Abstract
Objective:
Diazoxide is used to treat infants with persistent hypoglycemia, but the prevalence of its use and adverse effects are not well described. We report demographic and clinical characteristics of infants treated with diazoxide in neonatal intensive care units (NICUs).
Study design:
Retrospective cohort study of infants 24–41 weeks’ gestation admitted to 392 NICUs from 1997–2016, comparing characteristics between hypoglycemic infants exposed/not exposed to diazoxide. For diazoxide courses >1 day, we report percentages of infants starting diuretics and/or developing new ventilator/oxygen requirement during therapy.
Results:
Among 1,249,466 infants, 185,832 had hypoglycemia; 1066/185,832 (0.57%) received diazoxide. Diazoxide use increased over time (p=0.001). Infants receiving diazoxide varied from 0%−14.9% among centers. New diuretic courses were associated with 91/664 (14%), and new oxygen or ventilator requirement during therapy was associated with 64/556 (12%) and 34/647 (5%), respectively.
Conclusions:
Diazoxide use in NICU settings has increased over time. Infants receiving diazoxide commonly received diuretics.
INTRODUCTION
Hypoglycemia is common in newborns, and has been associated with long-term brain damage and neurocognitive delays.1–3 In some newborns, hypoglycemia resolves quickly as the transition is made from a continuous trans-placental supply of glucose to intermittent glucose exposure through feedings.4 In most infants, blood glucose levels improve spontaneously and hypoglycemia resolves within the first few postnatal days2,5; however, up to 15% of term infants experience more severe or persistent hypoglycemia.6 Neonatal risk factors for prolonged hypoglycemia include birth asphyxia, small or large birth weight for gestational age, and pre- and post-term birth.2 Among infants who continue to experience persistent hypoglycemia beyond the first few postnatal days, other causes of hypoglycemia may include transient hyperinsulinism, congenital hypopituitarism, and congenital hyperinsulinism.2,7 Transient neonatal hyperinsulinism is the most common of these conditions and may last several months; it usually resolves spontaneously by 6 months of age.7 There are currently no drugs approved by the U.S. Food and Drug Administration (FDA) for the treatment of transient neonatal hyperinsulinism.
Diazoxide is benzothiadiazide derivative previously studied as an antihypertensive medication and approved by the FDA for use in children and infants for a specific subset of conditions, including symptomatic hyperinsulinemic hypoglycemia due to leucine sensitivity (caused by missense mutations in the HADH and GLUD1 genes), islet cell hyperplasia, extrahepatic malignancy, islet cell adenoma, and adenomatosis.8 Diazoxide binds to KATP receptors on pancreatic β-cells, hyperpolarizes the cell membrane, and prevents release of insulin.1,7 Diazoxide is often used as a first-line treatment for all types of neonatal hyperinsulinemic hypoglycemia, including transient neonatal hyperinsulinism, despite the lack of a labeled indication for many of these conditions.4,9–11
Adverse effects associated with diazoxide therapy are common and include edema, hyperuricemia, tachycardia, hypertrichosis, leukopenia, feeding intolerance, pulmonary hypertension, and heart failure.1,4,7,8,12,15,16 In 2015, the FDA issued a warning regarding symptoms of pulmonary hypertension observed in infants exposed to diazoxide in 2015.17 Diazoxide can cause sodium retention and reduce free water clearance, leading to fluid retention requiring diuretic therapy.4,10,11,13,14
Few studies have reported the prevalence of these adverse effects of diazoxide in infants. In addition, few data exist regarding the number of infants who continue diazoxide following discharge from the neonatal intensive care unit (NICU). In this retrospective cohort analysis, we sought to characterize the prevalence and duration of diazoxide therapy in infants diagnosed with hypoglycemia in NICUs. We also examined demographic and clinical factors associated with diazoxide treatment and the prevalence of adverse events among treated infants.
METHODS
Study design and setting
We performed a retrospective cohort study of infants discharged from any of 392 NICUs managed by the Pediatrix Medical Group from 1997–2016. Data were obtained from the Pediatrix Medical Group Clinical Data Warehouse, an electronic medical record that functions as a tool for research and quality improvement. Demographic and clinical data were abstracted from the medical record, and all patient-identifying data were eliminated from this dataset.18 Small for gestational age (SGA) status and large for gestational age (LGA) status were determined using the Olsen definitions.19 The study was approved by the Duke Health Institutional Review Board with a waiver of informed consent.
Definitions
Hypoglycemic status was indicated by clinician diagnosis in the electronic progress note. New diuretics, oxygen, or ventilator exposure during diazoxide therapy (not including the start date of diazoxide) was defined as initiation following the start date of diazoxide. Specific diuretics included were acetazolamide, bumetanide, chlorothiazide, furosemide, hydrochlorothiazide, and spironolactone. Infants were classified as receiving diazoxide at discharge if they received diazoxide on the day of discharge or the day before discharge. Patent ductus arteriosus (PDA) was defined by clinician diagnosis. Sepsis was defined as a positive blood, cerebrospinal fluid, or catheterized urine culture, excluding typical contaminant organisms and including probable or definite coagulase-negative Staphylococcus infections.20 Necrotizing enterocolitis (NEC) was defined by clinician diagnosis of suspected or proven medical or surgical NEC. Pulmonary hypertension was defined by a new clinician diagnosis in progress notes or the new use of a pulmonary antihypertensive medication (inhaled nitric oxide, sildenafil, epoprostenol sodium, or bosentan) following the start date of diazoxide through the end of therapy.
Statistical analysis
We examined the prevalence and trend of the diagnosis of hypoglycemia and the use of diazoxide over time. We tested for significant change over time using a nonparametric test for trend21 We evaluated the prevalence of diazoxide use in infants diagnosed with hypoglycemia by center in centers that discharged ≥100 hypoglycemic infants during the study period. Among infants diagnosed with hypoglycemia, we reviewed the number treated with diazoxide. We compared the following demographic and clinical variables between hypoglycemic infants exposed and not exposed to diazoxide using the chi-square test: gestational age, birth weight, prenatal steroid use, sex, race/ethnicity, SGA, LGA, inborn, length of stay, and those that died. We also reviewed start dates of diazoxide administration and length of treatment for those courses with known end dates.
We reported outcomes following exposure to diazoxide including new diuretic exposure, ventilator exposure, or oxygen requirements among infants who were not receiving those therapies on the date that diazoxide was started. Among those with these outcomes, we reported the percentage of infants who developed PDA or sepsis during diazoxide therapy, factors which could also lead to worsening respiratory status. We also reported the percentage of infants who developed suspected or proven NEC during diazoxide therapy. We reported the number of infants diagnosed with pulmonary hypertension during diazoxide therapy. Of those hypoglycemic infants who were exposed to diazoxide and who were discharged home (i.e., did not die and were not transferred), we reported the percentage of infants discharged on diazoxide. We compared clinical characteristics between infants who were discharged on diazoxide and those who discontinued diazoxide prior to discharge.
P values <0.05 were considered significant. Statistical analysis was performed using Stata version 15 (College Station, TX).
RESULTS
A total of 1,249,466 infants were admitted to 392 NICUs during the study period. Of these, 185,832/1,249,466 (15%) infants were diagnosed with hypoglycemia. Among all infants, 1125/1,249,466 (0.09%) were exposed to diazoxide, and 1066 (95%) of these were also diagnosed with hypoglycemia. The percentage of infants treated with diazoxide and the percentage of infants with hypoglycemia increased significantly during the study period of 1997– 2016 (P=0.001 and P=0.04, respectively; Figure 1). The percentage of infants with hypoglycemia exposed to diazoxide varied from 0% to 14.9% among centers (Figure 2).
Figure 1.
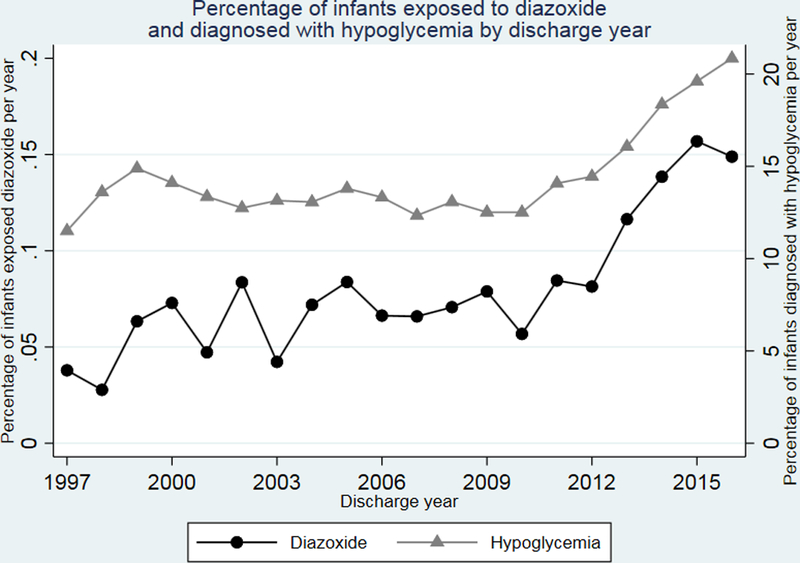
Percentage of infants 1) exposed to diazoxide and 2) diagnosed with hypoglycemia by discharge year.
Figure 2.
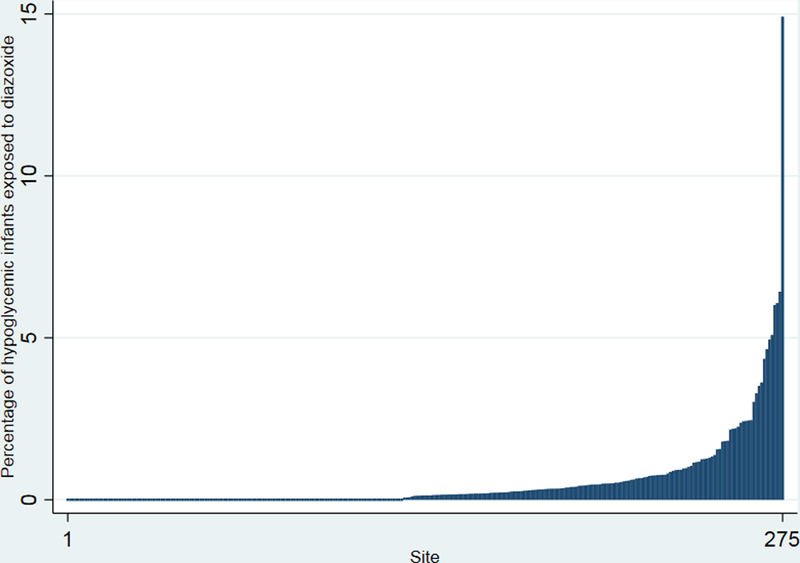
Percentage of infants exposed to diazoxide by site.
Among hypoglycemic infants, median (25th, 75th percentile) gestational age and birth weight were 37 weeks (34, 38) and 2683 g (1927, 3522), respectively in the group exposed to diazoxide and 36 weeks (33, 38) and 2513 g (1855, 3344), respectively, in the group not exposed to diazoxide. Median postnatal age at the start of diazoxide therapy was 6 days (2, 15). The median total diazoxide duration of exposure was 6 days (4, 10) for infants. On univariable analysis, the prevalence of diazoxide exposure differed significantly across gestational age, birth weight, and race/ethnicity groups, and male infants were more likely to be exposed (Table 1). Infants exposed to diazoxide were more likely to be SGA (29% vs. 17%; P< 0.001) or LGA (25% vs. 18%; P<0.001) compared with those not exposed. Antenatal steroid exposure was less common among infants exposed to diazoxide (21% vs. 30%; P<0.001).
>Table 1.
Demographics of hypoglycemic infants exposed to diazoxide.*
|
Exposed to diazoxide (n = 1066) |
Not exposed to diazoxide (n= 184,766) |
P value | |
|---|---|---|---|
| Gestational age (weeks) | <0.001 | ||
| <32 | 16 | 21 | |
| 33–36 | 31 | 38 | |
| >36 | 53 | 41 | |
| Birth weight (kg) | <0.001 | ||
| <1500 | 15 | 16 | |
| 1500–2499 | 28 | 34 | |
| 2500–3499 | 32 | 29 | |
| ≥ 3500 | 25 | 21 | |
| Prenatal steroids | 21 | 30 | <0.001 |
| Male sex | 62 | 56 | <0.001 |
| Race/ethnicity | <0.001 | ||
| White | 49 | 52 | |
| Black | 26 | 19 | |
| Hispanic | 18 | 23 | |
| Other | 7 | 6 | |
| Small for gestational age | 29 | 17 | <0.001 |
| Large for gestational age | 25 | 18 | <0.001 |
| Inborn | 67 | 90 | <0.001 |
| Died | 2 | 3 | 0.52 |
All values expressed as percentages.
A substantial number of infants received new exposure to diuretics, supplemental oxygen, or ventilation following the start of diazoxide therapy (Table 2). PDA or sepsis was also diagnosed in 10/91 infants with new exposure to diuretics, 7/64 infants with new supplemental oxygen, and 0/34 infants with new ventilator courses during diazoxide therapy. For infants exposed to diazoxide 55/1066 (5%) were also started on diuretics on the same date. In those exposed to diazoxide, 24/1066 (2%) were diagnosed with pulmonary hypertension at a median of 8 days (4, 18) following exposure. NEC was reported in 10/1066 following diazoxide exposure, and 9 of those infants were less than 37 weeks’ gestational age.
Table 2.
Use of diuretics and ventilator support during first course of diazoxide treatment among hypoglycemic infants
|
Started during therapy
n/N (%) |
|
|---|---|
| Diuretic exposure | 91/644 (14)* |
| Oxygen exposure | 64/566 (12)† |
| Ventilator requirement | 34/647 (5)‡ |
Total does not include 57 infants who were on a ventilator at the start of therapy.
Total does not include 43 infants who were on diuretics at the start of diazoxide therapy.
Total does not include 139 infants who were on oxygen at the start of therapy.
Of 1066 hypoglycemic infants exposed to diazoxide, 904 (84%) were discharged home, 141 (13%) were transferred, and 21 (2%) died. Of these, 68/868 (8%) were receiving diazoxide at discharge. Thirty-six infants were missing the end date of diazoxide and we could not determine whether the infant was discharged home on diazoxide. The 68 infants known to be on diazoxide at time of discharge were more likely to be >36 weeks of age than infants who discontinued diazoxide prior to discharge (47/68 [69%] vs. 426/798 [53%]; P=0.005). There was no significant difference in other demographic characteristics between infants discharged on diazoxide and infants who discontinued diazoxide prior to discharge (data not shown). The median course duration of diazoxide prior to discharge for infants discharged on diazoxide was 6 days (4, 9). Of 68 infants receiving diazoxide at discharge at 37 of 151 sites, 4 (6%) were discharged home on diuretics as well. The median length of stay for infants exposed to diazoxide was 19 days (12, 37).
DISCUSSION
In this study of the largest single cohort of newborn infants exposed to diazoxide, we found that the percentage of infants in the NICU treated with diazoxide increased significantly over the study period and some infants can experience significant morbidities. Most infants treated with diazoxide were late preterm and term infants, with only 16% of treated infants being ≤32 weeks gestational age. Most infants (92%) completed therapy with diazoxide prior to discharge.
The prevalence of diazoxide exposure increased over the study period and is much higher than the 1/50,000 incidence of congenital hyperinsulinism secondary to a genetic defect for which diazoxide use is approved.2,6 Our data also suggest there are variations in use by center. The center variation of 0–14.9% noted in our study cohort may reflect varying levels of comfort with off-label use of a drug for a problem with increasing prevalence.
The frequency of hypoglycemia as a diagnosis for NICU-admitted infants increased over the 18-year study period, almost doubling in the last 5 years. This could be attributable to the guidelines published by the American Academy of Pediatrics in 2011 on criteria and treatment of hypoglycemia in newborns.3 Even though the specific diagnosis of transient neonatal hypoglycemia was not available for the infants in our study, this diagnosis is far more likely to be the cause of hypoglycemia than genetic hyperinsulinism. A study of 218 infants with diazoxide-responsive hyperinsulinemic hypoglycemia was able to identify a genetic cause for only 27% of the infants.22 While these data provide some evidence to suggest that hyperinsulinemic hypoglycemia secondary to causes not described in the FDA-approved label may be responsive to diazoxide, further study is warranted to determine effectiveness.
We evaluated several risk factors linked with neonatal hypoglycemia for an association with diazoxide treatment, including gestational age, LGA and SGA status, and antenatal steroid use. In a single-center, randomized controlled trial of 514 infants, 51% of at-risk infants (including late preterm infants, infants of diabetic mothers, and LGA and SGA infants) experienced hypoglycemia.6 Approximately half of treated infants in our study were small or large for gestational age. Both SGA and LGA infants are among those at highest risk for neonatal hypoglycemia,3,23–28 and the number of LGA infants is expected to increase with the increasing prevalence of gestational diabetes.24,25 Birth timing is also an important factor affecting the glycemic status of the neonate, with preterm infants at higher risk of hypoglycemia. The number of late preterm births increased for the second straight year in a row, from 6.8% (2014) to 6.9% (2015) to 7.1% (2016).26 As the prevalence of each of these risk factors grows, more infants will be at risk for hypoglycemia.
Although transient neonatal hypoglycemia is responsive to diazoxide,7,10,11,22 the potential adverse effects of this therapy must be considered.29–31The success of diazoxide therapy in infants has been measured by its ability to maintain appropriate glucose levels and low levels of insulin at the end of an age-appropriate fast.1 Diazoxide causes sodium and chloride retention in the renal tubules and decreases bicarbonate excretion.29,30 Free water clearance is also reduced; the use of thiazide diuretics such as chlorothiazide can mitigate this action.30 In our study, 14% of infants received treatment with diuretics, 12% with supplemental oxygen, and 5% were exposed to ventilation support after initiating diazoxide therapy. Although we did not have data regarding the indication for diuretic therapy or respiratory intervention, these new exposures could have been secondary to the properties of diazoxide that promote fluid retention.
Several smaller studies have been performed to evaluate the safety of diazoxide, one of which was a 1989 observational study of diazoxide for the treatment of neonatal hypoglycemia. In a study of seven term infants who received doses ranging from 12–24 mg/kg/day, no excessive weight gain was reported.12 All infants in that study received the diuretic chlorothiazide during therapy. Two infants needed mechanical ventilation with substantial oxygen requirements after initiation of diazoxide. Each showed evidence of heart failure, defined as a new murmur, tachypnea, and tachycardia, which did not appear to be dose-dependent. All symptoms resolved within 2–4 days following discontinuation of diazoxide.12
A recent review of multiple case reports and case series evaluated side effects reported in 1216 infants with congenital hyperinsulinemia.10 Diazoxide was the most common medication prescribed (84%). Hypertrichosis was the most common adverse effect associated with diazoxide use, followed by fluid retention. The review did not report the number of infants who required escalation of respiratory support. The authors recommended combining a diuretic with higher-dose diazoxide therapy or if cardiac risk factors were present. Cardiotoxicity and primary pulmonary hypertension have been suspected in association with diazoxide use, but may be more attributable to the secondary fluid retention causing increased right ventricular preload.10 Up to 12% of infants experienced GI symptoms such as decreased appetite, nausea, and vomiting but the authors did not comment on the development of more serious conditions such as NEC. Our study did not find a strong association with the development of NEC following diazoxide exposure. While the population size of this review was comparable to our study, our study was specific to infants in NICUs, and included neonates with persistent hypoglycemia without the diagnosis of congenital hyperinsulinemia.
Diazoxide toxicity leading to cardiac and pulmonary dysfunction remains a concern. Our study found a small number of infants who were diagnosed with pulmonary hypertension following exposure to diazoxide. Other researchers have suspected lower serum protein levels and higher doses of protein-bound diazoxide may contribute to this diagnosis.12,30,33 A 2004 case study of an infant treated for prolonged hypoglycemia reported fluid overload, tachypnea, and cardiac and pulmonary failure associated with diazoxide therapy administered at a dose of 17 mg/kg/day, higher than the recommended dose range of 8–15 mg/kg/day.34
Another retrospective study of infants with transient hyperinsulinemic hypoglycemia found approximately 20% of the infants treated with diazoxide suffered from circulatory problems, defined as edema, low urine output, or reopening of the ductus arteriosus. The authors proposed that the fluid retention caused by diazoxide use and the agonist effect on the KATP channels needed for duct closure could contribute to this latter complication,31 which affected three infants in the review. Two of these were very low birth weight infants, both of whom received PDA ligation. Dosages of diazoxide were not available in that report. Very few instances of increased respiratory support in our study were associated with PDA or sepsis following diazoxide exposure.
The strengths of our study include the large sample size, which comprised >1M infants admitted to U.S. NICUs. The sample size enhanced our ability to detect specific adverse events and escalation of interventions following diazoxide exposure. Our results also highlight the incidence of new respiratory support requirements following exposure to diazoxide.
Limitations of our approach include a lack of dosage data, which left us unable to comment on the correlation of dosage with the adverse effects reported. We were unable to determine the indication for new diuretic therapy and respiratory support while on diazoxide therapy; it is possible that these occurrences were unrelated to diazoxide therapy. We were also unable to explore the effectiveness of diazoxide for the treatment of hypoglycemia by review of blood glucose levels, and we did not have information regarding the administration of other interventions (such as dextrose gel or glucose infusion rate) that may have affected the prevalence of diazoxide use. Because we were unable to quantify the resistance of hypoglycemia to intervention and the decision to prescribe diazoxide was likely affected by many such variables that were not available, we were unable to identify a reliable comparison group for the purpose of comparing prevalence of adverse events. However, our study provides preliminary data on the prevalence of adverse events among infants who receive diazoxide, which may be used the development of a future prospective study. In addition, our data do not include information gathered after hospital discharge, and we were therefore unable to examine the long-term effects of diazoxide for infants who require therapy in the NICU.
In conclusion, our study shows that diazoxide use in infants hospitalized in the nearly 400 U.S. NICUs included our study has increased during the interval from 1997 to 2016, and that infants receiving diazoxide commonly require treatment with diuretics. Randomized controlled trials or prospective cohort studies are needed to examine the effectiveness of diazoxide use in the treatment of hypoglycemia and to provide more detailed information on adverse effects associated with its use.
ACKNOWLEDGEMENTS
Source of funding
This work was funded under National Institute of Child Health and Human Development (NICHD) contract HHSN275201000003I (principal investigator: Benjamin) for the Pediatric Trials Network. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Dr. D.K. Benjamin receives support from the National Institutes of Health (award 2K24HD058735–06, National Institute of Child Health and Human Development (HHSN275201000003I), National Institute of Allergy and Infectious Diseases (HHSN272201500006I), ECHO Program (1U2COD023375–01), and the National Center for Advancing Translational Sciences (1U24TR001608–01); he also receives research support from Cempra Pharmaceuticals (subaward to HHSO100201300009C) and consulting payments from AstraZeneca, Cempra, Shionogi Inc., The Medicines Company, Allergan, Astellas Pharma, Cidara Therapeutics, Purdue Pharma, and UCB Biosciences, Inc. Dr. R. Benjamin serves on the Speaker’s Bureau for Pfizer. Dr. Clark is an employee of Pediatrix Medical Group, Inc. Dr. Cotten receives grant funding from the NIH (5U10 HD040492–16 and 1R01-EY025009–01A1). Dr. Greenberg receives salary support for research from NIH awards (HHSN 275201000003I, HHSN 272201300017I, HHSN200201253663), and from the Food and Drug Administration (HHSF223201610082C).
Footnotes
CONFLICT OF INTEREST
None of the authors notes a conflict of interest relevant to this manuscript.
REFERENCES
- 1.Güemes M, Hussain K. Hyperinsulinemic hypoglycemia. Pediatr Clin North Am 2015;62(4):1017–1036. [DOI] [PubMed] [Google Scholar]
- 2.Thornton PS, Stanley CA, De Leon DD, Harris D, Haymond MW, Hussain K, et al. Recommendations from the Pediatric Endocrine Society for Evaluation and Management of Persistent Hypoglycemia in Neonates, Infants, and Children. J Pediatr 2015;167(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamkin DH; Committee on Fetus and Newborn. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011;127(3):575–579. [DOI] [PubMed] [Google Scholar]
- 4.Sweet CB, Grayson S, Polak M. Management strategies for neonatal hypoglycemia. J Pediatr Pharmacol Ther 2013;18(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanley CA, Rozance PJ, Thornton PS, De Leon DD, Harris D, Haymond MW, et al. Re-evaluating “transitional neonatal hypoglycemia”: mechanism and implications for management. J Pediatr 2015;166(6):1520–5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr 2012;161(5):787–791. [DOI] [PubMed] [Google Scholar]
- 7.Hoe FM, Thornton PS, Wanner LA, Steinkrauss L, Simmons RA, Stanley CA. Clinical features and insulin regulation in infants with a syndrome of prolonged neonatal hyperinsulinism. J Pediatr 2006;148(2):207–212. [DOI] [PubMed] [Google Scholar]
- 8.National Library of Medicine. DaileyMed. Package insert: Proglycem-diazoxide suspension. October 2, 2015. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b16c7832-2fd9-49af-b923-1dc0d91fd6e2. Accessed Dec. 4, 2017.
- 9.Rozenkova K, Guemes M, Shah P, Hussain K. The diagnosis and management of hyperinsulinaemic hypoglycaemia. J Clin Res Pediatr Endocrinol 2015;7(2):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welters A, Lerch C, Kummer S, Marquard J, Salgin B, Mayatepek E, et al. Long- term medical treatment in congenital hyperinsulinism: a descriptive analysis in a large cohort of patients from different clinical centers. Orphanet J Rare Dis 10:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu S, Xu Z, Yan J, Liu M, Sun B, Li W, et al. The treatment effect of diazoxide on 44 patients with congenital hyperinsulinism. J Pediatr Endocrinol Metab 2012;25(11– 12):1119–1122. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Osba YK, Manasra KB, Mathew PM. Complications of diazoxide treatment in persistent neonatal hyperinsulinism. Arch Dis Child 1989;64(10):1496–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozance PJ. Update on neonatal hypoglycemia. Curr Opin Endocrinol Diabetes Obes 2014;21(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillies DR. Complications of diazoxide in the treatment of nesidioblastosis. Arch Dis Child 1985;60(5):500–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nebesio TD, Hoover WC, Caldwell RL, Nitu ME, Eugster EA. Development of pulmonary hypertension in an infant treated with diazoxide. J Pediatr Endocrinol Metab 2007;20(8):939–944. [DOI] [PubMed] [Google Scholar]
- 16.Demirel F, Unal S, Cetin, II, Esen I, Arasli A. Pulmonary hypertension and reopening of the ductus arteriosus in an infant treated with diazoxide. J Pediatr Endocrinol Metab 2011;24(7–8):603–605. [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. FDA Drug Safety Podcast: FDA warns about a serious lung condition in infants and newborns treated with Proglycem (diazoxide). July 16, 2015. https://www.fda.gov/Drugs/DrugSafety/DrugSafetyPodcasts/ucm455659.htm
- 18.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system--tools for “meaningful use” in continuous quality improvement. Clin Perinatol 2010;37(1):49–70. [DOI] [PubMed] [Google Scholar]
- 19.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics 2010;125(2):e214–24. [DOI] [PubMed] [Google Scholar]
- 20.Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK, Smith PB Jr., Manzoni P, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev 2012;88(Suppl 2):S69–74.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuzick J A Wilcoxon-type test for trend. Stat Med 1985;4(1):87–90. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan SE, Kapoor RR, Mali G, Cody D, Murphy N, Schwahn B, et al. Diazoxide- responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations. Eur J Endocrinol 2010;162(5):987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rozance PJ, Hay WW Jr, New approaches to management of neonatal hypoglycemia. Matern Health Neonatol Perinatol 2016;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erjavec K, Poljicanin T, Matijevic R. Impact of the implementation of new WHO diagnostic criteria for gestational diabetes mellitus on prevalence and perinatal outcomes: A population-based study. J Pregnancy 2016;2016:2670912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Global Health Observatory (GHO) data 2016 – Overweight and obesity. Available from: http://www.who.int/gho/ncd/risk_factors/overweight/en/. Accessed Dec. 4, 2017.
- 26.Martin JA, Hamilton BE, Osterman MJ. Births in the United States, 2016. NCHS Data Brief 2017. September;(287):1–8. [PubMed] [Google Scholar]
- 27.Collins JE, Leonard JV, Teale D, Marks V, Williams DM, Kennedy CR, et al. Hyperinsulinaemic hypoglycaemia in small for dates babies. Arch Dis Child 1990;65(10):1118–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto JM, Kallas-Koeman MM, Butalia S, Lodha AK, Donovan LE. Large-for- gestational-age (LGA) neonate predicts a 2.5-fold increased odds of neonatal hypoglycaemia in women with type 1 diabetes. Diabetes Metab Res Rev 2017;33(1). [DOI] [PubMed] [Google Scholar]
- 29.Bartorelli C, Gargano N, Leonetti G, Zanchetti A. Hypotensive and renal effects of diazoxide, a sodiumretaining benzothiadiazine compound. Circulation 1963;27:895– 903. [DOI] [PubMed] [Google Scholar]
- 30.Hutcheon DE, Barthalmus KS. Antihypertensive action of diazoxide. A new benzothiazine with antidiuretic properties. Br Med J 1962;2(5298):159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green TP. Systemic vasodilatation and renal sodium excretion: effects of hydralazine and diazoxide. Life Sci 1984;34(22):2169–2176. [DOI] [PubMed] [Google Scholar]
- 32.Tas E, Mahmood B, Garibaldi L, Sperling M. Liver injury may increase the risk of diazoxide toxicity: a case report. Eur J Pediatr 2015;174(3):403–406. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida K, Kawai M, Marumo C, Kanazawa H, Matsukura T, Kusuda S, et al. High prevalence of severe circulatory complications with diazoxide in premature infants. Neonatology 2014;105(3):166–171. [DOI] [PubMed] [Google Scholar]
- 34.Silvani P, Camporesi A, Mandelli A, Wolfler A, Salvo I. A case of severe diazoxide toxicity. Paediatr Anaesth 2004;14(7):607–609. [DOI] [PubMed] [Google Scholar]


