first 3 months of treatment, and thereafter as appropriate (including 2 weeks after any dosage adjustment) |
| Dosage adjustment based on fasting serum phosphorus levels | Serum phosphorus within normal range: no dosage change needed |
| Serum phosphorus > normal range: withhold the next dose and assess serum phosphorus in 4 weeks; once serum phosphorus is < normal range, restart burosumab at ≈ 50% of the dosage previously received (maximum 40 mg every 4 weeks); reassess in 2 weeks and adjust as required | |
| Do not adjust dosage more frequently than every 4 weeks | |
| How should burosumab be used in special populations? | |
| Women of child-bearing potential | Monitor serum phosphorus levels throughout pregnancy (lack of human data regarding risks) |
| Breast-feeding women | Consider the benefits of breastfeeding, the mother’s clinical need for treatment, and the potential adverse effects on the breastfed infant from burosumab or XLH (lack of data regarding risks) |
| Patients with renal impairment | Severe or end-stage renal disease: use is contraindicated |
| What other precautions should be taken with burosumab? | |
| Hyperphosphatemia | Serum phosphorus levels > ULN may ↑ the risk of nephrocalcinosis: interrupt treatment and/or ↓ dosage |
| Serious hypersensitivity or injection-site reactions | Discontinue burosumab and initiate appropriate medical treatment |
| Restless leg syndrome | Advise patients to contact their physician if symptoms of restless leg syndrome occur or worsen |
RRA reference range for age, ULN upper limit of normal, XLH X-linked hypophosphatemia, ↑ increase(d) greater, ↓ reduce/lower
What are the pharmacological properties of burosumab?
Pharmacodynamic profile
Burosumab has beneficial effects on serum phosphorus (measured as inorganic phosphate), and 1,25(OH)2D levels, as well as other pharmacodynamic outcomes. For example, in a phase 1 trial in adults with XLH, single doses of subcutaneous burosumab 0.3–1 mg/kg (n = 12) significantly (p < 0.05) increased serum phosphorus levels, the maximum rate of tubular phosphate reabsorption to the glomerular filtrate rate ratio (TmP/GFR), and serum 1,25(OH)2D levels relative to placebo (n = 4) [7], but did not increase serum calcium, intact parathyroid hormone (iPTH), or creatinine levels, 24-h urine calcium excretion, or fasting 24-h urine calcium/creatinine ratios [7]. In multi-dose pharmacodynamic or clinical studies in pediatric [8, 9] or adult [10–13] patients, the pharmacological effects of subcutaneous burosumab were consistent with those observed in the single-dose trial [7]. The effects of burosumab on pharmacodynamic parameters in the key clinical trials [8, 9, 13] are presented in more detail in the efficacy section.
Evidence from preclinical studies supports the use of burosumab in patients with XLH [14]. Anti-FGF23 antibodies increased serum phosphorus and 1,25(OH)2D levels, improved TmP/GFR, decreased urinary phosphate levels, did not decrease urinary calcium levels, increased the expression of type IIa sodium-phosphate co-transporters and 1α-hydroxylase in the kidney, while decreasing 24-hydroxylase expression, and significantly (p < 0.01) improved elongation of the long bones (femur and tibia during the growth period of PHEX-deficient mice), and improved bone mineralization in growing and adult mice [14].
Pharmacokinetic-pharmacodynamic profile
The pharmacokinetic profile of subcutaneous burosumab supports the dose adjustment of burosumab based on predose serum phosphorus levels, as there is a linear correlation between these factors in patients with XLH [10, 15]. Improvements from baseline in serum phosphorus levels and other outcomes in children aged 5–12 years indicated that administration every 2 weeks (the approved regimen in children; Table 1) was preferable to administration every 4 weeks in pediatric patients [8].
Table 2 presents the overall pharmacokinetic profile of burosumab in adult patients with XLH, which does not differ substantially from that in pediatric patients [5]. Although the pharmacokinetics of burosumab have not been studied in patients with renal or hepatic impairment, its use is contraindicated in patients with severe or end-stage renal disease (Table 1), due to the abnormal mineral metabolism associated with these conditions [5].
Table 2.
Summary of the pharmacokinetic profile of subcutaneous burosumab in adults with X-linked hypophosphatemia [5, 7, 10, 15]
| Parameter | Comments |
|---|---|
| Absorption | Absorbed slowly (mean tmax 8–11 daysa), with almost 100% bioavailability |
| Exposure and duration of activity | Dose proportional over the range of 0.1–2.0 mg/kg |
| In a population analysis of adults with XLH receiving dose-titrated burosumab every 28 days for 16 months, peak mean serum phosphorus levels progressively ↑ after the first 4 doses, with comparable peak phosphorus levels after doses 6–10, and a slight ↓ thereafter | |
| Pharmacokinetic–pharmacodynamic relationship | Direct relationship; serum concentrations of burosumab and phosphorus ↑ and ↓ in a parallel linear manner, and peak at approximately the same timepoint after each dose |
| Distribution | Apparent volume of distribution: 8 La (i.e., approximately the volume of plasma in adults with XLH), suggesting limited extravascular distribution |
| Metabolism | Exact pathway not known; expected to be broken into small peptides and amino acids via catabolic pathways |
| Hepatic mechanisms are unlikely to be involved | |
| Elimination | Clearance: low and body-weight dependent; apparent clearance 0.290 and 0.136 L/day in a typical 70-kg adult and 30-kg child with XLH, respectively |
| Mean terminal half-life: ≈ 19 daysa | |
| Excretion: not expected to be directly excreted due to its molecular size |
T max time to maximum serum concentration, XLH X-linked hypophosphatemia, ↑ increase, ↓ decrease
aIn a typical 70-kg adult with XLH receiving the approved starting dose of burosumab (i.e., 1 mg/kg)
What is the efficacy of burosumab in the treatment of XLH in children?
Subcutaneous burosumab (≈ 1.0 mg/kg) every 2 weeks significantly improved rickets severity and serum phosphorus levels in clinical trials in children aged 1–12 years with XLH. The two open-label, multicenter, phase 2 trials included 13 children with XLH aged 1–4 years [9], and 52 prepubescent children with XLH aged 5–12 years [8]. In the respective trials, 100 and 94% of children had radiographic evidence of rickets at baseline, and 100 and 96% had received prior conventional treatment with oral phosphates and active vitamin D (mean duration of conventional treatment 1.4 and 7 years [5, 8, 9]. Treatment with oral phosphates/active vitamin D was discontinued before starting burosumab [8, 9].
In the trial in younger children (mean age 2.9 years), patients received burosumab 0.8 mg/kg every 2 weeks, with increases up to 1.2 mg/kg every 2 weeks if serum phosphorus levels remained low (mean dosage 0.9 mg/kg) [9]. In the trial in older children (mean age 8.5 years), patients were randomized to receive burosumab 0.1 mg/kg every 2 weeks or 0.2 mg/kg every 4 weeks (n = 26 in each group); doses were titrated upwards to a maximum of 2.0 mg/kg (mean dosage 0.98 mg every 2 weeks, and 1.50 mg every 4 weeks at week 40) to achieve serum phosphorus levels of 3.5–5.0 mg/dL [8]. All patients completed at least 40 or 64 weeks of treatment, respectively, in the trials in younger [9] and older children [8]. All serum and urine samples were obtained during fasting [8]
The discussion in this article focuses on the results in the intent-to-treat (ITT) population of children receiving the approved regimen of burosumab every 2 weeks; results in the group receiving burosumab every 4 weeks are included in Table 3 for the sake of completeness. Some data are derived from the US prescribing information [5] and abstract reports [9, 16, 17].
Table 3.
Changes from baseline in primary and selected secondary pharmacodynamic outcomes in open-label phase 2 trials of subcutaneous burosumab in pediatric patients aged 1–4 or 5–12 years with X-linked hypophosphatemia
| Outcome | Burosumab every 2 wks in pts aged 1–4 years (n = 13) [5, 9, 16] | Dose-finding trial in pts aged 5–12 years [5, 8] | |
|---|---|---|---|
| Burosumab every 2 wks (n = 26) | Burosumab every 4 wks (n = 26) | ||
| Thacher Rickets Severity Score a (primary endpoint) | |||
| Mean score at BL (range) | 2.9 | 1.9 (0–4.5) | 1.7 (0–3.0) |
| Mean score at wk 40 (LSM change from BL; 95% CI) | 1.2 (− 1.7; − 2.0 to − 1.4)*** | 0.8 (− 1.1; − 1.3 to − 0.9)** | 1.1 (− 0.7)** |
| Mean score at wk 64 (LSM change from BL; 95% CI) | 0.8 (− 1.0; –1.2 to − 0.79) | 0.9 (− 0.8) | |
| Radiographic Global Impression of Change global score b | |||
| LSM change from BL in score at wk 40 (95% CI) | 2.3 (2.2–2.5)** | 1.7 (1.5–1.8) | 1.5 |
| LSM change from BL in score at wk 64 (95% CI) | 1.6 (1.4–1.8) | 1.6 | |
| Fasting serum phosphorus level (LLN 3.2 mg/dL) | |||
| Mean at BL (mg/dL) | 2.5 | 2.4 | 2.3 |
| Mean at wk 40 (mg/dL) | 3.5 | 3.4** | 2.8**c |
| Fasting TmP/GFR (LLN 3.42 mg/dL) | |||
| Mean at BL (mg/dL) | 2.2 | 2.0 | |
| Mean at wk 40 (mg/dL) | 3.3** | 3.0**c | |
| Fasting serum 1,25-dihydroxyvitamin D levels (LLN 43 pg/mL) | |||
| Mean at BL (pg/mL) | 41.3 | 41.4 | |
| Mean at wk 40 (pg/mL) | 70**c | 60**c | |
| Fasting serum total ALP activity (ULN 385 U/L) | |||
| Mean at BL (U/L) | 549 | 462 | 456 |
| Mean at wk 40 (U/L) | 335*** | 380c | 405c |
| Mean at wk 64 (U/L) | 354** | 385*c | |
ALP alkaline phosphatase, BL baseline, LLN lower limit of normal, LSM least-squares mean (estimated using models that accounted for Rickets Severity Score and other variables at BL), TmP/GFR maximum rate of tubular phosphate reabsorption/glomerular filtrate rate, ULN upper limit of normal, wk week
*p = 0.002, **p < 0.001, ***p < 0.0001 vs BL
aScores range from 0 to 10, with higher scores indicating more severe disease (blinded assessment of prespecified radiographic abnormalities at the wrist and knee at a single timepoint)
bScores range from − 3 to + 3, with negative scores indicating worsening, 0 no change, and positive scores healing (blinded side-by-side assessment of radiographs of the wrist and knee obtained at two timepoints)
cValue estimated from a figure
Changes in rickets severity
Burosumab once every 2 weeks significantly (p < 0.001) improved the severity of XLH-related rickets (primary endpoint), as shown by changes from baseline in total Thacher Rickets Severity Score (RSS) and Radiographic Global Impression of Change (RGI-C) score at week 40 (both trials) [8, 9], as well as at week 64 in the longer trial in older children (Table 3) [8]. At week 40, all 13 patients aged 1–4 years were considered to be RGI-C responders (defined as an RGI-C global score ≥ 2.0, which provides radiographic evidence of substantial healing) [9]. Of the 26 children aged 5–12 years receiving burosumab once every 2 weeks, 18 (69%) and 15 (58%) were RGI-C responders at weeks 40 and 64, respectively [8].
In a pooled subgroup analysis of children receiving burosumab every 2 or 4 weeks, improvements in rickets severity were consistent and statistically significant (p < 0.0001) in all age groups (i.e., 1–4, 5–7, 8–9, and 10–12 years), with least square mean (LSM) changes from baseline in total RSS of − 0.4 to − 1.7 at week 40 [16].
RSS wrist and knee scores, and RGI-C wrist and knee scores, significantly (p < 0.0001) improved from baseline at week 40 in children aged 5–12 years receiving burosumab every 2 weeks [8]. A modest improvement in RGI-C overall leg deformities was seen at week 64 in all patients. At weeks 40 and 64, improvements from baseline were also shown with regard to growth (assessed by standing height Z score) and exercise capacity [assessed by the 6-min walk test (6MWT)] in patients with impairment at baseline in recipients of burosumab every 2 weeks. Pediatric Outcomes Data Collection Instrument scores for pain, physical function, and global functioning improved at weeks 40 and 64 relative to baseline in the total population, as well as those with impaired global functioning at baseline (pooled data from the burosumab every 2- or 4-week groups) [8].
Radiographic outcomes in children aged 5–12 years receiving burosumab once every 2 weeks appeared to be better in those with relatively more severe rickets (i.e., baseline RSS total score ≥ 1.5) [8]. Improvements in total RSS and RGI-C scores were greater in patients with more severe rickets than in the overall treatment group at week 40 (LSM change from baseline − 1.7. vs − 1.1, and 2.02 vs 1.7, respectively) [8].
Changes in pharmacodynamic parameters
At week 40, burosumab every 2 weeks significantly (p < 0.001) improved mean serum phosphorus levels from baseline by 40% in children aged 1–4 years, and by 38% in those aged 5–12 years (Table 3) [8, 9]. In an initial analysis in 10 children aged 1–4 years, normal serum phosphorus levels were achieved by all patients at week 1 (mean increase 1.3 mg/dL), and by 80% at week 4 (mean increase 1.1 mg/dL) [17]. In older children, burosumab every 2 weeks improved mean serum phosphorus levels at all timepoints up to week 64, with the majority of patients achieving levels within the normal range (3.2–6.1 mg/dL) by week 6 [8]. Increases in mean serum phosphorus levels were sustained during treatment with burosumab every 2 weeks, but fluctuated during treatment every 4 weeks in this trial [8]. Serum phosphorus levels did not increase above the upper limit of normal (ULN) in any patient during these trials [8, 9].
Improvements from baseline in other pharmacodynamic parameters were also shown in both trials [8, 9]. In the trial in older children, mean TmP/GFR ratios significantly improved to close to the lower limit of normal (LLN) of 3.4 mg/dL, and mean serum 1,25(OH)2D levels improved to above the LLN of 43 pg/mL at weeks 40 (Table 3), with improvements being maintained at week 64 [8]. Mean serum total alkaline phosphatase (ALP) activity decreased by 36% to less than the ULN (385 U/L) at week 40 in the trial in younger children, and by 23% to less than the ULN at week 64 in the trial in older children [5] (Table 3).
Increases in mean serum phosphorus levels (range 0.3–1.2 mg/dL), and decreases in mean serum ALP activity (range − 54 to − 213 U/L; p < 0.05) were shown in children in all age groups (i.e., 1–4, 5–7, 8–9, and 10–12 years) by week 40 with burosumab every 2 or 4 weeks in the pooled subgroup analysis [16].
What is the efficacy of burosumab in the treatment of XLH in adults?
Phase 1/2 trial and extension studies
The clinical benefits of subcutaneous burosumab once every 4 weeks in the treatment of adults with XLH were initially shown in the following small open-label trial and extension studies:
Dose-escalation phase 1/2 trial (n = 28) [11, 18] At day 7 after dose 1, 2, 3, and 4 (i.e., 0.05, 0.1, 0.3, and 0.6 mg/kg), maximum fasting serum phosphorus levels within the normal range of 2.5–4.5 mg/dL were achieved by 15, 37, 74 and 88% of patients, respectively, compared with 3.7% of patients at baseline [11]. Relative to baseline at day 120, burosumab significantly (p < 0.05) improved XLH-related physical functioning [as assessed by the Western Ontario and McMaster Osteoarthritis Index (WOMAC)] and stiffness from baseline [as assessed by the physical component score of the Short Form-36 version 2 Health Survey (SF-36v2)] [18].
Extension of the phase 1/2 trial (n = 22) [11]. Patients received burosumab 0.1–1.0 mg/kg every 4 weeks for 12 months (mean time to first dose 53 days after the last dose in the dose-escalation phase trial). In each dose cycle, maximum and minimum fasting serum phosphorus levels were within the normal range in 58–85% and 25–43% of patients, respectively. Relative to pre-dose-escalation baseline, almost all post-dose peak serum phosphorus and 1,25(OH)2D levels, and peak and trough TmP/GFRs had increased to a significant (p < 0.05) extent.
Phase 2b extension study in participants of the previous trials (n = 20) [12] All patients received their first dose of burosumab > 12 months after their last dose in a previous trial. At week 24 of the extension, burosumab significantly (p < 0.005) increased mean serum phosphate, TmP/GFR, and serum 1,25(OH)2D levels from the extension baseline. Significant (p ≤ 0.05) improvements from baseline were also shown in mean WOMAC scores for pain, stiffness, and physical functioning, Short-Form Brief Pain Inventory (BPI) scores for worst pain, pain severity, and pain interference, the mean time to complete the Timed Up and Go test (assesses balance and agility) and the mean distance walked in the 6MWT.
Placebo-controlled phase 3 trial and extension
Subcutaneous burosumab 1.0 mg/kg every 4 weeks was more effective than placebo in improving clinical and pharmacodynamic outcomes in symptomatic adults with XLH [13]. The pivotal randomized, double-blind, placebo-controlled, 24-week multicenter [13] included 134 adults aged 19–66 years (mean 40.0 years) with XLH. Patients were required to have a serum phosphate concentration less than the LLN (i.e., < 2.5 mg/dL), a TmP/GFR of < 2.5 mg/dL, and a BPI worst pain score ≥ 4 [13]. Most patients enrolled in the trial had undergone orthopedic surgery (69%), had osteoarthritis (63%), had enthesopathy present on X-ray (99%), had evidence of nephrocalcinosis on renal ultrasound (54%), were experiencing severe pain (72%), and/or were using analgesics (68%) [13]. Over their lifetime, patients had received long-term conventional XLH treatment with phosphates and active vitamin D (mean duration 16.5 and 18.2 years, respectively). Treatment with agents that affect phosphorus metabolism (e.g., oral phosphates/active vitamin D) was discontinued ≥ 2 weeks before starting burosumab; oral vitamin D supplements (e.g., cholecalciferol, ergocalciferol) could be taken during the trial if serum 25-hydroxyvitamin D [25(OH)D] levels fell to < 20 ng/mL [13].
Following the 24-week double-blind phase of the trial, all patients from both arms entered an open-label 24-week extension study in which patients who previously received burosumab continued to receive burosumab (total of 48 weeks of burosumab), and those who previously received placebo switched to burosumab (placebo → burosumab; total duration of burosumab 24 weeks (available as abstracts) [19, 20]. Results in the double-blind and extension phases of the trial are reported for the ITT population. All serum and urine samples were obtained during fasting [13].
Changes in XLH-related signs and symptoms
Burosumab was associated with improvements in outcomes such as pain, stiffness, physical functioning, and healing of fracture/pseudofracture (Fx/PFx; Table 4) [13, 19, 20]. Relative to placebo at week 24, the change from baseline in WOMAC stiffness subscale score was significantly higher with burosumab than with placebo (Table 4). Changes from baseline in BPI worst pain and WOMAC physical function subscale at week 24 were nominally significantly (p < 0.05) better with burosumab than with placebo (Table 4), but did not achieve the significance level required using prespecified statistical testing [13]. At week 24 in both treatment groups, 6MWT results did not change from baseline to a significant extent [LSM change from baseline 5.9 m with burosumab vs − 5.7 m with placebo; treatment difference (TD) 11.63 m] [13].
Table 4.
Changes from baseline in key secondary and other outcomes with subcutaneous burosumab 1.0 mg/kg every 4 weeks (n = 68) vs placebo (n = 66) in adults with X-linked hypophosphatemia in a 48-week study (double-blind and extension phases)
| Outcome | Double-blind phase (wks 0–24) [5, 13] | Open-label extension phase (wks 25–48) [19, 20] | |
|---|---|---|---|
| Burosumab vs placebo | Continuous burosumab | Placebo → burosumab | |
| BPI worst pain a | |||
| LSM change from BL in score at wk 24 | − 0.79 vs − 0.32 (LSM TD − 0.46) | ||
| LSM change from BL in score at wk 48 | − 1.1†† | − 1.5†† | |
| WOMAC physical function subscale score b | |||
| LSM change from BL in score at wk 24 | − 3.11 vs 1.79 (LSM TD − 4.90) | ||
| LSM change from BL in score at wk 48 | − 7.8† | − 6.4† | |
| WOMAC stiffness subscale score c | |||
| LSM change from BL in score at wk 24 | − 7.87 vs 0.25 (LSM TD − 8.12*) | ||
| LSM change from BL in score at wk 48 | − 16.0†† | − 15.3†† | |
| Fx/PFx healing | |||
| % of pts with active Fx/PFx at BL (no. of Fx/PFx) | 47.1 vs 57.6 (65 vs 91) | ||
| % of Fx/PFx fully healed at wk 24 | 43.1 vs 7.7 (OR 16.8**) | ||
| % of Fx/PFx fully healed at wk 48 | 63 | 35 | |
| Fasting serum phosphate (normal range 2.5–4.5 mg/dL) | |||
| Mean at BL (mg/dL) | 2.0 vs 1.9 | ||
| Mean at midpoint of dose interval (wks 2–22) [mg/dL] | 3.2 vs 2.1 | ||
| Mean at end of the dosing interval (wks 4–24) [mg/dL] | 2.7 vs 2.0 | ||
| Mean at midpoint of dose interval at wk 46 (mg/dL) | 3.0 | 3.0 | |
| Mean at end of dose interval at wk 48 (mg/dL) | 2.4 | 2.5 | |
| Fasting TmP/GFR (normal range 2.5–4.2 mg/dL) | |||
| Mean at BL (mg/dL) | 1.7 vs 1.6 | ||
| Mean at wk 22 (peak effect) [mg/dL] | 2.7 vs 1.7 | ||
| Mean at wk 24 (trough effect) [mg/dL] | 2.2 vs 1.7 (LSM TD 0.43**) | ||
| Fasting serum 1,25-dihydroxyvitamin D levels (normal range 18–72 pg/mL) | |||
| Mean at BL (pg/mL) | 32.4 vs 33.5 | ||
| Mean at wk 22 (peak effect) [pg/mL] | 57.0 vs 34.9 (LSM TD 22.7**) | ||
BL baseline, BPI Short-Form Brief Pain Inventory, F/PF fracture/pseudofracture, LSM least-squares mean, OR odds ratio, pts patients, Placebo → burosumab, switch from placebo in double-blind phase to burosumab in extension phase (24 wks treatment with burosumab), TD treatment difference, mP/GFR maximum rate of tubular phosphate reabsorption/glomerular filtrate rate, wk week, WOMAC Western Ontario and McMaster Osteoarthritis Index
*p = 0.0012, **p < 0.001 vs placebo; † p < 0.001, †† p < 0.0001 vs BL
aScores range from 0 to 10, with a decrease from BL indicating less pain
bScore ranges from 0 to 68, with a decrease from BL indicating better physical function
cScores range from 0 to 8, with a decrease from BL indicating less stiffness
At week 48 (extension study), significant (p < 0.001) improvements from baseline in BPI worst pain, WOMAC physical function subscale, and WOMAC stiffness subscale scores were shown in the continuous and placebo → burosumab groups (Table 4) [19, 20]. Improvements in the placebo → burosumab group (i.e., 24 weeks of burosumab treatment) were broadly similar to those at week 24 in the continuous burosumab group (Table 4) [19, 20].
In patients with identified Fx/PFx at baseline, burosumab recipients were significantly (p < 0.001) more likely to have complete Fx/PFx healing than placebo recipients at week 24 (Table 4). At week 48, there was additional healing of Fx/PFx in the continuous burosumab group, and the proportion of fully healed Fx/PFx in the placebo → burosumab group was comparable to that at week 24 in the continuous burosumab group (Table 4) [20].
Changes in pharmacodynamic parameters
Mean serum phosphorus levels above the LLN at the midpoint of the dose interval (peak effect) was achieved by significantly (p < 0.001) more burosumab recipients than placebo recipients (94.1 vs 7.8%; primary endpoint) [13]. Moreover, mean serum phosphorus levels above the LLN at the end of the dose interval (i.e., just before the next dose; trough effect) were achieved by 67.6% of burosumab recipients compared with 6.1% of placebo recipients [13]. Mean peak and trough phosphorus levels in the burosumab group were consistently within the normal range from the first dose onwards [13]. Changes from baseline in mean serum TmP/GFR and 1,25(OH)2D were significantly (p < 0.001) higher with burosumab than with placebo (Table 4), but serum 25(OH)D levels did not change notably from baseline in either treatment group [13].
The beneficial effects of burosumab on serum phosphorus levels were generally maintained during the extension phase, with the 68 patients in the continuous burosumab group showing sustained normal phosphorus levels at 48 weeks of continuous treatment, and the 66 patients in the placebo → burosumab group achieving serum phosphate normalization after 24 weeks of burosumab treatment (Table 4) [19, 20].
At week 24, burosumab was significantly (p < 0.001) favored over placebo with regard to changes from baseline in serum levels of markers of bone formation (LSM TD in procollagen type I N-propeptide levels of 62 ng/mL) and bone resorption (LSM TD in carboxy-terminal cross-linked telopeptide of type I collagen levels of 190 pg/mL). However, there was no significant TD with regard to changes from baseline in serum bone-specific ALP levels (increase of 43% with burosumab and 33% with placebo; LSM TD 4.4 μg/L) [13].
What is the tolerability profile of burosumab?
Burosumab was generally well tolerated in pediatric and adult patients with XLH, with most treatment-emergent adverse events (TEAEs) being mild to moderate in severity [5, 8, 13, 16]. The descriptive tolerability profile in these patient groups are based on the data from the pooled phase 2 clinical trials in 65 children (mean duration of burosumab exposure 2.7 years; range 0.8–2.9 years) [5, 16], and the initial 24-week double-blind phase of the phase 3 trial in 68 adults receiving burosumab and 66 receiving placebo [5, 13]. No TEAEs led to treatment discontinuation, dose-limiting toxicity, or death [5, 8, 13, 16].
TEAEs that were reported in ≥ 10% of children with XLH receiving burosumab every 2 or 4 weeks in the pooled phase 2 trials included headache (60% of patients), local injection-site reactions (ISRs; including erythema, rash, bruising, pain, pruritus, swelling, discoloration, hematoma, hemorrhage, induration, macule, urticaria, and other reactions at the site of the injection; 59%), vomiting and pyrexia (each 48%), pain in extremity (42%), any type of rash (23%), toothache (22%), myalgia (15%), tooth abscess (17%), and dizziness/exertional dizziness (12%) [5]. Two children experienced a serious TEAE; one patient required hospitalization for fever/muscle pain that resolved within 1 day; the other had a dental abscess [16].
TEAEs were reported in almost all adults receiving burosumab or placebo in the first 24 weeks of the trial (94.1 and 92.4%, respectively) [13]. The most common TEAEs (incidence ≥ 10% and reported in at least three more patients with burosumab than with placebo) were back pain (15 and 9% of burosumab and placebo recipients, respectively), tooth abscess/infection (13 and 8%), headache/head discomfort (13 and 8%), restless syndrome (RLS)/related events (12 and 8%), and dizziness (10 and 6%) [5]. Serious TEAES were reported in four patients (two in each treatment groups), none of which were considered to be related to the study drug [13].
Reductions from baseline in vitamin D levels of any extent occurred in 32% of children and 12% of adults receiving burosumab, compared with 6% of adults receiving placebo [5]. There were no clinically meaningful changes from baseline in levels of serum/urine calcium, serum iPTH, nephrocalcinosis score, renal function, left ventricular mass index, vital signs, and/or other related parameters in children [8, 16] and adults [13] receiving burosumab.
Adverse events of specific interest
Dose changes, treatment interruption or treatment discontinuation may be required if certain adverse events (e.g., hyperphosphatemia, hypersensitivity reactions, ISRs, or RLS) occur during treatment with burosumab (Table 1). The following adverse events were of specific interest in patients with XLH receiving burosumab in the pooled phase 2 clinical trials in children [5, 16], and the double-blind phase of the phase 3 trial, or other studies, in adults [5, 13].
Hyperphosphatemia No cases of hyperphosphatemia were reported in pediatric patients [5, 16], whereas hyperphosphatemia requiring a protocol-specified dose reduction of 50% occurred in 7% of adults receiving burosumab; dose reduction controlled the hyperphosphatemia in all but one patient who required a second dose reduction [5].
ISRs Local ISRs were reported in 59% of children receiving burosumab [5], and ≈ 12% of adults receiving burosumab or placebo [5, 13]. ISRs were generally mild, occurred within 1 day of the injection, lasted ≈ 1–3 days, did not require treatment and resolved in almost all instances in both pediatric and adult patients [5]
Hypersensitivity reactions In pediatric patients, the potential hypersensitivity reactions that occurred most frequently with burosumab were rash (22%), injection-site rash (6%) and urticaria (5%) [5]. In adults, hypersensitivity events were experienced a similar proportion (≈ 6%) of burosumab and placebo recipients [5, 13].
RLS In adults, worsening of baseline or new-onset RLS was more common with burosumab than with placebo in the key trial (11.8 vs 6.1% of patients) [5, 13]. In other studies, worsening of baseline RLS in an adult with XLH led to discontinuation of burosumab, and resolution of the event [5].
Ectopic mineralization There were no reports of ectopic mineralization in children [8] or adults [13].
Spinal stenosis Of the six adults receiving burosumab who underwent spinal surgery in phase 2 or 3 studies, most cases appeared to involve the progression of pre-existing spinal stenosis; it is not known whether burosumab exacerbates spinal stenosis or spinal cord compression [5].
Immunogenicity
The effect of the presence of antibodies to burosumab on the efficacy or tolerability of burosumab is currently unknown, due to the limited sensitivity and specificity of the antibody assay [5]. Across the clinical trials of burosumab, up to 10% of patients with XLH had pre-existing anti-burosumab antibodies [5]. Children and adults who were anti-burosumab antibody-negative at the start of burosumab treatment did not develop antibodies post-baseline [5, 8, 13].
What is the current clinical role of burosumab in XLH?
Burosumab is an important new development in the treatment of XLH in children aged ≥ 1 year and adults due to the following factors:
Its novel mechanism of action Directly addresses the deficiency in serum phosphate levels caused by the excessive FGF23 activity in patients with XLH. By binding to FGF23 and inhibiting its signaling, burosumab increases renal tubular reabsorption and GI absorption of phosphate, thereby increasing serum phosphate levels and, ultimately, improving bone mineralization and decreasing bone disease (Fig. 1).
Its clinical effectiveness in treating XLH Significantly improves the severity of rickets and other XLH-related outcomes in pediatric patients, and XLH-related symptoms (e.g., pain, stiffness, physical functioning, and Fx/PFx healing) in adult patients.
Tolerability profile Well tolerated by children and adults with XLH, with most TEAEs being of mild to moderate severity. Certain adverse events (e.g., hyperphosphatemia, hypersensitivity reactions, ISRs, and RLS) may require dose changes, treatment interruption or treatment discontinuation (Table 1), but most events do not require intervention.
Acknowledgements
The manuscript was reviewed by: S. Fukumoto, Department of Molecular Endocrinology, Fujii Memorial Institute of Medical Sciences, Tokushima University, Tokushima, Japan; L.-N. Veilleux, Motion Analysis Center, Shriners Hospital for Children-Canada, Montréal, QC, Canada. During the peer review process, Ultragenyx Pharmaceutical Inc., the marketing-authorization holder of burosumab, was also offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
K.A. Lyseng-Williamson is an employee of Adis/Springer, is responsible for the article content and declares no conflicts of interest.
Footnotes
The original version of this article was revised due to a retrospective Open Access request.
Change history
11/12/2018
The article Burosumab in X-linked hypophosphatemia
References
- 1.Carpenter TO, Imel EA, Holm IA, et al. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26(7):1381–1388. doi: 10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon S, Crowley R. Developments in rare bone diseases and mineral disorders. Ther Adv Chronic Dis. 2018;9(1):51–60. doi: 10.1177/2040622317739538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukumoto S. Targeting fibroblast growth factor 23 signaling with antibodies and inhibitors, is there a rationale? Front Endocrinol (Lausanne). 2018;9:48. doi: 10.3389/fendo.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesher D, Oddy M, Darbar U, et al. Outcome of adult patients with X-linked hypophosphatemia caused by PHEX gene mutations. J Inherit Metab Dis. 2018;1–12. [DOI] [PMC free article] [PubMed]
- 5.Crysvita® (burosumab-twza) injection for subcutaneous use: US prescribing information. Novato (CA): Ultragenyx Pharmaceutical Inc.; 2018.
- 6.Crysvita (burosumab): summary of product characteristics. London: European Medicines Agency; 2018.
- 7.Carpenter TO, Imel EA, Ruppe MD, et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest. 2014;124(4):1587–1597. doi: 10.1172/JCI72829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378(21):1987–1998. doi: 10.1056/NEJMoa1714641. [DOI] [PubMed] [Google Scholar]
- 9.Portale A, Imel E, Whyte M, et al. Burosumab for X-linked hypophosphatemia (XLH): results from two pediatric phase 2 trials [abstract no. 525] Endocr Pract. 2018;24(Suppl 1):119–120. [Google Scholar]
- 10.Zhang X, Peyret T, Gosselin NH, et al. Population pharmacokinetic and pharmacodynamic analyses from a 4-month intradose escalation and its subsequent 12-month dose titration studies for a human monoclonal anti-FGF23 antibody (KRN23) in adults with X-linked hypophosphatemia. J Clin Pharmacol. 2016;56(4):429–438. doi: 10.1002/jcph.611. [DOI] [PubMed] [Google Scholar]
- 11.Imel EA, Zhang X, Ruppe MD, et al. Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab. 2015;100(7):2565–2573. doi: 10.1210/jc.2015-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruppe M, Peacock M, Weber T, et al. Clinical and radiographic characteristics of adult X-linked hypophosphatemia (XLH) in a cohort of patients treated with KRN23, an antibody to FGF23 [abstract no. MO0319]. In: 38th annual meeting of the American Society for Bone and Mineral Research. 2016.
- 13.Insogna KL, Briot K, Imel EA, et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res. 2018;33(8):1382–1393. doi: 10.1002/jbmr.3475. [DOI] [PubMed] [Google Scholar]
- 14.Aono Y, Yamazaki Y, Yasutake J, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–1888. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Imel EA, Ruppe MD, et al. Pharmacokinetics and pharmacodynamics of a human monoclonal anti-FGF23 antibody (KRN23) in the first multiple ascending-dose trial treating adults with X-linked hypophosphatemia. J Clin Pharmacol. 2016;56(2):176–185. doi: 10.1002/jcph.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Högler W, Carpenter TO, Imel E, et al. Burosumab, an anti-FGF23 monoclonal antibody, for X-linked hypophosphatemia (XLH): analysis by age from two phase 2 pediatric trials [abstract no. P086] Calcif Tissue Int. 2018;102(Suppl 1):S37. [Google Scholar]
- 17.Carpenter T, Imel E, Gottesman GS, et al. KRN23 effects on phosphate and vitamin d metabolism in children < 5 years old with X-linked hypophosphatemia (XLH) [abstract no. FC14] Horm Res Paediatr. 2017;88(Suppl 1):10–11. [Google Scholar]
- 18.Ruppe MD, Zhang X, Imel EA, et al. Effect of four monthly doses of a human monoclonal anti-FGF23 antibody (KRN23) on quality of life in X-linked hypophosphatemia. Bone Rep. 2016;5:158–162. doi: 10.1016/j.bonr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamenicky P, Lachmann R, Carpenter TO, et al. A phase 3 randomized, double-blind, placebo-controlled study investigating the efficacy and safety of burosumab, an anti-FGF23 antibody, in adult X-linked hypophosphatemia [abstract no. OC3.1] Endocrine Abstracts. 2018;56:64. [Google Scholar]
- 20.Lachmann R, Kamenicky P, Carpenter TO, et al. A phase 3 randomized, double-blind, placebo-controlled study investigating the efficacy and safety of burosumab, an anti-FGF23 antibody, in adult X-linked hypophosphatemia (XLH) [abstract no. PLO11] Calcif Tissue Int. 2018;102(Suppl 1):S6–S7. [Google Scholar]