Abstract
Aortic intramural hematoma (IMH) is a pathologic process with a clinical presentation identical to aortic dissection and associated with significant morbidity and mortality. Radiologists must be familiar with the imaging appearances of IMH as computed tomography (CT) plays a critical role in both diagnosis and patient management. The course of IMH is variable and the process may regress, remain stable, or progress in extent, and therefore imaging findings associated with a negative prognosis must be recognized and included in the formal radiology report. Potentially life-threatening complications and findings associated with IMH include hemopericardium and cardiac tamponade, coexisting aortic dissection, ulcer-like projection, intramural blood pool, and extension of hematoma along the pulmonary or coronary arteries, which are identifiable with aortic protocol CT. The purpose of this pictorial review is to provide the reader with an image-based review of the diagnostic criteria, related complications, and associated critical prognostic features in patients presenting with aortic IMH.
Aortic intramural hematoma (IMH) is a life-threatening pathologic process that radiologists must recognize and differentiate from the more commonly encountered aortic dissection. Clinically, aortic IMH is indistinguishable from aortic dissection, although IMH represents approximately 6%–20% of acute aortic syndromes and a higher incidence is reported in females and Asian cohorts (1).
IMH differs from aortic dissection in that it is caused by spontaneous hemorrhage within the media of the aortic wall, without associated intimal rupture or dissection flap (2). The immediate causes of IMH remain controversial although theories including spontaneous rupture of aortic vasa vasorum, bleeding from pathologic neovascularization, microscopic intimal tears and penetrating aortic ulcers (PAU) have been posited (2). This acute hemorrhagic process into the aortic media mandates that aortic syndrome CT protocols include a noncontrast series followed by an electrocardiography (ECG)-gated or ultra-high pitch CT angiogram, as the noncontrast sequence facilitates recognition of IMH as a hyperdense crescentic or circular region within a thickened aortic wall on noncontrast sequences, showing no enhancement after the administration of intravenous contrast (Fig. 1) (3, 4).
Figure 1. a, b.
Type A aortic intramural hematoma. Axial (a) and coronal (b) unenhanced CT images show a crescent-shaped hyperdense collection along the right aspect of the ascending aorta (arrows). Note a small volume hemopericardium.
The widely accepted Stanford classification system for aortic dissection is also applied to IMH and approximately 58% of IMHs are classified as Stanford type B (1). Patients with Stanford type A IMH are at increased risk for complications and have a higher mortality rate and these patients usually require surgical treatment, while those with Stanford type B IMH are treated medically or with endovascular repair (3, 4). However, as the natural history of IMH is variable and can be difficult to predict (it may resolve, stabilize, or progress to aneurysmal dilatation, dissection, or rupture) the prognosis and management of IMH is further dependent on several key morphologic features which may be recognized with CT imaging (3). The recommendation for imaging medically managed type B IMH follow-up is every 3 months during the first year, every 6 months during the second and third years, and annually thereafter (5). It is therefore imperative that radiologists understand the imaging appearance of IMH while recognizing and reporting relevant prognostic imaging features that may inform patient management. The purpose of this pictorial essay is to review the imaging manifestations, complications and prognostic features associated with IMH. A discussion on surgical or interventional management, and their possible complications is beyond the scope of this article.
Imaging protocol
Initial acquisition is a noncontrast CT through the chest. Following this, a CT angiogram acquisition spanning entire length of the aorta is obtained from the level of the clavicles to the femoral lesser trochanters. This is acquired at thin section (1 mm) and following the administration of a bolus of 70–100 mL of iodinated contrast media. Contrast should be injected at high rate. At our institution high concentration iodine contrast is employed (370 mg/mL) and injected at 4–5 mL/s. While image acquisition will greatly depend on hardware, ECG-gating or ultra-high pitch acquisition is recommended when available, as this will limit cardiac motion particularly at the aortic root.
Progression of IMH to classic aortic dissection
IMH may progress to classic acute aortic dissection (AAD) in 28% to 47% of patients, and this carries an associated risk of rupture in 20% to 45% of cases. Type A IMH is associated with progression to aortic dissection in 15%–87.5% of cases according to different series (4). CT imaging characteristics that may help to predict progression of IMH to overt dissection include type A aortic IMH, mean IMH thickness greater than 10–11 mm, compression of the true aortic lumen, a maximal ascending aortic diameter greater than 50 mm or descending aorta greater than 45 mm, and the presence of pericardial effusion (4, 6). The development of ulcer-like projections (discussed later in this review) has been found to predict development of focal dissection and is associated with a poorer prognosis (4).
Key imaging findings for identification of complicated IMH include the presence of hyperdense intramural thrombus on unenhanced images, as well as identification of a dissection flap and false lumen on CT angiography (Fig. 2).
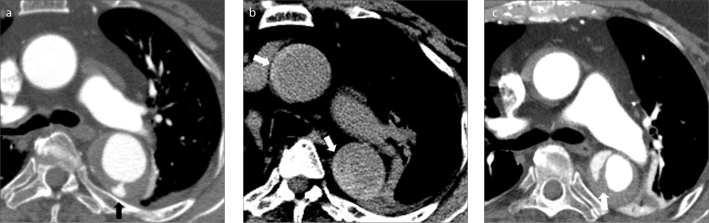
Figure 2. a–c.
Progression of type B IMH. Axial contrast-enhanced CTA (a) shows an intramural hematoma in the descending thoracic aorta with a small associated intimal tear and small collection of contrast in the false lumen (black arrow). Unenhanced and contrast-enhanced axial CT images (b, c) a few days later show retrograde progression of the IMH now involving the ascending thoracic aorta (b, white arrows); furthermore a frank dissection has developed in the descending thoracic aorta with a discrete intimal flap (c, white arrow). Case courtesy of Carlos S. Restrepo, MD.
Although surgical treatment is the standard for managing type A aortic dissection, there is still controversy about the appropriate treatment for type B IMH. Attention to development of aortic dissection during surveillance in patients with type B IMH is crucial. The detection of the previously mentioned predictors of progression may warrant an urgent change in the therapeutic strategy, implying emergent surgical or endovascular management (4).
Hemopericardium and cardiac tamponade
Type A IMH is associated with a higher rate of pericardial hemorrhage and tamponade than type A AAD (1). The reported incidence of pericardial effusion associated with IMH is variable and may reach up to 60%–66% (7). When type A IMH is complicated with aortic dissection, the incidence of hemopericardium increases (6). Cardiac tamponade is seen in approximately 31%–49% of cases of type A IMH, while only in 18%–30% of cases of type A AAD (7, 8).
The pathologic basis for development of pericardial effusion and cardiac tamponade in IMH patients is not completely understood. It is likely that wall thickness and elastic properties are affected to a higher degree in patients with IMH than in those with AAD; therefore, putting the former at an increased risk for periaortic hematoma, pericardial effusion, and aortic rupture (3, 8).
The central CT finding in hemopericardium is the presence of dense pericardial fluid in varying amounts (Fig. 1). Imaging manifestations of cardiac tamponade include flattening of the walls of the right ventricle and/or atrium, straightening of the interventricular septum, a dilated inferior vena cava, and reflux of intravenous contrast material into the inferior vena cava, azygos, and hepatic veins (Fig. 3).
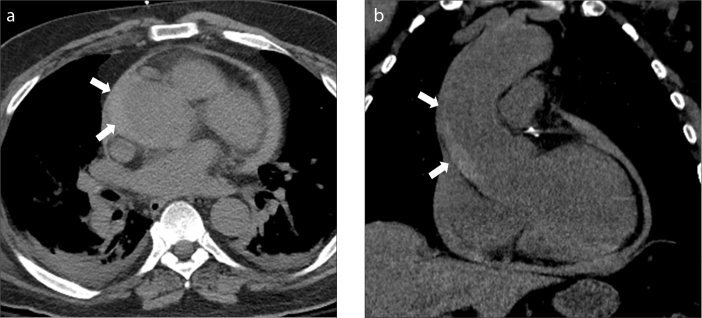
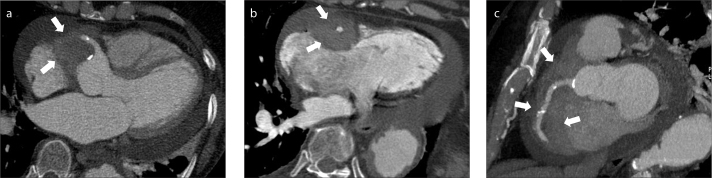
Figure 3. a–c.
Type A intramural hematoma complicated by hemopericardium and cardiac tamponade. Axial contrast-enhanced CT images through the ascending aorta (a), heart (b) and upper abdomen (c) show a large type intramural hematoma (a, arrow) with an associated large hemopericardium (b, asterisk). There is marked mass effect upon the right atrium (b, black arrow) along with flattening of the free wall of the right ventricle. Image (c) through the upper abdomen shows severe reflux of contrast media into the inferior vena cava and hepatic veins. The above findings constitute tamponade physiology.
Hematoma along the pulmonary arteries
Pulmonary artery extension of IMH is a complication of Stanford type A IMH, most commonly seen in severe or extensive IMH. The ascending aorta and main pulmonary artery share a common adventitial layer, which can be violated by an aortic IMH allowing extension of the extravasated blood into the wall of the main pulmonary artery. Anatomically, this process is more likely to occur in cases of rupture of the posterior wall of the ascending aorta (9).
On unenhanced CT, IMH involving the adventitia of the pulmonary artery appears as hyperattenuating hematoma extending along the surface of the main and branch pulmonary arteries (Fig. 4). This appearance may mimic chronic pulmonary embolism on contrast-enhanced CT. The enlarging hematoma may cause partial luminal narrowing of the pulmonary artery. Blood may extend along the pulmonary arterial system to reach the distal airways and may result in alveolar hemorrhage (9). This finding typically resolves spontaneously.
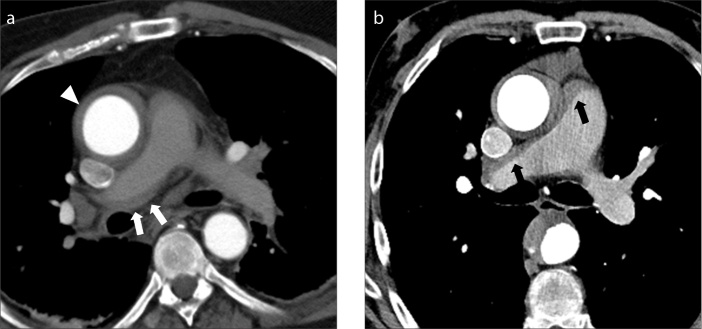
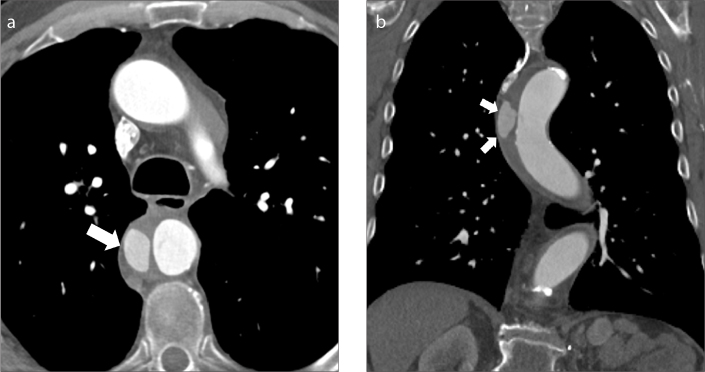
Figure 4. a, b.
Extension of intramural hematoma along the pulmonary arteries. Axial contrast-enhanced CT images in two different patients (a, b) show type A aortic intramural hematoma (a, arrowhead) and hyperdense collections along the main and right pulmonary arteries (a, b, arrows).
Hematoma along the coronary arteries
Extension of IMH along the walls of the coronary arteries is a rare complication. Few case reports of secondary extension to the coronary arteries have been published, most of them associated with aortic dissection. According to Neri et al. (10), coronary malperfusion in the setting of acute aortic dissection can be classified into three different types, which may be true in IMH as well: type A, coronary compression; type B, retrograde dissection; and type C, coronary artery detachment. Of all the patients included in the International Registry of Acute Aortic Dissection, 3.3% of the patients presented with ST-elevation myocardial infarction (STEMI) due to periaortic hematoma causing external compression of the coronaries (2).
Prompt recognition of acute coronary syndrome in IMH is of utmost importance, as primary coronary intervention and thrombolytic therapy are strictly contraindicated in these patients (2).
In some cases, the hematoma may dissect along the epicardial tissues, subjacent to the pericardium. This is seen as high attenuation fluid that may follow the course of the coronary arteries (Fig. 5).
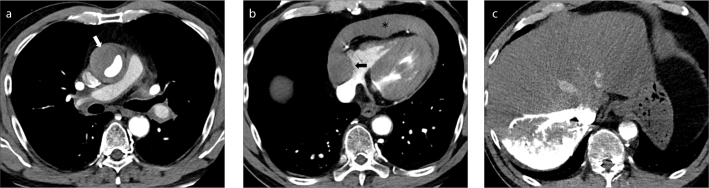
Figure 5. a–c.
Hematoma extension along the epicardial vessels in a patient with type A intramural hematoma. Axial contrast-enhanced CT images through the proximal (a), and mid (b) portions of the right coronary artery (RCA) and maximum intensity projection (MIP) multiplanar reformation (c) show high density fluid extending along much of the length of the RCA (arrows).
Ulcer-like projection
An ulcer-like projection (ULP) is a complication of IMH characterized by a new focus of intimal disruption in an area of high shear stress with no underlying atherosclerotic disease. ULPs are characteristically not seen at initial imaging, a feature that aids in distinction from penetrating atherosclerotic ulcer, which is always present at initial CT and is generally seen in patients with severe aortic atherosclerosis (4, 11). The mean rate of progression to ULP is approximately 25.3% (5).
On CT images, ULP can be seen as a localized blood-filled pouch protruding from the true lumen into the thrombosed aortic wall, enhancing to the same degree as the aortic lumen after contrast administration (12). These lesions characteristically have a wide connection with the aortic lumen (Fig. 6) (11, 12).
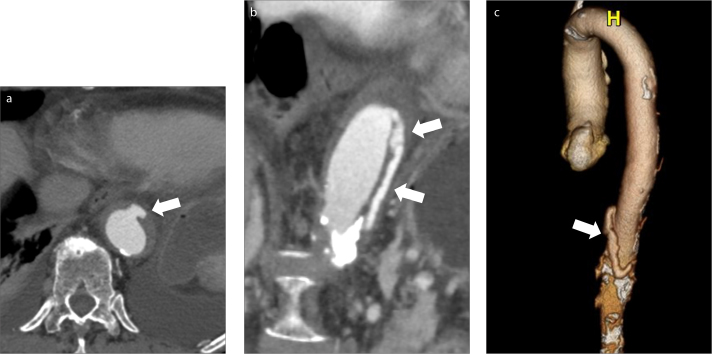
Figure 6. a–c.
Type B aortic intramural hematoma and ulcer-like projection (ULP). Axial contrast-enhanced CT image (a) through the descending aorta shows an outpouching off the aortic lumen in the region of the patient’s IMH (arrow). Double oblique image (b) and 3D surface rendering (c) show the caudal extension of the ULP, as it dissects through the aortic wall (arrows).
ULPs have been shown to be associated with an increased risk of complications of IMH including aneurysm formation, dissection and rupture (Fig. 6). The incidence of progression is directly proportional to the diameter, depth, and location of these lesions. A ULP with a diameter of more than 20 mm, depth of more than 10 mm, and those located in the ascending aorta and arch, are associated with highest risk (11, 12). In patients presenting with type B IMH, the more proximal the location of an ULP, the higher the risk for future aorta-related events. The recommended management in patients with ULP without persistent pain or signs of aortic rupture is medical treatment with close imaging follow-up every 3 months. Once the ULP has become stable, follow-up can be made every 6 months and annually after the third year. While indications for endovascular treatment are not well established, a ULP with a depth of >10 mm requires closer follow-up and those with a depth of >15 mm should prompt consideration for endovascular or surgical intervention (5, 13).
Intramural blood pool
An intramural blood pool (IBP) is defined as a focal contrast-enhancing collection within an intramural hematoma with a narrow or nondiscernible communication with the aortic lumen. These lesions are most commonly seen in the descending and abdominal aorta associated with acute IMH with a wall thickness greater than 10 mm and are believed to represent collections related to an aortic branch pseudoaneurysm or tear (12, 14).
Distinguishing features of an IBP from a ULP include a narrow communication with the aortic lumen, diameter usually less than 2 mm, and a definite communication between the IBP and an emerging intercostal or lumbar artery (Figs. 7, 8). The appearance of an aorta with IMH and intramural blood pools at multiple levels has been described as the “Chinese ring-sword sign” (14).
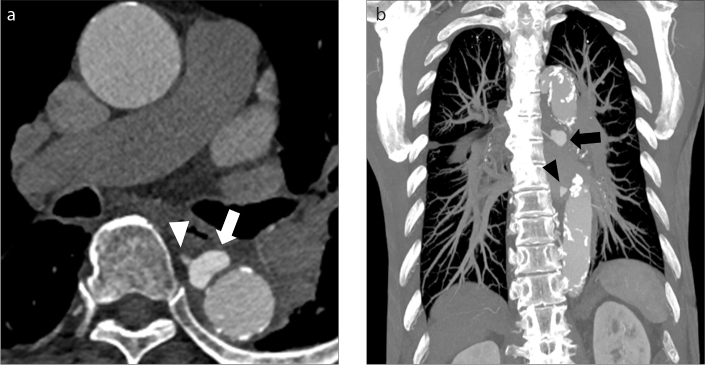
Figure 7. a, b.
Intramural blood pool. Axial (a) and coronal (b) CT images through the descending aorta in an 85-year-old man with type B aortic IMH obtained three weeks after initial presentation show a collection of contrast outside of the lumen but within the right aspect of the aortic wall (arrows). Notice there is no obvious communication with the aortic lumen.
Figure 8. a, b.
Intramural blood pools. Axial contrast-enhanced CT image (a) through the descending thoracic aorta shows an extraluminal collection of contrast within the aortic wall compatible with an IBP (arrow). Furthermore, the collection directly communicates with an intercostal artery (arrowhead). Coronal MIP image of the descending thoracic aorta (b) shows two separate intramural blood pools along the right aspect of the aorta (arrow and arrowhead).
The prognostic significance of IBPs remains uncertain but does not appear to be associated with an increased risk of IMH progression, need for surgery, or increased mortality. The presence of new IBP at follow-up CT could be a predictor of incomplete resorption of an IMH (3, 14).
Aneurysmal dilatation
The most frequent long-term complication of IMH is the development of aortic aneurysm, usually in the subacute or chronic stages of the disease. The subsequent development of fusiform aneurysms in IMH patients has been associated with larger aortic diameters in the acute phase of IMH, likely because of structural weakness of the media aggravated by mechanical stress. Evolution into fusiform aneurysms is also associated with the patient’s underlying atherosclerotic disease and presence of plaque ulceration (4). The development of saccular aneurysms from underlying ULPs is considered an early complication. From a pathologic standpoint, these saccular dilatations represent a direct communication between the aortic lumen and the media, as the intima is disrupted. In cases not associated with ULPs, the development of fusiform aneurysmal dilatation is considered a late complication secondary to structural weakness of the media (4).
Aneurysmal dilatation of the aorta in combination with IMH is considered an independent risk factor for adverse events such as expansion, progression to aortic dissection, rupture, incomplete resolution, need for surgery, and death. Its presence warrants close long-term imaging follow-up when no IMH regression is observed during the first 6 months of follow-up (3). Large series accept invasive intervention in patients with aortic enlargement (mean aortic diameter >55 mm or rapid enlargement) during follow-up (5).
Conclusion
The purpose of this pictorial essay was to familiarize the radiologist with imaging manifestations, complications, and prognostic features associated with IMH. While less common than aortic dissection syndromes, patients with IMH present with similar clinical symptoms, and CT imaging is critical in diagnosis, classification, and guiding prognosis and management. The radiologist therefore plays an important role in patient management and must be familiar with CT protocols, diagnostic criteria, classification, and prognostic factors necessary for optimal patient care.
Main points.
CT imaging findings that help predict progression of an aortic intramural hematoma (IMH) to overt dissection include type A aortic IMH, mean IMH thickness >10–11 mm, compression of the true aortic lumen, a maximal ascending aortic diameter >50 mm, descending aortic diameter >45 mm and the presence of pericardial effusion.
In the setting of a type A aortic intramural hematoma, a detailed evaluation for signs of tamponade physiology is warranted. CT imaging signs of cardiac tamponade include flattening of the walls of the right ventricle and/or atrium, straightening of the interventricular septum, a dilated inferior vena cava, and reflux of intravenous contrast material into the inferior vena cava, azygos, and hepatic veins.
Differentiation between ulcer-like projection and intramural blood pool is important as the former can progress to overt aortic dissection, aneurysm or rupture, requiring imaging follow-up, while the latter has not been shown to increase the risk of IMH progression or mortality.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Harris KM, Braverman AC, Eagle KA, et al. Acute aortic intramural hematoma: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2012;126:S91–96. doi: 10.1161/CIRCULATIONAHA.111.084541. [DOI] [PubMed] [Google Scholar]
- 2.Alomari IB, Hamirani YS, Madera G, Tabe C, Akhtar N, Raizada V. Aortic intramural hematoma and its complications. Circulation. 2014;129:711–716. doi: 10.1161/CIRCULATIONAHA.113.001809. [DOI] [PubMed] [Google Scholar]
- 3.Gutschow SE, Walker CM, Martinez-Jimenez S, Rosado-de-Christenson ML, Stowell J, Kunin JR. Emerging concepts in intramural hematoma imaging. Radiographics. 2016;36:660–674. doi: 10.1148/rg.2016150094. [DOI] [PubMed] [Google Scholar]
- 4.Chao CP, Walker TG, Kalva SP. Natural history and CT appearances of aortic intramural hematoma. Radiographics. 2009;29:791–804. doi: 10.1148/rg.293085122. [DOI] [PubMed] [Google Scholar]
- 5.Evangelista A, Czerny M, Nienaber C, et al. Interdisciplinary expert consensus on management of type B intramural haematoma and penetrating atherosclerotis ulcer. Eur J Cardiothorac Surg. 2015;47:209–217. doi: 10.1093/ejcts/ezu386. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Choi SJ, Kim JH, et al. Useful CT findings for predicting the progression of aortic intramural hematoma to overt aortic dissection. J Comput Assist Tomogr. 2001;25:295–299. doi: 10.1097/00004728-200103000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Uchida K, Imoto K, Takahashi M, Suzuki S, Isoda S, Sugiyama M, et al. Pathologic characteristics and surgical indications of superacute type a intramural hematoma. Ann Thorac Surg. 2005;79:1518–1521. doi: 10.1016/j.athoracsur.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita A, Fukui T, Tabata M, Sato Y, Takanashi S. Preoperative characteristics and surgical outcomes of acute intramural hematoma involving the ascending aorta: a propensity score-matched analysis. J Thorac Cardiovasc Surg. 2016;151:351–358. doi: 10.1016/j.jtcvs.2015.09.055. [DOI] [PubMed] [Google Scholar]
- 9.Shiau EL, Wu FZ, Huang YL, Wu MT. Aortic intramural hematoma with pulmonary artery extension mimics pulmonary embolism. Am J Emerg Med. 2013;31:1538 e3–4. doi: 10.1016/j.ajem.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 10.Neri E, Toscano T, Papalia U, et al. Proximal aortic dissection with coronary malperfusion: presentation, management and outcome. J Thorac Cardiovasc Surg. 2001;121:552–560. doi: 10.1067/mtc.2001.112534. [DOI] [PubMed] [Google Scholar]
- 11.Sueyoshi E, Matsuoka Y, Imada T, Okimoto T, Sakamoto I, Hayashi K. New development of an ulcerlike projection in aortic intramural hematoma: CT evaluation. Radiology. 2002;224:536–541. doi: 10.1148/radiol.2242011009. [DOI] [PubMed] [Google Scholar]
- 12.Kruse MJ, Johnson PT, Fishman EK, Zimmerman SL. Aortic intramural hematoma: review of high-risk imaging features. J Cardiovasc Comput Tomogr. 2013;7:267–272. doi: 10.1016/j.jcct.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Kitai T, Kaji S, Yamamuro A, et al. Impact of new development of ulcer-like projection on clinical outcomes in patients with type B aortic dissection with closed and thrombosed false lumen. Circulation. 2010;14:S74–80. doi: 10.1161/CIRCULATIONAHA.109.927517. [DOI] [PubMed] [Google Scholar]
- 14.Wu MT, Wang YC, Huang YL, et al. Intramural blood pools accompanying aortic intramural hematoma: CT appearance and natural course. Radiology. 2011;258:705–713. doi: 10.1148/radiol.10101270. [DOI] [PubMed] [Google Scholar]