Abstract
TiO2-supported PdAu bimetallic nanoparticles (NPs) with small size and good dispersity were prepared by the room-temperature ionic liquid-assisted bimetal sputtering, which is simple, environmentally friendly, and free of additives and byproducts. Pd/Au atomic ratio can be tuned by controlling the sputtering conditions simply. High catalytic activity was found in PdAu–NPs–TiO2 hybrids for solvent-free selective oxidation of 1-phenylethanol using O2 as the oxidant at the low temperature of 50 °C and low pressure of 1 atm. It was found that Pd/Au ratio strongly affected the catalytical activity, and the highest conversion of about 35 % and turnover frequency of about 421 h−1 were achieved at 1:1 of Pd/Au atomic ratio. The synergistic effect in PdAu NPs was also discussed based on the comprehensive characterization results. The present approach may offer an alternative platform for future development of green-chemistry compatible bimetallic nanocatalysts.
Keywords: PdAu nanoparticles, TiO2 nanosupport, Electronic structure
Introduction
The selective oxidation of primary alcohols to aldehydes/ketones is crucially important in both laboratory and commercial processes [1–4]. In homogeneous catalysis for conversion of alcohols to aldehydes, it is often needed to use organic solvents, which are typically expensive, toxic, and harmful to the environment [5]. Thus, great efforts have been made to design and synthesize alternative catalysts which can use molecular O2 as the oxidant. However, the use of O2 under severe conditions such as high temperature and high pressure could induce issues of overoxidation, decomposition products, and/or explosion risk. It is hence highly desired to realize the efficient catalysis free of solvents under mild conditions. Toward this target, metal nanoparticles (NPs) such as Au NPs [6–15], Pd NPs [16, 17] and PdAu bimetallic NPs [18–24] have been demonstrated to be promising catalysts for solvent-free selective oxidation of alcohols [8, 10, 11, 20, 21]. Especially, PdAu bimetallic NPs often exhibit superior catalytic performance than their monometallic counterparts due to the synergistic effects [18–24]. In order to maintain the small size and good dispersion of PdAu NPs, which are beneficial for the catalysis, nanosupports have been extensively utilized to stabilize the bimetallic NPs on top.
The conventional way to synthesize supported metal NPs involves reduction of corresponding metal salts, where additives such as capping agents and byproducts are always present [25–28]. In some cases, high-temperature processing is required as well [29, 30]. Recently, we have developed a more environmentally friendly strategy using the room-temperature ionic liquid (RTIL)-assisted sputtering to prepare supported metal NPs [31, 32], which is simple, clean, and totally free of additives and byproducts. By the green-chemistry compatible method, PdAu NPs are successfully decorated on TiO2 NPs which act as the nanosupport. The comprehensive results demonstrate the bimetallic nature of the PdAu NPs, and charge redistribution is observed among Pd, Au, and TiO2. The TiO2-supported bimetallic NPs show good catalytic capability for the selective oxidation reaction of 1-phenylethanol to acetophenone, which can occur near room temperature without a solvent. Furthermore, the catalytic activity of the PdAu NPs is significantly dependent on Pd/Au ratio, suggesting a strong correlation among the catalytic performance, bimetallic composition, and charge redistribution.
Experimental
Materials
Pd and Au targets for the sputtering were purchased from Hefei Kejing. 1-phenylethanol was purchased from Sinopharm Chemical Reagent. Pristine TiO2 NPs with an average diameter of 25 nm were acquired from Hangzhou Wanjing. The RTIL, 1-Butyl-3-methylimidazolium tetrafluoroborate ([BMIm][BF4], purity >99 %), was purchased from Shanghai Chengjie Chemical, which was dried in vacuum for 24 h before use.
Synthesis of TiO2-supported PdAu NPs
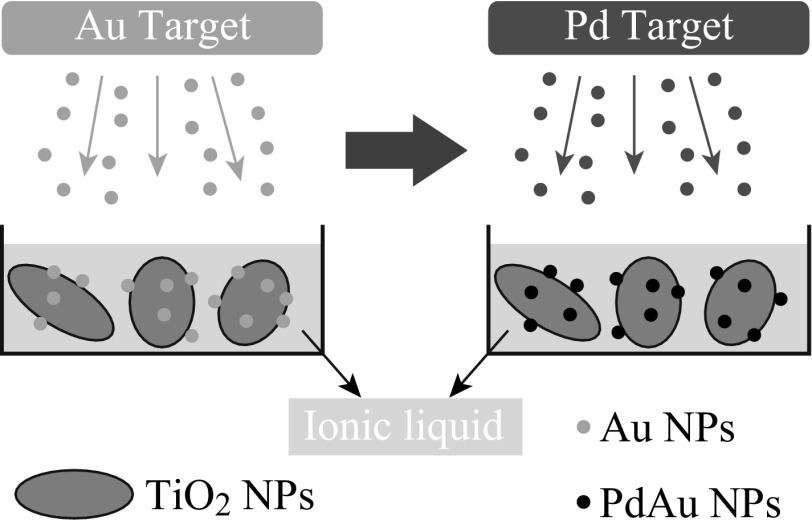
As depicted in Scheme 1, the TiO2-supported PdAu NPs were prepared by successive sputtering of Au and Pd onto the TiO2–RTIL suspension [31, 32]. Firstly, 10 mg TiO2 NPs were adequately dispersed into 10 mL [BMIm][BF4] with ultrasonication. Secondly, the TiO2–RTIL suspension was dropped into a clean stainless steel pot, and then Au was sputtered onto the suspension at room temperature in a desktop sputtering system (Quorum Technologies, equipped with a thickness monitor which is pre-calibrated by a surface profiler). Thirdly, Pd was sputtered onto the Au–TiO2–RTIL suspension by simply altering the metal target from Au to Pd. During the sputtering, Ar working pressure and deposition rate were kept at about 0.01 mbar and 0.2 Å s−1, respectively. The total sputtering time was controlled to be same for all the samples to get a same metal loading, e.g., 40 s for Au plus 40 s for Pd to the PdAu bimetallic NPs, and correspondingly 80 s for Au/Pd to the monometallic NPs. Eventually, the metal–NP–TiO2 hybrids were separated from [BMIm][BF4] by repeated high-speed centrifugation and decantation. Note that the current approach is a general method, which can be used to prepare various bimetallic NPs on diverse nanosupports [31, 32].
Scheme 1.
Preparation method for PdAu–NPs–TiO2 hybrids: Successive sputtering of Au and Pd onto RTIL of [BMIm][BF4], in which TiO2 NPs are pre-dispersed
Characterization of TiO2-supported PdAu NPs
The crystalline structures of the metal–NPs–TiO2 hybrids were characterized with X-ray diffraction (XRD, PANalytical Empyrean) with Cu Kα radiation. The microscopic structures of the metal–NPs–TiO2 hybrids were investigated with high-resolution transmission electron microscopy (HRTEM, FEI Quanta FRG 200F, operating at 200 kV) and high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM). The UV–Vis absorption measurements were carried out on a UV–Vis–NIR spectrophotometer (PerkinElmer, Lambda 750). The electronic structures of the metal–NPs–TiO2 hybrids were characterized with X-ray photo-emission spectroscopy (XPS, Kratos Axis Ultra DLD, monochromatic Al Kα). The X-ray absorption near-edge structure (XANES) measurements were performed at the BL14W1 beamline of the Shanghai Synchrotron Radiation Facility (SSRF).
Catalytic Reaction with TiO2-supported PdAu NPs
The solvent-free oxidation reaction of 1-phenylethanol using O2 was conducted in a glass flask pre-filled with 5 mL 1-phenylethanol and 8 mg hybrid catalyst. The mixture was stirred and heated using a magnetic stirrer with heating function. The system was equipped with a thermocouple to control the temperature and a reflux condenser to recover the vaporized solution. In each reaction run, the mixture was raised to 50 °C under stirring (1000 rpm). O2 was bubbled into the mixture at a constant flow rate (250 mL min−1) to initiate the reaction. After the reaction lasting 5 h, the reaction products were quantitatively analyzed with gas chromatograph (GC, Agilent).
The conversion, selectivity, and turnover frequency (TOF) are defined as follows:
where the amount of reactant and target product are measured by GC; the amount of total metal are approximated as the amount of Au and Pd atoms, which are measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES); D m is the dispersivity of metal; and t is the reaction time.
Results and Discussion
Microscopic Structure
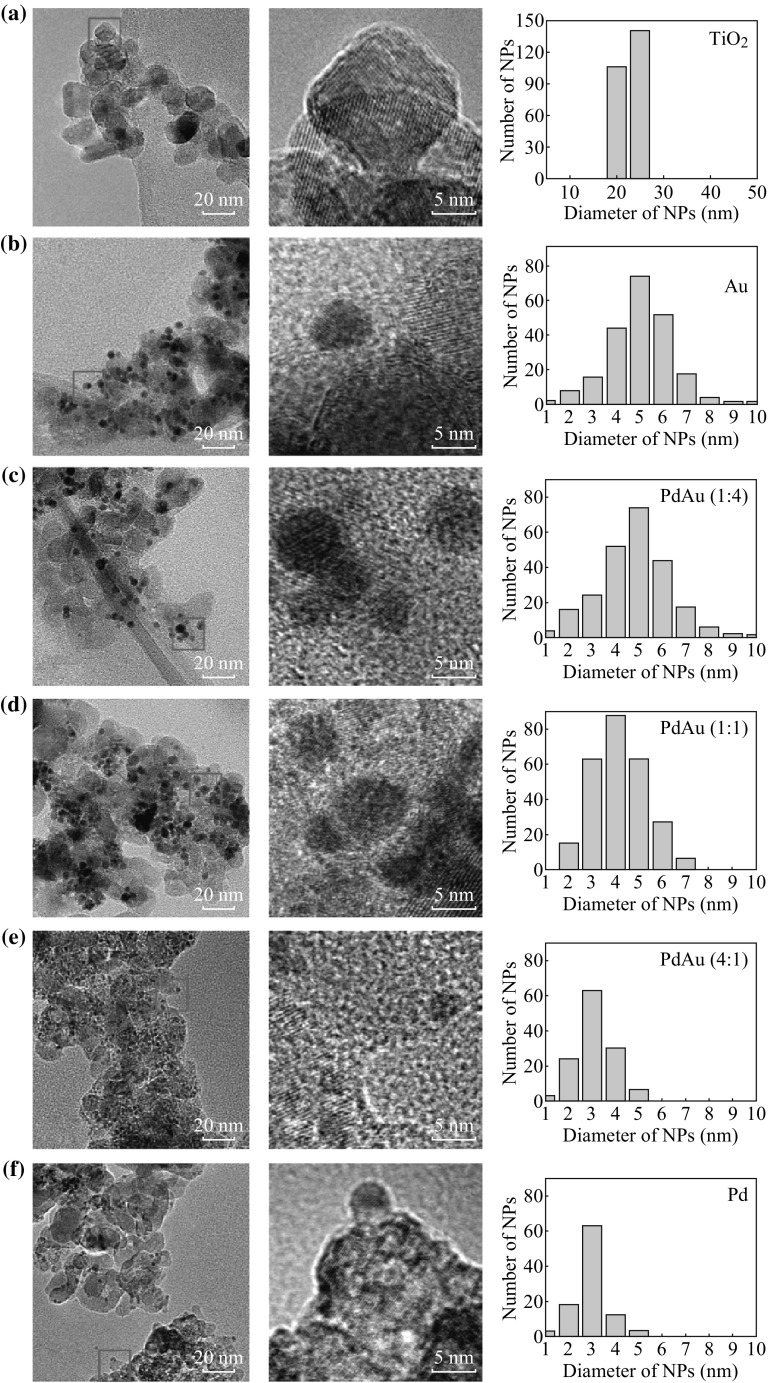
Commercially available TiO2 NPs with an average diameter of 25 nm were used as the nanosupport, and the morphology is shown in Fig. 1a. Utilizing the RTIL-assisted sputtering method, Au/Pd monometallic NPs or PdAu bimetallic NPs were decorated on the TiO2 support, where Pd/Au ratio (atomic ratio, unless otherwise noted) was controlled by adjusting the sputtering conditions of Au and Pd [32]. It was noted that the TiO2 support played a role of stabilizing metal NPs on top, otherwise the metal NPs will aggregate in cleaning process for removing [BMIm][BF4] if the support is absent [31]. Figure 1b–f displays the TEM images and metal nanoparticle size distributions of the Au–TiO2, PdAu (1:4)–TiO2, PdAu (1:1)–TiO2, PdAu (4:1)–TiO2, and Pd–TiO2 hybrids, respectively. It can be seen that the size of Au monometallic NPs is larger than that of Pd monometallic NPs, and the size of PdAu bimetallic NPs decreases gradually from about 5 to 3 nm upon increasing Pd/Au ratio.
Fig. 1.
TEM images and NPs size distributions of a TiO2 NPs as the support, b Au NPs on TiO2, c PdAu (1:4) NPs on TiO2, d PdAu (1:1) NPs on TiO2, e PdAu (4:1) NPs on TiO2, and f Pd NPs on TiO2. TEM images in the middle are magnified square region shown in the left
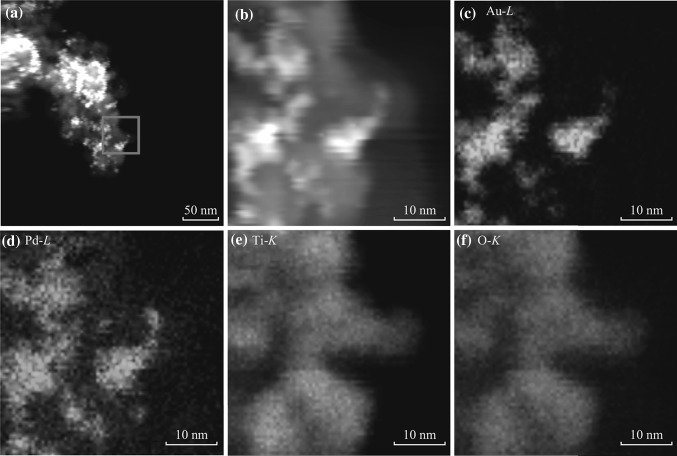
In order to clarify the microscopic structure of PdAu NPs, the elemental spatial distribution of Au and Pd is probed by mapping of the energy-dispersive spectroscopy (EDS). Figure 2a–d illustrates the HAADF-STEM images for the sample of PdAu (1:1)–TiO2, where Au and Pd spatial distributions are almost overlapped with each other. Similar HAADF-STEM results were observed for the samples with other Pd/Au ratios, and it suggests that Au and Pd are intermixed in the PdAu NPs due to atomic inter-diffusion [33–37]. The strong intermixing of Au and Pd can provide a large number of Au/Pd interfaces, which may be in favor of promoting catalytic activity of PdAu NPs [38]. On the other hand, the Ti and O spatial distributions are consistently uniform, as demonstrated in Fig. 2e, f.
Fig. 2.
HAADF-STEM images of PdAu (1:1) NPs on TiO2 a in large scale and b in selective small scale. Elemental mapping for c Au, d Pd, e Ti, and f O
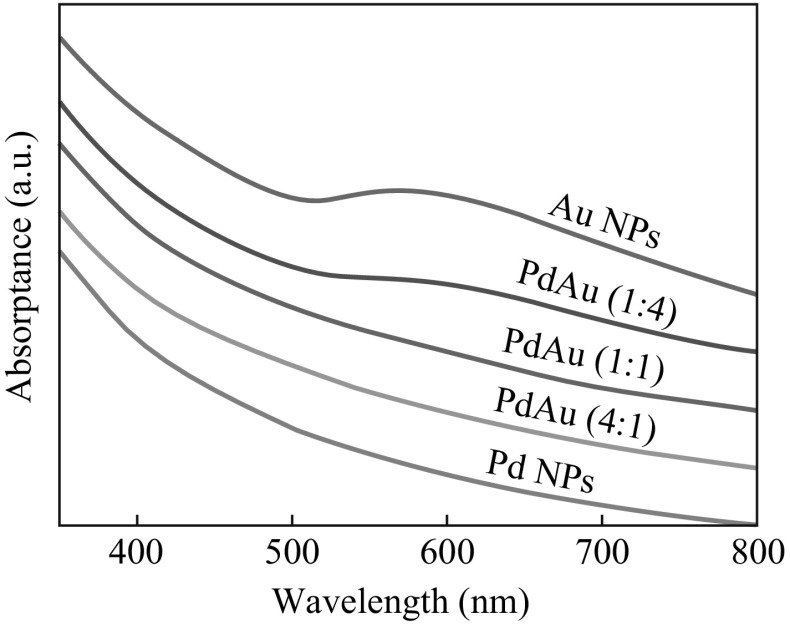
Figure 3 shows the UV–Vis absorption spectra of the TiO2-supported metal NPs with different Pd/Au ratios, in which the surface plasmon resonance (SPR) band of Au is around 570 nm. There is a small red-shift compared with the SPR band of typical Au NPs (~530 nm), which could be caused by the electronic structure modification of Au NPs due to the electron transfer from TiO2 to Au [39–43]. Upon addition of Pd, the SPR band gradually disappears. The disappearance of the SPR feature supports the HAADF-STEM-derived claim that Au and Pd are intermixed in the PdAu NPs. Therefore, the PdAu NPs are concluded to be bimetallic NPs rather than a physical mixture of separate Au and Pd monometallic NPs.
Fig. 3.
UV–Vis absorption spectra of TiO2-supported metal NPs with different Pd/Au atomic ratios
Electronic Structure
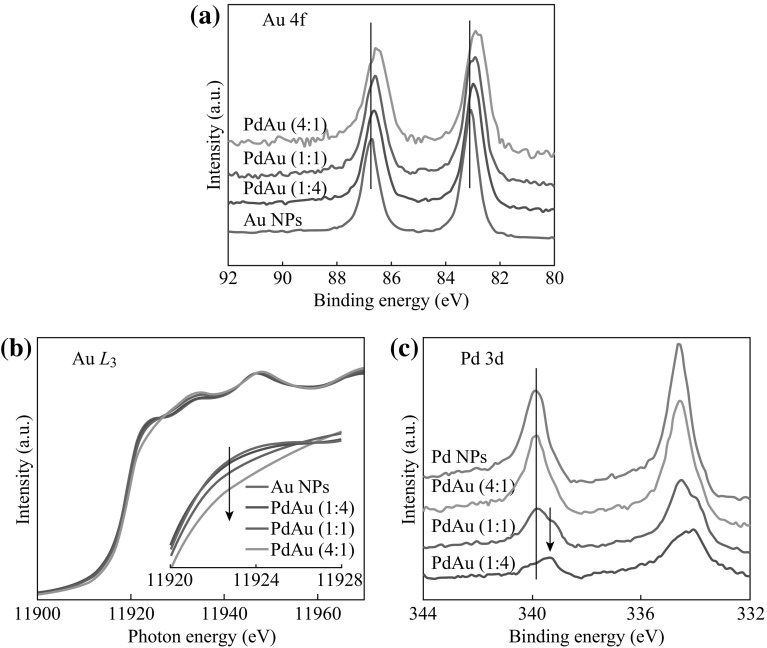
The charge redistribution in PdAu NPs is another bimetallic signature, which is investigated by XPS and XANES based on synchrotron radiation. Figure 4a shows the XPS Au 4f spectra, where the binding energy of Au 4f 7/2 peak for Au monometallic NPs is at about 83.0 eV. Considering the binding energy of bulk Au 4f 7/2 peak (typically at about 84.0 eV), there is a significant negative shift in binding energy (corresponding to electron gaining) for the Au NPs on TiO2. As TiO2 NPs often show n-type behavior arising from the oxygen vacancies, electron transfer from the TiO2 support to the Au NPs may be responsible for the binding energy shift [44]. Upon addition of Pd, the Au 4f peaks are further negatively shifted (up to 0.3 eV) with increasing Pd/Au ratio. It implies a strong interaction between Au and Pd [39, 44, 45], and electron transfer from Pd to Au may occur and result in a negative shift in binding energy. Furthermore, when Pd/Au ratio is increased, the full width at half maximum (FWHM) of the Au 4f peaks is broadened. This can be attributed to the size reduction of PdAu NPs with increasing Pd/Au ratio, as shown in Fig. 1.
Fig. 4.
a XPS Au 4f spectra, b XANES Au L 3-edge spectra, and c XPS Pd 3d spectra of TiO2-supported metal NPs with different Pd/Au atomic ratios. Inset in b is magnified whiteline region, where Au L 3-edge jump is normalized
Figure 4b shows the XANES Au L 3-edge spectra of the TiO2-supported metal NPs with different Pd/Au ratios, where the absorption jump (whiteline) region around 11,925 eV is magnified as the inset. The whiteline characterizes the dipole transition from Au 2p to 5d orbitals, and thus its intensity corresponds to the number of Au d-band holes. Compared with Au monometallic NPs, the whiteline intensity is decreased for PdAu bimetallic NPs, and the sample with higher Pd/Au ratio shows lower whiteline intensity. The trend of the whiteline change may be due to electron transfer from Pd to Au, which is in accordance with the XPS Au 4f results and literature [46].
On the other hand, the XPS Pd 3d spectra of the TiO2-supported metal NPs with different Pd/Au ratios are shown in Fig. 4c. The binding energy of Pd 3d 3/2 peak for Pd monometallic NPs is at about 339.8 eV, which is lower than that of bulk Pd at about 340.4 eV. Similar to the Au NPs on TiO2, the negative shift in binding energy can be ascribed to electron transfer from the n-type TiO2 support to the Pd NPs. Upon addition and promotion of Au, the original Pd feature is gradually weakened, and a new Pd feature at lower binding energy emerges and grows up. This could be a result of strong interaction between Au and Pd as observed in PdAu alloys [33, 45]. According to the above, the charge redistribution is present in the PdAu NPs, plausibly Pd losing s, p-electrons and getting d-electrons with net electron loss, while Au gaining net d-electrons [33, 43, 44, 46, 47].
Crystalline Structure
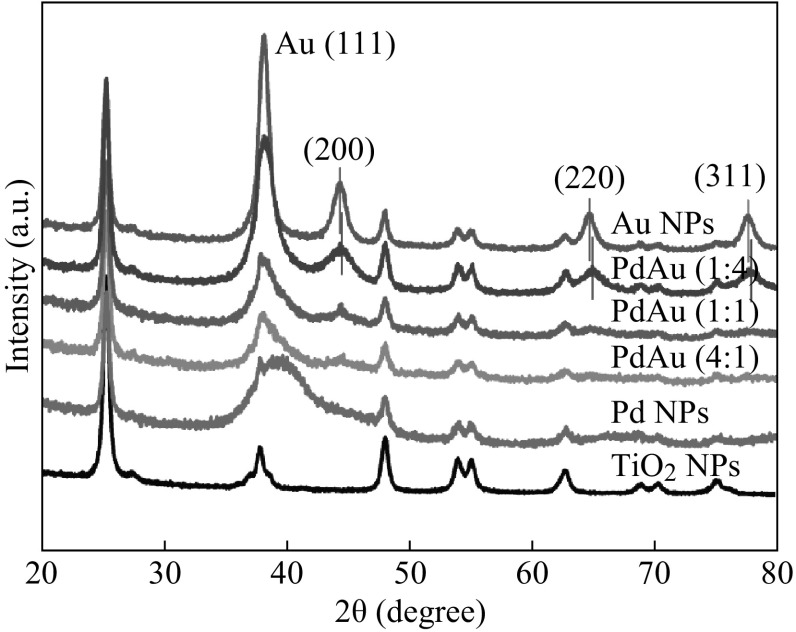
The XRD patterns of the metal–NPs–TiO2 hybrids are shown in Fig. 5. The TiO2 support shows the crystalline features of anatase phase, which remains identical 2θ positions in the hybrids. Thus, there is no phase change or crystallinity degradation of TiO2 after the metal NPs decoration. For monometallic NPs, the Au diffraction peaks are relatively sharp, whereas the Pd ones are broad or even absent. It is owing to the small size and resultant crystalline defects of the Pd NPs compared with the Au NPs [48, 49], which is consistent with the TEM results. Significantly, the Au diffraction peaks are slightly shifted toward larger 2θ side upon addition of Pd, and the Pd diffraction peaks gradually vanish upon the addition of Au. Both indicate the formation of PdAu bimetallic NPs rather than separate Au/Pd monometallic NPs, in good agreement with the above discussion based on the microscopic and electronic characterization results.
Fig. 5.
XRD patterns of TiO2 NPs (anatase) and TiO2-supported metal NPs with different Pd/Au atomic ratios
Catalytic Behavior
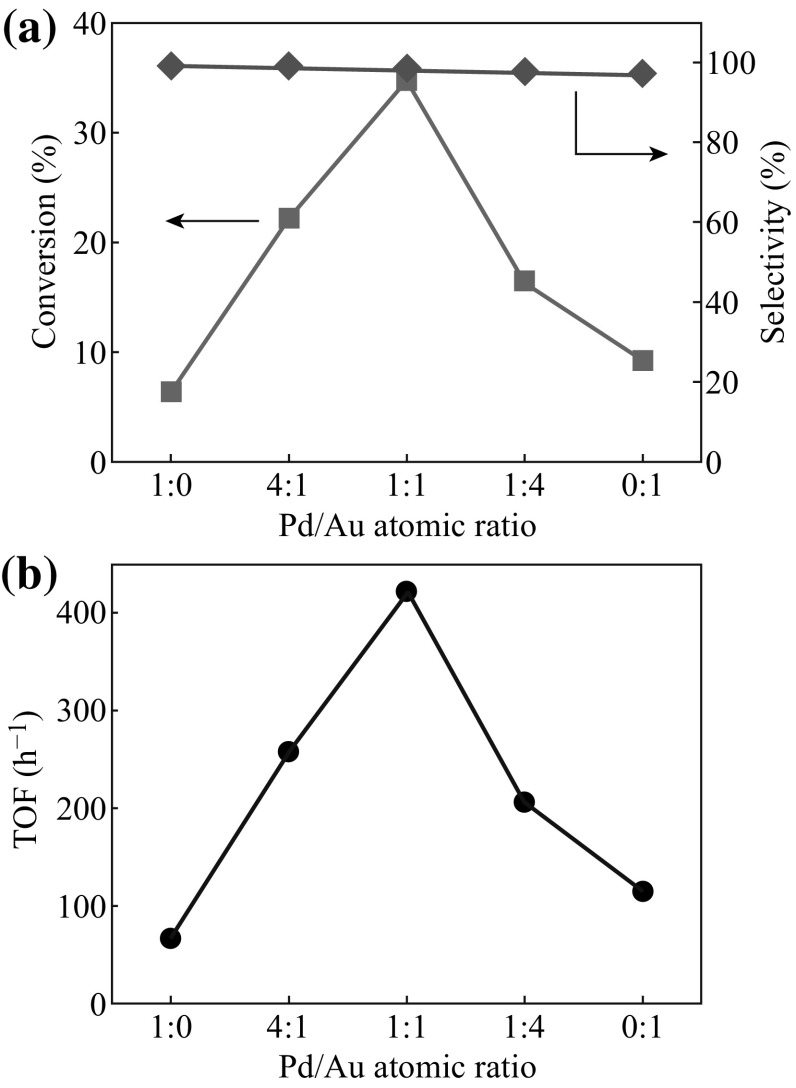
Utilizing the present RTIL-assisted sputtering method, PdAu bimetallic NPs on TiO2 with small size, bare surface, and controlled Pd/Au ratio are achieved. The catalytic activity for 1-phenylethanol oxidation of the metal–NPs–TiO2 hybrids is systematically studied, and the conversion and selectivity results are shown in Fig. 6a. Note that the catalytic reaction is realized using O2 as the oxidant at temperature as low as 50 °C and pressure as low as 1 atm. Furthermore, for a reliable comparison in catalytic activity, the total amount of sputtered metal is controlled to be the same for all the hybrids. Firstly, the Au monometallic NPs appear to be more active than the Pd monometallic NPs. Secondly, all the PdAu bimetallic NPs show higher activity compared with the monometallic counterparts. Thirdly, the conversion from 1-phenylethanol to acetophenone is strongly dependent on Pd/Au ratio, and it gets a maximum of about 35 % at 1:1 Pd/Au ratio. As a more catalytically relevant parameter, the highest TOF of ~421 h−1 is obtained at 1:1 Pd/Au ratio as well (Fig. 6b). Finally, the selectivity remains high irrespective of Pd/Au ratio.
Fig. 6.
Dependences of catalytic activity for 1-phenylethanol oxidation on Pd/Au atomic ratio in TiO2-supported metal NPs: a conversion (square) and selectivity (diamond); b turnover frequency (circle). The catalytic reaction is carried out at 323 K and 1 atm using O2 as the oxidant
The catalytic results suggest that the surface Au diluted by Pd is the active sites, and the synergistic effect can be understood in the following. On one hand, the size of the metal NPs is reduced with increasing Pd/Au ratio, which induces more surface area for the catalytic reaction. Furthermore, the dilution of Au by Pd generates more Au/Pd interface and isolated Au surface, which can promote the catalytic activity of Au (ensemble effect) [38, 50–52]. On the other hand, when Pd/Au ratio is high enough, the number of the Au active sites may decrease. In addition, owing to the charge redistribution effect in PdAu NPs, the d-hole depletion of Au is enhanced with increasing Pd/Au ratio. It may weaken the reactant adsorption on Au, which is adverse to the oxidation catalysis. As a consequence, the combined action of the above promotion and suppression effects shows the highest activity at 1:1 Pd/Au. The synergistic behavior indicates that Pd/Au ratio in PdAu NPs can be well tailored by the present means to optimize their catalytic performance.
Conclusion
A green-chemistry compatible approach toward functional PdAu–NPs–TiO2 hybrids is demonstrated, and the hybrids show high catalytic activity for solvent-free synthesis of acetophenone from 1-phenylethanol. The catalytic reaction is realized under mild conditions, using O2 as the oxidant at the low temperature of 50 °C and low pressure of 1 atm. The approach for the catalyst preparation is straightforward and totally free of additives and byproducts. All the microscopic structure, electronic structure, and crystalline structure characterizations indicate the bimetallic formation of the TiO2-supported PdAu NPs due to atomic inter-diffusion. The catalytic activity of the PdAu NPs exhibits a strong dependence on Pd/Au ratio, which is tunable using the present RTIL-assisted sputtering method.
Acknowledgments
The authors thank the BL14W1 beamline of the Shanghai Synchrotron Radiation Facility (SSRF) for providing the XANES beam time. This work was supported by the National Natural Science Foundation of China (No. 61274019), the Collaborative Innovation Center of Suzhou Nano Science & Technology, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- 1.Abad A, Almela C, Corma A, García H. Efficient chemoselective alcohol oxidation using oxygen as oxidant. Superior performance of gold over palladium catalysts. Tetrahedron. 2006;62(28):6666–6672. doi: 10.1016/j.tet.2006.01.118. [DOI] [Google Scholar]
- 2.Zaw K, Lautens M, Henry PM. Novel parallel path mechanism for the oxidation of allyl alcohol by aqueous palladium (ii) chloride. Organometallics. 1983;2(1):197–199. doi: 10.1021/om00073a051. [DOI] [Google Scholar]
- 3.Lee DG, Spitzer UA. Aqueous dichromate oxidation of primary alcohols. J. Org. Chem. 1970;35(10):3589–3590. doi: 10.1021/jo00835a101. [DOI] [Google Scholar]
- 4.Prabhakaran PV, Venkatachalam S, Ninan KN. Permanganate ion supported over crosslinked polyvinylamine as an oxidising agent for alcohols. Eur. Poly. J. 1999;35(9):1743–1746. doi: 10.1016/S0014-3057(98)00246-8. [DOI] [Google Scholar]
- 5.Muzart J. Palladium-catalysed oxidation of primary and secondary alcohols. Tetrahedron. 2003;59(31):5789–5816. doi: 10.1016/S0040-4020(03)00866-4. [DOI] [Google Scholar]
- 6.Prati I, Rossi M. Gold on carbon as a new catalyst for selective liquid phase oxidation of diols. J. Catal. 1998;176(2):552–560. doi: 10.1006/jcat.1998.2078. [DOI] [Google Scholar]
- 7.Enache D, Knight D, Hutchings G. Solvent-free oxidation of primary alcohols to aldehydes using supported gold catalysts. Catal. Lett. 2005;103(1–2):43–52. doi: 10.1007/s10562-005-6501-y. [DOI] [Google Scholar]
- 8.Tsunoyama H, Sakurai H, Negishi Y, Tsukuda T. Size-specific catalytic activity of polymer-stabilized gold nanoclusters for aerobic alcohol oxidation in water. JACS. 2005;127(26):9374–9375. doi: 10.1021/ja052161e. [DOI] [PubMed] [Google Scholar]
- 9.Abad A, Almela C, Corma A, Garcia H. Unique gold chemoselectivity for the aerobic oxidation of allylic alcohols. Chem. Commun. 2006;30:3178–3180. doi: 10.1039/b606257a. [DOI] [PubMed] [Google Scholar]
- 10.Tsunoyama H, Tsukuda T, Sakurai H. Synthetic application of pvp-stabilized au nanocluster catalyst to aerobic oxidation of alcohols in aqueous solution under ambient conditions. Chem. Lett. 2007;36(2):212–213. doi: 10.1246/cl.2007.212. [DOI] [Google Scholar]
- 11.Li H, Guan B, Wang W, Xing D, Fang Z, Wan X, Yang L, Shi Z. Aerobic oxidation of alcohol in aqueous solution catalyzed by gold. Tetrahedron. 2007;63(35):8430–8434. doi: 10.1016/j.tet.2007.05.117. [DOI] [Google Scholar]
- 12.Hu J, Chen L, Zhu K, Suchopar A, Richards R. Aerobic oxidation of alcohols catalyzed by gold nano-particles confined in the walls of mesoporous silica. Catal. Today. 2007;122(3–4):277–283. doi: 10.1016/j.cattod.2007.01.012. [DOI] [Google Scholar]
- 13.Tsunoyama H, Ichikuni N, Sakurai H, Tsukuda T. Effect of electronic structures of Au clusters stabilized by poly(n-vinyl-2-pyrrolidone) on aerobic oxidation catalysis. JACS. 2009;131(20):7086–7093. doi: 10.1021/ja810045y. [DOI] [PubMed] [Google Scholar]
- 14.Casanova O, Iborra S, Corma A. Biomass into chemicals: one pot-base free oxidative esterification of 5-hydroxymethyl-2-furfural into 2,5-dimethylfuroate with gold on nanoparticulated ceria. J. Catal. 2009;265(1):109–116. doi: 10.1016/j.jcat.2009.04.019. [DOI] [Google Scholar]
- 15.Liu H, Liu Y, Li Y, Tang Z, Jiang H. Metal–organic framework supported gold nanoparticles as a highly active heterogeneous catalyst for aerobic oxidation of alcohols. J. Phys. Chem. C. 2010;114(31):13362–13369. doi: 10.1021/jp105666f. [DOI] [Google Scholar]
- 16.Mifsud M, Parkhomenko KV, Arends IWCE, Sheldon RA. Pd nanoparticles as catalysts for green, sustainable oxidation of functionalized alcohols in aqueous media. Tetrahedron. 2010;66(5):1040–1044. doi: 10.1016/j.tet.2009.11.007. [DOI] [Google Scholar]
- 17.Ma Z, Yang H, Qin Y, Hao Y, Li G. Palladium nanoparticles confined in the nanocages of sba-16: enhanced recyclability for the aerobic oxidation of alcohols in water. J. Mol. Catal. A: Chem. 2010;331(1–2):78–85. doi: 10.1016/j.molcata.2010.08.006. [DOI] [Google Scholar]
- 18.Enache DI, Edwards JK, Landon P, Solsona-espriu B, Carley AF, Herzing AA, Watanabe M, Kiely CJ, Knight DW, Hutchings GJ. Solvent-free oxidation of primary alcohols to aldehydes using Au–Pd/TiO2 catalysts. Science. 2006;311(5759):362–365. doi: 10.1126/science.1120560. [DOI] [PubMed] [Google Scholar]
- 19.Dimitratos N, Lopez-sanchez J, Lennon D, Porta F, Prati L, Villa A. Effect of particle size on monometallic and bimetallic (Au, Pd)/c on the liquid phase oxidation of glycerol. Catal. Lett. 2006;108(3–4):147–153. doi: 10.1007/s10562-006-0036-8. [DOI] [Google Scholar]
- 20.Hou W, Dehm NA, Scott RWJ. Alcohol oxidations in aqueous solutions using Au, Pd, and bimetallic AuPd nanoparticle catalysts. J. Catal. 2008;253(1):22–27. doi: 10.1016/j.jcat.2007.10.025. [DOI] [Google Scholar]
- 21.Villa A, Janjic N, Spontoni P, Wang D, Su DS, Prati L. Au–Pd/AC as catalysts for alcohol oxidation: effect of reaction parameters on catalytic activity and selectivity. Appl. Catal. A: Gen. 2009;364(1–2):221–228. doi: 10.1016/j.apcata.2009.05.059. [DOI] [Google Scholar]
- 22.Marx S, Baiker A. Beneficial interaction of gold and palladium in bimetallic catalysts for the selective oxidation of benzyl alcohol. J. Phys. Chem. C. 2009;113(15):6191–6201. doi: 10.1021/jp808362m. [DOI] [Google Scholar]
- 23.Frank AJ, Rawski J, Maly KE, Kitaev V. Environmentally benign aqueous oxidative catalysis using AuPd/TiO2 colloidal nanoparticle system stabilized in absence of organic ligands. Green Chem. 2010;12(9):1615–1622. doi: 10.1039/c0gc00084a. [DOI] [Google Scholar]
- 24.Chen Y, Lim H, Tang Q, Gao Y, Sun T, Yan Q, Yang Y. Solvent-free aerobic oxidation of benzyl alcohol over Pd monometallic and Au–Pd bimetallic catalysts supported on SBA-16 mesoporous molecular sieves. Appl. Catal. A: Gen. 2010;380(1–2):55–65. doi: 10.1016/j.apcata.2010.03.026. [DOI] [Google Scholar]
- 25.Gong J, Mullins CB. Surface science investigations of oxidative chemistry on gold. Acc. Chem. Res. 2009;42(8):1063–1073. doi: 10.1021/ar8002706. [DOI] [PubMed] [Google Scholar]
- 26.Jian W, Xuxing L, Qiannan Z, Junwei Z, Qishun S, Li Z, Weihai N. Angle-resolved plasmonic properties of single gold nanorod dimers. Nano-Micro Lett. 2014;6(4):372–380. doi: 10.1007/s40820-014-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert L, Anming H, John P, Zhou YN. Palladium nanoparticles loaded on carbon modified TiO2 nanobelts for enhanced methanol electrooxidation. Nano-Micro Lett. 2013;5(3):202–212. doi: 10.1007/BF03353751. [DOI] [Google Scholar]
- 28.Fagen W, Yan X, Kunfeng Z, Dannong H. Preparation of palladium supported on ferric oxide nano-catalysts for carbon monoxide oxidation in low temperature. Nano-Micro Lett. 2014;6(3):233–241. doi: 10.1007/BF03353787. [DOI] [Google Scholar]
- 29.Pietron JJ, Stroud RM, Rolison DR. Using three dimensions in catalytic mesoporous nanoarchitectures. Nano Lett. 2002;2(5):545–549. doi: 10.1021/nl025536s. [DOI] [Google Scholar]
- 30.Somorjai GA, Park JY. Molecular factors of catalytic selectivity. Angew. Chem. Int. Ed. 2008;47(48):9212–9228. doi: 10.1002/anie.200803181. [DOI] [PubMed] [Google Scholar]
- 31.Liu CH, Mao BH, Gao J, Zhang S, Gao X, Liu Z, Lee ST, Sun XH, Wang SD. Size-controllable self-assembly of metal nanoparticles on carbon nanostructures in room-temperature ionic liquids by simple sputtering deposition. Carbon. 2012;50(8):3008–3014. doi: 10.1016/j.carbon.2012.02.086. [DOI] [Google Scholar]
- 32.Liu CH, Chen XQ, Hu YF, Sham TK, Sun QJ, Chang JB, Gao X, Sun XH, Wang SD. One-pot environmentally friendly approach toward highly catalytically active bimetal-nanoparticle-graphene hybrids. ACS Appl. Mater. Interface. 2013;5(11):5072–5079. doi: 10.1021/am4008853. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, White T, Li P, He C, Yu J, Yuan W, Han Y-F. Biphasic Pd–Au alloy catalyst for low-temperature co oxidation. JACS. 2010;132(30):10398–10406. doi: 10.1021/ja102617r. [DOI] [PubMed] [Google Scholar]
- 34.Edwards JK, Carley AF, Herzing AA, Kiely CJ, Hutchings GJ. Direct synthesis of hydrogen peroxide from H2 and O2 using supported Au–Pd catalysts. Faraday Discuss. 2008;138:225–239. doi: 10.1039/B705915A. [DOI] [PubMed] [Google Scholar]
- 35.Mizukoshi Y, Sato K, Konno TJ, Masahashi N. Dependence of photocatalytic activities upon the structures of Au/Pd bimetallic nanoparticles immobilized on TiO2 surface. Appl. Catal. B: Environ. 2010;94(3–4):248–253. doi: 10.1016/j.apcatb.2009.11.015. [DOI] [Google Scholar]
- 36.Wang Q, Cui X, Guan W, Zhang X, Liu C, Xue T, Wang H, Zheng W. A nanoflower shaped gold-palladium alloy on graphene oxide nanosheets with exceptional activity for electrochemical oxidation of ethanol. Microchim. Acta. 2014;181(3–4):373–380. doi: 10.1007/s00604-013-1119-z. [DOI] [Google Scholar]
- 37.Pasini T, Piccinini M, Blosi M, Bonelli R, Albonetti S, Dimitratos N, Lopez-sanchez JA, Sankar M, He Q, Kiely CJ, Hutchings GJ, Cavani F. Selective oxidation of 5-hydroxymethyl-2-furfural using supported gold-copper nanoparticles. Green Chem. 2011;13(8):2091–2099. doi: 10.1039/c1gc15355b. [DOI] [Google Scholar]
- 38.Cui C-H, Yu J-W, Li H-H, Gao M-R, Liang H-W, Yu S-H. Remarkable enhancement of electrocatalytic activity by tuning the interface of Pd–Au bimetallic nanoparticle tubes. ACS Nano. 2011;5(5):4211–4218. doi: 10.1021/nn2010602. [DOI] [PubMed] [Google Scholar]
- 39.Fang J, Cao SW, Wang Z, Shahjamali MM, Loo SCJ, Barber J, Xue C. Mesoporous plasmonic Au–TiO2 nanocomposites for efficient visible-light-driven photocatalytic water reduction. Int. J. Hydrog. Energy. 2012;37(23):17853–17861. doi: 10.1016/j.ijhydene.2012.09.023. [DOI] [Google Scholar]
- 40.Long MC, Jiang JJ, Li Y, Cao RQ, Zhang LY, Cai WM. Effect of gold nanoparticles on the photocatalytic and photoelectrochemical performance of au modified BiVO4. Nano-Micro Lett. 2011;3(3):171–177. doi: 10.1007/BF03353669. [DOI] [Google Scholar]
- 41.Dozzi MV, Saccomanni A, Altomare M, Selli E. Photocatalytic activity of NH4F-doped TiO2 modified by noble metal nanoparticle deposition. Photochem. Photobiol. Sci. 2013;12(4):595–601. doi: 10.1039/C2PP25175B. [DOI] [PubMed] [Google Scholar]
- 42.Han C, Yang X, Gao G, Wang J, Lu H, Liu J, Tong M, Liang X. Selective oxidation of methanol to methyl formate on catalysts of Au-Ag alloy nanoparticles supported on titania under UV irradiation. Green Chem. 2014;16(7):3603–3615. doi: 10.1039/c4gc00367e. [DOI] [Google Scholar]
- 43.Wang Y, Zheng J-M, Fan K, Dai W-L. One-pot solvent-free synthesis of sodium benzoate from the oxidation of benzyl alcohol over novel efficient AuAg/TiO2 catalysts. Green Chem. 2011;13(7):1644–1647. doi: 10.1039/c1gc15311k. [DOI] [Google Scholar]
- 44.Venezia AM, La parola V, Deganello G, Pawelec B, Fierro JLG. Synergetic effect of gold in Au/Pd catalysts during hydrodesulfurization reactions of model compounds. J. Catal. 2003;215(2):317–325. doi: 10.1016/S0021-9517(03)00005-8. [DOI] [Google Scholar]
- 45.Nascente PAP, Decastro SGC, Landers R, Kleiman GG. X-ray photoemission and auger energy shifts in some gold-palladium alloys. Phys. Rev. B. 1991;43(6):4659–4666. doi: 10.1103/PhysRevB.43.4659. [DOI] [PubMed] [Google Scholar]
- 46.Balcha T, Strobl JR, Fowler C, Dash P, Scott RWJ. Selective aerobic oxidation of crotyl alcohol using aupd core-shell nanoparticles. ACS Catal. 2011;1(5):425–436. doi: 10.1021/cs200040a. [DOI] [Google Scholar]
- 47.Cybula A, Priebe JB, Pohl M-M, Sobczak JW, Schneider M, Zielińska-Jurek A, Brückner A, Zaleska A. The effect of calcination temperature on structure and photocatalytic properties of Au/Pd nanoparticles supported on TiO2. Appl. Catal. B: Environ. 2014;152–153:202–211. doi: 10.1016/j.apcatb.2014.01.042. [DOI] [Google Scholar]
- 48.Wu M-I, Chen D-H, Huang T-C. Synthesis of Au/Pd bimetallic nanoparticles in reverse micelles. Langmuir. 2001;17(13):3877–3883. doi: 10.1021/la010060y. [DOI] [Google Scholar]
- 49.Cui CH, Li HH, Yu SH. A general approach to electrochemical deposition of high quality free-standing noble metal (Pd, Pt, Au, Ag) sub-micron tubes composed of nanoparticles in polar aprotic solvent. Chem. Commun. 2010;46(6):940–942. doi: 10.1039/B920705H. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Shao Y, Yin G, Lin Y. Electrostatic self-assembly of a Pt-around-Au nanocomposite with high activity towards formic acid oxidation. Angew. Chem. Int. Ed. 2010;49(12):2211–2214. doi: 10.1002/anie.200906987. [DOI] [PubMed] [Google Scholar]
- 51.Ponec V. Alloy catalysts: the concepts. Appl. Catal. A: Gen. 2001;222(1–2):31–45. doi: 10.1016/S0926-860X(01)00828-6. [DOI] [Google Scholar]
- 52.Liu P, Norskov JK. Ligand and ensemble effects in adsorption on alloy surfaces. Phys. Chem. Chem. Phys. 2001;3(17):3814–3818. doi: 10.1039/b103525h. [DOI] [Google Scholar]