Abstract
PURPOSE
We aimed to quantitatively evaluate volumetric metabolic tumor burden including metabolic tumor volume and total lesion glycolysis in different molecular subtypes of breast cancer using 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT).
METHODS
This study involved 99 female patients with pathologic diagnosis of primary breast cancer, who underwent 18F-FDG PET/CT before any therapy. Patients were divided into subtypes of luminal A, luminal B, ERBB2+, and basal-like based on the immunohistochemistry results. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) before and after correction for lean body mass were achieved and compared. Correlations between metabolic tumor burden and Ki-67 were analyzed and diagnostic performances of volumetric metabolic parameters were evaluated.
RESULTS
TLG values were significantly different between each molecular subtype, while MTV values were not. Values of TLG were significantly reduced after normalizing for lean body mass in each subtype. Both of them showed correlations with Ki-67 and presented high diagnostic ability in identifying patients with basal-like breast cancer from the rest. TLGs before and after normalizing for the lean body mass had similar diagnostic performances in differentiating patients of basal-like subtype from the rest.
CONCLUSION
Metabolic tumor burden could comprehensively reflect tumor metabolic differences of molecular subtypes of breast cancer, and it can serve to help differentiate patients with basal-like breast cancer.
Breast cancer is the most common cancer for women in the world and it is the second leading cause of cancer death among women according to the data of World Health Organization. Patients’ prognosis and therapeutic response are highly variable due to the heterogeneity of breast cancer, so that personalized targeted therapy is urgently needed to improve the efficiency of disease control. For more individualized and precise treatment, breast cancer is divided into four subtypes, i.e., luminal A, luminal B, ERBB2+ and basal-like (triple negative), according to tumor cells’ molecular and genetic information (1). Of the four subtypes, hormone receptor positive tumors (including luminal A and luminal B) tend to have better prognosis, while the basal-like tumors often have a poor prognosis. ERBB2+ subtype refers to human epidermal growth factor receptor 2 (HER2) positive tumors, which tend to be more aggressive and have a relatively poorer prognosis than the hormone receptor positive subgroups. Basal-like subtype seems to be worst in prognosis and therapy resistant (2–4).
Structural imaging is commonly used to detect breast lesions and describe the anatomic features but it has been well known that functional abnormalities including metabolic changes emerge much earlier than the morphologic changes of breast cancer. As one of the molecular imaging modalities, positron emission tomography/computed tomography (PET/CT), has greatly improved the sensitivity and specificity of diagnosis by providing both anatomical and metabolic information. In the last decade 18F-fluorodeoxyglucose (FDG) PET/CT has been widely used in clinical practice to characterize and stage tumors noninvasively. It is able to identify cancer in its early stage, reflect the glycolytic changes of tumors, and simultaneously evaluate the whole body’s responses to the tumors in vivo. Standardized uptake value (SUV), as a semiquantitative index in PET/CT to demonstrate the uptake of glucose in tumors/normal tissues, has been popularly accepted by nuclear physicians in daily use but it remains questionable because of several reasons. First, the semiquantitative SUVmax is a sensitive indicator of metabolic activity and tumor proliferation in breast cancer; however, it is the SUV on the highest image pixel, reflecting a single-pixel value of the maximum intensity of 18F-FDG activity in the tumor, ignoring the extent of metabolic abnormality and changes in the distribution of a tracer within the whole tumor mass (5, 6). Second, SUV is calculated based on the whole-body weight metric. Fat contributes to body weight without accumulating 18F-FDG in the fasting state, leading to overestimated SUV in obese patients compared with normal or underweight patients (7). Third, studies have reported that SUV could be influenced by many factors and SUVmax is not reliable and recommendable because of its poor reproducibility (3%±11%) (8–10). To overcome these controversies, researchers recommended the volume-based variables such as metabolic total volume (MTV) and total lesion glycolysis (TLG) to reflect the metabolic activities within the whole tumor mass; instead of the whole-body weight, the administered dose should be based on volume-based parameters that are corrected by the lean body mass (LBM) (11, 12).
Studies have reported differences of SUV-based variables and metabolic tumor burden in breast cancer (2, 3, 13). To our knowledge, few studies have addressed the changes and characteristics of metabolic tumor burden and LBM corrected quantitative volume-based parameters in different molecular subtypes of breast cancer. The purpose of this study was to retrospectively investigate the molecular subtypes of breast cancer with volumetric metabolic parameters on 18F-FDG PET/CT, including MTV and TLG before (bodyweight TLG, TLGBW) and after normalizing for LBM (TLGLBM). Associations between the quantitative parameters and tumor biological characteristics of primary breast cancer were evaluated as well. The performances of SUV before and after normalizing for LBM were both assessed as well.
Methods
Patient selection
Ninety-nine cases of primary breast cancer between January 2015 to January 2016 in our institute and hospital were reviewed. All of the patients were pathologically diagnosed as primary breast cancer by core needle biopsy and immunohistochemistry (IHC) test was performed consecutively. The patients had the examination of 18F-FDG PET/CT before operation and/or other therapeutic interventions. Each patient was required to sign the informed consent form before the examination. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee.
Molecular imaging acquisition and analysis
All 18F-FDG PET/CT scans were performed with a 64 multislice-detector PET/CT scanner (DiscoveryST, GE Healthcare). Patients fasted 4–5 hours before the examination. Blood glucose was examined and controlled under the level of 140 mg/dL prior to the injection of 18F-FDG (3.7 MBq/kg). CT scanning was performed with the following parameters: current 120–160 mA, voltage 120 kV, slice thickness 5 mm or 3.75 mm, and reconstruction interval 5 mm or 3.75 mm. Afterwards, PET images were acquired in the three-dimensional mode with 2 min per bed position without holding breath from head to the midthigh. The CT-based attenuation-corrected PET images were reconstructed using an iterative algorithm, with 128×128 matrix and 3.75 mm reconstruction slice thickness. All images, including CT, PET, and fusion images after partial volume effect correction, were reviewed by two nuclear medicine physicians with 7 and 10 years of experience in nuclear diagnosis, respectively. The masses in breasts were selected as the region of interest (ROI) and the largest lesion was chosen in patients with multifocal or multicentric foci. The average diameter (the average value of maximum and minimum diameter) of ROI was measured. Volume-based parameters including MTV, SUVmax, SUVmean (mean SUV), SULpeak (peak SUV normalized for LBM) and SULmean (mean SUV normalized for LBM) were obtained using PET VCAR, semiquantitative software that is imbedded in GE workstation (estimated threshold for discrimination of tumors was chosen as equaling to or more than 42% of SUVmax). TLGBW (TLGBW=MTV× SUVmean) and TLGLBM (TLGLBM=MTV× SULmean) were calculated based on above formulas, respectively. The performances of SUVmax and SULpeak were both assessed as references. Metastasis including ipsilateral or contralateral axillary and other lymph nodes and distant metastases were observed as well.
Categorization of molecular subtypes
According to the molecular characteristics-based classification in the 12th International Breast Cancer Conference in 2011, all patients were divided into four subtypes: 1) luminal A: hormone receptor ER and/or PR positive (ER or PR expression >1%), HER2 negative (“HER2 0” or “HER2+”), and low expression of Ki-67 (<14%); 2) luminal B: a) hormone receptor ER and/or PR positive, HER2/neu positive (“HER2+++”, or “HER2++” but “FISH+”), b) hormone receptor ER and/or PR positive, HER2/neu negative and high expression of Ki-67 (≥14%); 3) ERBB2 + : hormone receptors negative, HER2/neu positive; and 4) Basal-like (triple negative): hormone receptors and HER2 negative. Biologic prognostic parameters, including proliferation rate (Ki-67) and the expression level of p53, were obtained as well.
Statistical analysis
The software package SPSS 16.0 (SPSS Inc.) was used for statistical analysis and graphics. The inter-rater agreement rate was evaluated using the interclass correlation coefficient (ICC) on acquisition of data of all patients, with raters as independent variables. TLG before and after correction for LBM were compared using the pairwise t test. Group differences of volume-based parameters in each molecular subtype were compared using analysis of variance (ANOVA) for normally distributed data and Turkey comparison was further performed for post hoc test. If the variables were not normally distributed, Kruskal-Wallis test was performed instead of ANOVA followed by Mann Whitney U test for post hoc comparison. Interrelations between variables were performed using Pearson correlation analysis. Diagnostic performances of both TLGBW and TLGLBM were evaluated using the area under the curve (AUC) and receiver operating characteristic curve (ROC). A cutoff value was determined and the sensitivity, specificity and accuracy of this level were calculated at this single point of the ROC curve. Generally, the level of statistical significance was set at P < 0.05 (two-tailed) for ANOVA test. Bonferroni correction was performed for the Mann Whitney U test and the new level of statistical significance was set separately following the formula: 0.05/[N*(N−1)/2].
Results
All subjects in this study were female with breast cancer in single breast, with age ranging from 29 to 80 years (median, 52 years; mean, 52.0±9.9 years). Most patients (n=72, 72.7%) were between 40 and 60 years; number of patients younger than 40 years and older than 60 years were 11 (11.1%) and 16 (16.2%), respectively. Among the patients, 4 (4.3%) had other malignant cancers historically, such as cancer in the contralateral breast (n=1), colon (n=1), cervix (n=1), and stomach (n=1). According to the IHC results, luminal A, luminal B, ERBB2+, and basal-like accounted for 27.3%, 34.7%, 19.2%, and 19.2% of cases, respectively (Table). Metastasis of lymph nodes was found in 80.8% (n=80) of patients and distant metastases were observed in 23.2% (n=23) of patients.
Table.
Demographics and quantitative metabolic parameters of patients in molecular subtypes of breast cancer and pairwise comparisons of the metabolic tumor burden and metabolic parameters between each molecular subtype of breast cancer
| Group comparison | Pairwise comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Luminal A (LA) | Luminal B (LB) | ERBB2+ (HER2) | Basal-like (BL) | F/Z | P | Pairs | P | |
| n of subjects | |||||||||
| n (%) | 99 | 27 (27.3) | 34 (34.3) | 19 (19.2) | 19 (19.2) | _ | _ | _ | _ |
|
| |||||||||
| Age (years) | |||||||||
| Median (min–max) | 52 (29–80) | 57 (31–80) | 52 (29–75) | 52 (38–68) | 48 (33–63) | F=0.68 | 0.569 | _ | _ |
| Mean±SD | 51.89±9.95 | 54.15±11.77 | 51.47±10.26 | 51.00±7.76 | 50.37±8.66 | ||||
|
| |||||||||
| MTV (cm3) | |||||||||
| Median (min–max) | 10.27 (1.09–191.00) | 9.56 (1.96–107.00) | 12.35 (2.52–73.46) | 6.87 (1.09–73.11) | 17.31 (2.61–191.00) | Z=1.76 | 0.175 | _ | _ |
| Mean±SD | 21.57±28.29 | 20.55±26.68 | 19.75±18.53 | 13.98±18.53 | 33.89±45.31 | ||||
|
| |||||||||
| TLGBW | |||||||||
| Median (min–max) | 46.48 (2.64–1286.41) | 39.99 (5.16–394.65) | 58.33 (7.33–1161.43) | 24.11 (2.64–278.86) | 148.22 (8.30–1286.41) | LA-LB LA-HER2 LA-BL LB-HER2 LB-BL HER2-BL |
0.045 0.664 0.006* 0.041 0.164 0.006* |
||
| Mean±SD | 145.88±245.80 | 67.22±93.62 | 151.63±221.26 | 71.59±91.13 | 321.67±412.84 | Z=2.73 | 0.007* | ||
|
| |||||||||
| TLGLBM | |||||||||
| Median (min–max) | 32.93 (1.68–882.02) | 24.10 (3.56–281.12) | 39.20 (5.27–757.51) | 17.61 (1.68–199.84) | 105.42 (6.43–882.02) | LA-LB LA-HER2 LA-BL LB-HER2 LB-BL HER2-BL |
0.041 0.713 0.005* 0.051 0.138 0.005* |
||
| Mean±SD | 100.88±165.23 | 46.63±65.15 | 105.41±149.57 | 49.89±63.49 | 220.84±273.99 | Z=−2.80 | 0.006* | ||
|
| |||||||||
| SUVmax | |||||||||
| Median (min–max) | 9.80 (2.38–66.81) | 6.58 (2.50–42.93) | 10.76 (2.58–30.62) | 9.44 (2.38–27.35) | 15.79 (6.11–66.81) | LA-LB LA-HER2 LA-BL LB-HER2 LB-BL HER2-BL |
0.003* 0.177 <0.001* 0.161 0.069 0.007* |
||
| Mean±SD | 11.80±8.69 | 8.94±7.94 | 12.46±6.87 | 10.80±6.84 | 18.12±13.59 | Z=−4.03 | <0.001* | ||
|
| |||||||||
| SULpeak | |||||||||
| Median (min–max) | 4.95 (1.05–37.53) | 3.15 (1.19–8.85) | 5.42 (1.30–17.18) | 4.50 (1.05–14.44) | 8.11 (3.11–37.53) | LA-LB LA-HER2 LA-BL LB-HER2 LB-BL HER2-BL |
0.002* 0.200 <0.001* 0.111 0.107 0.005* |
||
| Mean±SD | 6.14±4.92 | 3.79±2.33 | 6.61±3.87 | 5.00±3.42 | 9.79±7.79 | Z=−4.18 | <0.001* | ||
SD, standard deviation; MTV, metabolic tumor volume; TLGBW, total lesion glycolysis of body weight; TLGLBM, total lesion glycolysis normalized for lean body mass; SUVmax, maximum standardized uptake value; SULpeak, peak SUV normalized for lean body mass.
Statistically significant difference.
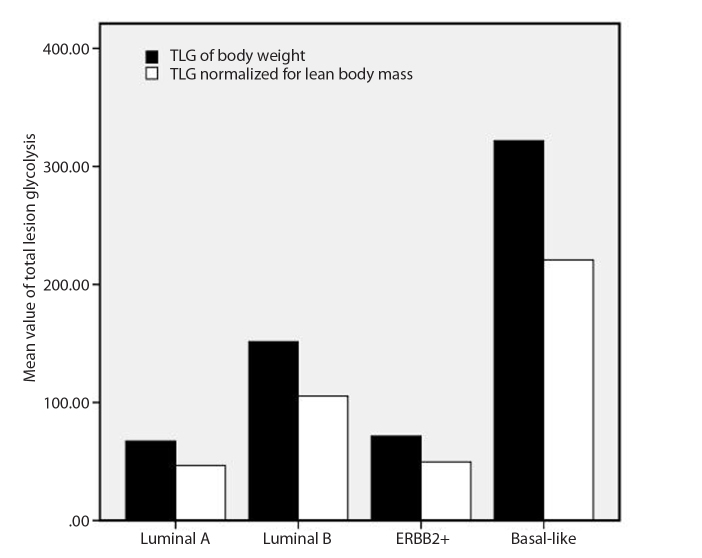
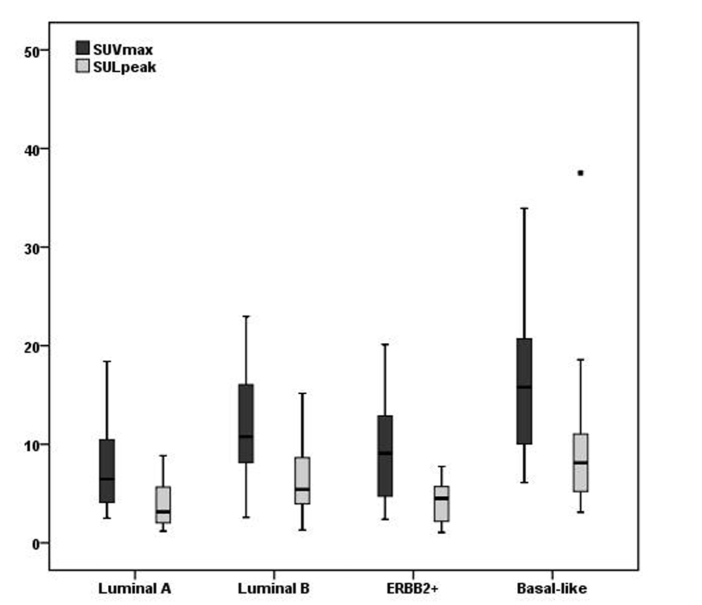
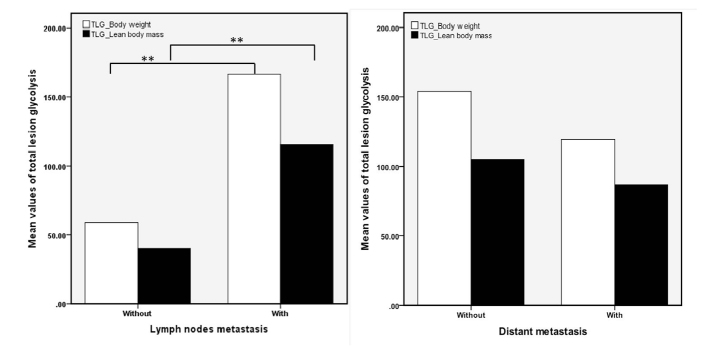
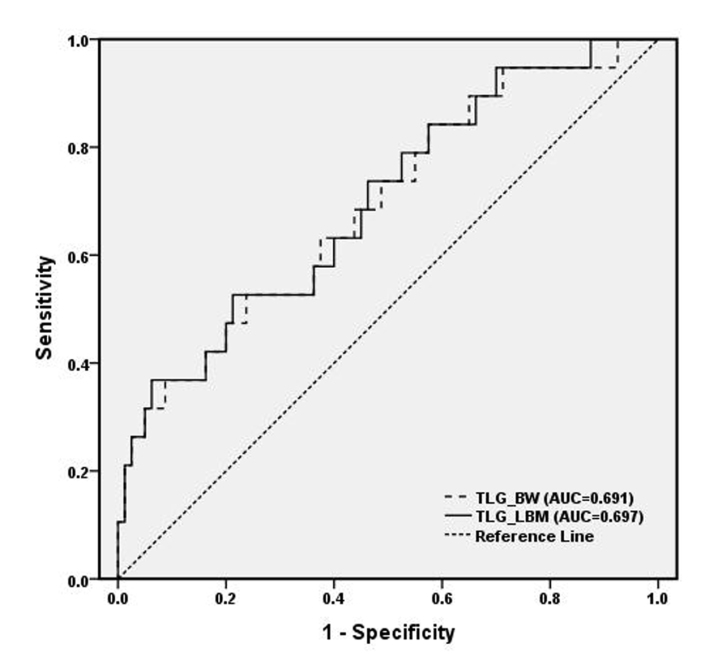
The inter-rater agreement analysis showed high reliability (ICC, 0.98; 95% CI, 0.81–0.94) for the measurement of MTV and TLG. The volume-based data showed that MTV differed in each molecular subgroup with large variability, but did not reach statistical significance, whereas the average size of the tumors was significantly different between the four subgroups (χ2=1.76, P = 0.175) (Table). A high correlation was found between MTV and size measurement (r=0.74, P < 0.001). Furthermore, pairwise comparisons of TLGBW (145.88±245.80) and TLGLBM (100.88±165.23) showed that the TLG values were significantly reduced after normalizing for LBM in all subjects (Z=−8.64, P < 0.001), as well as in each subtype (P = 0.001–0.006) (Fig. 1). Values of TLG before and after normalizing for LBM were both significantly different within the four molecular subtypes (Z=−2.73, P = 0.007 for TLGBW; Z=−2.80, P = 0.006 for TLGLBM) (Table). After normalizing for LBM, the SULpeak was lower than the SUVmax and it was significantly different between each subgroup (Fig. 2, Table). Significant group and pairwise differences were shown in TLGBW, TLGLBM, SUVmax and SULpeak between the luminal A and basal-like subgroups and between ERBB2+ and basal-like subgroups (details in the Table). The patients with lymph node involvement had significantly higher TLGBW (166.56±265.14 vs. 58.80±102.14, Z=−3.00, P = 0.003) and TLGLBM (115.32±178.12 vs. 40.06±67.53, Z=−2.95, P = 0.003) than those without lymph node metastases, whereas no significant difference of either TLGBW or TLGLBM was found between patients with and without distant organ metastases (Fig. 3). Correlation was found between TLGBW and the expression level of Ki-67 (r=0.26, P = 0.009) and between TLGLBM and Ki-67 after normalizing for LBM (r=0.27, P = 0.007), but without significant advantage in TLGBW or TLGLBM. However, neither TLGBW nor TLGLBM was associated with the expression level of p53. As for the diagnosing ability, the values of TLGBW and TLGLBM had similar performance in differentiating patients of basal-like subtype from those in non-basal-like subtypes with the AUC of 0.697 for TLGLBM (95% CI, 0.564–0.831) and 0.691 for TLGBW (95% CI, 0.556–0.827) (Fig. 4). Using the cutoff values of TLGLBM=101.46 and TLGBW=142.93, the sensitivity, specificity and accuracy values were 52.6%, 78.8%, 73.7%, and 52.6%, 76.3%, 71.7%, respectively. The sensitivity, specificity, and accuracy of SUVmax were 73.7%, 70.0%, and 69.7%, respectively, when using the cutoff value of SUVmax=11.6, whereas the sensitivity, specificity and accuracy of SULpeak were 73.7%, 67.5%, and 68.7%, respectively, when using the cutoff value of SULpeak=6.16.
Figure 1.
Comparison of total lesion glycolysis (TLG) before and after normalizing for lean body mass in different molecular subgroups of breast cancer.
Figure 2.
Differences of standardized uptake values before and after normalizing for lean body mass in different molecular subgroups of breast cancer. SUVmax, maximum standardized uptake value; SULpeak, peak SUV normalized for lean body mass.
Figure 3.
Differences of mean value of total lesion glycolysis in patients with and without lymph nodes (the left panel) and distant metastases (the right panel).
Figure 4.
The receiver operating characteristic curves of total lesion glycolysis before (TLG_BW) and after (TLG_LBM) normalizing for lean body mass to identify patients in the basal-like subtype.
Discussion
Studies have demonstrated that 18F-FDG PET/CT could be utilized to measure the metabolic abnormalities in patients with breast cancer (13). Our study made a retrospective analysis on metabolic tumor burden in IHC-defined molecular subtypes of primary breast cancer. They displayed different features of metabolic tumor volume and total lesion glycolysis with high heterogeneity. Among these subtypes, the basal-like group showed youngest age at diagnosis, largest MTV, and highest TLG.
Glucose uptake, which is a hallmark of cancers, increases with malignancy through up-regulation of membrane glucose transporters and improving the activity of hexokinase. It is usually evaluated on FDG-PET by calculating SUV in the tumor and SUVmax is the most commonly used parameter in clinical trials. However, tumor metabolic burden in terms of MTV and TLG have been reported to be capable of comprehensively reflecting glucose uptake within the whole tumor rather than a single-pixel value of 18F-FDG activity (SUVmax). They were adopted as the optimal parameters for therapeutic evaluation by PET Response Criteria in Solid Tumors (PERCIST) (14). Our data showed that the volume-based MTV could serve as a variable in the evaluation of initial breast cancer although the difference of MTV in each subtype did not reach statistical significance, with the lowest values of mean and median MTV in the subgroup of ERBB2+. MTV is able to reflect the metabolic volume, which is the FDG-avid volume in the tumor, rather than the size of the mass. It correlates with the size of the mass but provides more accurate measurement than the maximum or minimum diameters, especially for lesions with non-FDG-uptake necrosis inside.
Obesity is becoming an increasingly frequent problem in public health worldwide. Based on numerous meta-analyses, strong evidence supported the association of obesity with 11 types of cancer, including breast cancer, especially postmenopausal breast cancer (15, 16). Furthermore, being overweight was reported to increase risk of basal-like breast cancer and decrease risk of luminal A and B subtypes in premenopausal women (17). This fact makes it more prominent and important to apply LBM normalized FDG uptake values to avoid the adiposity-induced bias in evaluating the metabolic changes in breast cancer. SUL, the SUV normalized for LBM, has been recognized as the optimal parameter instead of SUV by PERCIST for therapeutic evaluation. In this study, SULpeak showed comparable performance with SUVmax, without significant advantage in the initial evaluation of breast cancer. Is TLG after correcting for LBM more reliable and feasible for clinical trials of breast cancer than non-corrected TLG? Are they also suitable for initial assessment of breast cancer? Our study showed that both TLGBW and TLGLBM could be used in the initial evaluation of metabolic changes and tumor glycolytic activity in different molecular subtypes of primary breast cancer other than reflecting response to therapy.
Ki-67 index is a proliferation-associated antigen and has been accepted as a reliable marker to measure tumor cell proliferation and prognosis (5). Studies have demonstrated that there was a significant correlation between 18F-FDG uptake and Ki-67 (18–21). Our data confirmed this positive relationship between Ki-67 and TLG, indicating that TLG might be correlated with the prognosis of breast cancer. It is well known that basal-like breast cancer is associated with aggressive behavioral features. In our study, people in this group demonstrated younger age at onset, larger size/volume, higher proliferation, and worst prognosis than the other subgroups, indicating the prominent importance of making an accurate differentiating diagnosis. Our data confirmed that both TLGBW and TLGLBM had high ability to distinguish patients of basal-like from those of non-basal-like. They had similar performance, but TLGLBM showed a slight superiority to TLGBW.
There are several limitations to this study. It was a retrospective cohort so that it had several unavoidable biases. A larger sample of patients with breast cancer needs to be included for a better understanding of the molecular imaging features in different IHC-based subtypes of breast cancer. In addition, therapeutic evaluation with the volume-based parameters should be added in further studies.
In conclusion, according to our data, the volumetric variables including MTV and TLG both showed good performances to reflect and evaluate metabolic changes and tumor glycolytic activity in different molecular subtypes of primary breast cancer. After normalizing for LNB, TLG can provide more accurate and reliable evaluation of the metabolic changes of breast cancer than those parameters based on body weight. In addition, TLG could serve as a tool to help differentiate patients with the basal-like subtype from the other subtypes of breast cancer.
Main points.
Different features of metabolic tumor volume and total lesion glycolysis were found with high heterogeneity in molecular subtypes of breast cancer.
Metabolic tumor burden could comprehensively reflect the metabolic differences of molecular subtypes of breast cancer.
Total lesion glycolysis could be used to help differentiate patients in basal-like subtype of breast cancer.
Footnotes
Financial disclosure
This work was funded by the China Postdoctoral Science Foundation (No.2015M571271) & the National Natural Science Foundation of China (No.81202795).
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia Vicente AM, Soriano Castrejon A, Leon Martin A, et al. Molecular subtypes of breast cancer: metabolic correlation with (1)(8)F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:1304–1311. doi: 10.1007/s00259-013-2418-7. [DOI] [PubMed] [Google Scholar]
- 3.Kitajima K, Fukushima K, Miyoshi Y, et al. Association between (1)(8)F-FDG uptake and molecular subtype of breast cancer. Eur J Nucl Med Mol Imaging. 2015;42:1371–1377. doi: 10.1007/s00259-015-3070-1. [DOI] [PubMed] [Google Scholar]
- 4.Wolff AC, HM, Hicks DG, Dowsett M, et al. College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger IA, Huser DM, Burger C, et al. Repeatability of FDG quantification in tumor imaging: averaged SUVs are superior to SUVmax. Nucl Med Biol. 2012;39:666–670. doi: 10.1016/j.nucmedbio.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Lodge MA, Chaudhry MA, Wahl RL. Noise considerations for PET quantification using maximum and peak standardized uptake value. J Nucl Med. 2012;53:1041–1047. doi: 10.2967/jnumed.111.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keramida G, Peters AM. Scaling of glomerular filtration rate and SUV for body size: the curious conflict of whole-body metric preferences. J Nucl Med. 2016;57:2028. doi: 10.2967/jnumed.116.176800. [DOI] [PubMed] [Google Scholar]
- 8.Hamberg LM, Hunter GJ, Alpert NM, et al. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35:1308–1312. [PubMed] [Google Scholar]
- 9.Laffon E, Cazeau AL, Monet A, et al. The effect of renal failure on 18F-FDG uptake: a theoretic assessment. J Nucl Med Technol. 2008;36:200–202. doi: 10.2967/jnmt.107.049627. [DOI] [PubMed] [Google Scholar]
- 10.Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804–1808. doi: 10.2967/jnumed.108.054239. [DOI] [PubMed] [Google Scholar]
- 11.Tahari AK, Chien D, Azadi JR, et al. Optimum lean body formulation for correction of standardized uptake value in PET imaging. J Nucl Med. 2014;55:1481–1484. doi: 10.2967/jnumed.113.136986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keramida G, Hunter J, Dizdarevic S, Peters AM. The appropriate whole-body index on which to base standardized uptake value in 2-deoxy-2-[(18)F]fludeoxyglucose PET. Br J Radiol. 2015;88:20140520. doi: 10.1259/bjr.20140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Vicente AM, Perez-Beteta J, Perez-Garcia VM, et al. Metabolic tumor burden assessed by dual time point [18F]FDG PET/CT in locally advanced breast cancer: relation with tumor biology. Mol Imaging Biol. 2017;19:636–644. doi: 10.1007/s11307-016-1034-x. [DOI] [PubMed] [Google Scholar]
- 14.Joo Hyun O, Lodge Martin A, Wahl Richard L. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology. 2016;280:576–584. doi: 10.1148/radiol.2016142043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munsell MF, Sprague BL, Berry DA, et al. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–136. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amadou A, Ferrari P, Muwonge R, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. 2013;14:665–678. doi: 10.1111/obr.12028. [DOI] [PubMed] [Google Scholar]
- 18.Koo HR, Park JS, Kang KW, et al. 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol. 2014;24:610–618. doi: 10.1007/s00330-013-3037-1. [DOI] [PubMed] [Google Scholar]
- 19.Groheux D, Giacchetti S, Moretti JL, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–435. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang CL, MacDonald LR, Rogers JV, et al. Positron emission mammography: correlation of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status and 18F-FDG. AJR Am J Roentgenol. 2011;197:W247–255. doi: 10.2214/AJR.11.6478. [DOI] [PubMed] [Google Scholar]
- 21.Koolen BB, Vrancken Peeters MJ, Wesseling J, et al. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2012;39:1830–1838. doi: 10.1007/s00259-012-2211-z. [DOI] [PubMed] [Google Scholar]