Abstract
Glucocorticoid receptor (GC), a founding member of the nuclear hormone receptor superfamily, is a glucocorticoid-activated transcription factor that regulates gene expression and controls the development and homeostasis of human podocytes. Synthetic glucocorticoids are the standard treatment regimens for proteinuria (protein in the urine) and nephrotic syndrome (NS) caused by kidney diseases. These include minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN) and immunoglobulin A nephropathy (IgAN) or subsequent complications due to diabetes mellitus or HIV infection. However, unwanted side effects and steroid-resistance remain major issues for their long-term use. Furthermore, the mechanism by which glucocorticoids elicit their renoprotective activity in podocyte and glomeruli is poorly understood. Podocytes are highly differentiated epithelial cells that contribute to the integrity of kidney glomerular filtration barrier. Injury or loss of podocytes leads to proteinuria and nephrotic syndrome. Recent studies in multiple experimental models have begun to explore the mechanism of GC action in podocytes. This review will discuss progress in our understanding of the role of glucocorticoid receptor and glucocorticoids in podocyte physiology and their renoprotective activity in nephrotic syndrome.
Keywords: Glucocorticoid receptor, Podocyte, Nephrotic syndrome, focal segmental glomerulosclerosis
1. GR Signaling
Glucocorticoid receptor (GR) is a founding member of the nuclear hormone receptor (NHRs) that control homeostasis, differentiation, proliferation and animal development. NHRs bind their cognate hormones and regulate the expression of a complex genetic network, in which their coordinated activity defines the physiological, hormonal responses. A key function of NRs is to mediate transcriptional regulation in response to hormones and other metabolic ligands through the recruitment of an array of positive and negative regulatory proteins, referred to as co-activators or co-repressors.
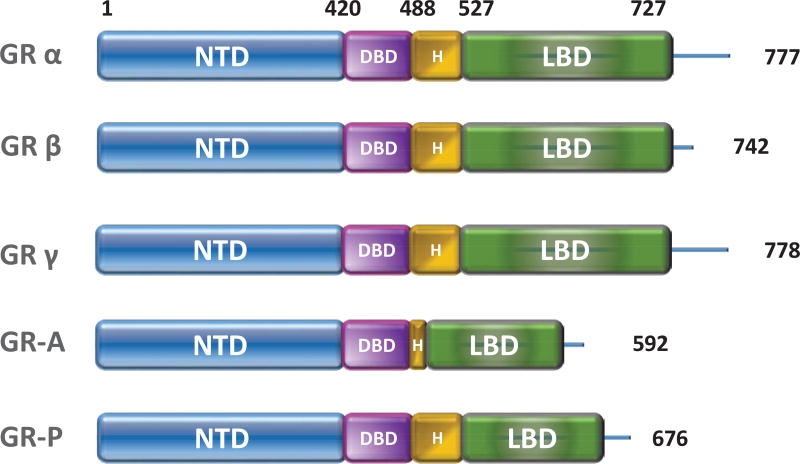
GRα is composed of four functional domains, the N-terminal ligand-independent transactivation domain (NTD) or activation function 1 (AF-1), the DNA-binding domain (DBD), the flexible hinge region and the ligand-binding domain (LBD). The LBD contains 12 helices including the ligand-binding pocket (helices 3, 4, 5 and 12) and the AF2 domain (Figure 1). Glucocorticoid binding to the hydrophobic pocket of the LBD triggers a conformational change, thereby unmasking the LBD from the AF2 domain followed by subsequent co-activator binding [1, 2]. The AF1 and AF2 domains have been shown to activate transcription through its interaction with the basal transcriptional machinery and transcriptional co-activators [3].
Figure 1. The GR family proteins.
Human GR harbors three functional domains: N-terminal domain (NTD), middle DNA-binding domain (DBD) and the C-terminal ligand-binding domain (LBD). The DBD and LBD are linked by the hinge region (HR). Alternative splicing of the NR3C1 (gene encoding GR) gene generates the isoforms GRα, GRβ, GRγ, GR-A, and GR-P, which differ in size and sequence of HR and/or LBD.
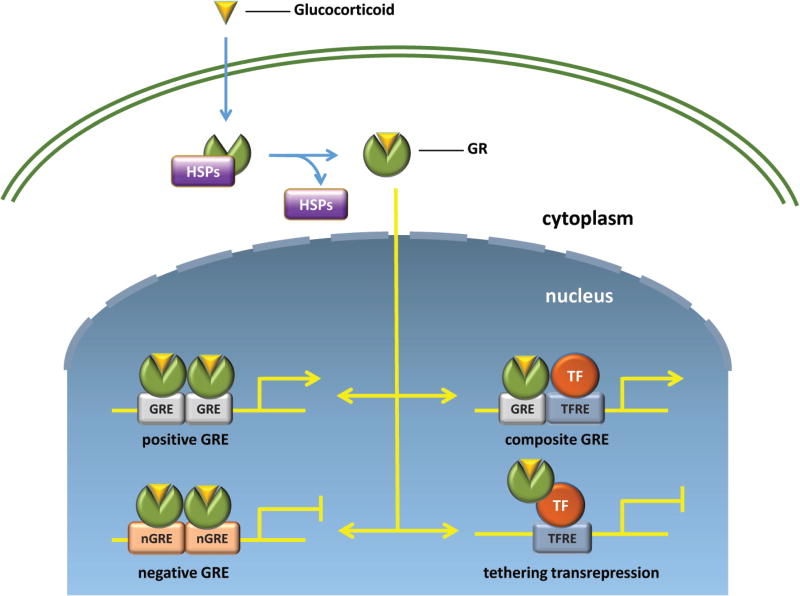
Glucocorticoid signaling is primarily dependent on GR-mediated transcription and protein synthesis [4]. In the absence of hormone, the GR resides in the cytoplasm as part of a large multiprotein complex that includes chaperone proteins such as HSP90 [5, 6]. Upon ligand binding, GR dissociates from the chaperone proteins and translocates into the nucleus, where it regulates transcription through multiple distinct modes of action (Figure 2). As a homodimer, it binds a cognate DNA sequence present in enhancers containing glucocorticoid response elements (GREs) to activate gene expression [7, 8]. In addition to homodimerization, GR also directly interacts with MR or AR to form heterodimers [9]. Furthermore, ligand-bound monomeric GR binds composite GC-responsive regions with additional transcription factors such as signal transducer and activator of transcription (STAT), and cAMP response element-binding protein (CREB) and potently induce glucocorticoid-mediated gene expression [10–12]. The recruitment of several coactivators, including histone modifying enzymes and chromatin modulators promotes chromatin remodeling and subsequent transcription initiation [13–16]. The GR homodimers also bind specific sequences called negative GREs (nGREs) in the promoter region of several target genes and repress their transcription [17]. Lastly, in contrast to the dimer, ligand-bound GR monomeric is capable of transrepressing transcription through its interactions with other transcriptional regulators, such as nuclear factor kappa B (NF-κB) and activating protein-1 (AP-1). These interactions block co-activator recruitment and promote co-repressor recruitment, thereby altering chromatin structure and repressing target gene expression [18–20].
Figure 2. Molecular mechanism of GR signaling pathways.
Glucocorticoids diffuse across the cell membrane to the cytosol, where they bind GR. Glucocorticoid binding promotes dissociation of GR from chaperone proteins (HSPs) and subsequent nuclear translocation. Once in the nucleus, GR forms hetero- or homodimers and interacts with DNA to control gene transcription. Ligand-bound GR can lead to either activation or repression of gene transcription. TF: transcription factor; GRE, glucocorticoid response element; nGRE, negative glucocorticoid response element; TFRE: transcription factor response element.
2. Glucocorticoids (GCs)
As a ligand-dependent transcription factor, the physiologic and pharmacologic action of GR is primarily mediated by the glucocorticoids (GC). The synthesis and release of GCs are under dynamic circadian regulation by the hypothalamic-pituitary-adrenal axis [21, 22].
Synthetic GCs are drugs that mimic the action of natural GCs. Dexamethasone (Dex), prednisone/prednisolone, and budesonide are the most commonly prescribed synthetic GCs [23, 24]. Synthetic GCs are prescribed for chronic inflammatory diseases, including autoimmune disorders, allergies, asthma and skin infections [25]. In addition to their anti-inflammatory properties, GCs have been used in conjunction with cancer chemotherapy to reduce side effects [26]. Importantly, synthetic GCs, such as Dex and prednisone/prednisolone, are therapeutically effective in treating nephrotic syndrome [27, 28]. Notably, it has been proposed that Dex can directly act on the glomerular podocytes contributing to its therapeutic effects [29].
3. GR Target Genes
Genome-wide analyses of GR-regulated genes and GR-binding sites in different cell types and tissues have recently been reported [30–32]. These experiments reveal the characteristics of genome-wide profiling of GR and genome-wide inventory of GR-binding sites. These results provide an exciting global view of the GR target genes and tissue-specific modes of GR action and potentially contribute to our understanding of glucocorticoid action. ChIP-seq studies showed that GR binding sites are not present in isolation but are often surrounded by binding motifs for other transcriptional factors such as AP-1 [33].
It is striking that GR selectively regulates transcription in a cell-specific manner. Chromatin accessibility is a significant contributor to the determination of the tissue-specific GR binding profiles and the primary determinant for tissue-specific chromatin accessibility is the cell type-specific expression of other transcription factors. Most GR target genes are involved in metabolism, signal transduction, inflammation and the immune response [34–36]. These GR target genes include both induced and repressed genes that are associated with known GC functions. Consistent with their ability to modulate the expression of inflammation-associated genes, GCs are widely used in medical therapy for immunosuppression and anti-inflammatory agents. However, GCs’ broad effects on different tissues can cause unwanted side effects such as bone loss and glucose dysregulation. It is hopeful that the information extracted from ChIP-seq and RNA-seq data in different tissues will provide mechanistic insights into a better understanding of GCs’ global effects and ultimately help develop agents that alleviate unwanted side effects.
4. Podocytes and Nephrotic Syndrome
4.1. Glomerular podocytes
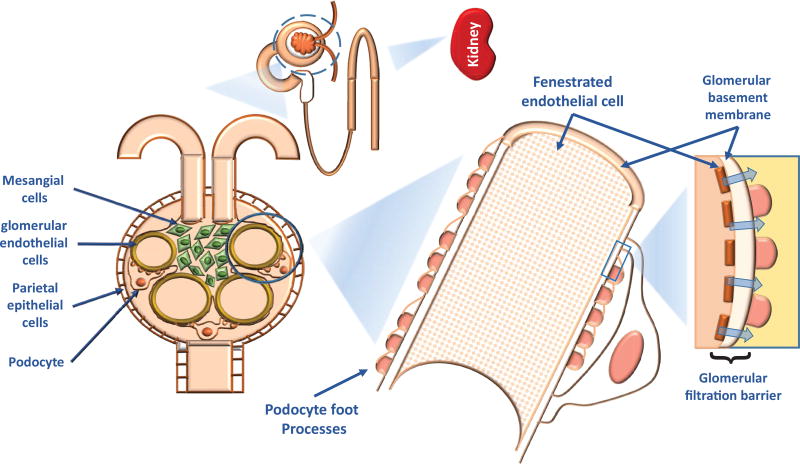
One of the crucial functions of the kidney is to remove toxins and metabolic waste while preventing proteins larger than albumin from entering the urine. The glomerulus is the functional unit required for blood plasma filtration and primary urine production [37]. Four distinct cell types assemble to form the glomerulus: glomerular endothelial cells, mesangial cells, podocytes, and parietal epithelial cells (PECs) (Figure 3, [38]). Podocytes are fully differentiated epithelial cells covering the outer surface of the glomerular basement member (GBM) and are critical for maintaining the integrity of the glomerular filtration barrier (GFB) [39]. The podocyte has a unique cell architecture that consists of an arborized cell body, primary processes, and secondary foot processes [40]. The long-interdigitated foot processes wrap around glomerular capillaries between adjacent podocytes and form the filtration slits, which are spanned by the slit diaphragms (SD), a highly specialized membrane-like cell-cell junctions. The cell body contains a nucleus and most of the cytoplasm, while the foot processes include primarily a dense network of actin filaments connected with an array of transmembrane proteins that link the SD and the GBM anchor proteins [41, 42]. The unique structure of the cell primary and secondary processes are maintained by the highly-organized cytoskeleton (Figure 3).
Figure 3. A diagram showing the structure and components of the renal glomerular filtration system, from the kidney to podocyte.
The glomerular filtration barrier consists of fenestrated endothelial cells, glomerular base membrane, and podocytes.
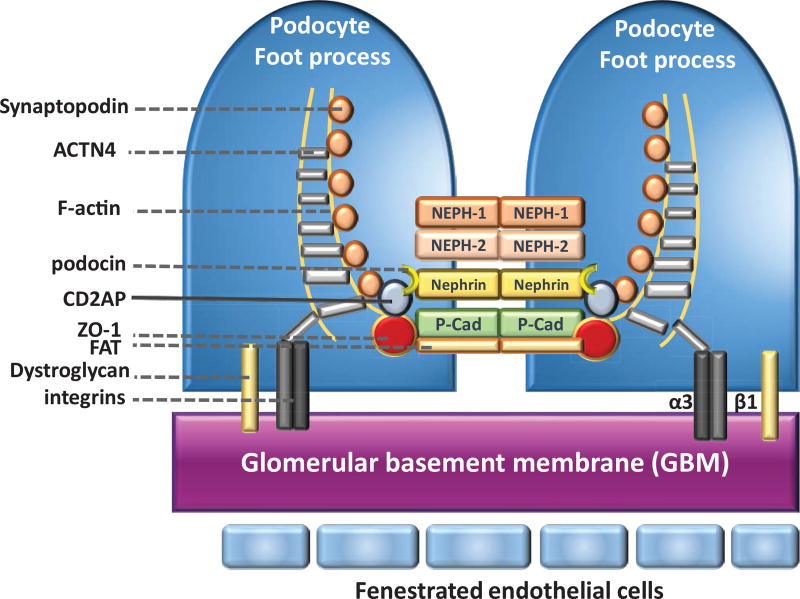
The highly-specialized podocytes SD structure is in charge of macromolecular filtering and connects the podocyte actin cytoskeleton to transmembrane proteins and receptors and regulates plasticity of the foot process. As such, SD is a unique structure mediating cell-cell interactions, and possibly relaying extracellular signaling stimuli [42]. A growing number of molecular components of mature SD have been identified, many of which are components of tight and adherent junctions. Both nephrin and podocin are podocyte-specific proteins that are found only in the SD [43–45]. Other proteins associated with this unique structure include CD2-associated protein (CD2AP), transient receptor potential channel 6 (TRPC6), alpha-actinin 4 (ACTN4), P-cadherin, FAT1, synaptopodin (Synpo), α- and β-catenin, zonula occludens-1 (ZO-1), nephrin homologue NEPH-1, and Wilms’ tumour suppressor 1 (WT1) [46–53] (Figure 4). These podocyte proteins are associated with survival, differentiation, and unique cytoskeleton-dependent morphology of the podocytes [54, 55].
Figure 4. A schematic diagram depicting components of the podocyte slit diaphragm and foot processes and slit diaphragm proteins.
Proteins that make up the SD between adjacent foot processes are depicted. Nephrin, NEPH1, NEPH2, P-cadherin, and FAT are membrane-spanning proteins that have large extracellular domains that are important for signaling events that determine the structural integrity of podocyte foot processes. These proteins include the slit diaphragm interact with intracellular adapter proteins, including CD2-AP, ZO-1, Synaptopodin, and ACTN4. The adapter proteins bind to filamentous actin (F-actin). The adhesion molecules dystroglycan and α3β1 integrin anchor the podocyte to the underlying glomerular basement membrane (GBM).
4.2. Podocyte injury
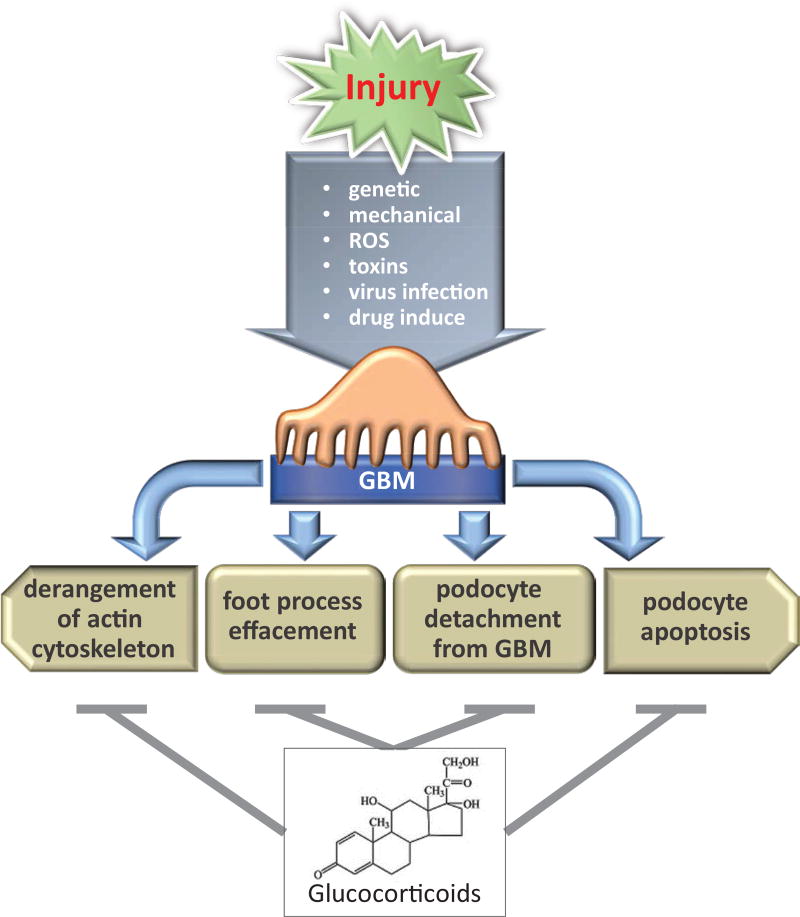
Podocytes play a critical role in the preservation of the integrity of the GFB under normal conditions and are the target of many forms of physiological stress and pathological states. Podocytes respond to genetic, mechanical, reactive oxygen species (ROS), immunological stresses, toxins, viral infection and drugs [56, 57]. Podocyte injury occurs when excessive stress disrupts homeostasis. The beginning of podocyte injury includes derangement of the actin cytoskeleton [58, 59], loss of SD proteins and structural integrity, which lead to subsequent foot process effacement and podocyte detachment from GBM or apoptosis [60, 61]. It is widely accepted that podocyte injury results in proteinuria and nephrotic syndrome (Figures 3 and 5).
Figure 5. The mechanism of the podocyte injury and the protective effected by glucocorticoids.
Several causes are known to contribute to podocyte injury. After the injury, podocytes can undergo cytoskeleton derangement, effacement, detachment or apoptosis. The mechanisms by which glucocorticoids exerts its renoprotective effect involve several mechanisms that protect podocyte from injury.
Upon injury, podocytes undergo apoptosis [62], which lead to a decrease in podocyte number. In the classical view, apoptosis has long been considered to be the primary cause of podocyte loss. Podocytes undergo apoptosis during the pathogenesis of the glomerular disease, as well as in mice exposed to PAN (puromycin aminonucleoside) treatment [62–64]. Podocyte detachment from the GBM is a terminal event in podocyte injuries, which can promote further glomerular damage [65–67]. The detachment of podocytes from GBM occurs in regions of sclerotic lesions of the glomerulus and consequently increases the appearance of podocytes and podocyte-associated molecules in urine.
4.3. Nephrotic syndrome
NS represents a term for a collection of conditions [68]. It is a kidney disorder that causes the body to excrete too much protein in the urine [69]. The key features of NS are proteinuria, hypoalbuminemia, hypercholesterolemia, and edema. In children, proteinuria is defined as more than 0.1g of urine protein per square meter of body-surface area per day (Note: proteinuria is age-dependent in the child, much higher in the neonate). In adults, the nephrotic syndrome is defined as a urine protein level of more than 3.5 g per day [70].
Based on kidney biopsies, NS patients can be diagnosed more specifically, including minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy and immunoglobulin A (IgA) nephropathy among others [71–74] (Table 1). FSGS can be further broadly classified as primary or adaptive. Primary FSGS is caused by monogenic alteration events, while adaptive FSGS, also referred to as secondary to FSGS, is associated with glomerular dysfunction associated with other diseases. This review will focus on primary FSGS. Normally, kidneys clear waste materials from the body and maintain a healthy balance of fluids and electrolytes in the blood. Upon the damages of the filtering units of the kidney, proteins that are usually kept in the plasma leak into the urine in large amounts. Various diseases, such as diabetes mellitus, hypertension, lupus erythematosus and viral infections, can damage glomeruli, resulting in proteinuria and NS [75–79]. Most NS in young children are idiopathic FSGS or frequently MCD, which is considered a less severe form of FSGS [80]. In adults, FSGS is the most common form of the glomerular disease [81] and a leading cause of the primary NS. FSGS accounts for 20% of NS in children and 40% in adults. FSGS is also the leading cause of glomerulonephritis-associated end-stage renal disease (ESRD) [82].
Table 1. The pathology and steroid responses for nephrotic syndrome.
MCD, minimal change disease; FSGS, only include primary (idiopathic); MN, (primary) membranous nephropathy; IgAN, IgA nephropathy; IST, immunosuppressive therapy; Effective, means decreased proteinuria and/or slowing the progression of renal function.
| Pathology | Steroid response | |
|---|---|---|
| MCD | foot processes effacement | Very good |
| FSGS | foot processes effacement and perihilar or sclerosis | Effective but may need other IST; relapse and resistant occurs |
| MN | Subepithelial deposition of the basement membrane on the outer surface of the capillary wall. | Effective, in combination with other IST |
| IgAN | IgA immune complex deposition in the mesangium | Effective, but with significant adverse effects |
FSGS is viewed as a podocyte disease or “podocytopathy” [83, 84]. This is because mutations in several genes encoding components of the SD, cell-membrane, actin-cytoskeleton and signal transduction complexes in podocytes are associated with FSGS [85–91]. More than 50 genes have also been identified as the disease-causing genes for NS [92–100]. The goal of NS therapy is to preserve kidney function and achieve remission of proteinuria [101, 102]. GCs are more effective for the treatment of MCD, but commonly require adjunctive therapy with additional agents for FSGS patients (Table 1). The calcineurin inhibitors, such as cyclosporine and tacrolimus, are widely used in the treatment of steroid-resistant NS (SRNS), of which the majority are FSGS [103, 104]. The significant effects of calcineurin inhibitors are stabilization of the podocyte actin cytoskeleton, and subsequent reduction in proteinuria, independent of its impact on the immune system.
Increasing evidence from patients and experimental models have implicated an essential role for the immune system in the pathogenesis of idiopathic nephrotic syndrome. Several excellent reviews have thoroughly discussed this topic [105, 106]. Indeed, the chimeric anti-CD20 monoclonal antibody, rituximab, originally used to treat many B cell lymphomas, has beneficial effects in ameliorating proteinuria [107]. Taken together, the use of immunosuppressive therapy in the treatment of non-genetic forms of NS suggests a role for the impaired immunity in the pathogenesis of NS.
The NF-κB transcription factor family of proteins plays a crucial role in the regulation of the induction and resolution of inflammation. Accumulated evidence suggests the involvement of NF-κB activation induced by pathogenic agents in experimental NS models and NS patients. NF-κB activation has been demonstrated in glomerular cells such as podocytes, mesangial cells, tubular and endothelial cells upon renal injury or after exposure to inflammatory stimuli both in vivo and in vitro [108–110]. Several NF-κB-inducible genes and their encoded proteins including angiotensin II and cytokines, such as IL-1, IL-8, E-selection and MCP-1 are associated with the progression of glomerulonephritis, tissue injury in nephrotoxicity and other renal diseases, including glycosylated IgA [111–119]. Dysregulation of the activity of canonical NF-κB, p50/p65 (RelA), in podocytes has pathogenic consequences in glomerular diseases [120]. For example, activation of NF-κB contributes to HIV-associated nephropathy (HIVAN) [121]. This aberrant NF-κB activation specifically has a role in enhancing the effects of the TNF family of receptors on podocytes including the activities of Fas/FasL and TNFR2 [122]. Other reports indicate that activation of the ERK pathway and subsequent nuclear translocation of NF-κB are necessary for Ang II-induced TRPC6 accumulation and podocyte apoptosis [123] and that NF-κB activity mediates PAN-induced glomerular injury and proteinuria [124]. Collectively, these observations indicate that NF-κB is an important mediator of pathogenic processes in glomerulopathies and that balanced NF-κB activity is critical to maintaining glomerular integrity and function. Because NF-κB family proteins are present in most renal and the immune cells, the ability of GCs to transrepress NF-κB transcriptional activity in these cell types contributes to their overall efficacy when treating NS [125, 126].
5. The Effects of Glucocorticoid Therapy on Nephrotic Syndrome
Glucocorticoids have an essential role in podocyte development and treatments for nephrotic syndrome [127]. The physiologic and pharmacologic actions of GCs are mediated by GRα [128–130]. Ligand-bound GR induces or represses the transcription of target genes through direct binding to DNA or association with other transcription factors. Glucocorticoids have been used as immunosuppressive drugs for many diseases by reducing inflammation [131]. It has been a long-established clinical practice to use GCs to treat kidney disease. Recent studies in multiple experimental models have begun to explore the direct and indirect effects of GCs in podocytes to better understand its renoprotective activity as well as its unwanted effects.
The remission rates of GC therapy of NS vary between patients, depending on age, initial renal function, and the pathological features of NS [70, 132]. Based on their steroid responsiveness, patients are classified as steroid-sensitive and steroid-resistant. Genetic mutations that affect glomerular podocyte function, such as NPHS1, NPHS2, and WT1 [133–137], account for most steroid-resistant cases and patients with genetic forms of steroid-resistance are less responsive to immunotherapeutic drugs. The circulating factor, soluble urokinase receptor, has been considered a cause for the development of SRNS [138, 139]. SRNS in adults has been defined as the persistence of symptoms after a 4-month trial of therapy and will inevitably progress to ESRD [140]. Alternative therapeutic strategies, including calcineurin inhibitor therapy, alkylating agents, and angiotensin-converting enzyme inhibitors, have been used to reduce proteinuria in steroid-resistant patients with FSGS [141].
Corticosteroid therapy has been used in childhood NS since the 1950s. GC therapy is more effective alone for children with MCD, but usually requires a combination with additional agents for adult NS [102, 142, 143] (Table 1). Children with NS are treated with oral prednisone for 2 to 3 months [81]. A combination of higher doses and increased duration of prednisone therapy can lead to enduring remission. Eighty percent of children with MCD respond to steroid therapy [143]. Thus, therapeutic decisions in children with SRNS are based on the underlying etiology [80, 144]. In contrast, adults with the NS usually undergo renal biopsy prior to the initiation of therapy. The renal biopsy is essential to determine the nature and severity of the glomerular processes and to clarify the type and causes of the glomerular nephropathy [145]. Patients whose biopsies demonstrate more cellular lesions are associated with a poor therapeutic response [146, 147]. Approximately 35% percent of adult patients fail to respond to initial steroid treatment and do not attain remission [102, 142]. A standard procedure for adults with FSGS is high dose glucocorticoid therapy for a significantly longer duration [148]. For patients who have a well-preserved renal function, initial high-dose prednisone is given for 3 to 4 months. However, complete remission rates for glucocorticoid therapy in adults with primary FSGS was quite disappointing [142, 148]. Consequently, there is less evidence to support steroid therapy for adaptive or genetic forms of adult FSGS patients. Thus, understanding the mechanism underlying steroid-resistance is an urgent matter for NS therapy.
5.1. The mechanisms underlying steroid-resistant nephrotic syndrome (SRNS)
Our current understanding of the mechanism underlying SRNS remains rather limited. This is in part due to the broad effects GCs have on multiple cell types through distinct mechanisms. Furthermore, the complexity and heterogeneity of SRNS make it difficult to establish correlations with genetic alterations. It is estimated that over 50 genes are associated with SRNS showing a different spectrum of phenotypes ranging from autosomal recessive to dominant and their onset from within 3 months after birth to late in adulthood [149]. Mutations in several early onset SRNS genes encode podocyte slit-diaphragm-associated proteins, indicating an important role for podocytes integrity in the pathogenesis of SRNS. Notably, several of these genes including NPHS1 [130], NPHS2 [150], TRPC6 [151] and CD2AP [152] are induced by Dex. However, Dex may have unwanted effects by inducing the expression of genes that promote further injury to glomeruli [153, 154]. Furthermore, mutations of ACTN4, which encodes a well-known cytoskeletal protein, are tightly associated with steroid-resistant FSGS. Our lab has recently reported that ACTN4 is a transcriptional coactivator for GR and FSGS-associated mutations are defective in GR-mediated transcriptional regulation [153]. Moreover, earlier reports indicated ACTN4 deficiency is found in multiple human primary glomerulopathies including sporadic FSGS, MCD, and IgA nephropathy [155–158]. It will be intriguing to learn whether other SRNS-associated genes play a physiological role in GR signaling networks or are GR downstream targets.
6. The Role of Glucocorticoids in Podocytes
6.1. The direct effects of GCs on podocytes
The glucocorticoid receptor, as well as the major GR transcriptional cofactors, are expressed in human podocytes [18, 127, 130]. In order to determine whether podocytes are the key cell type that responds to glucocorticoid therapy, recent studies in murine and human podocytes have shown that Dex directly regulates podocyte morphology and function (Figure 5). Mathieson et al. first evaluated the direct effect of Dex on immortalized human podocytes (HPCs) in vitro. Dex treatment (100 nM ~ 10 µM) up-regulated NPHS1 expression, and down-regulated VEGF, as well as CDKN1A (cyclin kinase inhibitor p21) and inflammation-associated cytokines, such as IL6. A proteomic analysis also identified proteins with known roles in protecting podocytes from injury and found them to be up-regulated by Dex in cultured murine podocytes [154]. These up-regulated proteins include proteins involved in the orchestration of the actin cytoskeleton and stress responses. Using microarray analyses, our lab showed that Dex induces SERPINE1 (encoding Plasminogen Activator Inhibitor Type 1 or PAI-1) and CCL20 mRNAs [153]. PAI-1 is present in trace amounts in healthy kidneys but increases in a wide variety of both acute and chronic diseased kidneys. Reduced PAI-1 activity has been shown to be protective of albuminuria and glomerulosclerosis in experimental diabetes [159], while CCL20 is upregulated in patients with progressive IgA nephropathy [160]. Thus, Dex potentially exhibits unwanted effects by inducing genes including SERPINE1 and CCL20, which may cause damage to podocytes or glomeruli. Our studies also uncovered that GR crosstalks with a broad range of signaling pathways, primarily the NF-κB, STAT and TGFβ, but also the inflammatory response, cell migration, and angiogenesis [161]. These data are consistent with the mechanism underlying transactivation and transrepression by GCs (Figure 2). GCs are considered to have immunosuppressive and anti-inflammatory effects. It exerts the anti-proteinuria effect not only by suppressing but also through protecting podocyte integrity. Recently, RNA-seq analysis revealed that Dex-regulated genes are linked to cytoskeleton-related processes, podocyte differentiation, pro-inflammatory cytokines and growth factors [162]. Collectively, these results advance our knowledge of the molecular mechanisms by which GCs exert their therapeutic effects on podocytes and potential targets for unwanted effects.
6.2. GCs and podocyte injury
GCs have significant effects on podocytes. Podocytes are therefore an important therapeutic target for the treatment of NS caused by genetic mutations or environmental stress. Current evidence suggests that GCs protect podocytes from experimental injuries induced by PAN, Adriamycin (ADR), or protein-overload [163, 164]. In an experimental podocyte injury model, up-regulation of TRPC6 was shown to contribute to Angiotensin II (Ang II)-induced podocyte injury [165]. Notably, Dex treatment significantly reduced PAN-induced TRPC6 expression in rat and cultured murine podocytes [166]. Furthermore, Agrawal et al. showed that GCs reduced PAN-induced proteinuria in rats, in part, by elevating the expression of glomerular synaptopodin and nephrin, and reduced COX-2 expression in rats [167]. Serum albumin overload in rats has also been reported to not only induce structural and pathological changes in podocytes [168, 169], but also increase pro-inflammatory genes COX-2, MCP-1, CXCL1, and the stress protein HSP25 expression in both rat glomeruli and cultured podocytes [170]. Similarly, GCs inhibit serum albumin-induced COX-2 expression via its transrepression activity on NF-κB. GCs are also implicated in activating glomerular antioxidant enzymes and protecting glomeruli from reactive oxygen species (ROS)-mediated injuries in PAN-induced nephrosis in rats [171]. Using zebrafish and cultured HPCs, a recent study demonstrated that GCs ameliorate PAN-induced podocyte injury by down-regulating caveolin-1 expression and overexpression of caveolin-1 impaired normal podocyte function [172]. In summary, podocyte injury can be relieved by GC treatment in animal models and cultured human podocytes, in part, through the ability of GCs to regulate its target gene expression.
6.3. GCs and actin-filament stabilization
As mentioned earlier, the podocyte actin cytoskeleton is a key component of the complex architecture of the slit diaphragm [37, 40]. GCs protect and enhance recovery of cultured murine podocytes through its ability to stabilize actin filaments [128]. Dex treatment induces a significant increase in the activity of the actin-regulating GTPase RhoA and thereby increases total cellular polymerized actin, stabilizing actin filaments, and blocking PAN-induced disruption of actin filaments [128, 173–175]. Additionally, a recent study in cultured podocytes indicated that Dex could protect podocytes from ADR-induced actin rearrangements [163]. These reports imply that the beneficial effects of GCs in treating renal disease, at least in part, results from enhancing the stability of podocyte actin filament.
6.4. GCs and podocyte apoptosis
One of the beneficial features of GC action is the prevention of podocyte apoptosis [164]. GCs inhibit apoptosis by restoring Bcl-2 expression, reducing p53 levels and inhibiting nuclear translocation of apoptosis-inducing factor (AIF) in PAN-treated cultured podocytes [164]. These activities are mediated, in part, by the blockade of PAN-mediated reduction of extracellular signal-regulated kinase (ERK) phosphorylation in response to Dex treatment [176]. PAN also inhibits PI3K/Akt signaling, and Dex treatment restores the PI3K/Akt signaling, which promotes the activity of anti-apoptotic proteins [152]. In another study, prednisone treatment was shown to reduce podocyte apoptosis. Dex also increased podocyte progenitors by activating ERK signaling in an FSGS mouse model induced by a cytotoxic anti-podocyte antibody [177]. Thus, GCs not only inhibit podocyte apoptosis but also increase the number of podocyte progenitors to prevent podocyte loss.
6.5. Animal knockout models
Renal epithelial cells include podocytes, parietal epithelial cells (PECs), and tubular cells. Using Pax8-Cre/GRf l/f l mice, Kuppe et al. generated kidney epithelial cells-specific Nr3c1 (GR) knockout mice [173]. These animals show no apparent abnormality in kidney development, indicating that renal epithelial GR is dispensable for kidney development. In a nephrotoxic serum (NTS)-induced glomerulonephritis (GN) mouse model, podocytes are injured and PECs become strongly activated. High-doses of GCs significantly improved NTS-induced renal dysfunction. Remarkably, Pax8-Cre/GRf l/f l mice are resistant to NTS-induced GN, showing no or little albuminuria or cellular crescent formation. This observation is accompanied by fewer activated PECs, suggesting that GR promotes NTS-induced activation of PECs. This beneficial effect is also observed in NTS-treated mice exposed to mifepristone, a partial GR antagonist. Taken together, these data demonstrate a role of GR in the pathogenesis of NTS-induced GN, possibly due to a role of GR in activating PECs.
Using a podocin-Cre transgene, Zhou et al. have established podocyte-specific GR knockout (pGRKO) mice [174]. Consistent with renal epithelial-specific pGRKO mice, these animals showed no developmental phenotype and did not develop proteinuria under physiological condition. However, upon a challenge with by lipopolysaccharides (LPS) or NTS, pGRKO mice demonstrated severe proteinuria compared to control littermates. These observations support a critical role of podocytes GR in the maintenance of kidney function in response to LPS- and NTS-induced glomerular injury.
The recent literature further demonstrates that podocyte Krüppel-Like Factor 15 (Klf15) [178] and serum- and glucocorticoid-inducible kinase 3 (SGK3) [179], both of which are Dex-inducible genes, play essential roles in GC-mediated beneficial effects in response to LPS- or PAN-induced podocyte injury. In summary, these studies demonstrate an essential role for podocyte GR in response to injury as well as in the therapeutic effects of GCs.
7. Conclusion
It has been a longstanding accepted protocol to use GCs to treat NS. GCs have beneficial effects on patients with NS due to their ability to stabilize actin-filaments and to protect podocytes from apoptosis. Nonetheless, steroid-resistance and unwanted side effects associated with GC treatments are unacceptable and are an issue that needs to be addressed. The fundamentals surrounding this central issue include: 1) the targets of the GCs in podocytes, 2) the complexity of the molecular mechanisms underlying pathogenesis of NS and how they respond to GCs differently, 3) the benefits of combination therapy and 4) the molecular mechanisms by which GCs regulate physiology of different cell types in the glomerulus.
With pGRKO mice and the newly developed NUTRAP (Nuclear tagging and Translating Ribosome Affinity Purification) mouse strain [180], identification of GR-regulated gene networks in podocytes has become possible. A better understanding of the function of podocyte GR target genes will undoubtedly provide insight into the pathogenesis and treatment of NS.
The Nephrotic Syndrome Study Network (NEPTUNE) is a collaborative consortium that aims to develop a translational research framework for NS. This database contains multiple molecular and clinical data sets associated with samples collected from adults and children with NS that include MCD, FSGS, and membranous nephropathy [181]. This provides an unmatched resource to understand the mechanisms and pathways involved in NS. Integration of the data sets across the genome-phenome continuum, quantitative histology, rigorous clinical phenotypes and clinical outcomes will enable clinicians and researchers to better study genetic mutations associated with human kidney diseases [182]. Notably, this clinical information including steroid sensitivity will provide a wealth of critical data that will allow basic scientists to formulate and test hypotheses and ultimately help develop effective treatments for NS patients.
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.Bledsoe RK, Montana VG, Stanley TB, et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110(1):93–105. doi: 10.1016/s0092-8674(02)00817-6. [DOI] [PubMed] [Google Scholar]
- 2.Bledsoe RK, Stewart EL, Pearce KH. Structure and Function of the Glucocorticoid Receptor Ligand Binding Domain. Vitamins & Hormones. 2004;68:49–91. doi: 10.1016/S0083-6729(04)68002-2. [DOI] [PubMed] [Google Scholar]
- 3.Garza AS, Khan SH, Moure CM, Edwards DP, Kumar R. Binding-folding induced regulation of AF1 transactivation domain of the glucocorticoid receptor by a cofactor that binds to its DNA binding domain. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0025875. Article ID e25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55(7):603–613. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandevyver S, Dejager L, Libert C. On the Trail of the Glucocorticoid Receptor: Into the Nucleus and Back. Traffic. 2012;13(3):364–374. doi: 10.1111/j.1600-0854.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 6.Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 7.Ricketson D, Hostick U, Fang L, Yamamoto KR, Darimont BD. A Conformational Switch in the Ligand-binding Domain Regulates the Dependence of the Glucocorticoid Receptor on Hsp90. Journal of Molecular Biology. 2007;368(3):729–741. doi: 10.1016/j.jmb.2007.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandler VL, Maler BA, Yamamoto KR. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- 9.Ou X-M, Storring JM, Kushwaha N, Albert PR. Heterodimerization of Mineralocorticoid and Glucocorticoid Receptors at a Novel Negative Response Element of the 5-HT1A Receptor Gene. The Journal of Biological Chemistry. 2001;276(17):14299–14307. doi: 10.1074/jbc.M005363200. [DOI] [PubMed] [Google Scholar]
- 10.Starick SR, Ibn-Salem J, Jurk M, et al. ChIP-exo signal associated with DNA-binding motifs provides insight into the genomic binding of the glucocorticoid receptor and cooperating transcription factors. Genome Research. 2015;25(6):825–835. doi: 10.1101/gr.185157.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miner JN, Diamond MI, andYamamoto KR. Joints in the regulatory lattice: composite regulation by steroid receptor-AP1 complexes. Cell Growth Differ. 1991;2:525–530. [PubMed] [Google Scholar]
- 12.Ratman D, Vanden Berghe W, Dejager L, et al. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Molecular and Cellular Endocrinology. 2013;380(1–2):41–54. doi: 10.1016/j.mce.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Khan SH, Ling J, Kumar R. TBP binding-induced folding of the Glucocorticoid receptor AF1 domain facilitates its interaction with Steroid Receptor Coactivator-1. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0021939. Article ID e21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonte C, Grenier J, Trousson A, et al. Involvement of β-catenin and unusual behavior of CBP and p300 in glucocorticosteroid signaling in Schwann cells. Proceedings of the National Acadamy of Sciences of the United States of America. 2005;102(40):14260–14265. doi: 10.1073/pnas.0506930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darimont BD, Wagner RL, Apriletti JW, et al. Structure and specificity of nuclear receptor-coactivator interactions. Genes & Development. 1998;12(21):3343–3356. doi: 10.1101/gad.12.21.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Wong J, Tsai SY, Tsai M-J, O’Malley BW. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Molecular and Cellular Biology. 2003;23(11):3763–3773. doi: 10.1128/MCB.23.11.3763-3773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronacher K, Hadley K, Avenant C, et al. Ligand-selective transactivation and transrepression via the glucocorticoid receptor: Role of cofactor interaction. Molecular and Cellular Endocrinology. 2009;299(2):219–231. doi: 10.1016/j.mce.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Hudson WH, Youn C, Ortlund EA. The structural basis of direct glucocorticoid-mediated transrepression. Nature Structural & Molecular Biology. 2013;20(1):53–58. doi: 10.1038/nsmb.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-κB suppression. The Journal of Experimental Medicine. 2006;203(1):7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bosscher K, Vanden Berghe W, Vermeulen L, Plaisance S, Boone E, Haegeman G. Glucocorticoids repress NF-kappa B-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proceedings of the National Acadamy of Sciences of the United States of America. 2000;97(8):3919–3924. doi: 10.1073/pnas.97.8.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. The FASEB Journal. 2009;23(5):1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 23.Baschant U, Lane NE, Tuckermann J. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nature Reviews Rheumatology. 2012;8(11):645–655. doi: 10.1038/nrrheum.2012.166. [DOI] [PubMed] [Google Scholar]
- 24.Buttgereit F. A fresh look at glucocorticoids how to use an old ally more effectively. Bull NYU Hosp Jt Dis. 2012;70(supplement 1):26–29. [PubMed] [Google Scholar]
- 25.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular and Cellular Endocrinology. 2011;335(1):2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba H, Pui C-H. Glucocorticoid use in acute lymphoblastic leukaemia. The Lancet Oncology. 2010;11(11):1096–1106. doi: 10.1016/S1470-2045(10)70114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haack D, Schärer K, Asam-Tauscher A, Vecsei P. Glucocorticoid receptors in idiopathic nephrotic syndrome. Pediatric Nephrology. 1999;13(8):653–656. doi: 10.1007/s004670050675. [DOI] [PubMed] [Google Scholar]
- 28.Carlotti APDCP, Franco PB, Elias LL, et al. Glucocorticoid receptors, in vitro steroid sensitivity, and cytokine secretion in idiopathic nephrotic syndrome. Kidney International. 2004;65(2):403–408. doi: 10.1111/j.1523-1755.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- 29.Clement LC, Avila-Casado C, Macé C, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nature Medicine. 2011;17(1):117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu C-Y, Mayba O, Lee JV, et al. Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0015188. Article ID e15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo T, Lew MJ, Mayba O, Harris CA, Speed TP, Wang J-C. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proceedings of the National Acadamy of Sciences of the United States of America. 2012;109(28):11160–11165. doi: 10.1073/pnas.1111334109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlenhaut NH, Barish GD, Yu RT, et al. Insights into Negative Regulation by the Glucocorticoid Receptor from Genome-wide Profiling of Inflammatory Cistromes. Molecular Cell. 2013;49(1):158–171. doi: 10.1016/j.molcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biddie SC, John S, Sabo PJ, et al. Transcription Factor AP1 Potentiates Chromatin Accessibility and Glucocorticoid Receptor Binding. Molecular Cell. 2011;43(1):145–155. doi: 10.1016/j.molcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshikawa N, Nagasaki M, Sano M, et al. Ligand-based gene expression profiling reveals novel roles of glucocorticoid receptor in cardiac metabolism. American Journal of Physiology-Endocrinology and Metabolism. 2009;296(6):E1363–E1373. doi: 10.1152/ajpendo.90767.2008. [DOI] [PubMed] [Google Scholar]
- 35.Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mechanisms of Ageing and Development. 2004;125(10–11):697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Tsurufuji S, Sugio K, Takemasa F. The role of glucocorticoid receptor and gene expression in the anti-inflammatory action of dexamethasone [20] Nature. 1979;280(5721):408–410. doi: 10.1038/280408a0. [DOI] [PubMed] [Google Scholar]
- 37.Schell C, Wanner N, Huber TB. Glomerular development—shaping the multi-cellular filtration unit. Seminars in Cell & Developmental Biology. 2014;36C:39–49. doi: 10.1016/j.semcdb.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Lennon R, Hosawi S. Glomerular cell crosstalk. Current Opinion in Nephrology and Hypertension. 2016;25(3):187–193. doi: 10.1097/MNH.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian X, Kim JJ, Monkley SM, et al. Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. The Journal of Clinical Investigation. 2014;124(3):1098–1113. doi: 10.1172/JCI69778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assady S, Wanner N, Skorecki KL, Huber TB. New insights into podocyte biology in glomerular health and disease. Journal of the American Society of Nephrology. 2017;28(6):1707–1715. doi: 10.1681/ASN.2017010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grahammer F, Schell C, Huber TB. The podocyte slit diaphragm - From a thin grey line to a complex signalling hub. Nature Reviews Nephrology. 2013;9(10):587–598. doi: 10.1038/nrneph.2013.169. [DOI] [PubMed] [Google Scholar]
- 42.Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Current Opinion in Nephrology and Hypertension. 2005;14(3):211–216. doi: 10.1097/01.mnh.0000165885.85803.a8. [DOI] [PubMed] [Google Scholar]
- 43.Kestilä M, Lenkkeri U, Männikkö M, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Molecular Cell. 1998;1(4):575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 44.Boute N, Gribouval O, Roselli S, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nature Genetics. 2000;24(4):349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 45.Schwarz K, Simons M, Reiser J, et al. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. The Journal of Clinical Investigation. 2001;108(11):1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi N-Y, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. The American Journal of Pathology. 2001;159(6):2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiser J, Polu KR, Möller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nature Genetics. 2005;37(7):739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiser J, Kriz W, Kretzler M, andMundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:18. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 49.Inoue T, Yaoita E, Kurihara H, et al. FAT is a component of glomerular slit diaphragms. Kidney International. 2001;59(3):1003–1012. doi: 10.1046/j.1523-1755.2001.0590031003.x. [DOI] [PubMed] [Google Scholar]
- 50.Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. The Journal of Cell Biology. 1990;111(3):1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. The Journal of Cell Biology. 1997;139(1):193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo J-K, Menke AL, Gubler M-C, et al. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Human Molecular Genetics. 2002;11(6):651–659. doi: 10.1093/hmg/11.6.651. [DOI] [PubMed] [Google Scholar]
- 53.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. Journal of the American Society of Nephrology. 2009;20(9):1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ha TS. Roles of adaptor proteins in podocyte biology. World Journal of Nephrology. 2013;2(1):1–10. doi: 10.5527/wjn.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jefferson JA, Alpers CE, Shankland SJ. Podocyte biology for the bedside. American Journal of Kidney Diseases. 2011;58(5):835–845. doi: 10.1053/j.ajkd.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pavenstädt H. Roles of the podocyte in glomerular function. American Journal of Physiology-Renal Physiology. 2000;278(2):F173–F179. doi: 10.1152/ajprenal.2000.278.2.F173. [DOI] [PubMed] [Google Scholar]
- 57.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. [PubMed] [Google Scholar]
- 58.Shirato I, Sakai T, Kimura K, Tomino Y, Kriz W. Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. The American Journal of Pathology. 1996;148(4):1283–1296. [PMC free article] [PubMed] [Google Scholar]
- 59.Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. The Journal of Clinical Investigation. 2001;108(2):289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwoh C, Shannon MB, Miner JH, Shaw A. Pathogenesis of Nonimmune Glomerulopathies. Annual Review of Pathology: Mechanisms of Disease. 2006;1(1):349–374. doi: 10.1146/annurev.pathol.1.110304.100119. [DOI] [PubMed] [Google Scholar]
- 61.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney International. 1998;54(3):687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 62.Sanwal V, Pandya M, Bhaskaran M, et al. Puromycin aminonucleoside induces glomerular epithelial cell apoptosis. Experimental and Molecular Pathology. 2001;70(1):54–64. doi: 10.1006/exmp.2000.2345. [DOI] [PubMed] [Google Scholar]
- 63.Kim YH, Goyal M, Kurnit D, et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney International. 2001;60(3):957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- 64.Shiiki H, Sasaki Y, Nishino T, et al. Cell proliferation and apoptosis of the glomerular epithelial cells in rats with puromycin aminonucleoside nephrosis. Pathobiology. 1998;66(5):221–229. doi: 10.1159/000028027. [DOI] [PubMed] [Google Scholar]
- 65.Whiteside C, Prutis K, Cameron R, Thompson J. Glomerular epithelial detachment, not reduced charge density, correlates with proteinuria in adriamycin and puromycin nephrosis. Laboratory Investigation. 1989;61(6):650–660. [PubMed] [Google Scholar]
- 66.Whiteside CI, Cameron R, Munk S, Levy J. Podocytic cytoskeletal disaggregation and basement-membrane detachment in puromycin aminonucleoside nephrosis. The American Journal of Pathology. 1993;142(5):1641–1653. [PMC free article] [PubMed] [Google Scholar]
- 67.Reiser J, Oh J, Shirato I, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and α3 integrin. The Journal of Biological Chemistry. 2004;279(33):34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 68.Cameron JS, andHicks J. The origins and development of the concept of a, nephrotic syndrome. Am J Nephrol. 2002;22:240–247. doi: 10.1159/000063768. [DOI] [PubMed] [Google Scholar]
- 69.Hull RP, Goldsmith DJA. Nephrotic syndrome in adults. British Medical Journal. 2008;336(7654):1185–1189. doi: 10.1136/bmj.39576.709711.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas DB, Franceschini N, Hogan SL, et al. Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney International. 2006;69(5):920–926. doi: 10.1038/sj.ki.5000160. [DOI] [PubMed] [Google Scholar]
- 71.Hama T, Nakanishi K, Shima Y, et al. Renal biopsy criterion in idiopathic nephrotic syndrome with microscopic hematuria at onset. Pediatric Nephrology. 2014;30(3):445–450. doi: 10.1007/s00467-014-2946-9. [DOI] [PubMed] [Google Scholar]
- 72.D’Agati V. Pathologic classification of focal segmental glomerulosclerosis. Seminars in Nephrology. 2003;23(2):117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 73.Cattran DC, Coppo R, Cook HT, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney International. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 74.Marshall S, Dressier R, D’Agati V. Membranous lupus nephritis with antineutrophil cytoplasmic antibody-associated segmental necrotizing and crescentic glomerulonephritis. American Journal of Kidney Diseases. 1997;29(1):119–124. doi: 10.1016/s0272-6386(97)90018-4. [DOI] [PubMed] [Google Scholar]
- 75.Kveder R, Kajtna-Koselj M, Rott T, Bren AF. Nephrotic syndrome in patients with diabetes mellitus is not always associated with diabetic nephropathy. Nephrology Dialysis Transplantation. 2001;16(6):86–87. doi: 10.1093/ndt/16.suppl_6.86. [DOI] [PubMed] [Google Scholar]
- 76.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. American Journal of Kidney Diseases. 2007;49(2):186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 77.Weening JJ, D’Agati VD, Schwartz MM. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Journal of the American Society of Nephrology. 2004;15(2):241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 78.Kimmel PL, Barisoni L, Kopp JB. Pathogenesis and treatment of HIV-associated renal diseases: lessons from clinical and animal studies, molecular pathologic correlations, and genetic investigations. Annals of Internal Medicine. 2003;139(3):214–226. [PubMed] [Google Scholar]
- 79.Weiner NJ, Goodman JW, Kimmel PL. The HIV-associated renal diseases: Current insight into pathogenesis and treatment. Kidney International. 2003;63(5):1618–1631. doi: 10.1046/j.1523-1755.2003.00901.x. [DOI] [PubMed] [Google Scholar]
- 80.Eddy AA, Symons JM. Nephrotic syndrome in childhood. The Lancet. 2003;362(9384):629–639. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 81.D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. The New England Journal of Medicine. 2011;365(25):2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 82.Kitiyakara C, Kopp JB, Eggers P. Trends in the epidemiology of focal segmental glomerulosclerosis. Seminars in Nephrology. 2003;23(2):172–182. doi: 10.1053/snep.2003.50025. [DOI] [PubMed] [Google Scholar]
- 83.Zenker M, MacHuca E, Antignac C. Genetics of nephrotic syndrome: New insights into molecules acting at the glomerular filtration barrier. Journal of Molecular Medicine. 2009;87(9):849–857. doi: 10.1007/s00109-009-0505-9. [DOI] [PubMed] [Google Scholar]
- 84.Büscher AK, Konrad M, Nagel M, et al. Mutations in podocyte genes are a rare cause of primary FSGS associated with ESRD in adult patients. Clinical Nephrology. 2012;78(1):47–53. doi: 10.5414/cn107320. [DOI] [PubMed] [Google Scholar]
- 85.Kaplan JM, Kim SH, North KN, et al. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nature Genetics. 2000;24(3):251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 86.Winn MP, Conlon PJ, Lynn KL, et al. Medicine: a mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308(5729):1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 87.Gigante M, Pontrelli P, Montemurno E, et al. CD2AP mutations are associated with sporadic nephrotic syndrome and focal segmental glomerulosclerosis (FSGS) Nephrology Dialysis Transplantation. 2009;24(6):1858–1864. doi: 10.1093/ndt/gfn712. [DOI] [PubMed] [Google Scholar]
- 88.Brown EJ, Schlöndorff JS, Becker DJ, et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nature Genetics. 2010;42(1):72–76. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mele C, Iatropoulos P, Donadelli R, et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. The New England Journal of Medicine. 2011;365(4):295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gbadegesin RA, Hall G, Adeyemo A, et al. Mutations in the gene that encodes the F-Actin binding protein anillin cause FSGS. Journal of the American Society of Nephrology. 2014;25(9):1991–2002. doi: 10.1681/ASN.2013090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ebarasi L, Ashraf S, Bierzynska A, et al. Defects of CRB2 cause steroid-resistant nephrotic syndrome. American Journal of Human Genetics. 2015;96(1):153–161. doi: 10.1016/j.ajhg.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park E, Kang HG, Choi YH, et al. Focal segmental glomerulosclerosis and medullary nephrocalcinosis in children with ADCK4 mutations. Pediatric Nephrology. 2017;32(9):1547–1554. doi: 10.1007/s00467-017-3657-9. [DOI] [PubMed] [Google Scholar]
- 93.Akilesh S, Suleiman H, Yu H, et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. The Journal of Clinical Investigation. 2011;121(10):4127–4137. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gee HY, Saisawat P, Ashraf S, et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. The Journal of Clinical Investigation. 2013;123(8):3243–3253. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vivante A, Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nature Reviews Nephrology. 2016;12(3):133–146. doi: 10.1038/nrneph.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Braun DA, Rao J, Mollet G, et al. Mutations in KEOPS-complex genes cause nephritic syndrome with primary microcephaly. Nature Genetics. 2017;49(10):1529–1538. doi: 10.1038/ng.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ozaltin F, Ibsirlioglu T, Taskiran EZ, et al. Disruption of PTPRO causes childhood-onset nephrotic syndrome. American Journal of Human Genetics. 2011;89(1):139–147. doi: 10.1016/j.ajhg.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hinkes B, Wiggins RC, Gbadegesin R, et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nature Genetics. 2006;38(12):1397–1405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- 99.Mucha B, Ozaltin F, Hinkes BG, et al. Mutations in the Wilms’ tumor 1 gene cause isolated steroid resistant nephrotic syndrome and occur in exons 8 and 9. Pediatric Research. 2006;59(2):325–331. doi: 10.1203/01.pdr.0000196717.94518.f0. [DOI] [PubMed] [Google Scholar]
- 100.Barua M, Brown EJ, Charoonratana VT, Genovese G, Sun H, Pollak MR. Mutations in the INF2 gene account for a significant proportion of familial but not sporadic focal and segmental glomerulosclerosis. Kidney International. 2013;83(2):316–322. doi: 10.1038/ki.2012.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gbadegesin R, Lavin P, Foreman J, Winn M. Pathogenesis and therapy of focal segmental glomerulosclerosis: An update. Pediatric Nephrology. 2011;26(7):1001–1015. doi: 10.1007/s00467-010-1692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chun MJ, Korbet SM, Schwartz MM, Lewis EJ. Focal segmental glomerulosclerosis in nephrotic adults: Presentation, prognosis, and response to therapy of the histologic variants. Journal of the American Society of Nephrology. 2004;15(8):2169–2177. doi: 10.1097/01.ASN.0000135051.62500.97. [DOI] [PubMed] [Google Scholar]
- 103.Cattran DC, Appel GB, Hebert LA, et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney International. 1999;56(6):2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 104.Bhimma R, Adhikari M, Asharam K, Connolly C. Management of steroid-resistant focal segmental glomerulosclerosis in children using tacrolimus. American Journal of Nephrology. 2007;26(6):544–551. doi: 10.1159/000097864. [DOI] [PubMed] [Google Scholar]
- 105.Pereira WDF, Brito-Melo GEA, Guimarães FTL, Carvalho TGR, Mateo EC, Silva ACSE. The role of the immune system in idiopathic nephrotic syndrome: a review of clinical and experimental studies. Inflammation Research. 2014;63(1):1–12. doi: 10.1007/s00011-013-0672-6. [DOI] [PubMed] [Google Scholar]
- 106.Colucci M, Corpetti G, Emma F, Vivarelli M. Immunology of idiopathic nephrotic syndrome. Pediatric Nephrology. 2018;33(4):573–584. doi: 10.1007/s00467-017-3677-5. [DOI] [PubMed] [Google Scholar]
- 107.Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Science Translational Medicine. 2011;3(85) doi: 10.1126/scitranslmed.3002231. Article ID 85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duque N, Gomez-Guerrero C, andEgido J. Interaction of IgA with Fc alpha receptors of human mesangial cells activates transcription factor nuclear factor-kappa B and induces expression and synthesis of monocyte chemoattractant protein-1, IL-8, and IFN-inducible protein 10. J Immunol. 1997;159:3474–3482. [PubMed] [Google Scholar]
- 109.Danilewicz M, andWagrowska-Danilewicz M. Tubular NF-#954;B is overexpressed in proteinuric patients with IgA nephropathy. Folia Histochem Cytobiol. 2012;50:93–98. doi: 10.2478/18702. [DOI] [PubMed] [Google Scholar]
- 110.Henke N, Schmidt-Ullrich R, Dechend R, et al. Vascular endothelial cell-specific NF-κB suppression attenuates hypertension-induced renal damage. Circulation Research. 2007;101(3):268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 111.Kiryluk K, Novak J. The genetics and immunobiology of IgA nephropathy. The Journal of Clinical Investigation. 2014;124(6):2325–2332. doi: 10.1172/JCI74475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coppo R, Amore A. Aberrant glycosylation in IgA nephropathy (IgAN) Kidney International. 2004;65(5):1544–1547. doi: 10.1111/j.1523-1755.2004.05407.x. [DOI] [PubMed] [Google Scholar]
- 113.Ruiz-Ortega M, Lorenzo Ó, Rupérez M, Blanco J, Egido J. Systemic Infusion of Angiotensin II into Normal Rats Activates Nuclear Factor-κB and AP-1 in the Kidney. The American Journal of Pathology. 2001;158(5):1743–1756. doi: 10.1016/s0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruiz-Ortega M, Ruperez M, Lorenzo O, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney International Supplements. 2002;62(82):S12–S22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- 115.Arumugam S, Sreedhar R, Thandavarayan RA, et al. Angiotensin receptor blockers: Focus on cardiac and renal injury. Trends in Cardiovascular Medicine. 2016;26(3):221–228. doi: 10.1016/j.tcm.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 116.Zhang F, Siow YL, O K. Hyperhomocysteinemia activates NF-κB and inducible nitric oxide synthase in the kidney. Kidney International. 2004;65(4):1327–1338. doi: 10.1111/j.1523-1755.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- 117.Tesch GH, Yang N, Yu H, et al. Intrinsic renal cells are the major source of interleukin-1beta synthesis in normal and diseased rat kidney. Nephrology Dialysis Transplantation. 1997;12(6):1109–1115. doi: 10.1093/ndt/12.6.1109. [DOI] [PubMed] [Google Scholar]
- 118.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. American Journal of Physiology-Renal Physiology. 2008;294(4):F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 119.Sakurai H, Hisada Y, Ueno M, Sugiura M, Kawashima K, Sugita T. Activation of transcription factor NF-κB in experimental glomerulonephritis in rats. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1996;1316(2):132–138. doi: 10.1016/0925-4439(96)00022-1. [DOI] [PubMed] [Google Scholar]
- 120.Martinka S, Bruggeman LA. Persistent NF-κB activation in renal epithelial cells in a mouse model of HIV-associated nephropathy. American Journal of Physiology-Renal Physiology. 2006;290(3):F657–F665. doi: 10.1152/ajprenal.00208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ross MJ. NF-B Regulates Fas-Mediated Apoptosis in HIV-Associated Nephropathy. Journal of the American Society of Nephrology. 2005;16(8):2403–2411. doi: 10.1681/ASN.2004121101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bruggeman LA, Drawz PE, Kahoud N, Lin K, Barisoni L, Nelson PJ. TNFR2 interposes the proliferative and NF-B-mediated inflammatory response by podocytes to TNF-α. Laboratory Investigation. 2011;91(3):413–425. doi: 10.1038/labinvest.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang H, Ding J, Fan Q, Liu S. RPC6 Up-Regulation in Ang II-Induced Podocyte Apoptosis Might Result from ERK Activation and NF-κB Translocation. Experimental Biology and Medicine. 2009;234(9):1029–1036. doi: 10.3181/0901-RM-11. [DOI] [PubMed] [Google Scholar]
- 124.Shimo T, Adachi Y, Yamanouchi S, et al. A novel nuclear factor κb inhibitor, dehydroxymethylepoxyquinomicin, ameliorates puromycin aminonucleoside-induced nephrosis in mice. American Journal of Nephrology. 2013;37(4):302–309. doi: 10.1159/000348803. [DOI] [PubMed] [Google Scholar]
- 125.Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cellular and Molecular Life Sciences. 2006;63(1):60–72. doi: 10.1007/s00018-005-5390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lill-Elghanian D, Schwartz K, King L, Fraker P. Glucocorticoid-induced apoptosis in early B cells from human bone marrow. Experimental Biology and Medicine. 2002;227(9):763–770. doi: 10.1177/153537020222700907. [DOI] [PubMed] [Google Scholar]
- 127.Schnenberger E, Ehrich JH, Haller H, Schiffer M. The podocyte as a direct target of immunosuppressive agents. Nephrology Dialysis Transplantation. 2011;26(1):18–24. doi: 10.1093/ndt/gfq617. [DOI] [PubMed] [Google Scholar]
- 128.Ransom RF, Lam NG, Hallett MA, Atkinson SJ, Smoyer WE. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney International. 2005;68(6):2473–2483. doi: 10.1111/j.1523-1755.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 129.Yan K, Kudo A, Hirano H, et al. Subcellular localization of glucocorticoid receptor protein in the human kidney glomerulus. Kidney International. 1999;56(1):65–73. doi: 10.1046/j.1523-1755.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 130.Xing C-Y, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney International. 2006;70(6):1038–1045. doi: 10.1038/sj.ki.5001655. [DOI] [PubMed] [Google Scholar]
- 131.Boumpas DT. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Annals of Internal Medicine. 1993;119(12):1198–1208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]
- 132.Stirling CM, Mathieson P, Boulton-Jones JM, et al. Treatment and outcome of adult patients with primary focal segmental glomerulosclerosis in five UK renal units. QJM: Monthly Journal of the Association of Physicians. 2005;98(6):443–449. doi: 10.1093/qjmed/hci072. [DOI] [PubMed] [Google Scholar]
- 133.Caridi G, Bertelli R, Carrea A, et al. Prevalence, genetics, and clinical features of patients carrying podocin mutations in steroid-resistant nonfamilial focal segmental glomerulosclerosis. Journal of the American Society of Nephrology. 2001;12(12):2742–2746. doi: 10.1681/ASN.V12122742. [DOI] [PubMed] [Google Scholar]
- 134.Philippe A, Nevo F, Esquivel EL, et al. Nephrin Mutations Can Cause Childhood-Onset Steroid-Resistant Nephrotic Syndrome. Journal of the American Society of Nephrology. 2008;19(10):1871–1878. doi: 10.1681/ASN.2008010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weber S, Gribouval O, Esquivel EL, et al. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney International. 2004;66(2):571–579. doi: 10.1111/j.1523-1755.2004.00776.x. [DOI] [PubMed] [Google Scholar]
- 136.Ruf RG, Schultheiss M, Lichtenberger A, et al. Prevalence of WT1 mutations in a large cohort of patients with steroid-resistant and steroid-sensitive nephrotic syndrome. Kidney International. 2004;66(2):564–570. doi: 10.1111/j.1523-1755.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 137.Yang Y, Zhao F, Tu X, Yu Z. Mutations in WT1 in boys with sporadic isolated steroid-resistant nephrotic syndrome. Genetics and Molecular Research. 2016;15(1) doi: 10.4238/gmr.15017559. Article ID 15017559. [DOI] [PubMed] [Google Scholar]
- 138.Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. The New England Journal of Medicine. 1996;334(14):878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 139.McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clinical Journal of the American Society of Nephrology. 2010;5(11):2115–2121. doi: 10.2215/CJN.03800609. [DOI] [PubMed] [Google Scholar]
- 140.Zagury A, Oliveira AL, Montalvão JA, et al. Steroid-resistant idiopathic nephrotic syndrome in children: long-term follow-up and risk factors for end-stage renal disease. Jornal Brasileiro de Nefrologia. 2013;35(3):191–199. doi: 10.5935/0101-2800.20130031. [DOI] [PubMed] [Google Scholar]
- 141.van Husen M, Kemper MJ. New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatric Nephrology. 2011;26(6):881–892. doi: 10.1007/s00467-010-1717-5. [DOI] [PubMed] [Google Scholar]
- 142.Hull R, Goldsmith D. Identifying and managing nephrotic syndrome in adults. Practitioner. 2008;252(30):32–34. [PubMed] [Google Scholar]
- 143.Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; Chichester, UK: 1996. [Google Scholar]
- 144.Lieberman K, V A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol. 1996;7:56–63. doi: 10.1681/ASN.V7156. [DOI] [PubMed] [Google Scholar]
- 145.Najafi CC, Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J. Significance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulonephritis. Kidney International. 2001;59(6):2156–2163. doi: 10.1046/j.1523-1755.2001.00730.x. [DOI] [PubMed] [Google Scholar]
- 146.Valeri A, Barisoni L, Appel GB, Seigle R, D’Agati V. Idiopathic collapsing focal segmental glomerulosclerosis: a clinicopathologic study. Kidney International. 1996;50(5):1734–1746. doi: 10.1038/ki.1996.493. [DOI] [PubMed] [Google Scholar]
- 147.Detwiler RK, Falk RJ, Hogan SL, Jennette JC. Collapsing glomerulopathy: a clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney International. 1994;45(5):1416–1424. doi: 10.1038/ki.1994.185. [DOI] [PubMed] [Google Scholar]
- 148.Korbet SM. Treatment of primary FSGS in adults. Journal of the American Society of Nephrology. 2012;23(11):1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 149.Preston R, Stuart HM, Lennon R. Genetic testing in steroid-resistant nephrotic syndrome: why, who, when and how? Pediatric Nephrology. doi: 10.1007/s00467-017-3838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moysiadis DK, Perysinaki GS, Bertsias G, et al. Early treatment with glucocorticoids or cyclophosphamide retains the slit diaphragm proteins nephrin and podocin in experimental lupus nephritis. Lupus. 2012;21(11):1196–1207. doi: 10.1177/0961203312451784. [DOI] [PubMed] [Google Scholar]
- 151.Yu S, Yu L. Dexamethasone resisted podocyte injury via stabilizing TRPC6 expression and distribution. Evidence-Based Complementary and Alternative Medicine. 2012;2012 doi: 10.1155/2012/652059. Article ID 652059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu S, Li Y. Dexamethasone inhibits podocyte apoptosis by stabilizing the PI3K/Akt signal pathway. BioMed Research International. 2013;2013 doi: 10.1155/2013/326986. Article ID 326986 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao X, Khurana S, Charkraborty S, et al. α actinin 4 (ACTN4) regulates glucocorticoid receptor-mediated transactivation and transrepression in podocytes. The Journal of Biological Chemistry. 2017;292(5):1637–1647. doi: 10.1074/jbc.M116.755546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ransom RF, Vega-Warner V, Smoyer WE, Klein J. Differential proteomic analysis of proteins induced by glucocorticoids in cultured murine podocytes. Kidney International. 2005;67(4):1275–1285. doi: 10.1111/j.1523-1755.2005.00205.x. [DOI] [PubMed] [Google Scholar]
- 155.Kimura M, Toyoda M, Kato M, et al. Expression of alpha-actinin-4 in human diabetic nephropathy. Internal Medicine. 2008;47(12):1099–1106. doi: 10.2169/internalmedicine.47.0352. [DOI] [PubMed] [Google Scholar]
- 156.Dai S, Wang Z, Pan X, et al. ACTN4 gene mutations and single nucleotide polymorphisms in idiopathic focal segmental glomerulosclerosis. Nephron Clinical Practice. 2009;111(2):c87–c94. doi: 10.1159/000191198. [DOI] [PubMed] [Google Scholar]
- 157.Dai S, Wang Z, Pan X, et al. Functional analysis of promoter mutations in the ACTN4 and SYNPO genes in focal segmental glomerulosclerosis. Nephrology Dialysis Transplantation. 2010;25(3):824–835. doi: 10.1093/ndt/gfp394. [DOI] [PubMed] [Google Scholar]
- 158.Liu Z, Blattner SM, Tu Y, et al. α-actinin-4 and CLP36 protein deficiencies contribute to podocyte defects in multiple human glomerulopathies. The Journal of Biological Chemistry. 2011;286(35):30795–30805. doi: 10.1074/jbc.M111.255984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang Y, Border WA, Yu L, Zhang J, Lawrence DA, Noble NA. A PAI-1 mutant, PAI-1R, slows progression of diabetic nephropathy. Journal of the American Society of Nephrology. 2008;19(2):329–338. doi: 10.1681/ASN.2007040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Villa L, Boor P, Konieczny A, et al. Late angiotensin II receptor blockade in progressive rat mesangioproliferative glomerulonephritis: New insights into mechanisms. The Journal of Pathology. 2013;229(5):672–684. doi: 10.1002/path.4151. [DOI] [PubMed] [Google Scholar]
- 161.Cheng X, Zhao X, Khurana S, Bruggeman LA, Kao H, Dryer SE. Microarray Analyses of Glucocorticoid and Vitamin D3 Target Genes in Differentiating Cultured Human Podocytes. PLoS ONE. 2013;8(4):e60213. doi: 10.1371/journal.pone.0060213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiang L, Hindmarch CCT, Rogers M, et al. RNA sequencing analysis of human podocytes reveals glucocorticoid regulated gene networks targeting non-immune pathways. Scientific Reports. 2016;6 doi: 10.1038/srep35671. Article ID 35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu H, Gao X, Xu H, et al. α-Actinin-4 is involved in the process by which dexamethasone protects actin cytoskeleton stabilization from adriamycin-induced podocyte injury. Nephrology. 2012;17(8):669–675. doi: 10.1111/j.1440-1797.2012.01645.x. [DOI] [PubMed] [Google Scholar]
- 164.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: Role of p53 and Bcl-2-related family proteins. Journal of the American Society of Nephrology. 2005;16(9):2615–2625. doi: 10.1681/ASN.2005020142. [DOI] [PubMed] [Google Scholar]
- 165.Nijenhuis T, Sloan AJ, Hoenderop JGJ, et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. The American Journal of Pathology. 2011;179(4):1719–1732. doi: 10.1016/j.ajpath.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Z, Wei X, Zhang Y, et al. NADPH oxidase-derived ROS contributes to upregulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cellular Physiology and Biochemistry. 2009;24(5–6):619–626. doi: 10.1159/000257517. [DOI] [PubMed] [Google Scholar]
- 167.Agrawal S, Chanley MA, Westbrook D, et al. Pioglitazone enhances the beneficial effects of glucocorticoids in experimental nephrotic syndrome. Scientific Reports. 2016;6 doi: 10.1038/srep24392. Article ID 24392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morigi M, Buelli S, Angioletti S, et al. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: Implication for permselective dysfunction of chronic nephropathies. The American Journal of Pathology. 2005;166(5):1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yoshida S, Nagase M, Shibata S, Fujita T. Podocyte injury induced by albumin overload in vivo and in vitro: involvement of TGF-beta and p38 MAPK. Nephron Experimental Nephrology. 2008;108(3):e57–e68. doi: 10.1159/000124236. [DOI] [PubMed] [Google Scholar]
- 170.Agrawal S, Guess AJ, Chanley MA, Smoyer WE. Albumin-induced podocyte injury and protection are associated with regulation of COX-2. Kidney International. 2014;86(6):1150–1160. doi: 10.1038/ki.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kawamura T, Yoshioka T, Bills T, Fogo A, Ichikawa I. Glucocorticoid activates glomerular antioxidant enzymes and protects glomeruli from oxidant injuries. Kidney International. 1991;40(2):291–301. doi: 10.1038/ki.1991.213. [DOI] [PubMed] [Google Scholar]
- 172.Wan X, Chen Z, Choi W-I, Gee HY, Hildebrandt F, Zhou W. Loss of epithelial membrane protein 2 aggravates podocyte injury via upregulation of caveolin-1. Journal of the American Society of Nephrology. 2016;27(4):1066–1075. doi: 10.1681/ASN.2014121197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kuppe C, Van Roeyen C, Leuchtle K, et al. Investigations of glucocorticoid action in GN. Journal of the American Society of Nephrology. 2017;28(5):1408–1420. doi: 10.1681/ASN.2016010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhou H, Tian X, Tufro A, Moeckel G, Ishibe S, Goodwin J. Loss of the podocyte glucocorticoid receptor exacerbates proteinuria after injury. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-10490-z. article no. 9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huang Z, Zhang L, Chen Y, et al. RhoA deficiency disrupts podocyte cytoskeleton and induces podocyte apoptosis by inhibiting YAP/dendrin signal. BMC Nephrology. 2016;17(1) doi: 10.1186/s12882-016-0287-6. article no. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Masaki T, Stambe C, Hill PA, Dowling J, Atkins RC, Nikolic-Paterson DJ. Activation of the extracellular-signal regulated protein kinase pathway in human glomerulopathies. Journal of the American Society of Nephrology. 2004;15(7):1835–1843. doi: 10.1097/01.asn.0000130623.66271.67. [DOI] [PubMed] [Google Scholar]
- 177.Zhang J, Pippin JW, Krofft RD, Naito S, Liu Z, Shankland SJ. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. American Journal of Physiology-Renal Physiology. 2013;304(11):F1375–F1389. doi: 10.1152/ajprenal.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mallipattu SK, Guo Y, Revelo MP, et al. Krüppel–Like Factor 15 Mediates Glucocorticoid-Induced Restoration of Podocyte Differentiation Markers. Journal of the American Society of Nephrology. 2016;28(1):166–184. doi: 10.1681/ASN.2015060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Peng L, Zhao H, Liu S, et al. Lack of serum- and glucocorticoid-inducible kinase 3 leads to podocyte dysfunction. The FASEB Journal. 2018;32(2):576–587. doi: 10.1096/fj.201700393RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roh HC, Tsai LT-Y, Lyubetskaya A, Tenen D, Kumari M, Rosen ED. Simultaneous Transcriptional and Epigenomic Profiling from Specific Cell Types within Heterogeneous Tissues In Vivo. Cell Reports. 2017;18(4):1048–1061. doi: 10.1016/j.celrep.2016.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gadegbeku CA, Gipson DS, Holzman LB, et al. Design of the nephrotic syndrome study network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney International. 2013;83(4):749–756. doi: 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sampson MG, Hodgin JB, Kretzler M. Defining nephrotic syndrome from an integrative genomics perspective. Pediatric Nephrology. 2015;30(1):51–63. doi: 10.1007/s00467-014-2857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]