Abstract
Objective:
Patients with poorly controlled seizures are at elevated risk of epilepsy-related morbidity and mortality. For patients with drug-resistant epilepsy that is focal in onset, epilepsy surgery is the most effective treatment available and offers a 50–80% cure rate. Yet it is estimated only 1% of epilepsy patients with drug-resistant disease undergo surgery in a timely fashion and delays to surgery completion are considerable. The aim of this study was to increase availability and decrease delay of surgical evaluation at our epilepsy center for patients with drug-resistant epilepsy by removing process barriers.
Methods:
For this quality improvement initiative, we convened a multidisciplinary team to construct a presurgical pathway process map and complete root cause analysis. This inquiry revealed that the current condition allowed patients to proceed through the pathway without centralized oversight. Therefore, we appointed an epilepsy surgery nurse manager, and under her direction multiple additional process improvement interventions were applied. We then retrospectively compared pre-intervention (2014–2015) and post-intervention (2016–2017) cohorts of patient undergoing the presurgical pathway. The improvement measures were patient throughput and pathway sojourn times. As a balancing measure, we considered the proportion of potentially eligible patients (epilepsy monitoring unit admissions) who ultimately completed epilepsy surgery.
Results:
Following our intervention, patient throughput was substantially increased for each stage of the presurgical pathway (32%−96% growth). However, patient sojourn times were not improved overall. No difference was observed in the proportion of possible candidates who ultimately completed epilepsy surgery.
Significance:
Although process improvement expanded the number of patients who underwent epilepsy surgical evaluation, we experienced concurrent prolongation of the time from pathway initiation to completion. Ongoing improvement cycles will focus on newly identified residual sources of bottleneck and delay.
Keywords: drug-resistant epilepsy, epilepsy surgery, quality improvement, process improvement
1. Introduction
While antiepileptic medication is effective in treating seizures for the majority of patients with epilepsy, around one third of patients will not achieve adequate seizure control with medication alone.[1] For patients with drug-resistant epilepsy that is focal in onset, surgical therapy offers a 50–80% cure rate; surgery can also palliate seizures for some patients with generalized epilepsy.[2–4] However, epilepsy surgery is inadequately offered to patients with drug-resistant seizures.[5,6] Only an estimated 1% of drug-resistant epilepsy patients undergo surgery in a timely fashion and there is a 20-year average delay from disease onset to surgical treatment.[6–8] Such delays are not benign for epilepsy surgery candidates, who experience diminished quality of life, unemployment, disability, comorbid psychological disease, and a 0.9% annual risk of sudden unexpected death in epilepsy (SUDEP).[8,9]
Recent growth of epilepsy surgery centers and new advancements in epilepsy surgical techniques have improved outcomes, decreased morbidity, and expanded patient eligibility. Yet these developments have not led to a parallel increase in the number of therapeutic epilepsy surgeries performed.[7] The basis for this profound underutilization of surgery, despite robust evidence of efficacy and explicit practice guidelines, is multifactorial. Patient perception and physician lack of knowledge are hypothesized to play a role.[3,10,11] Access, availability, and processes of care delivery are also potential barriers, as assessment for epilepsy surgery only occurs at specialized centers and the presurgical workup can be lengthy and burdensome to patients.[12,13]
To improve availability of epilepsy surgery at our center, we employed an epilepsy surgery nurse manager to coordinate and expedite the presurgical evaluation of patients with drug-resistant epilepsy. This study investigates the impact of our quality improvement initiative through comparison of pre-intervention and post-intervention patient cohorts.
2. Methods
2.1. Context
The setting for this work is a National Association of Epilepsy Centers (NAEC) Level 4 epilepsy center. As part of an academic medical center, a large proportion of our patients have drug-resistant epilepsy; we also see many patients in consultation for a second opinion on invasive therapies. To ascertain if surgical treatment would be beneficial, patients are referred to our epilepsy monitoring unit (EMU) for prolonged video electroencephalogram (EEG) monitoring with the goal of capturing multiple seizures. If it is determined from clinical and electrophysiological analysis that surgery may be an option, patients are recommended to complete several additional studies including: brain positron emission tomography (PET), neuropsychiatric testing, visual field testing, functional MRI (fMRI), and high-resolution brain MRI (if not already available). The results of these studies are then integrated with the full patient history into a formal case presentation in epilepsy surgery conference, which is attended by epileptologists, neurosurgeons, neuroradiologists, neuropsychologists, nurses, EEG technicians, and administrative staff. In that setting, it is again determined if the patient is a candidate for surgery and additional testing may be recommended prior to surgical planning, such as Wada testing, single photon emission computed tomography (SPECT), and magnetoencephalography (MEG). Finally, all this information is reviewed; if a surgical intervention is judged to have reasonably high likelihood of benefit and low associated risk, it will be recommended. Possible surgical procedures are: intracranial electrode implantation to further inform definitive treatment, lesionectomy, lobectomy, laser ablation, responsive neurostimulator (RNS) placement, and vagal nerve stimulator (VNS) placement. The primary neurologist, the primary neurosurgeon, and the patient determine the final care plan.
2.2. Evaluation
We convened a multidisciplinary team of key stakeholders: physicians, nurses, social workers, and administrative staff from the Division of Epilepsy and the Department of Neurosurgery. This team constructed a detailed presurgical pathway process map and completed a root cause analysis that focused on barriers and sources of delay. Careful evaluation revealed that in the current condition, patients proceeded through the evaluation process without centralized oversight. The steps of the pathway were not readily apparent to patients, physicians, or ancillary care providers. Patients were not clearly identified as participants of the presurgical pathway, and therefore could be lost to follow-up at some point during the workup. Lastly, multiple barriers to timely scheduling of EMU admissions, outpatient clinic appointments, outpatient studies, and neurosurgical procedures were identified.
2.3. Intervention
Starting in January 2016, an epilepsy surgery nurse manager began coordination of the presurgical pathway. Several changes were then implemented serially: An explicit, sharable presurgical pathway was created and published in Dorsata, a platform for electronic dissemination of care pathways that allows for integration with the medical record (www.dorsata.com) (Figure 1). A formal tracking system was devised for patients who were discharged from the EMU with the recommendation to be evaluated for surgery. Testing recommendations were routinely clarified and facilitated by the epilepsy surgery nurse manager and fellows. Attention was paid to restructuring the EMU admission process and optimizing EMU bed utilization. Additionally, consultation with the neurosurgeon was coordinated and the neurosurgical operating room schedule was optimized. Lastly, educational materials were developed for patients (Table 1).
Figure 1:
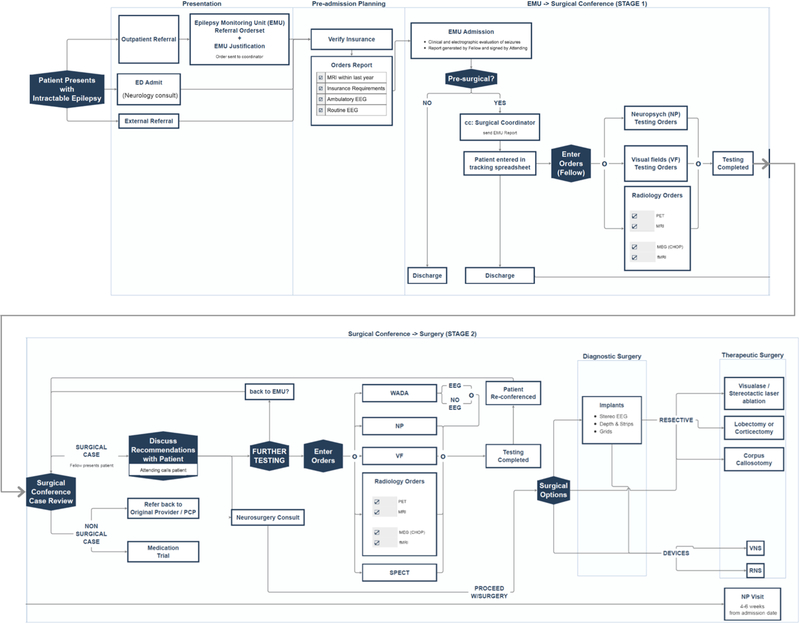
Presurgical Pathway
Table 1:
Root Cause Analysis and Targeted Interventions
| Problem | Countermeasure | Timeline |
|---|---|---|
| Lack of centralized oversight |
Employment of an epilepsy surgery nurse manager |
January 2016 |
| Poor pathway visibility for providers and vague steps |
Publishing of Dorsata pathway | January 2016 |
| Unclear identification of participants |
Tracking system for surgical candidates |
February 2016 |
| Confusion over testing recommendations |
Routine facilitation by nurse manager with contribution by epilepsy fellows |
March 2016 |
| Outpatient scheduling challenges and delays |
Routine facilitation by nurse manager |
April 2016 |
| Inefficient EMU utilization |
Restructuring EMU admission process and attention to EMU occupancy rates |
September 2016 |
| Surgical scheduling delays |
Adjustment to neurosurgery operating room schedule to accommodate greater volume |
October 2016 |
| Pathway components cryptic for patients |
Publication of epilepsy surgery patient guide |
December 2016 |
2.4. Measures
We examined three periods of the presurgical pathway process: 1) from initial EMU presurgical admission to presentation in epilepsy surgical conference (stage 1), 2) from epilepsy surgical conference to surgery completion (stage 2), and 3) from initial EMU presurgical admission to surgery completion (full pathway). For each period of the pathway, we measured both patient throughput and sojourn time. For patients with multiple EMU evaluations and/or conference presentations, we counted the first instance of each in which surgery was recommended. In the case of serial surgeries, such as with electrode implantation followed by lobectomy, the first epilepsy surgery date was utilized. We did not assess the percentage of patients completing each step of the pathway as our clinical documentation lacked sufficient detail to determine why patients did not advance toward surgery (e.g. a patient became well-controlled on antiepileptic medication, a patient decided against an invasive procedure, or a patient’s insurance coverage changed) and therefore we could not accurately specify the denominator. As a balancing metric, we assessed the proportion of all EMU admissions who ultimately completed epilepsy surgery-the goal of our initiative was not simply to perform more surgeries, but to perform more indicated surgeries by increasing the number of patients undergoing screening. Lastly, we noted the type of surgery performed: laser ablation, lobectomy/lesionectomy, RNS implantation, VNS implantation, and intracranial electrode implantation (category for patients who did not proceed to a definitive therapeutic procedure within the study timeframe).
2.5. Analysis
We performed a retrospective comparison of pre-intervention (2014–2015) and post-intervention (2016–2017) patients with drug-resistant epilepsy who underwent presurgical evaluation at our epilepsy center. To more accurately measure the impact of our intervention, we limited the cohorts to patients that completed a particular stage within their assigned two-year timeframe (2014–2015 or 2016–2017). For example, a patient who was presented in surgical conference in 2015 and then underwent surgery in 2016 was not included in our analysis. Additionally, patients may have had surgery without participation in this pathway, such as a patient undergoing VNS placement that did not require presentation in surgical conference. Therefore, this study does not represent a comprehensive assessment of all the epilepsy surgery patients at our center from 2014–2017.
We used descriptive statistics, the Wilcoxon rank sum test, and the chi-square test to compare patient throughput, sojourn times, and the proportion of patients advanced to surgery for the two cohorts. We employed run charts to assess our performance over time and look for evidence of non-random improvement with each intervention.[14] Statistical analysis was performed with Stata version 14.0. Statistical significance was defined as p<0.05.
2.6. Ethical Considerations
This quality improvement initiative was exempt from institutional review.
3. Results
In total, there were 546 patient admissions (median 23 per month, interquartile range 20–25) to the EMU in the pre-intervention period and 638 patient admissions (median 27 per month IQR 24–29; p<0.001) in the post-intervention period. Not all of these patients were recommended for surgical evaluation, and the subsequent results reported include only those patients categorized as potential surgical candidates at the time of EMU discharge.
Patient throughput was increased for each part of the pathway in the post-intervention period compared to the pre-intervention period. There was 36% growth in the EMU to conference presentation stage (50 vs. 68 patients), 92% growth in the conference to surgery completion stage (26 vs. 50 patients), and 43% growth for the full pathway of EMU to surgery completion (28 vs. 40 patients).
Sojourn time was unimproved for both stage 1 and stage 2 of the presurgical pathway, as illustrated by run chart assessment (Figures 2a and 2b). The median sojourn times for the pre-intervention and post-interventions cohorts were similar for stage 1 (66 days IQR 34–129 vs. 90 days IQR 52–128; p=0.22) and for stage 2 (115 days IQR 43–139 vs. 117 days IQR 60–167; p=0.51). However, the median duration of the full pathway was increased in the post-intervention period (164 days IQR 92–231 vs. 219 days IQR 153–298; p=0.02) (Figure 2c).
Figure 2: Sojourn Times.
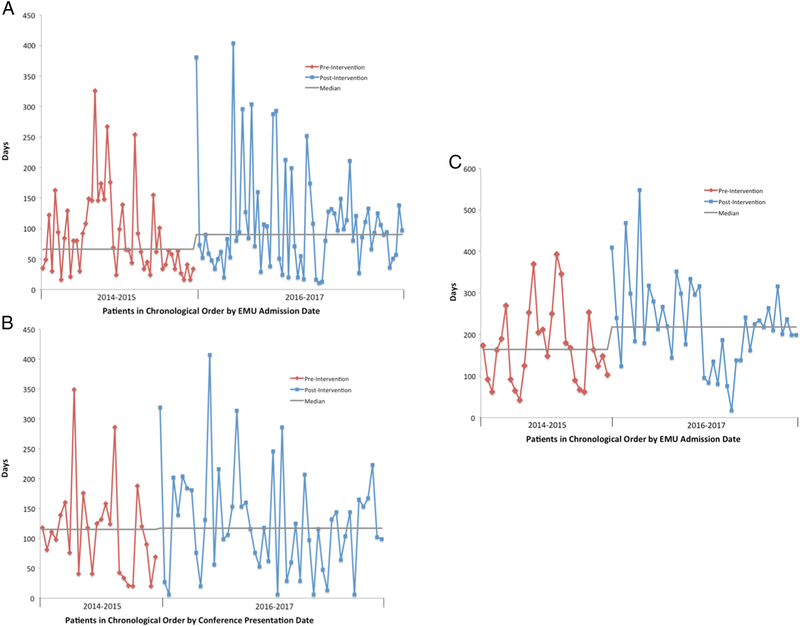
A) from EMU evaluation to conference presentation, B) from conference presentation to surgery completion, and C) from EMU evaluation to surgery completion.
The proportion of patients evaluated in the EMU who were advanced to surgery was similar across the two study periods: 5.1% (28/546) in the pre-intervention period and 6.3% (40/638; p=0.40) in the post-intervention period. The distribution of final surgical procedures performed differed between cohorts. In the pre-invention period, 5 patients (18%) had laser ablation, 11 (39%) had lobectomy/lesionectomy, 1 (4%) had RNS implantation, 7 (25%) had VNS implantation, and 4 (14%) had electrode implantation only. In the post-intervention period, 16 patients (40%) had laser ablation, 3 (7.5%) had lobectomy/lesionectomy, 7 (17.5%) had RNS implantation, 6 (15%) had VNS implantation, and 8 (20%) had electrode implantation only.
4. Discussion
Employment of an epilepsy surgery nurse manager and subsequent implementation of process improvements considerably increased the volume of patients evaluated for epilepsy surgery at our center. This growth was observed at each stage of the presurgical pathway, from EMU admission to conference presentation to epilepsy surgery completion. However, we did experience a concurrent prolongation of the average evaluation time.
Applying quality improvement methodology to assess barriers to care, we established that a multipronged approach was crucial in improving patient access to surgical evaluation. Investigation of the current condition identified a collection of key stakeholders including physicians, nurses, social workers, administrative staff, and patients. The major themes that emerged from our root cause analysis were: 1) supervision and oversight of the pathway, 2) visibility of the protocol and awareness of its components, and 3) logistics of scheduling. For other health systems that wish to apply a similar methodology to improve their presurgical process, we highly recommend careful consideration of all of these participants and themes when developing specific, responsive countermeasures appropriate to the local environment. While other types of professionals may be considered for the role of overseeing the presurgical pathway, the skillset necessary for this undertaking includes familiarity with complex neurological care, comfort with patient communication, sophisticated managerial skills, and experience with quality improvement methodology; we believe an epilepsy nurse manager is particularly well-suited to this job.
While the duration of evaluation for an individual patient was longer post-intervention (median 164 vs. 219 days), the clinical significance of <2 months prolongation is unknown. Providing a larger number of epilepsy patients access to surgery each year may well be worth the tradeoff of a slightly longer average individual evaluation time. Additionally, while increased patient throughput regrettably caused downstream bottlenecks, this did allow identification of secondary targets for optimization. We now have established several objectives for subsequent intervention. Timely scheduling is often delayed by patient preferences (e.g. a student who needs to wait for a school break), but some of these issues may improve as we continue to streamline the necessary workup, such as offering scheduling of all testing in a single day. Idiosyncratic requirements of different insurance companies are a common reason for delayed testing and procedures, and we are currently working with the financial department of our health system to simplify this process. For patients who already have a VNS in place, obtaining brain MRI becomes more involved and we are developing a standardized approach. Furthermore, the expanded patient volume that we were able demonstrate was vital in justifying mobilization of resources within the health system. For example, because of the exhibited growth, we have received support from the health system to purchase additional equipment for intracranial monitoring and expand the number of dedicated EMU beds. With this and other developments, we hypothesize seeing a decrease in average time to surgery for an individual patient within the next year or two.
Balancing measures are a critical component of quality improvement work and are designed to bring to light any unintended consequences of process manipulation. Epilepsy surgery appears to be underutilized, and while it is difficult to ascertain the magnitude of this problem, it has been estimated that around 1.5–3% of patients with a new diagnosis of epilepsy will require surgery.[13] Though drug-resistant epilepsy patients are overrepresented at our epilepsy center, we would not anticipate that a markedly greater percentage of our patients warrant epilepsy surgery. Additionally, no neurosurgical technique became newly available during this study (thus no expansion of patient eligibility between study periods) and our intention was to optimize the presurgical evaluation process not to increase surgical volume. Therefore, we were reassured to see that despite screening more patients, a comparable proportion of all EMU admissions completed surgery before and after intervention. A second worthwhile balancing measure would be the cost of our intervention, however formal cost analysis was outside the scope of this study. The addition of personnel and time spent on the presurgical pathway certainly comes at an expense, but this spending is likely justifiable both from the hospital’s perspective and from society’s perspective. Of note, a detailed analysis of cost was undertaken by our hospital prior to hiring our epilepsy nurse manager (including consideration of technical and professional rates for EEG studies, EMU care, epilepsy surgery, and outpatient visits as well as any additional staffing needs) and we found the economics to be favorable such that we could proceed with our intervention. Looking at only the largest contributors and not including downstream revenue (e.g. presurgical imaging, neuropsychological testing) the expense of an epilepsy nurse manager (annual salary ~$110,000) may be recovered through a small increase in the number of patients completing epilepsy surgery each year (average contribution margin per EMU hospitalization ~$7,000, average contribution margin per epilepsy surgery ~$11,000).
While the essential services, facilities, and staff for epilepsy surgical centers (level 3 and level 4) have been specified by the National Association of Epilepsy Centers (NAEC),[15] there is minimal guidance regarding the optimal structure for presurgical care delivery. Additionally, little evidence exists concerning either appropriate process metrics or benchmarks for length of evaluation. A previously published study reported presurgical evaluation durations of 5–9 months,[12] which is in keeping with our findings. Though it seems intuitive that faster is better, resources are limited and efficiency of care must be balanced with quality and cost.[16] Our results showed a statistically significant prolongation of evaluation time, but the interpretation of clinical significance is less straightforward. For future studies, it would be beneficial to develop standardized quality measures and time goals for presurgical evaluation.
The most notable limitation to this study was analysis of a heterogeneous patient population with incomplete knowledge of individual patient circumstances and provider considerations. It is possible that some of these unmeasured variables, rather than our QI intervention, influenced pace of advancement through the presurgical pathway. Similarly, we did not have comprehensive, accurate record of why and when patients dropped out. Additionally, there may have been external factors that impacted the pathway, such as changes in provider practices or insurance coverages/requirements. While our interventions occurred serially, we did not provide sufficient spacing between to truly unpack which factors contributed most substantially to patient throughput. Consideration of the outcome metric of epilepsy surgery outcome (seizure frequency and/or quality of life) would be ideal, but requires several years of follow-up and thus was impractical in the context of a quality improvement initiative.
Our study demonstrates that targeted attention to barriers in the presurgical evaluation process can increase patient throughput. However, further process improvement cycles are necessary as the resultant volume highlighted secondary bottlenecks. Development of standardized quality metrics and explicit time goals would be helpful for epilepsy centers invested in improving surgical evaluation for drug-resistant patients.
Highlights.
Barriers to epilepsy surgery can be detrimental to drug-resistant epilepsy patients
We employed an epilepsy surgery manager to facilitate presurgical evaluation
Volume of patients evaluated for surgery increased substantially (32%−96% growth)
Evaluation time lengthened, which revealed secondary targets for process improvement
Acknowledgement
We would like to thank Colleen Ryan, Josh Dahlerbruch, Karla Fausto, Katherine Deily, and Grace Eckels for their contributions.
Funding sources
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) T32 Award in Neurologic Clinical Epidemiology (T32-NS-061779) as well as the Mirowski Family Fund and the Family of Johnathan Rothberg.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None of the authors has any conflict of interest to disclose.
References
- [1].Kwan P, Brodie MJ. Early Identification of Refractory Epilepsy. N Engl J Med 2000;342:314–9. [DOI] [PubMed] [Google Scholar]
- [2].Schmidt D, Stavem K. Long-term seizure outcome of surgery versus no surgery for drug-resistant partial epilepsy: a review of controlled studies. Epilepsia 2009;50:1301–9. [DOI] [PubMed] [Google Scholar]
- [3].Jobst BC, Cascino GD. Resective Epilepsy Surgery for Drug-Resistant Focal Epilepsy. JAMA 2015;313:285. [DOI] [PubMed] [Google Scholar]
- [4].Englot DJ. A modern epilepsy surgery treatment algorithm: Incorporating traditional and emerging technologies. Epilepsy Behav 2018;80:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Haneef Z, Stern J, Dewar S, Engel J. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010;75:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Burneo JG, Shariff SZ, Liu K, Leonard S, Saposnik G, Garg AX. Disparities in surgery among patients with intractable epilepsy in a universal health system. Neurology 2016;86:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Engel J Why is there still doubt to cut it out? Epilepsy Curr 2013;13:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, et al. How long does it take for partial epilepsy to become intractable? Neurology 2003;60:186–90. [DOI] [PubMed] [Google Scholar]
- [9].Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol 2008;7:1021–31. [DOI] [PubMed] [Google Scholar]
- [10].Erba G, Messina P, Pupillo E, Beghi E, OPTEFF Group. Acceptance of epilepsy surgery among adults with epilepsy — What do patients think? Epilepsy Behav 2012;24:352–8. [DOI] [PubMed] [Google Scholar]
- [11].Dewar SR, Pieters HC. Perceptions of epilepsy surgery: A systematic review and an explanatory model of decision-making. Epilepsy Behav 2015;44:171– 8. [DOI] [PubMed] [Google Scholar]
- [12].Drees C, Sillau S, Brown M-G, Abosch A. Preoperative evaluation for epilepsy surgery. Neurol Clin Pract 2017;7:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jetté N, Sander JW, Keezer MR. Surgical treatment for epilepsy: the potential gap between evidence and practice. Lancet Neurol 2016;15:982–94. [DOI] [PubMed] [Google Scholar]
- [14].Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf 2011;20:46–51. [DOI] [PubMed] [Google Scholar]
- [15].Labiner DM, Bagic AI, Herman ST, Fountain NB, Walczak TS, Gumnit RJ, et al. Essential services, personnel, and facilities in specialized epilepsy centers--revised 2010 guidelines. Epilepsia 2010;51:2322–33. [DOI] [PubMed] [Google Scholar]
- [16].Kissick W Medicine’s Dilemmas: Infinite Need Versus Finite Resources New Haven and London: Yale University Press; 1994. [Google Scholar]


