ABSTRACT
Spinal cord injury (SCI) usually results in neurological damage. DGCR5 is closely related to neurological disorders, and this study aims to explore its role in neuronal apoptosis in acute SCI. The ASCI model was established in rats, and the Basso, Beattie, and Bresnahan (BBB) scoring was used to assess the neurological function. The expression of RNA and protein was quantified by quantitative real-time PCR (qRT-PCR) and western blotting, respectively. The oxygenglucose deprivation (OGD) was performed upon neurons and apoptosis was evaluated by flow cytometry. The interaction and binding between DGCR5 and PRDM5 was detected with RNA pull-down and RIP assay, respectively. DGCR5 was down-regulated in ASCI model rat and in neurons treated with hypoxia. Over-expression of DGCR5 inhibited neuronal apoptosis. Interaction between DGCR5 negatively regulated PRDM5 protein expression by binding and interacting with it. DGCR5 inhibited neuronal apoptosis through PRDM5. Over-expressed DGCR5 ameliorated ASCI in rat. DGCR5 suppresses neuronal apoptosis through directly binding and negatively regulating PRDM5, and thereby ameliorating ASCI.
KEYWORDS: Acute spinal cord injury, DGCR5, neuronal apoptosis, PR domain protein 5
Introduction
Spinal cord injury (SCI) is a kind of fatal injury that leads to neurological damage, necrosis, and dysfunction in patients [1]. Due to the irreversible change of neuronal construct and function, the high rate of disability and poor functional recovery are common in SCI, permanently affecting a patient’s quality of life [2]. As a severe traumatic injury, SCI occurs commonly in modern society because of heavy crash from traffic accidents, sports accidents, and falling from high buildings [3]. According to the biological response, SCI is usually divided into three stages: the acute SCI (ASCI), secondary SCI, and chronic SCI [4]. Exploration on the pathogenesis and regulatory mechanism of ASCI may help to develop satisfactory therapy and offer novel target for treatment of ASCI and neurological recovery.
PR (PRDI-BF1 and RIZ) domain proteins (PRDM) are a subfamily of the zinc finger proteins that modulate cellular processes such as cell growth, differentiation, and apoptosis as transcriptional factors. PRDM5 is a poorly characterized member of PRDM family that functions in various biological processes, including chromatin modification [5], corneal development [6], and lung cancer progression [7]. Knock-down of PRDM5 with short interfering RNA significantly decreased the level of active caspase-3 and inhibited neuronal apoptosis, revealing the crucial role of PRDM5 in ASCI and the subsequent central nervous system pathophysiology [8]. It also has been reported that inhibition of PRDM5 by miR-182 and miR-7a suppressed the neurons apoptosis and attenuated ASCI in rats [9], verifying the essential impact of PRDM5 on ASCI progression and the potential target for clinical therapy.
The DiGeorge syndrome-associated noncoding RNA, DGCR5, is an essential lncRNA highly expressed in brain with two distinct splice isoforms: AB051434 (5427 nt, six exons) and X91348 (1284nt, six exons) [10]. DGCR5 has been considered as a significant neural lncRNA that closely related to neurological disorders, and it has been proved to be down-regulated in Huntington’s disease [11]. Up-regulation of repressor element 1-silencing transcription factor (REST) contributed to neuronal death induced by the neurotoxicant PCB-95 [12]. As a REST target, DGCR5 is inhibited by REST through a proximal upstream binding site [10], implying its potential neuroprotective effect. In our preliminary study, we confirmed that PRDM5 was down-regulated by lncRNA through miRNA to inhibited neuronal apoptosis and alleviate ASCI. The binding site between DGCR5 and REST declares that DGCR5 could bind with protein directly, thus we undertook the present study to investigate whether DGCR5 interacts with PRDM5 to affect neuronal apoptosis and ASCI development. With the study completed, we hope to search for more therapeutic targets for ASDCI prevention and treatment.
Materials and methods
Establishment of ASCI model in rat
Our study on animals was approved by the Animal Care Committee of the First Affiliated Hospital of Zhengzhou University. All protocols of animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals proposed by the Chinese National Institutes of Health. Sprague-Dawley (SD) rats obtained from the laboratory animal center of the First Affiliated Hospital of Zhengzhou University were housed in control temperature (23–25°C) with ad libitum food and water. Rats were randomly divided into sham-operation control group (sham, n = 7) and ASCI group (n = 7). ASCI model was established in accordance with previously described protocol [9]. Rats were anesthetized with 10% chloral hydrate (3 ml/kg) by intraperitoneal injection. Back of the rat was shaved under the sterile condition to expose the T9-11 spinous process and vertebral segments. Then spinal cord crush were performed on the T10 spinous process of rats for 20s. Paralysis of the lower limbs was observed in a successful ASCI model. Except for inflicted crush injury, rats in the sham group were subjected with an accordant treatment.
Basso, Beattie, and Bresnahan (BBB) scoring
Neurological function of rats was evaluated at the 7th day post-surgery by using the Basso, Beattie, and Bresnahan (BBB) scoring, based on motor ability following ASCI [13]. BBB scores reflect a 21-point open field locomotor scale, where 0 indicates no locomotion and 21 normal motor functions. During the evaluation period, the hind limb movements, stability, coordination, stepping, trunk position, toe clearance, paw placement, and tail position of rats were analyzed individually by two blind observers. The mean value of the two observers’ scores was adopted.
Real-time quantitative RT-PCR (qRT-PCR)
Rats were sacrificed by injection of high-dose (200mg/kg) pentobarbital (Sigma, USA), and the spinal cord tissues were harvested. Total RNA was extracted by using the Trizol reagent (Invitrogen, USA) from spinal cord tissues or nerve cells according to the manufacturer’s manual. 2μg of total RNA was used for cDNA synthesis with a SuperScript Reverse Transcription Kit (Invitrogen, USA). Total cDNA was used for quantitative RT-PCR with SYBR Green Master Mix (Applied Biosystems, USA) on an ABI PRISM 7300 RT-PCR System (Applied Biosystems, USA). Design and synthesis of oligonucleotide primers of specific genes were done by Sangon Biotech (Shanghai, China). 2−∆∆Ct method was applied to determine the relative expression of RNA.
Western blotting
Spinal cord tissues or nerve cells were treated with RIPA lysis buffer (Beyotime, China) for protein extraction, with the concentration of protein measured by using a BCA protein assay kit (Beyotime, China). After separated with 12% SDS-PAGE, total protein was transferred onto the polyvinylidene difluoride (PVDF) membrane (Bio-Rad, USA). Then the membrane was blocked with Tris-buffered saline Tween-20 (TBST) containing 5% nonfat milk for 1 hour at RT, and subsequently incubated with the primary antibodies (Abcam, UK) of PRDM5, c-caspase-3, and β-actin (served as control) for 1 hour at RT. The membrane was incubated with HRP-bounded secondary antibodies for 1 hour at RT and the proteins were visualized with an ECL Plus Western Blotting Substrate (Thermo Fisher, USA).
Cell culture and hypoxia treatment
AGE1.HN and PC12 cells were maintained in DMEM medium (Gibco, USA) with 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin and 100 mg/mL streptomycin (Heclony, USA), under 37°C and 5% humidified CO2.
Hypoxia treatment was performed in AGE1.HN and PC12 cell lines for simulating SCI in vitro, as previously reported [14,15]. Oxygenglucose deprivation (OGD) was performed upon the above cells for hypoxia treatment in accordance with a previous study [16]. If brief, the medium DMEM without glucose (Gibco) was placed in a controlled airtight hypoxic chamber (Plas Labs, USA) for 2 h under an environment of 90% N2/5%CO2/5% H2 at 37°C. The oxygen concentration was monitored with an Anaerobic Indicator (Oxoid Ltd., UK). After cells washed with PBS, experimental hypoxic medium was added to the cell culture wells. OGD was induced by placing the plates in the hypoxic chamber, and cells were returned to a normal incubator for reperfusion and OGD media were replaced with normal DMEM medium after OGD completed.
Subcellular fractionation location
To validate that lncRNA DGCR5 is located in the nucleus of the AGE1.HN and PC12 cells, PARIS Kit (Life Technologies) was used to separate of nuclear and cytosolic fractions of cells according to the manufacturer’s instructions [17]. QRT-PCR was used to detect GAPDH, DGCR5, and U1 RNA levels in cytoplasm and nuclear fraction. GAPDH was used as cytoplasm control, and U1 was used as nuclear control. The relative rate of DGCR5, GAPDH and U1 in cytoplasm or nuclear part was presented as the percentage of the total RNA.
Flow cytometry (FCM)
Flow cytometer FACSCalibur (BD Biosciences) was used to determine neuronal apoptosis by using an Annexin V-FITC/propidium iodide (PI) cell apoptosis detection kit (Sigma, USA). AGE1.HN and PC12 cells (1x106) were treated by trypsin and washed twice with phosphate-buffered saline (PBS). Annexin V-FITC and PI were added and the cells were incubated for 30 min at 37℃. The stained nerve cells were analyzed by flow cytometry and examined by the Flow Jo V10 software (Tree Star Inc.).
RNA pull-down assay
RNA pull-down assay was conducted to determine the interaction between DGCR5 and PRDM5 protein. Briefly, the DNA probe complementary to DGCR5 was synthesized and biotinylated by GenePharma Co., Ltd (Shanghai, China). Performed according to the manufactures’ protocol, a Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher) was used to complete the RNA pull-down assay. The RNA-binding protein complexes were washed and eluted for western blot or qRT-PCR analysis.
RNA immunoprecipitation (RIP)
RIP was performed with the RNA-Binding Protein Immunoprecipitation Kit (Millipore) to verify the binding between DGCR5 and PRDM5 protein. The PC12 cells were lysed with lysis buffer and the cell lysis solutions were incubated with AGO2 antibody or normal mouse IgG. RNA-protein complexes were immunoprecipitated with protein A agarose beads and RNA was extracted by using Trizol (Invitrogen). The IP-western was used to detect AGO2 and PRDM5 protein level and qRT-PCR was performed to quantify the DGCR5.
Cell transfection
The pcDNA-DGCR5 and its control plasmid pcDNA were constructed and identified by Sangon Biotech (Shanghai, China) for cell transfection. The AGE1.HN and PC12 nerve cells were seeded in a 6-well plate and transfected with the above plasmids by Lipofectamine2000 (Invitrogen) according to the specification. The expression of DGCR5 after cell transfection was examined by qRT-PCR.
Over-expression of DGCR5 in ASCI model rat
To validate the role of DGCR5 in ASCI progression in vivo, ASCI rat model was established. 5μL pcDNA (n = 7) or pcDNA-DGCR5 (n = 7) was introduced into the ASCI model rats by intrathecal injection [18]. Neurological function of rats was evaluated at the 7th day post-surgery by using the BBB scoring. At the 14th day, rats were sacrificed and the spinal cord tissues were harvested for genes expression determination.
Statistical analysis
Data were expressed as mean ± standard deviation (SD), and statistically analyzed by SPSS 21.0 software (SPSS Inc.) Comparisons between two groups were completed by Student’s test. A value of P < 0.05 was considered statistically significant.
Results
Down-regulation of DGCR5 in ASCI model rat
To evaluate the expression level of DGCR5 and PRDM5 in ASCI, the ASCI rat model was constructed and the neurological function was assessed with BBB scoring. It showed that the BBB score was significantly decreased in ASCI rat, compared with the rat in sham group (Figure 1(a)). With the spinal tissue taken, the expression of DGCR5, PRDM5, and c-caspase3 was detected. Compared with the rat in sham group, the expression of DGCR5 in ASCI rat was markedly decreased (Figure 1(b)); while the level of PRDM5 and c-caspase3 was clearly increased (Figure 1(c)). The results indicated that the expression of DGCR5 and PRDM5 in ASCI was reduced and elevated, respectively; and the neuronal apoptosis was induced.
Figure 1.
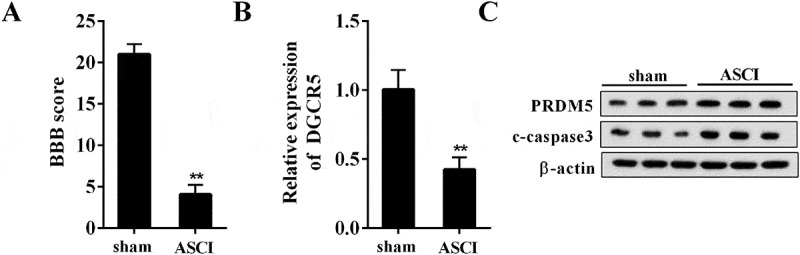
Down-regulation of DGCR5 in ASCI model rat. (a) Basso, Beattie, and Bresnahan (BBB) scoring was used to assess the neurological function of the ASCI model rats. (b) The expression of DGCR5 in spinal tissue was quantified by qRT-PCR. (c) The protein level of PRDM5 and c-caspase3 in spinal tissue was analyzed with western blot.
DGCR5 was down-regulated in neurons treated with hypoxia
To imitate the ASCI condition in vtro, neuron cells AGE1.HN and PC12 were treated with hypoxia. As shown in Figure 2(a), DGCR5 was down-regulated in AGE1.HN and PC12 cells treated with hypoxia; while the expression of PRDM5 and c-caspase3 was clearly promoted (Figure 2(b)). Simultaneously, the elevated protein expression of hypoxia-inducible factor 1α (HIF-1α) verified the success of cellular model after hypoxia treatment (Figure 2(c)). Relative DGCR5 levels in cell cytoplasm or nucleus of AGE1.HN and PC12 cells were detected, which demonstrated that DGCR5 was located in the nucleus of the above cell lines (Supplemental Figure 1). The results suggested hypoxia treatment suppressed the expression of DGCR5, but promoted PRDM5 expression and neuronal apoptosis.
Figure 2.
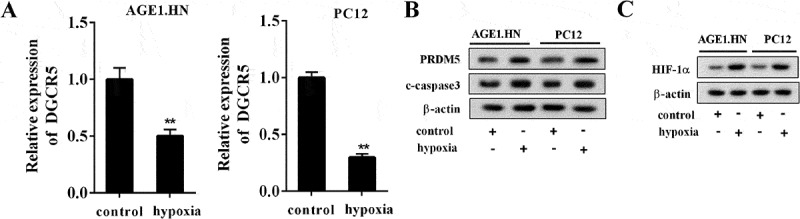
DGCR5 was down-regulated in neurons treated with hypoxia. (a) The expression of DGCR5 in AGE1.HN and PC12 neurons was measured by qRT-PCR. (b) The protein level of PRDM5 and c-caspase3 in AGE1.HN and PC12 neurons was detected with western blot.
Over-expression of DGCR5 inhibited neuronal apoptosis
To investigate the effect of DGCR5 expression on neuronal apoptosis, AGE1.HN and PC12 cells were divided into 4 groups, respectively: control, hypoxia, pcDNA, and pcDNA-DGCR5. After cell transfection, the AGE1.HN and PC12 cells were treated with hypoxia, and the apoptosis was measured. It revealed that hypoxia treatment led to extensive neuronal apoptosis, which was reversed by DGCR5 over-expression with pcDNA-DGCR5 transfection (Figure 3(a)). At the same time, DGCR5 over-expression repressed the c-caspase3 level (Figure 3(b)).
Figure 3.
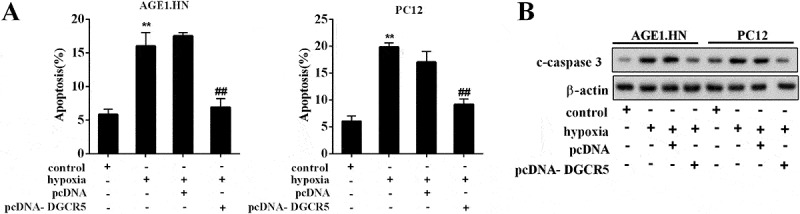
Over-expression of DGCR5 inhibited neuronal apoptosis. (a) Apoptosis of AGE1.HN and PC12 cells was evaluated with flow cytometry. (b) The protein level of c-caspase3 in AGE1.HN and PC12 neurons was determined by western blot.
Interaction between DGCR5 and PRDM5
In the RNA pull-down assay, AGO2 content in the pull-down complex of DGCR5 was analyzed by western blot. Compared with NC (negative control), PRDM5 was abundantly detected in the pull-down complex of DGCR5 (Figure 4(a)), affirming interaction between them. RIP assay was used to verify the binding between DGCR5 and PRDM5, with the AGO2 examined with IP-western. Compared with IgG, abundantly expressed DGCR5 and PRDM5 were observed in the AGO2 antibody (Figure 4(b)), confirming the binding between DGCR5 and PRDM5. The above findings demonstrated the binding and interaction between DGCR5 and PRDM5.
Figure 4.
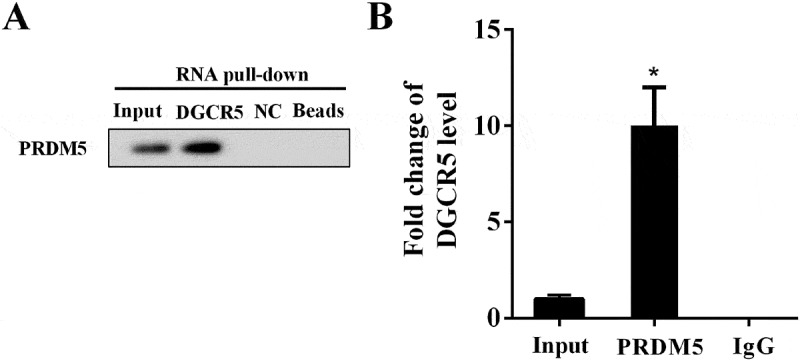
Interaction between DGCR5 and PRDM5. (a) The interaction between DGCR5 and PRDM5 was evaluated with RNA pull-down assay. (b) The binding condition between DGCR5 and PRDM5 was detected by RIP assay.
PRDM5 protein expression was negatively regulated by DGCR5
To explore the regulatory role of DGCR5 on PRDM5, AGE1.HN and PC12 cells were transfected with pcDNA or pcDNA-DGCR5. As shown in Figure 5(a), the level of PRDM5 protein was reduced by DGCR5 over-expression; but the expression of PRDM5 mRNA was not affected (Figure 5(b)). Cycloheximide (CHX) was generally used to restrain protein synthesis. With CHX (12.5μg/mL) treatment, DGCR5 facilitated the degeneration of PRDM5 protein in AGE1.HN and PC12 cells (Figure 5(c)).
Figure 5.
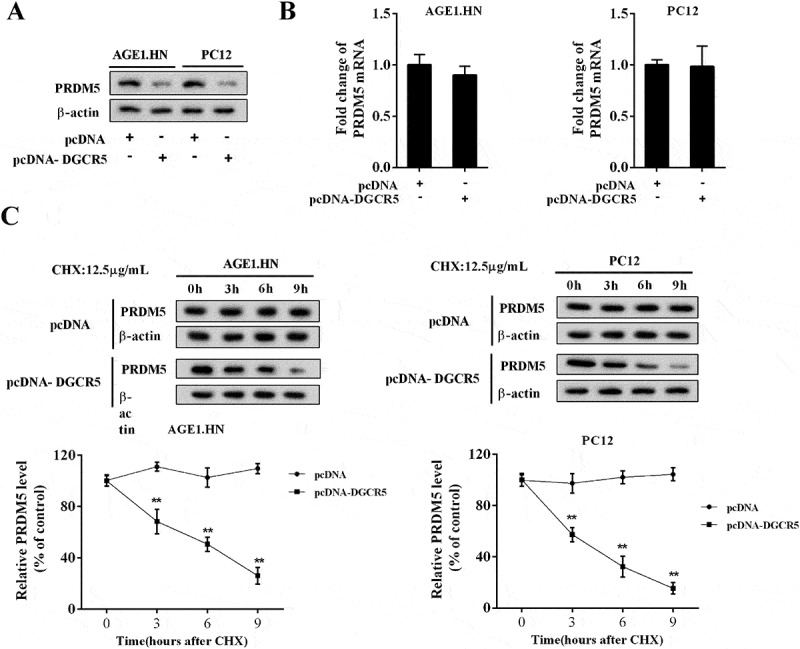
PRDM5 protein expression was negatively regulated by DGCR5. (a) The protein level of PRDM5 in AGE1.HN and PC12 neurons was analyzed with western blot. (b) The expression of PRDM5 mRNA in AGE1.HN and PC12 cells was quantified by qRT-PCR. (c) After treated with CHX (12.5μg/mL) for several hours, the protein level of PRDM5 in AGE1.HN and PC12 cells was examined by western blot.
DGCR5 inhibited neuronal apoptosis through PRDM5
To illuminate the regulatory mechanism of DGCR5 on neuronal apoptosis, the AGE1.HN and PC12 cells were respectively divided into three groups: pcDNA, pcDNA-DGCR5, pcDNA-DGCR5+pcDNA-PRDM5. After cell transfection, the AGE1.HN and PC12 cells were treated with hypoxia, and the apoptosis was evaluated. It showed that pcDNA-DGCR5 transfection suppressed apoptosis caused by hypoxia, which was reversed by pcDNA-PRDM5 transfection (Figure 6(a)). Similarly, the expression of c-caspase3 was inhibited with pcDNA-DGCR5 transfection, but inverted by pcDNA-PRDM5 transfection (Figure 6(b)). The above results revealed that DGCR5 inhibited neuronal apoptosis through PRDM5.
Figure 6.
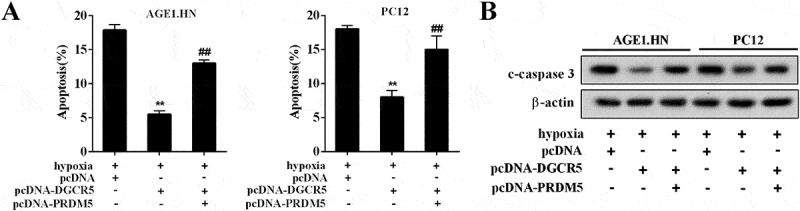
DGCR5 inhibited neuronal apoptosis through PRDM5. (a) Apoptosis of AGE1.HN and PC12 cells was evaluated with flow cytometry. (b) The protein level of c-caspase3 in AGE1.HN and PC12 neurons was determined by western blot.
Over-expressed DGCR5 ameliorated ASCI in rat
To validate the role of DGCR5 in ASCI progression in vivo, ASCI rat model was established. The pcDNA (n = 7) or pcDNA-DGCR5 (n = 7) was introduced into the ASCI model rats by intrathecal injection, and the neurological function of these rats was assessed by BBB scoring. Compared with the rats injected with pcDNA (n = 7), the BBB score of rats injected with pcDNA-DGCR5 (n = 7) was distinctly elevated (Figure 7(a)); and it implied that the remarkable effect of over-expressed DGCR5 on mitigating ASCI through repressing PRDM5 and neuronal apoptosis (Figure 7(b)).
Figure 7.
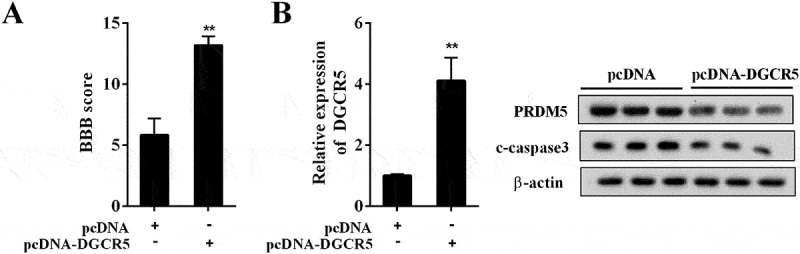
Over-expressed DGCR5 ameliorated ASCI in rat. (a) Basso, Beattie, and Bresnahan (BBB) scoring was used to assess the neurological function of the ASCI model rats after pcDNA or pcDNA-DGCR5 injected. (b) The expression of DGCR5 and PRDM5 protein in spinal tissue was quantified by qRT-PCR and western blot, respectively.
Discussion
ASCI is a devastating neurologic injury that leads to the loss of sensory, motor, autonomic function, and finally paraplegia, bring about huge economic burden in the worldwide. SCI results from primary and secondary injury mechanisms [19]. Primary injury refers to the immediate physical injury to the neural tissue by mechanical forces, with pathological changes including severed axons, direct mechanical damage to cells, and ruptured blood vessels [20]. Researches on cellular function, regulatory mechanism, and therapeutic target have attracted much attention. Recently, advances in cell transplantation and genetic engineering technologies are increasingly making it possible for effective treatment of ASCI, and numerous reports have claimed that regulation of gene expression plays a vital role in ASCI pathological changes [21]. Exploration on the underlying mechanism may contribute to impelling the clinical application of these technologies for ASCI and central nervous system injury.
Noncoding RNAs such as circulating microRNAs have been found out to be markedly increased in the serum relative to the severity of ASCI, which may be promising biomarkers for diagnose and predicting the severity of ASCI [22]. By using a microarray method in a contusion SCI mouse model, a dynamic lncRNA-mRNA network containing 264 lncRNAs and 949 mRNAs has been constructed to illuminate the interactions between the lncRNAs and mRNAs [23], verifying the differential expression of lncRNAs in contusion SCI. In this study, lncRNA DGCR5 was found to be down-regulated in ASCI. We revealed an inhibitory effect on neuronal apoptosis of DGCR5 through negatively regulating PRDM5, and thereby alleviating ASCI. Via targeting protein directly, the neuroprotective role of DGCR5 was emphasized, in keeping with its relationship with neurological disease and its down-regulation in neurodegenerative disease [11].
The noncoding transcripts lncRNAs have been proved to be associated with many biological processes, including autophagy [24], cell pluripotency [25], cell proliferation and apoptosis [26], and immune response[27]. Abnormal lncRNAs expression widely involves in disease occurrence and progression, such as cancer, aging, cardiovascular diseases, and neurological disorders [28], usually function as a competing endogenous RNA [29]. But the regulatory role of lncRNAs in ASCI has not been excavated yet. As a novel transcript identified in DiGeorge syndrome, DGCR5 is also implicated in some other diseases except for in nervous system. For instance, DGCR5 has been reported to be correlated with cancers progression or prognosis by regulating proliferation, migration, and invasion via targeting different miRNAs, including lung adenocarcinoma, lung cancer, and hepatocellular carcinoma [30–32]. In the present study, DGCR5 was initially discovered to be a crucial anti-apoptotic transcript in ASCI through binding with PRDM5 protein directly, unlike its previous mechanism of action with miRNAs. This study revealed the regulatory effect of lncRNA in ASCI for the first time, offering a novel perspective on ASCI pathological study and therapy development.
As a significant transcriptional factor, PRDM5 has been proved to mediate cell proliferation, invasion, differentiation, and apoptosis in cancers and brittle cornea syndrome [33,34]. The increased level of PRDM protein after ASCI and its pro-apoptotic effect on neurons has been elucidated in the previous study [8]. In the current study, PRDM5 was identified as the target of lncRNA DGCR5 for the first time, devoting itself to modulating neuronal apoptosis in ASCI. With the targeting effect of miRNAs and lncRNAs on PRDM5 far from clarified, we provided a new insight into the regulatory effect on PRDM5 by other non-coding RNAs in various disorders.
In conclusion, lncRNA DGCR5 exhibits an inhibitory effect on neuronal apoptosis through directly binding with PRDM5 protein and negatively regulating it, and thereby ameliorating ASCI. This study clarifies the targeting relationship between DGCR5 and PRDM5 protein, and emphasizes the influence of DGCR5 on ASCI pathological development via impacting neuronal apoptosis, providing novel targets and theoretic foundation for ASCI prevention and treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- [1].Cui M, Ma X, Sun J, Li F, et al. Effects of STAT3 inhibitors on neural functional recovery after spinal cord injury in rats. Biosci Trends. 2017;10(6):460–466. [DOI] [PubMed] [Google Scholar]
- [2].Bowes AL, PK Yip. Modulating inflammatory cell responses to spinal cord injury: all in good time. J Neurotrauma. 2014;31(21):1753–1766. [DOI] [PubMed] [Google Scholar]
- [3].Zhang YK, Liu JT, Peng ZW, Kuang F, et al. Different TLR4 expression and microglia/macrophage activation induced by hemorrhage in the rat spinal cord after compressive injury. J Neuroinflammation. 2013;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Witiw CD, MG Fehlings. Acute spinal cord injury. J Spinal Disord Tech. 2015;28(6):202–210. [DOI] [PubMed] [Google Scholar]
- [5].Duan Z, Person RE, Lee HH, MS Horwitz, et al. Epigenetic regulation of protein-coding and microRNA genes by the Gfi1-interacting tumor suppressor PRDM5. Mol Cell Biol. 2007;27(19):6889–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Micheal S, Khan MI, Islam F, AI den Hollander, et al. Identification of mutations in the PRDM5 gene in brittle cornea syndrome. Cornea. 2016;35(6):853–859. [DOI] [PubMed] [Google Scholar]
- [7].Tan SX, Hu RC, Liu JJ, WE Liu, et al. Methylation of PRDM2, PRDM5 and PRDM16 genes in lung cancer cells. Int J Clin Exp Pathol. 2014;7(5):2305–2311. [PMC free article] [PubMed] [Google Scholar]
- [8].Liu J, Wu W, Hao J, Zhang F, et al. PRDM5 expression and essential role after acute spinal cord injury in adult rat. Neurochem Res. 2016;41(12):3333–3343. [DOI] [PubMed] [Google Scholar]
- [9].Ling W, Xu X, Liu J.. A causal relationship between the neurotherapeutic effects of miR182/7a and decreased expression of PRDM5. Biochem Biophys Res Commun. 2017;490(1):1–7. [DOI] [PubMed] [Google Scholar]
- [10].Johnson R, Teh CH, Jia H, Lipovich L, et al. Regulation of neural macroRNAs by the transcriptional repressor REST. RNA. 2009;15(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Johnson R. Long non-coding RNAs in Huntington’s disease neurodegeneration. Neurobiol Dis. 2012;46(2):245–254. [DOI] [PubMed] [Google Scholar]
- [12].Guida N, Laudati G, Serani A, Formisano L, et al. The neurotoxicant PCB-95 by increasing the neuronal transcriptional repressor REST down-regulates caspase-8 and increases Ripk1, Ripk3 and MLKL expression determining necroptotic neuronal death. Biochem Pharmacol. 2017;142:229–241. [DOI] [PubMed] [Google Scholar]
- [13].Liu XZ, Sun X, Shen KP, ZX Xu, et al. Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/reperfusion injury. Neural Regen Res. 2017;12(7):1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Macks C, Gwak SJ, Lynn M, JS Lee. Rolipram-loaded polymeric micelle nanoparticle reduces secondary injury after rat compression spinal cord injury. J Neurotrauma. 2018;35(3):582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].He Y, Lv B, Huan Y, Yuan H, et al. Zhenbao pill protects against acute spinal cord injury via miR-146a-5p regulating the expression of GPR17. Biosci Rep. 2018;38(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lin HC, Narasimhan P, Liu SY, IR Lai, et al. Postconditioning mitigates cell death following oxygen and glucose deprivation in PC12 cells and forebrain reperfusion injury in rats. J Neurosci Res. 2015;93(1):140–148. [DOI] [PubMed] [Google Scholar]
- [17].Li W, Sun M, Zang C, et al. Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells. Cell Death Dis. 2016;7:e2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gu S, Xie R, Liu X, Che X, et al. Long coding RNA XIST contributes to neuronal apoptosis through the downregulation of AKT phosphorylation and is negatively regulated by miR-494 in rat spinal cord injury. Int J Mol Sci. 2017;18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schomberg D, Ahmed M, Miranpuri G, DK Resnick, et al. Neuropathic pain: role of inflammation, immune response, and ion channel activity in central injury mechanisms. Ann Neurosci. 2012;19(3):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ambrozaitis KV, Kontautas E, Spakauskas B, Vaitkaitis D. Pathophysiology of acute spinal cord injury. Medicina (Kaunas). 2006;42(3):255–261. [PubMed] [Google Scholar]
- [21].Varma AK, Das A, Wallace G, NL Banik, et al. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res. 2013;38(5):895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hachisuka S, Kamei N, Ujigo S, Ochi M, et al. Circulating microRNAs as biomarkers for evaluating the severity of acute spinal cord injury. Spinal Cord. 2014;52(8):596–600. [DOI] [PubMed] [Google Scholar]
- [23].Ding Y, Song Z, Liu J. Aberrant LncRNA expression profile in a contusion spinal cord injury mouse model. Biomed Res Int. 2016;2016:9249401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang K, Liu CY, Zhou LY, et al APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun. 2015;6:6779. [DOI] [PubMed] [Google Scholar]
- [25].Yang YW, Flynn RA, Chen Y, HY Chang, et al. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. Elife. 2014;3:e02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu WH, Zhang JB, Dang Z, KF Dou, et al. Long non-coding RNA URHC regulates cell proliferation and apoptosis via ZAK through the ERK/MAPK signaling pathway in hepatocellular carcinoma. Int J Biol Sci. 2014;10(7):664–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Heward JA, MA Lindsay. Long non-coding RNAs in the regulation of the immune response. Trends Immunol. 2014;35(9):408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kazemzadeh M, Safaralizadeh R. lncRNAs: emerging players in gene regulation and disease pathogenesis. J Genet. 2015;94(4):771–784. [DOI] [PubMed] [Google Scholar]
- [29].Yan B, Yao J, Liu JY, et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116(7):1143–1156. [DOI] [PubMed] [Google Scholar]
- [30].Dong HX, Wang R, Jin XY, et al. lncRNA DGCR5 promotes lung adenocarcinoma (LUAD) progression via inhibiting hsa-mir-22-3p. J Cell Physiol. 2018;233(5):4126–4136. [DOI] [PubMed] [Google Scholar]
- [31].Chen EG, Zhang JS, Xu S, et al. Long non-coding RNA DGCR5 is involved in the regulation of proliferation, migration and invasion of lung cancer by targeting miR-1180. Am J Cancer Res. 2017;7(7):1463–1475. [PMC free article] [PubMed] [Google Scholar]
- [32].Huang R, Wang X, Zhang W, et al. Down-regulation of LncRNA DGCR5 correlates with poor prognosis in hepatocellular carcinoma. Cell Physiol Biochem. 2016;40(3–4):707–715. [DOI] [PubMed] [Google Scholar]
- [33].Wang L, Qq D, Ss G, et al. PRDM5 promotes the proliferation and invasion of murine melanoma cells through up-regulating JNK expression. Cancer Med. 2016;5(9):2558–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lf P, Gallego-Pinazo R, Cl K, et al. Bruch’s membrane abnormalities in PRDM5-related brittle cornea syndrome. Orphanet J Rare Dis. 2015;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


