Introduction
Seasonal cyclicity is a ubiquitous feature of acute infectious diseases [1] and may be a ubiquitous feature of human infectious diseases in general, as illustrated in Tables 1–4. Each acute infectious disease has its own seasonal window of occurrence, which, importantly, may vary among geographic locations and differ from other diseases within the same location. Here we explore the concept of an epidemic calendar, which is the idea that seasonality is a unifying feature of epidemic-prone diseases and, in the absence of control measures, the local calendar can be marked by epidemics (Fig 1). A well-known example of a calendar marked by epidemics is that of the Northern Hemisphere, where influenza outbreaks occur each winter [2, 3] (hence the colloquial reference to winter as "the flu season"). In contrast, chickenpox outbreaks peak each spring [4, 5], and polio transmission historically occurred each summer [6].
Table 1. Seasonal drivers of human infectious diseases.
Drivers categorized as being related to (a) vector seasonality, (b) seasonality in nonhuman animal host (i.e., livestock, other domestic animals, or wildlife), (c) seasonal climate (e.g., temperature, precipitation, etc.), (d) seasonal nonclimatic abiotic environment (e.g., water salinity), (e) seasonal co-infection, (f) seasonal exposure and/or behavior and/or contact rate, (g) seasonal biotic environment (e.g., algal density in waterbodies).
| Infection/disease | Type | Seasonal driver(s) | Description |
|---|---|---|---|
| African sleeping sickness | Chronic | a | Tsetse fly distribution changes seasonally; expanded range during rainy season [7] |
| Anthrax | Acute | b | Zoonotic disease with seasonality reported in wildlife and livestock; seasonality varies among location and species [8] |
| Avian influenza | Acute | b | Winter in both humans and poultry (in Asia) [9, 10] |
| Bacterial Pneumonia | Acute | c, d, and e | Peaks in midwinter (in the US); it is associated with influenza [11] |
| Brucellosis | Acute | b | Spring and summer in wildlife and livestock; the timing relates to the birthing season; peaks in the summer in humans [12] |
| Buruli ulcer | Chronic | c | Varies by location; some studies have not observed seasonality [13] |
| Chagas disease | Acute and chronic | a | Peaks in spring and summer in countries with distinct seasons [14] |
| Chickenpox | acute | f | Peak in spring in the Northern and Southern Hemisphere [15] |
| Chikungunya | Acute | a | Rainy season when vector density peaks. [16] |
| Cholera | Acute | c, d, and g | Seasonality is stronger in countries further from the equator; outbreaks generally occur in warm months [17] |
| Crimean-Congo hemorrhagic fever | Acute | a | Seropositivity in livestock correlates with seasonal changes in tick parasitism; human cases correlate with livestock seropositivity [18] |
| Cryptosporidium | Acute | c | Increased risk of cryptosporidium associated with high ambient temperature and high rainfall [19] |
| Cutaneous leishmaniasis | Acute and chronic | a and b | Strong seasonal variation with elevated incidence from October to March (in Tunisia). Seasonality may be due to climate effects on the vector: blood-feeding sand flies [20] |
| Dengue fever | Acute | a | Rainy season (in Thailand) [21] |
| Diphtheria | Acute | f | Spring and summer (in Portugal) [22] |
| Dracunculiasis | Chronic | c, d, f, and g | Dry season (in Nigeria) [23] |
| Ebola | Acute | b | In wildlife the peak is in the dry season (in Gabon) [24] |
| Echinococcosis | Chronic | b | Exposure to livestock carrying the infection is seasonal [25] |
| Escherichia coli (pathogenic) | Acute | b | Seasonal in cattle; cattle are a source for human infection [26] |
| Foodborne trematodiases | Chronic | f | Exposure is seasonal due to seasonal ingestion of infected snails [27] |
| Genital herpes | Chronic | f | Elevated incidence in spring/summer and lower in winter (in the US) [28] |
| Gonorrhea | Chronic | f | Peak cases in the summer and autumn (in the US) [28] |
Since seasonal timing may differ among geographic areas, study location is indicated in parentheses.
Table 4. Seasonality of human infectious diseases (continued from Tables 1–3).
Drivers categorized as being related to (a) vector seasonality, (c) seasonal climate (e.g., temperature, precipitation, etc.), (h) seasonal flare-up/symptoms and/or remission/latency, (i) observed seasonal incidence with no hypotheses regarding drivers.
| Infection/disease | Type | Seasonal driver(s) | Description |
|---|---|---|---|
| TB | Chronic | c and h | Approximately 24% more TB notifications in the summer verses the winter (in the UK) [71] |
| Typhoid fever | Acute | i and c* | Peaks around July (in China) [72] |
| Viral meningitis | Acute | i | Higher in the summer, when enterovirus transmission peaks (in Israel) [73] |
| West Nile virus | Acute | a and c | Peaks July through August in the temperate zones of the Northern Hemisphere [74] |
| Yaws | Chronic | h | More cases in the wet season; hypothesized to be due to more clinical relapse during the wet season; transmission may be relatively constant throughout the year [75] |
| Yellow fever | Acute | a and c | Seasonal changes in the distribution and density of the vector Aedes aegypti; transmission peak was historically in autumn (in the Americas) [76] |
| Zika | Acute | a and c | Seasonal changes in incidence are expected to be driven by seasonal fluctuations in the vector population (the A. aegypti mosquito) [77] |
Since seasonal timing may differ among geographic areas, study location is indicated in parentheses.
*Indicated by author.
Abbreviation: TB, tuberculosis.
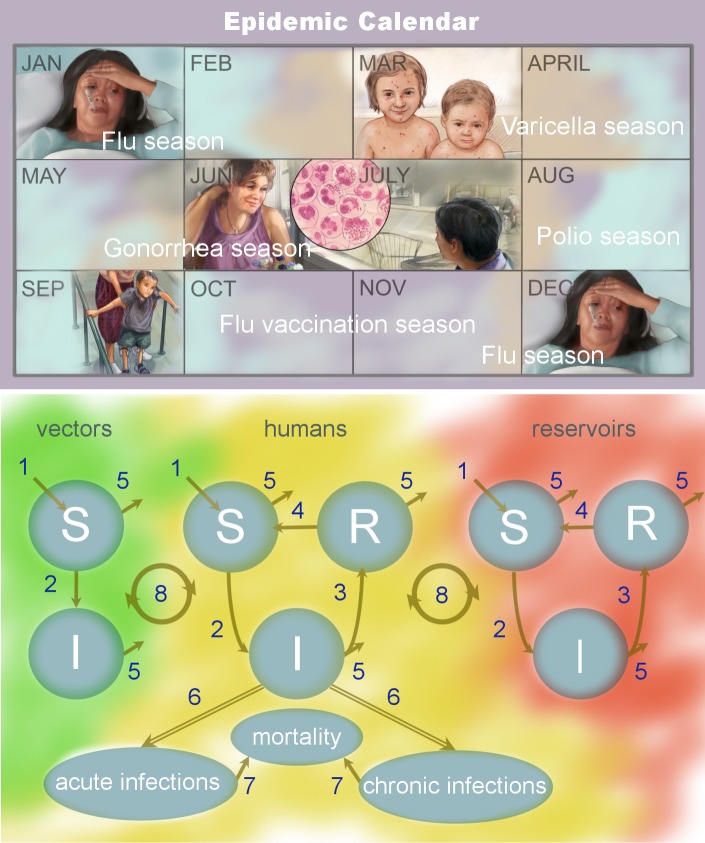
Fig 1. Epidemic calendar.
The concept of an epidemic calendar is illustrated in the top panel. Infectious diseases are seasonal, especially the occurrence of acute and epidemic-prone diseases. In any given population, infectious diseases are distributed throughout the year. Annual cycles of infectious disease are a ubiquitous feature of infection (Tables 1–4). The illustration depicts the wintertime seasonality of flu, springtime peaks of varicella (i.e., chickenpox), and the summertime occurrence of gonorrhea and polio, in the Northern Hemisphere. The bottom panel is a SIR schematic for the transmission of human infectious diseases, which include acute and chronic diseases, those that are vector-borne, and those that are zoonotic with animal reservoirs. The vector, human, and reservoir hosts populations are partitioned into individuals who are susceptible to infection, infected, and recovered and immune. Seasonality may enter into any of the eight key elements of the system: (1) susceptible recruitment via reproduction, (2) transmission, (3) acquired immunity and recovery, (4) waning immunity, (5) natural mortality, (6) symptomatology and pathology (which may be acute or chronic, depending on the disease), (7) disease-induced mortality, and (8) cross-species transmission. Disease illustrations reproduced from Google Medical Information. I, infected; R, recovered and immune; S, susceptible.
Seasonal variation in infectious disease transmission plays an important role in determining when epidemics happen; however, it is not the sole determinant. For instance, some infectious diseases with known seasonal transmission, such as pertussis and measles, can display multi-annual outbreaks, meaning their epidemics occur in multi-year intervals, such as every two or four years, rather than annually. This is because the timing of these epidemics is determined by a combination of (i) seasonal transmission and (ii) different processes shaping the number of susceptible individuals in the population, a sufficient number of which is a prerequisite for an outbreak.
Within the fields of infectious disease ecology and epidemic modeling, seasonal variation in transmission is known as seasonal forcing [78]. Over the past century, attention has been paid to detailing the cyclicity and mechanisms of seasonal forcing for a few diseases of public interest, such as measles, influenza, and cholera (e.g., see contemporary work by [3, 79, 80]). Despite these notable examples, disease seasonality has yet to be systemically and/or rigorously characterized for the majority of infections.
Here, I aim to motivate future studies of disease seasonality by drawing attention to the importance of seasonality in public health, medicine, and biology. I will explore documented seasonal cycles in human infections, including notifiable and neglected tropical diseases. I also aim to present a holistic view of hypothesized drivers of seasonality for each disease, with the caveat that, for the majority of infections, the current state of the science is insufficient to draw conclusions about seasonal timing, seasonal magnitude, and geographic variation in incidence. Although published data regarding disease seasonality are limited for individual diseases, collectively the body of work on disease seasonality is vast and reveals that infections—which may differ enormously in their pathology and/or ecology—coalesce via underlying seasonal drivers.
In order to explore documented seasonal cycles in human infections, the websites of the United States Centers for Disease Control and Prevention (CDC), World Health Organization (WHO), and the European Centre for Disease Prevention and Control were searched to compile a list of 60+ communicable diseases of public health interest. Careful attention was paid to include neglected tropical diseases that may be underrepresented in disease notification systems. For each infection, online WHO disease information pages were used to determine whether the disease is acute or chronic. When the nature of the infection could not be established from the WHO disease information page, this information was gathered from online CDC disease factsheets. Google scholar was then used to systematically search for information regarding disease seasonality. For each of the diseases, a search was conducted using "[disease name] AND season." When needed, I also added "AND human" to the search term for zoonotic diseases. When searches needed to be more specific, a search for "[disease name] AND seasonality" was conducted. Most diseases had very few papers that specifically focused on seasonality (based on their titles and abstracts); the most relevant paper(s) presented in top search results were used in Tables 1–4. The method employed here was meant to provide a broad overview of many infections as opposed to detailed information regarding any individual infection. A short description of the seasonality and hypothesized seasonal drivers were then summarized in Tables 1–4.
In the broadest sense, seasonal drivers can be separated into four categories: (1) environmental factors, (2) host behavior, (3) host phenology, and (4) exogenous biotic factors. These seasonal drivers may enter into disease transmission dynamics by way of hosts, reservoirs, and/or vectors. In surveying the literature to gauge the breadth of seasonal drivers acting upon human infectious disease systems (Tables 1–4), specific seasonal drivers were found to include (a) vector seasonality, (b) seasonality in nonhuman animal host (i.e., livestock, other domestic animals, or wildlife), (c) seasonal climate (e.g., temperature, precipitation, etc.), (d) seasonal nonclimatic abiotic environment (e.g., water salinity), (e) seasonal co-infection, (f) seasonal exposure and/or behavior and/or contact rate, (g) seasonal biotic environment (e.g., algal density in waterbodies), and (h) seasonal flare-ups/symptoms and/or remission/latency.
Environmental factors
Environmental factors, specifically climate conditions, are the seasonal drivers that have received the most attention. This may be because they often covary with seasonal disease incidence. Environmental drivers are abiotic conditions that influence transmission via their effects on hosts and/or parasites; classic examples are temperature and rainfall, which influence a variety of infectious diseases [81], but other examples include seasonal nonclimatic abiotic environmental conditions, such as water salinity, which may impact water-borne pathogens. Environmental factors can impact pathogen survival during transitions between hosts. Transitions can take place during short time windows (e.g., for droplet-transmitted infections) or long time windows (e.g., for parasites with environmental life stages). In addition to their impact on pathogens, environmental drivers can also influence host susceptibility to infection or vector population dynamics.
As for host susceptibility, environmental conditions can impact the host immune response and increase cells' susceptibility to infection [82] or pose seasonal challenges (such as food limitations) that leave hosts vulnerable to infection or pathology [83], which has been proposed to influence disease progression in individuals infected with HIV [35]. For directly transmitted infections, environmental conditions can be major drivers of cycles in incidence, with influenza and cholera transmission being notable examples (e.g., see [3, 80]). The effects of climate on flu transmission have been studied using population-level data coupled with transmission models, as well as empirical animal studies [84], to demonstrate the effects of temperature and humidity on transmission. Although climate conditions undoubtedly play a direct role in several directly transmitted infections, they may play a more nuanced role in vector-borne disease systems in which they modulate vector population dynamics and subsequently disease transmission. For example, in the case of African sleeping sickness (Table 1), the rainy season is hypothesized to modify tsetse fly distribution, which results in changes in human–tsetse fly contact and subsequently African sleeping sickness incidence; in this case, we can classify the seasonal driver as (1) vector seasonality alone or as (2) seasonal climate influencing vector seasonality and vector seasonality having a downstream effect on seasonal exposure. Abiotic and biotic seasonal drivers are therefore interconnected and not mutually exclusive.
Host behavior
Transmission seasonality is sometimes due to seasonal host behavior, specifically fluctuations in transmission-relevant host contact rates throughout the year. Seasonal host behavior not only includes seasonal behavior and/or exposure and/or contact rates in humans but also seasonality in nonhuman animal hosts (i.e., livestock, other domestic animals, or wildlife). The most well-known example of seasonal contact rates in humans is the aggregation of children in schools during school terms that results in elevated transmission of measles (e.g., [1]). Recent studies of seasonal contacts include the use of innovative data sources such as light-at-night satellite imagery and mobile phone data to infer human mobility patterns throughout the year [48, 59]. Data required to quantify seasonal variation in human contact rates are becoming increasingly available. For zoonotic diseases, however, it is contact with wildlife or livestock that can drive seasonal transmission, such as with anthrax, Crimean-Congo hemorrhagic fever, Ebola, echinococcosis, and others (Tables 1–4). Characterizing the seasonal interface among humans and wildlife/livestock poses unique challenges, especially when contacts are occurring in remote areas, which is likely the case with Ebola and echinococcosis (Table 1).
From an ecological perspective, wildlife systems offer a rich arena to study the diverse ways in which behavior impacts disease transmission. Understanding transmission within wildlife is not only relevant for zoonotic infections but is also important for conservation ecology (i.e., in order to protect populations from disease-induced declines, such as is currently experienced by Tasmanian devils, North American bats, and amphibians worldwide). More broadly, understanding the ecology of disease transmission in wildlife can lead to insights that may be applied to human health. Wildlife and urban human populations exist at opposite extremes of a continuum of exposure and/or subjection to natural environmental cycles. Due to the potentially more extreme effect of natural environmental cycles on wildlife disease systems—including climatic influences on wildlife behavior—wildlife could serve as a model for developing methods and conceptual frameworks needed to disentangle the contribution of environmental cycles and behavior from other drivers of disease transmission.
For example, wildlife may be a useful study system for sexually transmitted infections (STIs). Unlike humans, who might have moderate fluctuations in sexual contacts throughout the year [85], in some mammal species, there is a complete absence of sexual contact (and thus transmission) outside of the breeding season. I propose that isolation in time, such as this, is a much more extreme form of seasonal forcing than seen in human infectious disease systems. Isolation in time could also occur for parasites with other transmission modes, in addition to STIs. Although it is an unexplored area of research, the study of isolation in time may reveal pathogen metapopulation structure and parasite adaptations for surviving through transmission troughs. Discrete windows of transmission are likely to have evolutionary consequences for parasite life history. Cattadori and colleagues [86] pointed out that, when transmission is restricted to a short seasonal window, natural selection will favor parasites with "long-lived infective stages." I further speculate that transmission isolated in time will have dynamical consequences that make these disease systems unique from those with more continuous transmission cycles.
Sexual contact is not the only transmission-risk–elevating behavior that can display seasonality. Seasonal engagement in risk-taking behavior may occur in other disease contexts, including for infections transmitted during bouts of fighting. For example, the transmission of facial tumor disease among Tasmanian devils—which is caused by an infectious cancer—is facilitated by aggressive behavior. During a fight, the cancer cells from a facial tumor of an infected devil can be transferred into the wounds and mouth of a susceptible devil, resulting in infection. The Tasmanian devil contact network varies between the mating and nonmating seasons, and this could influence the transmission of this infectious cancer [87, 88]. Although mating and aggression can elevate disease risk, it is important to acknowledge that some behaviors can also mitigate disease risk. In wildlife, disease mitigation behaviors include grooming to remove ectoparasites (as observed in birds and primates) and self-medication (as observed in primates, birds, and monarch butterflies) [89, 90]. There are, however, very few studies of seasonality in risk-taking and risk-mitigation behavior. This is yet another area in which wildlife could serve as a useful model system.
As previously noted, mobility patterns are an aspect of host behavior that can also seasonally structure disease risk via the geographic localization of hosts. In humans, the most notable example is the movement of people in and out of cities. In Niger, for instance, the resulting changes in population density from migration is believed to be the primary driver of measles transmission seasonality [48]. Similarly, in wildlife, hosts seasonally engage with different aspects of their environment. For hosts that migrate or hibernate, contact with risky environments can be seasonal [91]. Migration and hibernation are part of host phenology, which has been implicated in several infectious disease systems [91–94].
Phenology
Host phenology includes host life history, annual cycles (e.g., migration and hibernation), and endogenous circannual rhythms (i.e., endogenously driven seasonal changes in physiology) [94]. Relevant host phenology includes, but is not limited to, seasonal changes in reproduction, seasonal restructuring of immunity, cycles of metabolism and body condition, hibernation, and migration. Phenology is not only a feature of hosts but also of reservoirs, vectors, and some parasites themselves (particularly helminths). Unlike environmental drivers and host behavior, which can affect diseases dynamics by (i) seasonally forcing transmission in hosts, reservoirs, and vectors, phenology can drive seasonality via additional mechanisms of action, which include the modulation of (ii) susceptible recruitment (via reproduction), (iii) susceptibility to infection, (iv) infectiousness, (v) the recovery rate, (vi) the mortality rate (both natural and disease-induced), and (vii) symptomatology and/or pathology.
Each of the seven mechanisms of action could leave a unique imprint in long-term incidence data, as proposed in [94]. These mechanisms and their drivers, therefore, would have different consequences for disease dynamics. Using models, such as that schematized in Fig 1, statistical inference and simulation studies could be conducted to identify the dynamical effects of various seasonal mechanisms acting in isolation and/or in combination. Simulation studies could provide a foundation for determining the types of data required for distinguishing among seasonal mechanisms and/or drivers and how factors such as resonance can influence the ability to detect seasonal drivers. For example, Martinez-Bakker and colleagues [94] used a simulation study to demonstrate the demographic and transmission regimes under which human birth seasonality is expected to have a meaningful impact on measles incidence in the face of strong seasonal forcing from transmission during school terms.
This challenge of identifying seasonal drivers and their mechanisms of action becomes greater when considering chronic infections, vector-borne diseases, and parasites with complex life histories and phenology of their own. In reviewing seasonal drivers of human disease systems for Tables 1–4, human phenology seemed to be particularly relevant for diseases that have seasonal flare-ups/symptoms and/or remission/latency; this includes some chronic infectious diseases, such as tuberculosis and yaws, along with Meningococcal disease, which is acute (Tables 2–4). Although the study of human phenology is a relatively new research area, phenology of vectors and nonhuman animals is well studied and could be the most common cause of vector seasonality and seasonality in nonhuman animal hosts, which impact diseases such as Zika (Table 4) and Middle East respiratory syndrome coronavirus (MERS-CoV) (Table 3).
Table 2. Seasonal drivers of human infectious diseases (continued from Table 1).
Drivers categorized as being related to (a) vector seasonality, (b) seasonality in nonhuman animal host (i.e., livestock, other domestic animals, or wildlife), (c) seasonal climate (e.g., temperature, precipitation, etc.), (f) seasonal exposure and/or behavior and/or contact rate, (g) seasonal biotic environment (e.g., algal density in waterbodies), (h) seasonal flare-up/symptoms and/or remission/latency, (i) observed seasonal incidence with no hypotheses regarding drivers.
| Infection/disease | Type | Seasonal driver(s) | Description |
|---|---|---|---|
| Haemophilus influenzae | Acute | i | Slightly elevated incidence in winter (in the US) [29] |
| Hepatitis A | Acute | f and i | Dry season (in Brazil) [30, 31] |
| Hepatitis B | Chronic | h | Seasonality is observed with elevated levels in spring and summer and/or autumn in some parts of the world, whereas there is lack of seasonality in other parts of the world [31, 32] |
| Hepatitis C | Acute and chronic | f | Seasonality observed in some countries and absent in others; spring and/or summer peaks in Egypt, China, and Mexico while there is a winter peak in India [31] |
| Hepatitis E | Acute | c | Waterborne outbreaks occur during the rainy season or following flooding (in China) [33] |
| Herpes zoster (shingles) | Acute and chronic | i and h* | Highest in August and lowest in winter (in Japan) [34] |
| HIV | Chronic | g | There is some evidence to suggest there is seasonal variation in the progression to AIDS; hypothesized to be related to seasonal nutritional deficiencies (study done in Uganda) [35] |
| Influenza | Acute | c | Winter (in the Northern Hemisphere) [36] |
| Japanese encephalitis | Acute | a | It is seasonal in the northern part of the tropical zone; outbreaks happen at the end of the rainy season, but there is no seasonal pattern in tropical regions [37] |
| Lassa fever | Acute | c | Increase in the number of Lassa fever cases during the dry season (in Nigeria) [38] |
| Legionellosis | Acute | c | Peaks during hot months and particularly during humid periods (in the US) [39] |
| Leishmania | Chronic | a | Transmitted by sand flies; domestic dogs are the main reservoir, and they are exposed during a discrete transmission season [40] |
| Leprosy | Chronic | b | Armadillos are the reservoir, and antibody prevalence is seasonal within them [41] |
| Leptospirosis | Acute | c | Peaks when there is hot weather; usually in a rainy period (on all continents) [42] |
| Lyme disease | Acute and Chronic | a | Peaks in summer around the time of maximal activity of the nymphal stage of the tick vector (in the US) [43] |
| Lymphatic filariasis | Chronic | a and c | Transmission is intensified during the rainy season [44] |
| Malaria | Acute | a | There is a spectrum of seasonal strength; some regions have strong seasonality and no seasonality in others [45] |
Since seasonal timing may differ among geographic areas, study location is indicated in parentheses.
*Indicated by author.
Table 3. Seasonality of human infectious diseases (continued from Tables 1 and 2).
Drivers categorized as being related to (a) vector seasonality, (b) seasonality in nonhuman animal host (i.e., livestock, other domestic animals, or wildlife), (c) seasonal climate (e.g., temperature, precipitation, etc.), (f) seasonal exposure and/or behavior and/or contact rate, (g) seasonal biotic environment (e.g., algal density in waterbodies), (h) seasonal flare-up/symptoms and/or remission/latency, (i) observed seasonal incidence with no hypotheses regarding drivers.
| Infection/disease | Type | Seasonal driver(s) | Description |
|---|---|---|---|
| Marburg | Acute | b | Seasonal incidence in bat reservoirs (in Uganda); seasonal peaks coincided with the twice-annual birthing season [46] |
| Measles | Acute | f | Elevated transmission driven by aggregation of children in school; seasonality in developing countries related to agricultural cycles [47, 48] |
| Meningococcal disease | Acute | c and h | Incidence varies seasonally in both tropical and temperate countries. Elevated incidence during the dry season (in sub-Saharan Africa). [49] |
| MERS-CoV | Acute | b | Introductions into humans are seasonal and are more frequent during the camel calving season. [50] |
| Onchocerciasis (river blindness) | Acute and chronic | a | Higher transmission potential in the rainy season when vector abundance and infection is elevated (in Nigeria) [51] |
| Pertussis | Acute | i and f* | Higher incidence June through October (in the US) [52] |
| Plague | Acute | a, b, c, f, and g | The seasonality varies among countries and is dependent on seasonality of reservoir and vector species and in some cases agricultural cycles [53] |
| Poliomyelitis | Acute | i, c*, and h* | Epidemics occurred during the summer (in the US) [6] |
| Rabies | Acute | b | Rabies is seasonal in bats, which are a source of human exposure [54] |
| RSV | Acute | i and c* | Peaks in winter months in temperate regions; less pronounced seasonality in the tropics [55] |
| Rift Valley fever | Acute | a and c | Associated with periods of heavy rainfall [56] |
| Rotavirus | Acute | i and c* | Geographical gradient in seasonality; peaks in December/January in the Southwest US and April/May in the Northeast US [57] |
| Rubella | Acute | f | Two seasonal peaks in transmission per year in Kenya; late-winter to early-summer peaks in the US [58, 59] |
| Salmonellosis | Acute | i | Increased number of isolates in the warm spring months (in Tunisia) [60] |
| Schistosomiasis | Chronic | b and c | Transmission is seasonal; two seasonal peaks per year (in Tanzania) [61] |
| Scrub typhus | Acute | a, c, and f | Seasonality depends on activity of vectors (i.e., chiggers) and humans. Seasonality varies geographically. Some areas (in Japan) have strong seasonal transmission, and others have relatively stable transmission [62] |
| Shigella | Acute | c | Elevated incidence in summer (in Massachusetts, US) [63] |
| Smallpox | Acute | c | Associated with dry weather [64] |
| Soil-transmitted helminth infections | Chronic | c and g | Hookworms undergo seasonal arrested development, which affects the acquisition of infection in humans; there is also seasonal acquisition of roundworm infections [65, 66] |
| Syphilis | Chronic | f | Higher incidence in summer (in China) [67] |
| Taeniasis (cysticercosis) | Chronic | b and f | Seropositivity varies seasonally in livestock, which are the source of human infection (in Romania) [68] |
| Tetanus | Acute | c and f | Peak in midsummer (in the US) [69] |
| Trachoma | Acute and chronic | a | More common in the wet season when the fly vector is most abundant (in Australia) [70] |
Since seasonal timing may differ among geographic areas, study location is indicated in parentheses.
*Indicated by author.
Abbreviations: MERS-CoV, Middle East respiratory syndrome coronavirus; RSV, Respiratory Syncytial Virus.
Exogenous biotic factors
In addition to abiotic, behavioral, and phenological features of host–parasite systems, hosts and their parasites are embedded within ecological communities that have additional seasonal aspects. We can refer to biotic factors driven by ecological communities as exogenous biotic factors because they are exogenous to any given host–parasite dyad, host–vector–parasite triad, or multi-host system. Exogenous biotic factors include (1) interactions that take place within hosts—specifically parasite–parasite interactions—and (2) interactions within the ecological community of hosts, reservoirs, and vectors.
During some co-infections, parasite–parasite interactions can occur directly or be mediated via the host immune system, when parasite species impact each other's population dynamics indirectly via their effect on the host immune system. Parasite–parasite interactions can result in parasite fitness being elevated (i.e., facilitation) or dampened (e.g., competition) [95–97]. The seasonality of individual infectious diseases likely imposes seasonal structure on co-infections and thus the presence of parasite–parasite interactions, as has been implicated in the seasonality of bacterial pneumonia (Table 1).
Outside of the host, community ecology becomes particularly relevant for disease transmission in multi-host systems, such as Lyme disease [95, 96, 98]. Community ecology is particularly important when there is heterogeneity in host and/or vector competence in a multi-species disease system. This is because the abundance of competent hosts and/or vectors can determine the transmissibility and maintenance of infection [98]. Each host and/or vector in a vector-borne or multi-host disease system has its own set of ecological interactions (e.g., competitive, commensal, parasitic, etc.) that can indirectly affect the disease of interest. The phenology of hosts, reservoirs, and vectors will give rise to seasonal changes in ecological community composition. Taken together, parasite–parasite interactions within hosts and ecological interactions outside hosts undoubtedly display seasonality, as do nearly all aspects of ecology, and this can influence the dynamics of diseases of public interest.
Understanding seasonality
Seasonality is an inherent feature of ecological systems, and seasonal incidence is a feature of both acute and chronic infectious diseases (Tables 1–4). It is, therefore, important to conceptualize the epidemic calendar (Fig 1) from the lens of "everything is seasonal." The utility of this lens is that it forces us to carefully consider mechanisms behind disease seasonality, thus preventing what could, in some cases, be the misleading establishment of correlative relationships between seasonal phenomena and infectious disease incidence. Focused attention on building theory that will provide a deeper understanding of seasonal mechanisms and learning how to identify imprints of seasonal drivers in disease data could bring rapid advancement to the field of disease seasonality. In general, if "everything is seasonal," then everything will covary (usually with some phase shifts). Therefore, seasonal covariance alone is not useful for establishing seasonal drivers. Instead, long-term parallel data of potential seasonal drivers and disease incidence should be confronted with mechanistic transmission models. There is evidence to suggest that the information contained in interannual variation and anomalous years holds a key to establishing causal inference of seasonal forcing [99].
A thought experiment can be used to illustrate how anomalous years and interannual variation could be used to establish causal mechanisms of disease seasonality. Let's consider a human disease with peak incidence in the summer, such as polio [6]. Because incidence peaks in summer, it would have a strong positive relationship with temperature, photoperiod (day length), and many other summer-related features of the environment and human populations. To highlight how noncausal seasonal factors could misleadingly correlate with disease incidence, we could quantify what would be a likely strong positive relationship between disease incidence and the sale of summer-related items that have nothing to do with transmission (e.g., bathing suit or ice cream sales). Let's imagine we build a transmission model for this infection and test three potential seasonal drivers (e.g., temperature, photoperiod, and bathing suit sales). We find that all three model variants capture the seasonal structure of the epidemics because they all contain a covariate with the necessary seasonal structure. However, if the disease displays (1) interannual variation in epidemic size and/or (2) anomalous years with differences in epidemic timing that cannot be explained by demography or susceptible recruitment dynamics, then only the model with data from the causal driver would improve our ability to predict the variation in incidence observed among years.
By confronting disease incidence data with transmission models and testing the relevance of various demographic, ecological, behavioral, and physiological covariates, we could identify potential seasonal drivers on the population level. For some seasonal drivers, such as seasonal changes in host immunity, we would, however, still be tasked with understanding the mechanism of action within the host. The effects of seasonal drivers are multi-layered. To better understand seasonality, we must work at multiple organizational levels of science. Geophysical factors, host population ecology, and within-host biology will need to be integrated in the practice of studying seasonality. As previously mentioned, many infectious diseases, which might differ greatly in multiple aspects of their biology, can share the same seasonal driver(s). An immediate way to advance the field of disease seasonality is to leverage the rich weekly and/or monthly datasets available for notifiable diseases and combine them with models and data on potential drivers. By coupling models and data, hypothesis testing can be done to assess seasonal drivers and their modes of action. These data and models can be applied to multiple disease systems in parallel. For instance, parallel study of the seasonal drivers of (a) flu, respiratory syncytial virus (RSV), bacterial pneumonia, and pertussis, or (b) polio, typhoid, and rotavirus, or (c) Zika, dengue, chikungunya, and yellow fever would be a logical start. Uncovering the mechanisms of seasonality for disease systems would empower the public health community to better control infection. This sentiment was shared in 1949 by polio epidemiologist H. Gear, who wrote: "It must be admitted that the reasons for the seasonal incidence of poliomyelitis remain obscure. When they have been elucidated perhaps much of the epidemiology of this disease will be solved" [100].
Funding Statement
Research reported in this publication was supported by the Office Of The Director, National Institutes Of Health of the National Institutes of Health under Award Number DP5OD023100. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Soper HE. The Interpretation of Periodicity in Disease Prevalence. Journal of the Royal Statistical Society. 1929;92(1):34–73. [Google Scholar]
- 2.Finkelman BS, Viboud C, Koelle K, Ferrari MJ, Bharti N, Grenfell BT. Global Patterns in Seasonal Activity of Influenza A/H3N2, A/H1N1, and B from 1997 to 2005: Viral Coexistence and Latitudinal Gradients. PLoS ONE. 2007;2(12):e1296 10.1371/journal.pone.0001296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. Absolute Humidity and the Seasonal Onset of Influenza in the Continental United States. PLoS Biol. 2010;8(2):e1000316 10.1371/journal.pbio.1000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.London WP, Yorke JA. Recurrent Outbreaks of Measles, Chickenpox and Mumps: I. Seasonal Variation in Contact Rates. American Journal of Epidemiology. 1973;98(6):453–468. [DOI] [PubMed] [Google Scholar]
- 5.Bakker KM, Martinez-Bakker ME, Helm B, Stevenson TJ. Digital Epidemiology Reveals Global Childhood Disease Seasonality and the Effects of Immunization. Proceedings of the National Academy of Sciences. 2016;113(24):6689–6694. 10.1073/pnas.1523941113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Bakker M, King AA, Rohani P. Unraveling the Transmission Ecology of Polio. PLoS Biol. 2015;13(6):e1002172 10.1371/journal.pbio.1002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight G. The Ecology of African Sleeping Sickness. Annals of the Association of American Geographers. 1971;61(1):23–44. [Google Scholar]
- 8.Chikerema SM, Pfukenyi DM, Matope G, Bhebhe E. Temporal and Spatial Distribution of Cattle Anthrax Outbreaks in Zimbabwe between 1967 and 2006. Tropical Animal Health and Production. 2012;44(1):63–70. 10.1007/s11250-011-9888-z [DOI] [PubMed] [Google Scholar]
- 9.Park AW, Glass K. Dynamic Patterns of Avian and Human Influenza in East and Southeast Asia. The Lancet Infectious Diseases. 2007;7(8):543–548. 10.1016/S1473-3099(07)70186-X [DOI] [PubMed] [Google Scholar]
- 10.Halvorson DA, Kelleher CJ, Senne DA. Epizootiology of Avian Influenza: Effect of Season on Incidence in Sentinel Ducks and Domestic Turkeys in Minnesota. Applied and Environmental Microbiology. 1985;49(4):914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim PE, Musher DM, Glezen WP, Rodriguez-Barradas MC, Nahm WK, Wright CE. Association of Invasive Pneumococcal Disease with Season, Atmospheric Conditions, Air Pollution, and the Isolation of Respiratory Viruses. Clinical Infectious Diseases. 1996;22(1):100–106. 10.1093/clinids/22.1.100 [DOI] [PubMed] [Google Scholar]
- 12.Gul S, Khan A. Epidemiology and Epizootology of Brucellosis: A Review. Pakistan Vet J. 2007;27(3):145–151. [Google Scholar]
- 13.Merritt RW, Walker ED, Small PLC, Wallace JR, Johnson PDR, Benbow ME, et al. Ecology and Transmission of Buruli Ulcer Disease: A Systematic Review. PLoS Negl Trop Dis. 2010;4(12):e911 10.1371/journal.pntd.0000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeledón R. Chagas' Disease: An Ecological Appraisal with Special Emphasis on its Insect Vectors. Annual Review of Entomology. 1981;26(51):101–133. 10.1146/annurev.en.26.010181.000533 [DOI] [PubMed] [Google Scholar]
- 15.Bakker KM, Martinez-Bakker ME, Helm B, Stevenson TJ. Digital Epidemiology Reveals Global Childhood Disease Seasonality and the Effects of Immunization. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(24). 10.1073/pnas.1523941113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pialoux G, Bernard-Alex G, Jaureguiberry S, Strobel M. Chikungunya, An Epidemic Arbovirosis. Lancet Infectious Diseases. 2007;7(5):319–327. 10.1016/S1473-3099(07)70107-X [DOI] [PubMed] [Google Scholar]
- 17.Emch M, Feldacker C, Sirajul SM, Ali M. Seasonality of Cholera from 1974 to 2005: A Review of Global Patterns. International Journal of Health Geographics. 2008;7:1–13. 10.1186/1476-072X-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo Hemorrhagic Fever: History, Epidemiology, Pathogenesis, Clinical Syndrome and Genetic Diversity. Antiviral Research. 2013;100(1):159–189. 10.1016/j.antiviral.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 19.Checkley W, White AC, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, et al. A Review of the Global Burden, Novel Diagnostics, Therapeutics, and Vaccine Targets for Cryptosporidium. The Lancet Infectious Diseases. 2015;15(1):85–94. 10.1016/S1473-3099(14)70772-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toumi A, Chlif S, Bettaieb J, Alaya NB, Boukthir A, Ahmadi ZE, et al. Temporal Dynamics and Impact of Climate Factors on the Incidence of Zoonotic Cutaneous Leishmaniasis in Central Tunisia. PLoS Negl Trop Dis. 2012;6(5):0–7. 10.1371/journal.pntd.0001633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Benthem BH, Vanwambeke SO, Khantikul N, Burghoorn-Maas C, Panart K, Oskam L, et al. Spatial Patterns of and Risk factors for Seropositivity for Dengue Infection. Am J Trop Med Hyg. 2005;72(2):201–208. 72/2/201 [pii]. [PubMed] [Google Scholar]
- 22.Gomes MC, Gomes JJ, Paulo AC. Diphtheria, Pertussis and Measles in Portugal Before and After Mass-Vaccination: A Time Series Analysis. European Journal of Epidemiology. 1999;15(October 1966):791–798. [DOI] [PubMed] [Google Scholar]
- 23.Huttly SR, Blum D, Kirkwood BR, Emeh RN, Okeke N, Ajala M, et al. The Imo State (Nigeria) Drinking Water Supply and Sanitation Project, 2. Impact on dracunculiasis, diarrhoea and nutritional status. Transactions of the Royal Society of Tropical Medicine & Hygiene. 1990;84(2):316–321. 10.1016/0035-9203(90)90300-4 [DOI] [PubMed] [Google Scholar]
- 24.Groseth A, Feldmann H, Strong JE. The Ecology of Ebola Virus. Trends in Microbiology. 2007;15(9):408–416. 10.1016/j.tim.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 25.Eckert J, Gemmell F, Meslin X, Pawlowski Z. WHO / OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; 2001. [Google Scholar]
- 26.Ferens WA, Hovde CJ. Escherichia coli O157:H7: Animal Reservoir and Sources of Human Infection. Foodborne Pathogens and Disease. 2011;8(4):465–87. 10.1089/fpd.2010.0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-Borne Trematodiases in Southeast Asia. Epidemiology, Pathology, Clinical Manifestation and Control. vol. 72 1st ed. Elsevier Ltd.; 2010. 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- 28.Wright RA, Judson FN. Relative and Seasonal Incidences of the Sexually Transmitted Diseases. A Two-Year Statistical Review. Sexually Transmitted Infections. 1978;54(6):433–440. 10.1136/sti.54.6.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisgard KM, Kao A, Leake J, Strebel PM, Perkins BA, Wharton M. Haemophilus Influenzae Invasive Disease in the United States, 1994–1995: Near Disappearance of a Vaccine-Preventable Childhood Disease. Emerging Infectious Diseases. 1998;4(2):229–237. 10.3201/eid0402.980210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bensabatb G, Stephen C, Soares MCP, Fiezds H, Maynard J. Epidemiological and Serological Studies of Acute Viral Hepatitis in Brazil's Amazon Basin. paho Bulletin. 1987;21(1):16–27. [PubMed] [Google Scholar]
- 31.Fares A. Seasonality of Hepatitis: A Review Update. Journal of Family Medicine and Primary Care. 2015;4(1):96 10.4103/2249-4863.152263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang SJ, Chen ZX, Jiang KP, Wu WK, Zhang CY, Gu YL. Effect of Seasonal Variation on the Clinical Course of Chronic Hepatitis B. Journal of Gastroenterology. 2006;41(January 1996):1107–1115. 10.1007/s00535-006-1903-1 [DOI] [PubMed] [Google Scholar]
- 33.Zhuang H, Cao XY, Liu CB, Wang GM. Epidemiology of Hepatitis E in China. Gastroenterologia Japonica. 1991;26(Suppl 3):135–8. [DOI] [PubMed] [Google Scholar]
- 34.Toyama N, Shiraki K, Dermatologists, of the Society of the Miyazaki Prefecture M. Epidemiology of Herpes Zoster and Its Relationship to Varicella in Japan: A 10-Year Survey of 48,388 Herpes Zoster Cases in Miyazaki Prefecture. Journal of Medical Virology. 2009;81:2053–2058. 10.1002/jmv.21599 [DOI] [PubMed] [Google Scholar]
- 35.Smallman-Raynor M, Cliff AD. Seasonality in Tropical AIDS: A Geographical Analysis. International Journal of Epidemiology. 1992;21(3). [DOI] [PubMed] [Google Scholar]
- 36.Shaman J, Kohn M. Absolute Humidity Modulates Influenza Survival, Transmission, and Seasonality. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3243–8. 10.1073/pnas.0806852106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umenai T, Krzysko R, Bektimirov TA, Assaad FA. Japanese Encephalitis: Current Worldwide Status. Bulletin of the World Health Organization. 1985;63(4):625–631. [PMC free article] [PubMed] [Google Scholar]
- 38.Oldstone MB, editor. Current Topics in Microbiology and Immunology Springer-Verlag; Berlin Heidelberg; 1987. http://link.springer.com/10.1007/978-3-642-65848-8. [Google Scholar]
- 39.Fisman D, Lim S, Wellenius G, Johnson C, Britz P, Gaskins M, et al. It's Not the Heat, It's the Humidity: Wet Weather Increases Legionellosis Risk in the Greater Philadelphia Metropolitan Area. The Journal of Infectious Diseases. 2005;192(12):2066–2073. 10.1086/498248 [DOI] [PubMed] [Google Scholar]
- 40.Oliva G, Scalone A, Manzillo VF, Gramiccia M, Pagano A, Di Muccio T, et al. Incidence and Time Course of Leishmania infantum Infections Examined by Parasitological, Serologic, and Nested-PCR Techniques in a Cohort of Naive Dogs Exposed to Three Consecutive Transmission Seasons. Journal of Clinical Microbiology. 2006;44(4):1318–1322. 10.1128/JCM.44.4.1318-1322.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truman RW, Job CK, Hastings RC, Kumaresan JA, Mcdonough CM. Seasonal and Spatial Trends in the Detectability of Leprosy in Wild Armadillos. Epidemiology and Infection. 1991;106(3):549–560. 10.1017/S0950268800067613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero E, Bernardo C, Yasuda P. Human Leptospirosis: A Twenty-Nine-Year Serological Study in Sao Paulo, Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo. 2003;45(5):245–248. 10.1590/S1517-83822002000300016 [DOI] [PubMed] [Google Scholar]
- 43.Piesman J, Mather TN, Dammin GJ, Telford SR, Lastavica CC, Spielman A. Seasonal Variation of Transmission Risk of Lyme Disease and Human Babesiosis. American Journal of Epidemiology. 1987;126(6):1187–9. [DOI] [PubMed] [Google Scholar]
- 44.Lindsay S, Thomas C. Mapping and Estimating the Population at Risk. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94(March):37–45. [DOI] [PubMed] [Google Scholar]
- 45.Carneiro I, Roca-Feltrer A, Griffin JT, Smith L, Tanner M, Schellenberg JA, et al. Age-Patterns of Malaria Vary with Severity, Transmission Intensity and Seasonality in Sub-Saharan Africa: A Systematic review and Pooled Analysis. PLoS ONE. 2010;5(2). 10.1371/journal.pone.0008988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amman BR, Carroll Sa, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, et al. Seasonal Pulses of Marburg Virus Circulation in Juvenile Rousettus aegyptiacus Bats Coincide with Periods of Increased Risk of Human Infection. PLoS Pathog. 2012;8(10). 10.1371/journal.ppat.1002877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conlan AJK, Grenfell BT. Seasonality and the Persistence and Invasion of Measles. Proceedings of the Royal Society B: Biological Sciences. 2007;274:1133–1141. 10.1098/rspb.2006.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bharti N, Tatem aJ, Ferrari MJ, Grais RF, Djibo A, Grenfell BT. Explaining Seasonal Fluctuations of Measles in Niger Using Nighttime Lights Imagery. Science. 2011;334(6061):1424–1427. 10.1126/science.1210554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenwood BM, Bradley AK, Blakebrough IS, Wali S, Whittle HC. Meningococcal Disease and Season in Sub-Saharan Africa. The Lancet. 1984;323(8390):1339–1342. 10.1016/S0140-6736(84)91830-0 [DOI] [PubMed] [Google Scholar]
- 50.Dudas G, Carvalho LM, Rambaut A, Bedford T. MERS-CoV Spillover at the Camel-Human Interface. eLife. 2018;7(e31257):1–37. 10.1101/173211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atting I, Ejezie G, Braide E, Opara K, Ekwe A. Seasonal Variations in Human Onchocerciasis Transmission by Black Flies (Simulium damnosum s. I.) in a Forest Area of Cross River State, Nigeria; 2005.
- 52.Farizo KM, Cochi SL, Zell ER, Brink EW, Wassilak SG P. Epidemiological Features of Pertussis in the United States. Clin Infec Dis. 1992;14(3):708–719. 10.1093/clinids/14.3.708 [DOI] [PubMed] [Google Scholar]
- 53.Ari T, Neerinckx S, Gage KL, Kreppel K, Laudisoit A, Leirs H, et al. Plague and Climate: Scales matter. PLoS Pathog. 2011;7(9):5–10. 10.1371/journal.ppat.1002160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.George DB, Webb CT, Farnsworth ML, O'Shea TJ, Bowen RA, Smith DL, et al. Host and Viral Ecology Determine Bat Rabies Seasonality and Maintenance. Proceedings of the National Academy of Sciences. 2011;108(25):10208–10213. 10.1073/pnas.1010875108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bloom-Feshbach K, Alonso WJ, Charu V, Tamerius J, Simonsen L, Miller Ma, et al. Latitudinal Variations in Seasonal Activity of Influenza and Respiratory Syncytial Virus (RSV): A Global Comparative Review. PLoS ONE. 2013;8(2):e54445 10.1371/journal.pone.0054445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. Climate and Satellite Indicators to Forecast Rift Valley Fever Epidemics in Kenya. Science. 1999;285(5426):397–400. [DOI] [PubMed] [Google Scholar]
- 57.Turcios RM, Curns AT, Holman RC, Pandya-Smith I, LaMonte A, Bresee JS, et al. Temporal and Geographic Trends of Rotavirus Activity in the United States, 1997–2004. Pediatric Infectious Disease Journal. 2006;25(5):451–454. 10.1097/01.inf.0000214987.67522.78 [DOI] [PubMed] [Google Scholar]
- 58.Reef SE, Redd SB, Abernathy E, Zimmerman L, Icenogle JP. The Epidemiological Profile of Rubella and Congenital Rubella Syndrome in the United States, 1998–2004: The Evidence for Absence of Endemic Transmission. Clinical infectious Diseases. 2006;43(Suppl 3):S126–S132. 10.1086/505944 [DOI] [PubMed] [Google Scholar]
- 59.Wesolowski A, Metcalf CJE, Eagle N, Kombich J, Grenfell BT, Bjørnstad ON, et al. Quantifying Seasonal Population Fluxes Driving Rubella Transmission Dynamics using Mobile Phone Data. Proceedings of the National Academy of Sciences. 2015;112(35):11114–11119. 10.1073/pnas.1423542112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aissa RB, Al-Gallas N, Troudi H, Belhadj N, Belhadj A. Trends in Salmonella Enterica Serotypes Isolated from Human, Food, Animal, and Environment in Tunisia, 1994–2004. Journal of Infection. 2007;55(4):324–339. 10.1016/j.jinf.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 61.Mazigo HD, Nuwaha F, Kinung SM, Morona D, Moira APD, Wilson S, et al. Epidemiology and control of human schistosomiasis in Tanzania. Parasites & Vectors. 2012;5(1):1 10.1186/1756-3305-5-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watt G, Parola P. Scrub Typhus and Tropical Rickettsioses. Current Opinion in Infectious Diseases. 2003;16(5):429–436. 10.1097/01.qco.0000092814.64370.70 [DOI] [PubMed] [Google Scholar]
- 63.Naumova EN, Jagai JS, Matyas B, DeMaria A, MacNeill IB, Griffiths JK. Seasonality in Six Enterically Transmitted Diseases and Ambient Temperature. Epidemiology and Infection. 2006;135(2):281–292. 10.1017/S0950268806006698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishiura H, Kashiwagi T. Smallpox and Season: Reanalysis of Historical Data. Interdisciplinary Perspectives on Infectious Diseases. 2009;2009:591935 10.1155/2009/591935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schad AGA, Chowdhury AB, Dean CG, Kochar VK, Nawalinski TA, Tonascia JA. Arrested Development in Human Hookworm Infections: An Adaptation to a Seasonally Unfavorable External Environment. Science. 1973;180(4085):502–504. 10.1126/science.180.4085.502 [DOI] [PubMed] [Google Scholar]
- 66.Anderson R, editor. Population Dynamics of Infectious Diseases: Theory and Applications Edited by Roy M. Anderson Reader in Parasite Ecology Imperial College, London University Springer-Seience-Business Media, B.V; 1982. [Google Scholar]
- 67.Zhang X, Zhang T, Pei J, Liu Y, Li X, Medrano-Gracia P. Time Series Modelling of Syphilis Incidence in China from 2005 to 2012. PLoS ONE. 2016;11(2):1–18. 10.1371/journal.pone.0149401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oleleu AM, Gherman CM, Blaga R, Gyorke A, Cozma V. Seroprevalence of Porcine Cysticercosis and Influence of Some Associated Risk Factors in Northwestern Romania. Acta Veterinaria Brno. 2016;85(2):121–126. 10.2754/avb201685020121 [DOI] [Google Scholar]
- 69.Heath CW, D JZM, Sherman IL. Tetanus in the United States, 1950–1960. AJPH. 1964; p. 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.da Cruz L, Dadour IR, McAllister IL, Jackson A, Isaacs T. Seasonal Variation in Trachoma and Bush Flies in Northwest Australian Aboriginal Communities. Clinical & Experimental Ophthalmology. 2002;30(2):80–83. [DOI] [PubMed] [Google Scholar]
- 71.Koh GCKW, Hawthorne G, Turner AM, Kunst H, Dedicoat M. Tuberculosis Incidence Correlates with Sunshine: An Ecological 28-Year Time Series Study. PLoS ONE. 2013;8(3):1–5. 10.1371/journal.pone.0057752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X, Liu Y, Yang M, Zhang T, Young AA, Li X. Comparative Study of Four Time Series Methods in Forecasting Typhoid Fever Incidence in China. PLoS ONE. 2013;8(5). 10.1371/journal.pone.0063116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levine H, Mimouni D, Zurel-Farber A, Zahavi A, Molina-Hazan V, Bar-Zeev Y, et al. Time Trends of Viral Meningitis Among Young Adults in Israel: 1978–2012. European Journal of Clinical Microbiology and Infectious Diseases. 2014;33(7):1149–1153. 10.1007/s10096-014-2057-3 [DOI] [PubMed] [Google Scholar]
- 74.Hayes EB, Komar N, Nasci RS, Montgomery SR, Leary DRO, Campbell GL. Epidemiolgy and Transmission Dynamics of West Nile Virus Disease. Emerging Infectious Diseases. 2005;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hackett CJ, Guthe T. Some Important Aspects of Yaws Eradication. Bulletin of the World Health Organization. 1956;15(6):869–896. [PMC free article] [PubMed] [Google Scholar]
- 76.Soper FL. Dynamics of Aedes aegypti Distribution and Density. Bulletin of the World Health Organization. 1967;36:536–538. [PMC free article] [PubMed] [Google Scholar]
- 77.Ferguson NM, Cucunubá ZM, Dorigatti I, Nedjati-Gilani GL, Donnelly CA, Basáñez MG, et al. Countering the Zika epidemic in Latin America. Science. 2016;353(6297):353–354. 10.1126/science.aag0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rohani P, Earn DJ, Grenfell BT. Opposite Patterns of Synchrony in Sympatric Disease Metapopulations. Science (New York, NY). 1999;286(5441):968–71. [DOI] [PubMed] [Google Scholar]
- 79.Mantilla-Beniers NB, Bjørnstad ON, Grenfell BT, Rohani P. Decreasing Stochasticity Through Enhanced Seasonality in Measles Epidemics. Journal of the Royal Society, Interface / the Royal Society. 2010;7(46):727–39. 10.1098/rsif.2009.0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bouma MJ, Pascual M. Seasonal and Interannual Cycles of Endemic Cholera in Bengal 1891–1940 in Relation to Climate and Geography. Hydrobiologia. 2001;460:147–156. 10.1023/A:1013165215074 [DOI] [Google Scholar]
- 81.Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. Seasonality and the Dynamics of Infectious Diseases. Ecology Letters. 2006;9(4):467–484. 10.1111/j.1461-0248.2005.00879.x [DOI] [PubMed] [Google Scholar]
- 82.Foxman EF, Storer Ja, Fitzgerald ME, Wasik BR, Hou L, Zhao H, et al. Temperature-Dependent Innate Defense Against the Common Cold Virus Limits Viral Replication at Warm Temperature in Mouse Airway Cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(3):827–832. 10.1073/pnas.1411030112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pedersen AB, Greives TJ. The Interaction of Parasites and Resources Cause Crashes in a Wild Mouse Population. The Journal of Animal Ecology. 2008;77(2):370–377. 10.1111/j.1365-2656.2007.01321.x [DOI] [PubMed] [Google Scholar]
- 84.Lowen AC, Mubareka S, Steel J, Palese P. Influenza Virus Transmission is Dependent on Relative Humidity and Temperature. PLoS Pathog. 2007;3(10):1470–1476. 10.1371/journal.ppat.0030151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinez-Bakker M, Bakker K, King AA, Rohani P. Human Birth Seasonality: Latitudinal Gradient and Interplay with Childhood Disease Dynamics. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1783). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cattadori IM, Boag B, Bjørnstad ON, Cornell SJ, Hudson PJ. Peak Shift and Epidemiology in a Seasonal Host-Nematode System. Proceedings of the Royal Society B: Biological Sciences. 2005;272:1163–1169. 10.1098/rspb.2004.3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamede RK, Bashford J, McCallum H, Jones M. Contact Networks in a Wild Tasmanian Devil (Sarcophilus harrisii) Population: Using Social Network Analysis to Reveal Seasonal Variability in Social Behaviour and its Implications for Transmission of Devil Facial Tumour Disease. Ecology Letters. 2009;12(11):1147–1157. 10.1111/j.1461-0248.2009.01370.x [DOI] [PubMed] [Google Scholar]
- 88.Bostanci A. A Devil of a Disease. Science. 2005;307(February):2005. [Google Scholar]
- 89.Mooring MS, Blumstein DT, Stoner CJ. The Evolution of Parasite-defence Grooming in Ungulates. Biological Journal of the Linnean Society. 2004;81:17–37. 10.1111/j.1095-8312.2004.00273.x [DOI] [Google Scholar]
- 90.Choisy M, de Roode JC. The Ecology and Evolution of Animal Medication: Genetically Fixed Response versus Phenotypic Plasticity. The American Naturalist. 2014;184 Suppl:S31–S46. 10.1086/676928 [DOI] [PubMed] [Google Scholar]
- 91.Altizer S, Bartel R, Han Ba. Animal Migration and Infectious Disease Risk. Science. 2011;331:296–302. 10.1126/science.1194694 [DOI] [PubMed] [Google Scholar]
- 92.Langwig KE, Frick WF, Reynolds R, Parise KL, Drees KP, Hoyt JR, et al. Host and Pathogen Ecology Drive the Seasonal Dynamics of a Fungal Disease, White-Nose Syndrome. Proceedings of the Royal Society B: Biological Sciences. 2015;282:20142335 10.1098/rspb.2014.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hill NJ, Ma EJ, Meixell BW, Lindberg MS, Boyce WM, Runstadler JA. Transmission of Influenza Reflects Seasonality of Wild Birds Across the Annual Cycle. Ecology Letters. 2016;19(8):915–925. 10.1111/ele.12629 [DOI] [PubMed] [Google Scholar]
- 94.Martinez-Bakker M, Helm B. The Influence of Biological Rhythms on Host-Parasite Interactions. Trends in Ecology and Evolution. 2015;30(6):314–326. 10.1016/j.tree.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 95.Pedersen AB, Fenton A. Emphasizing the Ecology in Parasite Community Ecology. Trends in Ecology and Evolution. 2006;22(3):133–139. 10.1016/j.tree.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 96.Ezenwa VO, Jolles AE. From Host Immunity to Pathogen Invasion: The Effects of Helminth Coinfection on the Dynamics of Microparasites. Integrative and Comparative Biology. 2011;51(4):540–551. 10.1093/icb/icr058 [DOI] [PubMed] [Google Scholar]
- 97.Cattadori IM, Boag B, Hudson PJ. Parasite Co-Infection and Interaction as Drivers of Host Heterogeneity. International Journal for Parasitology. 2008;38(3–4):371–380. 10.1016/j.ijpara.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 98.Johnson PTJ, De Roode JC, Fenton A. Why Infectious Disease Research Needs Community Ecology. Science. 2015;349(6252). 10.1126/science.1259504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez PP, King AA, Yunus M, Faruque ASG, Pascual M. Differential and Enhanced Response to Climate Forcing in Diarrheal Disease due to Rotavirus Across a Megacity of the Developing World. Proceedings of the National Academy of Sciences. 2016;113(15):4092–4097. 10.1073/pnas.1518977113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gear J. The Virus of Poliomyelitis: Its Distribution and Methods of Spread. The Journal of the Royal Sanitary Institute. 1949;69(3):149–153. 10.1177/146642404906900301 [DOI] [PubMed] [Google Scholar]