Abstract
The metanephros is the functional organ in adult amniotes while the mesonephros degenerates. However, parallel tubulogenetic events are thought to exist between mesonephros and metanephros. Mesonephric tubules are retained in males and differentiate into efferent ducts of the male reproductive tract. By examining the murine mesonephric expression of markers of distinct stages and regions of metanephric nephrons during tubule formation and patterning, we provide further evidence to support this common morphogenetic mechanism. Renal vesicle, early proximal and distal tubule, loop of Henle and renal corpuscle genes were expressed by mesonephric tubules. Vip, Slc6a20b and Slc18a1 were male-specific. In contrast, mining of the GUDMAP database identified candidate late mesonephros-specific genes, 10 of which were restricted to the male. Amongst the male-specific genes are candidates for regulating ion/fluid balance within the efferent ducts, thereby regulating sperm maturation and genes marking tubule-associated neurons potentially critical for normal male reproductive tract function.
Keywords: mesonephros, metanephros, nephron, gene expression, efferent duct, male reproductive tract
Introduction
In vertebrates, three distinct excretory organs can develop from the intermediate mesoderm (IM). The most basic of these, the pronephros, functions during development in some species but is replaced by the mesonephros as the functional postnatal excretory organ of amphibians and fish. Higher organisms develop a third pair of excretory organs, the metanephros. While the pronephros and mesonephros form in amniotes, both regress leaving the metanephros as the functional postnatal kidney. The induction of these organs involves two paired nephric ducts (ND; Wolffian ducts) which arise from the IM posterior to the forelimb bud (8.0 dpc, days post coitum, in mice). In mice, the mesonephros arises from the rostral IM around 9.0 dpc in response to signals from the ND, while the metanephros forms at the most caudal end of the ND adjacent to the hindlimb at 10.5 dpc (Saxen, 1987; Sainio et al., 1997; Sainio and Raatikainen-Ahokas, 1999). Between 9.0 and 10.5 dpc, mesenchymal condensations parallel with the ND develop within the mesonephros, representing the first signs of mesonephric tubule formation (Smith et al., 1991; Sainio et al., 1997; see Figure 1 A). These epithelial tubules have been classified into cranial tubules (Figure 1 A, MTcr), which have a connection with the ND, and caudal tubules (Figure 1 A, MTca) located within the mesenchyme and not connected to the ND (Smith et al., 1991; Sainio et al., 1997). While it was initially proposed that the cranial tubules developed as outgrowths from the ND, fate-mapping has shown that both cranial and caudal tubules develop via a mesenchyme-to-epithelial transition (MET) within the mesonephric mesenchyme (Mugford et al., 2008). This suggests a very similar process of tubule formation to the metanephric nephrons.
Figure 1. Key molecules in the formation of the mesonephric and metanephric tubules.

A. Schematic (left) illustrating the position of the urogenital tract (UGT) within the 10.5 dpc embryo (hindlimb visible) and the mesonephroi and kidneys of the 12.5–13.5 dpc UGT (right). At 10.5 dpc, S-shaped cranial MT (MTcr) are connected to the nephric duct (ND). Vesicle-shaped caudal MT (MTca) are located within the mesenchyme (Smith et al., 1991). At 12.5–13.5 dpc, the paramesonephric duct (PMD) is present and 18–26 highly convoluted MT extend from the cranial tip to half-way down the gonad (Smith et al., 1991; Sainio et al., 1997). B. Enlarged schematic showing the expression of key genes within early mesonephros and metanephros. Wnt9b secreted by the nephric duct (ND) and ureteric bud (UB) into the mesonephric (MesoM) and metanephric (MM) mesenchyme induces a common, tubule promoting developmental program, including expression of Wnt4, Fgf8 and Lhx1 within both early epithelial mesonephric tubules (MT) and renal vesicles (RV) (boxed region). Eya1, Six2 and Six1 are transiently expressed in the MesoM at 9.5 dpc and downregulated at 10.5 dpc. G, gonad; CM, cap mesenchyme; ET, early tubules (renal vesicle, S-shaped body); UT, ureteric tip; white arrowhead, MT connecting tubule.
While the mesonephros degenerates from 13.5 dpc, some elements of this organ persist. In the male, the cranial mesonephric tubules develop into the efferent ducts, the anterior ND develops into the epididymis and vas deferens and the caudal ND into the seminal vesicles. The tubule-derived efferent ducts play a role in sperm maturation and are essential for male fertility. In both sexes, the ND is also involved in the induction of the paramesonephric duct (PMD) or Müllerian duct, which arises at the rostral tip of each mesonephros at 12.5 dpc and extends caudally in parallel to the ND (reveiewed by Yin and Ma, 2005; Klattig and Englert, 2007; Massé et al., 2009) (Figure 1 A). In males, the PMD degenerates. In contrast, in females, the ND degenerates and the PMD gives rise to the oviducts, uterine horns and upper part of the vagina (Saxen, 1987; Sainio et al., 1997; Sainio and Raatikainen-Ahokas, 1999; Tilmann and Capel, 2002; Vize et al., 2002). While the development of the metanephric kidney has been extensively studied, little is known about the molecular mechanisms involved in the formation of the mesonephric tubules and the subsequent regulation of efferent duct differentiation and maturation in the male reproductive tract.
Our current understanding of the molecular basis of mesonephric development is drawn from studies on metanephric development. Mutant mice displaying metanephric defects frequently show mesonephric defects due to the significant overlap in the pathways involved. For example, induction of both the metanephric and mesonephric tubules is dependent on the ND (Saxen, 1987; Grobstein, 1953; Carroll et al., 2005) with Wnt signaling responsible for the initial induction of MET in both tubule types. Wnt9b is secreted by the ND (Carroll et al., 2005) and initiates the tubulogenic program involving Fgf8 (Perantoni et al., 2005; Grieshammer et al., 2005) and Wnt4 (Stark et al., 1994; Vainio et al., 1999) in the nascent tubules of both organs (see Figure 1 B). Hence, loss of Wnt9b results in a complete loss of all mesonephric tubules and the loss of metanephric MET. Other genes common to the formation of both tubule types include Pax2 (Torres et al., 1995; Bouchard et al., 2002; Grote et al., 2006; Narlis et al., 2007), Lhx1 (Kobayashi et al., 2004; Shawlot and Behringer, 1995), Osr1 (Wang et al., 2005; James et al., 2006), Sall1 (Nishinakamura et al., 2001; Ott et al., 2001), Six1 (Xu et al., 2003; Kobayashi et al., 2007) and Wt1 (Sainio et al., 1997). Despite this congruence, the mature tubules that develop in each organ are distinctly different, with only the metanephric tubules of the kidney forming functional excretory nephrons in the mouse.
One obvious divergence in structure between the metanephros and mesonephros lies in the branching collecting duct of the metanephros, a structure that does not exist within the mesonephros. Indeed, expression of Gdnf is restricted to the metanephric mesenchyme (MM) and it is this ligand that induces the upregulation of Ret and its co-receptor Gfra1 in the adjacent ND to initiate ureteric bud outgrowth. This distinction in developmental patterning between metanephric and mesonephric development is in part due to the expression of the Hox11 paralogs, Hoxa11, Hoxc11 and Hoxd11. Loss of these transcription factors within the IM results in failure to express crucial factors in the caudal IM, including Gdnf and Six2. The result is renal agenesis without affecting the mesonephros (Wellik et al., 2002; Mugford et al., 2008). Conversely, when Hoxd11 is ectopically expressed in the mesonephric mesenchyme, Six2 is ectopically activated, inducing additional tubule formation in the mesonephros without induction of Gdnf expression (Mugford et al., 2008).
While it has been proposed that there is considerable overlap in the processes of tubule formation and patterning between mesonephros and metanephros, there is debate on how similar these processes are. Some studies have suggested that mesonephric tubules lack a juxtaglomerular apparatus and segments of the loop of Henle (Vize et al., 2002), while glomerulus-like structures are rare and restricted to MTcr (Smith et al., 1991; Sainio et al., 1997). However, other studies revealed mesonephric tubular expression of some but not all markers of differentiated loop of Henle segments with markers of more distal segments absent (Mugford et al., 2008), leaving open the question of parallels in developmental programs between the two tissues. In this study, we have investigated this question further taking advantage of our recent studies defining an atlas of gene expression during metanephric development (Brunskill et al., 2008). From that atlas, we have previously identified and validated genes enriched in the renal vesicle (RV), the first stage of nephron development (Georgas et al., 2009), and defined a set of anchor genes specifically expressed in distinct developmental stages or compartments of the metanephric nephrons (Thiagarajan et al., 2011). Here we reexamine the expression of these RV-enriched and tubular compartment anchor genes during murine mesonephric development from 10.5 to 13.5 dpc using wholemount in situ hybridization. Our results extend the number of genes expressed during both mesonephric and metanephric tubule formation and patterning. The strong congruence between RV and early mesonephric tubule expression supports the existence of a common pathway for tubule initiation and primary patterning between the two organs. A review of the Genitourinary Development Molecular Atlas Project (GUDMAP; www.gudmap.org; McMahon et al., 2008) database looking for evidence of differences between these pathways identified 9 early mesonephric-specific genes and 4 metanephric-specific genes. While these 9 genes were differentially expressed in mesonephric and not metanephric tubules, they were expressed in other metanephric structures, including UB and MM. The metanephric-specific genes were not expressed in any stage of mesonephric tubule development. Of note, a subset of 3 mesonephric/metanephric tubule anchor genes showed expression specific to male mesonephric tubules at 13.5 dpc. A further 10 genes expressed specifically in male mesonephric tubules were also identified via a review of GUDMAP database. This suggests a sexually dimorphic divergence in the differentiation of mesonephric tubular structures, presumably reflecting specific requirements for efferent duct function.
Results
Metanephric renal vesicle genes are expressed in early mesonephric tubules but not retained in mature mesonephric tubules
Using a set of genes up-regulated in the renal vesicle (RV) of the developing nephron at 15.5 dpc (Georgas et al., 2009), we reexamined the expression of these genes using wholemount in situ hybridization (WISH) at two earlier stages in both the metanephros and mesonephros (10.5 dpc and 12.5 and/or 13.5 dpc). At 10.5 dpc, early mesonephric tubules are visible and the metanephric mesenchyme (MM) is first observed flanking the caudal ND. From 12.5 to 13.5 dpc, early nephrons are readily identifiable within the metanephros and more mature mesonephric tubules are present within the mesonephroi of both sexes. The resulting expression patterns were annotated using the anatomical ontology generated for the mouse urogenital system (Little et al., 2007). Of the RV-enriched genes expressed in early nephrons at 15.5 dpc (Georgas et al., 2009) 21 genes were identified in the early nephrons of the 12.5–13.5 dpc kidney, and all of these were also expressed in 10.5 dpc mesonephric tubules (Table 1). Representative images are shown in Figure 2. These included genes previously shown to distinguish distal RV (Dkk1, Greb1, Lhx1, Papss2, Pcsk9, Pou3f3, Jag1, Wnt4), proximal RV markers (Tmem100, Wt1) and markers of the early connecting segment of the RV (Lhx1, Ltbp1, Pax8, Pou3f3). Where possible, the mesonephric tubules were subdivided into MTcr and MTca based on the presence (MTcr) or absence (MTca) of a connecting segment joining the MT to the ND. The majority of genes showed expression in both types, with the exception of Npy which appeared to be specific to MTca. This may simply reflect the earlier stage of development of MTca with respect to MTcr. Of note, Pou3f3 (Brn1), an early marker of the connecting segment in the metanephric kidney, was more strongly expressed in MTcr, again possibly reflecting a more advanced stage of development and / or a connection with the ND. While MTcr and MTca differ in the appearance of a direct connection with the ND, the question of whether these represent distinct tubular populations or a temporal developmental series (cranial more advanced than caudal) has not been determined. However, it has been stated that MTca regress leaving the cranial MTcr to differentiate into the efferent ducts of the epididymis, thereby providing a connection with the testis via the rete testis (Sainio et al., 1997). Dkk1, Lhx1 and Pdgfa were the only genes expressed in the ND/mesenchyme-derived connecting tubule component of the cranial MT connected to the ND and all three were also expressed in the ND itself. Several other genes also showed expression in the ND (Pax8, Pcsk9, Pou3f3, Ano1, Wnt4) or in the mesonephric mesenchyme (Greb1, Ltbp1, Wnt4, Wt1). At this early stage in metanephric development, only the MM is present, the ureteric bud has not invaded and there are no nephrons. Several genes were present in the MM at 10.5 dpc including Greb1, Papss2, Tmem100 and Wt1, however only Wt1 was maintained in the cap mesenchyme later in metanephric development (Georgas et al., 2009). In contrast Cdh4 and Pax8, expressed in the cap mesenchyme at 15.5 dpc (Georgas et al., 2009), were absent in the MM at 10.5 dpc.
TABLE 1.
Expression Profile of Early Metanephric Tubule Renal Vesicle Markers in the Mesonephrosa
| Gene Symbol (MGI) | WISH 10.5 dpc UG system | WISH 12.5–13.5 dpc kidney | SISH 15.5 dpc kidney | Female 12.5–13.5 dpc mesonephros | Male 12.5–13.5 dpc mesonephros | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MT Cranial | MT Caudal | Other structures | Early tubules | PA | RV | CSB | SSB | III | IV | MT | Other structures | MT | Other structures | |
| Ano1 | + | + | ND | + | ? | + | + | + | + RC | RC | − | M | − | M |
| Cbln4 | ? | + | G, UB | + | + | + | + | + | + | − | − | PMD | − | PMD |
| Cdh4 | + | + | − | + | + | + | + | + | + | + | − | PMD (12.5) | − | PMD |
| Cpb2 | + | + | − | + | − | + | + | + | + | + | + | − | + | − |
| Ctxn3 | + | + | − | + | * | * | * | * | * | * | − | M | + | − |
| Dkk1 | + & CT | + | ND, UB | + & RI | ? | + | + | + | + | − | + | − | + | PMD |
| Gja1 | + | + | − | + | + | + | + | + | − | − | − | PMD | − | ND, PMD |
| Greb1 | + | ? | Me, MM, UB | + | + | + | + | + | + | − | − | M, ND, PMD | ne | ne |
| Jag1 | ? | + | − | + & V | − | + | + | + | + | + RC* | − | PMD | − | PMD |
| Lhx1 | + & CT | + | ND, UB | + | + | + | + | + | + | + | + & CT | ND, PMD | + & CT | ND, PMD |
| Ltbp1 | ? | + | Me | + | − | + | + | + | + RC | RC | − | M, PMD | − | M, PMD |
| Npy | − | + | − | + | − | + | + | − | − | − | − | PMD | − | PMD |
| Papss2 | + | + | MM | + | − | + | + | + | + | + | − | PMD | − | PMD |
| Pax8 | + | + | ND, UB | + | + | + | + | + | + RC | + | ne | ne | ne | ne |
| Pcsk9 | ? | + | ND | + | − | + | + | + | + | + | − | − | − | − |
| Pdgfa | + & CT | ? | G, ND | + | ne | ne | ne | ne | ne | ne | + | PMD | − | PMD |
| Pou3f3 | + | ? | ND, UB | + & UT | − | + | + | + | + | + | ne | ne | ne | ne |
| Svopl | + | + | − | + | − | + | + | + | + | + | − | PMD | − | PMD |
| Tmem100 | + | + | UB, MM | + & V | + | + | + | + | − | − | − | − | − | − |
| Wnt4 | + | + | G, ND, Me | + | + | + | + | + | − | − | − | M | − | M |
| Wt1 | ? | ? | Me, MM, G | + | + | + | + | + | RC | RC | ne | ne | ne | Ne |
Marker genes of the metanephric renal vesicle were examined in the UG system at 10.5 dpc and in the male and female mesonephros and metanephros at 12.5–13.5 dpc by WISH. This table summarizes their whole-mount expression patterns and the previously published SISH expression patterns in the 15.5 dpc kidney (Georgas et al., 2009). MT at 10.5 dpc were divided into cranial and caudal. In whole-mount kidneys at 12.5–13.5 dpc expression in the early tubules may include renal vesicle (RV), comma-shaped body (CSB), and/or S-shaped body (SSB). Published SISH data include metanephric kidney tubule expression across all stages of nephrogenesis including pretubular aggregate (PA), RV, CSB, SSB, stage III capillary loop nephrons, and stage IV maturing nephrons. MT, mesonephric tubule; CT, connecting tubule of mesonephric tubules; G, gonad; ND, nephric duct (mesonephric portion); Me, mesonephric mesenchyme; MM, metanephric mesenchyme; UB, ureteric bud location (ND metanephric portion); RI, renal interstitium; V, vasculature; UT, ureteric tip; RC, renal corpuscle; PMD, paramesonephric duct; ?, uncertain expression; ne, not examined; U, ubiquitous; UG, urogenital; dpc, days post coitum.
Spotted expression in metanephros for Ctxn3.
RC indicates expression of Jag1 in the arterioles.
Figure 2. Gene expression patterns of early nephron renal vesicle markers in the kidney and early mesonephros.

Examples of genes identified as markers of the renal vesicle in the 15.5 dpc kidney and co-expressed in the early mesonephric tubules (MT) at 10.5 dpc. Wholemount in situ hybridization (WISH) of 10.5 dpc embryos sagitally bisected down the midline to reveal the urogenital system and kidneys at 13.5 dpc are shown. The rostral mesonephros is shown with expression in MT and in the early tubules of kidneys indicated (arrowheads). Additional sites of expression in the kidney were seen for Dkk1 in a subset of the renal interstitium (arrow) and Jag1 in the renal arterial system (arrow). Lhx1 and Dkk1 also showed expression in the nephric duct (dotted arrows) and the tubular component of the cranial mesonephric tubules which connect these tubules to the duct (white arrowheads). Additional images including the whole urogenital system are available on the GUDMAP website (www.gudmap.org, see Supplementary Table 4).
RV-enriched genes were also examined at 12.5–13.5 dpc to see if their expression was maintained in more developed mesonephric tubules (Table 1). Only 5/18 genes examined at these stages (Cpb2, Ctxn3, Dkk1, Lhx1, Pdgfa) were expressed in the more mature mesonephric tubules (Figure 3). While the majority of RV genes showed continued expression in more developed nephron tubules of the kidney, most of these genes did not persist in the mesonephric tubules. As well as expression in the mesonephric tubules, these RV-enriched genes were also expressed in PMD (12 genes), ND (3 genes) or mesonephric mesenchyme (5 genes) (Table 1).
Figure 3. Gene expression patterns of renal vesicle markers co-expressed in mesonephric tubules at 12.5–13.5 dpc.
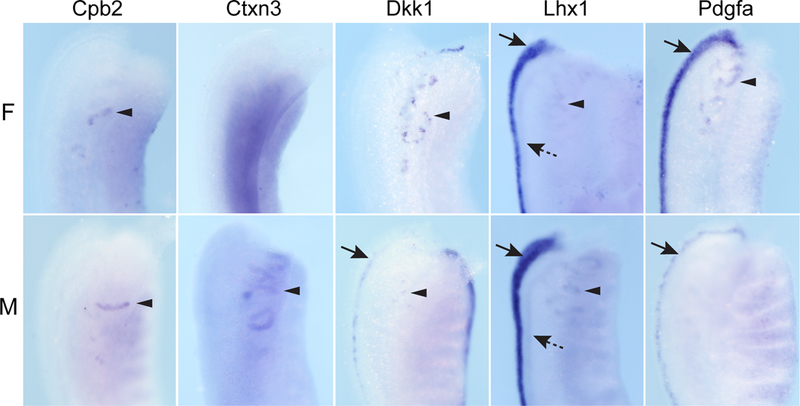
Examples of genes identified as markers of the renal vesicle in the 15.5 dpc kidney and co-expressed in the mesonephric tubules (MT) at 12.5–13.5 dpc. Wholemount in situ hybridization (WISH) of female (F) and male (M) mesonephros-gonads are shown from the dorsal side and focused on the MT (arrowheads) in the rostral half of the mesonephros. Expression in the nephric duct (ND, dotted arrows) or paramesonephric duct (PND, arrows) is also indicated. Lhx1 also showed expression in the tubular component of the cranial mesonephric tubules which connect these tubules to the duct. Additional images and annotated expression patterns of these genes in the whole urogenital system, including the ovary and testis, are available on the GUDMAP website (www.gudmap.org, see Supplementary Table 4).
Early proximal tubule metanephric genes are expressed in more mature mesonephric tubules
To investigate congruence in proximal tubular patterning in mesonephric versus metanephric tubules, we then examined the expression pattern of a set of 13 genes previously identified as specific to the early proximal tubule (EPT) segment of the developing kidney (Thiagarajan et al., 2011). In addition, expression of Scnn1b, a marker of early distal tubule, and one marker of renal corpuscle (Vip) were also examined. While most genes examined (11/13) were also expressed in the 13.5 dpc mesonephric tubules, only Acaa1b was expressed in the early mesonephric tubules (Table 2, Figure 4). Our previous analysis suggested that none of these genes were expressed before stage III nephron (Thiagarjan et al., 2011). As anticipated, no expression was seen in the 13.5 dpc metanephros. By 13.5 dpc, gene expression was detected within the mesonephric tubules for markers from all three proximal tubular segments (S1–S3). Of note, expression of two proximal tubule genes (Slc6a20b, Slc18a1) was restricted to the male mesonephros at 13.5 dpc (Table 2, Figure 4).
TABLE 2.
Expression Profile of Proximal and Distal Tubule and Renal Corpuscle Kidney Markers in the Mesonephrosa
| Gene symbol (MGI) | WISH 10.5 dpc urogenital tract | WISH 13.5 dpc female mesonephros | WISH 13.5 dpc male mesonephros | WISH 13.5 dpc Kidney | SISH 15.5 dpc kidney | SISH adult kidney | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| MT | Other structures | MT | Other structures | MT | Other structures | Nephron tubules | III | IV | Mature IV | |
| Aadac | − | − | + | − | + | − | − | + | S2–3 EPT, LOH | − |
| Acaa1b | + & CT | MesoM, ND, MM | + | M? | + | M? | ? | + | EPT, LOH, EDT | S2 PT |
| Entpd5 | P | (ND?) | +* | − | + | ND | − | − | S1–2 EPT | ? |
| Fbp1 | − | − | + | − | + | − | − | − | S1–2 EPT | S1–3 PT |
| Gpd1 | ? | MesoM, ND, MM | + | − | + | PMD | − | + RC | EPT, LOH, EDT, RC | S2 PT |
| Myo15b | − | − | + | − | + | − | − | + | EPT, LOH, EDT | − |
| Slc18a1 | ne | ne | − | − | + | − | − | − | S1–2 EPT | S1–2 PT |
| Slc27a2 | − | − | + | PMD | + | PMD (ND?) | − | − | S1–3 EPT | S2–3 PT |
| Slc3a1 | − | − | ? | PMD | ? | ? | ? | − | S2–3 EPT | S2–3 PT |
| Slc6a20b | ? | MesoM (MM?) | − | − | + | ND | − | − | S2 EPT | S2–3 PT |
| Tcn2 | − | MesoM, MM (ND?) | + | M | + | M | ? | − | S1–3 EPT | S1–3 PT |
| Ttr | − | ? | − | M ? | − | M ? | − | − | S2–3 EPT, LOH | S3 PT |
| Unc5cl | − | ? | + | M ? | + | M ? | − | + | S1–3 EPT, LOH | − |
| Scnn1b | + & CT | ND | + | ND ? | + | − | − | − | EDT (CCD, MCD) | ne |
| Vip | ne | ne | − | − | + | − | − | − | arterioles stage IV RC | − |
Marker genes of the metanephric early proximal and distal tubule and renal corpuscle were examined in the urogenital system at 10.5 dpc and in the male and female mesonephros and metanephros at 13.5dpc by WISH. This table summarizes their whole-mount expression patterns and the previously published SISH expression patterns in the 15.5 dpc and adult kidney Thiagarjan et al., 2011). In whole-mount kidneys at 13.5 dpc, expression in the early tubules may include renal vesicle (RV), comma-shaped body (CSB) and/or S-shaped body (SSB). Published SISH data include metanephric kidney expression in tubules and RC of stage III capillary loop nephrons, segments 1–3 (S1–3) of EPT and other tubule segments of stage IV maturing and stage IV mature nephrons.
Entpd5 female shows only very weak expression in the MT. EPT, early proximal tubule; LOH, immature loop of Henle; EDT, early distal tubule; MT, mesonephric tubule; ND, nephric duct (mesonephric portion); Me, mesonephric mesenchyme; MM, metanephric mesenchyme; RC, renal corpuscle; PMD, paramesonephric duct; ?, uncertain expression; dpc, days post coitum.
Figure 4. Gene expression patterns of proximal and distal tubule kidney markers in the mesonephros.
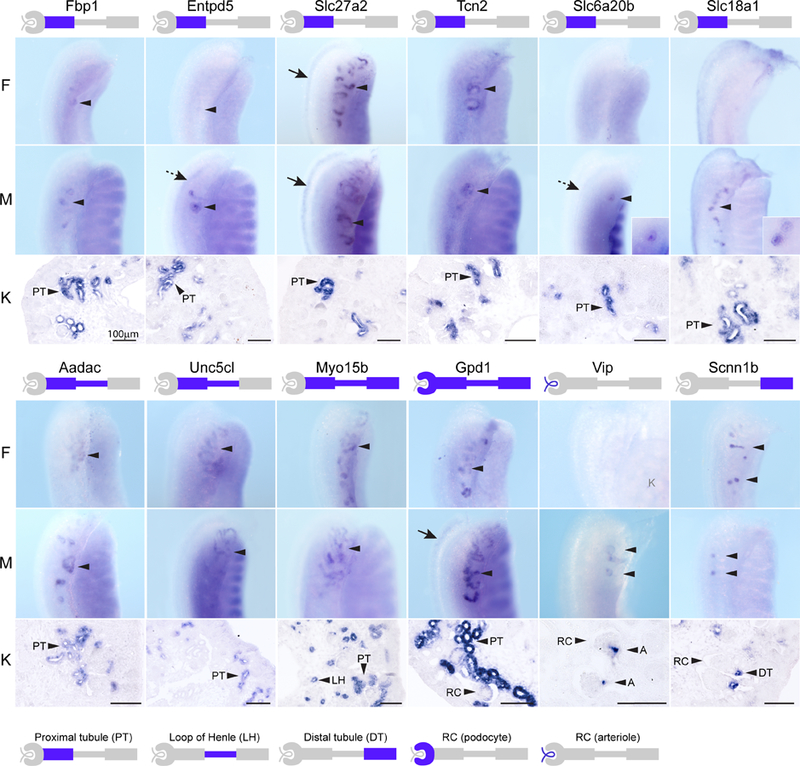
Examples of genes identified as markers of the early proximal tubule, early distal tubule or renal corpuscle of the kidney at 15.5 dpc and co-expressed in the mesonephric tubules (MT) at 12.5–13.5 dpc. Wholemount in situ hybridization (WISH) of female (F) and male (M) mesonephros-gonads are shown from the dorsal side and focused on the MT (arrowheads) in the rostral half of the mesonephros. Expression in the nephric duct (ND, dotted arrow) or paramesonephric duct (PND, arrows) is also indicated. Section ISH of 15.5 dpc kidneys are shown below and the expression patterns in the segments of the nephron are indicated in the schematic below each gene symbol. K, kidney; PT, early proximal tubule; LH, immature loop of Henle; DT, early distal tubule; RC, renal corpuscle; A, arteriole. All scale bars = 100μm. Additional images and annotated expression patterns of these genes in the whole urogenital system including the ovary and testis are available on the GUDMAP website (www.gudmap.org, see Supplementary Table 4).
Scnn1b has previously been described in the medullary collecting duct, however it is also expressed in the cortical collecting duct and early distal tubules of stage IV nephrons at 15.5 dpc (Thiagarajan et al., 2011). Due to its late onset in the distal tubule, it was not present in the kidney tubules at 13.5 dpc, however it was detected in the ureteric tree. Scnn1b was also expressed in both early and late mesonephric tubules, the connecting segment of MTcr and in the ND (Table 2, Figure 4). In the kidney, Vip is specifically expressed in the juxtaglomerular arterioles at 15.5 dpc (Thiagarajan et al., 2011). In the mesonephros, expression was restricted to the male mesonephric tubules at 13.5 dpc (Table 2, Figure 4).
Identification of additional markers of male efferent tubules
In order to identify further examples of genes synexpressed in the mesonephros and metanephros and identify potential mesonephric-specific genes, we mined the GUDMAP database using a series of Boolean anatomy searches. First we searched for genes common to the early and late mesonephric tubules as well as kidney. This identified a further 14 synexpressed genes (Cd24a, Clp1, Enpep, Epha4, Itga4, Lama1, Lamb1–1, Npnt, Osr2, Pcnt, Pepd, Rhoa, Tgfbr1 and Tmem147; Supplementary Table 1, Supplementary Figure 1). Five genes (Lama1, Lamb1–1, Rhoa, Tgfbr1, Clp1) were expressed in the connecting tubule segment of the mesonephric tubules from 10.5 dpc at both stages, whereas Pcnt expression in this structure was restricted to 10.5 dpc (Supplementary Figure 1). Again using Boolean anatomy queries we identified another set of 14 genes specifically expressed in more mature MT at 12.5–13.5 dpc and absent from kidney tubules at the same stage (Table 3). Interestingly, these mesonephric-specific candidates included 10 genes displaying male-specific gene expression within the mesonephros (Figure 5). Such genes may be involved in the early development of the male epididymis and / or efferent tubules.
TABLE 3.
Expression Profile of Male-Specific Mesonephric Tubule Markers a
| Gene symbol (MGI) | WISH 12.5–13.5 dpc kidney | Female 12.5–13.5 dpc mesonephros | Male 12.5–13.5 dpc mesonephros | ||
|---|---|---|---|---|---|
| Early tubule | MT | Other structures | MT | Other structures | |
| Male-specific MT expression | |||||
| Adam12 | − | − | − | + | (ND?) |
| Ascl1 | − | − | − | + | − |
| B230310I20 | − | − | − | + | − |
| Casq2 | − | − | − | + | − |
| D630049N15 | − | − | − | + | − |
| Gap43 | − | − | − | + | − |
| Mtap1b | − | − | − | + | PMD |
| Slc9a2 | − | − | − | + | ND |
| Spdya | − | − | − | + | − |
| Usp9x | − | ? | − | + | − |
| Male and female MT expression | |||||
| Smoc2 | RI | ? | ND, M | + | ND, M |
| Pmaip1 | − | + | (M?) | + | (ND?) |
| Expression in the MT and kidney | |||||
| Ide | ? (kidney) | − | − | + | ND |
| Cthrc1 | - (Utree) | + & CT | ND, PMD | + & CT | ND, PMD |
Genes identified from the GUDMAP database as expressed in the mesonephric tubules and absent from the kidney tubules. This table summarizes the expression patterns available on the GUDMAP Web site (www.gudmap.org).
Regional expression in the metanephros likely to include early tubule. MT, mesonephric tubule; CT, connecting tubule; M, mesonephric mesenchyme; ND, nephric duct (mesonephric portion); MM, metanephric mesenchyme; Utree, ureteric tree; RI, renal interstitium; PMD, paramesonephric duct; ?, uncertain expression; ne, not examined; dpc, days post coitum.
Figure 5. Expression patterns of male-specific mesonephric tubule genes.
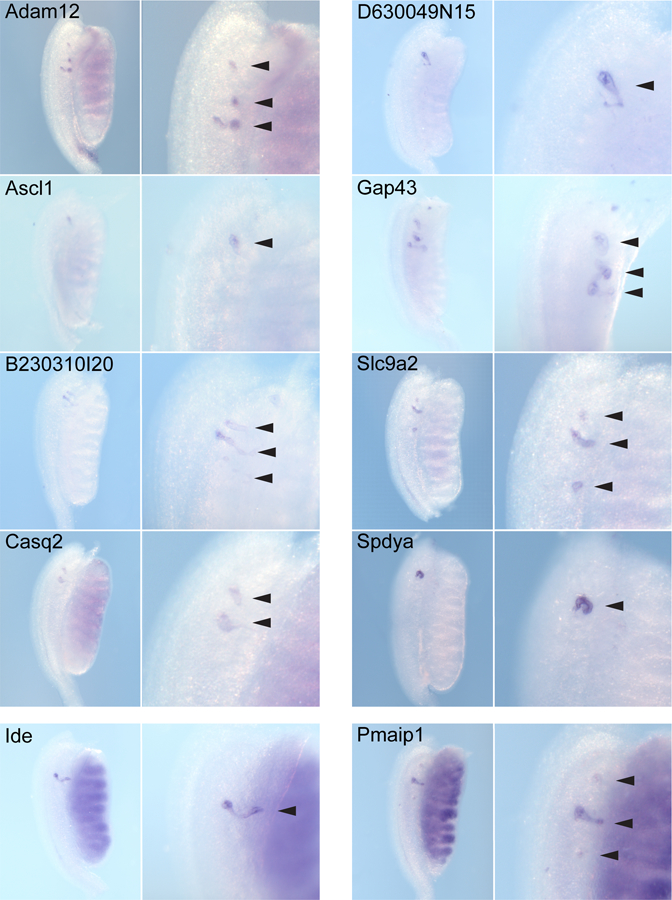
Examples of genes identified in a search of the GUDMAP database for mesonephric-specific tubular expression. Genes exclusively expressed in the mesonephric tubules (MT) of the male at 13.5 dpc were identified (Adam12, Ascl1, B230310I20, Casq2, D630049N15, Gap43, Slc9a2, Spdya) as well as a few genes expressed in male and female MT (Pmaip1) or in the male MT and in the kidney (Ide). Wholemount in situ hybridization (WISH) images of male mesonephros-gonads are shown from the dorsal side with the rostral mesonephros enlarged to show expression in the MT (arrowheads). Additional images for the male and all female images are available on the GUDMAP website together with the text-annotated expression patterns of these genes in the whole urogenital tract including expression in the ovary and testis (www.gudmap.org, see Supplementary Table 4).
Identification of candidate mesonephric- and metanephric-specific tubular genes
A search of the database analysing genes examined in the urogenital tract at 10.5 dpc was performed to identify genes congruent or discordant in expression between the early mesonephros and metanephros. A further 9 genes expressed in the mesonephric tubules of the 10.5 dpc mesonephros were identified (Adamts5, Ccne2, Ccdc91, Col9a1, Crym, Mfap3, Mtap, Rps6ka6, Vcan, Supplementary Table 2, Supplementary Figure 2). However, these genes were also expressed in early metanephric structures, including the UB and MM at 10.5dpc and later at 12.5–13.5 dpc in non-nephron structures within the metanephros, including the interstitium, cap mesenchyme and ureteric tree (Supplementary Table 2).
Conversely, 4 candidate metanephric-specific genes (Cdh11, Gcnt1, Hapln1, Hoxa10) were identified, whose expression was absent from both early and late mesonephric tubules (Supplementary Table 3, Supplementary Figure 3). Of particular note, Hoxa10 was strongly MM-specifc at 10.5dpc and showed expression in early tubules as well as in CM and renal intersititum at 13.5dpc and was validated at 15.5dpc. Cdh11 was also strongly expressed in the MM but was also detected in the mesonephric mesenchyme. This gene has previously been identified as specific to the developing metanephros at 10.5 dpc (Challen et al., 2004). In contrast, Gcnt1 and Hapln1 were restricted to the more mature tubules of metanephric nephrons.
Discussion
Although both excretory organs are composed of epithelial tubules, the molecular distinctions between the development of the mesonephros and metanephros are poorly understood. The work presented in this study represents the most thorough comparison of tubule formation in both organs performed to date. We have examined gene expression in the mesonephric tubules just after they first appear at 10.5 dpc and later at 12.5–13.5 dpc in both males and females. Markers of early nephrogenesis expressed in renal vesicles of the metanephros were also expressed in early mesonephric tubules at 10.5 dpc. In concordance with Mugford et al. (2008), we found that specific markers of the more differentiated metanephric nephron segment, the early proximal tubule were also found in 12.5–13.5 dpc mesonephric tubules. This included markers of the proximal convoluted tubules (S1 and S2) and proximal straight tubules (S3). Metanephric markers of the immature loop of Henle, early distal tubule and juxtaglomerular arteriole were also expressed in mature mesonephric tubules.
Further evidence supporting a shared early morphogenetic pathway was the discovery of very few genes restricted to the tubules of either the mesonephros or metanephros. Of the candidate organ specific genes identified in this study, some were expressed in non-tubular structures within both organs, suggesting congruence in developmental processes outside of tubular patterning. A small number of genes were identified that showed metanephric tubule specificity, including Cdh11 and Hoxa10. Cdh11 has been identified as such before in an expression profiling comparison between 10.5 dpc metanephric mesenchyme and adjacent intermediate mesodermal structures (mesonephros and early gonad of the same timepoint) (Challen et al., 2004). Similarly, prior studies have noted the metanephric-specific expression of the Hox11 transcription factors, Hoxa11, Hoxc11 and Hoxd11 (Wellik et al., 2002; Mugford et al., 2008). The overlapping expression patterns of 28 Hox genes, including Hoxa10 and the Hox11 paralogs, have previously been described in the mouse metanephros (Patterson and Potter, 2004) suggesting potentially redundant roles for these genes in nephrogenesis and patterning in the metanephric kidney.
During embryogenesis the mesonephros regresses, however in males the cranial mesonephric tubules persist and differentiate into the efferent ducts of the male epididymis, connecting it to testis via the rete testis. In the adult male, the major role of the mature efferent ducts is the concentration of sperm via the reabsorption of testicular fluid. This process, essential for the maturation of sperm, involves ion transporters and aquaporin water channel proteins, the expression of which is controlled by oestrogen and androgen (Hess, 2002; Hess et al., 2002; Oliveira et al., 2005). When genes involved in the reabsorption process or its control are mutated, such as estrogen receptor 1 α (Esr1) or Na+/H+ exchanger NHE3 (Slc9a3), tissue swelling occurs as a result of a defect in fluid reabsoprtion at the efferent ducts, leading to liquid and sperm accumulation and male sterility (Zhou et al., 2001; Schultheis et al., 1998a; Hess et al., 1997). A similar outcome results from the disruption of Lgr4 in male knockout mice. These mice display a postnatal developmental defect of the epididymis and efferent ducts, which are less convoluted and severely hypoplastic (Mendive et al., 2006). Disruption of estrogen receptor 1 α function leads to the altered expression of ion transporters and aquaporin 1 (Aqp1) in the efferent ducts (Lee et al., 2000; Zhou et al., 2001; Oliveira et al., 2002). Of note, Slc9a3 and Aqp1 are also expressed by the renal proximal convoluted tubule and function in fluid homeostasis in the kidney, where reaborption defects also occur when these genes are mutated (Schultheis et al., 1998b; Ma et al., 1998). Lgr4 is also expressed in the kidney and null mutations result in renal hypoplasia with a reduced number of functional nephrons and polycystic kidneys, which occur as a result of a defect in renal development (Kato et al., 2006). In this study, we have shown the continued expression of a number of nephron segment-specific genes in the mesonephric tubules forming the male efferent ducts. It is possible that many of the proximal tubule genes expressed in the efferent ducts also play functional roles in the reabsorption of fluid. We observed the male specific expression of Slc6a20b, Slc18a1 and vasoactive intestinal peptide (Vip). The neurotransmitter transporter Slc6a20b, when expressed in the proximal tubule, acts as an amino acid transporter critical for the reclamation of proline and hydroxyproline (Broer et al., 2009). Slc6a20b, along with other transporters and ion channels, has been shown to be downregulated in humans with non-obstructive azoospermia (Dubé et al., 2008). Vip is known to function as an erectile neurotransmitter, with receptors for this peptide in the corpus cavernosum of the penis (Zhang et al., 2010). While previously isolated from epididymis (Osterhoff et al., 1997), the onset of expression of this gene in the efferent tubules has not previously been reported. Of note, Slc18a1 plays a role in vesicular monoamine transport. Its role in kidney or efferent duct is unclear, but it is known to play a role in presynaptic neurotransmitter packaging in the nervous system (Eiden et al., 2004; Erickson et al., 1996).
In addition to the continued expression of nephron segment marker genes in the mesonephric tubules, we have also identified a set of genes specific to the 12.5–13.5 dpc male mesonephric tubules and possibly involved in the formation and/or function of the efferent ducts. Male-specific genes Spdya and Usp9x are both involved in the cell cycle and may play a role in the development and differentiation of the efferent ducts in the male. Of particular interest was the expression of a cluster of neural genes, including Ascl1, Gap43 and Mtap1b, in the region of the male mesonephric tubules/efferent ducts. Nothing is known about the innervation of the epididymis / efferent ducts, but all three of these genes showed concurrent expression in the developing ganglionic complex surrounding the primitive bladder and in developing ganglia associated with medial sympathetic innervation along the dorsal aorta (data not shown, see supplementary Table 4). This evidence supports the likelihood that these genes mark the initiation of innervation of the testis / epididymis/ efferent ducts. Showing a similar expression pattern along the dorsal aorta were the male-specific mesonephric tubule genes Slc9a2, D630049N15 and Casq2 (see supplementary Table 4). However these genes have not previously been associated with the nervous system. Slc9a2 is a Na+/H+ exchanger (NHE2), an isoform of Slc9a3 (NHE3), and also known to be expressed in proximal convoluted tubules, as well as in thick ascending loop of Henle (distal straight tubules) of adult mice (Choi et al., 2000; Sun et al., 1997) and in the distal convoluted tubules of the adult human kidney (Ghishan et al., 1995). Although male mice with knockout mutations in Slc9a3 are infertile, homozygous mice with null mutations in Slc9a2 do not breed well, however the cause of their abnormal fertility has not been published (Schultheis et al., 1998a; Schultheis et al., 1998b; Choi et al., 2000). Slc9a3 knockout mice also show defects in fluid reabsorption in the kidney leading to reduced blood pressure, however Slc9a2−/− mice show no obvious defects in renal function (Schultheis et al., 1998a; Schultheis et al., 1998b; Choi et al., 2000). In addition to the Na+/H+ exchangers several other ion transporters are known to be present in the efferent ducts including ATPase Na+/K+ transporting α 1 polypeptide, Atp1a1 (Ilio and Hess, 1992), Chloride anion exchanger, Slc26a3 (Lee et al., 2001) and the chloride channel Cftr (Lee et al., 2001; Leung et al., 2001). In this study, in addition to identifying genes involved in transmembrane ion transport (Slc9a2, Slc6a20b, Slc18a1) male-specific mesonephric tubule genes showed common gene ontology functions in metal ion binding (Adam12, Casq2, Ide), and may also have functional roles in testicular fluid homeostasis in the male efferent ducts.
In summary, these data provide further information on genes potentially involved in both mesonephric tubule formation as well as male efferent duct development. They serve to support the conclusion that tubular morphogenesis in the mesonephros strongly parallels that defined in the metanephros.
Experimental Procedures
Tissue collection and processing
All animal work contributing to this manuscript was conducted according to all state, national and international guidelines. Animal ethics approval was provided by AEEC3 of The University of Queensland. Embryos at 10.5 dpc (TS17), 12.5 or 13.5 dpc (TS20 or 21) and 15.5 dpc (TS23) were collected from adult pregnant female outbred CD1 mice sacrificed by cervical dislocation. A normal variation of ±0.5 days was observed. The urogenital system was examined at 10.5 dpc by dissecting embryos transversely below the forelimbs and longitudinally down the midline to expose the urogenital system. In all images the caudal end is on the right and the hindlimb is visible. Whole urogenital tracts (including primitive bladder, mesonephros, gonad, ureter and kidney) were collected from both male and female embryos at 12.5 and/or 13.5 dpc and kidneys were collected at 15.5 dpc. All tissues were fixed in 4% paraformaldehyde (PFA) in PBS at 4°C overnight, dehydrated in methanol/PBTX for wholemount in situ hybridization (WISH) or ethanol/water for section in situ hybridization (SISH) and stored in alcohol (100% methanol at −20°C for WISH; 70% ethanol at 4°C for SISH). Paraffin-embedding and sectioning of kidneys was performed as described previously (Rumballe et al., 2008).
In situ hybridization
Detailed protocols for riboprobe generation, WISH and SISH are available on the GUDMAP website (http://www.gudmap.org/Research/Protocols/Little.html) and have been described previously (Georgas et al., 2008; Little et al., 2007; Rumballe et al., 2008). In brief, digoxigenin (DIG)-labelled antisense riboprobes were generated by PCR and purified using Roche Quick Mini Spin Columns. SISH was performed on 7µm paraffin sectioned and de-waxed 15.5 dpc kidneys using a Tecan Freedom Evo 150 robot and WISH performed using a BioLane HTI Robot. Hybridization of riboprobe (0.2μg/ml WISH; 0.3–0.5µg/ml SISH) was performed at 64ºC for 10 hours for WISH and 65°C overnight for SISH. Chromogenic substrates NBT and BCIP were used for WISH and BM Purple for SISH, with incubations of 2–120 hours at 25ºC. Once the signal had reached optimal intensity the tissues were washed (1% Triton in PBS for WISH; PBS for SISH) and fixed in 4% PFA for 10 mins at 25°C in order to preserve the in situ hybridization signal. SISH slides were mounted in aqueous mounting medium and WISH tissues stored in PBS at 4°C.
Photography of WISH and SISH and annotation of expression
Wholemount tissues were photographed using a Nikon SMZ1500 research stereomicroscope system with a Nikon DXM1200f, colour 12 megapixel digital camera and ACT-2U Image Application Software and Adobe Photoshop CS2. Sectioned kidneys were scanned automatically using the semi-automated .slide System from Olympus and Soft Imaging Systems (BX51 microscope, digital CCD camera, motorized scanning stage and workstation, automated slide loader and .slide software) and representative images captured using Olyvia software (Soft Imaging Systems, Olympus) and Adobe Photoshop CS2. Gene expression patterns were annotated following standard procedures developed for the GUDMAP database (http://www.gudmap.org) and using the published text-based anatomical ontology of the mouse urogenital system (Little et al., 2007). Annotated expression patterns, riboprobe details and WISH images are available on the GUDMAP website (www.gudmap.org) and the GUDMAP Accession IDs for each gene are listed in Supplementary Table 4.
Boolean anatomy searches of the GUDMAP database
Boolean queries were used to search for genes expressed in the metanephric and mesonephric tubules. We restricted searches to expression patterns of the same probe sequence (TS17, TS20–21 WISH entries and TS23 and adult SISH entries from the Little laboratory) in order to minimize any discrepancies which may have been seen due to sequence differences. Genes expressed in both tubule types were identified using the example Boolean query string for three anatomical terms below and replacing the metanephros term in each case; for example genes expressed in early nephron and mesonephric tubule were identified using the Boolean query string; GUDMAP: p{in “early nephron GROUP” TS20..TS21} OR p{in “mesonephric tubule of female” TS20..TS21} OR p{in “mesonephric tubule of male” TS20..TS21}. The following metanephros terms were also searched; “cortical renal tubule” TS23..TS28; “immature loop of Henle” TS23..TS23; “s-shaped body”, “renal vesicle” and “late tubule”. We then looked for genes with entries for both types of tubule when the data returned from the query was sorted by Gene. Genes expressed in only one tubule type utilized the same query strings as those above with not detected for one type of tubule, with the following example; genes not detected in early nephron and present in mesonephric tubules had the Boolean query string GUDMAP: nd{in “early nephron GROUP” TS20..TS21} OR p{in “mesonephric tubule of female” TS20..TS21} OR p{in “mesonephric tubule of male” TS20..TS21}. Not present in the metanephros, GUDMAP: nd{in “metanephros” TS17..TS28} OR p{in “mesonephric tubule of female” TS20..TS21} OR p{in “mesonephric tubule of male” TS20..TS21}. Common genes were also searched using the same metanephros terms above and replacing the mesonephric tubule TS20–21 terms with the early mesonephric tubule at 10.5 dpc, “mesonephric tubule (TS17–TS18)” for example; GUDMAP: p{in “cortical renal tubule” TS23..TS28} OR p{in “mesonephric tubule” TS17..TS17}.
Supplementary Material
Acknowledgements
We thank Dave Tang, Darrin Taylor and Sean Grimmond, Institute for Molecular Bioscience, The University of Queensland, for local database support. We thank Jane Armstrong, Jane Brennan, Sue Lloyd-MacGilp, Chris Armit and Jamie Davies, within the GUDMAP Editorial office, Edinburgh University, Scotland and Derek Houghton, Ying Cheng, Xingjun Pi, Mehran Sharghi, Simon Harding and Duncan Davidson within the GUDMAP Database Development office, MRC Human Genetics Unit, Western General Hospital, Scotland, for their support and assistance in annotating and uploading data. This work was supported by NIH NIDDK grant to ML (DK070136). MHL is a Principal Research Fellow of the National Health and Medical Research Council of Australia.
References
- Bouchard M, Souabni A, Mandler M, Neubüser A, Busslinger M. 2002. Nephric lineage specification by Pax2 and Pax8. Genes Dev 16(22):2958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer A, Balkrishna S, Kottra G, Davis S, Oakley A, Bröer S. 2009. Sodium translocation by the iminoglycinuria associated imino transporter (SLC6A20). Mol Membr Biol 26(5):333–46. Epub 2009 Jul 30. [DOI] [PubMed] [Google Scholar]
- Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS. 2008. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell 15(5):781–91. Erratum in: Dev Cell. 2009 Mar;16(3):482. Yu, Jing [added]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. 2005. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9(2):283–92. [DOI] [PubMed] [Google Scholar]
- Challen GA, Martinez G, Davis M, Teasdale R, Grimmond S, Little MH. 2004. Identifying the molecular phenotype of renal progenitor cells. J Am Soc Nephrol 15(9):2344–57 [DOI] [PubMed] [Google Scholar]
- Choi JY, Shah M, Lee MG, Schultheis PJ, Shull GE, Muallem S, Baum M. 2000. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest 105(8):1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé E, Hermo L, Chan PT, Cyr DG. 2008. Alterations in gene expression in the caput epididymides of nonobstructive azoospermic men. Biol Reprod 78(2):342–51. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Schäfer MK, Weihe E, Schütz B.2004. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch 447(5):636–40. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. 1996. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A 93(10):5166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgas K, Rumballe B, Wilkinson L, Chiu HS, Lesieur E, Gilbert T, Little MH. 2008. Use of dual section mRNA in situ hybridisation/immunohistochemistry to clarify gene expression patterns during the early stages of nephron development in the embryo and in the mature nephron of the adult mouse kidney. Histochem Cell Biol 130(5):927–42. [DOI] [PubMed] [Google Scholar]
- Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, Taylor D, Grimmond SM, Potter SS, McMahon AP, Little MH. 2009. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332(2):273–86. [DOI] [PubMed] [Google Scholar]
- Ghishan FK, Knobel SM, Summar M 1995. Molecular cloning, sequencing, chromosomal localization, and tissue distribution of the human Na+/H+ exchanger (SLC9A2). Genomics 30: 25–30. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Cebrián C, Ilagan R, Meyers E, Herzlinger D, Martin GR. 2005. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132(17):3847–57. [DOI] [PubMed] [Google Scholar]
- Grobstein C 1953. Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science 118(3054):52–5. [DOI] [PubMed] [Google Scholar]
- Grote D, Souabni A, Busslinger M, Bouchard M. 2006. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133(1):53–61. [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. 1997. A role for oestrogens in the male reproductive system. Nature (London) 390:509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA. 2002. The efferent ductules: structure and functions. In: Robaire B, Hinton B, editors. The Epididymis: From Molecules to Clinical Practice Kluwer Academic/Plenum; New York:49–80. [Google Scholar]
- Hess RA, Zhou Q, Nie R. The role of estrogens in the endocrine and paracrine regulation of the efferent ductules, epididymis and vas deferens. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice Kluwer Academic/Plenum; New York:317–338. [Google Scholar]
- Ilio KY, Hess RA. 1992. Localization and activity of Na+, K+-ATPase in the ductuli efferentes of the rat. Anat Rec 234:190–200. [DOI] [PubMed] [Google Scholar]
- James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. 2006. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133:2995–3004. [DOI] [PubMed] [Google Scholar]
- Kato S, Matsubara M, Matsuo T, Mohri Y, Kazama I, Hatano R, Umezawa A, Nishimori K. 2006. Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron Exp Nephrol 104(2):e63–75. [DOI] [PubMed] [Google Scholar]
- Klattig J, Englert C. 2007. The Müllerian duct: recent insights into its development and regression. Sex Dev 1(5):271–8. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Shawlot W, Kania A, Behringer RR. 2004. Requirement of Lim1 for female reproductive tract development. Development 131(3):539–49. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kawakami K, Asashima M, Nishinakamura R. 2007. Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mech. Dev 124:290–303. [DOI] [PubMed] [Google Scholar]
- Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. 2000. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod 63:1873–1880. [DOI] [PubMed] [Google Scholar]
- Lee KH, Finnigan-Bunick C, Bahr J, Bunick D. 2001. Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) alpha and ER beta. Biol Reprod 65:1534–1541. [DOI] [PubMed] [Google Scholar]
- Leung GPH, Tse CM, Cheng Chew SB, Wong PYD. 2001. Expression of multiple Na+/H+ exchanger isoforms in cultured epithelial cells from rat efferent duct and cauda epididymidis. Biol Reprod 64:482–490. [DOI] [PubMed] [Google Scholar]
- Little MH, Brennan J, Georgas K, Davies JA, Davidson DR, Baldock RA, Beverdam A, Bertram JF, Capel B, Chiu HS, Clements D, Cullen-McEwen L, Fleming J, Gilbert T, Herzlinger D, Houghton D, Kaufman MH, Kleymenova E, Koopman PA, Lewis AG, McMahon AP, Mendelsohn CL, Mitchell EK, Rumballe BA, Sweeney DE, Valerius MT, Yamada G, Yang Y, Yu J. 2007. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr Patterns 7(6):680–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. 1998. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem 273(8):4296–9. [DOI] [PubMed] [Google Scholar]
- Massé J, Watrin T, Laurent A, Deschamps S, Guerrier D, Pellerin I. 2009. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol 53(2–3):411–24. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, Zhang P. 2008. GUDMAP: the genitourinary developmental molecular anatomy project. J. Amer. Society Nephrol 19(4):667–671. [DOI] [PubMed] [Google Scholar]
- Mendive F, Laurent P, Van Schoore G, Skarnes W, Pochet R, Vassart G. 2006. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol 290(2):421–34. [DOI] [PubMed] [Google Scholar]
- Mugford JW, Sipilä P, Kobayashi A, Behringer RR, McMahon AP. 2008. Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev Biol 319(2):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. 2007. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J. Am. Soc. Nephrol 18:1121–1129. [DOI] [PubMed] [Google Scholar]
- Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, Katsuki M, Asashima M, Yokota T. 2001. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128:3105–3115. [DOI] [PubMed] [Google Scholar]
- Ott T, Parrish M, Bond K, Schwaeger-Nickolenko A, Monaghan AP. 2001. A new member of the spalt like zinc finger protein family, Msal-3, is expressed in the CNS and sites of epithelial/mesenchymal interaction. Mech. Dev 101:203–207. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Zhou Q, Carnes K, Nie R, Kuehl DE, Jackson GL, Franca LR, Nakai M, Hess RA. 2002. ER function in the adult male rat: short- and long-term effects of the antiestrogen ICI 182,780 on the testis and efferent ductules, without changes in testosterone. Endocrinology 143:2399–2409. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, França LR, Hermo L, Hess RA. 2005. Aquaporin-1 and −9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol. Cell 97(6):385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhoff C, Ivell R, Kirchhoff C. 1997. Cloning of a human epididymis-specific mRNA, HE6, encoding a novel member of the seven transmembrane-domain receptor superfamily. DNA Cell Biol 16(4):379–89. [DOI] [PubMed] [Google Scholar]
- Patterson LT and Potter SS. 2004. Atlas of Hox gene expression in the developing kidney. Dev Dyn 229:771–79. [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. 2005. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132(17):3859–71. [DOI] [PubMed] [Google Scholar]
- Rumballe B, Georgas K, Little MH. 2008. High-throughput paraffin section in situ hybridisation and dual immunohistochemistry. Cold Spring Harb Protoc 10.1101/pdb.prot5030. [DOI] [PMC free article] [PubMed]
- Sainio K, Hellstedt P, Kreidberg JA, Saxén L, Sariola H. 1997. Differential regulation of two sets of mesonephric tubules by WT-1. Development 124(7):1293–9. [DOI] [PubMed] [Google Scholar]
- Sainio K, Raatikainen-Ahokas A. 1999. Mesonephric kidney--a stem cell factory? Int J Dev Biol 43(5):435–9. [PubMed] [Google Scholar]
- Saxén L, Sariola H. 1987. Early organogenesis of the kidney. Pediatr Nephrol 1(3):385–92. [DOI] [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. 1998a. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19(3):282–5. [DOI] [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. 1998b. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest 101(6):1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawlot W, Behringer RR. 1995. Requirement for Lim1 in head-organizer function. Nature 374(6521):425–30. [DOI] [PubMed] [Google Scholar]
- Smith C, Mackay S. 1991. Morphological development and fate of the mouse mesonephros. J Anat 174:171–84. [PMC free article] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G and McMahon AP. 1994. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372:679–683. [DOI] [PubMed] [Google Scholar]
- Sun AM, Liu Y, Dworkin LD, Tse CM, Donowitz M, Yip KP. 1997. Na+/H+ exchanger isoform 2 (NHE2) is expressed in the apical membrane of the medullary thick ascending limb. J Membr Biol 160(1):85–90. [DOI] [PubMed] [Google Scholar]
- Thiagarajan R, Georgas K, Rumballe B, Lesieur E, Chiu H , Taylor D, Tang D, Little MH, Grimmond SM. Identification of anchor genes during kidney development defines ontological relationships, molecular subcompartments and regulatory pathways. PLoS ONE 6(2): e17286 10.1371/journal.pone.0017286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilmann C, Capel B. 2002. Cellular and molecular pathways regulating mammalian sex determination. Recent Prog Horm Res 57:1–18. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. 1995. Pax-2 controls multiple steps of urogenital development. Development 121:4057–4065. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. 1999. Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409. [DOI] [PubMed] [Google Scholar]
- Vize PD, Woolf AS, Bard JBL. 2002. The kidney: from normal development to congenital diseases Boston: Academic Press, Amsterdam. [Google Scholar]
- Wang Z, Orlowski J, and Shull GE. 1993. Primary structure and functional expression of a novel gastrointestinal isoform of the rat Na/H exchanger. J. Biol. Chem 268:11925–11928. [PubMed] [Google Scholar]
- Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. 2005. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev. Biol 288, 582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellik DM, Hawkes PJ, Capecchi MR. 2002. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev 16(11):1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D 2003. Six1 is required for the early organogenesis of mammalian kidney. Development 130:3085–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Ma L. 2005. Development of the mammalian female reproductive tract. J Biochem 137(6):677–83. [DOI] [PubMed] [Google Scholar]
- Zhang MG, Shen ZJ, Zhang CM, Wu W, Gao PJ, Chen SW, Zhou WL. 2010. Vasoactive intestinal polypeptide, an erectile neurotransmitter, improves erectile function more significantly in castrated rats than in normal rats. BJU Int 10.1111/j.1464-410X.2010.09901.x. [DOI] [PubMed]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. 2001. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci 98(24):14132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


