Abstract
PURPOSE
Anal fistula is an abnormal tract or cavity between the anal canal and perianal skin. Surgical treatment of anal fistulas requires the identification of the course of the primary and secondary tracts and their relation with the sphincter musculature in order to appropriately manage them and drain any abscess. Physical examination alone is not as accurate as imaging modalities in detecting these features of the fistula, and recurrences are usually due to missed or inadequately managed infective components. Magnetic resonance imaging (MRI) is the preferred imaging modality for detecting anal fistulas, but which patient group should undergo preoperative MRI is a matter of debate. The aim of this study was to determine the contribution of MRI in the surgical management of anal fistulas.
METHODS
Medical records of patients who underwent surgery for primary anal fistula and preoperative MRI in our University Hospitals from January 1, 2008 to April 15, 2018 were collected anonymously and retrospectively. Any discrepancies between operative findings and MRI reports were noted. Two study groups were formed as per the contribution of preoperative MRI: significant and nonsignificant contribution groups. The significant contribution group included patients with secondary (blind) tracts, horseshoe fistulas, or abscesses undiagnosed at physical examination and examination under anesthesia; those with the location of the internal orifice different from that identified by physical examination; or those with the grade of the fistula assessed to be more advanced after preoperative MRI.
RESULTS
The total number of surgeries was 136. Mean patient age was 43±13 years. There were 106 males. In total, 47 patients suffered from recurrent fistulas. MRI contribution to clinical evaluation was significant in 33.8% of the patients. MRI more frequently provided significant information for complex fistulas than for simple fistulas. Significant preoperative MRI contribution was more frequent if the external opening was more than 2 cm away from the anal canal or when a horseshoe fistula was present.
CONCLUSION
Our study is valuable in linking physical examination findings with preoperative MRI findings. The distance of the external opening from the anal canal was not studied in the literature; our findings support the use of MRI for fistulas with external opening located more than 2 cm from the anus. These fistulas also tend to be complex and have a higher grade. In recurrent cases, MRI contributes not only by establishing the fistula anatomy but also by identifying possible sphincter damage.
Fistula is a nonanatomic connection between two epithelized surfaces. Fistula-in-ano, also referred to as the anal fistula, is an abnormal tract or cavity between the anal canal and perianal skin.
Parks et al. (1) for the first time attempted to classify anal fistulas. Parks’ classification is based on perioperative physical findings and is composed of four grades, namely intersphincteric, transsphincteric, suprasphincteric, and extrasphincteric, with the inter- and transsphincteric grades further divided into subcategories (Table 1). Standard Practice Task Force (SPTF), by the American Society of Colon and Rectal Surgeons, classifies anal fistulas as “simple” and “complex”; the latter category identifying the increased risk for incontinence after surgery (Table 1) (2).
Table 1.
Commonly used fistula classifications
| Parks | SPTF | St. James Hospital |
|---|---|---|
1- Intersphincteric
4- Extrasphincteric fistula |
Simple Complex
|
Grade 1: Simple linear intersphincteric fistula Grade 2: Intersphincteric fistula with an abscess or secondary tract Grade 3: Simple linear transsphincteric fistula Grade 4: Transsphincteric fistula with an abscess or secondary tract in the ischiorectal or ischioanal fossa Grade 5: Supralevator or translevator disease |
SPTF, standard practice task force.
The characteristics of anal fistulas that should be noted during physical examination include the external opening(s), internal opening, main tract, lateral burrowings from the main tract, and presence of other diseases complicating the fistula (3).
As the major cause of fistula-in-ano is cryptoglandular infection, abscess formation is not unusual. Proper manipulations, such as curettage and drainage of blind sinuses, abscess cavities, and accessory tracts, are the key for successful treatment. Physical examination alone may not be sufficient in detecting these features of the fistula, and imaging modalities play a very important complementary role (4).
Fistulography, computed tomography (CT), endoanal ultrasonography (EUS), and magnetic resonance imaging (MRI) may be used to delineate anal fistulas (5). Fistulography has not gained popularity because of its very poor diagnostic accuracy (6). Low soft tissue contrast and need for cannulating the fistula to increase the contrast are the main causes that decrease the utility of CT in the assessment of anal fistulas (7). In a meta-analysis, EUS yielded comparable results to MRI (8), but poorer results also exist in the literature probably due to operator dependency of this technique (5).
MRI use in anal fistulas was first reported in the early 1990s (9). In that initial report, MRI showed 87.5% concordance with the surgery. MRI has the ability to differentiate soft tissues, identify tracts outside the anal canal, and demonstrate the images compatible with the surgically relevant plane (10, 11). The Association of Coloproctology of Great Britain and Ireland (12) defined MRI as an imaging technique with high sensitivity and specificity for the diagnosis of the primary fistula tract and recommended this technique for imaging assessment of the complex or recurrent fistulas.
Owing to high soft tissue resolution of MRI, localization of the site of internal opening of anal fistula, definition of the primary and secondary tracts and their relationships with the sphincter muscles, and presence of horseshoe fistulas and abscesses can be more accurately depicted preoperatively compared with physical examination (13). A classification based on MRI findings alone was also developed by St. James Hospital (Table 1) (Figs. 1–5) (14).
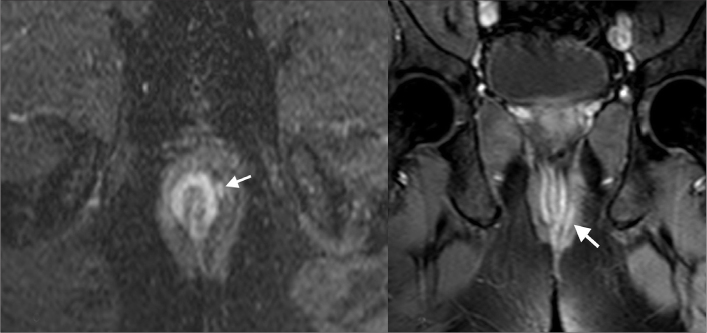
Figure 1.
St. James grade 1 fistula. Axial and coronal T2-weighted images reveals intersphincteric fistula with hyperintense appearance (arrows).
Figure 2.
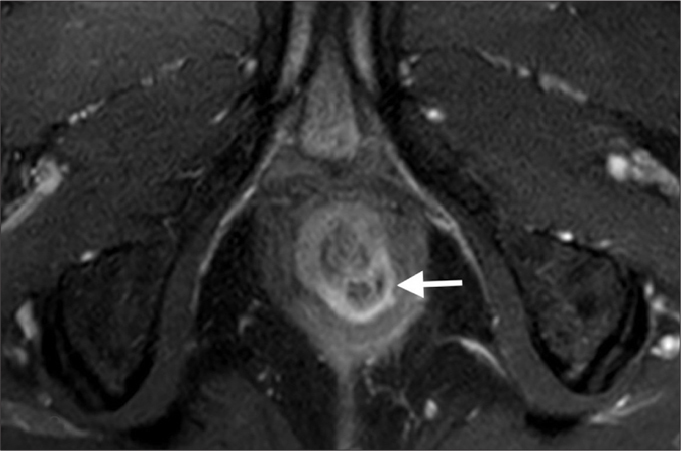
St. James grade 2 fistula. Axial contrast-enhanced T1-weighted image reveals an abscess (arrow) in intersphincteric space resulting from interhemispheric fistula.
Figure 3.
St. James grade 3 fistula. Axial contrast-enhanced T1-weighted image reveals a fistula (arrows) traversing internal and external sphincters.
Figure 4.
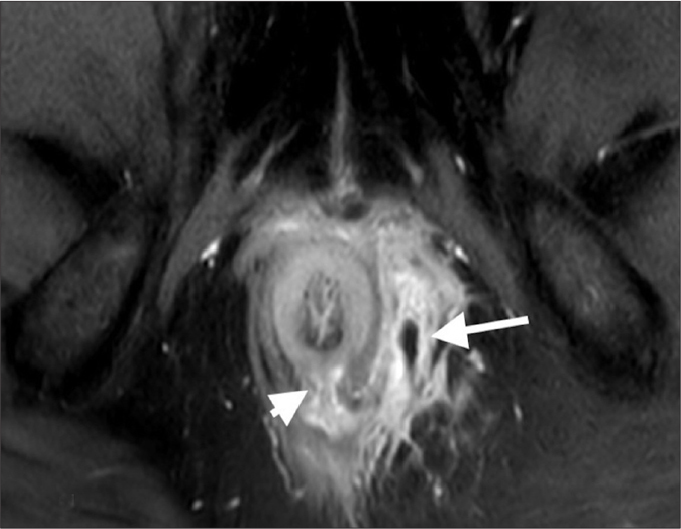
St. James grade 4 fistula. Axial contrast-enhanced T1-weighted image reveals a transsphincteric anal fistula (arrowhead) at 6 o’clock position with accompanying abscess (arrow).
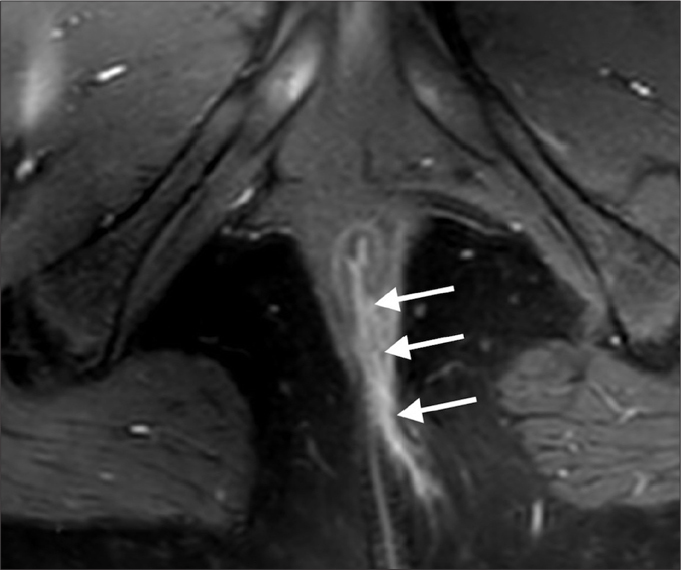
Figure 5.
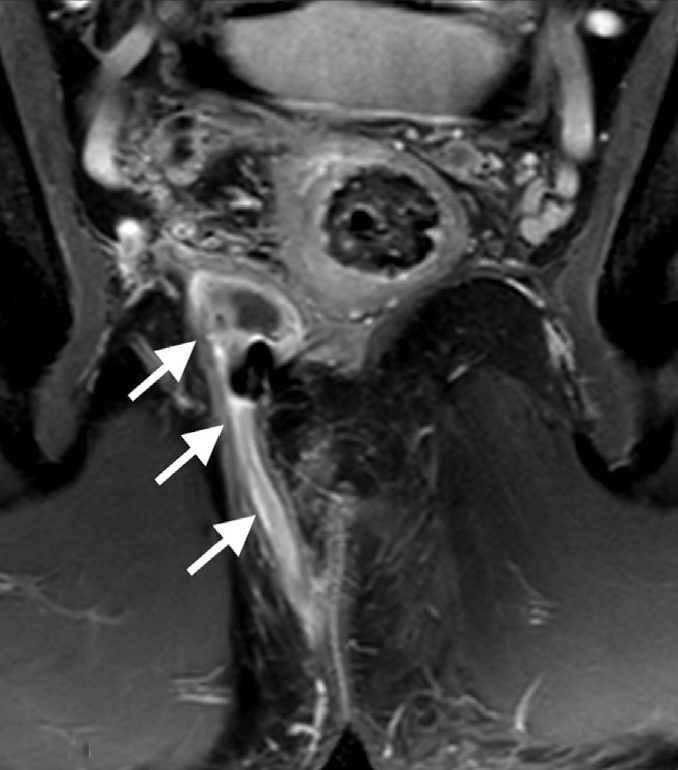
St. James grade 5 fistula. Coronal contrast-enhanced T1-weighted image reveals suprasphincteric fistula (arrows) extending superiorly to the proximal rectum level.
Many studies have compared the accuracy of different MRI techniques to operative findings. These were mainly blinded studies, in which the surgeon was not aware of MRI findings at the time of surgery (15–18). Buchanan et al. (19) have shown that surgeons’ awareness of MRI results prior to fistula surgery resulted in decrease in the recurrence rate of anal fistulas. In a later study, Buchanan et al. (20) has found that in 10% of the primary fistulas, surgical treatment plan based on examination under anesthesia was changed after the results of MRI were provided to the surgeon. Beets-Tan et al. (21) have designed a study in which MRI results were provided to the surgeon just before the surgery was about to be concluded, and in 21% of these cases, the surgeon decided to continue the surgery based on the additional information provided by MRI.
For anal fistulas, it remains unclear whether a surgical management plan drawn after physical examination and history analysis will benefit from preoperative MRI, providing additional information that could have not been identified even during surgery. Therefore, this study aims to determine the group of patients for which MRI is more likely to provide important complementary information leading to an extended initial surgical management of the fistula.
Methods
Patients and methods
Our study cohort comprised patients who underwent surgery for primary anal fistula and preoperative MRI in our institution from January 1, 2008 to April 15, 2018. Data were retrospectively collected from a database management system, including the surgery and physical examination notes derived from the personal identifiers, which were retrieved from the electronic records department of the hospital. Patients who had developed fistulas due to Crohn’s disease or patients who underwent preoperative MRI in another institution were excluded. This study was approved by the Clinical Research Ethical Committee of our institution (ref no: KA-180055).
All surgeries were performed by or under the supervision of surgeons with at least 5 years of experience. Age, sex, examination under anesthesia findings, and Parks and SPTF classifications were obtained from operation notes, while St. James Hospital classification and any complementary radiological information, such as abscess or secondary tract formation, were derived from MRI reports assessed by specialists of the Abdominal Radiology Group.
Any discrepancies between surgical findings and MRI reports were noted. Two study groups were formed as per the contribution of preoperative MRI: significant and nonsignificant contribution groups. The significant contribution group identified patients with secondary (blind) tracts, horseshoe fistulas, or abscesses undiagnosed at physical examination and examination under anesthesia; those with the location of the internal orifice different from that identified by physical examination; or those with the grade of the fistula assessed to be more advanced after preoperative MRI.
MRI technique
Patients were examined with four different MRI scanners, three of them were 1.5 Tesla (T) MRI scanner (Symphony TIM, Siemens; SignaHDxt GE Medical Systems; Achieva dStream, Phillips Healthcare) and one was 3 T MRI scanner (Ingenia, Phillips Healthcare). Pelvic phased array coils were used to obtain images. Patients were scanned in the supine position. No special bowel preparation with oral or rectal contrast agents was used. MRI examination was started with sagittal fast spin-echo (FSE) T2-weighted sequence, which provided an overview of the pelvis showing the extent and axis of the anal canal. Following sequences included oblique axial and coronal fat-suppressed T2-weighted and oblique axial and coronal fat-suppressed T1-weighted images after the injection of 0.1 mmol/kg of gadolinium-based contrast agent (gadoterate meglumine). Axial and coronal oblique images of the anal canal were acquired with proper multiplanar prescription to obtain ideal images in accordance with the axis of the anal canal. Field of view of magnetic resonance images included levator ani muscles and supralevator planes since these anatomical sites may also be affected in the clinical course of anal fistula.
MRI assessment
Diagnosis of anal fistulas on MRI was based on the visualization of signal intensities (isointense to hyperintense on T2-weighted images, peripherally enhanced on contrast-enhanced T1-weighted images), shape (linear or oval structure surrounded by an irregular area), and extension of fistula. All fistulas were classified according to St. James Hospital classification system (Table 1) (14). The radial site of the internal opening was defined according to clock position (6 o’clock posterior). Secondary extensions and accompanying abscesses were defined by their anatomical location such as intersphincteric, extrasphincteric, ischioanal, or ischiorectal. Fluid collections with peripheral contrast enhancement and visualized as the extension of fistula were defined as abscess. The abscesses extending to the either side of the anal canal were defined as horseshoe abscesses.
Statistical analysis
For the primary endpoint, the study aims to determine the clinical characteristics (history and physical examination) that are likely to benefit from preoperative MRI. The study cohort of 136 patients (categorized into significant vs. nonsignificant MRI contribution groups) provides 80% power with 5% type-I error level to statistically identify significant differences ranging between 15% and 25% for the clinical findings observed in these two groups. As a secondary endpoint, the concordance between the classification schemes with and without the use of information from MRI (Parks and St. James classifications, respectively) was analyzed.
Descriptive statistics were provided as mean and standard deviation for age and as percentages for the categorical variables. The concordance between the two grading schemes was analyzed using Kendall’s tau test. The difference between groups was analyzed using chi-square or Fisher’s test for nominal variables and Mantel–Haenszel test for ordinal variables. A P value of <0.05 was used as the cutoff to infer statistical significance.
Results
The total number of eligible patients was 136. Mean patient age was 43±13 years. There were 106 males (77.9%). In total, 47 patients suffered from recurrent fistulas (34.6%). In nine patients (eight grade 1 and one grade 2), MRI failed to identify any fistulas. MRI was concordant with operative findings in 83.1% of the patients. Details of patients and their clinical characteristics are summarized in Table 2.
Table 2.
Demographic and clinical characteristics of patients
| n (%) | ||
|---|---|---|
| Age, years (mean±SD) | 43±13 | |
|
| ||
| Gender | Female | 30 (22.1) |
| Male | 106 (77.9) | |
|
| ||
| St James Hospital classification | Grade 0 | 9 (6.6) |
| Grade 1 | 45 (33.1) | |
| Grade 2 | 25 (18.4) | |
| Grade 3 | 34 (25) | |
| Grade 4 | 16 (11.8) | |
| Grade 5 | 7 (5.1) | |
|
| ||
| Sphincter damage | None | 116 (85.3) |
| Sphincter damage | 20 (14.7) | |
|
| ||
| SPTF classification | Simple | 57 (41.9) |
| Complex | 79 (58.1) | |
|
| ||
| Concordance of MRI with PE | 0 | 22 (16.2) |
| 1 | 114 (83.8) | |
|
| ||
| Number of external openings | 1 | 113 (83.1) |
| 2 | 17 (12.5) | |
| 3 | 4 (2.9) | |
| 4 | 2 (1.5) | |
|
| ||
| External opening >2 cm | <2 cm | 49 (36) |
| >2 cm | 87 (64) | |
|
| ||
| Horseshoe fistula | Absent | 124 (91.2) |
| Present | 12 (8.8) | |
|
| ||
| Blind tract | Absent | 112 (83) |
| Present | 23 (17) | |
|
| ||
| Parks classification | Grade 1 | 58 (42.6) |
| Grade 2 | 63 (46.3) | |
| Grade 3 | 7 (5.1) | |
| Grade 4 | 8 (5.9) | |
|
| ||
| Treatment | Fistulotomy | 68 (50) |
| Seton | 63 (46.3) | |
| Sphincteroplasty | 3 (2.2) | |
| Colostomy | 2 (1.5) | |
|
| ||
| Abscess formation | Absent | 91 (66.9) |
| Present | 45 (33.1) | |
| Operational finding only | 10 (22.2) | |
| MRI finding only | 16 (35.6) | |
| Identified by both | 19 (42.2) | |
| All abscesses | 45 (100) | |
|
| ||
| Recurrent case | No | 89 (65.4) |
| Yes | 47 (34.6) | |
|
| ||
| Impact on the operation | No effect | 90 (66.2) |
| Changed the operation | 46 (33.8) | |
| Total | 136 (100) | |
SD, standard deviation; SPTF, standard practice task force; MRI, magnetic resonance imaging; PE, physical examination.
MRI contribution to clinical evaluation was significant in 33.8% (46/136) of the patients. MRI more frequently provided significant information for complex fistulas than for simple fistulas (54.4% vs. 5.2%, P < 0.001). Proportion of patients with significant MRI contribution increased with increasing Parks grade (grade 1, 5.2%; grade 2, 47.6%; grade 3, 85.7%; grade 4, 87.5%). Significant preoperative MRI contribution was also more frequent if the external opening was more than 2 cm away from the anal canal (10.2% vs. 47.1%, P < 0.001) and when a horseshoe fistula was present (30.6% vs. 66.7%, P = 0.021). Although not statistically significant, the contribution of MRI was slightly more for recurrent fistulas than for primary fistulas (42.6% significant contribution vs. 29.2%, P = 0.11) (Table 3).
Table 3.
Association of clinical findings with significant contribution of MRI on surgical management
| Impact of MRI on the operation | |||||
|---|---|---|---|---|---|
| No effect n (%) |
Significant n (%) |
Total n (%) |
P | ||
| SPTF classification | Simple | 54 (94.7) | 3 (5.3) | 57 (100) | <0.001 |
| Complex | 36 (45.6) | 43 (54.4) | 79 (100) | ||
|
| |||||
| Parks classification | Grade 1 | 55 (94.8) | 3 (5.2) | 58 (100) | <0.001a |
| Grade 2 | 33 (52.4) | 30 (47.6) | 63 (100) | 0.024b | |
| Grade 3 | 1 (14.3) | 6 (85.7) | 7 (100) | 1.00c,d | |
| Grade 4 | 1 (12.5) | 7 (87.5) | 8 (100) | ||
|
| |||||
| External opening >2 cm | No | 44 (89.8) | 5 (10.2) | 49 (100) | <0.001 |
| Yes | 46 (52.9) | 41 (47.1) | 87 (100) | ||
|
| |||||
| Recurrent case | No | 63 (70.8) | 26 (29.2) | 89 (100) | 0.11 |
| Yes | 27 (57.4) | 20 (42.6) | 47 (100) | ||
|
| |||||
| Horseshoe fistula | Absent | 86 (69.4) | 38 (30.6) | 124 (100) | 0.021d |
| Present | 4 (33.3) | 8 (66.7) | 12 (100) | ||
|
| |||||
| Number of external openings | 1 | 78 (69) | 35 (31) | 113 (100) | 0.23d |
| 2 | 8 (47.1) | 9 (52.9) | 17 (100) | ||
| 3 | 3 (75) | 1 (25) | 4 (100) | ||
| 4 | 1 (50) | 1 (50) | 2 (100) | ||
| Total | 90 (66.2) | 46 (33.8) | 136 (100) | ||
MRI, magnetic resonance imaging; SPTF, standard practice task force.
Grade 1 vs. others;
Grade 2 vs. 3 and 4;
Grade 3 vs. 4;
Fisher exact test.
MRI detected blind tracts in 23 cases (16.9%). Blind tract frequency increased with increasing Parks grades (0% for grade 1, 23% for grade 2, 28% for grade 3, and 75% for grade 4). Abscess formation was present in 45 patients (33.1%), and approximately 35.6% of these abscesses were discovered on only MRI, whereas 22.2% of the abscesses were detected operatively. MRI also identified damaged sphincteric musculature (Fig. 6) in 36.2% of the recurrent cases (17/47).
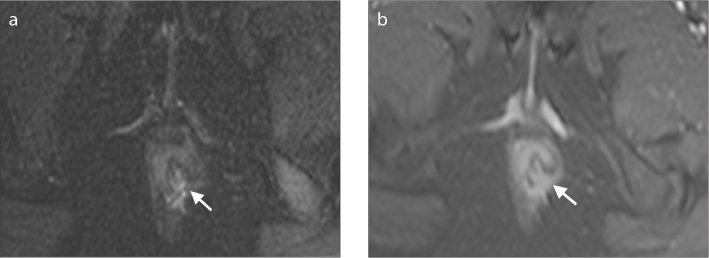
Figure 6. a, b.
Axial T2-weighted (a) and contrast-enhanced T1-weighted (b) images reveal the discontinuity of external sphincter (arrows) representing sphincter defect which resulted from previous surgery.
The concordance between St. James Hospital grade and Parks classification was 0.79 (Kendall’s tau-b test, P < 0.001).
Discussion
The surgical treatment of anal fistulas requires identification of the course of the primary and secondary tracts and their relation with the sphincteric musculature in order to properly manage the fistula and drain any present abscess. Physical examination alone may not be enough to delineate these features (4) and recurrence is usually due to missed infective foci at the first surgery (5, 22). MRI is the most accurate imaging tool to define anal canal anatomy and anal fistulas (23, 24). With 136 patients, our study presents one of the largest series in the literature and identifies the group of patients for which the radiologic evaluation of the fistula using MRI significantly contributes to the surgical management of the disease. In our study, for nearly one-third of the patients, MRI provided important additional information. Detection of higher Parks grades, distance of external opening of the fistula from the anal canal, horseshoe fistulas, and complex fistulas are indicative of significant MRI contribution following clinical evaluation.
Garg et al. (25) in a study evaluating MRI contribution to surgical management in 229 patients have reported that MRI added significant information in patients with additional tracts, horseshoe tracts, supralevator extension, unsuspected abscess, and multiple internal openings. Using these parameters, they concluded that MRI added significant information to 46.7% of the surgeries.
In an article by Beets-Tan et al. (21), when the researchers delivered MRI results to the surgeon just before his decision to conclude the surgery, the surgeon decided to continue the surgery in 21% (12/56) of the patients based on information obtained from the MRI. In another study, MRI results changed the surgical management in 40% of the fistula cases caused by Crohn’s disease and 24% of the recurrent fistula cases. Our study excludes patients with Crohn’s disease, which is an accepted indication for MRI and thus identifies clinical findings associated with significant MRI contribution in primary fistulas.
A relatively smaller study of 40 patients by Mullen et al. (26) has shown that in cases where it was able to correctly identify the anatomical detail of the fistula, establish the need of extended surgery, correlate with EUS, or rule out a suspected fistula, MRI positively contributed to the surgical management of the patient. They concluded that such a positive contribution of MRI could be as high as 85% if used in selected cases. Positive contribution of MRI to clinical assessment has also been shown in studies by Chapple et al. (27) and Spencer et al. (28), which demonstrated that compared with initial surgical exploration, MRI findings were a better predictor of both satisfactory surgery and need for re-operation.
In our study, MRI proved to change the operation when it delineated fistula characteristics, which could not be identified by physical examination or when the fistula grade was assessed to be higher than that of Parks classification after MRI assessment. With these criteria, MRI changed the operation in 33% of the cases. This ratio was 85% and 87% for Parks grade 3 and 4, respectively. We have also shown a significant contribution of MRI in detecting complex fistulas. This is mainly related to the increased incidence of blind tracts in Parks grade 3 and 4 or complex fistulas. The Association of Coloproctology of Great Britain and Ireland (2) recommends preoperative MRI for recurrent and complex fistulas. The parameters for complex fistulas are listed in Table 1. Particularly for the primary fistulas, predicting whether a fistula is complex or not preoperatively may be difficult with physical examination alone (29). Our experience is that if the external opening is farther away from the anal canal, the fistula tends to have a more complex course. Similarly, in our research, the benefit of MRI was significantly more for fistulas in which external opening was more than 2 cm far from the anal canal. Subgroup analysis revealed that blind tracts were more frequent in this group (25.3% vs. 2%). In some fistulas, the location of the external opening may be the only physical examination finding in the clinical settings; thus, our finding may be important to justify a preoperative MRI for this group of patients.
We found 79% concordance between St James Hospital grade and Parks classification. This confirms that the two assessments are correlated but not equally informative. The correlation of MRI findings with operative findings was investigated in other studies (17, 18, 20, 28) and ranged from 89% to 100%.
Missed infective features of fistulas are the main causes of their recurrence (30), and these are not easy to detect on physical examination. Buchanan et al. (4) in their work comparing physical examination, ultrasonography, and MRI have shown that physical examination revealed 36% of the abscess or horseshoe fistulas, whereas MRI detected 88% of those. In our study, 8 of the 12 horseshoe fistulas were detected on MRI. Our findings also show that 35.6% of the abscesses were not detected during the surgery. These findings emphasize the importance of MRI in revealing the infective features of fistula.
Recurrence of anal fistulas is currently the only widely accepted indication for preoperative MRI evaluation. In our study, we observed that MRI significantly contributed to 42.6% of the cases (20/47). When combined with our finding that MRI significantly contributes to the management of anal fistulas in more than 85% of the Parks grade 3 and 4 patients, this identifies high grade as a stronger indication than recurrence for preoperative MRI.
Among our cases, MRI detected sphincter damage in 36.2% of the patients, and none of these patients presented with other clinical findings, such as incontinence. Trying to identify complex parameters may require extensive dissection during surgery, which increases the probability of sphincter damage (27). Identifying the defective portion of the anal sphincter by MRI assures the surgeon to be more careful during this dissection, and the surgeon feels more confident if the recurrent fistula is related to the defective portion of the sphincter.
Few studies have pointed out the false negative results of the MRI (27, 31, 32). MRI failed to identify nine fistulas in our study. Three of these fistulas were recurrent but all were simple (eight grade 1 and one grade 2). MRI may misdiagnose thin fistulas as fibrous tracts in the absence of inflammatory findings, yielding false negative results, and surgical therapy is never solely based on MRI findings, particularly for simple fistulas.
The limitation of this study is that the information is evaluated retrospectively, representing our past experience with preoperative MRI for primary fistulas. Although we can precisely identify the cases for which MRI provided additional information to the clinical evaluation and surgical exploration findings using the criteria given in the methods section, we could not define prospectively for which patients the surgical management has definitely changed. Fistulas caused by inflammatory bowel diseases were excluded from the study to investigate the added value of preoperative MRI in the group of patients encountered most frequently during clinical practice.
In conclusion, our study is valuable in linking the findings of preoperative physical examination and surgical exploration with preoperative MRI findings for the surgical management of anal fistulas. We assessed and demonstrated for the first time in the literature the added value of MRI for fistulas with external opening located more than 2 cm from the anal canal. Moreover, we identified other useful indications for MRI, such as the complex and higher grade fistulas. In recurrent cases, MRI contributes not only by establishing the fistula anatomy but also by identifying possible sphincter damage. Therefore, we propose the inclusion of MRI in the preoperative surgical assessment of anal fistulas when they are recurrent, complex, high grade, or when the external opening is located more than 2 cm from the anal canal.
Main points.
MRI is an accurate imaging modality to delineate anal fistula characteristics.
MRI contribution to surgical management is mostly observed for grade 3 and 4 fistulas.
Fistulas with the external opening located more than 2 cm from the anal canal tend to be complex, and MRI is a helpful tool in the preoperative surgical assessment of these fistulas.
For recurrent fistulas, preoperative MRI is useful to reveal any sphincter defect.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 2.Whiteford MH, Kilkenny J, 3rd, Hyman N, et al. Practice parameters for the treatment of perianal abscess and fistula-in-ano (revised) Dis Colon Rectum. 2005;48:1337–1342. doi: 10.1007/s10350-005-0055-3. [DOI] [PubMed] [Google Scholar]
- 3.Goodsall DH, Miles WE. Diseases of the anus and rectum. London: Longmans, Green; 1900. pp. 92–173. [Google Scholar]
- 4.Buchanan GN, Halligan S, Bartram CI, Williams AB, Tarroni D, Cohen CR. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004;233:674–681. doi: 10.1148/radiol.2333031724. [DOI] [PubMed] [Google Scholar]
- 5.Halligan S, Stoker J. Imaging of fistula in ano. Radiology. 2006;239:18–33. doi: 10.1148/radiol.2391041043. [DOI] [PubMed] [Google Scholar]
- 6.Kuijpers HC, Schulpen T. Fistulography for fistula-in-ano. Is it useful? Dis Colon Rectum. 1985;28:103–104. doi: 10.1007/BF02552656. [DOI] [PubMed] [Google Scholar]
- 7.Liang C, Lu Y, Zhao B, Du Y, Wang C, Jiang W. Imaging of anal fistulas: comparison of computed tomographic fistulography and magnetic resonance imaging. Korean J Radiol. 2014;15:712–723. doi: 10.3348/kjr.2014.15.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui MR, Ashrafian H, Tozer P, et al. A diagnostic accuracy meta-analysis of endoanal ultrasound and MRI for perianal fistula assessment. Dis Colon Rectum. 2012;55:576–585. doi: 10.1097/DCR.0b013e318249d26c. [DOI] [PubMed] [Google Scholar]
- 9.Lunniss PJ, Armstrong P, Barker PG, Reznek RH, Phillips RK. Magnetic resonance imaging of anal fistulae. Lancet. 1992;340:394–396. doi: 10.1016/0140-6736(92)91472-K. [DOI] [PubMed] [Google Scholar]
- 10.Halligan S, Buchanan G. MR imaging of fistula-in-ano. Eur J Radiol. 2003;47:98–107. doi: 10.1016/S0720-048X(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 11.Joyce M, Veniero JC, Kiran RP. Magnetic resonance imaging in the management of anal fistula and anorectal sepsis. Clin Colon Rectal Surg. 2008;21:213–219. doi: 10.1055/s-2008-1081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JG, Farrands PA, Williams AB, et al. The treatment of anal fistula: ACPGBI position statement. Colorectal Dis. 2007;9( Suppl 4):18–50. doi: 10.1111/j.1463-1318.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- 13.Zbar AP, Armitage NC. Complex perirectal sepsis: clinical classification and imaging. Tech Coloproctol. 2006;10:83–93. doi: 10.1007/s10151-006-0258-1. [DOI] [PubMed] [Google Scholar]
- 14.Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20:623–635. doi: 10.1148/radiographics.20.3.g00mc15623. [DOI] [PubMed] [Google Scholar]
- 15.Beckingham IJ, Spencer JA, Ward J, Dyke GW, Adams C, Ambrose NS. Prospective evaluation of dynamic contrast enhanced magnetic resonance imaging in the evaluation of fistula in ano. Br J Surg. 1996;83:1396–1398. doi: 10.1002/bjs.1800831022. [DOI] [PubMed] [Google Scholar]
- 16.Holzer B, Rosen HR, Urban M, Anzbock W, Schiessel R, Hruby W. Magnetic resonance imaging of perianal fistulas: predictive value for Parks classification and identification of the internal opening. Colorectal Dis. 2000;2:340–345. doi: 10.1046/j.1463-1318.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- 17.Sofic A, Beslic S, Sehovic N, Caluk J, Sofic D. MRI in evaluation of perianal fistulae. Radiol Oncol. 2010;44:220–227. doi: 10.2478/v10019-010-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaefer O, Lohrmann C, Langer M. Assessment of anal fistulas with high-resolution subtraction MR-fistulography: comparison with surgical findings. J Magn Reson Imaging. 2004;19:91–98. doi: 10.1002/jmri.10436. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan G, Halligan S, Williams A, et al. Effect of MRI on clinical outcome of recurrent fistula-in-ano. Lancet. 2002;360:1661–1662. doi: 10.1016/S0140-6736(02)11605-9. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan GN, Halligan S, Williams AB, et al. Magnetic resonance imaging for primary fistula in ano. Br J Surg. 2003;90:877–881. doi: 10.1002/bjs.4125. [DOI] [PubMed] [Google Scholar]
- 21.Beets-Tan RG, Beets GL, van der Hoop AG, et al. Preoperative MR imaging of anal fistulas: Does it really help the surgeon? Radiology. 2001;218:75–84. doi: 10.1148/radiology.218.1.r01dc0575. [DOI] [PubMed] [Google Scholar]
- 22.Colon TSPTFASo. Surgeons R. Practice parameters for treatment of fistula-in-ano. Diseases of the Colon & Rectum. 1996;39:1361–1372. doi: 10.1007/BF02054525. [DOI] [PubMed] [Google Scholar]
- 23.Maier AG, Funovics MA, Kreuzer SH, et al. Evaluation of perianal sepsis: comparison of anal endosonography and magnetic resonance imaging. J Magn Reson Imaging. 2001;14:254–260. doi: 10.1002/jmri.1181. [DOI] [PubMed] [Google Scholar]
- 24.Sahni VA, Ahmad R, Burling D. Which method is best for imaging of perianal fistula? Abdom Imaging. 2008;33:26–30. doi: 10.1007/s00261-007-9309-y. [DOI] [PubMed] [Google Scholar]
- 25.Garg P, Singh P, Kaur B. Magnetic resonance imaging (MRI): operative findings correlation in 229 fistula-in-ano patients. World J Surg. 2017;41:1618–1624. doi: 10.1007/s00268-017-3886-x. [DOI] [PubMed] [Google Scholar]
- 26.Mullen R, Deveraj S, Suttie SA, Matthews AG, Yalamarthi S. MR imaging of fistula in ano: indications and contribution to surgical assessment. Acta Chir Belg. 2011;111:393–397. doi: 10.1080/00015458.2011.11680780. [DOI] [PubMed] [Google Scholar]
- 27.Chapple KS, Spencer JA, Windsor AC, et al. Prognostic value of magnetic resonance imaging in the management of fistula-in-ano. Dis Colon Rectum. 2000;43:511–516. doi: 10.1007/BF02237196. [DOI] [PubMed] [Google Scholar]
- 28.Spencer JA, Chapple K, Wilson D, Ward J, Windsor AC, Ambrose NS. Outcome after surgery for perianal fistula: predictive value of MR imaging. AJR Am J Roentgenol. 1998;171:403–406. doi: 10.2214/ajr.171.2.9694464. [DOI] [PubMed] [Google Scholar]
- 29.Abou-Zeid AA. Anal fistula: intraoperative difficulties and unexpected findings. World J Gastroenterol. 2011;17:3272–3276. doi: 10.3748/wjg.v17.i28.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723–729. doi: 10.1007/BF02054434. [DOI] [PubMed] [Google Scholar]
- 31.Myhr GE, Myrvold HE, Nilsen G, Thoresen JE, Rinck PA. Perianal fistulas: use of MR imaging for diagnosis. Radiology. 1994;191:545–549. doi: 10.1148/radiology.191.2.8153337. [DOI] [PubMed] [Google Scholar]
- 32.Chauhan NS, Sood D, Shukla A. Magnetic resonance imaging (MRI) characterization of perianal fistulous disease in a rural based tertiary hospital of North India. Pol J Radiol. 2016;81:611–617. doi: 10.12659/PJR.899315. https://doi.org/10.12659/PJR.899315. [DOI] [PMC free article] [PubMed] [Google Scholar]