Abstract
Background
The anti-programmed death receptor-1 (anti–PD-1) pembrolizumab is approved as first-line monotherapy for metastatic non-small cell lung cancer (mNSCLC) with PD-ligand 1 (PD-L1) tumor expression ≥50%. Most studies comparing PD-L1 results by immunohistochemistry (IHC) assay type have been conducted by prespecified and, in most cases, highly experienced, trained pathologists; however, knowledge is limited regarding the current use and concordance of PD-L1 assays in the real-world clinical setting. Our aim was to study the distribution of PD-L1 tumor expression by IHC assay type among patients with mNSCLC in US oncology practices.
Methods
This retrospective observational study utilized de-identified, longitudinal data from a large US electronic medical record database. Eligible patients were adults (≥18 years) with histologically/cytologically confirmed initial diagnosis of metastatic or recurrent NSCLC from October 2015 through December 2017. We determined PD-L1 testing trends and distribution of PD-L1 tumor expression (percentage of tumor cells staining for PD-L1) by IHC assay type.
Results
The 12,574 eligible patients (mean age, 69 years) included 6,620 (53%) men and 86% with positive smoking history. Of 4,868 evaluable tests, 3,799 (78%), 195 (4%), 165 (3%), and 709 (15%) used the Agilent 22C3 pharmDx, Agilent 28–8 pharmDx, Ventana PD-L1 (SP142) Assay, and laboratory-developed tests (LDTs, including SP263), respectively. The percentages of tests scoring PD-L1 tumor expression of ≥50% were 33%, 32%, 10%, and 23%, respectively. Measured PD-L1 tumor expression varied across the four assay types (χ2 p < 0.001) and across three assay types excluding SP142 (p < 0.001), with no significant difference between 22C3 and 28–8 assays (p = 0.96). The PD-L1 testing rate increased from 18% in the fourth quarter of 2015 to 71% in the fourth quarter of 2017.
Conclusions
In the real-world clinical setting, we observed that measured PD-L1 tumor expression is concordant using the 22C3 and 28–8 assays; however, the SP142 assay and LDTs appear discordant and could underestimate high PD-L1 positivity. Further study is needed to evaluate the association between PD-L1 tumor expression and response to therapy.
Introduction
The introduction of anti-programmed death receptor-1 (anti-PD-1) antibodies, pembrolizumab and nivolumab, and the anti-programmed death-ligand 1 (anti-PD-L1) antibodies, such as atezolizumab and durvalumab, has dramatically changed the treatment landscape for non-small cell lung cancer (NSCLC). Currently in the US, pembrolizumab is the only one of these agents approved by the US Food and Drug Administration (FDA) for first-line monotherapy of metastatic NSCLC (for tumors negative for epidermal growth factor receptor/anaplastic lymphoma kinase [EGFR/ALK] genomic alterations). In the second line setting, pembrolizumab, nivolumab, and atezolizumab are all approved for advanced and metastatic NSCLC therapy upon disease progression after platinum-based chemotherapy and treatment for EGFR/ALK genomic alterations, if indicated.
Pembrolizumab therapy is approved in conjunction with a companion diagnostic, the PD-L1 IHC 22C3 pharmDx test (Agilent Technologies, Carpinteria, CA), for identifying those patients who are likely to benefit from pembrolizumab [1–5]. As first-line therapy, pembrolizumab is approved as monotherapy for metastatic NSCLC with high tumor expression of PD-L1 (tumor proportion score [TPS] of ≥50%), and in combination with pemetrexed and carboplatin for metastatic NSCLC with nonsquamous histology regardless of PD-L1 expression level. In addition, pembrolizumab is approved as second-line monotherapy for advanced or metastatic NSCLC with PD-L1 TPS ≥1%. However, testing for PD-L1 tumor expression is not a labeled requirement for use of nivolumab and atezolizumab, although FDA-approved drug-specific complementary PD-L1 diagnostic assays were developed for use in clinical trials, namely, the Agilent PD-L1 IHC 28–8 pharmDx for nivolumab and the Ventana PD-L1 (SP142) Assay (Ventana Medical Systems, Tucson, AZ) for atezolizumab [6–9]. Another available PD-L1 assay is the Ventana PD-L1 (SP263) Assay, developed for use with durvalumab, a PD-L1 antibody FDA-approved for treating urethelial carcinoma and unresectable Stage III NSCLC. In addition, some centers are using laboratory-developed tests (LDTs) for PD-L1 that employ various combinations of primary antibodies and IHC autostainer platforms [6,10,11].
The utilization of different PD-L1 IHC assays, as well as the different PD-L1 tumor expression cut-points used in clinical trials, raised concerns in the oncology community as early as 2015 regarding the concordance among these assays and the optimal approach for measuring PD-L1 [12–14]. The Blueprint PD-L1 IHC Assay Comparison Project is an industrial-academic partnership seeking to harmonize IHC PD-L1 testing [10]. Results of Blueprint phase 1, a feasibility study evaluating the analytical comparability of four assays (22C3, 28–8, SP263, and SP142) assessed independently by three experts, indicated that PD-L1 tumor expression (cell staining) was concordant for the 22C3, 28–8, and SP263 assays, while the SP142 assay consistently showed fewer stained tumor cells [10]. The Blueprint study phase 2 confirmed the findings of phase 1 using real-world routine clinical samples from 81 patients, with concordant results as read by 25 trained pathologists [15]. Several other validation studies have reported similar findings [6,11,16–19].
Recent evidence suggests, however, that, while the 22C3 and 28–8 assays may be interchangeable, potential differences can occur among laboratories and individual pathologists in applying specific PD-L1 cut-points, particularly when using LDTs that are not properly validated [11,18] and particularly at lower cut-points [16,17]. The need to standardize PD-L1 testing is highly relevant in the clinical setting, where clinicians must choose the optimal treatment regimen for their patients with NSCLC. Evidence from a recent meta-analysis indicates improved overall response rates with anti-PD-1/anti-PD-L1 therapy in NSCLC when used to treat chemotherapy-naïve patients as compared with patients previously treated with chemotherapy [20], highlighting the importance of identifying patients who will benefit from first-line anti-PD-1/anti-PD-L1 therapy.
Prior studies comparing PD-L1 results by IHC assay type have been conducted by prespecified and, in most cases, highly experienced, trained pathologists. However, knowledge is limited regarding the current use and concordance of PD-L1 assays in the real-world clinical setting. The objectives of this retrospective study were to study the distribution of PD-L1 tumor expression by assay type among patients with metastatic NSCLC treated at US oncology practices and to assess recent PD-L1 testing patterns.
Methods
Data source
This was a retrospective cohort study utilizing longitudinal electronic health record data from the Flatiron Health database [21]. At the time of our study, this database included de-identified data from active records of more than 2 million patients with cancer in the United States. Both structured and unstructured patient-level data are included in the Flatiron database, which is refreshed monthly. The structured data include laboratory values, limited biomarker information, and prescribed drugs, while unstructured data include information taken from unstructured documents, such as physician’s notes in the medical record and detailed biomarker, radiology, and pathology reports.
Study design and patient population
The population for this study was derived from patients with advanced NSCLC represented in the Flatiron Health database, which has the following inclusion criteria: (1) diagnosis of advanced NSCLC, confirmed via review of unstructured documentation, and (2) at least two clinical visits documented in the Flatiron dataset on or after January 1, 2011.
Patients who received an initial diagnosis of metastatic or recurrent NSCLC from October 2, 2015 (the first US FDA approval date for pembrolizumab in second-line treatment of metastatic NSCLC with PD-L1 TPS ≥50% [22]), through December 31, 2017 (most recent data cut from Flatiron Health at the time of this analysis), were included in the study. Eligible patients were 18 years or older at the time of diagnosis, which was histologically or cytologically confirmed. Patients were excluded if they had a PD-L1 tumor expression test date recorded before October 2, 2015.
This was a non-interventional study using anonymized data; therefore, informed consent was not possible or necessary. No patient identifying information was accessible during the study, and the data in the Flatiron Health database are protected against breach of confidentiality [21].
Study outcome measures
We evaluated PD-L1 testing information for all eligible patients, including the IHC assay type and the distribution of PD-L1 tumor expression by IHC assay type. The assays included were three FDA-approved IHC assays (Agilent 22C3 pharmDx, Agilent 28–8 pharmDx, Ventana PD-L1 SP142 assay) and all LDTs pooled (including Ventana SP263). We defined the test date as the results date, and PD-L1 tumor expression as the percentage of tumor cells staining for PD-L1. At the time of the study, the database did not capture results for immune cell staining seen with the SP142 assay. Patients could have more than one PD-L1 test and/or assay type.
Statistical analyses
We used descriptive statistics to summarize patient demographic and clinical characteristics at baseline and the percentage of patients tested, by PD-L1 assay type. For patient baseline characteristics, continuous and count variables were compared using a t test, and binary and categorical variables were compared using the χ2 test.
The χ2 test was used to compare PD-L1 tumor expression, categorized as <1%, 1–49%, and ≥50%, (1) across four assay types including the SP142, (2) across three assay types (22C3, 28–8, and LDTs, which also included the SP263) excluding the SP142, and (3) between two assay types (22C3 and 28–8).
Statistical analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC).
Results
Patients
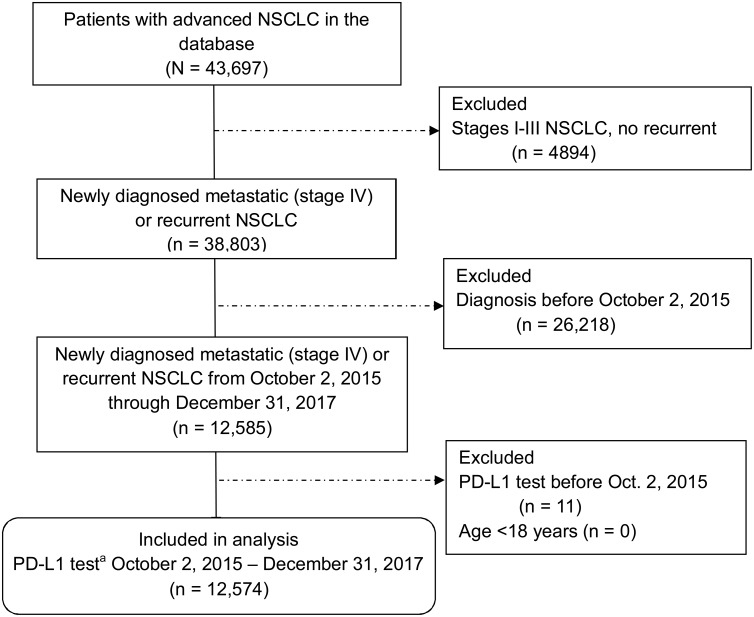
Of 12,585 patients with metastatic or recurrent NSCLC diagnosis on or after October 2, 2015, through December 31, 2017, we identified 12,574 (>99%) patients eligible for the study (Fig 1), ranging in age from 20–85 years (mean 69 years) and including 6620 (53%) men (Table 1). Overall, 9048 (72%) patients had nonsquamous NSCLC, 2821 (22%) had squamous NSCLC, and 705 (6%) had NSCLC not otherwise specified (NOS).
Fig 1. Selection of eligible patients with metastatic or recurrent non-small cell lung cancer from the database.
NSCLC, non–small-cell lung cancer; PD-L1, programmed death-ligand 1. aAll testing for PD-L1 was required to be on or after October 2, 2015; however, patients were not required to have a PD-L1 test to be eligible for the study.
Table 1. Baseline demographic and clinical characteristics of patients with metastatic or recurrent NSCLC by PD-L1 testing status.
| Characteristic | All patients (N = 12,574) |
Tested for PD-L1 (n = 6024) |
Not tested for PD-L1 (n = 6550) |
p Valuea |
|---|---|---|---|---|
| Age at metastatic diagnosis, years | ||||
| Mean (SD) | 69.2 (10.0) | 68.8 (10.3) | 69.5 (9.8) | <0.001 |
| ≤65 years | 4317 (34.3) | 2163 (35.9) | 2154 (32.9) | <0.001 |
| >65 years | 8257 (65.7) | 3861 (64.1) | 4396 (67.1) | |
| Male sex | 6620 (52.6) | 3127 (51.9) | 3493 (53.3) | 0.11 |
| Race, data availableb | 10,945 (87.0) | 5238 (87.0) | 5707 (87.1) | |
| White | 8364 (76.4) | 3952 (75.4) | 4412 (77.3) | 0.15 |
| Black | 1035 (9.5) | 509 (9.7) | 526 (9.2) | |
| Asian | 357 (3.3) | 183 (3.5) | 174 (3.0) | |
| Other | 1189 (10.9) | 594 (11.3) | 595 (10.4) | |
| Smoking history, data availableb | 12,484 (99.3) | 5993 (99.5) | 6491 (99.1) | |
| Positive smoking history | 10,778 (86.3) | 5105 (85.2) | 5673 (87.4) | <0.001 |
| Stage available at first diagnosisb | 12,123 (96.4) | 5868 (97.4) | 6255 (95.5) | |
| Stage II or lower | 1768 (14.6) | 679 (11.6) | 1089 (17.4) | <0.001 |
| Stage III/IIIA | 1133 (9.3) | 349 (5.9) | 784 (12.5) | |
| Stage IV | 9222 (76.1) | 4840 (82.5) | 4382 (70.1) | |
| Histology | ||||
| Nonsquamous cell carcinoma | 9048 (72.0) | 4,596 (76.3) | 4452 (68.0) | <0.001 |
| Squamous cell carcinoma | 2821 (22.4) | 1144 (19.0) | 1677 (25.6) | |
| NSCLC NOS | 705 (5.6) | 284 (4.7) | 421 (6.4) | |
| Tested for EGFR/ALK | 8870 (70.5) | 5214 (86.6) | 3656 (55.8) | <0.001 |
| Practice type | ||||
| Academic | 1159 (9.2) | 555 (9.2) | 604 (9.2) | 0.99 |
| Community | 11,415 (90.8) | 5469 (90.8) | 5946 (90.8) |
Data presented as No. (%) unless otherwise noted. Percentages may not add up to 100% because of rounding. ALK, anaplastic lymphoma kinase rearrangement; EGFR, epidermal growth factor receptor mutation; NSCLC NOS, non-small cell lung cancer, not otherwise specified; PD-L1, programmed death-ligand 1.
at test used for continuous and count variables, and χ2 test for binary and categorical variables, comparing patients tested vs. not tested for PD-L1.
bReported percentages for race, smoking history, and stage pertain to patients with available data.
A total of 6024 (48%) patients had one or more tests for PD-L1 at any time from October 2, 2015, through December 31, 2017; the remaining 6550 (52%) patients in the study cohort were not tested for PD-L1 status. Patients whose NSCLC was tested for PD-L1 were more likely to have NSCLC initially diagnosed at stage IV (83% vs. 70% of those not tested; p < 0.001), of nonsquamous histology (76% vs. 68%; p < 0.001), and tested for EGFR mutation and ALK rearrangement (87% vs. 56%; p < 0.001; Table 1). Other statistically significant differences between patients who were tested for PD-L1 and those not tested appeared not to be clinically significant, for example, 36% vs. 33% of those with vs. without PD-L1 test, respectively, were age 65 years or younger.
PD-L1 results by assay type
The 6024 patients had a total of 7031 PD-L1 tests, of which 4868 tests (69%) were included in the analyses, and 2163 tests (31%) were excluded because of missing assay type (n = 749), missing PD-L1 tumor expression data (n = 645), or missing assay type and results (n = 769). The 22C3 assay was the most common one used, comprising 78% of available assay results (Table 2). The LDTs, taken as a group, were the second most common assays used (15%), followed by the 28–8 assay (4%), and, least commonly, the SP142 assay (3%; Table 2).
Table 2. PD-L1 biomarker immunohistochemical (IHC) assay results by assay typea.
| PD-L1 tumor expression, categorized | FDA-approved IHC assay | LDTsc (n = 709) |
||
|---|---|---|---|---|
| 22C3 (n = 3799) |
28–8 (n = 195) |
SP142b (n = 165) |
||
| <1% | 1469 (38.7) | 76 (39.0) | 96 (58.2) | 260 (36.7) |
| 1–49% | 1085 (28.6) | 57 (29.2) | 52 (31.5) | 289 (40.8) |
| ≥50% | 1245 (32.8) | 62 (31.8) | 17 (10.3) | 160 (22.6) |
Data presented as No. (%). Some patients had more than one test and are represented in more than one column. FDA, Food and Drug Administration; LDT, laboratory-developed test; PD-L1, programmed death-ligand 1.
aχ2 test: (1) p < 0.001 for comparing results across all 4 assay types: 22C3, 28–8, SP142, and LDTs (including the SP263); (2) p < 0.001 comparing results across three assay types (22C3, 28–8, and LDTs), excluding the SP142 assay; (3) p = 0.96 comparing results between 22C3 and 28–8.
bSP142 results represent the percentage of tumor cells staining for PD-L1 (immune cell staining not included).
cLDTs included 45 tests using the SP263, with 23 (51%), 13 (29%), and 9 (20%) showing PD-L1 tumor expression of <1%, 1–49%, and ≥50%, respectively.
Measured PD-L1 tumor expression, categorized as <1%, 1–49%, and ≥50%, varied significantly across the four assay types (χ2 p < 0.001) and across three assay types excluding the SP142 assay (χ2 p < 0.001). However, there was no significant difference between the 22C3 and 28–8 assays for PD-L1 tumor expression as categorized (χ2 p = 0.96; Table 2). A PD-L1 tumor expression of ≥50% was recorded approximately one-third of the time with the 22C3 and 28–8 assays, less frequently with LDTs (23%), and 10% of the time with the SP142. Of the 45 tests using the SP263 assay, 9 (20%) had recorded PD-L1 tumor expression ≥50% (results included with LDTs; see Table 2).
PD-L1 testing trends
The percentage of patients whose NSCLC was tested for PD-L1 increased during the study period from 18% in the fourth quarter of 2015 to 71% in the third quarter of 2017 (Table 3). The 22C3 was the most common assay used in each quarter, with LDTs representing the second largest category. Assay type was not recorded for approximately 20% of tests in each quarter (Table 3).
Table 3. PD-L1 testing trends over time, by assay type.
| Year and Quarter | No. Patients | Patients Tested, No. (% patients) | Number of PD-L1 Tests | PD-L1 IHC Assay Type,a No. (% assays) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 22C3 Assay | 28–8 Assay | SP-142 Assay | SP-263 Assay | LDT | Unknown Assay Type | ||||
| 2015Q4 | 1511 | 275 (18.2) | 326 | 127 (39.0) | 17 (5.2) | 3 (0.9) | 2 (0.6) | 91 (27.9) | 86 (26.4) |
| 2016Q1 | 1716 | 364 (21.2) | 411 | 207 (50.4) | 15 (3.6) | 5 (1.2) | 3 (0.7) | 74 (18.0) | 107 (26.0) |
| 2016Q2 | 1532 | 384 (25.1) | 443 | 239 (54.0) | 22 (5.0) | 8 (1.8) | 1 (0.2) | 68 (15.3) | 105 (23.7) |
| 2016Q3 | 1625 | 563 (34.6) | 680 | 372 (54.7) | 35 (5.1) | 13 (1.9) | 4 (0.6) | 106 (15.6) | 150 (22.1) |
| 2016Q4 | 1425 | 881 (61.8) | 1066 | 579 (54.3) | 37 (3.5) | 55 (5.2) | 7 (0.7) | 149 (14.0) | 239 (22.4) |
| 2017Q1 | 1435 | 1049 (73.1) | 1247 | 773 (62.0) | 40 (3.2) | 51 (4.1) | 10 (0.8) | 141 (11.3) | 232 (18.6) |
| 2017Q2 | 1365 | 1043 (76.4) | 1197 | 791 (66.1) | 28 (2.3) | 18 (1.5) | 8 (0.7) | 97 (8.1) | 255 (21.3) |
| 2017Q3 | 1229 | 940 (76.5) | 1071 | 686 (64.1) | 21 (2.0) | 16 (1.5) | 19 (1.8) | 97 (9.1) | 232 (21.7) |
| 2017Q4 | 736 | 525 (71.3) | 590 | 402 (68.1) | 9 (1.5) | 3 (0.5) | 11 (1.9) | 53 (9.0) | 112 (19.0) |
| Total | N = 12,574 | N = 6024 (47.9) | N = 7031 | N = 4176 (59.4) | N = 224 (3.2) | N = 172 (2.4) | N = 65 (0.9) | N = 876 (12.5) | N = 1518 (21.6) |
IHC, immunohistochemical; LDT, laboratory-developed test; PD-L1, programmed death-ligand 1; Q, quarter.
aSome patients had more than one test and/or type of assay and could be represented in more than one assay type column.
Discussion
We found no significant difference in measured PD-L1 tumor expression between the 22C3, the most common assay type, and the 28–8 assay, in this observational study of PD-L1 testing patterns for patients with metastatic or recurrent NSCLC at US oncology practices. These findings indicate that the 22C3 and 28–8 can be used interchangeably for measuring PD-L1 tumor expression in NSCLC. Most importantly, however, the results of LDTs and particularly the SP142 assay appeared discordant, with proportionately fewer PD-L1 tumor expression results of ≥50%, suggesting that some cases of NSCLC with high PD-L1 tumor expression could have been missed.
Recent study results have indicated that pembrolizumab administered in the first-line setting may be more effective than in the second-line or later setting, as demonstrated in the KEYNOTE-024 trial and a recent large meta-analysis [3,20]. Therefore, in the clinical setting, correctly identifying patients with NSCLC PD-L1 tumor expression score ≥50% and thus eligible for first-line therapy with pembrolizumab is imperative.
Our study evaluated concordance of PD-L1 assays in real-world oncology practice where most patients are treated. This enabled us to include almost 5000 NSCLC samples, a much higher number than those examined in prior quality-controlled, laboratory-based comparative studies (from 21 to 493 samples) [6,10,11,15–19,23,24]. Our findings support the results of prior work, including the Blueprint study phases 1 and 2, which found that the SP142 consistently underperforms as compared with the 22C3 and the 28–8 [10,15].
We included the SP263 in the LDT category because the SP263 was not approved for use in NSCLC in the US during the study. Only 45 tests used the SP263, too few from which to draw conclusions. In Blueprint phases 1 and 2 [10,15], as well as in other prior studies [11,16–18], the results of the SP263 were considered to be aligned with those of the 22C3 and/or the 28–8, while in two studies, the SP263 stained more tumor cells on some samples relative to the other assays [19,24].
We found that LDTs constituted a substantial proportion of all PD-L1 assays (15%) included in the database. The overall percentage of tumors with measurable PD-L1 tumor expression (≥1%) using LDTs (63%) was similar to that using the 22C3 (61%) and the 28–8 (61%); however, the proportion with PD-L1 ≥50% was much lower (23% vs. 33% and 32%, respectively), suggesting that the LDTs as a group could be less sensitive IHC assays. Two recent studies examining interlaboratory and interassay concordance both found that locally developed LDTs produced variable results, some aligned and some not aligned with those of the 22C3, 28–8, and SP263 [11,18]. Other studies report good concordance among the 22C3 and 28–8 and specific validated LDTs [23,25]. These results together with ours suggest that all LDTs should be carefully validated and that clinicians should exercise caution in relying on LDTs for PD-L1 IHC assays.
The implementation of NSCLC testing recommendations in the clinical setting can be challenging, particularly as NSCLC guideline recommendations have expanded to testing for molecular biomarkers other than EGFR/ALK genomic alterations, including ROS1 and BRAF, in addition to PD-L1 [26,27]. Primary lung tumors often have low tumor cellularity, and obtaining adequate tissue quantity and quality for both histological subtyping of NSCLC as well as molecular and biomarker testing may be difficult [27].
Strengths of this observational study include the large sample size, the very recent, up-to-date findings, and the use of a large, well-maintained database drawing on longitudinal medical records from a geographically diverse patient population in the US [21]. Our results reflect current clinical practice in the US, with PD-L1 readings by numerous different pathologists. Not surprisingly, we found that the percentage of patients whose NSCLC was tested for PD-L1 increased substantially from the fourth quarter of 2015 through the fourth quarter of 2017.
Several limitations of this study should be considered in interpreting our analyses. The Flatiron data primarily reflect real-world clinical practices at community oncology centers (~90% of patients in this study), with fewer patients seen or beginning treatment at academic medical centers, for example. We lacked some information that would have been useful to assess the generalizability of our findings, including the types of testing laboratories, handling of diagnostic material, and the specimens themselves—whether histologic or cytologic, and whether newly obtained or archived specimens. Moreover, data were missing regarding assay type or results for 31% of the recorded tests. Finally, the study dataset did not include response data associated with the PD-L1 test results.
Continuing follow-up over time with more complete characterization of PD-L1 test specimens will be important to supplement our findings. The rapid increase in rates of PD-L1 testing, as observed in this study, will facilitate future study of the impact of PD-L1 assay type on the reported level of PD-L1 tumor expression. In addition, information about patient outcomes associated with PD-L1 tumor expression levels and chosen therapies in the real-world setting is of great interest. Other areas for further research include identifying and addressing barriers to PD-L1 testing in clinical practice.
In conclusion, our findings suggest that the 22C3 and 28–8 assays can be used interchangeably for measuring PD-L1 tumor expression in metastatic or recurrent NSCLC. Instead, use of the SP142 assay or an LDT for IHC PD-L1 screening is associated with less reliable capture of PD-L1 positivity, particularly at the higher cut-point of ≥50% PD-L1 tumor expression. This could result in a missed opportunity for first-line monotherapy with an anti-PD-1/anti-PD-L1 agent and optimizing outcomes for patients with metastatic NSCLC.
Acknowledgments
We gratefully acknowledge the help of Qian Xia (Merck & Co., Inc., Kenilworth, NJ, USA) for providing statistical programming support. Medical writing and editorial assistance was provided by Elizabeth V. Hillyer, DVM. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Data Availability
These are third-party data from Flatiron Health database. Flatiron Health is subject to the requirements of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) including appropriate de-identification. Practically speaking, in order to share select data and data elements in adherence with the PLOS ONE policies on sharing data and materials, it will be necessary to first define the methods of storage, transmission, access rights, and scope of intended use prior to making any such data available, for the purposes of compliance with de-identification and data protection specifications and requirements under HIPAA. The authors do not have permission to give public access to the study dataset. Please refer any questions or requests regarding data used in this manuscript to Melissa Tucker (mtucker@flatiron.com) and include Dr. Amy Abernethy (amy@flatiron.com) on the email request. The authors accessed the data in the same way as the instructions provided and they did not have any special access privileges that others would not have.
Funding Statement
This study was funded by Merck & Co., Inc., Kenilworth NJ, USA. The funder of the study participated in the study design, data analysis, decision to publish, and preparation of the manuscript. The funder also provided support in the form of salaries for authors PDP, FXL, XC, XC, and TB. All authors, including those employed by Merck, participated in the data interpretation and writing of the report. All authors had full access to study results and had final responsibility for the decision to submit for publication.
References
- 1.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 4.Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016;2:1217–22. 10.1001/jamaoncol.2016.0639 [DOI] [PubMed] [Google Scholar]
- 5.Khunger M, Hernandez AV, Pasupuleti V, Rakshit S, Pennell NA, Stevenson J, et al. Programmed cell death 1 (PD-1) ligand (PD-L1) expression in solid tumors as a predictive biomarker of benefit from PD-1/PD-L1 axis inhibitors: A systematic review and meta-analysis. JCO Precis Oncol. 2017;1:1–15. 10.1200/PO.16.00030 [DOI] [PubMed] [Google Scholar]
- 6.Buttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35:3867–76. 10.1200/JCO.2017.74.7642 [DOI] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208–22. 10.1016/j.jtho.2016.11.2228 [DOI] [PubMed] [Google Scholar]
- 11.Adam J, Le Stang N, Rouquette I, Cazes A, Badoual C, Pinot-Roussel H, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol. 2018;29:953–8. 10.1093/annonc/mdy014 [DOI] [PubMed] [Google Scholar]
- 12.Kerr KM, Tsao MS, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR. Programmed death-ligand 1 immunohistochemistry in lung cancer: In what state is this art? J Thorac Oncol. 2015;10:985–9. 10.1097/JTO.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2:46–54. 10.1001/jamaoncol.2015.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in lung cancer. J Thorac Oncol. 2016;11:964–75. 10.1016/j.jtho.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsao M, Kerr K, Yatabe Y, Hirsch FR. PL 03.03 Blueprint 2: PD-L1 immunohistochemistry comparability study in real-life, clinical samples. J Thorac Oncol. 2017;12:S1606 Abstract. 10.1016/j.jtho.2017.09.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchetti A, Barberis M, Franco R, De Luca G, Pace MV, Staibano S, et al. Multicenter comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J Thorac Oncol. 2017;12:1654–63. 10.1016/j.jtho.2017.07.031 [DOI] [PubMed] [Google Scholar]
- 17.Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scott M, Scorer P, et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res. 2017;23:3585–91. 10.1158/1078-0432.CCR-16-2375 [DOI] [PubMed] [Google Scholar]
- 18.Scheel AH, Baenfer G, Baretton G, Dietel M, Diezko R, Henkel T, et al. Interlaboratory concordance of PD-L1 immunohistochemistry for non-small-cell lung cancer. Histopathology. 2018;72:449–59. 10.1111/his.13375 [DOI] [PubMed] [Google Scholar]
- 19.Hendry S, Byrne DJ, Wright GM, Young RJ, Sturrock S, Cooper WA, et al. Comparison of four PD-L1 immunohistochemical assays in lung cancer. J Thorac Oncol. 2018;13:367–76. 10.1016/j.jtho.2017.11.112 [DOI] [PubMed] [Google Scholar]
- 20.Khunger M, Jain P, Rakshit S, Pasupuleti V, Hernandez AV, Stevenson J, et al. Safety and efficacy of PD-1/PD-L1 inhibitors in treatment naïve and chemotherapy refractory patients with non-small cell lung cancer: a systematic review and meta-analysis. Clin Lung Cancer. 2018;19:e335–e48. 10.1016/j.cllc.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 21.Flatiron Health. Flatiron Health database. https://flatiron.com/real-world-evidence/.
- 22.Pai-Scherf L, Blumenthal GM, Li H, Subramaniam S, Mishra-Kalyani PS, He K, et al. FDA approval summary: Pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist. 2017;22:1392–9. 10.1634/theoncologist.2017-0078. 10.1634/theoncologist.2017-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3:1051–8. 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheel AH, Dietel M, Heukamp LC, Johrens K, Kirchner T, Reu S, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol. 2016;29:1165–72. 10.1038/modpathol.2016.117 [DOI] [PubMed] [Google Scholar]
- 25.Ilie M, Juco J, Huang L, Hofman V, Khambata-Ford S, Hofman P. Use of the 22C3 anti-programmed death-ligand 1 antibody to determine programmed death-ligand 1 expression in cytology samples obtained from non-small cell lung cancer patients. Cancer Cytopathol. 2018;126:264–74. 10.1002/cncy.21977 [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer, version 2.2108 (updated December 19, 2017). http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiley CT, Le Quesne J, Santis G, Sharpe R, de Castro DG, Middleton G, et al. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet. 2016;388:1002–11. 10.1016/S0140-6736(16)31340-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These are third-party data from Flatiron Health database. Flatiron Health is subject to the requirements of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) including appropriate de-identification. Practically speaking, in order to share select data and data elements in adherence with the PLOS ONE policies on sharing data and materials, it will be necessary to first define the methods of storage, transmission, access rights, and scope of intended use prior to making any such data available, for the purposes of compliance with de-identification and data protection specifications and requirements under HIPAA. The authors do not have permission to give public access to the study dataset. Please refer any questions or requests regarding data used in this manuscript to Melissa Tucker (mtucker@flatiron.com) and include Dr. Amy Abernethy (amy@flatiron.com) on the email request. The authors accessed the data in the same way as the instructions provided and they did not have any special access privileges that others would not have.