ABSTRACT
High secretion of interleukin (IL)-6 from white adipose tissue may contribute to metabolic complications in obesity. We have recently shown that IL-6-type cytokine signaling in adipocytes is involved in the development of obesity-associated hepatic insulin resistance and steatosis. In addition, we revealed that adipocyte-specific IL-6 signaling ameliorates glucose metabolism in obesity via enhancing insulin secretion. Mechanistically, IL-6 induces the release of free fatty acid (FFA) and leptin from adipocytes thereby affecting liver metabolism and pancreatic β-cell function, respectively. This commentary further discusses the role of adipocyte-specific IL-6-type cytokine signaling in the regulation of FFA and leptin release. In particular, we outline depot-specific differences in IL-6-induced basal release of the two aforementioned factors. Moreover, we provide evidence that insulin’s effect on the release of FFA and leptin is adipose depot-dependent. We conclude that adipose depot-specific targeting of the IL-6 signaling pathway may be a novel approach to blunt obesity-associated metabolic complications.
KEYWORDS: gp130, insulin resistance, glucose tolerance, high fat diet, obesity, fat depot
The role of adipocyte-specific IL-6-type cytokine signaling in glucose metabolism
The prevalence of obesity and associated diseases such as insulin resistance and type 2 diabetes are increasing worldwide.1,2 High secretion of interleukin (IL)-6 from adipose tissue may contribute to obesity-induced insulin resistance and, consequently, the development of type 2 diabetes.3 To induce its intracellular signaling pathways, IL-6 either binds to its soluble or membrane-bound receptor. Subsequently, the assembled IL-6 ligand/receptor complex associates with a homodimer of glycoprotein 130 (gp130), which is a signal transducing protein of all IL-6-type cytokine family members.4 Using adipocyte-specific gp130 knockout mice (gp130Δadipo), we recently demonstrated that IL-6-type cytokine signaling in visceral adipocytes contributes to the development of obesity-associated hepatic insulin resistance and steatosis.5 Mechanistically, obesity-induced IL-6-type cytokine signaling induced basal free fatty acid (FFA) release in mesenteric adipocytes of high fat diet (HFD)-fed mice. Consequently, increased portal FFA flux induced hepatic insulin resistance and lipid accumulation. In humans, omental IL-6 mRNA expression correlated negatively with insulin sensitivity and positively with liver lipid accumulation,5 supporting a role for visceral IL-6 in the development of obesity-associated hepatic complications. Besides this pathological role of IL-6-type cytokine signaling in adipocytes, we have recently unraveled a physiological role of the latter in improving glucose tolerance in obesity.6 In vivo and in vitro experiments revealed that IL-6 stimulates leptin release from adipocytes thereby inducing glucagon-like peptide-1 (GLP-1) release from enteroendocrine cells and subsequent insulin secretion from pancreatic β-cells. In particular, HFD-fed gp130Δadipo mice were characterized by impaired glucose tolerance accompanied by reduced circulating leptin, GLP-1 and insulin levels. Importantly, administration of the GLP-1 receptor antagonist exendin 9–39 blunted the observed difference in glucose tolerance between control and knockout mice, indicating a critical involvement of GLP-1 in the observed phenotype. Ex vivo, basal leptin release in epididymal adipocytes isolated from HFD-fed gp130Δadipo mice was reduced compared to control (gp130F/F) mice. Moreover, collected supernatant from gp130-depleted adipocytes reduced GLP-1 secretion from cultured enteroendocrine cells leptin-dependently. Taken together, IL-6-type cytokine signaling induces the release of FFA and leptin from adipocytes in obesity thereby affecting glucose metabolism. While IL-6-induced FFA release induces hepatic insulin resistance and steatosis, IL-6-mediated leptin secretion improves glucose tolerance in obesity via inducing insulin secretion. Such opposing effects may suggest that inhibition of IL-6 signaling in adipocytes may not offer an appropriate approach to treat obesity-associated metabolic complications.
Depot-specific differences of adipocyte-specific IL-6-type cytokine signaling
Metabolism of adipocytes may vary depot-dependently in mice and men.7–9 However, it remains largely unknown whether the stimulatory effect of IL-6 on FFA10,11 and leptin12 release differs between adipose depots. Our data indicate that adipocyte-specific IL-6-type cytokine signaling affects FFA and leptin release depot-specifically. In fact, basal FFA release was reduced in portal-drained mesenteric but not in systemically-drained epididymal adipocytes isolated from HFD-fed gp130Δadipo mice.5 In addition, insulin’s ability to suppress FFA release was ameliorated in mesenteric but not epididymal adipocytes isolated from HFD-fed knockout compared to control mice.5 Consequently, portal but not systemic FFA levels were reduced in HFD-fed gp130Δadipo mice. In contrast, basal leptin release was reduced in epididymal adipocytes isolated from of HFD-fed gp130Δadipo mice, resulting in reduced systemic leptin levels.6 To elucidate whether insulin’s effect on leptin release is dependent on IL-6 cytokine signaling, we assessed leptin release in epididymal adipocytes isolated from HFD-fed gp130F/F and gp130Δadipo mice treated with or without insulin. Of note, insulin is an important physiological regulator of leptin release in adipocytes.13,14 In fact, insulin increases the release of leptin in 3T3-L1 adipocytes and epididymal adipose tissue of lean rodents.15,16 In epididymal adipocytes isolated from HFD-fed control (gp130F/F) mice, insulin had no stimulatory effect on leptin release, indicating evolved insulin resistance (Figure 1). In contrast, insulin stimulated the release of leptin in adipocytes isolated from HFD-fed gp130Δadipo mice. Hence, lack of IL-6-type cytokine signaling partly protects adipocytes from HFD-induced impairment in insulin-stimulated leptin release. In summary, IL-6-type cytokine signaling affects FFA release in mesenteric adipocytes while influencing leptin release in epididymal adipocytes (Table 1).
Figure 1.
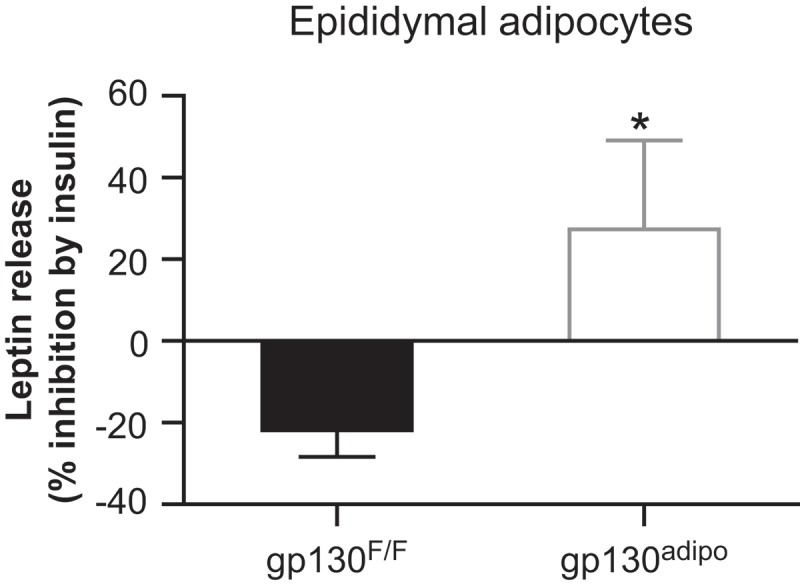
The effect ofDepot-specific differences of adipocyte insulin on leptin release in epididymal adipocytes isolated from HFD-fed gp130F/F and gp130Δadipo mice. Isolated adipocytes were incubated with or without 100 nM insulin for 1 hour and leptin release was assessed. Data correspond to the same mice (n = 8) included in a previously published paper.6 Values are expressed as mean ± SEM. *p < 0.05 (Student’s t test).
Table 1.
Metabolism of adipocyte isolated from HFD-fed gp130Δadipo mice.
| Epididymal adipocytes | Mesenteric adipocytes | |
|---|---|---|
| Basal FFA release | = | ↓ |
| Basal leptin release | ↓ | n.d. |
| Insulin effect on FFA release | = | ↑ |
| Insulin effect on leptin release | ↑ | n.d. |
n.d. not determined
Conclusion and future perspectives
IL-6 is a pleotropic cytokine that exerts diverse and opposing roles in different cells and tissues.17 In line, our findings indicate that IL-6 affects FFA and leptin release in adipocytes depot-specifically. We cannot exclude that other IL-6-type cytokines may modulate the release of leptin and/or FFA from adipocytes. However, among six tested IL-6-type cytokines, only IL-6 significantly induced leptin release from isolated adipocytes.6 In support for a role of IL-6-induced FFA release in hepatic dys-function, IL-6 infusion induced liver insulin resistance via adipose tissue lipolysis in rats.18 Importantly, IL-6-mediated FFA release may be restrained to visceral adipose tissue.5 Accordingly, targeting IL-6 signaling specifically in visceral adipocytes may be a promising strategy to protect the liver from obesity-associated hepatic insulin resistance and steatosis without reducing circulating leptin levels and, consequently, GLP-1 mediated insulin release.6 Although we did not provide evidence that inhibition of IL-6 signaling in mesenteric adipocytes reduces leptin release, we postulate that such reduction may not affect leptin levels in systemic circulation since mesenteric fat-secreted factors may reach systemic circulation only in minor amounts.19 Moreover, production and secretion of leptin is higher in systemically compared to portally-drained depots.20,21 In contrast to its action in visceral adipocytes, IL-6 may have beneficial effects in systemically-drained adipose tissue and, consequently, reduces obesity-induced metabolic complications. In particular, IL-6 activation in subcutaneous adipose tissue may induce leptin-mediated GLP-1 release in obesity. As GLP-1 is an important inducer of glucose-stimulated insulin release in vivo, this may be a useful strategy to prevent the development of type 2 diabetes in insulin resistant patients.22,23 In conclusion, adipose depot-specific targeting of the IL-6 signaling pathway may be a novel approach to blunt obesity-associated metabolic complications.
Funding Statement
This work was supported by the Gottfried und Julia Bangerter-Rhyner-Stiftung [n.a.]; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung [#310030-179344].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. [DOI] [PubMed] [Google Scholar]
- 3.Allen TL, Febbraio MA. IL6 as a mediator of insulin resistance: fat or fiction? Diabetologia. 2010;53:399–402. [DOI] [PubMed] [Google Scholar]
- 4.White UA, Stephens JM. The gp130 receptor cytokine family: regulators of adipocyte development and function. Curr Pharm Des. 2011;17:340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wueest S, Item F, Lucchini FC, Challa TD, Muller W, Bluher M, et al. Mesenteric Fat Lipolysis Mediates Obesity-Associated Hepatic Steatosis and Insulin Resistance. Diabetes. 2016;65:140–148. [DOI] [PubMed] [Google Scholar]
- 6.Wueest S, Laesser CI, Boni-Schnetzler M, Item F, Lucchini FC, Borsigova M, et al. IL-6-Type Cytokine Signaling in Adipocytes Induces Intestinal GLP-1 Secretion. Diabetes. 2018;67:36–45. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Smith U. Adipose tissue distribution and risk of metabolic disease: does thiazolidinedione-induced adipose tissue redistribution provide a clue to the answer? Diabetologia. 2007;50:1127–1139. [DOI] [PubMed] [Google Scholar]
- 8.Wueest S, Schoenle EJ, Konrad D. Depot-specific differences in adipocyte insulin sensitivity in mice are diet- and function-dependent. Adipocyte. 2012;1:153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wueest S, Yang X, Liu J, Schoenle EJ, Konrad D. Inverse regulation of basal lipolysis in perigonadal and mesenteric fat depots in mice. Am J Physiol Endocrinol Metab. 2012;302:E153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio MA, et al. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am J Physiol Endocrinol Metab. 2005;288:E155–62. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Ju D, Zhang M, Yang G. Interleukin-6 stimulates lipolysis in porcine adipocytes. Endocrine. 2008;33:261–269. [DOI] [PubMed] [Google Scholar]
- 12.Trujillo ME, Sullivan S, Harten I, Schneider SH, Greenberg AS, Fried SK. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J Clin Endocrinol Metab. 2004;89:5577–5582. [DOI] [PubMed] [Google Scholar]
- 13.Tsai M, Asakawa A, Amitani H, Inui A. Stimulation of leptin secretion by insulin. Indian J Endocrinol Metab. 2012;16:S543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried SK, Ricci MR, Russell CD, Laferrere B. Regulation of leptin production in humans. J Nutr. 2000;130:3127S–31S. [DOI] [PubMed] [Google Scholar]
- 15.Barr VA, Malide D, Zarnowski MJ, Taylor SI, Cushman SW. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138:4463–4472. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Ali Y, Lim CY, Hong W, Pang ZP, Han W. Insulin-stimulated leptin secretion requires calcium and PI3K/Akt activation. Biochem J. 2014;458:491–498. [DOI] [PubMed] [Google Scholar]
- 17.Fuster JJ, Walsh K. The good, the bad, and the ugly of interleukin-6 signaling. EMBO J. 2014;33:1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004;113:1530–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell CD, Petersen RN, Rao SP, Ricci MR, Prasad A, Zhang Y, et al. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am J Physiol. 1998;275:E507–15. [DOI] [PubMed] [Google Scholar]
- 21.Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. [DOI] [PubMed] [Google Scholar]
- 22.Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159–169. [DOI] [PubMed] [Google Scholar]
- 23.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. [DOI] [PubMed] [Google Scholar]


