Abstract
Background
Anemia in pregnancy is a major public health concern worldwide, especially in developing countries. Thus, there is a need of having current information and local data on the prevalence of anemia and associated factors during pregnancy to help inform preventive programmes. The aim of this study was to assess the prevalence of anemia and associated factors among pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia.
Methods
An institution based cross-sectional study was conducted at Debre Markos Referral Hospital in July and August 2016. A total of 234 randomly-selected pregnant women took part in the study. Data on sociodemographic factors, environmental and sanitation factors, reproductive factors, and nutrition related characteristics were collected using a structured questionnaire. Hemoglobin level was determined using hematological analyzer (Cell Dyn 1800) machine. The stool sample was collected to identify intestinal parasitic infections. Statistical analysis was done using logistic regression. The p value of less than 0.05 at 95% confidence interval was considered statistically significant.
Results
The overall prevalence of anemia among pregnant women was 11.5% (95% CI: 8.2%– 14.9%). The result of multivariable analysis revealed that, coffee consumption [AOR = 2.91; 95% CI (1.63, 8.78)], and hookworm infection [AOR = 2.65; 95% CI (1.48, 4.72)] were factors significantly associated with anemia among pregnant women.
Conclusion
Anemia is of public health concern among pregnant women in the study area. All pregnant women coming to antenatal clinics should be screened and treated routinely for intestinal parasitic infection. Pregnant women should limit coffee consumption, and avoid drinking coffee with meals.
Background
Anemia in pregnancy is a major public health concern worldwide, especially in developing countries[1, 2]. Anemia is characterized by a decline in the concentration of circulating erythrocytes or hemoglobin (Hgb) and impairment in the oxygen transporting capacity[3]. The World Health Organization (WHO) defines anemia in pregnancy as a hemoglobin levels less than 11g/dl[4]. According to WHO, anemia is considered of a public health concern if the prevalence of anemia is 5% or higher[2]. Anemia in pregnancy affects almost half of all pregnant women (41.8%) globally[2, 5]. The WHO estimated that anemia affects about 56% of pregnant women in low and middle income countries, with the highest prevalence in Africa [2, 6–8].
The causes of anemia during pregnancy are multifactorial and include inadequate dietary intake leading to deficiencies in iron, vitamin B12, folate and vitamin A, increased iron requirements due to physiological demands during pregnancy, poor iron bioavailability, intestinal parasitic infections, malaria, hemoglobinopathies, chronic infections like TB and HIV[9–17]. In Sub Saharan Africa inadequate intake of diets rich in iron is shown to be the main cause of anemia among pregnant women[13].
A number of studies suggest possible adverse effects of maternal anemia on pregnancy and child outcomes, including prolonged labor, postpartum hemorrhage, intrauterine growth restriction, intrauterine deaths, preterm delivery, low birth weight, low APGAR score, fetal anemia, increased perinatal mortality, and impaired physical and cognitive development of children[6, 18–23]. Anemia during pregnancy has also been associated with adverse health effects for mother, including fatigue, reduced work productivity, impaired immune function, and increased risk of maternal morbidity and mortality[2, 6, 23, 24]. Some studies have shown that anemia is responsible for about 20% maternal deaths in Africa[25].
Ethiopian Demographic and Health Surveys(EDHS) 2011 reported that 22% of pregnant women were anemic[26]. Other studies conducted in different parts of Ethiopia also reported high prevalence of anemia among pregnant women[27–34]. All the studies consistently indicated the public health significance of maternal anemia in the country. The government has strengthened different interventions to reduce the burden of maternal anemia. However, the outcome of these interventions has not yet knocked mitigating effect on the prevalence of maternal anemia in Ethiopia. In Ethiopia, a number of studies assessed anemia and its determinants, and came up with a significant variation in prevalence of anemia, and divergent and equivocal risk factors. Therefore, there is a need of having current information and local data on the prevalence of anemia and associated factors during pregnancy to help inform preventive programmes. Thus, this study aims to determine the prevalence of anemia and associated factors among pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia.
Methods
Study design and setting
An institution based cross-sectional study was conducted at Debre Markos Referral Hospital in July and August 2016. Debre Markos Referral Hospital is situated at Debre Markos town, East Gojjam administrative zone, 300 km from Addis Ababa, the capital city of Ethiopia. The hospital provides health service to over 3.5 million populations in its catchments, the hospital has 132 beds for inpatients service and the hospital also provides health services for outpatients. Debre Markos town lies on the average at 2, 630 meters above sea level. Over 100,000 populations reside in the town[35, 36]. The study populations were all pregnant women who attend antenatal care (ANC) at Debre Markos Referral Hospital.
Sample size and sampling technique
Single population proportion formula was used in determining adequate sample-size for estimating the prevalence of anemia. The sample-size of 234 pregnant women was computed with the assumptions of 95% confidence level, 5% margin of error, 16.6% expected prevalence of anemia[34], and 10% non-response rate. Systematic random sampling technique was used to select the study subjects from antenatal clinic during the data collection period. According to the Hospital report, on average, 30–40 pregnant women visit the ANC daily, and 804 pregnant women have been enrolled to ANC at the Hospital. Since the sample size was determined as 234, a sampling interval of 3 was used to select study participants. Of the first three pregnant women, one woman was randomly selected by using lottery method. Eventually, every 2nd pregnant women were selected to participate in the study until the required sample size of 234 pregnant women was obtained.
Data collection methods
Questionnaire
Data on sociodemographic factors, environmental and sanitation factors, reproductive factors, and nutrition related characteristics were collected using a structured and pretested questionnaire through a face to face interview. The section of the questionnaire on dietary diversity was adopted from Food and Nutrition Technical Assistance (FANTA) indicator guideline and modified for local context[37]. Other parts of the questionnaire were taken from standard DHS questionnaire, and developed by the principal investigators. The questionnaire was administered using local language (Amharic). The content validity of the tool was assessed based on the conceptual framework of the study by relevant professionals, and the reliability of the tool was checked via test-retest method. The tool was pretested on 5% of the total sample outside the study area. During the pre-test, the tool was assessed for its clarity, accuracy of the knowledge measured and comprehensiveness, readability and the optimal time for completing the interview. Modifications were done based on the result. Five data collectors (three clinical mid wives and two laboratory technicians) and one supervisor were recruited. Two days intensive training was given for both data collectors and supervisor by principal investigators prior to the data collection regarding the objective of the study, confidentiality of information, and techniques of interview. To assure quality of the data, the data collection process was followed daily by the supervisor and principal investigators. The dietary diversity (DD) level was assessed using 24-hour recall method. The pregnant women were asked whether they had taken any food from predefined food categories in a day before the survey. Dietary diversity scores were computed based on FAO guidelines[38]. Accordingly, the level of Dietary Diversity Score (DDS) was classified into low (DDS ≤3), medium (DDS of 4 or 5), or high (DDS ≥6).
Mid upper arm circumference (MUAC) measurement
Mid upper arm circumference was measured halfway between the tip of the shoulder (olecranon process) and the tip of the elbow (acromion process) to the nearest 0.1 cm. The measurement was taken at the mid-point on the relaxed non-dominant hand, without any clothing and with optimal tape tension following the standard instructions and steps[39]. Undernutrition was defined as MUAC less than 22 cm[40].
Laboratory analysis
Blood sample collection and hemoglobin level determination
Venous blood was collected from each pregnant woman, using stainless steel needles and plain tubes (SARSTEDT MonovetteR, Germany). Hemoglobin level was determined using hematological analyzer (Cell Dyn 1800, PD, USA) machine. Anemia was defined as a hemoglobin level of less than 11 g/dl. Anemia was classified into three categories as mild (10–10.9 g/dl), moderate (7–9.9 g/dl) and severe (less than 7 g/dl)[41]. Hemoglobin values were adjusted for altitude according to the formulae recommended by Center for Disease Prevention and Control(CDC)[42].
Stool specimen collection and examination
Stool samples were collected from pregnant women using clean, dry and leak-proof cupped plastic container following standard procedures. The stool samples were masked, coded, and processed for parasitological examination. Direct wet-mount and formaldehyde-ether sedimentation method were used for stool examination[43, 44]. The WHO guide for diagnosis of intestinal parasitosis was used as an identification reference[45].
Data processing and analysis
Data were entered using EPI-INFO version 7 software. Data screening and analysis were carried out using SPSS version 20. Descriptive analysis was done using mean, frequency and percentage. Logistic regression analyses were used in controlling potential confounders. Independent variables significantly associated with the dependent variable in simple regression models were exported to multiple regression models for adjustment. The Odds Ratio (OR) with 95% Confidence Interval (CI) was used to measure the strength of association between anemia and independent variables. The fitness of logistic regression model was assessed using Hosmer-Lemeshow statistic. The collinearity effect was tested using the Variance Inflation Factor (VIF) for all independent variables. The p value of less than 0.05 at 95% CI was considered statistically significant.
Ethical consideration
Ethical clearance was obtained from ethical review committee of Debre Markos University prior to data collection. Informed written consent was obtained from the study participants before enrolment in the study after the nature of the study was fully explained. Numerical codes instead of names were used in all laboratory forms and questionnaires. Nutrition education was given to all study participants. Pregnant women who were found to have anemia received iron-folate supplementation and counselling according to National treatment guidelines. Pregnant women who were infected with intestinal parasites were given Albendazole tablet.
Results
Socio-demographic characteristics of study subjects
All 234 pregnant women, initially planned for the study were volunteered to take part in the study, with a response rate of 100%. The mean age (+/-standard deviation) of the study participants was 26.4 years (+/-4.8 years). The vast majority of the respondents were Amhara in ethnicity (97.9%) and orthodox (92.7%) in religion. Nearly half, (43.2%) of the study participants’ occupations were housewife and one-third, (34.6%) had no formal education (Table 1).
Table 1. Socio-demographic characteristics of the study participants, Northwest Ethiopia, 2016.
(n = 234).
| Characteristics | Frequency (n) | Percent (%) | |
|---|---|---|---|
| Age(years) |
15–24 | 73 | 31.2 |
| 25–34 | 124 | 53.0 | |
| ≥35 | 37 | 15.8 | |
| Marital status |
Married | 222 | 94.9 |
| Single | 12 | 5.1 | |
| Religion | Orthodox | 217 | 92.7 |
| Muslim | 14 | 6.0 | |
| Protestant | 2 | 0.9 | |
| Catholic | 1 | 0.4 | |
| Ethnicity |
Amhara | 229 | 97.9 |
| Oromo | 3 | 1.3 | |
| Tigre | 2 | 0.8 | |
| Educational status |
No formal education | 81 | 34.6 |
| Primary education | 12 | 5.2 | |
| High school education | 43 | 18.4 | |
| Certificate and above | 98 | 41.9 | |
| Occupation | House wife | 101 | 43.2 |
| Farmer | 17 | 7.3 | |
| Merchant | 29 | 12.4 | |
| Government employee | 73 | 31.2 | |
| Daily laborer | 14 | 5.9 | |
| Family size | ≤ 3 | 149 | 63.7 |
| 4–6 | 69 | 29.5 | |
| >6 | 16 | 6.8 | |
| Monthly income |
Low | 46 | 19.6 |
| Medium | 149 | 63.7 | |
| High | 39 | 16.7 | |
Environmental and sanitation factors
Table 2 summarizes environmental and sanitation characteristics of the study participants. The majority of study participants, 228(97.4%) had toilet facilities. The greater number of study participants, 216(94.7%) use pit latrine. The water source for the majority, 169(72.2%) of study subjects was tap water.
Table 2. Environmental and sanitation characteristics of pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia, 2016.
| Characteristics | Frequency (n) | Percent (%) | |
|---|---|---|---|
| Source of drinking water | Tab | 169 | 72.2 |
| Well | 10 | 4.3 | |
| Spring | 55 | 23.5 | |
| Possession of toilet facility | Yes | 228 | 97.4 |
| No | 6 | 2.6 | |
| Types of latrine | Pit latrine | 216 | 94.7 |
| Water flush | 7 | 3.1 | |
| Public | 5 | 2.2 | |
Reproductive health factors
Table 3 summarizes reproductive health factors of study participants. More than half, 138 (59%) of the pregnant women were in the third trimester at a time of data collection. The mean gestational age (±SD) of the study participants was 24.2±9.2 weeks. More than half, 137 (58.5%) of the study participants were multi gravida. Among 137 women who gave at least a birth in the previous 5 years of the survey, in 50 (36.5%) of the cases the birth interval was less than the recommended 24 months.
Table 3. Reproductive health factors among pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia, 2016.
| Characteristics | Frequency (n) | Percent (%) | |
|---|---|---|---|
| Gravida | Primi | 97 | 41.5 |
| Multi | 137 | 58.5 | |
| History of abortion | Yes | 13 | 5.6 |
| No | 221 | 94.4 | |
| History of still birth | Yes | 21 | 9.0 |
| No | 213 | 91.0 | |
| Birth interval(year) | < 1 | 32 | 23.4 |
| 1–2 | 18 | 13.1 | |
| > 2 | 87 | 63.5 | |
| Trimester | First | 37 | 15.8 |
| Second | 59 | 25.2 | |
| Third | 138 | 59 | |
| Menstrual character |
Regular | 192 | 82.1 |
| Irregular | 42 | 17.9 | |
Nutrition related characteristics
The staple diets for the majority, (72.6%) of the study subjects were plant-based foods (made of teff). Daily meal frequency was three times for the majority of the study subjects (62.8%). More than half of study participants, 134 (57.3%) had low dietary diversity score (≤3 food groups). The commonly and frequently consumed food groups were a starchy staple, 100% and legumes, 173(73.9%). Only about one-fifth, 51(21.8%) pregnant women were consumed diet of animal origin in the reference period. More than one-third (35.5%) of the pregnant women were undernourished (MUAC < 22 cm) (Table 4).
Table 4. Nutrition related Characteristics of pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia, 2016.
| Characteristics | Frequency (n) | Percent (%) | |
|---|---|---|---|
| Main staple diet |
Teff | 170 | 72.6 |
| Maize | 60 | 25.6 | |
| Sorghum | 2 | 0.9 | |
| Wheat | 2 | 0.9 | |
| Number of meals/day | < 3 | 17 | 7.3 |
| 3 | 147 | 62.8 | |
| >3 | 70 | 29.9 | |
| Family food source | Grow their own | 72 | 30.8 |
| Buy/purchase | 161 | 68.8 | |
| Subsidies/food aid | 1 | 0.4 | |
| Nutritional education during pregnancy | Yes | 200 | 85.5 |
| No | 34 | 14.5 | |
| Dietary Diversity |
Low | 134 | 57.3 |
| Medium | 53 | 22.6 | |
| High | 47 | 20.1 | |
| Frequency of coffee intake per day | ≤ 3 coffee cups (≤ 210ml) | 214 | 91.2 |
| > 3 coffee cups (>210ml) | 20 | 8.8 | |
| MUAC | Undernourished (MUAC < 22 cm) |
96 |
41.0 |
| Normal(≥22cm) | 138 | 59.0 | |
Clinical factors
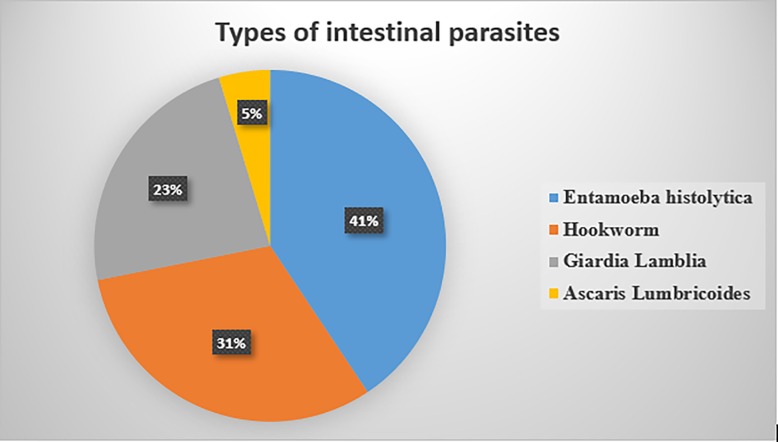
More than quarter, 64(27.4%) of pregnant women were infected with one or more intestinal parasites. The most common parasites observed were Entamoeba histolytica 26(40.6%) and Hookworm 20(31.2%) (Fig 1). Only 108(46.2%) of pregnant women were taking deworming. Of all respondents, 167 (71.4%) took iron-folate supplement at least once in the preceding four weeks of the survey. However, only 72(43.1%) reported full compliance with the supplement in the reference period. A significant number, 19(8.1%) of the pregnant women were positive for HIV (Table 5).
Fig 1. Prevalence and types of intestinal parasites among pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia, 2016.
Table 5. Clinical factors among pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia, 2016.
| Characteristics | Frequency (n) | Percent (%) | |
|---|---|---|---|
| Intestinal Parasite | yes | 64 | 27.4 |
| No | 170 | 72.6 | |
| Deworming | Yes | 108 | 46.2 |
| No | 126 | 53.8 | |
| HIV | Yes | 19 | 8.1 |
| No | 215 | 91.9 | |
| Iron supplement | Yes | 167 | 71.4 |
| No | 67 | 28.6 | |
Prevalence of anemia
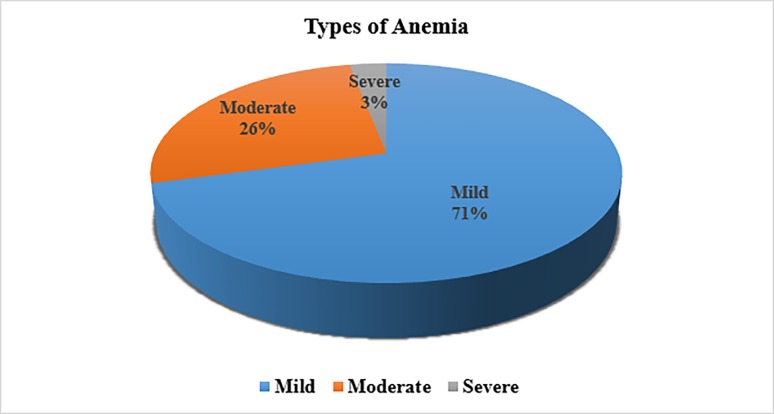
The study finding showed that the overall prevalence of anemia among pregnant women was 11.5% (95% CI: 8.2%– 14.9%). Among anemic pregnant women, 74.7% were mildly, 26.3% were moderately, and 3% were severely anemic (Fig 2). The mean hemoglobin concentration level (±SD) among the study participants was 12.65 (±2.82) g/dl.
Fig 2. Distribution and severity of anemia among pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia, 2016.
Factors associated with anemia
Table 6 summarizes factors associated with anemia among pregnant women. A multivariable analysis in a form of logistic regression was employed to identify risk factors of anemia among pregnant women. In the bivariate analysis, anemia was significantly associated with trimester of pregnancy, birth interval, nutrition education during pregnancy, iron supplement, frequency of coffee consumption, MUAC and hookworm infection. The multivariable logistic regression analysis revealed that coffee consumption and hookworm infection were predictors of anemia.
Table 6. Factors associated with anemia among pregnant women.
| Predictors | Anemia | COR (95%CI) | AOR (95% CI) | P- values | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Trimester | |||||
| First | 14 | 23 | 1 | ||
| Second | 26 | 33 | 0.96(0.33, 2.78) | ||
| Third | 76 | 62 | 2.06 (0.97, 6.38) | ||
| Birth interval(year) | |||||
| < 1 | 18 | 14 | 2.16(1.11, 3.38) | ||
| 1–2 | 10 | 8 | 0.89(0.29, 2.65) | ||
| >2 | 51 | 36 | 1 | ||
| Nutrition education during pregnancy | |||||
| Yes | 109 | 91 | 1 | ||
| No | 21 | 13 | 2.63 (0.96, 4.51) | ||
| Coffee consumption | |||||
| ≤ 3 coffee cups (≤ 210ml) | 98 | 116 | 1 | ||
| > 3 coffee cups (>210ml) | 13 | 7 | 4.03(1.89, 9.95) | 2.91(1.63, 8.78) | 0.001 |
| Iron supplement | |||||
| Yes | 76 | 91 | 1 | ||
| No | 38 | 29 | 1.70 (1.09–3.23) | ||
| MUAC | |||||
| Normal | 80 | 58 | 1 | ||
| Undernourished | 54 | 42 | 1.89 (1.16, 3.93) | ||
| Hookworm infection | |||||
| No | 101 | 113 | 1 | 1 | |
| Yes | 12 | 8 | 3.74 (1.68, 6.83) | 2.65 (1.48, 4.72) | 0.001 |
Hookworm infection was found to be significantly associated with anemia after adjustment for potential confounders. Pregnant women who had hookworm infection were two and half times at greater risk of being anemic as compared to pregnant women with no infection [AOR = 2.65; 95% CI (1.48–4.72)]. The study also witnessed significant association between coffee intake and maternal anemia. Coffee intake was associated with lower level of hemoglobin. Compared to pregnant women who consumed ≤ 3 coffee cups per day, the risk of anemia was three times higher among those who consumed > 3 coffee cups per day [AOR = 2.91; 95% CI (1.63–8.78)].
Discussion
In the current study, 11.5% of pregnant women had anemia based on low hemoglobin levels. According to WHO, anemia is considered of a public health problem if the prevalence of anemia is greater than 5%[4]. Accordingly, with the prevalence of 11.5%, anemia is of public-health concern in the study area. The prevalence determined in this study was considerably lower as compared to other studies conducted in different parts of Ethiopia that were reported 29%[46],31.6%[27], (32.8%)[47], (36.6%)[48], (39.9%)[49], 52%[29] and 56.8%[33]. The prevalence of anemia reported in the current study is also lower than the national prevalence that reported 22%[26]. The current study showed that prevalence of anemia among pregnant women in the country is decreasing as compared to earlier studies. The current study showed that prevalence of anemia among pregnant women in the country is decreasing as compared to earlier studies. This may imply an improvement in maternal nutrition and care during pregnancy. The low prevalence of anemia in the current study may be due to the fact that the interventions employed to address anemia in pregnancy such as; advancements in the quality of antenatal care and every pregnant woman is given iron supplement, deworming, malaria prophylaxis, and mosquito nets. Moreover, traditional variation in feeding habits and cooking a few varieties of food, seasonal difference in data collection, low purchasing power of food, and the role of religious traditions in the Ethiopian diet is very relevant. Further, the study area is one of the districts which has surplus production and a diversified food item produced; as a result, they had a chance to consume of iron rich foods and iron absorption promoters in the diet.
Hookworm infection was significantly associated with an increased risk of anemia in pregnant women. The role of hookworm as risk factor of anemia is consistent with the findings of the past studies[30–32, 34, 46, 48, 50–54]. Hookworm infection may cause anemia by reducing dietary intake, mal-absorption and endogenous nutrient loss. The hookworm also ingests blood by attaching to the mucosa of the upper small intestine, which may cause bleeding within the gastrointestinal tract causing chronic anemia in pregnancy[55–60]. The current study has shown that hookworm contributed to a significant proportion of the anemia in this population, suggesting that all pregnant women should be screened and treated for intestinal parasitic infection during antenatal care visits. Anti-helminthic treatment should be carried out among pregnant women because anti‑helminthic therapy is inexpensive, effective, and safe to administer during pregnancy[61–63].
The study finding indicated a negative association between coffee intake and maternal anemia. Previous study conducted in Costa Rica also supported the finding[64]. Coffee drinking affects iron bioavailability and due to its potency as an inhibitor of absorption is likely to aggravate anemia at times of increased physiological need or when dietary iron intake is precarious[65]. Coffee is known to contain tannin which can potentially interfere with iron absorption[66]. However, empirical evidences are scanty. Further studies with strong study design should be conducted in this direction.
Age, residence, educational status, occupation, family size, monthly income, source of drinking water, latrine availability, gravida, parity, child spacing, trimester of pregnancy, menstrual character, presence of pica, staple diet, frequency of feeding, undernutrition, low dietary diversity, and HIV did not show significant association with anemia. This is may be due to the widespread nature of anemia in the community. A more in-depth understanding of anemia among pregnant women would require further study, using strong study designs with larger sample size. The major limitation of the present study was the cross sectional nature of its design as we can’t establish causal relationships between anemia and the independent variables. Secondly, assessment of dietary intake depends on the 24-hour recall method, which may not accurately reflect their past feeding experience.
Conclusion
Anemia in pregnancy is of public health concern among pregnant women in the study area. In this study maternal coffee consumption and hookworm infection during pregnancy are key predisposing factors to maternal anemia. Hence, a more comprehensive and community-wide deworming intervention should be performed. All pregnant women coming to antenatal clinics should be screened and treated routinely for intestinal parasitic infection. Anti‑helminthes should be given as prophylaxis to adolescent and young adult women, before their reproductive career. Stool analysis and nutrition intervention should be strongly inculcated into the routine maternity services during antenatal care. We also suggest sustained health education on the dietary intake, access to health care, clean water, hygiene and sanitation for women of reproductive age group particularly, pregnant women. Iron-folate supplementation combined with de-worming will also have affirmative input. The inhibiting effects of coffee on iron absorption can be partially prevail by the concurrent intake of vitamin C rich foods and diet of animal origin. Moreover, pregnant women should limit coffee consumption, and avoid drinking coffee with meals. Causal relationships between maternal coffee consumption during pregnancy and anemia should be further investigated with strong study design.
Supporting information
(PDF)
Acknowledgments
Special thanks go to entire study subjects who volunteered for the study. We acknowledge Debre Markos University for funding the study. We are grateful for Debre Markos Referral Hospital staffs for their full cooperation and vital assistance during data collection and conducting the laboratory analysis.
Abbreviations
- ANC
Ante Natal Care
- CDC
Center of Disease Control
- DD
Dietary Diversity
- DDS
Dietary Diversity Score
- DHS
Demographic and Health Survey
- EDHS
Ethiopian Demographic and Health Survey
- ETB
Ethiopian Birr
- FANTA
Food and Nutrition Technical Assistance
- FAO
Food and Agriculture Organization of the United Nations
- G/DL
Gram/ Deciliter
- Hgb
Hemoglobin
- OR
Odds Ratio
- SD
Standard Deviation
- WHO
World Health Organization
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Debre Markos University for the data collection material. However, the University had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. : Maternal and child undernutrition and overweight in low-income and middle-income countries. The lancet 2013, 382(9890):427–451. [DOI] [PubMed] [Google Scholar]
- 2.Bd Benoist, McLean E, Egll I, Cogswell M: Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemiaGeneva: WHO; 2008. [Google Scholar]
- 3.McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B: Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public health nutrition 2009, 12(4):444–454. 10.1017/S1368980008002401 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization: Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers Geneva: WHO, 2001. [Google Scholar]
- 5.Badham J, Zimmermann MB, Kraemer K: The guidebook nutritional anemia: Sight and Life Press; Basel, Switzerland; 2007. [Google Scholar]
- 6.Allen LH: Anemia and iron deficiency: effects on pregnancy outcome–. The American journal of clinical nutrition 2000, 71(5):1280–1284. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization: Micronutrient deficiencies: Iron deficiency anaemia Geneva: World Health Organization, 2008. [Google Scholar]
- 8.Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian S: Anaemia in low-income and middle-income countries. The Lancet 2011, 378(9809):2123–2135. [DOI] [PubMed] [Google Scholar]
- 9.Msuya SE, Hussein TH, Uriyo J, Sam NE, Stray-Pedersen B: Anaemia among pregnant women in northern Tanzania: prevalence, risk factors and effect on perinatal outcomes. Tanzania journal of health research 2011, 13(1):33–39. [DOI] [PubMed] [Google Scholar]
- 10.Okube OT, Mirie W, Odhiambo E, Sabina W, Habtu M: Prevalence and factors associated with anaemia among pregnant women attending antenatal clinic in the second and third trimesters at pumwani maternity hospital, Kenya. Open Journal of Obstetrics and Gynecology 2016, 6(01):16. [Google Scholar]
- 11.Brooker S, Hotez PJ, Bundy DA : Hookworm-related anaemia among pregnant women: a systematic review. PLoS neglected tropical diseases 2008, 2(9):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClure EM, Meshnick SR, Mungai P, Malhotra I, King CL, Goldenberg RL, et al. : The association of parasitic infections in pregnancy and maternal and fetal anemia: a cohort study in coastal Kenya. PLoS neglected tropical diseases 2014, 8(2):2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ononge S, Campbell O, Mirembe F: Haemoglobin status and predictors of anaemia among pregnant women in Mpigi, Uganda. BMC research notes 2014, 7(1):712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antelman G, Msamanga GI, Spiegelman D, Urassa EJ, Narh R, Hunter DJ, et al. : Nutritional factors and infectious disease contribute to anemia among pregnant women with human immunodeficiency virus in Tanzania. The Journal of nutrition 2000, 130(8):1950–1957. 10.1093/jn/130.8.1950 [DOI] [PubMed] [Google Scholar]
- 15.Uneke C, Duhlinska D, Igbinedion E: Prevalence and public-health significance of HIV infection and anaemia among pregnant women attending antenatal clinics in south-eastern Nigeria. Journal of health, population, and nutrition 2007, 25(3):328. [PMC free article] [PubMed] [Google Scholar]
- 16.Verhoeff FH, Brabin BJ, Chimsuku L, Kazembe P, Broadhead RL: An analysis of the determinants of anaemia in pregnant women in rural Malawi—a basis for action. Annals of Tropical Medicine & Parasitology 1999, 93(2):119–133. [DOI] [PubMed] [Google Scholar]
- 17.Bondevik G, Eskeland B, Ulvik R, Ulstein M, Lie R, Schneede J, et al. : Anaemia in pregnancy: possible causes and risk factors in Nepali women. European Journal of Clinical Nutrition 2000, 54(1):3 [DOI] [PubMed] [Google Scholar]
- 18.Idowu O, Mafiana C, Dopu S: Anaemia in pregnancy: a survey of pregnant women in Abeokuta, Nigeria. African health sciences 2005, 5(4):295–299. 10.5555/afhs.2005.5.4.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidanto HL, Mogren I, Lindmark G, Massawe S, Nystrom L: Risks for preterm delivery and low birth weight are independently increased by severity of maternal anaemia. SAMJ: South African Medical Journal 2009, 99(2):98–102. [PubMed] [Google Scholar]
- 20.Haggaz AD, Radi EA, Adam I: Anaemia and low birthweight in western Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene 2010, 104(3):234–236. 10.1016/j.trstmh.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 21.Lone FW, Qureshi RN, Emanuel F: Maternal anaemia and its impact on perinatal outcome. Tropical Medicine & International Health 2004, 9(4):486–490. [DOI] [PubMed] [Google Scholar]
- 22.Adam I, Babiker S, Mohmmed AA, Salih MM, Prins MH, Zaki ZM: Low body mass index, anaemia and poor perinatal outcome in a rural hospital in eastern Sudan. Journal of Tropical Pediatrics 2007, 54(3):202–204. 10.1093/tropej/fmm110 [DOI] [PubMed] [Google Scholar]
- 23.Scholl TO, Hediger ML : Anemia and iron-deficiency anemia: compilation of data on pregnancy outcome. The American journal of clinical nutrition 1994, 59(2):492–501. [DOI] [PubMed] [Google Scholar]
- 24.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. : Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. The Lancet Global Health 2013, 1(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olusanya O: The importance of social class in voluntary fertility control in a developing country. WAJM 1985, 4:205–212. [Google Scholar]
- 26.Central Statistical Agency (Ethiopia) and ICF International: Ethiopia demographic and health survey 2011. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ICF International 2012, 430.
- 27.Gebremedhin S, Enquselassie F, Umeta M: Prevalence and correlates of maternal anemia in rural Sidama, Southern Ethiopia. African journal of reproductive health 2014, 18(1):44–53. [PubMed] [Google Scholar]
- 28.Jufar AH, Zewde T: Prevalence of anemia among pregnant women attending antenatal care at tikur anbessa specialized hospital, Addis Ababa Ethiopia. Journal of Hematology & Thromboembolic Diseases. 2014;2(125):2. [Google Scholar]
- 29.Mihiretie H, Fufa M, Mitiku A, Bacha C, Getahun D, Kejela M, et al. : Magnitude of anemia and associated factors among pregnant women attending antenatal care in Nekemte health center, Nekemte, Ethiopia. Journal of Medical Microbiology & Diagnosis 2015, 4(3):1. [Google Scholar]
- 30.Lebso M, Anato A, Loha E: Prevalence of anemia and associated factors among pregnant women in Southern Ethiopia: A community based cross-sectional study. PloS one 2017, 12(12):0188783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kefiyalew F, Zemene E, Asres Y, Gedefaw L: Anemia among pregnant women in Southeast Ethiopia: prevalence, severity and associated risk factors. BMC research notes 2014, 7(1):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Getachew M, Yewhalaw D, Tafess K, Getachew Y, Zeynudin A: Anaemia and associated risk factors among pregnant women in Gilgel Gibe dam area, Southwest Ethiopia. Parasites & vectors 2012, 5(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Addis Alene K, Mohamed Dohe A: Prevalence of anemia and associated factors among pregnant women in an urban area of eastern Ethiopia. Anemia, 2014:7 10.1155/2014/561567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alem M, Enawgaw B, Gelaw A, Kena T, Seid M, Olkeba Y: Prevalence of anemia and associated risk factors among pregnant women attending antenatal care in Azezo Health Center Gondar town, Northwest Ethiopia. J Interdiscipl Histopathol 2013, 1(3):137–144. [Google Scholar]
- 35.Debre Markos Referral Hospital: Information and statistics office. Debre Markos Referral Hospital, Debre Markos, 2016.
- 36.Federal Democratic Republic of Ethiopia Central Statistical Agency: Population projection of Ethiopia for all regions at Wereda level from 2014–2017. Federal Democratic Republic of Ethiopia Central Statistical Agency, 2013.
- 37.Swindale A, Bilinsky P: Household dietary diversity score (HDDS) for measurement of household food access: indicator guide Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2006. [Google Scholar]
- 38.Kennedy G, Ballard T, Dop MC: Guidelines for measuring household and individual dietary diversity: Rome: Food and Agriculture Organization of the United Nations; 2011. [Google Scholar]
- 39.Cogill B: Anthropometric indicators measurement guide. 2003.
- 40.Ferro-Luzzi A, James W: Adult malnutrition: simple assessment techniques for use in emergencies. British Journal of Nutrition 1996, 75(1):3–10. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization: Prevention and management of severe anaemia in pregnancy: report of a technical working group Geneva: WHO, 1993. [Google Scholar]
- 42.Nestel P: Adjusting hemoglobin values in program surveys Washington, DC: International Nutritional Anemia Consultative Group. International Life Sciences Institute, 2002. [Google Scholar]
- 43.Casemore D, Armstrong M, Sands R: Laboratory diagnosis of cryptosporidiosis. Journal of Clinical Pathology 1985, 38(12):1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridley D, Hawgood B : The value of formol-ether concentration of faecal cysts and ova. Journal of Clinical Pathology 1956, 9(1):74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization: Bench aids for the diagnosis of intestinal parasites Geneva: World Health Organization; 1994. [Google Scholar]
- 46.Ejeta E, Alemnew B, Fikadu A, Fikadu M, Tesfaye L, Birhanu T, et al. : Prevalence of anaemia in pregnant womens and associated risk factors in Western Ethiopia. Food Science and Quality Management. 2014; 31:82–91. [Google Scholar]
- 47.Bekele A, Tilahun M, Mekuria A: Prevalence of anemia and Its associated factors among pregnant women attending antenatal care in health institutions of Arba Minch town, Gamo Gofa Zone, Ethiopia: A Cross-sectional study. Anemia 2016;2016:9 10.1155/2016/1073192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gedefaw L, Ayele A, Asres Y, Mossie A: Anaemia and associated factors among pregnant women attending antenatal care clinic in Walayita Sodo town, Southern Ethiopia. Ethiopian journal of health sciences 2015, 25(2):155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obse N, Mossie A, Gobena T: Magnitude of anemia and associated risk factors among pregnant women attending antenatal care in Shalla Woreda, West Arsi Zone, Oromia Region, Ethiopia. Ethiopian journal of health sciences 2013, 23(2):165–173. [PMC free article] [PubMed] [Google Scholar]
- 50.Onoh R, Lawani O, Ezeonu P, Nkwo P, Onoh T, Ajah L: Predictors of anemia in pregnancy among pregnant women accessing antenatal care in a poor resource setting in South Eastern Nigeria. Sahel Medical Journal 2015, 18(4):182. [Google Scholar]
- 51.Gyorkos TW, Gilbert NL, Larocque R, Casapía M : Trichuris and hookworm infections associated with anaemia during pregnancy. Tropical Medicine & International Health 2011, 16(4):531–537. [DOI] [PubMed] [Google Scholar]
- 52.Nurdia D, Sumarni S, Hakim M, Winkvist A: Impact of intestinal helminth infection on anemia and iron status during pregnancy: a community based study in Indonesia. Southeast Asian journal of tropical medicine and public health. 2001;32(1):14–22. [PubMed] [Google Scholar]
- 53.Shah BK, Baig LA: Association of anemia with parasitic infestation in pregnant Nepalese women: Results from a hospital-based study done in eastern Nepal. J Ayub Med Coll Abbottabad 2005, 1:5–9. [PubMed] [Google Scholar]
- 54.Mengist HM, Zewdie O, Belew A: Intestinal helminthic infection and anemia among pregnant women attending ante-natal care (ANC) in East Wollega, Oromia, Ethiopia. BMC research notes 2017, 10(1):440 10.1186/s13104-017-2770-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee AI, Okam MM: Anemia in pregnancy. Hematology/Oncology Clinics 2011, 25(2):241–259. 10.1016/j.hoc.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 56.Roche M, Layrisse M: The nature and causes of “hookworm anemia”. The American journal of tropical medicine and hygiene 1966, 15(6):1032–1102. [PubMed] [Google Scholar]
- 57.Layrisse M, Roche M: The relationship between anemia and hookworm infection. Results of surveys of rural Venezuelan population. American journal of hygiene 1964, 79(3):279–301. [DOI] [PubMed] [Google Scholar]
- 58.Layrisse M, Aparcedo L, Martínez-Torres C, Roche M: Blood loss due to infection with Trichuris trichiura. The American journal of tropical medicine and hygiene 1967, 16(5):613–619. [DOI] [PubMed] [Google Scholar]
- 59.Pritchard D, Quinnell R, Moustafa M, McKean PG, Slater A, Raiko A, et al. : Hookworm (Necator americanus) infection and storage iron depletion. Transactions of the Royal Society of Tropical Medicine and Hygiene 1991, 85(2):235–238. [DOI] [PubMed] [Google Scholar]
- 60.Crompton D, Whitehead R: Hookworm infections and human iron metabolism. Parasitology 1993, 107(1):137–145. [DOI] [PubMed] [Google Scholar]
- 61.Allen H, Crompton DW, de Silva N, LoVerde PT, Olds GR: New policies for using anthelmintics in high risk groups. Trends in parasitology 2002, 18(9):381–382. [DOI] [PubMed] [Google Scholar]
- 62.Stephenson L, Latham M: Hookworm. Curr Treat Options Infect Dis 2003, 5:291–299. [Google Scholar]
- 63.Olds GR : Administration of praziquantel to pregnant and lactating women. Acta tropica 2003, 86(2–3):185–195. [DOI] [PubMed] [Google Scholar]
- 64.Munoz L, Lönnerdal B, Keen CL, Dewey KG: Coffee consumption as a factor in iron deficiency anemia among pregnant women and their infants in Costa Rica. The American journal of clinical nutrition 1988, 48(3):645–651. 10.1093/ajcn/48.3.645 [DOI] [PubMed] [Google Scholar]
- 65.World Health Organization: Requirements of Vitamin A, Iron, Folate, and Vitamin B12: Report of a joint FAO/WHO Expert Consultation. Rome: FAO, 1988. [Google Scholar]
- 66.Savolainen: Tannin content of tea and coffee. Journal of Applied Toxicology 1992, 12(3):191–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.