Dear Editor,
We recently reported genetic and epigenetic aberrations in the telomerase reverse transcriptase (TERT) gene in follicular thyroid tumors, further supporting the notion that expression of TERT could constitute a prognostic tool for these lesions [1]. We also showed that screening for TERT aberrancies showed a high specificity for distinguishing follicular thyroid carcinoma (FTC) and follicular tumor of uncertain malignant potential (FT-UMP) from follicular thyroid adenoma (FTA). Screening for such molecular changes (TERT promoter mutations and/or aberrant methylation, copy number gain, and mRNA expression) is, however, not as well established as immunohistochemistry (IHC) in most clinical pathology laboratories. In addition, a reliable TERT antibody has not yet showed satisfactory results in thyroid cancer, with conflicting results in the interpretation of the staining patterns. Moreover, these previous studies were not correlated to various TERT gene aberrancies, thereby preventing an essential correlation to underlying genetics. Our aim with this study was to evaluate if TERT immunoreactivity correlates to TERT promoter mutations or mRNA expression in FTCs, to see whether TERT IHC could be used as a surrogate marker for the causal genetic analyses that previously have been shown to correlate to a malignant phenotype in follicular tumors. The cohort comprised of 51 FTC cases (denoted T1-T51) operated at Karolinska University Hospital, Stockholm, Sweden, between 2014 and 2016. Of these, 27 were widely invasive (WIFTCs), 17 were minimally invasive (MIFTC) and seven were encapsulated angio-invasive (EAIFTC) according to the 2017 WHO classification algorithm (Table 1). T1-T28 were denoted as part of the validation cohort with known TERT promoter mutational status and TERT mRNA expression from our previous study [1], and an additional 23 cases (T29-T51) without available genetic information were retrieved from our pathology registries as an expansion cohort. Informed consent was obtained from all individual participants included in the study, and clinical follow-up was retrieved for all patients. Out of 51 FTCs, 38 cases included corresponding normal thyroid tissue on the same slide which allowed comparison of the immunoreactivity with non-tumorous thyroid tissue. All cases were formalin-fixed paraffin-embedded (FFPE) and cut into 4 μm thick sections and stained using the Envision+ Dual Link System-HRP (DAB+) (DAKO, Carpenteria, CA, USA). The tissue slides were incubated with the monoclonal primary antibody anti-telomerase reverse transcriptase (ab32020, Abcam, Cambridge, UK) for 30 min at a dilution of 1:100. As a positive control, primary FUCH-1 (fibroblast cells infected with TERT [2] with approximately 10% expressing the protein) were used and BJ cells from human foreskin (ATCC® CRL-2522™, Manassas, VA, USA) were used as a negative control. The optimal primary antibody concentration for our experiments was determined through serial dilutions using positive and negative controls (data not shown). The tissue slides were counterstained with hematoxylin and subsequently evaluated independently by two experienced pathologists and graded as displaying “negative/no immunoreactivity,” “weak immunoreactivity,” or “strong immunoreactivity.” The cell component that showed immunoreactivity was also taken into consideration and described as “nuclear,” “cytoplasmic,” or “perinuclear.”
Table 1.
TERT immunoreactivity in follicular thyroid carcinoma
| TERT immunoreactivity | ||||||
|---|---|---|---|---|---|---|
| FTC no. | WHO 2017 subtype | Cohort | Tumor tissue | Corresponding normal thyroid tissue | TERT promotor mutation | TERT mRNA expression |
| T1 | WIFTC | Validation | Weak perinuclear | N/A | Y | Y |
| T2 | MIFTC | Validation | Weak perinuclear | N/A | Y | Y |
| T3 | WIFTC | Validation | Weak perinuclear | Weak perinuclear | Y | Y |
| T4 | WIFTC | Validation | Weak perinuclear | Negative | Y | Y |
| T5 | WIFTC | Validation | Weak perinuclear | N/A | N | Y |
| T6 | WIFTC | Validation | Weak perinuclear | Weak perinuclear | Y | Y |
| T7 | Oxy WIFTC | Validation | Strong perinuclear | Weak perinuclear | N | Y |
| T8 | MIFTC | Validation | Strong perinuclear | N/A | Y | Y |
| T9 | MIFTC | Validation | Strong perinuclear | N/A | N | Y |
| T10 | MIFTC | Validation | Strong perinuclear | Negative | N | Y |
| T11 | WIFTC | Validation | Negative | Negative | Y | Y |
| T12 | WIFTC | Validation | Negative | Weak perinuclear | Y | Y |
| T13 | WIFTC | Validation | Negative | N/A | Y | Y |
| T14 | MIFTC | Validation | Weak perinuclear | Weak perinuclear | N | N |
| T15 | WIFTC | Validation | Weak perinuclear | Weak perinuclear | N | N |
| T16 | EAIFTC | Validation | Strong perinuclear | Negative | N | N |
| T17 | MIFTC | Validation | Strong perinuclear | Weak perinuclear | N | N |
| T18 | WIFTC | Validation | Strong perinuclear | Weak perinuclear | N | N |
| T19 | MIFTC | Validation | Strong perinuclear | Weak perinuclear | N | N |
| T20 | WIFTC | Validation | Strong perinuclear | N/A | N | N |
| T21 | EAIFTC | Validation | Strong perinuclear | Negative | N | N |
| T22 | Oxy EAIFTC | Validation | Strong perinuclear | Weak perinuclear | N | N |
| T23 | WIFTC | Validation | Negative | Weak perinuclear | N | N |
| T24 | WIFTC | Validation | Negative | Negative | N | N |
| T25 | EAIFTC | Validation | Negative | N/A | N | N |
| T26 | WIFTC | Validation | Negative | Weak perinuclear | N | N |
| T27 | WIFTC | Validation | Negative | Weak perinuclear | N | N |
| T28 | MIFTC | Validation | Negative | Negative | N | N |
| T29 | MIFTC | Expansion | Weak perinuclear | N/A | N/A | N/A |
| T30 | EAIFTC | Expansion | Weak perinuclear | Weak perinuclear | N/A | N/A |
| T31 | WIFTC | Expansion | Weak perinuclear | Negative | N/A | N/A |
| T32 | EAIFTC | Expansion | Weak perinuclear | Negative | N/A | N/A |
| T33 | MIFTC | Expansion | Weak perinuclear | Weak perinuclear | N/A | N/A |
| T34 | WIFTC | Expansion | Weak perinuclear | Weak perinuclear | N/A | N/A |
| T35 | MIFTC | Expansion | Weak perinuclear | Weak perinuclear | N/A | N/A |
| T36 | MIFTC | Expansion | Weak perinuclear | Weak perinuclear | N/A | N/A |
| T37 | Oxy MIFTC | Expansion | Weak perinuclear | Weak perinuclear | N/A | N/A |
| T38 | MIFTC | Expansion | Weak perinuclear | N/A | N/A | N/A |
| T39 | WIFTC | Expansion | Weak perinuclear | N/A | N/A | N/A |
| T40 | MIFTC | Expansion | Strong perinuclear | Negative | N/A | N/A |
| T41 | MIFTC | Expansion | Strong perinuclear | Weak perinuclear | N/A | N/A |
| T42 | WIFTC | Expansion | Strong perinuclear | Negative | N/A | N/A |
| T43 | Oxy WIFTC | Expansion | Strong perinuclear | Negative | N/A | N/A |
| T44 | Oxy WIFTC | Expansion | Strong nuclear /cytoplasmic | N/A | N/A | N/A |
| T45 | WIFTC | Expansion | Strong perinuclear | Negative | N/A | N/A |
| T46 | Oxy EAIFTC | Expansion | Strong perinuclear | Weak perinuclear | N/A | N/A |
| T47 | WIFTC | Expansion | Strong perinuclear | Negative | N/A | N/A |
| T48 | WIFTC | Expansion | Strong perinuclear | Weak perinuclear | N/A | N/A |
| T49 | WIFTC | Expansion | Negative | Weak perinuclear | N/A | N/A |
| T50 | MIFTC | Expansion | Negative | Weak perinuclear | N/A | N/A |
| T51 | WIFTC | Expansion | Negative | N/A | N/A | N/A |
EAIFTC encapsulated angio-ivasive follicular thyroid carcinoma, MIFTC minimally invasive follicular thyroid carcinoma, Oxy oxyphilic variant, WIFTC widely invasive follicular thyroid carcinoma, Y yes, N no, N/A not available
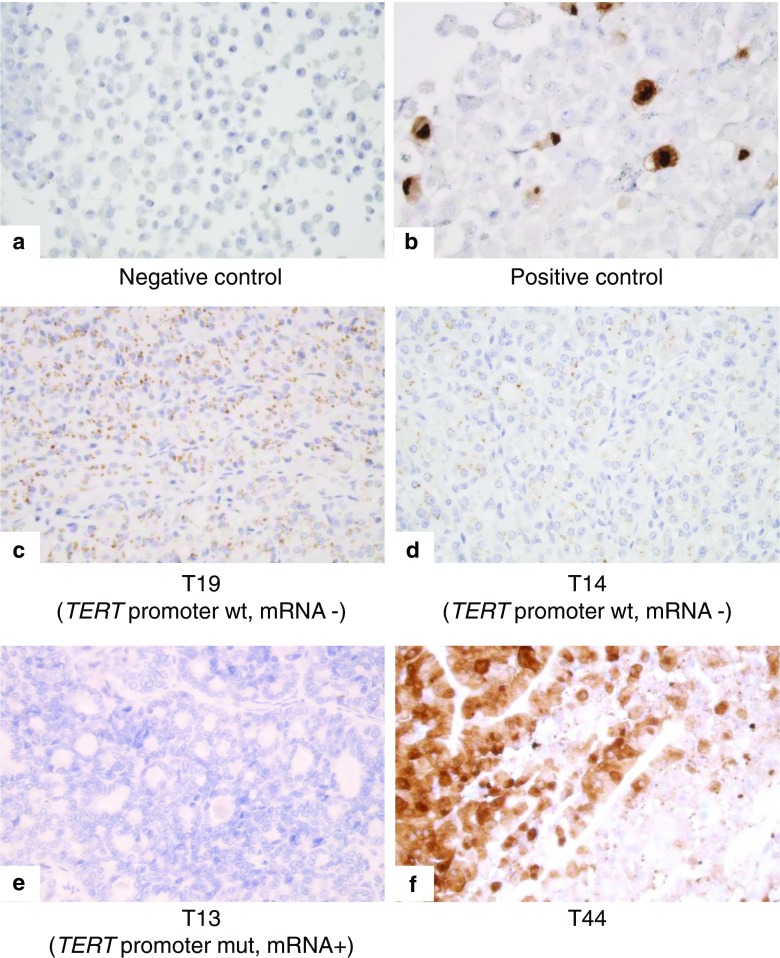
The FUCH-1 TERT expressing cells showed strong nuclear and cytoplasmic immunoreactivity whereas the BJ cells showed no immunoreactivity (Fig. 1). Among the FTCs, 20 (39%) showed strong immunoreactivity, 19 (37%) showed weak immunoreactivity and 12 (24%) cases showed negative immunoreactivity (Table 1). A single FTC case (T44) showed nuclear and cytoplasmic staining, whereas all remaining positively stained cases displayed a perinuclear “Golgi-pattern” type of staining of unknown significance (Fig. 1). In the validation cohort, 10 out of 13 FTC cases with known TERT mRNA expression displayed either weak or strong TERT immunoreactivity, providing a robust sensitivity for the method. However, among the 15 FTCs in the validation cohort without measurable TERT mRNA levels, nine showed either weak or strong TERT immunoreactivity, whereas six were negative—thereby reducing the specificity of the method considerably. Corresponding and adjacent normal thyroid tissues displayed weak perinuclear immunoreactivity in 24 (63%) of cases, and the remaining 14 (37%) showed no immunoreactivity. For statistical purposes, cases with weak immunoreactivity and strong immunoreactivity were collectively regarded as “positive immunoreactivity.” There were no correlations between positive TERT immunoreactivity and TERT promoter mutational status or TERT mRNA expression (p = 1.000 and p = 0.435 respectively). Furthermore, when consulting follow-up data of the 51 FTC patients included (data not shown), no increased risk for persistence/recurrences was noted in cases with positive TERT immunoreactivity (p = 0.586).
Fig. 1.
TERT immunoreactivity in controls and follicular thyroid cancer a TERT staining of BJ cells shows an expected negative immunoreactivity (× 400 magnification) b TERT staining of FUCH1 cells infected with TERT shows strong immunoreactivity in both the nucleus and the cytoplasm of approxiamtely 10% of the cells (× 400 magnification) c TERT staining of FTC case T19 (validation cohort case with known absence of TERT promoter mutations and mRNA expression) displaying strong perinuclear immunoreactivity (× 400 magnification) d TERT staining of FTC case T14 (validation cohort case with known absence of TERT promoter mutations and mRNA expression) showing weak perinuclear immunoreactivity (× 400 magnification) e TERT staining of FTC case T13 (validation cohort case with an established TERT promoter mutation and mRNA expression) with negative immunoreactivity (× 400 magnification) f TERT staining of FTC case T44 (from the expansion cohort) with strong immunoreactivity both in the nucleus and cytoplasm (× 400 magnification). This was the only case with positive nuclear staining except for the positive control
For the first time, we here describe the staining patterns of TERT immunohistochemistry in FTCs, in addition to attempting to correlate the immunoreactivity to established genetic parameters with a known coupling to worse prognosis. All FTCs with positive immunoreactivity except a single case showed an exclusive perinuclear staining which was unexpected when considering the nuclear staining of the positive control in addition to the conventional nuclear role of telomerase. However, recent studies have indeed identified predominant cytoplasmic TERT-staining patterns in other tumor types, for example in hepatocellular carcinoma where cases with cytoplasmic staining surprisingly showed a better overall prognosis [3]. TERT IHC have also been studied in papillary thyroid carcinoma where the protein is hypothesized to shift between subcellular localizations in response to oxidative stress and irradiation [4]. In that study, a “polar” localization was observed in some cases which is in line with our observed perinuclear staining. Moreover, the finding of weak perinuclear staining in the majority of adjacent normal thyroid tissues is puzzling, and might indicate that low cytoplasmic levels of TERT could imply an unknown “house-keeping” function apart from the known roles of TERT in cancer.
The fact that the immunoreactivity in our cohort did not show any correlation to TERT mRNA expression in the validation cohort indicates that TERT IHC may not be an ideal surrogate marker for prognostic purposes. While our previous findings indicate that the increase in TERT mRNA expression is mainly explained by promoter mutations and aberrant methylation, as well as copy number variations of the TERT gene—this was not mirrored by TERT immunoreactivity and indicates that the TERT protein expression displays a much more complex regulation [1]. Several studies have recently been assessing the regulation of TERT, in which further evidence of microRNA regulating mechanisms have been discovered [5]. The lack of concordance between TERT mRNA levels and TERT immunoreactivity might suggest post-translational regulation at work, and, therefore, microRNAs targeting TERT should constitute ideal candidates for future research purposes in FTCs. Alternatively, the protein turn-over could be discordant with the overall TERT mRNA levels in FTCs, arguing for pulsatile expressional cycles of the protein without correlation to the mRNA pool. A third explanation might be that the TERT gene aberrancies and subsequent mRNA expression is subclonal, meaning that the area selected for IHC might not represent the area responsible for TERT mRNA production.
In conclusion, TERT immunoreactivity does not seem to be a satisfactory marker for prognostication in FTC. Furthermore, the expression pattern and the lack of correlation to mRNA expression indicate a more complex regulation machinery in FTCs that urges to be further studied.
Acknowledgements
The authors would like to thank Dr. Monika Ehnman for providing control tissue for the study. The authors would also like to thank Ms. Lisa Ånfalk for assistance with acquiring tissue from the KI biobank.
Funding Information
This study was supported by grants from the Swedish Cancer Society and the Swedish Society for Medical Research.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Paulsson JO, Mu N, Shabo I, et al. TERT aberrancies: a screening tool for malignancy in follicular thyroid tumours. Endocr.-Relat Cancer. 2018;25:723–733. doi: 10.1530/ERC-18-0050. [DOI] [PubMed] [Google Scholar]
- 2.Scholl FA, Betts DR, Niggli FK, Schafer BW. Molecular features of a human rhabdomyosarcoma cell line with spontaneous metastatic progression. Br. J. Cancer. 2000;82:1239–1245. doi: 10.1054/bjoc.1999.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang W, Zhou WP, Li C, et al. Promoter mutations and cellular distribution of telomerase in non-clear cell and clear cell hepatocellular carcinoma. Oncotarget. 2017;8:26288–26297. doi: 10.18632/oncotarget.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muzza M, Colombo C, Rossi S, et al. Telomerase in differentiated thyroid cancer: Promoter mutations, expression and localization. Mol. Cell Endocrinol. 2015;399:288–295. doi: 10.1016/j.mce.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Farooqi Ammad, Mansoor Qaisar, Alaaeddine Nada, Xu Baojun. MicroRNA Regulation of Telomerase Reverse Transcriptase (TERT): Micro Machines Pull Strings of Papier-Mâché Puppets. International Journal of Molecular Sciences. 2018;19(4):1051. doi: 10.3390/ijms19041051. [DOI] [PMC free article] [PubMed] [Google Scholar]