Abstract
Current clinical treatment regimens, including many emergent immune strategies (e.g. checkpoint inhibitors) have done little to affect the devastating course of pancreatic ductal adenocarcinoma (PDA). Clinical trials for PDA often employ multi-modal treatment, and have started to incorporate stromal-targeted therapies, which have shown promising results in early reports. Focused ultrasound (FUS) is one such therapy that is uniquely equipped to address local and systemic limitations of conventional cancer therapies as well as emergent immune therapies for PDA. FUS methods can non-invasively generate mechanical and/or thermal effects that capitalize on the unique oncogenomic/proteomic signature of a tumor. Potential benefits of FUS therapy for PDA include: 1) emulsification of targeted tumor into undenatured antigens in situ, increasing dendritic cell maturation, and increasing intra-tumoral CD8+/ T regulatory cell ratio and CD8+ T cell activity; 2) reduction in intra-tumoral hypoxic stress; 3) modulation of tumor cell membrane protein localization to enhance immunogenicity; 4) modulation of the local cytokine milieu toward a Th1-type inflammatory profile; 5) up-regulation of local chemoattractants; 6) remodeling the tumor stroma; 7) localized delivery of exogenously packaged immune-stimulating antigens, genes and therapeutic drugs. While not all of these results have been studied in experimental PDA models to date, the principles garnered from other solid tumor and disease models have direct relevance to the design of optimal FUS protocols for PDA. In this review, we address the pertinent limitations in current and emergent immune therapies that can be improved with FUS therapy for PDA.
Keywords: ultrasound, therapy, pancreatic, cancer, immune
Introduction
Pancreatic cancer responds poorly to many conventional and emergent immune therapies
Pancreatic ductal adenocarcinoma (PDA), commonly known as pancreatic cancer, typically presents as metastatic or unresectable disease and has an overall 5-year survival rate of less than 8% [1]. It has recently been estimated that by 2030, PDA will be the second most common cause of cancer-related mortality – indicating the limited impact of cancer therapy research to date on the clinical course of the disease [2]. The poor response of PDA to conventional therapies is thought to be in part due to a dense matrix of tumor stromal cells that fosters: 1) high interstitial fluid pressure (~99mmHg, versus 10mmHg in normal pancreas), that results in collapsed vasculature and hypoxia, and 2) an immunosuppressive microenvironment, impeding the endogenous immune system from eradicating the tumor [3–9]. Exogenous immune-modulating immunotherapies have garnered significant attention in recent years due to early successes in clinical trials for treatment of hematologic cancers and a limited number of solid tumors (e.g. melanoma, non-small-lung cancer, clear cell renal cell carcinoma, head and neck squamous cell carcinoma, urothelial cancer), but have unique obstacles to overcome in treatment of many other solid tumors, including PDA [10]. Clinical trials of many emergent immune strategies, such as immune checkpoint inhibitors and treatments targeting macrophages and myeloid-derived suppressor cells, have yielded few objective responses in PDA patients [11–16].
The importance of immune infiltrate, hypoxia, and the PDA stroma
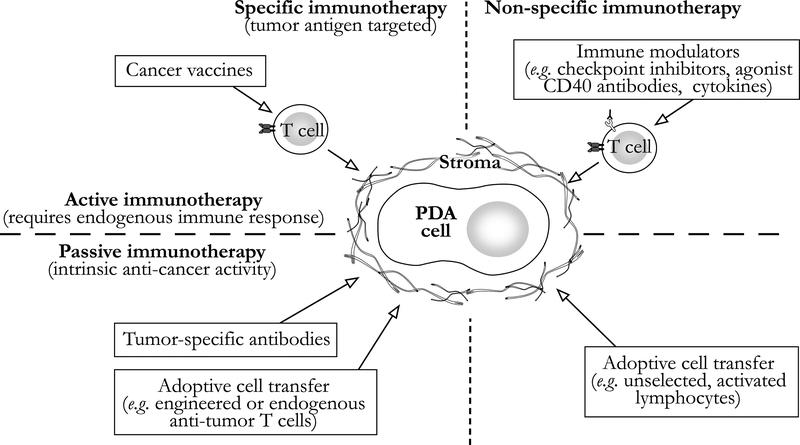
Tumor-antigen specific, endogenous CD8+ T cells are often present in the circulation and bone marrow of PDA patients, and CD8+ T cells capable of T helper (Th)-1, anti-tumor functional response (e.g. IFN-gamma) dominate the immune cell infiltrate of resected tumor specimens [8, 16–20]. These latter findings contrast with those observed in genetically engineered mouse models of PDA, where CD8+ T cells remain relatively scarce within the tumor compared to immunosuppressive cells [9, 21–24]. This discrepant picture of the immune response may be due to the relatively rapid tumor development after oncogene activation in genetically engineered animals versus the prolonged genetic evolution of human PDA [25]. Regardless, despite the presence of an effector T-cell-rich infiltrate in human tumors, meaningful anti-tumor activity is most commonly not observed. For a graphical overview of the categorization of immune therapies employed in the treatment of PDA, please see Figure 1.
Figure 1.
Classification systemof immunotherapies for pancreatic ductal adenocarcinoma (PDA). The tumor stroma impedes these therapies withmultiple immunosuppressive mechanisms described in the text. Stromal-directed treatments, such as focused ultrasound, have shown promising results in early clinical trials and may provide substantial clinical benefit as immuno-adjuvant therapy.
Notable barriers to effective cytotoxic T-cell activity in PDA tumors include: 1) the poorly vascularized, profoundly hypoxic and acidic tumor microenvironment and advanced desmoplastic stroma that correlate with more aggressive tumor phenotypes [26–32]; 2) the immunosuppressive activity of FOXP3+ regulatory T cells (Treg), CD11b+ myeloid-derived suppressor cell (MDSC), and tumor-associated macrophages (TAM) within the PDA stromal matrix [33]. Increased hypoxia [34–36], and increased ratio of immunosuppressive versus effector memory CD8+ T cells [17, 33] are poor prognostic indicators in PDA, and are closely inter-related. Hypoxic zones within tumors have been shown to: 1) foster localized accumulation and differentiation of immune inhibitory cell lines (e.g. Treg, TAM, MDSC) [37]; 2) promote immunosuppressive activity such as selective upregulation of PD-L1 expression on MDSCs and tumor cells [38, 39]; 3) decrease production of IFN-gamma and IL-2 [40, 41]; 4) diminish cytotoxic T-cell performance with tumor microenvironments in vivo [42–46]. Indeed, accumulation of intracellular hypoxia-inducible factors (e.g. HIF-1α) are correlated with poor tumor differentiation and fibrotic foci in PDA [47, 48] and have been shown to promote pancreatic cancer stem cell (CSC) expansion, including CD133+ CSCs that are known to determine the metastatic phenotype of individual tumors through HIF-1α-dependent activation of the Notch signaling pathway [34, 49–53]. Treatments that decrease the hypoxic stress of the PDA microenvironment could significantly improve endogenous immune responses and immunotherapy efficacy, and have also been shown to improve response to radiotherapy and chemotherapy [53–55].
Tregs play a particularly important role in modulating the immune activity in the PDA microenvironment. There is debate and ongoing research regarding the most important features of Tregs and their interactions that help to enable tumor evasion of immune eradication; however expansion of Tregs in peripheral blood and tumor tissue has been shown to correlate with poor prognosis [56–58]. Th1 (T-bet+), Th2 (GATA-3+), and Th17 (ROR-gamma-t+) have recently been shown to be not only T helper subtypes, but also dynamic phenotypes of Treg cells [59, 60]. GATA3 expression has been shown to be critical to Treg functions during inflammation, and PDA is associated with chronic inflammation [61, 62]. The ratio of GATA3+/T-Bet+ infiltrating-lymphoid cells in human PDAs has been shown to correlate inversely with survival, implying an association between Th2 dominance (increased IL-5 and IL-13 levels) and disease progression [63–65]. In addition, patients with PDA have expanded peripheral ROR-gamma-t+ Tregs that induce both Th17 (e.g. IL-17, IL-6) and Th2 (e.g. IL-4, IL-13, IL-33) responses – effectively suppressing anti-tumor T cell activity while promoting chronic inflammation [60]. This finding correlates with resected human PDA specimens demonstrating expanded intra-tumoral Tregs and Th17 cells [17]. Thus the hybrid, dynamic, Th17/Th2-response-inducing Treg phenotype appears to be an important pro-carcinogenic driver of the PDA immuno-microenvironment [66].
PDA stromal remodeling strategies, such as selective depletion of specific subsets of immune-suppressor cells within the tumor stroma, have promoted endogenous T cell activity against PDA in genetically engineered pre-clinical models, and unmasked the benefit of complimentary checkpoint-inhibitor therapy [21, 22, 67]. These results are distinctly different from efforts to selectively ablate stromal fibroblasts, which appeared to evoke a more aggressive disease [68, 69], but similar to those achieved with targeted depletion of hyaluronic acid (HA; a naturally occurring glycosaminoglycan that is produced by PDA cells and is highly concentrated in the tumor extracellular space). Decreasing intra-tumoral HA concentration via intravenous recombinant enzyme administration lowered interstitial fluid pressure, increased intra-tumoral vessel diameter, improved chemotherapy delivery, and significantly prolonged survival in KrasLSL-G12D/+;Trp53LSL-R172H/+;p48Cre/+ (KPC) mice (a genetically engineered model of PDA known to closely mimic the human disease) [5, 70]. In a recent phase 1B clinical trial, enzymatic HA depletion doubled overall survival when combined with chemotherapy in patients with high HA, stage IV PDA [4]. In addition, as described in greater detail below, a recent phase 1 clinical trial of relatively low-intensity focused ultrasound (FUS) therapy designed to minimize thermal effects, directed at the tumor and stroma in combination with chemotherapy, doubled median overall survival in patients with inoperable PDA versus chemotherapy alone [71]. Thus, stromal-targeted therapies appear to be an important component of evolving multi-modal PDA treatment regimens. The mechanisms underlying the efficacy of adjuvant stromal-targeted treatments are incompletely understood, but may be driven by reduction of intra-tumoral interstitial fluid pressure (e.g. targeted HA reduction), anti-tumor immune effects (e.g. selective immune- suppressor cell depletion), or a combination of these two (e.g. FUS therapy).
Passive Specific Immune Therapies
“Passive specific” immune therapies are exogenously engineered or expanded with tumor-specificity, and passively infused to mediate immediate anti-tumor immune activity. These include engineered or expanded tumor-specific T cells and antibodies. Ex-vivo expansion of endogenous anti-tumor T cells for adoptive transfer is discussed in greater detail below. Adoptive transfer of genetically engineered T cells, incorporating either a cloned T cell receptor (TCR) or synthetic chimeric antigen receptor (CAR), can improve anti-tumor immune function, and (with careful selection of target antigens) cause minimal off-target or on-target-off-tumor toxicity. Recent advances in high-throughput patient lymphocyte testing as well as sequencing of tumor transcriptomes and whole-exomes have enabled wide survey of tumor-specific antigens recognized by autologous T cells and facilitated a surge in clinical implementation of adoptive cell transfer [72]. CAR T cells directed against the B cell surface receptor CD19 have been effective in treating leukemia and lymphoma in early clinical trials [73–78], and research to translate this success to solid tumor models, including PDA, is gaining momentum.
MUC1 and mesothelin are two PDA-associated antigens that have been targeted with adoptive therapies. An early clinical trial in Japan employed adoptive transfer of peripherally-derived MUC1 targeted cytotoxic T cells for post-operative treatment of 20 patients with resectable PDA. They observed a 19.4% 3 year median overall survival, increased peripherally circulating cytotoxic T cells, and decreased peripherally circulating Tregs (p<0.05) [79]. Similarly targeted CAR T cells have now been developed in the United States and were successfully employed in subcutaneous PDA mouse models [80]. Endogenous CD8+ T cell titers with activity specific to mesothelin have been correlated with longer overall survival times in patients with advanced PDA [81, 82]. Engineered T cells with an affinity-enhanced TCR for mesothelin have been employed pre-clinically in KPC mice. Despite relatively transient anti-tumor activity, with bi-weekly, serial infusions, adoptive transfer of these cells in a blinded, placebo-controlled study in the KPC model led to: 1) stromal involution (including death of fibroblasts), 2) improved vessel patency, and 3) doubled overall survival time, without evidence of significant off-tumor tissue injury [23]. Results such as these have laid the foundation for multiple phase I/II clinical trials. Additional trials are currently recruiting patients to study the effect of tumor infiltrating lymphocytes in advanced cases of solid tumor-based malignancies that include pancreatic cancer [83, 84].
Active Specific Immune Therapies
“Active specific” immune therapies include vaccines and immunomodulatory agents designed to eventually lead to expansion of endogenous tumor-specific T cells. Numerous “cancer vaccines” have been employed for treatment of PDA – modest successes have been seen in early trials of whole-cell, dendritic cell and telomerase peptide vaccines (see two recent excellent reviews on this subject for more comprehensive discussion) [85, 86]. The most relevant example to the topic of this review is “whole cell” vaccine, designed to bolster the endogenous immune response by recruiting the host’s antigen presenting cells (APCs) to the site of vaccination for T cell cross-priming / MHC class I presentation of a broad array of tumor antigens. For PDA treatment, these agents have had modest successes. In phase I/II clinical trials, intra-dermal vaccination of advanced-PDA patients with two irradiated, allogeneic pancreatic tumor cell lines, genetically engineered to secrete granulocyte-macrophage colony-stimulating factor to induce chemotaxis of APCs to the injection site has: 1) “Primed” T cells against a broad array of PDA antigens, including mesothelin [81, 82, 87, 88]; 2) significantly prolonged patient survival (from 4.6 to 9.7 months) when combined with low-dose cyclophosphamide and a “booster” of recombinant bacterial-vector for mesothelin expression in the cytosol of infected APC’s [81]. A proposed mechanism for this latter study result from Le et al is induction of T cell trafficking from the periphery to central tissues by stimulatory cytokines released in response to the bacterial vector [81, 89]. A recent Phase 2b trial examining this same mesothelin “boosted” vaccine, however, revealed no significant difference in overall survival versus standard of care, and an ongoing trial is now examining its benefit in combination with a checkpoint inhibitor [90, 91]. While most human PDAs express mesothelin, and this is often used as a marker of tumor specific immune response, the degree of overexpression remains highly individualized. PDAs have relatively few coding mutations compared to more immunogenic cancers, and a recent study of genome-wide mutations from 99 informative PDA tumors revealed substantial heterogeneity [92, 93]. These factors provide significant challenge to the formulation of a single effective exogenous “vaccine” to bolster anti-tumor endogenous immune activity for PDA patients.
Non-Specific Immune Therapies and Immunomodulation
As early as the 1990’s, clinical trials in Japan employed non-specific, activated peripheral lymphocytes for adoptive therapy in patients with advanced cancer, including PDA [94]. This practice has evolved with technologic advances enabling more tumor-specific adoptive therapies (as described above) but other non-specific immunomodulatory therapies, such as checkpoint inhibitors, have gained favor. As previously discussed, Th2/Th17 Treg activity represents a significant barrier to effective anti-tumor immune response and immunotherapies for PDA. Agents that promote Th1-type anti-tumor inflammatory activity (e.g. IFN-gamma) versus Th2-type immunosuppressive activity within the tumor microenvironment may greatly expand the therapeutic potential of PDA specific immunotherapies [16, 95–98]. By modulating the background CD4+ T cell population response, “immune priming” strategies might allow for rescue of CD8+ T cell anti-tumor activity, greater anti-tumor effect of passive specific therapies, and more durable immune response toward tumor eradication. For example, combining agonist CD40 monoclonal antibodies (designed to foster APC maturation and up-regulate Th1 chemokine expression) with chemotherapy and checkpoint inhibitors has improved endogenous CD8+ T cell-mediated tumor rejection and long-term tumor free survival in both subcutaneous, and genetically engineered PDA murine models [67, 99].
Beyond direct cytotoxicity, both passive and active specific immune therapies can also elicit anti-tumor systemic immunomodulation. A recent meta-analysis of specific immunotherapy trials employed in treatment of pancreatic cancer revealed that circulating IFN-gamma levels were significantly higher post-treatment versus pre-treatment (4 trials with 81 patients; pooled mean difference of 3.75 IU/mL; p=0.01), and circulating IL-4 levels were significantly lower (2 trials with 55 patients; pooled mean difference of −1.85 IU/mL; p<0.0001) [100]. Anti-tumor immunomodulatory effects are most likely maximized with multi-modal non-specific and specific immune therapies. Indeed, many of the shortcomings of tumor specific immunotherapies (e.g. poor or heterogeneous expression of immunogenic antigens, local immunosuppressive mechanisms) may be overcome by appropriate combination with non-specfic therapies [101], or therapies that demonstrate both specific and non-specific effects, such as focused ultrasound.
Focused Ultrasound Treatment for Pancreatic Cancer
Thermal ablation
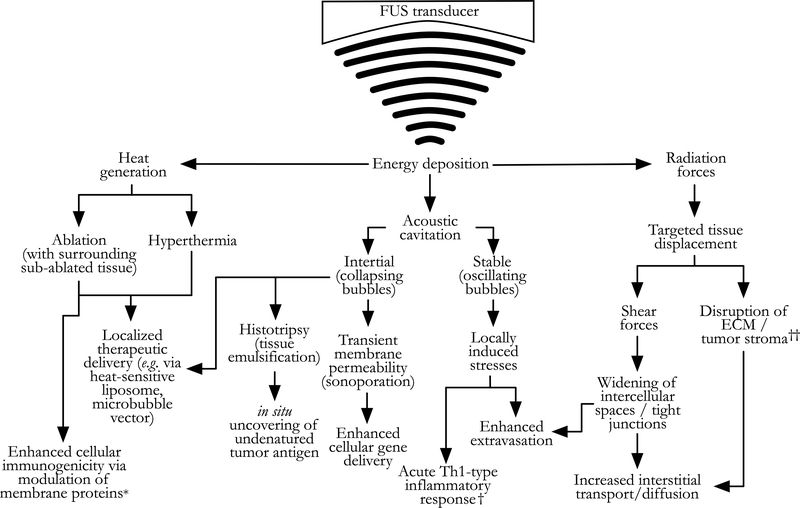
Please see Figure 2 for a schematic overview of the mechanisms that focused ultrasound (FUS) therapy employs to achieve anti-tumor effects. Thermally-ablative (coagulative-necrosis inducing) FUS has long been used clinically outside the United States as both primary and palliative therapy for pancreatic cancer. The majority of reported treatments have taken place in China since the late 1990’s, and more recent case series have emerged from Korea, Japan, and European groups [103]. Typical study endpoints have included safety and feasibility confirmation, tumor volume reduction, and pain relief [104–110]. A comprehensive review of all clinical treatment series employing palliative FUS therapy for pancreatic cancer treatment is beyond the scope of this paper; but we direct the reader to an excellent recent meta-analysis by Dababou et al that examined 23 studies on this topic and showed an overall pain reduction in 81% of treated patients (95% CI: 76–86) [111]. A recent, moderately powered (n=689) retrospective analysis from China revealed an independent median overall survival benefit from ablative FUS treatment for patients with unresectable PDA who also received a variety of multi-modal treatment regimens, without severe adverse events (7.1 versus 5 months; p=0.005) [112]. A smaller retrospective series (n=38) from China found that an ablative FUS protocol with prolonged lower-power heat deposition had a greater median overall survival benefit versus a rapid heating protocol (10.3 versus 6.0 months; p=0.018) [113]. A small number of randomized clinical trials have examined the survival benefit of ablative FUS therapy. Li et al recently reported significant improvement in median overall survival time and progression-free survival (10.3 versus 6.6 months; n=120; p<0.001) in patients with unresectable PDA randomized to receive ablative FUS treatment and chemotherapy, versus chemotherapy alone [114]. A smaller randomized clinical trial by Lv et al found similar survival benefit (8.9 versus 5.5 months median overall survival; n=45; p<0.05) [115]. While these results are encouraging, multiple recent systematic reviews have found a relative paucity of randomized clinical trials evaluating the clinical survival benefit achieved with FUS ablative therapy for pancreatic cancer. Further studies are needed to improve the quality of evidence for its appropriate inclusion in multimodal treatment regimens [116–118].
Figure 2.
Schematic depiction of selected mechanisms underlying the anti-tumor effects of non-invasive energy deposition via focused ultrasound (FUS). These mechanisms are not mutually exclusive, and indeed, co-exist on a continuum; however, FUS treatment protocols can be adjusted to maximize some mechanisms and minimize others. Most commonly, this involves minimizing heat generation relative to cavitation and radiation forces. This figure is adapted with significant revision froma review by Victor Frenkel, [102], where the reader will find a more comprehensive discussion of these mechanisms as they relate to localized delivery of therapeutics to solid tumors, beyond the immuno-adjuvant effects reviewed here. *Immunogenic cellular membrane protein modulation has also been observed with lowintensity/minimal-thermal protocols. †Acute T-helper (Th)1-type inflammatory responses have also been observed with thermal-emphasis protocols. ‡Disruption of the extracellularmatrix (ECM) / tumor stroma is likely the sequela of both radiative forces and acoustic cavitation activity. See text and Table 1 for details.
In addition to the locally destructive thermal effects of FUS, recent data suggests potential immunomodulatory effects of FUS for PDA. Specifically, in a small series (n=15) of patients with advanced PDA by Wang et al, post-FUS-treatment blood samples showed increased percentages of circulating CD3+, and CD4 T cells (in 66% of patients), a higher CD4+/CD8+ T cell ratio, and enhanced NK cell activity [119]. These findings were confirmed in a recent meta-analysis of 3022 clinical cases of FUS-thermally-ablated PDA [120]. One possible mechanism to explain these findings it that thermal ablation delivers sub-lethal heat exposure to peripheral, surviving tumor cells and can thus cause upregulation and surface expression of heat shock proteins (HSPs). HSPs are intracellular molecular chaperones that can bind tumor peptide antigens and have long been recognized as potent stimulators of tumor immunogenicity via antigen-presenting cells of the endogenous immune system (dendritic cells, macrophages, CD4+ T cells) [121–127]. Although studies of HSP expression specific to pancreatic cancer cell lines have not yet been published, preliminary results have been presented, and this topic is being actively pursued [128].
The abscopal effect
Orsi et al recently presented preliminary results from a small clinical series where they observed diminished size in tumor metastases (the abscopal effect) following local thermally-ablative, palliative FUS treatment of patients with advanced PDA (4/46 patients) [129]. Similar effects have been observed by others [114, 130–132]. In all cases, patients received systemic chemotherapy concurrently with FUS treatments, but chemotherapy had been ineffective prior to initiation of FUS therapy. In theory, such systemic responses could be due to the above described favorable, local and systemic immunomodulatory effects observed following FUS therapy, however strong and durable immune responses have not been observed following tumor ablation as monotherapy in clinical trials.
Beyond thermal ablation
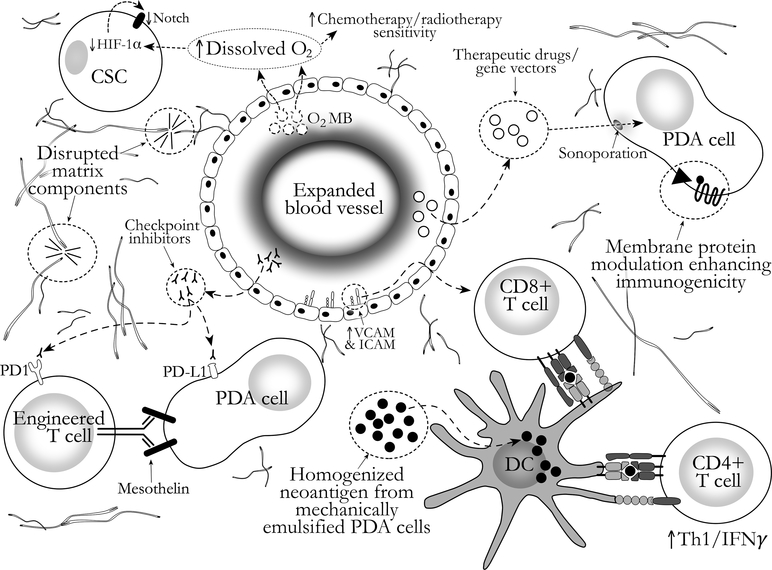
FUS treatment settings (e.g. pulse duration and repetition frequency, acoustic power, focal pressure, transducer frequency) can be customized to achieve a variety of effects in target tissues. Indeed, the versatility of FUS is what sets it apart from alternative methods for targeted coagulative necrosis (e.g. radiofrequency, microwave, laser). Predominantly pre-clinical study of FUS treatment for pancreatic cancer has advanced beyond thermal ablation. Modern FUS transducers can precisely (at ~1mm focus), non-invasively generate targeted, sub-ablative hyperthermia, as well as mechanical and pressure-related effects (such as cavitation of gas and vapor bubbles, acoustic radiation force, and microstreaming) to achieve several desirable results in the treatment of PDA, including those summarized in Figure 2 and Table 1. Of note, not all of these results (facets of immunomodulation, mechanical tumor homogenization, exogenous antigen delivery) have been specifically studied in PDA models to date. However, assuming some degree of independence of FUS effects from tissues targeted, the general principles garnered from other tumor and disease models have direct relevance to the design of optimal FUS protocols for PDA treatment. See Figure 3 for a graphical depiction of these mechanisms in compliment to other PDA therapies.
Table 1 –
Summary of selected pre-clinical focused ultrasound effects relevant to immuno-adjuvant PDA treatment
| Focused Ultrasound Effect | FUS Protocol(s) | Tissue(s) Targeted | References |
|---|---|---|---|
| • Increase penetration and diffusion of drugs into tumor | Pulsed | PDA | [134–136] |
| • Disrupt tumor stroma | Pulsed | PDA | [135] |
| • Reduce intra-tumoral hypoxic stress via augmented oxygen delivery (e.g. oxygen-gas-loaded microbubbles) | Pulsed | PDA | [54, 55] |
| • Modulate tumor cell membrane protein localization to enhance immunogenicity (e.g. immediate downregulation of CD47, a suppressor of phagocytic activity; upregulation of HSP70, calreticulin, CD40, CD80 and CD86) | Thermal (CD47), low-intensity | PDA (CD47), lung / breast / prostate cancer | [128, 140] |
| •Modulate local and systemic cytokine milieu toward a Th1-type inflammatory profile (e.g. increase in IFN-gamma, TNF-alpha) | Pulsed, thermal, low-intensity | RCC, prostate cancer, dystrophic muscle, HCC | [137–139, 154, 155] |
| • Up-regulate local chemoattractants (cytokines, chemokines, trophic factors, VCAM and ICAM) | Pulsed†, low -intensity | Normal, ischemic and dystrophic muscle | [137, 141–143] |
| • Mechanically homogenate / liquefy targeted tissue into cellular debris – releasing large quantities of undenatured tumor antigen in situ | Histotripsy, pulsed | Muscle, liver, prostate cancer, colon adenocarcinoma, melanoma | [144–148, 150, 158–160] |
| • Increase local dendritic cell maturation | Pulsed, thermal | HCC, prostate cancer, colon cancer | [157, 161, 162] |
| • Increase intra-tumoral CD8+/ T regulatory cell ratio and CD8+ T cell activity | Histotripsy, pulsed, thermal | RCC, prostate cancer, colon cancer, HCC | [139, 152, 154, 155, 161] |
| • Facilitate delivery of exogenously packaged immune-stimulating antigen or genes (e.g. via microbubbles, liposomes, sonoporation) | Pulsed, low-intensity | Colon cancer, HCC, prostate cancer, ovarian cancer, melanoma, lymphoma | [163–170] |
FUS = focused ultrasound; PDA = pancreatic ductal adenocarcinoma; RCC = Renal cell carcinoma; HCC = hepatocellular carcinoma; VCAM = vascular cell adhesion protein; ICAM = intercellular adhesion molecule.
Aicher et al. [142] applied targeted ultrasound to muscle via a shockwave lithotripter device using a low duty cycle, similar in some regard to pulsed protocols used with FUS transducers.
Figure 3.
Conceptual diagram of focused ultrasound effects for augmentation of pancreatic ductal adenocarcinoma (PDA) treatment. See text for details. CSC = Cancer stem cell; DC = dendritic cell; HIF = hypoxia-inducible factor; O2 MB = Oxygen-gas-loaded microbubble.
Pulsed focused ultrasound
Pulsed FUS protocols have been used in early clinical trials [71] and pre-clinical studies [133–136] for PDA treatment to augment tumor drug delivery, and recently presented pre-clinical results show favorable immunomodulatory effects from similar treatments [128]. “Pulsed” protocols typically include a relatively short pulse duration (e.g. ~1ms) and low pulse repetition frequency (e.g. 1 Hz; duty cycle 0.1%) to minimize temperature effects while harnessing the mechanical effects of FUS. Specifically, pulses often employ spatial-average pulse-average acoustic intensities sufficient to generate peak focal pressure levels to achieve cavitation, or utilize lower intensities combined with microbubble contrast agents. Pulsed FUS treatments in combination with doxorubicin chemotherapy in the KPC model have: 1) disrupted the stromal collagen architecture of treated PDA; 2) increased intra-tumoral doxorubicin concentrations up to 4.5-fold versus intravenous chemotherapy alone [135]. An early phase I clinical trial from Norway by Dimcevski et al examined 10 patients with inoperable PDA who were treated with a pulsed FUS protocol at low intensity, with exogenously administered microbubbles, designed to minimize thermal deposition while facilitating stable cavitation. This treatment, in combination with gemcitabine infusion, doubled the median overall survival compared to gemcitabine treatment alone (17.6 months versus 8.9 months) [71]. Preliminary results from pre-clinical studies in PDA models, as well as studies applying pulsed or low-intensity FUS treatment protocols to non-PDA tumors and tissues support an acute post-treatment immunomodulatory effect toward Th1-type inflammation, upregulation of localized cell recruitment factors and tumor-cell-surface immunogenic proteins, and increase in local CD8+/T regulatory ratio [128, 137–143]. Anti-tumor effects achieved with pulsed FUS protocols in pre-clinical studies are summarized in Table 1.
Boiling and cavitation-cloud histotripsy
“Histotripsy” is a specific type of non-thermal, pulsed FUS method designed to non-invasively, mechanically homogenize target tissue into subcellular debris without thermal denaturation of proteins through gas or vapor bubble activity – a mechanism distinct from thermally-induced coagulative necrosis [144, 145]. Targeted histotripsy treatments of tumors could thus uncover large quantities of undenatured tumor antigen in situ. Histotripsy can be accomplished through application of: 1) repetitive, short duration pulses of FUS with shock fronts to targeted tissues, generating transient, millimeter-sized vapor bubbles that mechanically disrupt tissues (a.k.a. “boiling” histotripsy – a misnomer of sorts, as thermal contribution to the tissue effect is negligible) [146, 147]; 2) application of extremely high magnitude peak negative pressures to induce abundant cavitation events (a.k.a. “cavitation cloud” histotripsy) [148, 149]. To our knowledge, the use of histotripsy FUS methods on pancreatic tumors has not previously been reported. However, our group has recently successfully employed this technique in feasibility tests in the KPC model (unpublished work). In addition, preliminary work from our institution has suggested Th1-type immunomodulatory effects in a rat model of renal cell carcinoma with boiling histotripsy treatment associated with release of the damage-associated molecular pattern (DAMP) HMGB1 into the plasma in vivo (an important factor in inciting the acute inflammatory response in the context of acute tissue trauma) and increased CD8+ T cells in both the treated and contralateral kidneys [150–152]. Pre-clinical studies exploring the anti-tumor effects of histotripsy treatments are summarized in Table 1.
Endogenous T-cell therapy and focused ultrasound
“Endogenous T-cell therapy” relies on isolation and expansion of often low-frequency endogenous T cells that are reactive to tumor antigens from patient’s peripheral blood for ex-vivo expansion and adoptive transfer back into the patient. This method of adoptive cell transfer therapy is appealing given: 1) its flexible, personalized tumor-antigen result (particularly appealing for PDA patients, who are more likely to need rare, personalized peptide targets due to the relatively sparse and heterogeneous coding mutations described above); 2) minimally invasive method of cell acquisition; 3) ability to generate both effector T cells and central memory type T cells; 4) lack of regulatory hurdles and logistical barriers associated with clinical application of T cell receptor engineering [153]. FUS has already been employed pre-clinically as an in situ method of priming the endogenous immune system to generate more effective, tumor-specific cytotoxic T cells for ex-vivo expansion and adoptive transfer. Two recent studies employed a thermally ablative FUS method to generate endogenous tumor-antigen-primed cytotoxic T cells in a subcutaneous murine model of hepatocellular carcinoma and observed: 1) increased Th1-type inflammatory response (e.g. increased levels of TNF-alpha and IFN-gamma) and cytotoxicity (e.g. percentage of LDH release above baseline from co-culture of effector and target tumor cells, greater frequency of MHC class I tetramer/CD8+ cells) from lymphocytes collected 14 days post-treatment from the spleens of ablative-FUS treated animals versus sham and control groups); 2) significantly prolonged overall survival rate at 60 days in subsequent HCC-animals infused with T cells derived from the randomly assigned FUS-treated animals versus sham or control (86% versus 33% and 16%, respectively; log-rank p<0.0001) [154, 155].
Predominantly mechanical FUS methods may be more effective than thermal in promoting systemic anti-tumor endogenous immune response. Xing et al found that melanoma metastasis rates were decreased and overall survival increased in both thermally and mechanically FUS-treated animals versus control, when allowing 2 days between treatment and amputation of the limb bearing the primary tumor (sufficient time for dendritic cell infiltration and migration), but found greater benefit in the mechanical-FUS treatment group versus the thermal-FUS group [156]. Similar results were reported by Hu et al in a murine colon adenocarcinoma model – where mechanical FUS treatment resulted in significantly greater dendritic cell activation versus thermal treatments [157]. In combination with the above-described endogenous T cell activation, these results demonstrate: 1) the importance of the APC-to-cytotoxic-T-cell mechanism in FUS-induced anti-tumor endogenous immune effects; 2) that mechanical FUS methods may have greater immune benefit – either for use in generation of effective endogenous T cell subsets for subsequent ex vivo expansion and adoptive transfer, or for complimentary systemic endogenous immune activity to other therapies. The relatively recently developed histotripsy FUS methods may prove to be an integral component of optimized PDA FUS treatment protocols in this regard.
Concluding Remarks
Clinical cancer immunotherapy has rapidly progressed over the past decade, with several new agents approved by the FDA (including immune checkpoint therapy to promote cytotoxic T cell-mediated anti-tumor activity) and the first and most promising CAR T cells for treatment of hematologic malignancy pending FDA review. Even so, efficacy in treatment of PDA has been marginal, likely based on the elaborate immunosuppressive barriers encountered in the tumor microenvironment. Non-invasive FUS treatment methods designed to maximize non-thermal effects have shown promising early results at improving drug delivery to PDA and prolonging overall clinical survival in combination with chemotherapy. In addition, FUS treatment has the potential among similarly effective PDA stromal-directed therapies to directly and favorably modulate the endogenous immune response to the tumor. Thus, FUS methods may provide both beneficial changes to the treated PDA microenvironment and systemic endogenous immune effects that improve response to metastatic lesions and recurrent disease. Future research will help to precisely define optimal FUS protocols for augmentation of multi-modal PDA treatment regimens, including immune checkpoint inhibitors and adoptive cell transfer.
Acknowledgements
Dr. Maloney is supported in part through a National Institutes of Health, National Research Service Award training grant held by the University of Washington Gastroenterology Division (grant number 5 T32 DK00772). This sponsor had no role in the collection, analysis, and interpretation of data included in this review, nor did they contribute to the writing of this article.
Funding
No specific funding was received to support the writing of this article.
Footnotes
Declaration of Interest
The authors report to conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Ezekiel Maloney, University of Washington / Department of Radiology.
Tanya Khokhlova, University of Washington / Department of Medicine / Division of Gastroenterology.
Venu G. Pillarisetty, University of Washington / Department of Surgery.
George R. Schade, University of Washington / Department of Urology.
Elizabeth A. Repasky, Department of Immunology / Roswell Park Cancer Institute, Buffalo, NY 14263.
Yak-Nam Wang, University of Washington / Applied Physics Laboratory.
Lorenzo Giuliani, University of Rome “La Sapienza”.
Matteo Spring, University of Rome “La Sapienza”.
Joo Ha Hwang, University of Washington / Department of Medicine / Division of Gastroenterology, Box 359773, 325 Ninth Avenue, Seattle, WA 98104.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 3.DuFort CC, DelGiorno KE, Carlson MA, Osgood RJ, Zhao C, Huang Z, et al. Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase. Biophys J 2016;110(9):2106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res 2016;22(12):2848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21(3):418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013;62(1):112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamburrino A, Piro G, Carbone C, Tortora G, Melisi D. Mechanisms of resistance to chemotherapeutic and anti-angiogenic drugs as novel targets for pancreatic cancer therapy. Front Pharmacol 2013;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 2012;21(6):822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007;67(19):9518–27. [DOI] [PubMed] [Google Scholar]
- 10.Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366(26):2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res 2010;16(6):1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanford DE, Porembka MR, Panni RZ, Mitchem JB, Belt BA, Plambeck-Suess SM, et al. A Study of Zoledronic Acid as Neo-Adjuvant, Perioperative Therapy in Patients with Resectable Pancreatic Ductal Adenocarcinoma. J Cancer Ther 2013;4(3):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33(8):828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013;36(7):382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331(6024):1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibuya KC, Goel VK, Xiong W, Sham JG, Pollack SM, Leahy AM, et al. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PLoS One 2014;9(5):e96565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryschich E, Notzel T, Hinz U, Autschbach F, Ferguson J, Simon I, et al. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res 2005;11(2 Pt 1):498–504. [PubMed] [Google Scholar]
- 19.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 2012;21(6):836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz-Winnenthal FH, Volk C, Z’Graggen K, Galindo L, Nummer D, Ziouta Y, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res 2005;65(21):10079–87. [DOI] [PubMed] [Google Scholar]
- 21.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110(50):20212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 2014;63(11):1769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stromnes IM, Schmitt TM, Hulbert A, Brockenbrough JS, Nguyen HN, Cuevas C, et al. T Cells Engineered against a Native Antigen Can Surmount Immunologic and Physical Barriers to Treat Pancreatic Ductal Adenocarcinoma. Cancer Cell 2015;28(5):638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer - science driving clinical progress. Nat Rev Cancer 2005;5(6):459–67. [DOI] [PubMed] [Google Scholar]
- 25.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467(7319):1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Capello M, Fredolini C, Racanicchi L, Piemonti L, Liotta LA, et al. Proteomic analysis reveals Warburg effect and anomalous metabolism of glutamine in pancreatic cancer cells. J Proteome Res 2012;11(2):554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013;496(7443):101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149(3):656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Q, Jurisica I, Do T, Hedley DW. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res 2011;71(8):3110–20. [DOI] [PubMed] [Google Scholar]
- 30.Hiraoka N, Ino Y, Sekine S, Tsuda H, Shimada K, Kosuge T, et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br J Cancer 2010;103(7):1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiser-Erkan C, Erkan M, Pan Z, Bekasi S, Giese NA, Streit S, et al. Hypoxia-inducible proto-oncogene Pim-1 is a prognostic marker in pancreatic ductal adenocarcinoma. Cancer Biol Ther 2008;7(9):1352–9. [DOI] [PubMed] [Google Scholar]
- 32.Erkan M, Reiser-Erkan C, Michalski CW, Deucker S, Sauliunaite D, Streit S, et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia 2009;11(5):497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2014;2(7):616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto O, Shimizu K, Semba S, Chiba S, Ku Y, Yokozaki H, et al. Hypoxia induces tumor aggressiveness and the expansion of CD133-positive cells in a hypoxia-inducible factor-1alpha-dependent manner in pancreatic cancer cells. Pathobiology 2011;78(4):181–92. [DOI] [PubMed] [Google Scholar]
- 35.Lu Y, Hu J, Sun W, Duan X, Chen X. Hypoxia-mediated immune evasion of pancreatic carcinoma cells. Mol Med Rep 2015;11(5):3666–72. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz DL, Powis G, Thitai-Kumar A, He Y, Bankson J, Williams R, et al. The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects. Mol Cancer Ther 2009;8(4):947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, et al. Hypoxia: a key player in antitumor immune response. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Cell Physiol 2015;309(9):C569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 2014;74(3):665–74. [DOI] [PubMed] [Google Scholar]
- 39.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014;211(5):781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol 2001;167(11):6140–9. [DOI] [PubMed] [Google Scholar]
- 41.Lukashev D, Klebanov B, Kojima H, Grinberg A, Ohta A, Berenfeld L, et al. Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol 2006;177(8):4962–5. [DOI] [PubMed] [Google Scholar]
- 42.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol 2005;3(6):e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiel M, Caldwell CC, Kreth S, Kuboki S, Chen P, Smith P, et al. Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS One 2007;2(9):e853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lardner A The effects of extracellular pH on immune function. J Leukoc Biol 2001;69(4):522–30. [PubMed] [Google Scholar]
- 45.Nakagawa Y, Negishi Y, Shimizu M, Takahashi M, Ichikawa M, Takahashi H. Effects of extracellular pH and hypoxia on the function and development of antigen-specific cytotoxic T lymphocytes. Immunol Lett 2015;167(2):72–86. [DOI] [PubMed] [Google Scholar]
- 46.Redegeld F, Filippini A, Sitkovsky M. Comparative studies of the cytotoxic T lymphocyte-mediated cytotoxicity and of extracellular ATP-induced cell lysis. Different requirements in extracellular Mg2+ and pH. J Immunol 1991;147(10):3638–45. [PubMed] [Google Scholar]
- 47.Couvelard A, O’Toole D, Leek R, Turley H, Sauvanet A, Degott C, et al. Expression of hypoxia-inducible factors is correlated with the presence of a fibrotic focus and angiogenesis in pancreatic ductal adenocarcinomas. Histopathology 2005;46(6):668–76. [DOI] [PubMed] [Google Scholar]
- 48.Couvelard A, O’Toole D, Turley H, Leek R, Sauvanet A, Degott C, et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer 2005;92(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1(3):313–23. [DOI] [PubMed] [Google Scholar]
- 50.Zhu H, Wang D, Liu Y, Su Z, Zhang L, Chen F, et al. Role of the Hypoxia-inducible factor-1 alpha induced autophagy in the conversion of non-stem pancreatic cancer cells into CD133+ pancreatic cancer stem-like cells. Cancer Cell Int 2013;13(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu H, Wang D, Zhang L, Xie X, Wu Y, Liu Y, et al. Upregulation of autophagy by hypoxia-inducible factor-1alpha promotes EMT and metastatic ability of CD133+ pancreatic cancer stem-like cells during intermittent hypoxia. Oncol Rep 2014;32(3):935–42. [DOI] [PubMed] [Google Scholar]
- 52.Maeda K, Ding Q, Yoshimitsu M, Kuwahata T, Miyazaki Y, Tsukasa K, et al. CD133 Modulate HIF-1alpha Expression under Hypoxia in EMT Phenotype Pancreatic Cancer Stem-Like Cells. Int J Mol Sci 2016;17(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuen A, Diaz B. The impact of hypoxia in pancreatic cancer invasion and metastasis. Hypoxia (Auckl) 2014;2:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McEwan C, Kamila S, Owen J, Nesbitt H, Callan B, Borden M, et al. Combined sonodynamic and antimetabolite therapy for the improved treatment of pancreatic cancer using oxygen loaded microbubbles as a delivery vehicle. Biomaterials 2016;80:20–32. [DOI] [PubMed] [Google Scholar]
- 55.McEwan C, Owen J, Stride E, Fowley C, Nesbitt H, Cochrane D, et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J Control Release 2015;203:51–6. [DOI] [PubMed] [Google Scholar]
- 56.Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, et al. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One 2014;9(3):e91551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto T, Yanagimoto H, Satoi S, Toyokawa H, Hirooka S, Yamaki S, et al. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas 2012;41(3):409–15. [DOI] [PubMed] [Google Scholar]
- 58.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006;12(18):5423–34. [DOI] [PubMed] [Google Scholar]
- 59.Yu F, Sharma S, Edwards J, Feigenbaum L, Zhu J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat Immunol 2015;16(2):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chellappa S, Hugenschmidt H, Hagness M, Line PD, Labori KJ, Wiedswang G, et al. Regulatory T cells that co-express RORgammat and FOXP3 are pro-inflammatory and immunosuppressive and expand in human pancreatic cancer. Oncoimmunology 2016;5(4):e1102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest 2011;121(11):4503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans A, Costello E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front Physiol 2012;3:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 2011;208(3):469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tassi E, Gavazzi F, Albarello L, Senyukov V, Longhi R, Dellabona P, et al. Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol 2008;181(9):6595–603. [DOI] [PubMed] [Google Scholar]
- 65.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004;4(8):583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wormann SM, Diakopoulos KN, Lesina M, Algul H. The immune network in pancreatic cancer development and progression. Oncogene 2014;33(23):2956–67. [DOI] [PubMed] [Google Scholar]
- 67.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res 2015;3(4):399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25(6):719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25(6):735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7(5):469–83. [DOI] [PubMed] [Google Scholar]
- 71.Dimcevski G, Kotopoulis S, Bjanes T, Hoem D, Schjott J, Gjertsen BT, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release 2016;243:172–81. [DOI] [PubMed] [Google Scholar]
- 72.Tran E, Robbins PF, Rosenberg SA. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol 2017;18(3):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116(20):4099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118(18):4817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365(8):725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385(9967):517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33(6):540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawaoka T, Oka M, Takashima M, Ueno T, Yamamoto K, Yahara N, et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep 2008;20(1):155–63. [PubMed] [Google Scholar]
- 80.Posey AD, Schwab RD Jr., Boesteanu AC, Steentoft C, Mandel U, Engels B, et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016;44(6):1444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33(12):1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med 2004;200(3):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.National Cancer Institute; National Institutes of Health Clinical Center. CAR T cell receptor immunotherapy targeting mesothelin for patients with metastatic cancer In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2000- [cited 17.03.16]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01583686 NLM Identifier: NCT01583686. [Google Scholar]
- 84.National Cancer Institute; National Institutes of Health Clinical Center. Immunotherapy using tumor infiltrating lymphocytes for patients with metastatic cancer In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2000- [cited 17.03.16]. Available from: https://clinicaltrials.gov/show/NCT01174121 NLM Identifier: NCT01174121. [Google Scholar]
- 85.Thind K, Padrnos LJ, Ramanathan RK, Borad MJ. Immunotherapy in pancreatic cancer treatment: a new frontier. Therap Adv Gastroenterol 2017;10(1):168–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kotteas E, Saif MW, Syrigos K. Immunotherapy for pancreatic cancer. J Cancer Res Clin Oncol 2016;142(8):1795–805. [DOI] [PubMed] [Google Scholar]
- 87.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg 2011;253(2):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res 2008;14(5):1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res 2012;18(3):858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Le DT, Ko AH, Wainberg ZA, Picozzi VJ, Kindler HL, Wang-Gillam A, et al. Results from a phase 2b, randomized, multicenter study of GVAX pancreas and CRS-207 compared to chemotherapy in adults with previously-treated metastatic pancreatic adenocarcinoma (ECLIPSE Study). Gastrointestinal Cancers Symposium; January 19, 2017; San Francisco, California. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29 -. Identifier NCT02243371, GVAX Pancreas Vaccine (With CY) and CRS-207 With or Without Nivolumab; 2016 Aug 31 [cited 17.05.24] Available from: https://clinicaltrials.gov/ct2/show/NCT02243371.
- 92.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499(7457):214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012;491(7424):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Egawa K Immuno-cell therapy of cancer in Japan. Anticancer Res 2004;24(5C):3321–6. [PubMed] [Google Scholar]
- 95.Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol 2013;4:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480(7378):480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 2017;36(4):439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arina A, Karrison T, Galka E, Schreiber K, Weichselbaum RR, Schreiber H. Transfer of Allogeneic CD4+ T Cells Rescues CD8+ T Cells in Anti-PD-L1-Resistant Tumors Leading to Tumor Eradication. Cancer Immunol Res 2017;5(2):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luheshi NM, Coates-Ulrichsen J, Harper J, Mullins S, Sulikowski MG, Martin P, et al. Transformation of the tumour microenvironment by a CD40 agonist antibody correlates with improved responses to PD-L1 blockade in a mouse orthotopic pancreatic tumour model. Oncotarget 2016;7(14):18508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen J, Xiao-Zhong G, Qi XS. Clinical Outcomes of Specific Immunotherapy in Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. J Immunol Res 2017;2017:8282391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Monjazeb AM, Hsiao HH, Sckisel GD, Murphy WJ. The role of antigen-specific and non-specific immunotherapy in the treatment of cancer. J Immunotoxicol 2012;9(3):248–58. [DOI] [PubMed] [Google Scholar]
- 102.Frenkel V Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev 2008;60(10):1193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia 2015;31(3):302–9. [DOI] [PubMed] [Google Scholar]
- 104.Li PZ, Zhu SH, He W, Zhu LY, Liu SP, Liu Y, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hepatobiliary Pancreat Dis Int 2012;11(6):655–60. [DOI] [PubMed] [Google Scholar]
- 105.Orsi F, Zhang L, Arnone P, Orgera G, Bonomo G, Vigna PD, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol 2010;195(3):W245–52. [DOI] [PubMed] [Google Scholar]
- 106.Sung HY, Jung SE, Cho SH, Zhou K, Han JY, Han ST, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas 2011;40(7):1080–6. [DOI] [PubMed] [Google Scholar]
- 107.Wang K, Chen Z, Meng Z, Lin J, Zhou Z, Wang P, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia 2011;27(2):101–7. [DOI] [PubMed] [Google Scholar]
- 108.Xiong LL, Hwang JH, Huang XB, Yao SS, He CJ, Ge XH, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP 2009;10(2):123–9. [PubMed] [Google Scholar]
- 109.Marinova M, Rauch M, Mucke M, Rolke R, Gonzalez-Carmona MA, Henseler J, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol 2016;26(11):4047–56. [DOI] [PubMed] [Google Scholar]
- 110.Zhao H, Yang G, Wang D, Yu X, Zhang Y, Zhu J, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs 2010;21(4):447–52. [DOI] [PubMed] [Google Scholar]
- 111.Dababou S, Marrocchio C, Rosenberg J, Bitton R, Pauly KB, Napoli A, et al. A meta-analysis of palliative treatment of pancreatic cancer with high intensity focused ultrasound. J Ther Ultrasound 2017;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ning ZY, Cheng CS, Xie J, Chen QW, Xu LT, Zhuang LP, et al. A retrospective analysis of survival factors of high intensity focused ultrasound (HIFU) treatment for unresectable pancreatic cancer. Discov Med 2016;21(118):435–45. [PubMed] [Google Scholar]
- 113.Zhao J, Zhao F, Shi Y, Deng Y, Hu X, Shen H. The efficacy of a new high intensity focused ultrasound therapy for locally advanced pancreatic cancer. J Cancer Res Clin Oncol 2017. [DOI] [PubMed] [Google Scholar]
- 114.Li X, Wang K, Zheng L, Meng Z. Retrospective analysis of high intensity focused ultrasound combined with S-1 in the treatment of metastatic pancreatic cancer after failure of gemcitabine. Am J Cancer Res 2016;6(1):84–90. [PMC free article] [PubMed] [Google Scholar]
- 115.Lv W, Yan T, Wang G, Zhao W, Zhang T, Zhou D. High-intensity focused ultrasound therapy in combination with gemcitabine for unresectable pancreatic carcinoma. Ther Clin Risk Manag 2016;12:687–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li CC, Wang YQ, Li YP, Li XL. High-intensity focused ultrasound for treatment of pancreatic cancer: a systematic review. J Evid Based Med 2014;7(4):270–81. [DOI] [PubMed] [Google Scholar]
- 117.Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol 2014;20(9):2267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rombouts SJ, Vogel JA, van Santvoort HC, van Lienden KP, van Hillegersberg R, Busch OR, et al. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surg 2015;102(3):182–93. [DOI] [PubMed] [Google Scholar]
- 119.Wang X, Sun J. High-intensity focused ultrasound in patients with late-stage pancreatic carcinoma. Chin Med J (Engl) 2002;115(9):1332–5. [PubMed] [Google Scholar]
- 120.Zhou Y High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pract 2014;2014:205325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu Z, Yang XY, Liu Y, Morse MA, Lyerly HK, Clay TM, et al. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. Biochem Biophys Res Commun 2005;335(1):124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hundt W, O’Connell-Rodwell CE, Bednarski MD, Steinbach S, Guccione S. In vitro effect of focused ultrasound or thermal stress on HSP70 expression and cell viability in three tumor cell lines. Acad Radiol 2007;14(7):859–70. [DOI] [PubMed] [Google Scholar]
- 123.Hundt W, Steinbach S, Burbelko M, Kiessling A, Rominger M, O’Connell-Rodwell CE, et al. Induction of luciferase activity under the control of an hsp70 promoter using high-intensity focused ultrasound: combination of bioluminescence and MRI imaging in three different tumour models. Technol Cancer Res Treat 2011;10(2):197–210. [DOI] [PubMed] [Google Scholar]
- 124.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet 2003;362(9382):469–76. [DOI] [PubMed] [Google Scholar]
- 125.Wang X, Ji J, Zhang H, Fan Z, Zhang L, Shi L, et al. Stimulation of dendritic cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget 2015;6(42):44688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology 2003;110(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bajzert J, Stefaniak T. [Heat shock protein HSP60 and the perspective for future using as vaccine antigens]. Postepy Hig Med Dosw (Online) 2015;69:1149–68. [DOI] [PubMed] [Google Scholar]
- 128.Ter Haar G, Mouratidis P, Repasky E. Definition of basic properties of physical immunotherapy in pancreatic cancer using HIFU and immune checkpoint inhibition. 5th International Symposium on Focused Ultrasound; 30 August 2016; North Bethesda, MD Unpublished results. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5123388/pdf/40349_2016_Article_76.pdf [accessed 17.05.06]. [Google Scholar]
- 129.Orsi F, Bonomo G, Della Vigna P, Mauri G, Varano G. The “abscopal” effect after USgHIFU treatment of advanced pancreatic cancer. 5th International Symposium on Focused Ultrasound; 30 August 2016; North Bethesda, MD. Unpublished results. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5123388/pdf/40349_2016_Article_76.pdf [accessed 17.05.06]. [Google Scholar]
- 130.Ungaro A, Orsi F, Casadio C, Galdy S, Spada F, Cella CA, et al. Successful palliative approach with high-intensity focused ultrasound in a patient with metastatic anaplastic pancreatic carcinoma: a case report. Ecancermedicalscience 2016;10:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dimitrov D, Andreev T, Feradova H, Ignatov B, Zhou K, Johnson C, et al. Multimodality treatment by FOLFOX plus HIFU in a case of advanced pancreatic carcinoma. A case report. JOP 2015;16(1):66–9. [DOI] [PubMed] [Google Scholar]
- 132.Lee JY, Choi BI, Ryu JK, Kim YT, Hwang JH, Kim SH, et al. Concurrent chemotherapy and pulsed high-intensity focused ultrasound therapy for the treatment of unresectable pancreatic cancer: initial experiences. Korean J Radiol 2011;12(2):176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee ES, Lee JY, Kim H, Choi Y, Park J, Han JK, et al. Pulsed high-intensity focused ultrasound enhances apoptosis of pancreatic cancer xenograft with gemcitabine. Ultrasound Med Biol 2013;39(11):1991–2000. [DOI] [PubMed] [Google Scholar]
- 134.Li T, Chen H, Khokhlova T, Wang YN, Kreider W, He X, et al. Passive cavitation detection during pulsed HIFU exposures of ex vivo tissues and in vivo mouse pancreatic tumors. Ultrasound Med Biol 2014;40(7):1523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li T, Wang YN, Khokhlova TD, D’Andrea S, Starr F, Chen H, et al. Pulsed High-Intensity Focused Ultrasound Enhances Delivery of Doxorubicin in a Preclinical Model of Pancreatic Cancer. Cancer Res 2015;75(18):3738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu MH, Lee JY, Kim HR, Kim BR, Park EJ, Kim HS, et al. Therapeutic Effects of Microbubbles Added to Combined High-Intensity Focused Ultrasound and Chemotherapy in a Pancreatic Cancer Xenograft Model. Korean J Radiol 2016;17(5):779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burks SR, Ziadloo A, Hancock HA, Chaudhry A, Dean DD, Lewis BK, et al. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One 2011;6(9):e24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kramer G, Steiner GE, Grobl M, Hrachowitz K, Reithmayr F, Paucz L, et al. Response to sublethal heat treatment of prostatic tumor cells and of prostatic tumor infiltrating T-cells. Prostate 2004;58(2):109–20. [DOI] [PubMed] [Google Scholar]
- 139.Liu HL, Hsieh HY, Lu LA, Kang CW, Wu MF, Lin CY. Low-pressure pulsed focused ultrasound with microbubbles promotes an anticancer immunological response. J Transl Med 2012;10:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Skalina K, Guha C. Stress response of cancer cells after low intensity focused ultrasound. 5th International Symposium on Focused Ultrasound; 30 August 2016; North Bethesda, MD. Unpublished results. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5123388/pdf/40349_2016_Article_76.pdf [accessed 17.05.06]. [Google Scholar]
- 141.Tebebi PA, Burks SR, Kim SJ, Williams RA, Nguyen BA, Venkatesh P, et al. Cyclooxygenase-2 or tumor necrosis factor-alpha inhibitors attenuate the mechanotransductive effects of pulsed focused ultrasound to suppress mesenchymal stromal cell homing to healthy and dystrophic muscle. Stem Cells 2015;33(4):1173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 2006;114(25):2823–30. [DOI] [PubMed] [Google Scholar]
- 143.Burks SR, Ziadloo A, Kim SJ, Nguyen BA, Frank JA. Noninvasive pulsed focused ultrasound allows spatiotemporal control of targeted homing for multiple stem cell types in murine skeletal muscle and the magnitude of cell homing can be increased through repeated applications. Stem Cells 2013;31(11):2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simon JC, Sapozhnikov OA, Khokhlova VA, Wang YN, Crum LA, Bailey MR. Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound. Phys Med Biol 2012;57(23):8061–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang YN, Khokhlova T, Bailey M, Hwang JH, Khokhlova V. Histological and biochemical analysis of mechanical and thermal bioeffects in boiling histotripsy lesions induced by high intensity focused ultrasound. Ultrasound Med Biol 2013;39(3):424–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Canney MS, Khokhlova VA, Bessonova OV, Bailey MR, Crum LA. Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound. Ultrasound Med Biol 2010;36(2):250–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khokhlova TD, Canney MS, Khokhlova VA, Sapozhnikov OA, Crum LA, Bailey MR. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. J Acoust Soc Am 2011;130(5):3498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu Z, Fowlkes JB, Rothman ED, Levin AM, Cain CA. Controlled ultrasound tissue erosion: the role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am 2005;117(1):424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maxwell AD, Wang TY, Cain CA, Fowlkes JB, Sapozhnikov OA, Bailey MR, et al. Cavitation clouds created by shock scattering from bubbles during histotripsy. J Acoust Soc Am 2011;130(4):1888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schade GR, Wang YN, S’Andrea S, Hwang JH, Liles WC, Bailey MR. Initial assessment of boiling histotripsy ablation of renal cell carcinoma in the Eker rat. International Society of Therapeutic Ultrasound; 18 April 2015; Utrecht, Netherlands. Unpublished results. Available from: http://www.istu.org/events/ann2015/abstractBook.pdf [accessed 17.05.06]. [Google Scholar]
- 151.Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol 2013;55(1):76–82. [DOI] [PubMed] [Google Scholar]
- 152.Schade GR, Wang YN, Pillarisetty V, Hwang JH, Khokhlova VA, Bailey MR, et al. Characterizing the immune response to boiling histotripsy ablation of renal carcinoma in the Eker rat. 5th International Symposium on Focused Ultrasound; 30 August 2016; North Bethesda, MD. Unpublished results. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5123388/pdf/40349_2016_Article_76.pdf [accessed 17.05.06]. [Google Scholar]
- 153.Yee C, Lizee G, Schueneman AJ. Endogenous T-Cell Therapy: Clinical Experience. Cancer J 2015;21(6):492–500. [DOI] [PubMed] [Google Scholar]
- 154.Xia JZ, Xie FL, Ran LF, Xie XP, Fan YM, Wu F. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol 2012;38(8):1363–71. [DOI] [PubMed] [Google Scholar]
- 155.Ran LF, Xie XP, Xia JZ, Xie FL, Fan YM, Wu F. Specific antitumour immunity of HIFU-activated cytotoxic T lymphocytes after adoptive transfusion in tumour-bearing mice. Int J Hyperthermia 2016;32(2):204–10. [DOI] [PubMed] [Google Scholar]
- 156.Xing Y, Lu X, Pua EC, Zhong P. The effect of high intensity focused ultrasound treatment on metastases in a murine melanoma model. Biochem Biophys Res Commun 2008;375(4):645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med 2007;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chevillet JR, Khokhlova TD, Giraldez MD, Schade GR, Starr F, Wang YN, et al. Release of Cell-free MicroRNA Tumor Biomarkers into the Blood Circulation with Pulsed Focused Ultrasound: A Noninvasive, Anatomically Localized, Molecular Liquid Biopsy. Radiology 2017;283(1):158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khokhlova VA, Fowlkes JB, Roberts WW, Schade GR, Xu Z, Khokhlova TD, et al. Histotripsy methods in mechanical disintegration of tissue: towards clinical applications. Int J Hyperthermia 2015;31(2):145–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khokhlova TD, Wang YN, Simon JC, Cunitz BW, Starr F, Paun M, et al. Ultrasound-guided tissue fractionation by high intensity focused ultrasound in an in vivo porcine liver model. Proc Natl Acad Sci U S A 2014;111(22):8161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang X, Yuan F, Liang M, Lo HW, Shinohara ML, Robertson C, et al. M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer. PLoS One 2012;7(7):e41632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Y, Deng J, Feng J, Wu F. Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound. World J Gastroenterol 2010;16(28):3584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Unga J, Hashida M. Ultrasound induced cancer immunotherapy. Adv Drug Deliv Rev 2014;72:144–53. [DOI] [PubMed] [Google Scholar]
- 164.Sakakima Y, Hayashi S, Yagi Y, Hayakawa A, Tachibana K, Nakao A. Gene therapy for hepatocellular carcinoma using sonoporation enhanced by contrast agents. Cancer Gene Ther 2005;12(11):884–9. [DOI] [PubMed] [Google Scholar]
- 165.Zolochevska O, Xia X, Williams BJ, Ramsay A, Li S, Figueiredo ML. Sonoporation delivery of interleukin-27 gene therapy efficiently reduces prostate tumor cell growth in vivo. Hum Gene Ther 2011;22(12):1537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Suzuki R, Oda Y, Utoguchi N, Namai E, Taira Y, Okada N, et al. A novel strategy utilizing ultrasound for antigen delivery in dendritic cell-based cancer immunotherapy. J Control Release 2009;133(3):198–205. [DOI] [PubMed] [Google Scholar]
- 167.Oda Y, Suzuki R, Otake S, Nishiie N, Hirata K, Koshima R, et al. Prophylactic immunization with Bubble liposomes and ultrasound-treated dendritic cells provided a four-fold decrease in the frequency of melanoma lung metastasis. J Control Release 2012;160(2):362–6. [DOI] [PubMed] [Google Scholar]
- 168.Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release 2010;142(2):245–50. [DOI] [PubMed] [Google Scholar]
- 169.Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Suppression of melanoma growth and metastasis by DNA vaccination using an ultrasound-responsive and mannose-modified gene carrier. Mol Pharm 2011;8(2):543–54. [DOI] [PubMed] [Google Scholar]
- 170.Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy. Biomaterials 2010;31(30):7813–26. [DOI] [PubMed] [Google Scholar]