Abstract
Aim: Osteoglycin is one of proteoglycans that are biologically active components of vascular extracellular matrix. However, the role of osteoglycin in atherosclerosis remains unclear.
Methods: We investigated plasma osteoglycin levels and the presence, severity, and lesion morphology of coronary artery disease (CAD) in 462 patients undergoing elective coronary angiography.
Results: Of 462 patients, 245 had CAD. Osteoglycin levels were higher in patients with CAD than without CAD (median 29.7 vs. 25.0 ng/mL, P < 0.05). However, osteoglycin levels did not differ among patients with one-vessel, two-vessel, or three-vessel disease (30.8, 30.6, and 29.4 ng/mL, respectively) and did not correlate with the number of stenotic segments. Among 245 CAD patients, 41 had complex coronary lesions, and 70 had total occlusion, of whom 67 had good collateralization. Between 70 patients with occlusion and 175 without occlusion, osteoglycin levels did not differ (30.4 vs. 29.5 ng/mL). Notably, osteoglycin levels were lower in 41 patients with complex lesions than in 204 without such lesions (24.2 vs. 31.6 ng/mL, P < 0.02). In multivariate analysis, osteoglycin levels were an independent factor for complex lesion but not for CAD. Odds ratio for complex lesion was 0.80 (95%CI = 0.67–0.96) for each 10 ng/mL increase in osteoglycin levels (P < 0.02).
Conclusion: Although plasma osteoglycin levels were high in patients with CAD, they did not correlate with the severity of CAD and were not an independent factor for CAD. Notably, osteoglycin levels were low in patients with complex lesions and were a factor for complex lesions, suggesting that osteoglycin plays a role in coronary plaque stabilization.
Keywords: Coronary artery disease, Osteoglycin, Plaque stabilization
Introduction
The structure and composition of vascular extracellular matrix (ECM) plays an important role in atherosclerosis1). Osteoglycin, also known as mimecan, is one of small leucine-rich proteoglycans that are biologically active components of ECM and are involved in a wide range of biological processes such as inflammation, fibrosis, and cell proliferation2–4). Although the main function of osteoglycin in ECM is recognized to regulate collagen fibrillonegesis5, 6), the role of osteoglycin in atherosclerosis remains unclear. In rabbit atherosclerotic plaques, osteoglycin mRNA was down-regulated in media and up-regulated in activated endothelium and neointima2). In human coronary atherosclerotic plaques, osteoglycin mRNA was down-regulated in media and intimal smooth muscle cells (SMCs)7). Decreased expression of osteoglycin was also shown in aortic aneurysm8). Notably, in carotid endarterectomy samples, osteoglycin was shown to be down-regulated in hemorrhagic plaques compared with fibrotic plaques9). The down-regulation of osteoglycin may lead to plaque instability. However, Zhang et al.10) reported conflicting findings that overexpression of osteoglycin inhibits aortic SMC proliferation and enhances apoptosis. Furthermore, in a rabbit femoral artery ligation model, down-regulation of osteoglycin was required for the development of collateral arteries11). Fergandez et al.12) reported that apoE-osteoglycin double knockout mice developed severe atherosclerosis with aneurysm, medial degeneration, inflammation, and leukocyte extravasation, whereas Moncayo et al.13) showed that they did not have severe atherosclerotic lesions. The role of osteoglycin in the process of atherosclerosis therefore remains controversial.
Regarding blood osteoglycin levels, few studies have investigated the association between blood osteoglycin levels and coronary artery disease (CAD). One study14) reported serum osteoglycin levels to be high in 78 patients with CAD. Another study15) showed that serum osteoglycin levels were lower in CAD patients with total occlusion and good collateralization than in those with poor collateralization. We examined the associations between plasma osteoglycin levels and the presence, severity, and lesion morphology of CAD in 462 patients undergoing elective coronary angiography.
Methods
Study Patients
We measured plasma osteoglycin levels in 462 consecutive patients undergoing elective coronary angiography for suspected CAD at Tokyo Medical Center from July 2008 to March 2016. Any patients with acute myocardial infarction or class Ⅲ unstable angina by Braunwald's classification16), or those with a history of percutaneous coronary intervention or cardiac surgery, were excluded. Hypertension was defined as blood pressures of ≥ 140/90 mmHg or on drugs, and 277 (60%) patients were taking relevant drugs. Hyperlipidemia was defined as a low-density lipoprotein (LDL)-cholesterol level of > 140 mg/dL or on drugs, and 175 (38%) were taking statins. Diabetes mellitus (a fasting plasma glucose level of ≥ 126 mg/dL or on treatment) was present in 124 (27%), and 167 (36%) were smokers (≥ 10 pack-years). Our study was approved by the institutional ethics committee. After written informed consent was obtained, overnight fasting blood samples were taken on the morning of the day of coronary angiography.
Measurement of Plasma Osteoglycin Levels
Blood samples were collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes, and only the plasma was stored at −80°C. Plasma osteoglycin levels were measured by an enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (Human OGN ELISA Kit; Elabscience Biotechnology, Houston, USA) at Ochanomizu University according to manufacturer's instructions. This kit was developed for the measurement of osteoglycin levels in both serum and plasma. The minimum level detected by this assay was 0.75 ng/mL. The intra-assay and inter-assay coefficients of variation were 9.9% and 9.9%, respectively.
Coronary Angiography
Coronary angiograms were recorded on a cineangiogram system (Philips Electronics Japan, Tokyo). CAD was defined as at least one coronary artery having > 50% luminal diameter stenosis. The severity of coronary atherosclerosis was represented as the number of > 50% stenotic vessels and the numbers of > 50% and > 25% stenotic segments. According to Ambrose's classification17), coronary stenotic lesions were classified as complex if they had sharp overhanging edges, irregular borders, or intraluminal lucency. Collaterals to total occlusion were classified as poor (grades 0 and 1) and good (2 and 3) collateralization by the Rentrop scoring system18). Furthermore, coronary artery segments were defined as 29 segments by the CASS classification. All angiograms were evaluated by a single cardiologist (Y.M.), blinded to clinical and laboratory data.
Statistical Analysis
Differences between two groups were evaluated by unpaired t-test for parametric variables, by Mann–Whitney U test for nonparametric variables, and by chi-squared test for categorical variables. Differences among more than or equal to three groups were evaluated by analysis of variance with Scheffe's test for parametric variables, by Kruskal–Wallis test with Steel–Dwass test for nonparametric variables, and by the chi-squared test for categorical variables. Correlations between osteoglycin levels and the severity of CAD were evaluated by Spearman's rank correlation test. A multiple logistic regression analysis was used to elucidate independent associations between osteoglycin levels and CAD or complex lesions. A P-value of < 0.05 was considered to be statistically significant. Results are presented as the mean ± SD or the median value.
Results
Of 462 study patients, CAD was found in 245 (one-vessel [1-VD], n = 102; two-vessel [2-VD], n = 69; three-vessel disease [3-VD], n = 74). Compared with 217 patients without CAD, 245 with CAD were older and had a male predominance and higher prevalence of hypertension, diabetes, hyperlipidemia, and smoking (Table 1). Plasma osteoglycin levels were higher in patients with CAD than in those without CAD (median 29.7 vs. 25.0 ng/mL, P < 0.05). However, there was no marked difference in osteoglycin levels among the 1-VD, 2-VD, and 3-VD groups (30.8, 30.6, and 29.4 ng/mL, respectively; P = 0.94) (Fig. 1). Furthermore, no correlation was found between osteoglycin levels and the numbers of > 50% and > 25% stenotic segments. To elucidate independent association between osteoglycin levels and CAD, variables (age, gender, hypertension, hyperlipidemia, statin use, diabetes, smoking, and osteoglycin levels) were entered into a multiple logistic regression model. However, osteoglycin levels were not a significant factor for CAD independent of atherosclerotic risk factors.
Table 1. Clinical characteristics and plasma osteoglycin levels.
| CAD(−) | CAD | |||||
|---|---|---|---|---|---|---|
| (n = 217) | P value | (n = 245) | Complex lesion (+) | Complex lesion (−) | ||
| CAD(−) | (n = 41) | Complex lesion | (n = 204) | |||
| vs. CAD | (+) vs. (−) | |||||
| Age (years) | 65 ± 12 | < 0.001 | 70 ± 10 | 69 ± 10 | 0.63 | 70 ± 10 |
| Gender (male) | 138 (64%) | < 0.05 | 183 (75%) | 32 (78%) | 0.80 | 151 (74%) |
| Hypertension | 134 (62%) | < 0.001 | 196 (80%) | 31 (76%) | 0.60 | 165 (81%) |
| SBP (mmHg) | 131 ± 21 | 0.14 | 133 ± 22 | 134 ± 21 | 0.35 | 133 ± 22 |
| Diabetes mellitus | 31 (14%) | < 0.001 | 93 (38%) | 15 (37%) | 0.50 | 87 (43%) |
| Smoking | 65 (30%) | < 0.025 | 102 (42%) | 59 (40%) | 0.60 | 11 (37%) |
| Hyperlipidemia | 87 (40%) | < 0.001 | 150 (61%) | 24 (59%) | 0.90 | 126 (62%) |
| Statin | 55 (25%) | < 0.001 | 120 (49%) | 16 (39%) | 0.30 | 104 (51%) |
| LDL-C (mg/dL) | 111 ± 28 | 0.06 | 115 ± 33 | 127 ± 28 | < 0.05 | 113 ± 34 |
| HDL-C (mg/dL) | 58 ± 15 | < 0.001 | 51 ± 13 | 52 ± 12 | 0.39 | 51 ± 13 |
| Osteoglycin (ng/mL) | 25 [13.2, 41.3] | < 0.05 | 29.7 [15.7, 46.3] | 24.2 [12.5, 35.8] | < 0.02 | 31.6 [17.7, 49.9] |
| 1-VD | 102 (42%) | 12 (29%) | 0.20 | 90 (44%) | ||
| 2-VD | 69 (28%) | 11 (27%) | 0.99 | 58 (28%) | ||
| 3-VD | 74 (30%) | 18 (44%) | 0.10 | 56 (27%) |
Data are presented as the mean ± SD or the number (%) of patients, except for osteoglycin levels, which are presented as the median value and interquartile range (IQR).
SBP indicates systolic blood pressure; LDL-C, LDL-cholesterol; and HDL-C, HDL-cholesterol.
Fig. 1.
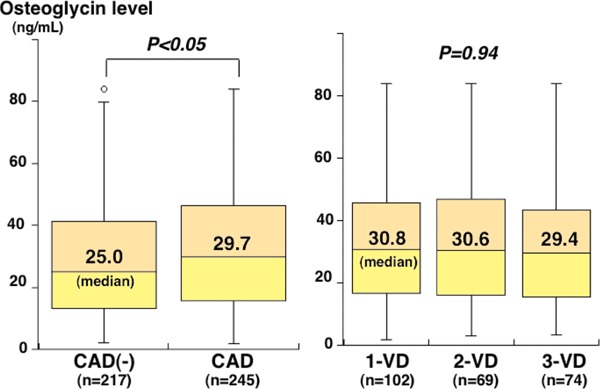
Plasma osteoglycin levels and the presence and severity of CAD
Plasma osteoglycin levels were higher in patients with CAD than in those without CAD (P < 0.05), but there was no marked difference in osteoglycin levels among the 1-VD, 2-VD, and 3-VD groups (P = 0.94). The central line represents the median, and the box represents the 25th to 75th percentiles. The whiskers represent the lowest and highest value in the 25th percentile minus 1.5 interquartile range (IQR) and 75th percentile plus 1.5 IQR, respectively.
Among 245 patients with CAD, 41 (17%) had complex coronary lesions, and 70 (29%) had total occlusion, of whom 67 (96%) had good collateralization. Between 70 CAD patients with total occlusion and 175 without occlusion, osteoglycin levels did not differ (30.4 vs. 29.5 ng/mL, P = 0.85) (Fig. 2). Notably, osteoglycin levels in 41 CAD patients with complex lesions were lower than in 204 CAD patients without such lesions (24.2 vs. 31.6 ng/mL, P < 0.02) (Table 1, Fig. 2). However, osteoglycin levels tended to be lower in patients with complex lesions than in patients without CAD, but this difference did not reach statistical significance. Osteoglycin levels were higher in CAD patients without such lesions than in patients without CAD (P < 0.02) (Fig. 2). In multivariate analysis of the 245 CAD patients, osteoglycin levels were an independent factor associated with complex lesions. The odds ratio for complex lesion was 0.80 (95%CI = 0.67–0.96) for a 10 ng/mL increase in osteoglycin levels (P < 0.02). However, among the 462 study patients, osteoglycin levels were not a significant factor for the presence of complex lesions.
Fig. 2.
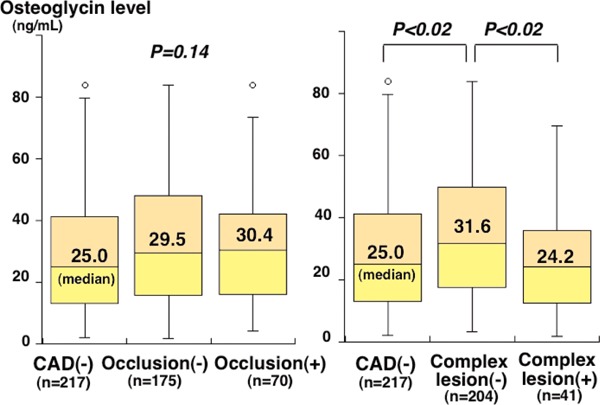
Plasma osteoglycin levels and the presence of total occlusion and complex lesions
Plasma osteoglycin levels did not differ between CAD patients with and without total occlusion (P = 0.85) (left). Notably, osteoglycin levels were significantly lower in CAD patients with complex lesions than in those without such lesions (P < 0.02). However, osteoglycin levels in patients with complex lesions were not different from those in patients without CAD (P = 0.38) (right). The central line represents the median, and the box represents the 25th to 75th percentiles. The whiskers represent the lowest and highest value in the 25th percentile minus 1.5 IQR and 75th percentile plus 1.5 IQR, respectively.
Although patients with class Ⅲ unstable angina were excluded, 17 and 24 of the 245 CAD patients were classes Ⅰ and Ⅱ unstable angina, respectively. However, there was no significant difference in osteoglycin levels between 41 patients with class Ⅰ or Ⅱ unstable angina and 204 patients with stable CAD (31.9 vs. 29.5 ng/mL, P = 0.32).
Discussion
In our study, plasma osteoglycin levels were high in patients with CAD. However, they did not correlate with the severity of CAD and were not an independent factor for CAD. Notably, osteoglycin levels were low in patients with complex coronary lesions and were an independent factor associated with complex lesions. Osteoglycin may play a role in coronary plaque stabilization.
Regarding blood osteoglycin levels in patients with CAD, Hu et al.14) measured serum osteoglycin levels in 158 patients undergoing coronary angiography. They reported osteoglycin levels to be higher in 78 patients with CAD than in 80 without CAD and to correlate with Gensini score (r = 0.79). However, patients with diabetes or hypertension were excluded from that study, suggesting that their patients were highly selected among patients undergoing angiography. In human coronary atherosclerotic lesions, osteoglycin mRNA was down-regulated in the medial and intimal SMCs7). In contrast, overexpression of osteoglycin was shown to inhibit SMC proliferation and to enhance apoptosis8). Therefore, high levels of serum osteoglycin in patients with CAD may represent an adaptive mechanism aimed at preventing the progression of coronary atherosclerosis. We investigated plasma osteoglycin levels in 462 patients undergoing coronary angiography. We also found that osteoglycin levels were higher in 245 patients with CAD than in 217 without CAD. However, no correlation was found between osteoglycin levels and the number of > 50% stenotic coronary vessels or the numbers of > 50% and > 25% stenotic segments. Furthermore, osteoglycin levels were not a significant factor for CAD independent of atherosclerotic risk factors. As shown in Fig. 1, there were some overlaps in osteoglycin levels between patients with and without CAD. Plasma osteoglycin levels may reflect not only coronary atherosclerosis but also atherosclerosis in other vascular beds. Hence, plasma osteoglycin levels are high in patients with CAD, but they are unlikely to be a good biomarker reflecting the presence or severity of CAD.
Cheng et al.19) investigated plasma osteoglycin levels in 88 patients with major adverse cardiovascular events (MACE) among 768 patients undergoing coronary angiography, of whom 52% had angiography for acute coronary syndromes. They documented osteoglycin levels to be high in patients with MACE. However, the same group had previously reported that osteoglycin was down-regulated in hemorrhagic plaques, so-called unstable plaques, than fibrotic plaques in carotid endarterectomy samples9). In apoE-osteoglycin double-knockout mice, severe atherosclerosis with medial degeneration and inflammation was also reported12). These findings suggest that the downregulation of osteoglycin may lead to plaque instability. In our study, 41 (17%) of the 245 patients with CAD had complex coronary lesions, which are known to be associated with plaque instability and to be predictive of coronary events17, 20). Notably, we found that plasma osteoglycin levels were lower in patients with complex coronary lesions than in CAD patients without complex lesions and that low levels of osteoglycin were an independent factor associated with complex lesions among CAD patients. Our results thus suggest that low levels of plasma osteoglycin may play a role in coronary plaque instability, such as complex coronary lesions. Furthermore, plasma osteoglycin levels were higher in CAD patients without complex lesions than in CAD patients with complex lesions and patients without CAD. High osteoglycin levels in CAD patients without complex lesions may have been aimed at coronary plaque stabilization as well as preventing the progression of coronary atherosclerosis. Moreover, because CAD patients with complex lesions had low levels of osteoglycin in plasma, osteoglycin may be a therapeutic target in coronary plaque instability and may be used to treat patients with complex lesions to stabilize coronary plaques.
In rabbits, down-regulation of osteoglycin was associated with the development of collateral arteries11). Shen et al.15) investigated serum osteoglycin levels and coronary collateralization in 559 CAD patients with chronic total occlusion. They reported that osteoglycin levels were lower in 350 patients with good collateralization than in 209 with poor collateralization. In our study, 70 (29%) of the 245 CAD patients had total occlusion, but 67 (96%) of 70 patients with total occlusion had good collateralization. Between 70 patients with total occlusion and 175 CAD patients without occlusion, no marked difference was found in osteoglycin levels.
Our study has several limitations. First, in our study, angiography was used to evaluate coronary atherosclerosis. Coronary angiography cannot visualize plaques and only shows the severity and morphology of stenotic lesions. In our study, IVUS or OCT, which can visualize coronary plaques, were not always performed. Moreover, because CAD was defined as at least one coronary artery having > 50% luminal diameter stenosis on angiograms, some patients without CAD had mild stenosis, such as < 50% stenosis. This is one of major study limitations. Second, osteoglycin is abundantly expressed in normal vessels and atherosclerotic lesions as well as in cornea, skin, and cartilage and, in lower amounts, in myocardium, skeletal muscle, cerebelum, intestine, and kidney21). However, because we did not measure osteoglycin levels in the coronary sinus, our study did not provide any information about the main sources of osteoglycin in CAD patients. Third, in our study, 41 (17%) of 245 patients with stable CAD had complex coronary lesions. This prevalence of complex lesions was similar to that reported by Ambrose et al.17) (10% to 20% of patients with stable CAD). However, the small number of patients with complex lesions (n = 41) is a major study limitation. Moreover, no patient with acute coronary syndrome was included in our study. A further study in patients with acute coronary syndrome is needed to elucidate the potential role of osteoglycin in this syndrome. Fourth, our study was cross-sectional in nature and could not establish causality, because it only depicted some associations and proposed some hypotheses. To determine the prognostic values of osteoglycin levels as well as complex lesions, further prospective studies are needed in a larger number of patients with stable CAD. Finally, our study was in Japanese patients undergoing coronary angiography, who are generally considered to be a highly select population at high risk for CAD. Our results therefore may not be applicable to the general or other ethnic populations.
In conclusion, plasma osteoglycin levels were high in patients with CAD. However, they were not associated with the severity of CAD and were not an independent factor for CAD. Notably, osteoglycin levels were low in patients with complex lesions and were an independent factor associated with complex lesions. Our results suggest that osteoglycin may play a role in coronary plaque stabilization.
Funding
This study was supported in part by a grant from Honjo International Scholarship Foundation. Financial funding was also provided by Bayer Yakuhin Ltd., Daiichi Sankyo Co., and Pfizer Japan Inc.; however, these sponsors had no role in the design, analysis, or interpretation of the study.
Conflict of Interest
Our study has no conflict of interest to disclose.
References
- 1). Orr AW, Hastings NE, Blackman BR, Wamhoff BR: Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res, 2010; 47: 168-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Fernández B, Kampmann A, Pipp F, Zimmermann R, Schaper W: Osteoglycin expression and localization in rabbit tissues and atherosclerotic plaques. Mol Cell Biochem, 2003; 246: 3-11 [PubMed] [Google Scholar]
- 3). Merline R, Schaefer RM, Schaefer L: The matricellular functions of small leucine-rich proteoglycans (SLRPs). J Cell Commun Signal, 2009; 3: 323-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Deckx S, Heymans S, Papageorgiou AP: The diverse functions of osteoglycin: a deceitful dwarf, or a master regulator of disease? FASEB J, 2016; 30: 2651-2661 [DOI] [PubMed] [Google Scholar]
- 5). Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, Song M, Fox N, Conrad GW: Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis, 2002; 8: 407-415 [PubMed] [Google Scholar]
- 6). Ge G, Seo NS, Liang X, Hopkins DR, Hook M, Greenspan DS: Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis. J Biol Chem, 2004; 279: 41626-41633 [DOI] [PubMed] [Google Scholar]
- 7). Shanahan CM, Cary NR, Osbourn JK, Weissberg PL: Identification of osteoglycin as a component of the vascular matrix. Differential expression by vascular smooth muscle cells during neointima formation and in atherosclerotic plaques. Arterioscler Thromb Vasc Biol, 1997; 17: 2437-2447 [DOI] [PubMed] [Google Scholar]
- 8). Matsumoto K, Maniwa T, Tanaka T, Satoh K, Okunishi H, Oda T: Proteomic analysis of calcified abdominal and thoracic aortic aneurysms. Int J Mol Med, 2012; 30: 417-429 [DOI] [PubMed] [Google Scholar]
- 9). Malaud E, Merle D, Piquer D, Molina L, Salvetat N, Rubrecht L, Dupaty E, Galea P, Cobo S, Blanc A, Saussine M, Marty-Ané C, Albat B, Meilhac O, Rieunier F, Pouzet A, Molina F, Laune D, Fareh J: Local carotid atherosclerotic plaque proteins for the identification of circulating biomarkers in coronary patients. Atherosclerosis, 2014; 233: 551-558 [DOI] [PubMed] [Google Scholar]
- 10). Zhang HJ, Wang J, Liu HF, Zhang XN, Zhan M, Chen FL: Overexpression of mimecan in human aortic smooth muscle cells inhibits cell proliferation and enhances apoptosis and migration. Exp Ther Med, 2015; 10: 187-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Kampmann A, Fernandez B, Deindl E, Thomas Kubin T, Pipp F, Eitenmuller I, Hoefer IE, Wolfgang Schaper W, Zimmermann R: The proteoglycan osteoglycin/ mimecan is correlated with arteriogenesis. Mol Cell Biochem, 2009; 322: 15-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Fernández B, Fernández MC, Moncayo J, Duran AC, Such M, Sans-Coma V: Absence of mimecan causes medial damage associated with atherosclerotic lesions in apoE-deficient mice. FASEB J, 2009; 23: Suppl 640.1 [Google Scholar]
- 13). Moncayo-Arlandi J, Lopez-García A, Fernandez MC, Duran AC, Fernandez B: Osteoglycin deficiency does not affect atherosclerosis in mice. Atherosclerosis, 2014; 237: 418-425 [DOI] [PubMed] [Google Scholar]
- 14). Hu Y, Liu J, Zhao Q, Xu P, Chen Y, Zhou H, Li X: Correlation between mimecan expression and coronary artery stenosis in patients with coronary heart disease. Int J Clin Exp Med, 2015; 8: 21641-21646 [PMC free article] [PubMed] [Google Scholar]
- 15). Shen Y, Ding FH, Zhang RY, Zhang Q, Lu L, Shen WF: Association of serum mimecan with angiographic coronary collateralization in patients with stable coronary artery disease and chronic total occlusion. Atherosclerosis, 2016; 252: 75-81 [DOI] [PubMed] [Google Scholar]
- 16). Braunwald E: Unstable angina: a classification. Circulation, 1989; 80: 410-414 [DOI] [PubMed] [Google Scholar]
- 17). Ambrose JA, Israel DH: Angiography in unstable angina. Am J Cardiol, 1991; 68: 78B-84B [DOI] [PubMed] [Google Scholar]
- 18). Rentrop KP, Cohen M, Blanke H, Phillips RA: Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol, 1985; 5: 587-592 [DOI] [PubMed] [Google Scholar]
- 19). Cheng JM, Akkerhuis KM, Meilhac O, Oemrawsingh RM, Garcia-Garcia HM, van Geuns RJ, Piquer D, Merle D, du Paty E, Galéa P, Jaisser F, Rossignol P, Serruys PW, Boersma E, Fareh J: Circulating osteoglycin and NGAL/MMP9 complex concentrations predict 1-year major adverse cardiovascular events after coronary angiography. Arterioscler Thromb Vasc Biol, 2014; 34: 1078-1084 [DOI] [PubMed] [Google Scholar]
- 20). Wilson RF, Holida MD, White CW: Quantitative angiographic morphology of coronary stenoses leading to myocardial infarction or unstable angina. Circulation, 1986; 73: 286-293 [DOI] [PubMed] [Google Scholar]
- 21). Deckx S, Heymans S, Papageorgiou AP: The diverse functions of osteoglycin: a deceitful dwarf, or a master regulator of disease? FASEB J, 2017; 30: 2651-2661 [DOI] [PubMed] [Google Scholar]


