Abstract
A centralized deposit of adiposity increases the risk of cardiometabolic diseases. Several anthropometric markers can be used to characterize fat distribution. In the case of severe obesity, several markers, such as hip and waist circumference, are prone to measurement error. Conversely, neck circumference is easy to obtain. The aim was to determine the best surrogate marker of obesity-related cardiometabolic diseases from: body mass index (BMI), waist, hip and neck circumferences and waist-to-hip ratio.
Methods
Data from women (n = 305, aged 43 [34; 53] years-old, BMI 44.2 [40.8; 48.2] kg/m2) included in the Severe Obesity Outcome Network (SOON) cohort were analyzed (i) to evaluate collinearity between the different anthropometric markers, (ii) to compare the association of markers with hypertension, type 2 diabetes, obstructive sleep apnea syndrome (OSAS) and other cardiometabolic risks.
Results
Hip, waist and neck circumferences correlated with BMI with respectively less collinearity (r = 0.70, r = 0.59 and r = 0.37, respectively, p<0.001) whereas waist-to-hip ratio was not correlated (r = 0.11, p = 0.072). Waist and neck circumferences were significantly associated with hypertension, type 2 diabetes and OSAS in univariate logistic regressions, waist-to-hip ratio with hypertension and type 2 diabetes. Hip circumference was inversely correlated with type 2 diabetes (OR 0.970 (95CI: 0.948; 0.991) p = 0.006). BMI was only linked to OSAS (OR 1.092 (95CI: 1.043; 1.143) p<0.001). Neck circumference was the only marker significantly associated with all cardiometabolic risk markers (HOMA-IR, apnea-hypopnea index, Log Triglycerides/HDL-c, alanin-aminotransferase, aspartate-aminotransferase, gammaglutamyl transpeptidase).
Conclusions
Neck circumference appears the most appropriate anthropometric marker to identify the fat distribution associated with high cardiometabolic risk in women with severe obesity.
Introduction
Obesity affects physical, psychological and social well-being. Although there is a relationship between obesity and cardiometabolic diseases, the risk of developing such diseases is variable. In the 1950’s studies showed that obesity-related cardiovascular and metabolic comorbidities were associated with a centralized deposit of adiposity [1]. Excess visceral fat is associated with a loss of the capacity to stock excessive calories within subcutaneous adipose tissue, inducing an ectopic fat deposition in the liver, muscles, heart etc. that leads to metabolic syndrome and cardiometabolic diseases [2]. Patients with a central obesity distribution have therefore a higher risk of hypertension, type 2 diabetes, obstructive sleep apnea syndrome (OSAS) and non-alcoholic fatty liver diseases (NAFLD). The risk of cardiovascular diseases is also high [3]. Conversely, obese patients with peripheral or subcutaneous adiposity are usually “metabolically healthy”, and even protected against metabolic diseases because subcutaneous adiposity is both a marker of, and a contributor to, a high level of insulin sensitivity [4, 5].
Waist circumference and waist-to-hip ratio are well established as useful indicators of visceral fat accumulation. These simple anthropometric measurements can detect subjects at risk in the primary care setting. However, waist circumference increases with increasing body weight. Progressively higher median waist values are associated with increasing body mass index (BMI), although for any given BMI value, the variation in waist circumference is considerable. Thus it is difficult to define a single cut-off value for abdominal obesity across a large range of BMIs [6]. Waist-to-hip ratio is not limited in the same way since the collinearity of waist and hip circumferences with BMI is neutralized by dividing one by the other [7]. However, the measurement of waist and hip circumference in patients with severe obesity is hampered by technical issues. The measurement of waist circumference is often complicated by the lumbar lordosis and measurement of hip circumference is difficult due to the abdominal fat apron. Neck circumference is used as an anthropometric marker to detect patients at risk of sleep apnea syndrome [8]; however, this marker also appears to be associated with metabolic disorders [9, 10].
The aim of this study was to compare the level of association of different anthropometric markers with cardiometabolic diseases and cardiometabolic risk markers in women with severe obesity. We sought to determine which of the following parameters was the best surrogate to identify women with a high risk of obesity related diseases: BMI, waist, hip and neck circumferences, and waist-to-hip ratio. In addition, we assessed whether waist-to-height and neck-to-height ratios improved the results obtained with waist and neck circumferences, respectively.
Materials and methods
The severe obesity outcome network (SOON) cohort recruited adult patients volunteering for bariatric surgery from the 1st of September 2013, at the Grenoble Alpes University Hospital, France. The population studied was mainly of Caucasian people living in France. Inclusion criteria were based on French recommendations; adults aged 18–65 years-old with class III obesity (body mass index (BMI) ≥40 kg/m2) or class II obesity (40>BMI ≥35 kg/m2) with at least one obesity-related complication (type 2 diabetes, hypertension, OSAS or nonalcoholic steatohepatitis). Exclusion criteria were contraindications to bariatric surgery: pregnancy, psychotic disease and alcohol or drug addictions. The present work analyzed the data from the 305 women included by September 2016. The present study was reviewed and approved by an institutional review board (ethics committee: “Comité de protection des personnes Sud-Est V”, IRB 6705) before the study began. All patients signed informed consent for their inclusion. The SOON cohort is registered on clinical trials (NCT02264431).
Study design
Observational, transversal study.
Anthropometric measures
Height, weight, and hip, waist and neck circumference were measured according to standardized procedures at the greater trochanter, iliac crest, and cricoid, respectively [11]. Circumferences were measured with the participant in a standing position using a non-extensible, flexible anthropometric tape.
Plasma, glucose/insulin homeostasis profile
Women were considered to have type 2 diabetes either based on current medication for diabetes or by HbA1c > 6.5%, fasting plasma glucose above 7 mmol/L over two separate analyses, or according to the results of a 75g oral glucose tolerance test (OGTT) with fasting glucose ≥7 mmol/L or 120 min OGTT-glucose ≥11.1 mmol/L [12]. Insulin resistance was assessed with HOMA-IR index, based on fasting plasma and insulin levels [13].
Plasma lipid/lipoprotein profile
Plasma HDL-cholesterol, triglycerides, VLDL-cholesterol and LDL-cholesterol were determined according to standardized procedures [14, 15].
Blood pressure
Hypertension was determined either by current use of anti-hypertensive drugs or by a mean systolic blood pressure above 140 mmHg and/or a diastolic blood pressure above 90 mmHg found during three blood pressure measurements taken 3 min apart on the non-dominant arm, with an appropriate-sized cuff after the patient had been sitting for 5 min [16].
Sleep apnea syndrome
OSAS was determined either by current use of continuous positive airway pressure therapy or an oral mandibular advancement device, or by the results of nocturnal polygraphy. The recordings were scored according to standardized methods, described in the American Academy of Sleep Medicine (AASM) Manual for Scoring Sleep and Associated Events [17]. Patients with an apnea+hypopnea index (AHI) >30 apnea + hypopnea events per hour were considered to have severe OSAS.
Functional liver tests
Functional liver enzymes, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gammaglutamyl transpeptidase (GT) were evaluated.
Statistical analysis
Descriptive data are reported as medians [interquartile range] for continuous variables and as numbers (%) for qualitative variables.
Univariate correlations were analyzed using non-parametric Spearman tests to assess collinearity between anthropometric markers. A weighted local regression line (LOESS) was added on graphic representations.
Univariate logistic regressions were performed to address the relationship between anthropometric markers and obesity related diseases (Type 2 diabetes, hypertension, OSAS). The linearity of each variable was tested using a generalized additive model (proc GAM in SAS). One outlier for waist-to-hip ratio conducted to perform a sensitivity analysis for logistic regressions involving waist-to-hip ratio. Multiple logistic regressions were then performed including age, tobacco, alcohol consumption and the presence of depression as covariates in the models.
Univariate correlations were performed using non-parametric Spearman tests to measure the relationship between anthropometric markers and continuous cardiometabolic risk markers (log TG/HDL, HOMA-IR, AST, ALT, GT, AHI).
Models were performed with imputed data according to the FCS logistic regression method, using 10 data sets to deal with missing data (less than 10% in all cases). For patients previously diagnosed and treated for severe OSAS, AHI was missing. Imputation of data for AHI in these patients was performed using the subpopulation of women who were found to have OSAS on nocturnal polygraphy.
The significance level was set at p<0.05. All analyses were performed with the SAS statistical package version 9.4 (SAS Institute, Cary, NC, USA).
Results
The characteristics of the women included are reported in Table 1. In these mainly middle-aged women, the prevalence of obesity-related diseases was 26.6% for type 2 diabetes, 28.0% for hypertension and 29.9% for severe OSAS.
Table 1. Patient characteristics.
| N = 305 | |
|---|---|
| Age (years) | 43 [34; 53] |
| Female gender n (%) | 305 (100.0) |
| Anthropometric markers | |
| Body weight (kg) | 117 [107; 129] |
| BMI (kg/m2) | 44.2 [40.8; 48.2] |
| Waist circumference (cm) | 123 [115; 130.5] |
| Hip circumference (cm) | 134 [125; 143] |
| Neck circumference (cm) | 40.2 [38; 42] |
| Waist-to-hip ratio | 0.91 [0.85; 0.98] |
| Neck-to-height | 0.25 [0.23; 0.26] |
| Waist-to-height | 0.76 [0.71; 0.81] |
| Obesity related diseases | |
| Hypertension n (%) | 85 (28.0) |
| Systolic blood pressure (mmHg) | 123 [116; 133] |
| Diastolic blood pressure (mmHg) | 70 [63; 77] |
| Diabetes n (%) | 81 (26.6) |
| HOMA-IR | 3.6 [2.3; 6.4] |
| Sleep apnea syndrome | |
| Mild n (%) | 102 (34.6) |
| Moderate n (%) | 63 (21.4) |
| Severe n (%) | 88 (29.8) |
| IAH 3% (event/h) | 13.8 [7.1; 26] |
| Number of associated comorbidities* | |
| 0 n (%) | 145 (47.5) |
| 1 n (%) | 83 (27.2) |
| 2 n (%) | 60 (19.7) |
| 3 n (%) | 17 (5.6) |
| Lipid/lipoprotein profile | |
| Triglycerides (mmol/L) | 1.5 [1.1; 2] |
| HDL-c (mmol/L) | 1.2 [1; 1.4] |
| LDL-c (mmol/L) | 2.9 [2.4; 3.6] |
| Log Tg/HDL-c | 0.4 [0.1; 0.6] |
| Liver functional tests | |
| ALT (UI/L) | 31 [23.5; 44] |
| AST (UI/L) | 18 [14; 25] |
| GT (UI/L) | 31 [19; 53] |
Data are medians (IQR) for continuous variables and n (%) for qualitative variables. Abbreviations: BMI: body mass index; HOMA-IR: HOMA-Index Resistance; Tg: triglycerides; AHI: apnea-hypopnea index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GT: gammaglutamyl transpeptidase.
*The comorbidities taken into account are hypertension, type 2 diabetes, severe sleep apnea syndrome.
Collinearity among anthropometric markers
Waist and hip circumferences were highly collinear with BMI (r = 0.59 p<0.001 and r = 0.70 p<0.001, respectively). Neck circumference and BMI were collinear but with a smaller r coefficient (r = 0.37 p<0.001), whereas waist-to-hip ratio was unrelated to BMI (r = 0.11 p = 0.072). Waist-to-height and neck-to-height ratios demonstrated similar results to waist and neck circumferences, respectively (r = 0.61, p<0.001 and r = 0.37, p<0.001, respectively).
Waist circumference and waist-to-hip ratio were also collinear with hip circumference (r = 0.37 and r = 0.53, respectively, p<0.001 for both). Neck circumference was the only anthropometric marker that was collinear with other markers of central adiposity (r = 0.51 with waist circumference and r = 0.34 with waist-to-hip ratio, p<0.001) whereas it was unrelated to hip circumference (r = 0.11, p = 0.084), a marker of subcutaneous adiposity.
Association between anthropometric markers and obesity related diseases
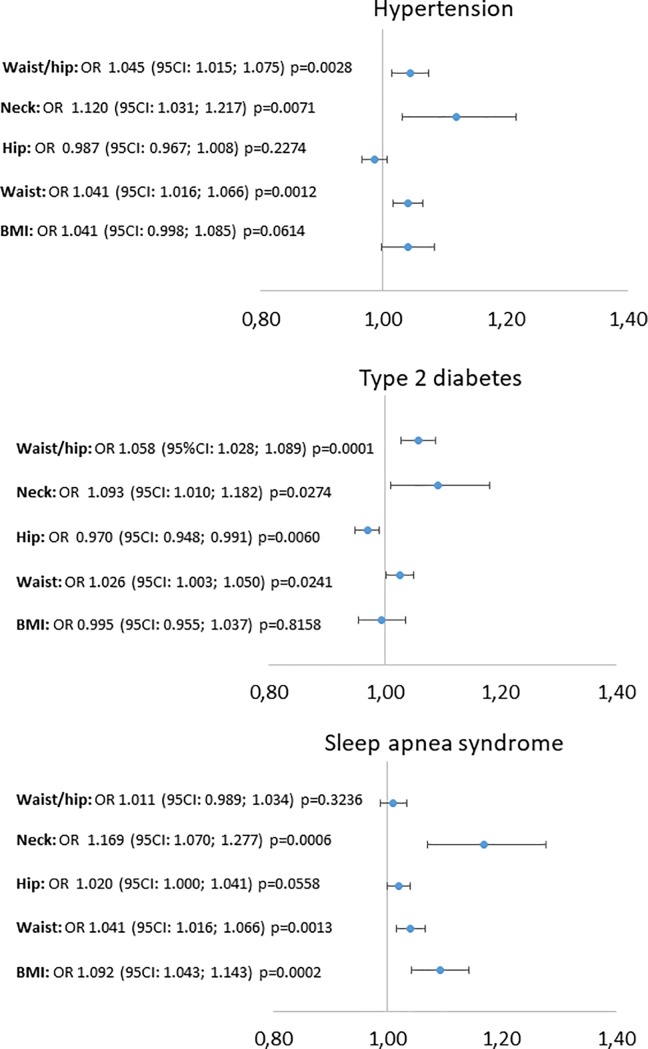
Univariate logistic regressions are reported in Fig 1. Waist and neck circumferences were significantly associated with hypertension, type 2 diabetes and OSAS. Hip circumference was unrelated to hypertension or OSAS and inversely associated with type 2 diabetes. The logistic regressions with waist-to-hip ratio were influenced by an outlier waist-to-hip ratio of 1.99, due to an extremely large abdominal apron. A sensitivity analysis without this patient led to a significant association of waist-to-hip with hypertension and type 2 diabetes but not with OSAS. These results are reported in Fig 1. BMI was significantly associated with OSAS but not with hypertension or type 2 diabetes. The results for waist-to-height and neck-to-height ratios were similar to those for waist and neck circumferences: OR 1.077 (95CI: 1.035; 1.121), p<0.0001 and OR 1.221 (95CI: 1.073; 1.389), p = 0.002, respectively for association with hypertension; OR 1.054 (95CI: 1.015; 1.093), p = 0.006 and OR 1.179 (95CI: 1.044; 1.331), p = 0.008 for association with diabetes; OR 1.058 (95CI: 1.017; 1.100), p = 0.003 and OR 1.223 (95CI: 1.072; 1.395) p = 0.008 for association with OSAS. The areas under the curve for these logistic regressions are reported in Table 2.
Fig 1. Univariate logistic regressions with anthropometric markers as independent variables and obesity related diseases as dependent variables.
Abbreviation: BMI: body mass index; OR: odd ratio; OSAS: Obstructive sleep apnea syndrome.
Table 2. Areas under the curve of univariate logistic regressions.
| Waist circ. | Hip circ. | Waist-to-hip | Neck circ. | Waist circ.-to-height | Neck circ.-to-height | |
|---|---|---|---|---|---|---|
| Hypertension | 0,77 | 0,74 | 0,78 | 0,76 | 0.78 | 0.76 |
| Type 2 diabetes | 0,67 | 0,67 | 0,72 | 0,66 | 0.68 | 0.67 |
| OSAS | 0,77 | 0,76 | 0,75 | 0,78 | 0.76 | 0.77 |
Abbreviation: OSAS: obstructive sleep apnea; Circ.: circumference.
To test whether such associations were independent of potential covariates, multiple logistic regressions were then performed including age, tobacco, alcohol consumption and the presence of depression as covariates in the models. Results were essentially the same than in univariate logistic analyses. These models are reported in Table 3.
Table 3. Multivariable logistic regressions for obesity related diseases.
| B-estimate | Standard error | OR (95%CI) | P values | |
|---|---|---|---|---|
| Hypertension | ||||
| BMI | 0.0431 | 0.0223 | 1.044 (0.999;1.091) | 0.0534 |
| Neck circumference | 0.1215 | 0.0446 | 1.129 (1.035; 1.232) | 0.0064 |
| Waist circumference | 0.0407 | 0.0129 | 1.042 (1.015; 1.068) | 0.0017 |
| Hip circumference | -0.0103 | 0.0109 | 0.990 (0.969; 1.011) | 0.3450 |
| Waist/hip raio | 0.0385 | 0.0154 | 1.039 (1.008; 1.071) | 0.0123 |
| Neck circ./height | 0.2019 | 0.0691 | 1.224 (1.069; 1.401) | 0.0035 |
| Waist circ./height | 0.0718 | 0.0212 | 1.074 (1.031; 1.120) | 0.0007 |
| Type 2 diabetes | ||||
| BMI | -0.00046 | 0.0221 | 1.000 (0.957; 1.044) | 0.9836 |
| Neck circumference | 0.0856 | 0.0429 | 1.089 (1.001; 1.185) | 0.0462 |
| Waist circumference | 0.0260 | 0.0122 | 1.026 (1.002; 1.051) | 0.0330 |
| Hip circumference | -0.0278 | 0.0117 | 0.973 (0.951; 0.995) | 0.0171 |
| Waist/hip raio | 0.0505 | 0.0150 | 1.052 (1.021; 1.083) | 0.0008 |
| Neck circ./height | 0.1475 | 0.0664 | 1.159 (1.018; 1.320) | 0.0263 |
| Waist circ./height | 0.0495 | 0.0201 | 1.051 (1.010; 1.093) | 0.0139 |
| Obstructive sleep apnea syndrome | ||||
| BMI | 0.0843 | 0.0239 | 1.088 (1.038; 1.140) | 0.0004 |
| Neck circumference | 0.2015 | 0.0483 | 1.223 (1.113; 1.345) | <0.0001 |
| Waist circumference | 0.0433 | 0.0131 | 1.044 (1.018; 1.071) | 0.0009 |
| Hip circumference | 0.0170 | 0.0107 | 1.017 (0.996; 1.039) | 0.1137 |
| Waist/hip raio | 0.0140 | 0.0123 | 1.014 (0.990; 1.039) | 0.2556 |
| Neck circ./height | 0.2480 | 0.0714 | 1.281 (1.114; 1.474) | 0.0005 |
| Waist circ./height | 0.0589 | 0.0210 | 1.061 (1.018; 1.105) | 0.0050 |
Abbreviations: Circ: circumference; BMI: body mass index; CI: confidence interval. Multiple logistic regressions were performed including age, tobacco, alcohol consumption and the presence of depression as covariates in the models.
Correlations between anthropometric and cardiometabolic risk markers
Spearman correlations are reported in Table 4. Neck circumference (as well as neck-to-height ratio) was the only anthropometric marker that strongly correlated with all cardiometabolic risk parameters, particularly with functional liver enzymes, i.e. ALT, AST and GT. No significant correlation was found between the different anthropometric markers and the LDL-c levels.
Table 4. Univariate Spearman correlations between anthropometric parameters and cardiometabolic risk markers.
| BMI | Neck circ. | Waist circ. | Hip circ. | Waist/hip | Neck circ./height | Waist circ./height | |
|---|---|---|---|---|---|---|---|
| HOMA-IR | r = 0.07 | r = 0.34 | r = 0.30 | r = 0.14 | r = 0.37 | r = 0.34 | r = 0.32 |
| p = 0.230 | p<0.001 | p<0.001 | p = 0.018 | p<0.001 | p<0.001 | p<0.001 | |
| AHI | r = 0.16 | r = 0.23 | r = 0.11 | r = 0.05 | r = 0.05 | r = 0.29 | r = 0.18 |
| p = 0.010 | p<0.001 | p = 0.079 | p = 0.448 | p = 0.431 | p<0.001 | p = 0.003 | |
| Log Tg/HDL | r = 0.00 | r = 0.24 | r = 0.14 | r = 0.08 | r = 0.22 | r = 0.22 | r = 0.15 |
| p = 0.966 | p<0.001 | p = 0.018 | p = 0.167 | p<0.001 | p<0.001 | p = 0.008 | |
| ALT | r = 0.05 | r = 0.24 | r = 0.08 | r = 0.21 | r = 0.28 | r = 0.24 | r = 0.09 |
| p = 0.351 | p<0.001 | p = 0.147 | p<0.001 | p<0.001 | p<0.001 | p = 0.123 | |
| AST | r = 0.06 | r = 0.14 | r = 0.07 | r = 0.19 | r = 0.26 | r = 0.17 | r = 0.10 |
| p = 0.315 | p<0.001 | p = 0.212 | p = 0.001 | p<0.001 | p = 0.040 | p = 0.092 | |
| GGT | r = 0.04 | r = 0.36 | r = 0.17 | r = 0.04 | r = 0.18 | r = 0.34 | r = 0.17 |
| p = 0.450 | p<0.001 | p = 0.003 | p = 0.515 | p = 0.002 | p<0.001 | p = 0.004 |
Abbreviations: Circ: circumference; BMI: body mass index; HOMA-IR: HOMA-Index Resistance; Tg: triglycerides; AHI: apnea-hypopnea index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gammaglutamyl transpeptidase.
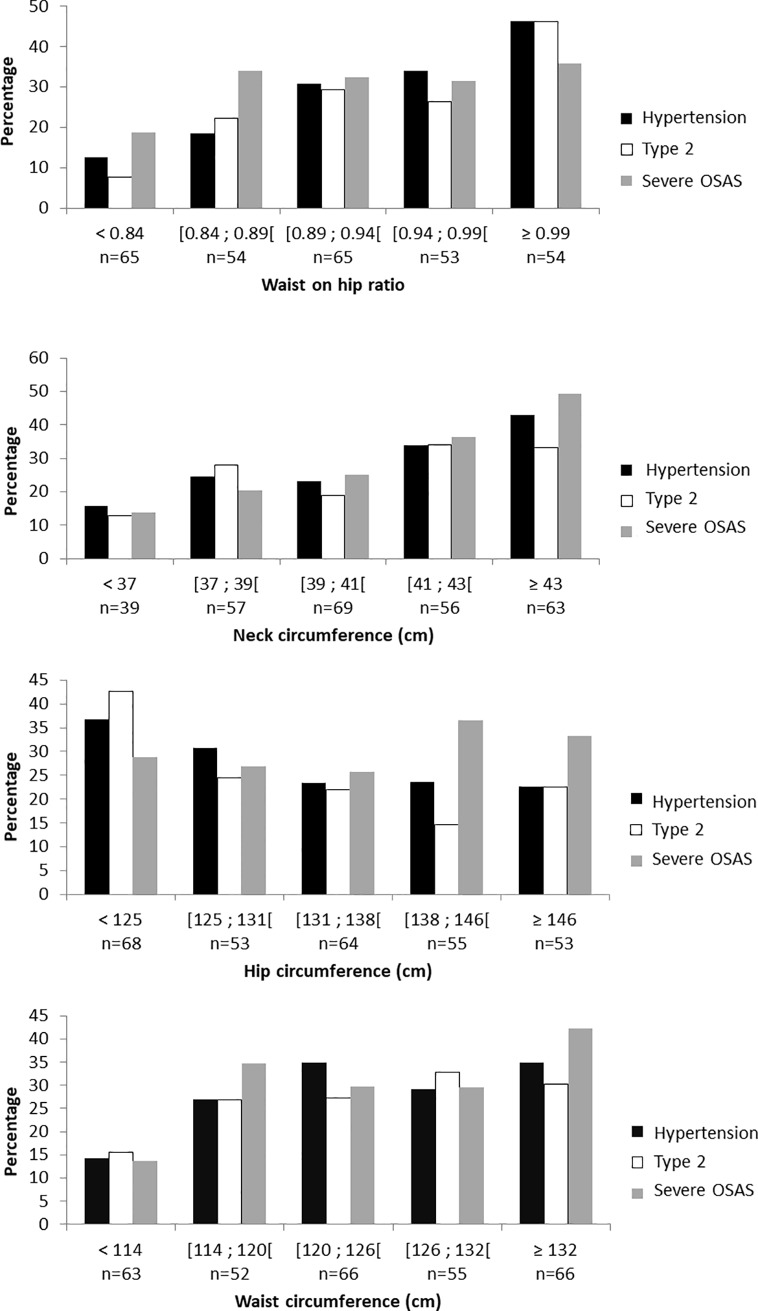
Prevalence of obesity related diseases according to neck circumference
We reported the prevalence of hypertension, type 2 diabetes and severe OSAS according to the different anthropometric markers, divided into quintiles (Fig 2). The prevalence of hypertension progressively increased from 16 to 43%, type 2 diabetes from 13 to 33% and severe OSAS from 14 to 49% across increasing neck circumference quintiles.
Fig 2. Prevalence of obesity related diseases according to quintiles of anthropometric markers.
Abbreviation: OSAS: Obstructive sleep apnea syndrome.
Discussion
The present work studied women with class II or class III obesity who were volunteering for bariatric surgery. The results demonstrated that waist-to-hip ratio was the only anthropometric marker that was unrelated to BMI. The other markers were all collinear with BMI, although neck circumference was less strongly related than waist and hip circumferences. In addition, neck circumference was collinear with markers of central obesity (waist circumference and waist-to-hip ratio) but not with a marker of subcutaneous adiposity (hip circumference).
This study therefore showed that neck circumference was as valid as waist circumference and waist-to-hip ratio for the identification of women with a high cardiometabolic risk profile. Moreover, it is much easier to measure in routine clinical practice in women with severe obesity.
Reliability of anthropometric markers in the context of severe obesity
In the present work, hip circumference, a marker of subcutaneous adiposity, was not correlated with any metabolic markers and was inversely associated with type 2 diabetes. Previous studies have shown that hip circumference was inversely associated with the risk of incident type 2 diabetes in men and women [18]. Subcutaneous adipose tissue, measured by abdominal computed tomography, was also found to be inversely correlated with type 2 diabetes [4]. An increase in hip circumference reflects an expansion in low body subcutaneous adipose tissue. This subcutaneous fat depot is diametrically opposed to visceral fat. In epidemiological studies, it has been associated with a lower cardiac risk factor burden [19] and lower risk of incident cardiovascular diseases [20] and cancer [21]. This low body subcutaneous fat may impart a protective effect by acting as a metabolic buffer to the influx of dietary lipids and protecting other tissues from the lipotoxicity caused by lipid overflow and ectopic fat deposition [5]. In addition, human obese visceral and subcutaneous adipose tissues secretomes are clearly distinct. Visceral fat secretome leads to a low-grade inflammation profile whereas subcutaneous secretome favors insulin sensitivity [22].
Waist circumference has been shown to be a less reliable marker of metabolic abnormalities in men [23] and women [24] with severe obesity, compared to those with lower or normal BMI scores. This could be attributed to the contribution of abdominal subcutaneous adipose tissue, which appears to be protective for metabolic conditions [25]. However, other studies have also found the well-known relationship between waist circumference and cardiometabolic risk profile in patients with severe obesity [26, 27]. The present work confirmed that in women with severe obesity, waist circumference was associated with hypertension, type 2 diabetes and OSAS, as well as with HOMA-IR and Log TG/HDL, both markers of insulin resistance.
Waist-to-hip ratio and waist circumference, were associated with an increased risk of cardiovascular mortality in women from the Nurses' Health Study prospective cohort [28], as well as in other cohorts of women [29]. In the present study, waist-to-hip ratio in women with severe obesity was a reliable marker for the identification of hypertension, type 2 diabetes (but not OSAS) and other cardiometabolic risk markers. The stronger reliability of this measure over waist circumference alone could be related to the neutralization of abdominal subcutaneous adiposity by the hip circumference denominator, which adjusts waist circumference for subcutaneous adiposity [30]. For the same reason, waist-to-hip ratio is unaffected by increases in BMI, across large ranges of BMI, as confirmed by the present results. However, these results need to be considered according to the ease and accuracy of the physical measurements. It is common clinical experience that waist and hip measurements are subject to errors in patients with severe obesity. This was underlined in our cohort by the outlier data for the patient who had a very high waist-to-hip ratio (1.99) because of a very large abdominal apron. Neck circumference could therefore be a clinically relevant anthropometric marker of central obesity in patients with severe obesity.
Previous evidence regarding neck circumference
Neck circumference was first identified as a risk marker for OSAS through its use as an index of upper airway restriction by fat deposits in the neck [31]. It has been identified as a relevant tool for clinical screening for OSAS through the STOP-BANG questionnaire [32], which was found to be the most sensitive for the detection of OSAS [8]. In addition, neck circumference was found to be correlated with the visceral adipose tissue, measured by computed tomography in 3307 men (r = 0.63, p<0.001) and women (r = 0.74, p<0.001) in the Framingham health study [33]. Neck circumference was found to be linked with cardiometabolic risk factors after correction for BMI and visceral fat. In other cross-sectional studies, neck circumference was also associated with atherogenic dyslipidemia [34], inflammatory markers (PAI-1) [35], insulin resistance [36], coronary artery disease [37], carotid wall intima-media thickness [38] and NAFLD [39, 40].
Prospective studies have shown that changes in neck circumference are associated with changes in blood pressure [41] and incident type 2 diabetes [42, 43]. Finally, in a cohort of 12,151 patients with a high risk of cardiovascular disease, higher values of neck circumference predicted a higher incidence of fatal and non-fatal cardiovascular events [44].
Thus, neck circumference appears to be a relevant anthropometric marker for the identification of patients with a high cardiometabolic risk associated with central adiposity. Only two previous studies have addressed the question of the relevance of this marker in the context of severe obesity. Assyov et al. [45] found that neck circumference was superior to waist circumference for the identification of a high-risk cardiometabolic profile in 205 men and women with a mean BMI of 36.9 (6.2) kg/m2. A cut-off neck circumference value of 37 cm in women was 74% sensitive and 62% specific to identify a risk of type 2 diabetes. Cizza et al. [46] found that neck circumference was associated with metabolic syndrome and OSAS in 102 men and women with a mean BMI of 38.6 (6.5) kg/m2, who were identified as short sleepers (<6.5h/night).
Finally, we assessed whether waist-to-height and neck-to-height ratios yielded better results than waist and neck circumferences based on the hypothesis that dividing by height would reduce the variability. Waist-to-height ratio was previously used as a marker of central adiposity in childhood obesity [47]. We found similar results for waist-to-height ratio as for waist circumference, as previously demonstrated in adult women [48]. Accordingly, neck-to-height ratio was previously used in children [49] and adults [50], mainly to screen for sleep apnea. Similarly to our results, neck-to-height ratio did not improve the association observed with neck circumference.
Study strengths and limitations
The present study sought the easiest and most clinically relevant anthropometric marker for the identification of a high-risk profile for cardiometabolic diseases in women with severe obesity. The study sample was large and the cardiometabolic characteristics of the women were well-characterized. The results are limited by the cross-sectional design of the study, thus it was not possible to determine if anthropometric markers were good predictors of incident metabolic diseases and cardiovascular events in this population, as has been demonstrated in subjects with lower BMIs. The sex ratio of obese patients volunteering for bariatric surgery is largely in favor of women (76% in our cohort). Thus, too few men were included in our cohort to allow the same analyses to be performed in men. However, it would be of interest to study whether the present results are extendable to men in future work. Finally, the population studied was mainly Caucasian thus the results cannot be extrapolated to other ethnicities.
Conclusion
The present work showed that waist-to-hip ratio and neck circumference have low collinearity with BMI in women with severe obesity. Both markers demonstrated significant associations with prevalent hypertension and type 2 diabetes. Neck circumference was also linked to OSAS. Both were collinear with all other markers of cardiometabolic risk, in particular functional liver tests. Because of the ease of measurement of neck circumference in routine clinical practice, we propose neck circumference to be the best anthropometric marker in women with severe obesity. Further investigation regarding the ability of neck circumference to predict incident cardiometabolic diseases in this population should be carried out. Future studies should evaluate whether a reduction in neck circumference after bariatric surgery is a good marker of cardiometabolic improvement.
Supporting information
(XLS)
Acknowledgments
We thank Johanna Robertson, native English medical writer, for critically reviewing the English language and Matthieu Lesgoirres, clinical research professional implicated in the SOON cohort.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was funded by the Fond AGIR pour les maladies chroniques and Agence Régionale pour la Santé Rhône-Alpes. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Heady JA, Morris JN and Raffle PA. Physique of London busmen; epidemiology of uniforms. Lancet. 1956. 271:569–70. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP and Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006. 444:881–7. 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 3.Sperling LS, Mechanick JI, Neeland IJ, Herrick CJ, Despres JP, Ndumele CE, et al. The CardioMetabolic Health Alliance: Working Toward a New Care Model for the Metabolic Syndrome. J Am Coll Cardiol. 2015. 66:1050–67. 10.1016/j.jacc.2015.06.1328 [DOI] [PubMed] [Google Scholar]
- 4.Smith JD, Borel AL, Nazare JA, Haffner SM, Balkau B, Ross R, et al. Visceral Adipose Tissue Indicates the Severity of Cardiometabolic Risk in Patients with and without Type 2 Diabetes: Results from the INSPIRE ME IAA Study. J Clin Endocrinol Metab. 2012. [DOI] [PubMed] [Google Scholar]
- 5.Neeland IJ, Poirier P and Despres JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 2018. 137:1391–1406. 10.1161/CIRCULATIONAHA.117.029617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Despres JP. Excess visceral adipose tissue/ectopic fat the missing link in the obesity paradox? J Am Coll Cardiol. 2011. 57:1887–9. 10.1016/j.jacc.2010.10.063 [DOI] [PubMed] [Google Scholar]
- 7.Picon PX, Leitao CB, Gerchman F, Azevedo MJ, Silveiro SP, Gross JL, et al. [Waist measure and waist-to-hip ratio and identification of clinical conditions of cardiovascular risk: multicentric study in type 2 diabetes mellitus patients]. Arq Bras Endocrinol Metabol. 2007. 51:443–9. [DOI] [PubMed] [Google Scholar]
- 8.Chiu HY, Chen PY, Chuang LP, Chen NH, Tu YK, Hsieh YJ, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med Rev. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Onat A, Hergenc G, Yuksel H, Can G, Ayhan E, Kaya Z, et al. Neck circumference as a measure of central obesity: associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin Nutr. 2009. 28:46–51. 10.1016/j.clnu.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 10.Ahbab S, Ataoglu HE, Tuna M, Karasulu L, Cetin F, Temiz LU, et al. Neck circumference, metabolic syndrome and obstructive sleep apnea syndrome; evaluation of possible linkage. Med Sci Monit. 2013. 19:111–7. doi: 10.12659/MSM.883776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MJ Marfell-Jones AS, JH de Ridder. International standards for anthropometric assessment. Wellington, New Zealand; 2012.
- 12.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007. 30:753–9. 10.2337/dc07-9920 [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF and Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–9. [DOI] [PubMed] [Google Scholar]
- 14.Burstein M and Samaille J. [On a rapid determination of the cholesterol bound to the serum alpha- and beta-lipoproteins.]. Clin Chim Acta. 1960. 5:609 [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI and Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. 18:499–502. [PubMed] [Google Scholar]
- 16.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014. 311:507–20. 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 17.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012. 8:597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003. 77:1192–7. 10.1093/ajcn/77.5.1192 [DOI] [PubMed] [Google Scholar]
- 19.Manolopoulos KN, Karpe F and Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond). 2010. 34:949–59. [DOI] [PubMed] [Google Scholar]
- 20.Neeland IJ, Turer AT, Ayers CR, Berry JD, Rohatgi A, Das SR, et al. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015. 65:2150–1. 10.1016/j.jacc.2015.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Pandey A, Ayers C, Beg MS, Lakoski SG, Vega GL, et al. An Analysis of Individual Body Fat Depots and Risk of Developing Cancer: Insights From the Dallas Heart Study. Mayo Clin Proc. 2017. 92:536–543. 10.1016/j.mayocp.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roca-Rivada A, Bravo SB, Perez-Sotelo D, Alonso J, Castro AI, Baamonde I, et al. CILAIR-Based Secretome Analysis of Obese Visceral and Subcutaneous Adipose Tissues Reveals Distinctive ECM Remodeling and Inflammation Mediators. Sci Rep. 2015. 5:12214 10.1038/srep12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemieux I, Drapeau V, Richard D, Bergeron J, Marceau P, Biron S, et al. Waist girth does not predict metabolic complications in severely obese men. Diabetes Care. 2006. 29:1417–9. 10.2337/dc06-0441 [DOI] [PubMed] [Google Scholar]
- 24.Drapeau V, Lemieux I, Richard D, Bergeron J, Tremblay A, Biron S, et al. Waist circumference is useless to assess the prevalence of metabolic abnormalities in severely obese women. Obes Surg. 2007. 17:905–9. [DOI] [PubMed] [Google Scholar]
- 25.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ and Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009. 32:1068–75. 10.2337/dc08-2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perea V, Jimenez A, Flores L, Ortega E, Coves MJ and Vidal J. Anthropometric indexes outperform bioelectrical impedance analysis-derived estimates of body composition in identification of metabolic abnormalities in morbid obesity. Surg Obes Relat Dis. 2013. 9:648–52. 10.1016/j.soard.2012.05.010 [DOI] [PubMed] [Google Scholar]
- 27.Zazai R, Wilms B, Ernst B, Thurnheer M and Schultes B. Waist circumference and related anthropometric indices are associated with metabolic traits in severely obese subjects. Obes Surg. 2014. 24:777–82. 10.1007/s11695-013-1141-6 [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Rexrode KM, van Dam RM, Li TY and Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008. 117:1658–67. 10.1161/CIRCULATIONAHA.107.739714 [DOI] [PubMed] [Google Scholar]
- 29.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E and Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed). 1984. 289:1257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalton M, Cameron AJ, Zimmet PZ, Shaw JE, Jolley D, Dunstan DW, et al. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. 2003. 254:555–63. [DOI] [PubMed] [Google Scholar]
- 31.Davies RJ and Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990. 3:509–14. [PubMed] [Google Scholar]
- 32.Chung F, Abdullah HR and Liao P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest. 2016. 149:631–8. 10.1378/chest.15-0903 [DOI] [PubMed] [Google Scholar]
- 33.Preis SR, Massaro JM, Hoffmann U, D'Agostino RB, Sr., Levy D, Robins SJ, et al. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J Clin Endocrinol Metab. 2010. 95:3701–10. 10.1210/jc.2009-1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallianou NG, Evangelopoulos AA, Bountziouka V, Vogiatzakis ED, Bonou MS, Barbetseas J, et al. Neck circumference is correlated with triglycerides and inversely related with HDL cholesterol beyond BMI and waist circumference. Diabetes Metab Res Rev. 2013. 29:90–7. 10.1002/dmrr.2369 [DOI] [PubMed] [Google Scholar]
- 35.Jamar G, Pisani LP, Oyama LM, Belote C, Masquio DC, Furuya VA, et al. Is the neck circumference an emergent predictor for inflammatory status in obese adults? Int J Clin Pract. 2013. 67:217–24. 10.1111/ijcp.12041 [DOI] [PubMed] [Google Scholar]
- 36.Liang J, Teng F, Li Y, Liu X, Zou C, Wang Y, et al. neck circumference and insulin resistance in Chinese adults: the Cardiometabolic Risk in Chinese (CRC) Study. Diabetes Care. 2013. 36:e145–6. 10.2337/dc13-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zen V, Fuchs FD, Wainstein MV, Goncalves SC, Biavatti K, Riedner CE, et al. Neck circumference and central obesity are independent predictors of coronary artery disease in patients undergoing coronary angiography. Am J Cardiovasc Dis. 2012. 2:323–30. [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenquist KJ, Massaro JM, Pencina KM, D'Agostino RB, Beiser A, O'Connor GT, et al. Neck circumference, carotid wall intima-media thickness, and incident stroke. Diabetes Care. 2013. 36:e153–4. 10.2337/dc13-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Wang N, Han B, Chen Y, Zhu C, Chen Y, et al. Neck circumference as an independent indicator to non-alcoholic fatty liver disease in non-obese men. Nutr Metab (Lond). 2015. 12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang BX, Zhu MF, Wu T, Zhou JY, Liu Y, Chen XL, et al. Neck circumference, along with other anthropometric indices, has an independent and additional contribution in predicting fatty liver disease. PLoS One. 2015. 10:e0118071 10.1371/journal.pone.0118071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Noun LL and Laor A. Relationship between changes in neck circumference and changes in blood pressure. Am J Hypertens. 2004. 17:409–14. 10.1016/j.amjhyper.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 42.Preis SR, Pencina MJ, D'Agostino RB, Sr., Meigs JB, Vasan RS and Fox CS. Neck circumference and the development of cardiovascular disease risk factors in the Framingham Heart Study. Diabetes Care. 2013. 36:e3 10.2337/dc12-0738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho NH, Oh TJ, Kim KM, Choi SH, Lee JH, Park KS, et al. Neck Circumference and Incidence of Diabetes Mellitus over 10 Years in the Korean Genome and Epidemiology Study (KoGES). Sci Rep. 2015. 5:18565 10.1038/srep18565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai Y, Wan X, Li X, Jin E and Li X. Neck circumference and future cardiovascular events in a high-risk population—A prospective cohort study. Lipids Health Dis. 2016. 15:46 10.1186/s12944-016-0218-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assyov Y, Gateva A, Tsakova A and Kamenov Z. A comparison of the clinical usefulness of neck circumference and waist circumference in individuals with severe obesity. Endocr Res. 2017. 42:6–14. 10.3109/07435800.2016.1155598 [DOI] [PubMed] [Google Scholar]
- 46.Cizza G, de Jonge L, Piaggi P, Mattingly M, Zhao X, Lucassen E, et al. Neck circumference is a predictor of metabolic syndrome and obstructive sleep apnea in short-sleeping obese men and women. Metab Syndr Relat Disord. 2014. 12:231–41. 10.1089/met.2013.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou D, Yang M, Yuan ZP, Zhang DD, Liang L, Wang CL, et al. Waist-to-Height Ratio: a simple, effective and practical screening tool for childhood obesity and metabolic syndrome. Prev Med. 2014. 67:35–40. 10.1016/j.ypmed.2014.06.025 [DOI] [PubMed] [Google Scholar]
- 48.Hajian-Tilaki K, Heidari B, Hajian-Tilaki A, Firouzjahi A and Bagherzadeh M. The discriminatory performance of body mass index, waist circumference, waist-to-hip ratio and waist-to-height ratio for detection of metabolic syndrome and their optimal cutoffs among Iranian adults. J Res Health Sci. 2014. 14:276–81. [PubMed] [Google Scholar]
- 49.de Sousa Caixeta JA, Saramago AM, de Cacia Pradella-Hallinan ML, Moreira GA, Tufik S and Fujita RR. Waist-to-height ratio distinguish obstructive sleep apnea from primary snoring in obese children. Sleep Breath. 2015. 19:231–7. 10.1007/s11325-014-1001-1 [DOI] [PubMed] [Google Scholar]
- 50.Banhiran W, Junlapan A, Assanasen P and Chongkolwatana C. Physical predictors for moderate to severe obstructive sleep apnea in snoring patients. Sleep Breath. 2014. 18:151–8. 10.1007/s11325-013-0863-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.