Abstract
Objective
Bupivacaine, a local anaesthetic substance, is used as a regional-anaesthesia agent. Lidocaine, a sodium channel blocker, is used in combination with epinephrine for regional anaesthesia. We aimed to evaluate the effects of lidocaine with epinephrine (LE) at different doses on bupivacaine-induced cardiotoxicity in rats.
Methods
In our study, 24 Wistar albino rats were divided into four groups: I) Control; II) LE, 1 mg kg−1; III) LE, 3 mg kg−1 and IV) LE, 6 mg kg−1. Intravenous bupivacaine was administered at a dose of 3 mg kg−1 min−1 to the anaesthetized rats in all groups until cardiac asystole was achieved. LE was administered at the doses of 1, 3 and 6 mg kg−1 min−1 using infusion, simultaneously with bupivacaine. The asystole time and 75% decrement time in mean arterial blood pressure (MABP) were determined. P-Q, Q-T and QRS intervals were measured using electrocardiography (ECG) recordings.
Results
LE significantly increased the asystole time and 75% decrement time in MABP at the doses of 3 and 6 mg kg−1 compared to the control group (p<0.05) and significantly increased these values at the dose of 1 mg kg−1 compared to the control and other treatment groups (p<0.05). LE abolished the prolongation of P-Q, Q-T and QRS intervals in ECG recordings at the dose of 1 mg kg−1 (p<0.05).
Conclusion
These results reveal that LE has a protective effect against bupivacaine cardiotoxicity. In clinical application, the simultaneous application of LE and bupivacaine may reduce the risk of cardiotoxicity due to bupivacaine.
Keywords: Bupivacaine, cardiotoxicity, lidocaine with epinephrine, rat
Introduction
Bupivacaine is an amino-amide derivative and a lipophilic local anaesthetic agent. Bupivacaine is widely used in labour analgesia, postoperative pain and peripheral nerve blocks (1). Bupivacaine is a reliable anaesthetic agent with limited side effects when used in appropriate doses (2). However, it is known that it can cause sudden death due to cardiotoxicity when given intravenously (iv) in the systemic circulation during regional anaesthesia applications (3).
A lipid emulsion is used in clinics to prevent bupivacaine cardiotoxicity (4). However, experimental studies have been conducted to investigate more effective treatment methods to prevent bupivacaine cardiotoxicity. In these studies, the effect of dobutamine, levosimendan, clonidine, dexmedetomidine and dopexamine on bupivacaine cardiotoxicity has been investigated (5–8).
Lidocaine is an amino-amide derivative local anaesthetic agent. Lidocaine is widely used in infiltration anesthesia, central neuraxial anaesthesia and peripheral nerve blocks (9). It is used in combination with lidocaine bupivacaine to obtain an early anaesthetic effect in the clinic (10).
A few studies investigating the effect of lidocaine on bupivacaine cardiotoxicity have been conducted. In these studies, conflicting results have been obtained, and the issue is controversial. Fujita et al. (11) showed that lidocaine showed a protective effect against bupivacaine toxicity by decreasing the ventricular fibrillation (VF) in their study conducted on anesthetised pigs. Krikava et al. (12) showed that lidocaine reduced the QRS prolongation in ECG caused by bupivacaine toxicity. With these results, they suggested that lidocaine had a protective effect against bupivacaine cardiotoxicity by inhibiting the decrease in the intraventricular impulse transmission rate caused by bupivacaine. In their study with an isolated rabbit heart, Simon et al. (13) reported that lidocaine did not reduce the increase in the QRS prolongation caused by bupivacaine and that it had no protective effect on bupivacaine cardiotoxicity.
Lidocaine is combined with epinephrine, which has a vasoconstrictor effect, to improve its effect in clinical practice (14). The aim of this study was to investigate the effects of different doses of epinephrine+lidocaine on bupivacaine cardiotoxicity in anaesthetised rats.
Methods
Animals
In our study, 24 adult male Wistar albino rats weighing between 250 g and 330 g were used. Experimental animals were purchased from the Kobay Experimental Animals Laboratory ind.trade.co.ltd. (Çankaya, Ankara, Turkey). The animals were raised in a room under suitable conditions (21±2°C temperature, 45%–65% humidity, and 12-hour light/dark cycle). The rats were able to drink as much tap water as they wanted, and they were fed with standard rat pellet food. The experimental procedures applied in our study were discussed and approved by the Experimental Animals Local Ethics Committee (Protocol no: 2016-21-02/03) of the Zonguldak Bulent Ecevit University.
Surgical procedures
The rats were anaesthetised with 75 mg kg−1 ketamine and 8 mg kg−1 xylazine administered intraperitoneally (ip) (Sigma Aldrich, St. Louis, MO, USA). The body temperature of the rats was kept consistent at 37±1°C by placing the rectal probe on the heating plate (RTC-9404 Ankara, Turkey). The trachea was cannulated, and the animal was connected to the ventilator (respiratory volume 1.5 mL for 100 g, respiratory frequency 60 min−1; SAR-830, Life Sciences, USA). The right jugular vein was cannulated for the administration of the drugs, and the right carotid artery for the measurement of blood pressure (blood pressure measurement device, SS 13 L, California, USA). Subcutaneous bipolar electrodes were placed, and the ECG was monitored (data collection system, Biopac MP35, California, USA). The ECG and blood pressure data recorded during the experiment were stored on a computer for subsequent analyses (Acer A114-31, Newtaipei, Taiwan). A waiting period of 15 minutes was given for the animals to be stabilised after the surgery. For the measurement of arterial blood gases, 0.1 mL of blood was drawn. Instead, 0.1 mL of physiological saline solution was administered ip. The arterial pH, pO2 and pCO2 values were measured using a blood gas meter (Nova Biomedical Company, Phox Plus L, Walthom, USA). All active agents were administered through the jugular vein with an infusion pump (small animal infusion pump, Legato 100, USA).
Experimental groups and pharmacotherapy
A total of 24 animals were divided into four groups:
Group I (n=6): control
Group II (n=6): lidocaine with epinephrine 1 mg kg−1,
Group III (n=6): lidocaine with epinephrine 3 mg kg−1
Group IV (n=6): lidocaine with epinephrine 6 mg kg−1
An infusion of iv Bupivacaine (20 ml of Marcaine 0.5%, Astra Zeneca, Istanbul, Turkey) was given to all animals from all groups until the occurrence of cardiac asystole. In the drug groups, lidocaine with epinephrine (Vilcam 50 mL, Vils, Ankara, Turkey) was started to be administered simultaneously with the infusion of bupivacaine at the doses of 1 mg kg−1 min−1, 3 mg kg−1 min−1 and 6 mg kg−1 min−1, respectively.
The basal heart rate (HR) values and the mean arterial blood pressure (MABP) were calculated using the stored ECG and blood pressure records (data collection system, MP 35, Biopak, Goleta, California, USA). After bupivacaine administration, the period until asystole was observed, and the amount of bupivacaine consumed until the formation of asystole and the time until the first ventricular arrhythmia (ventricular premature contraction, ventricular tachycardia and VF) were calculated. The time necessary for the HR and MABP to decrease at the rates of 50% and 75% in reference to the basal values was detected. The QRS, P–Q, R–R and Q–T lengths were calculated at the baseline, and at the 1st, 3rd, 5th and 7th minute in ECG. The increased HR changes the measured Q–T lengths by shortening them. Therefore, the QTc value, which is the corrected form of Q–T values, was calculated according to the Bazett formula to eliminate the effect of the HR on Q–T (15).
Statistical analysis
The data were analysed using the GraphPad programme (GraphPad Prism version 5, San Diego, CA, USA). A one-way analysis of variance and Tukey test were used in the inter-group comparison of the obtained data. Student’s t-test was used to compare the basal values with the data measured during infusion in all groups. The data were given as the mean±standard error. The p-value <0.05 was considered to be statistically significant.
Results
No significant difference was observed among the groups in terms of body weight, HR, MABP, arterial blood pH, pO2 and pCO2 values recorded before bupivacaine administration (p>0.05) (Table 1).
Table 1.
Parameters recorded before bupivacaine administration
| Control | Lidocaine with Epinephrine 1 mg kg−1 | Lidocaine with Epinephrine 3 mg kg−1 | Lidocaine with Epinephrine 6 mg kg−1 | |
|---|---|---|---|---|
| Weight (g) | 306±8 | 290±7 | 280±13 | 289±5 |
| Heart rate/minute | 291±16 | 296±5 | 351±33 | 317±17 |
| Mean blood pressure (mmHg) | 103±14 | 118±2 | 109±2 | 112±4 |
| Arterial blood pH | 7±0.05 | 7±0.01 | 7±0.02 | 7±0.01 |
| Arterial blood pO2 (mmHg) | 182±0.8 | 148±0.2 | 149±0.1 | 153±0.3 |
| Arterial blood pCO2 (mmHg) | 31±0.4 | 29±0.2 | 33±0.3 | 32±0.3 |
After the administration of bupivacaine, the duration of asystole, the 50% and 75% reduction in MABP, and bupivacaine consumption values significantly increased compared to control in the groups of lidocaine with epinephrine at the doses of 3 and 6 mg kg−1 (p<0.05) (Table 2 and Figure 1). These values increased significantly in the group of lidocaine with epinephrine at the dose of 1 mg kg−1 compared to other groups (p<0.05) (Table 2 and Figure 1). In the group of lidocaine with epinephrine at the dose of 1 mg kg−1, the time of first arrhythmia and the time to the 50% and 75% reduction in the HR significantly increased compared to the other groups (p<0.05) (Table 2 and Figure 1).
Table 2.
Parameters recorded after bupivacaine administration
| Control | Lidocaine with Epinephrine 1 mg kg−1 | Lidocaine with Epinephrine 3 mg kg−1 | Lidocaine with Epinephrine 6 mg kg−1 | |
|---|---|---|---|---|
| QRS >20% (sn) | 154±1 | 167±1 | 163±5 | 156±3 |
| First arrhythmia (sec) | 207±12 | 621±70*,#,† | 256±24 | 243±48 |
| Heart rate <50% (sec) | 329±57 | 891±63*,#,† | 519±14 | 404±66 |
| Heart rate <75% (sec) | 479±44 | 1510±113*,#,† | 898±71 | 779±88 |
| Mean arterial pressure <50% (sec) | 318±31 | 923±91* | 760±85* | 736±112* |
| Mean arterial pressure <75% (sec) | 474±38 | 1656±66*,#,† | 1011±78* | 860±91* |
| Asystole duration (sec) | 593±46 | 1727±77*,#,† | 1061±81* | 923±72* |
| Bupivacaine consumption (mg) | 1.87±0.16 | 4.98±0.1*,#,† | 2.91±0.1* | 2.56±0.2* |
p<0.05 according to control group,
p<0.05 according to the group of lidocaine with epinephrine 3 mg kg−1
p<0.05 according to the group of lidocaine with epinephrine 6 mg kg−1
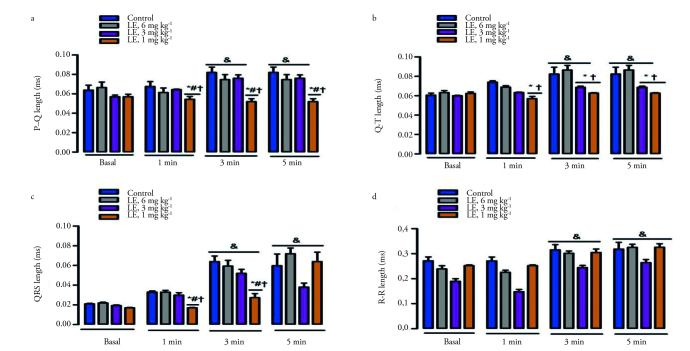
Figure 1.
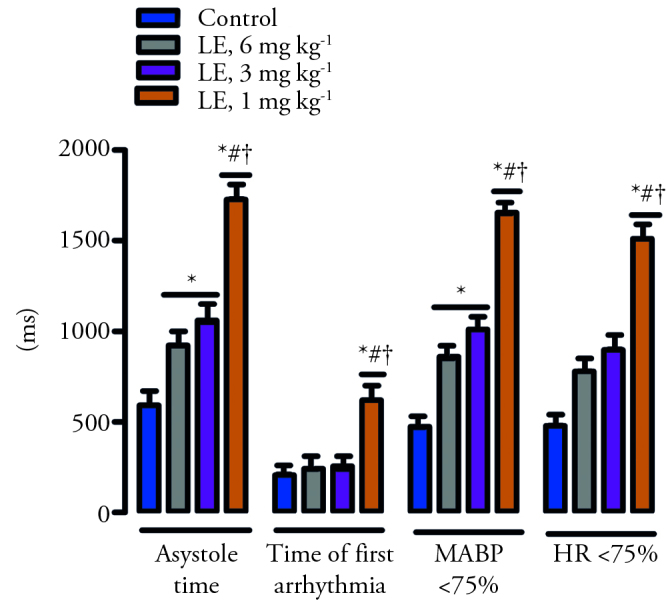
The effect of lidocaine with epinephrine on asystole, initial arrhythmia and MABP<75% and HR<75%. *p<0.05 according to the control group; #p<0.05 according to the group of lidocaine with epinephrine (L+E) 3 mg kg−1; +p<0.05 according to the group of lidocaine with epinephrine 6 mg kg−1. MABP, The duration of the 75% reduction in the mean arterial blood pressure; HR<75%, The duration of the 75% reduction in heart rate. Data are given as the mean±standard error.
The QRS, R–R and QTc values significantly increased in all groups at the 3rd and 5th minute in comparion with the basal values (p<0.05) (Table 3 and Figure 2). The P–Q and Q–T lengths increased significantly at the 3rd, 5th and 7th minute in the control group, and they also increased in the groups of lidocaine with epinephrine at the doses of 3 and 6 mg kg−1 compared to the basal values (p<0.05). However, there was no significant difference in terms of these values in the group in which lidocaine with epinephrine was administered at a dose of 1 mg kg−1 (Table 3 and Figure 2).
Table 3.
Changes in recorded electrocardiographic intervals after bupivacaine administration
| Basal | 1 | 3 | 5 | 7 | |
|---|---|---|---|---|---|
| P–Q | |||||
| Control | 0.063±0.005 | 0.067±0.005 | 0.082±0.005& | 0.082±0.005& | 0.082±0.005& |
| L+E 1 mg kg−1 | 0.057±0.002 | 0.054±0.002*,#,† | 0.052±0.002*,#,† | 0.052±0.002*,#,† | 0.052±0.002*,#,† |
| L+E 3 mg kg−1 | 0.057±0.001 | 0.064±0.001 | 0.076±0.003& | 0.076±0.003& | 0.076±0.003& |
| L+E 6 mg kg−1 | 0.066±0.005 | 0.061±0.004 | 0.074±0.005& | 0.074±0.005& | 0.074±0.005& |
| QRS | |||||
| Control | 0.021±0.0005 | 0.021±0.0005 | 0.064±0.0056& | 0.051±0.0112& | 0.057±0.0059& |
| L+E 1 mg kg−1 | 0.017±0.0003 | 0.017±0.0003*,#,† | 0.027±0.0040*# † & | 0.058±0.0098& | 0.090±0.0086& |
| L+E 3 mg kg−1 | 0.019±0.0005 | 0.020±0.0007 | 0.052±0.0040& | 0. 038±0.0041& | 0.067±0.0075& |
| L+E 6 mg kg−1 | 0.021±0.0009 | 0.021±0.0009 | 0.059±0.0059& | 0.071±0.0058& | 0.080±0.0036& |
| R–R | |||||
| Control | 0.271±0.015 | 0.271±0.015 | 0.306±0.024& | 0.611±0.101& | 0.824±0.047& |
| L+E 1 mg kg−1 | 0.252±0.002 | 0.252±0.002 | 0.242±0.003& | 0.288±0.023& | 0.315±0.034*,& |
| L+E 3 mg kg−1 | 0.189±0.010 | 0.195±0.009 | 0.221±0.007& | 0.317±0.033& | 0.396±0.041*,& |
| L+E 6 mg kg−1 | 0.239±0.012 | 0.245±0.008 | 0.287±0.047& | 0.356±0.082& | 0.439±0.111*,& |
| Q–T | |||||
| Control | 0.061±0.002 | 0.068±0.001 | 0.082±0.007& | 0.082±0.007& | 0.082±0.007& |
| L+E 1 mg kg−1 | 0.062±0.001 | 0.057±0.002*† | 0.062±0.003*† | 0.062±0.003*† | 0.062±0.003*† |
| L+E 3 mg kg−1 | 0.060±0.003 | 0.063±0.001 | 0.068±0.001*† & | 0.068±0.001*† & | 0.068±0.001*† & |
| L+E 6 mg kg−1 | 0.063±0.002 | 0.062±0.006 | 0.087±0.004& | 0.087±0.004& | 0.087±0.004& |
| QTc | |||||
| Control | 0.117±0.006 | 0.120±0.005 | 0.149±0.008& | 0.149±0.008& | 0.149±0.008& |
| L+E 1 mg kg−1 | 0.123±0.002 | 0.130±0.004 | 0.139±0.007& | 0.139±0.007& | 0.139±0.007& |
| L+E 3 mg kg−1 | 0.129±0.004 | 0.136±0.003 | 0.145±0.003& | 0.145±0.003& | 0.145±0.003& |
| L+E 6 mg kg−1 | 0.130±0.005 | 0.125±0.003 | 0.167±0.009& | 0.167±0.009& | 0.167±0.009& |
p<0.05 according to control group
p<0.05 according to the group of lidocaine with epinephrine 3 mg kg−1
p<0.05 according to the group of lidocaine with epinephrine 6 mg kg−1
p<0.05 according to the basal values
Figure 2.
The effect of lidocaine with epinephrine on the P–Q (A), Q–T (B), QRS (C) and R–R (D) lengths. *p<0.05 according to the control group; #p<0.05 according to the group of lidocaine with epinephrine (L+E) 3 mg kg−1; +p<0.05 according to the group of lidocaine with epinephrine 6 mg kg−1; &p <0.05 according to the basal values.
The P–Q length in the 1st, 3rd, 5th and 7th minute in the rats from the lidocaine with epinephrine 1 mg kg−1 group showed a significant decrease in comparison to all other groups; the Q–T length showed a significant decrease in comparison to the control group and the group of lidocaine with epinephrine 6 mg kg−1. Lidocaine with epinephrine significantly reduced the Q–T length at the dose of 3 mg kg−1 at the 3rd, 5th and 7th minute in comparison to the control group and the groups of lidocaine with epinephrine 6 mg kg−1 (p<0.05) (Table 3 and Figure 2). The QRS length measured at the 1st and 3rd minute in the group of lidocaine with epinephrine 1 mg kg−1 significantly decreased (p<0.05) (Table 3 and Figure 2). The R–R interval measured at the 7th minute in all groups given lidocaine with epinephrine showed a significant decrease compared to the control group (p<0.05) (Table 3).
Discussion
To the best of our knowledge, it was first reported in our study that lidocaine with epinephrine had a protective effect against bupivacaine toxicity and that it increased the asystole duration and improved haemodynamic parameters. Lidocaine with epinephrine was found to have an effect on bupivacaine cardiotoxicity by providing improvement in these parameters at the doses of 1, 3 and 6 mg kg−1. However, this effect was found to be the highest at the dose of 1 mg kg−1. The effect of lidocaine with epinephrine or different doses of lidocaine against bupivacaine cardiotoxicity has not been investigated. Therefore, there is no similar study in which these results can be compared in the literature. In our study, lidocaine with epinephrine administered at a dose of 1 mg kg−1 increased the time when the first arrhythmia was seen. Similarly, Fujita et al. (11) showed that lidocaine increased the threshold value of VF in bupivacaine cardiotoxicity.
In our study, bupivacaine significantly increased the P–Q, Q–T, R–R and QRS lengths compared to basal values. This result suggests that bupivacaine may have reduced the rate of impulse conduction in the heart. This effect may be due to the inhibitory effect of bupivacaine on fast sodium, ether-a-go-go potassium, and T-type and L-type calcium ion channels (16–19). Lidocaine with epinephrine eliminated an increase in these values at a dose of 1 mg kg−1. This result can be explained by the fact that lidocaine with epinephrine blocks the effect of bupivacaine to decrease the rate of intracardiac impulse conduction. In accordance with our study, Lefrant et al. (20) in a similar study conducted on anesthetised pigs, showed that lidocaine reduced the increament in the QRS and P-Q lengths induced by bupivacaine.
Clarkson and Hondeghem (16) compared the effect of bupivacaine and lidocaine on the cardiac impulse conduction in a study using the voltage clamp technique in guinea pig ventricular muscle cells. In this study, it has been reported that bupivacaine and lidocaine block these channels by binding to the fast sodium channels. However, it has been shown that lidocaine disperses from channel receptors faster than bupivacaine. Clarkson and Hondeghem (21) report in their other studies that lidocaine is replaced by bupivacaine by showing a competitive inhibition over these channels. The investigators have suggested that lidocaine reduces the toxicity of bupivacaine because it dissociates faster from the fast sodium channels. In our study, lidocaine may have reduced the cardiotoxicity caused by bupivacaine through a similar mechanism.
Different from our study, Simon et al. (13) showed in an in vitro study conducted with a rabbit heart that lidocaine was not effective in reducing the QRS prolongation caused by bupivacaine. The difference in results can be explained by the administration time of the anaesthetic agents used in these studies. In our study, bupivacaine and lidocaine with epinephrine were given concurrently, which was consistent with the competitive inhibition hypothesis of Clarkson and Hondeghem (21). Simon et al. (13) initiated the bupivacaine infusion 10 minutes before the lidocaine infusion.
Conclusion
These results suggest that a low dose (1 mg kg−1) of lidocaine with epinephrine in rats may have a protective effect against bupivacaine cardiotoxicity. However, this result should be supported by further research in order to be transferred to clinical practice.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Animal Research Local Ethical Committee of Bülent Ecevit University (Date:02.03.2016, protocol no: 2016-21-02/03).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – E.G.; Design – E.G.; Supervision – E.G.; Resources – E.G.; Materials – D.Ç.; Data Collection and/or Processing – E.G.; Analysis and/or Interpretation – D.Ç.; Literature Search – E.G.; Writing Manuscript – E.G.; Critical Review – E.G.; Other – E.G.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: This study was finacially supported by Bülent Ecevit University Coordinatorship of Scientific Research Projects (Project no: 2016-84906727-02).
References
- 1.Kayhan Z. Lokal/Bölgesel anestezi yöntemleri: Klinik Anestezi. 2. Basım. İstanbul: Logos Yayıncılık; 1997. pp. 270–3. [Google Scholar]
- 2.Cooper MH, McClure JH. Anesthesia chapter from saving mothers lives reviewing maternal deaths to make pregnancy safer. Br J Anaesth. 2008;100:17–22. doi: 10.1093/bja/aem344. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg GL. Treatment of local anesthetic systemic toxicity (LAST) Reg Anesth Pain Med. 2010;35:188–93. doi: 10.1097/AAP.0b013e3181d246c3. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg GL. Lipid infusion therapy: translation to clinical practice. Anesth Analg. 2008;106:1340–2. doi: 10.1213/ane.0b013e31816a6c09. [DOI] [PubMed] [Google Scholar]
- 5.Kandemir U, Maltepe F, Ugurlu B, Gokmen N, Celik A. The effects of levosimendan and dobutamine in experimental bupivacaine-induced cardiotoxicity. BMC Anesthesiol. 2013;13:28. doi: 10.1186/1471-2253-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilir A, Yelken B, Kaygisiz Z, Senturk Y. The effects of dopexamine in bupivacaine and ropivacaine induced cardiotoxicity in isolated rat heart. Saudi Med J. 2006;27:1194–8. [PubMed] [Google Scholar]
- 7.Hanci V, Karakaya K, Yurtlu S, Hakimoglu S, Can M, Ayoglu H, et al. Effects of dexmedetomidine pretreatment on bupivacaine cardiotoxicity in rats. Reg Anesth Pain Med. 2009;34:565–8. doi: 10.1097/AAP.0b013e3181bfbe35. [DOI] [PubMed] [Google Scholar]
- 8.Gulec S, Aydin Y, Uzuner K, Yelken B, Senturk Y. Effects of clonidine pre-treatment on bupivacaine and ropivacaine cardiotoxicity in rats. Eur J Anaesthesiol. 2004;21:205–9. doi: 10.1097/00003643-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kayaalp O. Tıbbi Farmakoloji. 10. Basım. Ankara: Hacettepe Taş Kitapcılık; 2002. [Google Scholar]
- 10.Křikava I, Nováková M, Ševčík P. The effects of trimecaine on bupivacaine induced cardiotoxicity in the isolated rat heart: a pilot study. Physiol Res. 2008;57:18. doi: 10.33549/physiolres.932014. [DOI] [PubMed] [Google Scholar]
- 11.Fujita Y, Endoh S, Yasukawa T, Sarı A. Lidocaine increases the ventricular fibrillation threshold during bupivacaine-induced cardiotoxicity in pigs. Br J Anaesth. 1998;80:218–22. doi: 10.1093/bja/80.2.218. [DOI] [PubMed] [Google Scholar]
- 12.Krikava I, Jarkovsky J, Stourac P, Novakova M, Sevcik P. The Effects of Lidocaine on Bupivacaine-induced cardiotoxicity in the isolated Rat Heart. Physiol Res. 2010;59:65–9. doi: 10.33549/physiolres.932014. [DOI] [PubMed] [Google Scholar]
- 13.Simon L, Kariya N, Pelle-Lancien E, Mazoit JX. Bupivakaine-Induced QRS Prolongation is Enhanced by Lidocaine and by Phenytoin in Rabbit Hearts. Anesth Analg. 2002;94:203–7. doi: 10.1097/00000539-200201000-00039. [DOI] [PubMed] [Google Scholar]
- 14.Sinnott CJ, Cogswell LP, III, Johnson A, Strichartz GR. On the mechanism by which epinephrine potentiates lidocaine’s peripheral nerve block. Anesthesiol. 2003;98:181–8. doi: 10.1097/00000542-200301000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Bazett HC. An analysis of the time relationships of electrocardiograms. Ann Noninvasive Electrocardiol. 2006;2:177–94. doi: 10.1111/j.1542-474X.1997.tb00325.x. [DOI] [Google Scholar]
- 16.Clarkson CW, Hondeghem LM. Mechanism for bupivacaine depression of cardiac conduction: Fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology. 1985;62:396–405. doi: 10.1097/00000542-198504000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Sintra GL, Carrupt PA, Abriel H, Daina A. Block of the hERG channel by bupivacaine: electrophysiological and modeling insights towards stereochemical optimization. Eur J Med Chem. 2011;46:3486–98. doi: 10.1016/j.ejmech.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Wen X, Xu S, Liu H, Zhang Q, Liang H, Yang C, et al. Neurotoxicity induced by bupivacaine via T-type calcium channels in SH-SY5Y cells. PLoS One. 2013;8:e62942. doi: 10.1371/journal.pone.0062942. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Rossner KL, Freese KJ. Bupivacaine inhibition of L-type calcium current in ventricular cardiomyocytes of hamster. Anesthesiology. 1997;87:926–34. doi: 10.1097/00000542-199710000-00028. [DOI] [PubMed] [Google Scholar]
- 20.Lefrant JY, Muller L, de La Coussaye JE, Lalourcey L, Ripart J, Peray PA, et al. Hemodynamic and cardiac electrophysiologic effects of lidocaine-bupivacaine mixture in anesthetized and ventilated piglets. Anesthesiology. 2003;98:96–103. doi: 10.1097/00000542-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson CW, Hondeghem LM. Evidence for a specific receptor site for lidocaine, quinidine, and bupivacaine associated with cardiac sodium channels in guinea pig ventricular myocardium. Circ Res. 1985;56:496–506. doi: 10.1161/01.RES.56.4.496. [DOI] [PubMed] [Google Scholar]