Abstract
Treatments for Cocaine Use Disorder (CUD) are variably effective, and there are no FDA-approved medications. One approach to developing new treatments for CUD may be to investigate and target poor prognostic signs. One such sign is anhedonia (i.e. a loss of pleasure or interest in non-drug rewards), which predicts worse outcomes in existing CUD treatments. Inflammation is thought to underlie anhedonia in many other disorders, but the relationship between anhedonia and inflammation has not been investigated in CUD. Therefore, we assessed peripheral genome-wide gene expression in n = 48 individuals with CUD with high (n = 24) vs. low (n = 24) levels of anhedonia, defined by a median split of self-reported anhedonia. Our hypothesis was that individuals with high anhedonia would show differential gene expression in inflammatory pathways. No individual genes were significantly different between the low and high anhedonia groups when using t-tests with a stringent false discovery rate correction (FDR-corrected p < 0.05). However, an exploratory analysis identified 166 loci where t-tests suggested group differences at a nominal p < 0.05. We used DAVID, a bioinformatics tool that provides functional interpretations of complex lists of genes, to examine representation of this gene list in known pathways. It confirmed that mechanisms related to immunity were the top significant associations with anhedonia in the sample. Further, the two top differentially expressed genes in our sample, IRF1 and GBP5, both have primary inflammation and immune functions, and were significantly negatively correlated with total scores on our self-report of anhedonia across all 48 subjects. These results suggest that prioritizing development of anti-inflammatory medications for CUD may pay dividends, particularly in combination with treatment-matching strategies using either phenotypic measures of anhedonia or biomarkers of inflammatory gene expression to individualize treatment.
Introduction
Cocaine use disorder (CUD) affects approximately 913,000 people aged 12 or older in the United States, and constitutes a substantial burden on the health care system [1,2]. Many treatments have been attempted for CUD, including psychosocial therapies and various pharmaceutical interventions. However, psychosocial interventions show variable efficacy, and to date, no pharmaceutical interventions are FDA approved. Thus, further investigation of the psychopathological manifestations and causes of CUD is needed to develop more effective therapies. One approach may be to identify symptoms associated with poor treatment outcomes, and investigate the mechanisms underlying these symptoms. A better understanding of the biological and psychological underpinnings of these poor prognostic signs may critically assist the development of novel effective treatments for CUD.
One potential key symptom in addictive disorders in general, and CUD in particular, is anhedonia. Anhedonia is defined as a decrease in pleasure capacity; here we use it to refer more specifically to a loss of pleasure or interest in non-drug rewards. Anhedonia is common in addictive disorders, including cocaine, but also alcohol, opiate, amphetamine, and cannabis disorders [3,4]. Across substances of abuse, the presence of anhedonia correlates with withdrawal symptom severity, craving, and likelihood of relapse, suggesting anhedonia may predict a more difficult course of addiction and worse treatment outcomes [3]. Several studies support the hypothesis that anhedonia is a poor prognostic sign in substance use disorders. For example, lifetime presence of anhedonia significantly predicts continued smoking during smoking cessation treatment, even after adjusting for the potential impact of co-occurring depressed mood [5]. Another study found that opiate dependent individuals who were less aroused by pleasant, non-drug related pictures were more likely to continue using heroin during treatment [6]. Most pertinent to CUD, low positive mood, measured using the Profile of Mood States (which could represent anhedonia), and the presence of anhedonia at treatment outset, as assessed using the Snaith-Hamilton Pleasure Scale, have been associated with poorer outcomes in CUD treatment [7,8]. Therefore, understanding and addressing the pathological basis of anhedonia in CUD may help produce more effective treatments for CUD.
A significant body of literature suggests that inflammation, or excessive immune activity, may underlie anhedonia across disorders [9–11]. During acute inflammatory events, such as a cold, circulating inflammatory mediators produce “sickness behaviors” such as reduced activity, which serve the adaptive function of conserving energy and speeding healing [11]. However, maladaptive chronic low-grade inflammation appears to be present in many psychiatric disorders, and has been implicated in symptoms of anhedonia seen in depression, schizophrenia, and cancer-related fatigue, among other conditions [9,12–14].
The neurological and biological mechanisms of anhedonia in CUD are not known, but one possibility is that cocaine use creates inflammation, which produces anhedonia. Some literature indicates inflammation is elevated in cocaine users, although findings are not fully consistent. In one study comparing crack cocaine users to healthy controls, users showed higher levels of the inflammatory mediators IL-1β, TNFα, and IL-10 [15]. A similar study indicated increases in IL-6 in cocaine users compared to healthy controls, although IL-10 was decreased in cocaine users [16]. Female crack cocaine users at the outset of treatment showed higher levels of IL-10, IL-4, and IL-6, which gradually moved closer to reference values throughout detoxification, indicating inflammation declines in conjunction with cocaine use [17]. A similar study of cocaine-using adolescents also found elevated IL-6 and IL-10 at treatment admission; however, in this study values did not change over treatment [18]. A study examining stress responses in cocaine users showed decreased basal IL-10 but enhanced TNFα responses to stress compared to social drinking controls [19]. However, other studies have either shown no differences in inflammatory biomarkers between individuals with CUD and controls [20], only shown differences in individuals with additional risk factors such as early life trauma [21], or even suggested immunosuppression in cocaine users [22]. Taken together, these mixed results suggest inflammation may be present only in a subset of cocaine users. We hypothesize these may be the same individuals who show anhedonia and more difficult clinical courses.
This study assessed peripheral genome-wide gene expression in individuals with CUD who were also displaying high levels of anhedonia, compared to individuals with CUD and low anhedonic symptoms. We examined gene expression in peripheral blood mononuclear cells, with the hypothesis that individuals with CUD and high levels of anhedonia would show different expression of genes in inflammatory pathways, compared to individuals with CUD and low levels of anhedonia. We assessed genome-wide expression for several reasons. First, gene expression shows promise as a peripheral biomarker of alterations in brain functioning, with studies suggesting that peripheral changes in expression, particularly in inflammatory pathways, relate to changes in brain tissue [23]. Further, a previous study in individuals with alcohol abuse discovered changes in the immune signaling pathways of alcohol abusers compared to healthy controls, suggesting this technique is sensitive to inflammatory changes in addiction [24]. We selected genome-wide analysis coupled with a bioinformatics approach because, as a complex condition, it is likely gene expression alterations in CUD are not limited to a few candidate genes, but rather to complex gene networks and potentially the accumulation of several unfavorable changes. Although the inflammation-anhedonia hypothesis has a strong theoretical basis, this exploratory approach was judged appropriate for an initial hypothesis-generating study that is the first to examine the biological underpinnings of anhedonia in CUD.
Materials and methods
Participants
Participants were 48 individuals with Cocaine Dependence per DSM-IV or CUD of at least moderate severity per DSM-5 (this study bridged the transition from DSM-IV to DSM-5) attending screening sessions used to determine eligibility for both treatment and non-treatment studies at a center focused on treatment development for addictions. All participants provided separate written informed consent for data collection during the screening, including the blood draw and gene expression analyses, and all procedures were carried out in accordance with the Declaration of Helsinki and with the approval of the local Institutional Review Board at the University of Texas Health Science Center at Houston. Screening consisted of a Structured Clinical Interview for DSM-IV or DSM-5 [25,26] and Addiction Severity Index–Lite [27] administered by a master’s level clinician, a physical exam conducted by a physician, an electrocardiogram, laboratory tests, and completion of several self-report forms, including self-report of anhedonia. Criteria for inclusion in the gene expression study were: 1. No major inflammatory/medical conditions (e.g. tuberculosis, Hepatitis C, HIV, diabetes, active infection, cancer) with the exception of hypertension or high cholesterol; 2. No psychoactive or daily medication use, except for hypertension or high cholesterol medications; 3. No psychotic disorders; 4. If female, not pregnant, breastfeeding, peri- or post-menopausal, having a hysterectomy, or taking oral contraceptives. Participants with other psychiatric or substance use disorders were included, as significant comorbidity is typical in this population.
Self-report of anhedonia
Our measure of anhedonia was the Snaith-Hamilton Pleasure Scale (SHAPS), which assesses ability to enjoy non-drug rewards [28]. The SHAPS is a 14-item, self-report scale that asks participants to indicate on a scale from 0 = "Strongly Agree" to 3 = "Strongly Disagree" their ability to enjoy 14 normally pleasurable events (“I would enjoy my favorite television or radio program” or “I would get pleasure from helping others”). Scores range from 0 to 42 with higher scores indicating greater anhedonia. The SHAPS is one of the most widely used self-reports of anhedonia, with good psychometric characteristics [29], and previous validation in a substance dependent population [30]. A median split of SHAPS scores was used to create high and low anhedonia groups for the gene expression analysis.
Genome-wide expression analysis
Peripheral blood was collected from fasting participants in the morning (between 8am-12pm), by venipuncture into Heparin-containing vacutainers. This was followed by isolation of peripheral blood mononuclear cells with Ficoll-Paque (GE Healthcare, Little Chalfont, UK) density gradient centrifugation, as described by the manufacturer. Isolated cells (up to 3 x 106 per vial) were resuspended in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA) containing 5% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) and stored at -80°C until further processed. RNA isolation was performed with the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. RNA quantification was performed on NanoDrop (Thermo Fisher Scientific) and the integrity of RNA samples was confirmed by assessing the RNA Integrity Number (RIN) using the RNA 6000 Nano Kit (Agilent, Santa Clara, CA) on Bioanalyzer (Agilent). Total RNA samples were converted into biotin-labeled cRNAs with the TargetAmp Nano Labeling Kit (Epicentre, Madison, WI), quantified on NanoDrop, and hybridized (750ng) to the HumanHT-12 v4 Expression BeadChip (Illumina, San Diego, CA), according to the manufacturer’s instructions. BeadChips were scanned on an iScan microarray reader (Illumina) immediately after the hybridization protocol. Raw scan data were uploaded into the GenomeStudio software v2011.1 (Illumina), where they were initially background subtracted and quantile normalized using the Gene Expression Module v1.0. Microarray data has been deposited into the NCBI GEO database, accession number GSE116833.
Real-time quantitative PCR
The top two differentially expressed genes between groups were selected for validation using real time quantitative PCR. Briefly, RNA samples (160 ng) were initially converted into cDNA using the High Capacity cDNA Synthesis Kit (Life Technologies, Carlsbad, CA) and later diluted 5 times for the PCR reactions. Amplifications of Interferon Regulatory Factor 1 (IRF1) and Guanylate Binding Protein 5 (GBP5) were performed in 12 μL-reactions using inventoried FAM-MGB-labeled TaqMan Gene Expression Assays (Hs00971965_m1 and Hs00369472_m1 for IRF1 and GBP5, respectively) and the VIC-MGB_PL-labelled beta-2-microglobulin (B2M) as endogenous control (Hs00187842_m1). PCR reactions were run on a QuantStudio 7 Flex Real-Time PCR System (Life Technologies) with each sample assayed in triplicate. Data were analyzed by the 2(-Delta Delta C(T)) method [31].
Statistical analysis
Between-group t-tests and chi-squared tests with a significance level of p < 0.05 were used to evaluate for possible group differences that might represent confounds. Genome-wide expression levels were compared between low and high anhedonia groups using GenomeStudio software v2011.1 (Illumina). Differential expression analysis between low and high anhedonia groups was performed using the low anhedonia as the reference group, ‘Illumina custom’ as the error model, and multiple testing correction using the Benjamini and Hochberg procedure to control for FDR. In addition to this stringent analysis, an exploratory analysis was performed to identify differentially expressed genes with nominal p-values < 0.05. After checking for the normality of data on IBM SPSS Statistics 25 using Shapiro-Wilk’s test and histogram visualization, we also investigated the correlation between the top-ranked differentially expressed genes and total SHAPS scores with the Spearman rank correlation coefficient. Differentially expressed genes were analyzed for their collective functional annotation on Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.8 [32]. The enriched functional annotation terms were selected based on a false discovery rate (FDR) cutoff < 0.05.
Results
Demographic data from CUD patients by group are shown in Table 1. Groups did not significantly differ on any demographic, substance use or medical/psychiatric variables tested (p > 0.05 for all comparisons). Race was collapsed into African-American vs. all others due to the small number of participants in other racial categories. In our sample the overall mean for the SHAPS was 11.68 (SD = 7.67), and the median split was at 11.5. This is similar to previous reports in individuals with substance use disorders [30], which tend to be above those of healthy adults, but below individuals with depression. Twenty seven percent of the sample met a previously established clinical cutoff for the presence of significant anhedonia on the SHAPS [28].
Table 1. Demographic variables by group.
| Variables | Low Anhedonia Group (N = 24) M (SD) or N (%) |
High Anhedonia Group (N = 24) M (SD) or N (%) |
Statistic |
|---|---|---|---|
| Demographic | |||
| Female gender | 5 (21%) | 6 (25%) | χ2(1) = 0.12, p = 0.73 |
| Age | 44.12 (8.87) | 47.50 (8.53) | t(46) = -1.34, p = 0.19 |
| African-American race | 17 (71%) | 20 (83%) | χ2(1) = 1.06, p = 0.30 |
| Years of education | 13.54 (1.84) | 12.75 (1.89) | t(46) = 1.47, p = 0.15 |
| Substance Use | |||
| # of days cocaine use past month | 15.17 (9.12) | 16.04 (8.98) | t(46) = -0.34, p = 0.74 |
| # of years using cocaine | 14.42 (10.61) | 16.17 (9.30) | t(46) = -0.61, p = 0.55 |
| Cocaine+ UDS day of draw | 16 Yes (67%) | 19 Yes (79%) | χ2(1) = 0.95, p = 0.33 |
| Other Current SUD diagnosis | 7 Yes (29%) | 9 Yes (38%) | χ2(1) = 0.38, p = 0.54 |
| Medical/Psychiatric | |||
| Hypertension | 4 (17%) | 4 (17%) | χ2(1) = 0.00, p = 1.00 |
| Cholesterol mg/dl | 165.58 (30.10) | 182.92 (46.66) | t(46) = -1.48, p = 0.15 |
| Fasting glucose mg/dl | 85.21 (9.95) | 84.04 (8.19) | t(46) = 0.44, p = 0.66 |
| Regular use of medications | 9 (38%) | 7 (29%) | χ2(1) = 0.38, p = 0.54 |
| Other psychiatric diagnosis | 6 (25%) | 7 (29%) | χ2(1) = 0.11, p = 0.75 |
Cocaine + UDS–Cocaine positive urine drug screen; SUD–Substance Use Disorder (including Alcohol Use Disorder); mg/dl–milligrams per deciliter.
No gene was significantly different between groups after correction for multiple testing (FDR-corrected p > 0.05 for all comparisons). We found 166 differentially expressed genes or putative loci between groups in the exploratory analysis (nominal p < 0.05), of which 41 were up-regulated and 125 were down-regulated in the high anhedonia group (Table 2).
Table 2. Differentially expressed genes in peripheral blood mononuclear cells from cocaine use disorder patients with low and high anhedonia (ranked by nominal p-value).
| SYMBOL | Low anhedonia AVG | High anhedonia AVG | Nominal p-value | Fold change | Definition |
|---|---|---|---|---|---|
| IRF1 | 1857.4 | 1365.6 | 0.00221 | 0.735 | Homo sapiens interferon regulatory factor 1 (IRF1), mRNA. |
| GBP5 | 1246.3 | 798.6 | 0.00288 | 0.640 | Homo sapiens guanylate binding protein 5 (GBP5), mRNA. |
| KCTD10 | 162.4 | 115.3 | 0.00479 | 0.709 | Homo sapiens potassium channel tetramerisation domain containing 10 (KCTD10), mRNA. |
| PF4V1 | 98.5 | 226.1 | 0.00506 | 2.295 | Homo sapiens platelet factor 4 variant 1 (PF4V1), mRNA. |
| C1orf71 | 774.6 | 1000.9 | 0.00529 | 1.292 | Homo sapiens chromosome 1 open reading frame 71 (C1orf71), mRNA. |
| PID1 | 199.9 | 304.7 | 0.00673 | 1.524 | Homo sapiens phosphotyrosine interaction domain containing 1 (PID1), mRNA. |
| NLRC5 | 92.4 | 69.6 | 0.00766 | 0.753 | Homo sapiens NLR family, CARD domain containing 5 (NLRC5), mRNA. |
| SRXN1 | 480.8 | 377.7 | 0.0082 | 0.785 | Homo sapiens sulfiredoxin 1 homolog (S. cerevisiae) (SRXN1), mRNA. |
| CXCL2 | 541.9 | 235.1 | 0.00897 | 0.433 | Homo sapiens chemokine (C-X-C motif) ligand 2 (CXCL2), mRNA. |
| SLC40A1 | 428.3 | 555.8 | 0.01012 | 1.297 | Homo sapiens solute carrier family 40 (iron-regulated transporter), member 1 (SLC40A1), mRNA. |
| PPP1R16B | 620.9 | 499.1 | 0.0111 | 0.803 | Homo sapiens protein phosphatase 1, regulatory (inhibitor) subunit 16B (PPP1R16B), mRNA. |
| PDIA4 | 46.6 | 31.9 | 0.01143 | 0.684 | Homo sapiens protein disulfide isomerase family A, member 4 (PDIA4), mRNA. |
| C1orf21 | 43.3 | 23.1 | 0.01242 | 0.533 | Homo sapiens chromosome 1 open reading frame 21 (C1orf21), mRNA. |
| LAP3 | 1230.5 | 920.8 | 0.01302 | 0.748 | Homo sapiens leucine aminopeptidase 3 (LAP3), mRNA. |
| LOC441408 | 94.4 | 73 | 0.01321 | 0.773 | PREDICTED: Homo sapiens hypothetical LOC441408, transcript variant 1 (LOC441408), mRNA. |
| TMEM170A | 161.8 | 127.6 | 0.01329 | 0.788 | Homo sapiens transmembrane protein 170A (TMEM170A), mRNA. |
| PRIC285 | 761.8 | 556.4 | 0.01379 | 0.730 | Homo sapiens peroxisomal proliferator-activated receptor A interacting complex 285 (PRIC285), transcript variant 2, mRNA. |
| FCHSD2 | 231.3 | 188.6 | 0.01382 | 0.815 | Homo sapiens FCH and double SH3 domains 2 (FCHSD2), mRNA. |
| PRC1 | 71.5 | 51.6 | 0.01388 | 0.721 | Homo sapiens protein regulator of cytokinesis 1 (PRC1), transcript variant 2, mRNA. |
| SH3BGRL2 | 285 | 425 | 0.01453 | 1.49 | Homo sapiens SH3 domain binding glutamic acid-rich protein like 2 (SH3BGRL2), mRNA. |
| CD55 | 284.9 | 226.8 | 0.01486 | 0.796 | Homo sapiens CD55 molecule, decay accelerating factor for complement (Cromer blood group) (CD55), mRNA. |
| PDK4 | 228.5 | 371.1 | 0.01529 | 1.624 | Homo sapiens pyruvate dehydrogenase kinase, isozyme 4 (PDK4), mRNA. |
| FLJ33590 | 87.2 | 58.4 | 0.0153 | 0.669 | Homo sapiens hypothetical protein FLJ33590 (FLJ33590), mRNA. |
| SNCA | 208.6 | 277.4 | 0.01562 | 1.329 | Homo sapiens synuclein, alpha (non A4 component of amyloid precursor) (SNCA), transcript variant NACP112, mRNA. |
| SPATS2L | 177.6 | 122 | 0.0163 | 0.686 | Homo sapiens spermatogenesis associated, serine-rich 2-like (SPATS2L), transcript variant 2, mRNA. |
| STAT1 | 2174 | 1456.3 | 0.01725 | 0.669 | Homo sapiens signal transducer and activator of transcription 1, 91kDa (STAT1), transcript variant alpha, mRNA. |
| TGFBR3 | 1661.2 | 1222.6 | 0.01767 | 0.735 | Homo sapiens transforming growth factor, beta receptor III (TGFBR3), mRNA. |
| ARL4C | 208.3 | 163.1 | 0.01783 | 0.783 | Homo sapiens ADP-ribosylation factor-like 4C (ARL4C), mRNA. |
| RIPK2 | 1123.6 | 846.9 | 0.01787 | 0.753 | Homo sapiens receptor-interacting serine-threonine kinase 2 (RIPK2), mRNA. |
| IFITM3 | 3464.5 | 1863.3 | 0.01793 | 0.537 | Homo sapiens interferon induced transmembrane protein 3 (1-8U) (IFITM3), mRNA. |
| DUSP5 | 1234.6 | 950.5 | 0.01852 | 0.769 | Homo sapiens dual specificity phosphatase 5 (DUSP5), mRNA. |
| C17orf97 | 48.9 | 88.5 | 0.01864 | 1.809 | Homo sapiens chromosome 17 open reading frame 97 (C17orf97), mRNA. |
| CYLN2 | 52.7 | 73 | 0.01948 | 1.385 | Homo sapiens cytoplasmic linker 2 (CYLN2), transcript variant 2, mRNA. |
| RGS18 | 1318.6 | 1720.1 | 0.01965 | 1.304 | Homo sapiens regulator of G-protein signaling 18 (RGS18), mRNA. |
| BCAT1 | 61.4 | 86.3 | 0.02051 | 1.405 | Homo sapiens branched chain aminotransferase 1, cytosolic (BCAT1), mRNA. |
| TAP1 | 3242.5 | 2637.6 | 0.0215 | 0.813 | Homo sapiens transporter 1, ATP-binding cassette, sub-family B (MDR/TAP) (TAP1), mRNA. |
| HERC5 | 955.6 | 644.8 | 0.02163 | 0.674 | Homo sapiens hect domain and RLD 5 (HERC5), mRNA. |
| APOBEC3H | 51.9 | 30.8 | 0.02165 | 0.593 | Homo sapiens apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3H (APOBEC3H), mRNA. |
| KLF5 | 24.2 | 11.1 | 0.02191 | 0.458 | Homo sapiens Kruppel-like factor 5 (intestinal) (KLF5), mRNA. |
| LBA1 | 311.2 | 255.4 | 0.02252 | 0.820 | Homo sapiens lupus brain antigen 1 (LBA1), mRNA. |
| ZMYND15 | 94.3 | 68.5 | 0.02335 | 0.726 | Homo sapiens zinc finger, MYND-type containing 15 (ZMYND15), transcript variant 2, mRNA. |
| HERC6 | 295.6 | 215.7 | 0.02347 | 0.729 | Homo sapiens hect domain and RLD 6 (HERC6), transcript variant 1, mRNA. |
| PFN4 | 4 | 14.4 | 0.02409 | 3.6 | Homo sapiens profilin family, member 4 (PFN4), mRNA. |
| KIAA1600 | 473.5 | 393.3 | 0.02432 | 0.830 | Homo sapiens KIAA1600 (KIAA1600), mRNA. |
| RGS1 | 643.7 | 370.8 | 0.02467 | 0.576 | Homo sapiens regulator of G-protein signaling 1 (RGS1), mRNA. |
| SLC25A28 | 1486.8 | 1259.1 | 0.02474 | 0.846 | Homo sapiens solute carrier family 25, member 28 (SLC25A28), mRNA. |
| NAPB | 134.8 | 109.6 | 0.0249 | 0.813 | Homo sapiens N-ethylmaleimide-sensitive factor attachment protein, beta (NAPB), mRNA. |
| C20orf3 | 718.3 | 606.4 | 0.02518 | 0.844 | Homo sapiens chromosome 20 open reading frame 3 (C20orf3), mRNA. |
| USP18 | 44 | 16.8 | 0.02551 | 0.3818 | Homo sapiens ubiquitin specific peptidase 18 (USP18), mRNA. |
| NCOA7 | 853.7 | 674.2 | 0.02568 | 0.789 | Homo sapiens nuclear receptor coactivator 7 (NCOA7), mRNA. |
| MYBPC3 | 43.6 | 30.2 | 0.02598 | 0.692 | Homo sapiens myosin binding protein C, cardiac (MYBPC3), mRNA. |
| DGKE | 25.8 | 14.5 | 0.02606 | 0.562 | Homo sapiens diacylglycerol kinase, epsilon 64kDa (DGKE), mRNA. |
| LOC100132503 | 68.6 | 88.3 | 0.02656 | 1.287 | PREDICTED: Homo sapiens similar to ring finger protein 208 (LOC100132503), mRNA. |
| LOC400759 | 126.3 | 70 | 0.02685 | 0.554 | Homo sapiens similar to Interferon-induced guanylate-binding protein 1 (GTP-binding protein 1) (Guanine nucleotide-binding protein 1) (HuGBP-1) (LOC400759) on chromosome 1. |
| LOC197135 | 201.7 | 128.4 | 0.02699 | 0.636 | PREDICTED: Homo sapiens hypothetical LOC197135, transcript variant 5 (LOC197135), mRNA. |
| REC8 | 251.1 | 194.2 | 0.02707 | 0.773 | Homo sapiens REC8 homolog (yeast) (REC8), transcript variant 1, mRNA. |
| NFIL3 | 946 | 707.4 | 0.02725 | 0.747 | Homo sapiens nuclear factor, interleukin 3 regulated (NFIL3), mRNA. |
| LOC729580 | 88.3 | 69 | 0.02736 | 0.781 | PREDICTED: Homo sapiens hypothetical LOC729580 (LOC729580), mRNA. |
| C16orf33 | 389.5 | 327.8 | 0.02783 | 0.841 | Homo sapiens chromosome 16 open reading frame 33 (C16orf33), mRNA. |
| CD8A | 2691 | 2060.5 | 0.02813 | 0.765 | Homo sapiens CD8a molecule (CD8A), transcript variant 2, mRNA. |
| LRRC57 | 122.4 | 99.2 | 0.02817 | 0.810 | Homo sapiens leucine rich repeat containing 57 (LRRC57), mRNA. |
| CD83 | 1912 | 1345.2 | 0.0283 | 0.703 | Homo sapiens CD83 molecule (CD83), transcript variant 1, mRNA. |
| 673.5 | 556.7 | 0.02839 | 0.826 | full-length cDNA clone CS0CAP005YH21 of Thymus of Homo sapiens (human) | |
| 248 | 194.4 | 0.02872 | 0.783 | Homo sapiens cDNA clone IMAGE:5277162 | |
| F13A1 | 659.8 | 875 | 0.02876 | 1.326 | Homo sapiens coagulation factor XIII, A1 polypeptide (F13A1), mRNA. |
| NECAP1 | 563.1 | 473.3 | 0.02878 | 0.840 | Homo sapiens NECAP endocytosis associated 1 (NECAP1), mRNA. |
| 12.1 | 23.6 | 0.02931 | 1.950 | Human tissue plasminogen activator mRNA, partial cds | |
| 31.1 | 19.7 | 0.02958 | 0.633 | Homo sapiens cDNA FLJ35432 fis, clone SMINT2002311 | |
| LOC648470 | 345.6 | 281.4 | 0.02995 | 0.814 | PREDICTED: Homo sapiens similar to Caspase-4 precursor (CASP-4) (ICH-2 protease) (TX protease) (ICE(rel)-II) (LOC648470), mRNA. |
| GBP4 | 760.3 | 544.7 | 0.03006 | 0.716 | Homo sapiens guanylate binding protein 4 (GBP4), mRNA. |
| EPSTI1 | 1084.5 | 642.8 | 0.03101 | 0.592 | Homo sapiens epithelial stromal interaction 1 (breast) (EPSTI1), transcript variant 2, mRNA. |
| FAM50B | 52.2 | 72.4 | 0.03109 | 1.386 | Homo sapiens family with sequence similarity 50, member B (FAM50B), mRNA. |
| HSH2D | 257.2 | 202.8 | 0.03133 | 0.788 | Homo sapiens hematopoietic SH2 domain containing (HSH2D), mRNA. |
| LOC644590 | 328.2 | 276.6 | 0.03143 | 0.842 | PREDICTED: Homo sapiens similar to EVIN1 (LOC644590), mRNA. |
| PSME2 | 2178.8 | 1812.5 | 0.03168 | 0.831 | Homo sapiens proteasome (prosome, macropain) activator subunit 2 (PA28 beta) (PSME2), mRNA. |
| LOC649864 | 54.2 | 39.7 | 0.03174 | 0.732 | PREDICTED: Homo sapiens similar to HLA class I histocompatibility antigen, A-29 alpha chain precursor (MHC class I antigen A*29) (Aw-19), transcript variant 1 (LOC649864), mRNA. |
| KDELC2 | 146.1 | 119.6 | 0.03215 | 0.818 | Homo sapiens KDEL (Lys-Asp-Glu-Leu) containing 2 (KDELC2), mRNA. |
| UAP1 | 293 | 245.3 | 0.03287 | 0.837 | Homo sapiens UDP-N-acteylglucosamine pyrophosphorylase 1 (UAP1), mRNA. |
| CLCF1 | 102.4 | 80.9 | 0.03289 | 0.790 | Homo sapiens cardiotrophin-like cytokine factor 1 (CLCF1), transcript variant 1, mRNA. |
| IBSP | 9.4 | 19.9 | 0.03293 | 2.117 | Homo sapiens integrin-binding sialoprotein (bone sialoprotein, bone sialoprotein II) (IBSP), mRNA. |
| C9orf82 | 97.9 | 78.6 | 0.03309 | 0.802 | Homo sapiens chromosome 9 open reading frame 82 (C9orf82), mRNA. |
| PGA5 | 5.4 | 19.8 | 0.03319 | 3.666 | Homo sapiens pepsinogen 5, group I (pepsinogen A) (PGA5), mRNA. |
| ITM2C | 776.2 | 638.7 | 0.03364 | 0.822 | Homo sapiens integral membrane protein 2C (ITM2C), transcript variant 2, mRNA. |
| TRIM22 | 976.5 | 775.9 | 0.03373 | 0.794 | Homo sapiens tripartite motif-containing 22 (TRIM22), mRNA. |
| SNORD114-2 | 24.1 | 35.6 | 0.03385 | 1.477 | Homo sapiens small nucleolar RNA, C/D box 114–2 (SNORD114-2), small nucleolar RNA. |
| LAPTM4B | 67.1 | 89 | 0.03442 | 1.326 | Homo sapiens lysosomal protein transmembrane 4 beta (LAPTM4B), mRNA. |
| FAM46C | 1498.2 | 1164.3 | 0.03446 | 0.777 | Homo sapiens family with sequence similarity 46, member C (FAM46C), mRNA. |
| SLAMF7 | 138.5 | 104 | 0.03478 | 0.750 | Homo sapiens SLAM family member 7 (SLAMF7), mRNA. |
| KBTBD8 | 200.4 | 163.8 | 0.03491 | 0.817 | Homo sapiens kelch repeat and BTB (POZ) domain containing 8 (KBTBD8), mRNA. |
| IRF4 | 185.1 | 152.3 | 0.03537 | 0.822 | Homo sapiens interferon regulatory factor 4 (IRF4), mRNA. |
| FFAR2 | 63.5 | 35.4 | 0.03551 | 0.557 | Homo sapiens free fatty acid receptor 2 (FFAR2), mRNA. |
| TDRD9 | 47.7 | 66.9 | 0.03554 | 1.402 | Homo sapiens tudor domain containing 9 (TDRD9), mRNA. |
| FBXO6 | 295.5 | 232 | 0.03571 | 0.785 | Homo sapiens F-box protein 6 (FBXO6), mRNA. |
| IL12A | 34.6 | 21.7 | 0.03571 | 0.627 | Homo sapiens interleukin 12A (natural killer cell stimulatory factor 1, cytotoxic lymphocyte maturation factor 1, p35) (IL12A), mRNA. |
| GALM | 148.3 | 120.8 | 0.036 | 0.814 | Homo sapiens galactose mutarotase (aldose 1-epimerase) (GALM), mRNA. |
| PATL2 | 182 | 116.5 | 0.03614 | 0.640 | PREDICTED: Homo sapiens misc_RNA (PATL2), miscRNA. |
| IFI44 | 973.5 | 556.6 | 0.03638 | 0.571 | Homo sapiens interferon-induced protein 44 (IFI44), mRNA. |
| TNFRSF21 | 156.3 | 111.6 | 0.03707 | 0.714 | Homo sapiens tumor necrosis factor receptor superfamily, member 21 (TNFRSF21), mRNA. |
| GNG11 | 899.5 | 1303.2 | 0.03713 | 1.448 | Homo sapiens guanine nucleotide binding protein (G protein), gamma 11 (GNG11), mRNA. |
| TMEM88 | 43.6 | 27.2 | 0.03735 | 0.623 | Homo sapiens transmembrane protein 88 (TMEM88), mRNA. |
| NRGN | 1413 | 1980.4 | 0.03751 | 1.401 | Homo sapiens neurogranin (protein kinase C substrate, RC3) (NRGN), mRNA. |
| LOC100133583 | 1081.4 | 870.6 | 0.03818 | 0.805 | PREDICTED: Homo sapiens similar to major histocompatibility complex, class II, DQ beta 1, transcript variant 2 (LOC100133583), mRNA. |
| GABARAPL1 | 536.9 | 417.1 | 0.03826 | 0.776 | Homo sapiens GABA(A) receptor-associated protein like 1 (GABARAPL1), mRNA. |
| PTGFRN | 13.2 | 24 | 0.03829 | 1.818 | Homo sapiens prostaglandin F2 receptor negative regulator (PTGFRN), mRNA. |
| PDXK | 436.9 | 514.6 | 0.0385 | 1.177 | Homo sapiens pyridoxal (pyridoxine, vitamin B6) kinase (PDXK), mRNA. |
| ATG2A | 339.2 | 287.2 | 0.0385 | 0.846 | Homo sapiens ATG2 autophagy related 2 homolog A (S. cerevisiae) (ATG2A), mRNA. |
| TAGAP | 330.2 | 259 | 0.0393 | 0.784 | Homo sapiens T-cell activation RhoGTPase activating protein (TAGAP), transcript variant 2, mRNA. |
| NAT8B | 127.4 | 184.4 | 0.03936 | 1.447 | Homo sapiens N-acetyltransferase 8B (GCN5-related, putative, gene/pseudogene) (NAT8B), mRNA. |
| C19orf33 | 23.3 | 42.5 | 0.0396 | 1.824 | Homo sapiens chromosome 19 open reading frame 33 (C19orf33), mRNA. |
| MYLIP | 1777.6 | 1479.2 | 0.03961 | 0.832 | Homo sapiens myosin regulatory light chain interacting protein (MYLIP), mRNA. |
| ANKRD27 | 113.7 | 93.1 | 0.03962 | 0.818 | Homo sapiens ankyrin repeat domain 27 (VPS9 domain) (ANKRD27), mRNA. |
| PATL1 | 853.9 | 731.7 | 0.03985 | 0.856 | Homo sapiens protein associated with topoisomerase II homolog 1 (yeast) (PATL1), mRNA. |
| MCL1 | 1439.3 | 1263.9 | 0.03995 | 0.878 | Homo sapiens myeloid cell leukemia sequence 1 (BCL2-related) (MCL1), transcript variant 1, mRNA. |
| LOC643733 | 107.4 | 87.4 | 0.04028 | 0.813 | PREDICTED: Homo sapiens hypothetical LOC643733 (LOC643733), mRNA. |
| S1PR5 | 811.2 | 591 | 0.04044 | 0.728 | Homo sapiens sphingosine-1-phosphate receptor 5 (S1PR5), mRNA. |
| GNLY | 3203.7 | 2308.7 | 0.04079 | 0.720 | Homo sapiens granulysin (GNLY), transcript variant NKG5, mRNA. |
| C17orf56 | 96.3 | 77.7 | 0.04085 | 0.806 | Homo sapiens chromosome 17 open reading frame 56 (C17orf56), mRNA. |
| PCNT | 594.2 | 500.2 | 0.04122 | 0.841 | Homo sapiens pericentrin (PCNT), mRNA. |
| GFI1 | 217.8 | 168.2 | 0.04128 | 0.772 | Homo sapiens growth factor independent 1 transcription repressor (GFI1), mRNA. |
| GLTPD1 | 36.1 | 48.6 | 0.04149 | 1.346 | Homo sapiens glycolipid transfer protein domain containing 1 (GLTPD1), mRNA. |
| SLC25A4 | 154.7 | 126.6 | 0.04161 | 0.818 | Homo sapiens solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 4 (SLC25A4), nuclear gene encoding mitochondrial protein, mRNA. |
| HBEGF | 839.1 | 550.7 | 0.0417 | 0.656 | Homo sapiens heparin-binding EGF-like growth factor (HBEGF), mRNA. |
| FAM179A | 128.1 | 91.4 | 0.04173 | 0.713 | Homo sapiens family with sequence similarity 179, member A (FAM179A), mRNA. |
| TNFAIP3 | 2436.9 | 1817.1 | 0.04194 | 0.745 | Homo sapiens tumor necrosis factor, alpha-induced protein 3 (TNFAIP3), mRNA. |
| GBP2 | 3234.4 | 2664.8 | 0.04267 | 0.823 | Homo sapiens guanylate binding protein 2, interferon-inducible (GBP2), mRNA. |
| TRIM11 | 186.6 | 156.9 | 0.04292 | 0.840 | Homo sapiens tripartite motif-containing 11 (TRIM11), mRNA. |
| MAFB | 3522 | 2710.3 | 0.04307 | 0.769 | Homo sapiens v-maf musculoaponeurotic fibrosarcoma oncogene homolog B (avian) (MAFB), mRNA. |
| CEBPA | 755.7 | 907.2 | 0.04315 | 1.200 | Homo sapiens CCAAT/enhancer binding protein (C/EBP), alpha (CEBPA), mRNA. |
| GBP1 | 592.5 | 334.1 | 0.0434 | 0.563 | Homo sapiens guanylate binding protein 1, interferon-inducible, 67kDa (GBP1), mRNA. |
| FANCG | 154.3 | 128.9 | 0.04358 | 0.835 | Homo sapiens Fanconi anemia, complementation group G (FANCG), mRNA. |
| C1orf166 | 215.1 | 181.7 | 0.04383 | 0.844 | Homo sapiens chromosome 1 open reading frame 166 (C1orf166), mRNA. |
| OTUD1 | 563.3 | 444.8 | 0.04392 | 0.789 | PREDICTED: Homo sapiens OTU domain containing 1 (OTUD1), mRNA. |
| RNASE6 | 712.7 | 850.7 | 0.04399 | 1.193 | Homo sapiens ribonuclease, RNase A family, k6 (RNASE6), mRNA. |
| IL18R1 | 326.3 | 274.9 | 0.04408 | 0.842 | Homo sapiens interleukin 18 receptor 1 (IL18R1), mRNA. |
| FAM82A2 | 661.2 | 567.8 | 0.04481 | 0.858 | Homo sapiens family with sequence similarity 82, member A2 (FAM82A2), mRNA. |
| ITPRIP | 823.8 | 701 | 0.04497 | 0.850 | Homo sapiens inositol 1,4,5-triphosphate receptor interacting protein (ITPRIP), mRNA. |
| TM6SF1 | 158.5 | 196.8 | 0.04506 | 1.241 | Homo sapiens transmembrane 6 superfamily member 1 (TM6SF1), mRNA. |
| SH2D2A | 111.2 | 91.5 | 0.04507 | 0.822 | Homo sapiens SH2 domain protein 2A (SH2D2A), mRNA. |
| TBC1D8 | 164.6 | 133.6 | 0.04512 | 0.811 | Homo sapiens TBC1 domain family, member 8 (with GRAM domain) (TBC1D8), mRNA. |
| KBTBD2 | 780.8 | 668.5 | 0.04523 | 0.856 | Homo sapiens kelch repeat and BTB (POZ) domain containing 2 (KBTBD2), mRNA. |
| LOC728835 | 1550.2 | 788.3 | 0.04542 | 0.508 | PREDICTED: Homo sapiens similar to cytokine, transcript variant 3 (LOC728835), mRNA. |
| DDX60 | 282.4 | 208.8 | 0.04543 | 0.739 | Homo sapiens DEAD (Asp-Glu-Ala-Asp) box polypeptide 60 (DDX60), mRNA. |
| HLA-G | 1282.7 | 1105.5 | 0.04544 | 0.861 | Homo sapiens HLA-G histocompatibility antigen, class I, G (HLA-G), mRNA. |
| TXNDC11 | 263.6 | 223.9 | 0.04555 | 0.849 | Homo sapiens thioredoxin domain containing 11 (TXNDC11), mRNA. |
| SYTL1 | 359.6 | 307 | 0.04565 | 0.853 | Homo sapiens synaptotagmin-like 1 (SYTL1), mRNA. |
| CCL4L1 | 1781.5 | 845.5 | 0.04659 | 0.474 | Homo sapiens chemokine (C-C motif) ligand 4-like 1 (CCL4L1), mRNA. |
| PARP12 | 374.2 | 299.3 | 0.04667 | 0.799 | Homo sapiens poly (ADP-ribose) polymerase family, member 12 (PARP12), mRNA. |
| ACTN1 | 1248.3 | 1617.6 | 0.04701 | 1.295 | Homo sapiens actinin, alpha 1 (ACTN1), mRNA. |
| TST | 683.6 | 805.1 | 0.04719 | 1.177 | Homo sapiens thiosulfate sulfurtransferase (rhodanese) (TST), nuclear gene encoding mitochondrial protein, mRNA. |
| ZNF366 | 8.7 | 17.7 | 0.04733 | 2.034 | Homo sapiens zinc finger protein 366 (ZNF366), mRNA. |
| CCL2 | 335.1 | 178.2 | 0.04737 | 0.531 | Homo sapiens chemokine (C-C motif) ligand 2 (CCL2), mRNA. |
| RNF10 | 415.4 | 350.3 | 0.04757 | 0.843 | Homo sapiens ring finger protein 10 (RNF10), mRNA. |
| FCER1A | 1459.3 | 1873.7 | 0.04808 | 1.283 | Homo sapiens Fc fragment of IgE, high affinity I, receptor for; alpha polypeptide (FCER1A), mRNA. |
| IFI44L | 662.6 | 264.8 | 0.04823 | 0.399 | Homo sapiens interferon-induced protein 44-like (IFI44L), mRNA. |
| C1orf177 | 26.2 | 37.2 | 0.04823 | 1.419 | Homo sapiens chromosome 1 open reading frame 177 (C1orf177), mRNA. |
| OAS3 | 435.9 | 272.1 | 0.04838 | 0.624 | Homo sapiens 2'-5'-oligoadenylate synthetase 3, 100kDa (OAS3), mRNA. |
| LOC728006 | 140.1 | 170.1 | 0.04866 | 1.214 | PREDICTED: Homo sapiens hypothetical protein LOC728006 (LOC728006), mRNA. |
| ATP6V0A1 | 489.6 | 577.4 | 0.04873 | 1.179 | Homo sapiens ATPase, H+ transporting, lysosomal V0 subunit a1 (ATP6V0A1), transcript variant 3, mRNA. |
| SLC2A1 | 494.2 | 421.3 | 0.04882 | 0.852 | Homo sapiens solute carrier family 2 (facilitated glucose transporter), member 1 (SLC2A1), mRNA. |
| SPOCD1 | 23.6 | 42.4 | 0.04902 | 1.796 | Homo sapiens SPOC domain containing 1 (SPOCD1), mRNA. |
| IDI1 | 435.2 | 348.6 | 0.04916 | 0.801 | Homo sapiens isopentenyl-diphosphate delta isomerase 1 (IDI1), mRNA. |
| SERPINF1 | 56.9 | 40.1 | 0.04927 | 0.704 | Homo sapiens serpin peptidase inhibitor, clade F (alpha-2 antiplasmin, pigment epithelium derived factor), member 1 (SERPINF1), mRNA. |
| CDC45L | 32 | 18.4 | 0.04934 | 0.575 | Homo sapiens CDC45 cell division cycle 45-like (S. cerevisiae) (CDC45L), mRNA. |
| KLHDC5 | 203.5 | 247.7 | 0.04958 | 1.217 | Homo sapiens kelch domain containing 5 (KLHDC5), mRNA. |
| KLRG1 | 407.4 | 294.4 | 0.0497 | 0.722 | Homo sapiens killer cell lectin-like receptor subfamily G, member 1 (KLRG1), mRNA. |
| LOC728276 | -1.5 | 6.5 | 0.04973 | 4.333 | PREDICTED: Homo sapiens similar to Lithostathine 1 precursor (Pancreatic stone protein 1) (PSP) (Pancreatic thread protein 1) (PTP) (Islet of Langerhans regenerating protein 1) (REG 1) (LOC728276), mRNA. |
AVG–average. Fold change values lower than 1 represent down-regulation of the gene in the high anhedonia group compared to low anhedonia group, while values higher than 1 represent up-regulation.
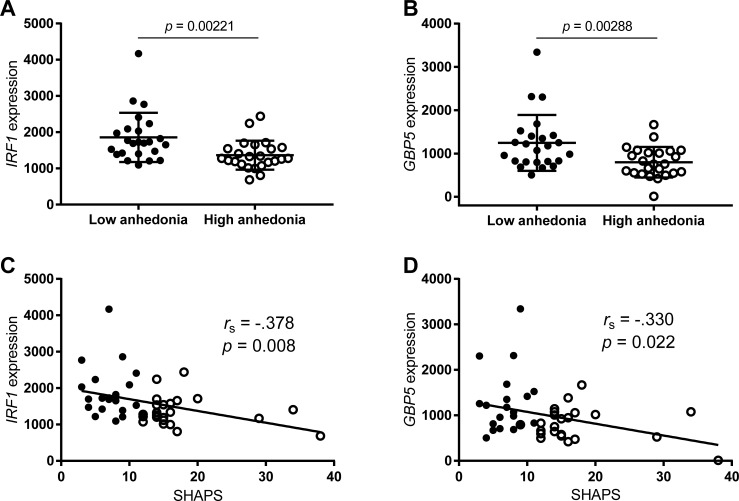
The top differentially expressed genes were IRF1 (p = 0.00221) and GBP5 (p = 0.00288), which were both significantly downregulated in the high anhedonia group (Fig 1A and 1B). Significant correlations were found between microarray expression of both genes and the total SHAPS score (IRF1 –rs = -0.378, p = 0.008; GBP5 –rs = -0.330, p = 0.022, Fig 1C and 1D). Findings from the microarray were partly validated by qPCR, with measures of IRF1 and GBP5 obtained from both methods showing significant correlations (S1 Fig). However, the relationships between these individual genes and anhedonia did not sustain statistical significance when gene expression was measured by qPCR data, although trends were similar (IRF1 –Mann-Whitney U = 210, p = 0.108; GBP5 –U = 197, p = 0.061; S2 Fig).
Fig 1. Gene expression of interferon regulatory factor 1 (IRF1) and guanylate binding protein 5 (GBP5) in peripheral blood mononuclear cells from patients with cocaine use disorder with low and high symptoms of anhedonia.
A and B) Between-group comparison of IRF1 (A) and GBP5 (B) expression values. Dots represent individual values for each subject and lines represent mean ± standard deviation. Comparisons were made with Mann-Whitney U tests. C and D) Spearman’s rank-order correlation between total Snaith-Hamilton Pleasure Scale (SHAPS) scores and the expression of IRF1 (C) and GBP5 (D).
Pathway analysis performed on DAVID showed that the 166 differentially expressed genes were enriched for mechanisms related to defense response to virus, antiviral defense, immunity, interferon-gamma-mediated signaling pathway, among others (Table 3). Functional annotation clustering analysis was also performed to reduce the redundancy of our findings and confirmed mechanisms related to immunity as the top-relevant pathways associated with anhedonia in our sample (S1 Table).
Table 3. Pathway analysis (functional annotation) of the nominally differentially expressed genes (n = 166) between cocaine use disorder patients with low and high anhedonia.
| Category | Term | Count | % | P-value | FDR |
|---|---|---|---|---|---|
| GOTERM_BP_DIRECT | defense response to virus | 13 | 9,6 | 2,1E-9 | 3,2E-6 |
| UP_KEYWORDS | Antiviral defense | 11 | 8,1 | 3,8E-9 | 4,8E-6 |
| UP_KEYWORDS | Immunity | 18 | 13,3 | 2,2E-8 | 2,8E-5 |
| GOTERM_BP_DIRECT | interferon-gamma-mediated signaling pathway | 8 | 5,9 | 6,9E-7 | 1,1E-3 |
| GOTERM_BP_DIRECT | immune response | 15 | 11,1 | 1,7E-6 | 2,6E-3 |
| GOTERM_BP_DIRECT | type I interferon signaling pathway | 7 | 5,2 | 6,0E-6 | 9,3E-3 |
| UP_KEYWORDS | Innate immunity | 11 | 8,1 | 7,3E-6 | 9,1E-3 |
Gene-term enrichment performed on DAVID v. 6.8 (https://david.ncifcrf.gov/). The most relevant (overrepresented) biological terms associated with the list of differentially expressed genes were identified by the DAVID functional annotation chart. Enrichments were calculated with a modified Fisher’s exact test controlled for false discovery rate (FDR).
Discussion
In this study, we examined genome-wide gene expression in peripheral blood mononuclear cells, with the hypothesis that participants with CUD and high levels of anhedonia would display a unique expression of inflammatory pathways, compared to individuals with CUD and low anhedonic symptoms. No individual genes were determined to be significantly different between the low and high anhedonia groups using stringent controls for false discovery rate. However, an exploratory analysis to identify differentially expressed genes with nominal p < 0.05 found 166 putative loci that differed between groups. DAVID, a bioinformatics tool that provides functional interpretations of complex lists of genes, was utilized to examine representation of these top differentially expressed genes in known gene pathways. It confirmed that mechanisms related to immunity were the most pertinent pathways corresponding with anhedonia in our sample. The two top differentially expressed genes in our sample were chosen for additional validation. These were IRF1 and GBP5; genes related to transcriptional regulation, tumor response, inflammation, and innate immunity. Expression of both genes significantly correlated with total scores on our self-report of anhedonia, further substantiating the relationship of these gene expression changes to anhedonia.
Several studies have linked cocaine use to inflammatory mechanisms [15,33]. However, our results suggest that some CUD patients display greater changes in inflammatory markers than others, suggesting a more complex and heterogeneous association between CUD and inflammation. In particular, these alterations in immune functioning were associated with a poor prognostic sign for CUD, anhedonia. Based on these results, it seems possible that a subset of patients with CUD present a pro-inflammatory genetic profile that interacts with cocaine use or other associated environmental factors to promote an anhedonic (and treatment-resistant) phenotype. Although our groups did not differ on basic demographics or measures of drug use, these changes may also be reflective of subtler differences in intensity or frequency of cocaine use, unmeasured environmental or psychosocial factors, biological and genetic differences, or gene by environment interactions, as seen with other genetic variants in addiction [34,35]. Our study is the first to provide preliminary evidence for the hypothesis that anhedonia in CUD is related to inflammatory changes in gene-expression, but a more in-depth assessment in a larger sample size that could include examination of potential gene vs. environment interactions is warranted.
As hypothesized, the top-ranked pathways associated with the differentially expressed genes in our sample were all linked to inflammatory mechanisms. Further, both top differentially expressed genes have immune-related functions. IRF1 serves as a transcriptional activator of several genes involved in both innate and acquired immune responses, as well as in tissue response to inflammation, cell proliferation, and programmed cell death [36–38]. The relationship of IRF1 to cocaine use is unknown, but it could plausibly be modulated by dopamine-enhancing properties of cocaine and the cellular signaling pathways that are activated by both D1- and D2-like dopamine receptors after cocaine use [39]. Dopamine receptor D1 is known to activate protein kinase A (PKA), which in turn can serine-phosphorylate IRF1 [40]. Once phosphorylated, IRF1 has been shown to activate IFN-α/β promoters to induce their endogenous expressions [41–43], ultimately affecting the immune/inflammatory response [44]. Moreover, cocaine use has been associated with alterations in the levels of leptin [45], which is an adipokine produced by both the adipose tissue and immune cells and participates in innate and adaptive immunity [46]. Interestingly, leptin has been shown to promote the activation and recruitment of a transcriptional complex between IRF1 and CREB [47], suggesting another layer of regulation by which cocaine could interfere with IRF-1 mechanisms and ultimately regulate inflammation. Similarly, GBP5’s expression is not only induced by IFN-γ [48], but is also an activator of the NLRP3 inflammasome [49], with key roles in innate immunity and overall inflammation [50]. There is no reported evidence of a direct effect of cocaine or the activation of dopamine receptors on the expression or function of GBP5, limiting the further interpretation of the relationship of this factor to cocaine use. Our study is the first to report altered levels of these gene transcripts both in CUD and in relation to anhedonia. Both genes were found to be downregulated in high anhedonia CUD patients compared to those with low anhedonia, suggesting a dysfunction in inflammatory mechanisms in these patients. Interestingly, IRF-1 is also downregulated by stress-related hormones [51], consistent with hypotheses that drug use and stress produce similar and synergistic effects on immune responses [52], and hypotheses linking excessive inflammatory responses to stress with anhedonia [53]. Further, one study found that chronic methamphetamine administration in mice resulted in downregulation of GBP-5 expression in the nucleus accumbens. Together these findings support our results by suggesting that although the identification of these transcripts in CUD is novel, they are affected in consistent directions by other stressors, including other stimulant drugs.
There has been increasing recognition of the value of using genome-wide transcription profiles to drive medication development in addiction, with examples in both alcohol use disorder [54] and methamphetamine use disorder [55]. Although our top gene candidates are not part of the “druggable genome” that can be directly targeted with already-developed compounds, our results nevertheless suggest critical future directions for CUD treatment research. Specifically, medication development efforts in CUD have often focused on agonist strategies using stimulant medications [56], but these medications may themselves have inflammatory effects, resulting in poor outcomes for individuals with an anhedonic/inflammatory profile. Our research suggests that prioritizing anti-inflammatory medications in development for stimulant use disorders, such as pioglitazone [57] and ibudilast [58], may pay dividends, particularly in combination with treatment-matching strategies using either the anhedonic phenotype or biomarkers of inflammatory gene expression to personalize treatment. Interestingly, a recent study found that an improvement in the hedonic tone induced by repetitive transcranial magnetic stimulation (rTMS) in CUD patients was associated with a reduction in the craving for cocaine [59]. A future direction for research may be to explore whether these improvements are associated with inflammatory alterations, and whether the inflammatory genes identified in our study may be able to predict outcomes in treatments like this that are directed at ameliorating anhedonia in CUD patients.
Our results need to be interpreted in light of some limitations. First, the relationship between peripheral and central changes in gene expression and/or inflammation is ultimately unknown. Although research suggests some substantive correspondences between peripheral and central gene expression [23], our top candidates are not among those transcripts with confirmed peripheral/central relationships. It is also the case that we do not know the degree to which the observed mRNA alterations relate to altered production of inflammatory compounds in the brain or periphery. Future studies should focus on the correlation of our identified inflammatory markers between blood and brain to allow a further discussion and more accurate interpretation of our findings, especially as they may relate to neuroinflammation or systemic inflammation. Although prior studies suggest a relationship between increased inflammation and anhedonia, we found downregulation of two genes generally presumed to be pro-inflammatory. In the absence of more direct measures of the state of either peripheral or central immune functioning in these participants, these results do not allow us to directly infer the presence of inflammation, only that immune functioning appears to differ between our groups. There are also limitations inherent in measuring genome-wide expression via a microarray, which is restricted to the probes available in the Beadchip and may not capture the full snapshot of gene expression alterations in CUD [60]. In addition, our analysis did not allow for the detection of specific transcripts derived from the same gene, which might have masked important information regarding alternative splicing mechanisms and their potential involvement in anhedonia [23]. A network-based approach assessing the interaction between several genes and transcripts detected by next-generation sequencing techniques might provide more comprehensive and biologically-relevant results in the future. In this sense, to actually confirm our microarray results and explore their full clinical implications, the findings obtained in this analysis (which were only partially replicated by qPCR) require further biological validation by the measurement of protein levels, the targeted assessment of proteins belonging to the same signaling pathways, and replication in independent cohorts. We also did not test for differences in blood cell type composition between the two groups [61], which, if present, could also confound our results to some extent. In addition, we included some patients presenting with highly typical medical and psychiatric comorbidities of CUD in our groups, including those with hypertension, high cholesterol, and non-psychotic psychiatric disorders such as depression and PTSD in our analysis. All of these conditions are thought to be associated with chronic inflammation [62,63]. While we carefully matched the groups on these potential confounders, we did not have the sample size needed to statistically rule out that they may have influenced our results. We are also not able to establish a direction of causality for relationships between anhedonia, CUD and inflammation–in the absence of either longitudinal data or temperament measures of anhedonia that are assumed to be more stable (e.g. [64] we cannot establish whether anhedonia precedes or results from either CUD or inflammation. Another limitation is that our small sample size was not powered to yield FDR-corrected results, raising the possibility type I errors. Although we attempted to protect against this to some extent by taking a bioinformatics approach that tested the likelihood of a pattern of changes appearing, use of larger sample sizes in follow-up studies will be important for replication and validation of our preliminary findings, as well as for allowing a statistical correction for the potential confounding clinical variables described above. Likewise, future analyses would significantly benefit from the inclusion of a healthy control group, allowing for further interpretations of the size of gene expression changes between high and low anhedonia groups and the analysis of the potential interaction between the CUD diagnosis and anhedonia. Last, as discussed earlier, integration of these analyses with genotype data (e.g. by expression quantitative trait loci analyses) will be particularly important to assess for the involvement of genotypic differences.
Conclusions
In summary, we found that the poor prognostic symptom of anhedonia in CUD is associated with changes in peripheral gene expression in pathways related to inflammatory mechanisms and immune response. This is consistent with evidence from other psychiatric disorders suggesting that anhedonia is related to inflammation, and suggests that anti-inflammatory strategies should be explored in treatment-resistant CUD. Our results form part of an exciting new body of research that uses genome-wide changes in expression to highlight novel directions for treatment research, identifying mechanisms that may not have been a focus of past treatment development efforts.
Supporting information
Both IRF1 (A) and GBP5 (B) show statistically significant correlations between both methods (Spearman’s rank order correlation tests) in our sample of cocaine use disorder patients with low (n = 24) and high anhedonia (n = 24).
(TIF)
A and B) Between-group comparison of IRF1 (A) and GBP5 (B) expression values. Dots represent individual values (normalized for the expression of beta-2-microglobulin (B2M) and calculated by the delta delta Ct method) for each subject and lines represent mean ± standard deviation. Comparisons were made with Mann-Whitney U tests. C and D) Spearman’s rank-order correlation between total Snaith-Hamilton Pleasure Scale (SHAPS) scores and the expression of IRF1 (C) and GBP5 (D) measured by quantitative real-time PCR.
(TIFF)
(DOCX)
Data Availability
Microarray data has been deposited into the NCBI GEO database, accession number GSE116833.
Funding Statement
This work was supported by the National Institutes of Health [grant numbers K08 DA040006 to M.W., P50 DA009262 to J.S.]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. (HHS Publication No. SMA 15–4927, NSDUH Series H-50). Retrieved from http://www.samhsa.gov/data/. 2015.
- 2.Degenhardt L, Baxter AJ, Lee YY, Hall W, Sara GE, Johns N, et al. The global epidemiology and burden of psychostimulant dependence: Findings from the Global Burden of Disease Study 2010. Drug Alcohol Depend. 2014;137: 36–47. 10.1016/j.drugalcdep.2013.12.025 [DOI] [PubMed] [Google Scholar]
- 3.Garfield JBB, Lubman DI, Yücel M. Anhedonia in substance use disorders: A systematic review of its nature, course and clinical correlates. Aust N Z J Psychiatry. 2013;48: 36–51. 10.1177/0004867413508455 [DOI] [PubMed] [Google Scholar]
- 4.Hatzigiakoumis DS, Martinotti G, Di Giannantonio M, Janiri L, Giannantonio MD, Janiri L. Anhedonia and Substance Dependence: Clinical Correlates and Treatment Options. Front psychiatry. 2011;2: 10 10.3389/fpsyt.2011.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW. Anhedonia, depressed mood, and smoking cessation outcome. J Consult Clin Psychol. 2014;82: 122–129. 10.1037/a0035046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubman DI, Yücel M, Kettle JWL, Scaffidi A, MacKenzie T, Simmons JG, et al. Responsiveness to drug cues and natural rewards in opiate addiction: Associations with later Heroin use. Arch Gen Psychiatry. American Medical Assn: US; 2009;66: 205–213. 10.1001/archgenpsychiatry.2008.522 [DOI] [PubMed] [Google Scholar]
- 7.Wardle MC, Vincent JN, Suchting R, Green CE, Lane SD, Schmitz JM. Anhedonia Is Associated with Poorer Outcomes in Contingency Management for Cocaine Use Disorder. J Subst Abuse Treat. 2016/09/21. Elsevier Inc.; 2017;72: 32–39. 10.1016/j.jsat.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall SM, Havassy BE, Wasserman DA. Effects of commitment to abstinence, positive moods, stress, and coping on relapse to cocaine use. J Consult Clin Psychol. US: American Psychological Association; 1991;59: 526–532. 10.1037/0022-006X.59.4.526 [DOI] [PubMed] [Google Scholar]
- 9.Felger JC, Lotrich FE. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246: 199–229. 10.1016/j.neuroscience.2013.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: Role of dopamine. Neuropsychopharmacology. Nature Publishing Group; 2017;42: 216–241. 10.1038/npp.2016.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. Nature Publishing Group; 2008;9: 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015/05/20. 2015;300: 141–154. 10.1016/j.neuroscience.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 13.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65: 732–741. 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. 2014;37: 39–46. 10.1016/j.tins.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narvaez JCM, Magalhães P V., Fries GR, Colpo GD, Czepielewski LS, Vianna P et al. Peripheral toxicity in crack cocaine use disorders. Neurosci Lett. Elsevier Ireland Ltd; 2013;544: 80–84. 10.1016/j.neulet.2013.03.045 [DOI] [PubMed] [Google Scholar]
- 16.Moreira FP. Cocaine abuse and effects in the sérum levels of cytokines IL-6 and IL-10. Drug Alcohol Depend. 2015;158: 181–185. 10.1016/j.drugalcdep.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 17.Levandowski ML, Viola TW, Prado CH, Wieck A, Bauer ME, Brietzke E, et al. Distinct behavioral and immunoendocrine parameters during crack cocaine abstinence in women reporting childhood abuse and neglect. Drug Alcohol Depend. 2016;167: 140–148. 10.1016/j.drugalcdep.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 18.Pianca TG, Rosa RL, Ceresér KMM, de Aguiar BW, de Abrahão RC, Lazzari PM, et al. Differences in biomarkers of crack-cocaine adolescent users before/after abstinence. Drug Alcohol Depend. 2017;177: 207–213. 10.1016/j.drugalcdep.2017.03.043 [DOI] [PubMed] [Google Scholar]
- 19.Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, et al. Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol Clin Exp. John Wiley & Sons, Ltd; 2012;27: 156–166. 10.1002/hup.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narendran R, Lopresti BJ, Mason NS, Deuitch L, Paris J, Himes ML, et al. Cocaine abuse in humans is not associated with increased microglial activation: An 18-kDa translocator protein positron emission tomography imaging study with [11C]PBR28. J Neurosci. 2014;34: 9945–9950. 10.1523/JNEUROSCI.0928-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levandowski ML, Viola TW, Wearick-Silva LE, Wieck A, Tractenberg SG, Brietzke E, et al. Early life stress and tumor necrosis factor superfamily in crack cocaine withdrawal. J Psychiatr Res. 2014/03/19. Elsevier Ltd; 2014;53: 180–186. 10.1016/j.jpsychires.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 22.Irwin MR, Olmos L, Wang M, Valladares EM, Motivala SJ, Fong T, et al. Cocaine Dependence and acute cocaine induce decreases of monocyte proinflammatory cytokine expression across the diurnal period: Autonomic mechanisms. J Pharmacol Exp Ther. 2007;320: 507–515. 10.1124/jpet.106.112797 [DOI] [PubMed] [Google Scholar]
- 23.Tylee DS, Kawaguchi DM, Glatt SJ. On the outside, looking in: A review and evaluation of the comparability of blood and brain “-omes.” Am J Med Genet Part B Neuropsychiatr Genet. 2013;162: 595–603. 10.1002/ajmg.b.32150 [DOI] [PubMed] [Google Scholar]
- 24.Fox HC, Milivojevic V, Angarita GA, Stowe R, Sinha R. Peripheral immune system suppression in early abstinent alcohol-dependent individuals: Links to stress and cue-related craving. J Psychopharmacol. 2017/07/05. 2017;31: 883–892. 10.1177/0269881117691455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, Williams JB, Karg RS, Spitzer RL. Strutured clinical interview for DSM-5—Research Version Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JB. Strutured clinical interview for DSM-IV axis I disorders New York: Biometrics Research Department; 1996. [Google Scholar]
- 27.McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. J Nerv Ment Dis. 1980;168: 26–33. [DOI] [PubMed] [Google Scholar]
- 28.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone. The Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995/07/01. 1995;167: 99–103. 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- 29.Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: A psychometric analysis of three anhedonia scales. J Clin Psychol. Wiley Subscription Services, Inc., A Wiley Company; 2006;62: 1545–1558. 10.1002/jclp.20327 [DOI] [PubMed] [Google Scholar]
- 30.Franken IHA, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: Further validation of the Snaith–Hamilton Pleasure Scale (SHAPS). J Affect Disord. 2007;99: 83–89. 10.1016/j.jad.2006.08.020 [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 32.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. Nature Publishing Group; 2008;4: 44. [DOI] [PubMed] [Google Scholar]
- 33.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: Mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012;134: 219–245. 10.1016/j.pharmthera.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 34.Yuferov V, Ji F, Nielsen DA, Levran O, Ho A, Morgello S, et al. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology. 2009;34: 1185–97. 10.1038/npp.2008.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wetherill L, Schuckit MA, Hesselbrock V, Xuei X, Liang T, Dick DM, et al. Neuropeptide Y receptor genes are associated with alcohol dependence, alcohol withdrawal phenotypes, and cocaine dependence. Alcohol Clin Exp Res. 2008;32: 2031–40. 10.1111/j.1530-0277.2008.00790.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong MJ, Stang MT, Liu Y, Gao J, Ren B, Zuckerbraun BS, et al. Interferon Regulatory Factor 1 (IRF-1) induces p21WAF1/CIP1 dependent cell cycle arrest and p21WAF1/CIP1 independent modulation of survivin in cancer cells. Cancer Lett. 2012;319: 56–65. 10.1016/j.canlet.2011.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao J, Senthil M, Ren B, Yan J, Xing Q, Yu J, et al. IRF-1 transcriptionally upregulates PUMA, which mediates the mitochondrial apoptotic pathway in IRF-1-induced apoptosis in cancer cells. Cell Death Differ. 2010;17: 699–709. 10.1038/cdd.2009.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kröger A, Köster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002;22: 5–14. 10.1089/107999002753452610 [DOI] [PubMed] [Google Scholar]
- 39.Jonkman S, Kenny PJ. Molecular, cellular, and structural mechanisms of cocaine addiction: A key role for MicroRNAs. Neuropsychopharmacology. 2013. 10.1038/npp.2012.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF Family of Transcription Factors as Regulators of Host Defense. Annu Rev Immunol. Annual Reviews; 2001;19: 623–655. 10.1146/annurev.immunol.19.1.623 [DOI] [PubMed] [Google Scholar]
- 41.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity. 2000; 10.1016/S1074-7613(00)00053-4 [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi T, Takaoka A. The interferon-α/β system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. Elsevier Current Trends; 2002;14: 111–116. 10.1016/S0952-7915(01)00305-3 [DOI] [PubMed] [Google Scholar]
- 43.Honda K, Takaoka A, Taniguchi T. Type I Inteferon Gene Induction by the Interferon Regulatory Factor Family of Transcription Factors. Immunity. 2006. 10.1016/j.immuni.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 44.Zhang X-J, Jiang D-S, Li H. The interferon regulatory factors as novel potential targets in the treatment of cardiovascular diseases. Br J Pharmacol. 2015; 10.1111/bph.12881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinotti G, Montemitro C, Baroni G, Andreoli S, Alimonti F, Di Nicola M, et al. Relationship between craving and plasma leptin concentrations in patients with cocaine addiction. Psychoneuroendocrinology. Pergamon; 2017;85: 35–41. 10.1016/J.PSYNEUEN.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nature Reviews Rheumatology. 2017. 10.1038/nrrheum.2016.209 [DOI] [PubMed] [Google Scholar]
- 47.Sahu S, Ganguly R, Raman P. Leptin augments recruitment of IRF-1 and CREB to thrombospondin-1 gene promoter in vascular smooth muscle cells in vitro. Am J Physiol Physiol. 2016; 10.1152/ajpcell.00068.2016 [DOI] [PubMed] [Google Scholar]
- 48.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, et al. GBP5 Promotes NLRP3 inflammasome assembly and immunity in mammals. Science (80-). 2012; 10.1126/science.1217141 [DOI] [PubMed] [Google Scholar]
- 49.Schroder K, Tschopp J. The Inflammasomes. Cell. 2010;140: 821–832. 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 50.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336: 481–5. 10.1126/science.1217141 [DOI] [PubMed] [Google Scholar]
- 51.Juszczak GR, Stankiewicz AM. Glucocorticoids, genes and brain function. Prog Neuro-Psychopharmacology Biol Psychiatry. Elsevier; 2017;82: 136–168. 10.1016/j.pnpbp.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 52.Frank MG, Watkins LR, Maier SF. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: Potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 2011;25: S21–S28. 10.1016/j.bbi.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140: 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferguson LB, Ozburn AR, Ponomarev I, Metten P, Reilly M, Crabbe JC, et al. Genome-wide expression profiles drive discovery of novel compounds that reduce binge drinking in mice. Neuropsychopharmacology. Nature Publishing Group; 2017; 10.1038/npp.2017.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niu T, Li J, Wang J, Ma JZ, Li MD. Identification of novel signal transduction, immune function, and oxidative stress genes and pathways by topiramate for treatment of methamphetamine dependence based on secondary outcomes. Front Psychiatry. Frontiers; 2017;8: 271 10.3389/fpsyt.2017.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mariani JJ, Levin FR. Psychostimulant treatment of cocaine dependence. Psychiatr Clin North Am. 2012;35: 425 10.1016/j.psc.2012.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmitz JM, Green CE, Hasan KM, Vincent J, Suchting R, Weaver MF, et al. PPAR-gamma agonist pioglitazone modifies craving intensity and brain white matter integrity in patients with primary cocaine use disorder: a double-blind randomized controlled pilot trial. Addiction. 2017/05/13. 2017;112: 1861–1868. 10.1111/add.13868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ray LA, Roche DJ, Heinzerling K, Shoptaw S. Opportunities for the development of neuroimmune therapies in addiction. Int Rev Neurobiol. 2014/09/02. 2014;118: 381–401. 10.1016/B978-0-12-801284-0.00012-9 [DOI] [PubMed] [Google Scholar]
- 59.Pettorruso M, Spagnolo PA, Leggio L, Janiri L, Di Giannantonio M, Gallimberti L, et al. Repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex may improve symptoms of anhedonia in individuals with cocaine use disorder: A pilot study. Brain Stimul. Elsevier; 2018;11: 1195–1197. 10.1016/J.BRS.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 60.Zhao S, Fung-Leung W-P, Bittner A, Ngo K, Liu X. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. Zhang S-D, editor. PLoS One. 2014;9: e78644 10.1371/journal.pone.0078644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer C, Diehn M, Alizadeh AA, Brown PO. Cell-type specific gene expression profiles of leukocytes in human peripheral blood. BMC Genomics. 2006;7: 115 10.1186/1471-2164-7-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pietri P, Tousoulis CV and D. Inflammation and Arterial Hypertension: From Pathophysiological Links to Risk Prediction [Internet]. Current Medicinal Chemistry. 2015. pp. 2754–2761. 10.2174/0929867322666150420104727 [DOI] [PubMed] [Google Scholar]
- 63.Shapiro MD, Fazio S. From Lipids to Inflammation: New Approaches to Reducing Atherosclerotic Risk. Circ Res. 2016; 10.1161/CIRCRESAHA.115.306471 [DOI] [PubMed] [Google Scholar]
- 64.Martinotti G, Cloninger CR, Janiri L. Temperament and Character Inventory Dimensions and Anhedonia in Detoxified Substance-Dependent Subjects. Am J Drug Alcohol Abuse. 2008;34: 177–183. 10.1080/00952990701877078 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Both IRF1 (A) and GBP5 (B) show statistically significant correlations between both methods (Spearman’s rank order correlation tests) in our sample of cocaine use disorder patients with low (n = 24) and high anhedonia (n = 24).
(TIF)
A and B) Between-group comparison of IRF1 (A) and GBP5 (B) expression values. Dots represent individual values (normalized for the expression of beta-2-microglobulin (B2M) and calculated by the delta delta Ct method) for each subject and lines represent mean ± standard deviation. Comparisons were made with Mann-Whitney U tests. C and D) Spearman’s rank-order correlation between total Snaith-Hamilton Pleasure Scale (SHAPS) scores and the expression of IRF1 (C) and GBP5 (D) measured by quantitative real-time PCR.
(TIFF)
(DOCX)
Data Availability Statement
Microarray data has been deposited into the NCBI GEO database, accession number GSE116833.