Abstract
Background:
Rising healthcare costs have led to increased focus on the need to achieve a higher “value of care.” As value-maximization efforts expand to include more complex surgical patients, evidence to support meaningful implementation of complication-based initiatives is lacking. The objective of this study was to compare incremental costs of complications following major gastrointestinal (GI) resections for organ-specific malignant neoplasia using nationally-representative data.
Methods:
National (Nationwide) Inpatient Sample data, 2001-2014, were queried for adult (≥18y) patients undergoing major resections for malignant neoplasia. Based on system-based complications considered relevant to the long-term treatment of GI disease, stratified differences in risk-adjusted incremental hospital costs and complication probabilities were compared. Differences in surgical outcomes and costs over time were also assessed.
Results:
A total of 293,967 patients were included, weighted to represent 1,408,117 patients nationwide. One-fourth (26.1%, 95%CI: 25.7-26.4%) experienced ≥1 pre-discharge complication (range: 45.3% esophagectomy to 24.0% rectal resection). Resultant annual risk-adjusted incremental hospital costs totaled $540 million nationwide (19.5% of the overall cost of care and an average of $20,900 per-patient). Costs varied substantially with both cancer/resection type and complication group, ranging from $76.7 million for colectomies with infectious complications to $0.2 million for rectal resections with urinary complications. For each resection type, infectious ($154.7 million), GI ($85.5 million), and pulmonary ($77.9 million) complications were among the most significant drivers of increased hospital cost.
Conclusions:
Quantifying and comparing the impact of complications on an indication-specific level in more complex patients offers an important step toward allowing providers/payers to meaningfully prioritize the design of novel and adaptation of existing value-maximization approaches.
Keywords: complication, value, quality, cost, oncology, cancer, infection
Introduction
In 2015, healthcare spending in the United States (US) reached a record high of $3.2 trillion1—a number that is projected to rise by an average of 5.8% per year,2 grow at a rate nearly five-times that of the current US total gross domestic product (GDP),3 and reach >20% of the US GDP by 2025.2 Already a source of national concern, rising healthcare costs have led to increased focus on the need to achieve a higher “value of care.”4 Defined as the sum of quality and outcomes divided by cost,4 the concept of value represents a somewhat nebulous notion of the need to achieve a better understanding of the association between costs and quality. Balance between cost and quality, combined with an awareness of how they affect patient outcomes, is critical when considering cost-reduction strategies capable of simultaneously maximizing the quality of care.5-10
Prior approaches to improve value in surgery have increased awareness of healthcare spending and changed how providers conceptualize efforts to account for quality.10 However, many value-maximization approaches, including pay-for-performance (receipt of financial/non-financial (dis)incentives based on outcomes achieved in line with predetermined metrics), have been widely criticized.8, 10-12 Government programs13 based on pay-for-performance metrics have led to questions about which hospitals and patients they most affect.14-16 Other approaches, such as the use of bundled payments (payment for an episode of care versus individual line items)17-19 and accountable care organizations (groups of hospitals/providers who coordinate their efforts)20-22 are thought to have the potential to be viable surgical reimbursement models.18 Although, future studies and “frameworks to characterize the strategic advantages and disadvantages” of each are needed in order to understand their full impact.22 Lacking such a framework, evidence supporting effective prioritization remains scarce at a time where the success of value-maximization depends on an ability to account for what leads to the growing and high healthcare costs observed.10
Among cancer patients where annual spending is projected to reach $173 billion dollars within three years,23 relative increases in index hospital costs upwards of 50% have been reported when complications occur.24 For general surgery patients, complications are thought to play an equally critical role, increasing both hospital costs and the probability of poor patient outcomes.6,7 Expanding efforts to promote value within both groups will have to take into account complications in order to allocate resources in evidence-based ways. The challenge lies in knowing how it ought to be done. Prior efforts to guide value-maximization around complications in surgery have often been limited by a lack of standardized definitions and complication groupings. These data have created only a limited understanding of the interplay between complications, costs, and outcomes on a national scale.
Thus, today, as the focus of surgical value-maximization begins to expand from its initial emphasis on colectomy and sub-specialty CABG and THA/TKA care to include a broader array of surgical procedures,25,26 including those associated with surgical oncology,27-32 research is needed in order to understand the impact of complications and guide the development of novel and adaptation of existing value-maximization approaches in meaningful ways. The objective of this study was to provide a framework to help achieve this end by comparing incremental costs of complications following major gastrointestinal (GI) resections for organ-specific malignant neoplasia using nationally-representative data.
Methods
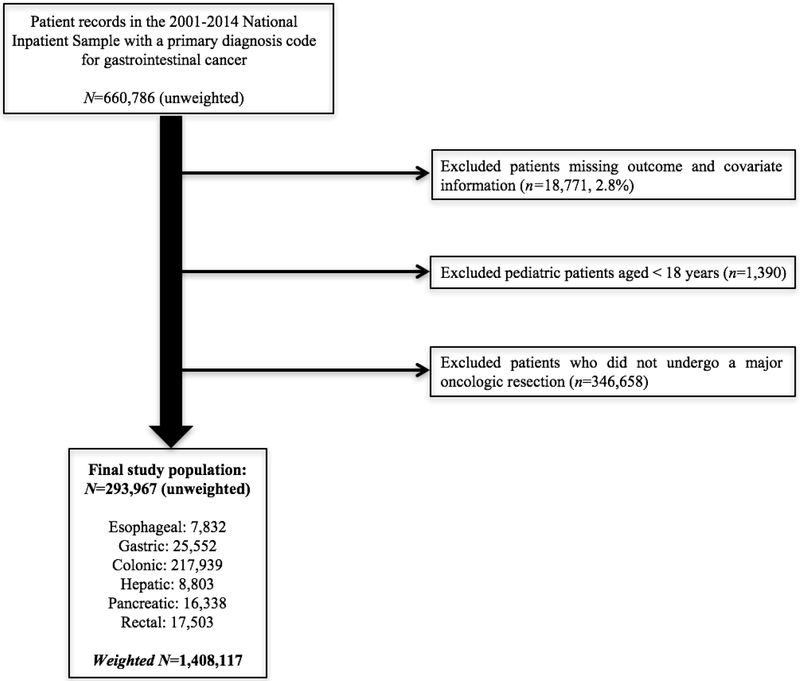
Data from the 2001-2014 National (Nationwide) Inpatient Sample (NIS) were queried for hospitalizations with primary International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes for malignant neoplasia (Supplemental Table 1). Identified hospitalizations were grouped by organ-site into esophageal, gastric, colonic, hepatic, pancreatic, and rectal cancer cases. Hospitalizations were excluded if they were missing outcome or covariate information (total 2.8%). Hospitalizations for patients aged <18y and for patients who did not undergo a major surgical resection based on reported ICD-9-CM procedure codes were also excluded (Supplemental Table 2; Figure 1). Missing information for race/ethnicity was addressed using a missing indicator variable to account the approximately 20% of states in NIS that do not report race/ethnicity.
Figure 1.
Schematic of inclusion and exclusion criteria
NIS is the largest publically-available all-payer inpatient database in the US, yielding national estimates of inpatient stays.33 Unweighted, it contains data from >7 million annual hospital visits. HCUP-defined design-weights are used to approximate national estimates, accounting for >36 million hospitalizations each year. Prior to 2012, NIS represented a 20% stratified-sample of hospitals selected based on geographic region, ownership-control, urban/rural location, teaching status, and number of hospital beds. Since 2012, the database has become a 20% stratified sample of hospital discharges from the majority of hospitals in the US.33 Application of trend weights allows for consistent reporting of nationally-weighted estimates across both iterations of database years (2001-2011, 2012-2014). Available data-elements include information on patient age, sex, race/ethnicity, primary payer, length of stay (LOS), total charges, disposition, hospital characteristics, and up to 25 ICD-9-CM diagnosis and 15 ICD-9-CM procedure codes.
Information abstracted on patient and hospital characteristics included: age (18-25, 26-35, 36-45, 46-55, 56-65, 66-75, >75y), sex, NIS-defined race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic other, unreported/unknown), insurance status (private, government, uninsured), NIS-defined income quartile, elective versus emergent/urgent indication, primary diagnosis, calculated Charlson Comorbidity Index (CCI: 0, 1-2, ≥3), year, hospital volume (high, low; NIS-defined large, small/medium bedsize), hospital teaching-location (rural, urban teaching, urban non-teaching), and hospital region (Northeast, Midwest, South, West). Patients were assessed for the occurrence of complications using physiological system-based codes defined in conjunction with the research department of Minnesota Gastroenterology, P.A. These codes were chosen with the specific intention of creating a set of ICD-9-CM system-based complications relevant to the long-term treatment of patients undergoing GI procedures.10 Complication groups included: mechanical wounds (e.g. hematoma, seroma), infections (e.g. sepsis/septicemia), complications during surgery (e.g. accidental puncture or laceration complicating surgery), and urinary (e.g. postoperative urinary tract infection), pulmonary (e.g. ventilator associated pneumonia, post-procedural aspiration pneumonia), gastrointestinal (e.g. postoperative ileus, postoperative small bowel obstruction), cardiovascular (e.g. postoperative deep vein thrombosis), and systemic (e.g. postoperative shock: septic or hypovolemic) complications. Corresponding secondary diagnosis codes are presented in Supplementary Table 3. Hospitalized patients included in the final analysis were dichotomized based on occurrence of individual complication groups and ≥1 complication(s) overall.
Total index hospital costs in 2017 US dollars (USD) served as the study’s primary outcome measure. NIS-provided total hospital charges in 2001-2014 USD were converted to total hospital costs using cost-to-charge ratios. Values were converted to 2017 USD using annual hospital consumer-price-indices. Secondary measures included assessment of surgical outcomes: non-routine discharge (other than to home under self-care), in-hospital mortality, and index LOS.
Statistical analyses
Differences in patient- and hospital-level factors were compared between patients with and without complications using Chi-squared tests. Binary outcomes were compared using crude and risk-adjusted logistic regression to give odds ratios (OR) and 95% confidence intervals (95%CI). The continuous outcomes, total index hospital cost and LOS, were both right-skewed. To account for the non-normal distributions of these outcome measures, modified Park tests were used to determine appropriate modeling strategies. Both conformed to natural logarithm-transformed gamma distributions. Crude and risk-adjusted generalized linear models based on these parameters were used to model the continuous distributions. Calculation of post-estimation average marginal effects yielded predicted mean differences and 95%CI for each.
Risk-adjusted incremental costs and complication probabilities for each cancer/resection type and complication group were calculated in an analogous manner. Determination of incremental effects included risk-adjustment for the potential influence of multiple complications by adding the occurrence of other complications to the complication group-specific model in order to yield isolated, complication group-specific effects. Aggregate measures of the total national burden were obtained by combining risk-adjusted information on complication group prevalence and incremental cost.
All models were checked for multicollinearity and appropriateness of fit. The models were weighted with NIS-provided design-weights (including corrected trend weights from 2001-2011, strata sampling from 2001-2014, and hospital clustering from 2001-2014) using survey commands in Stata in order to account for patient clustering within hospitals and attain nationally-weighted effects. Data analyses were performed using Stata Statistical Software: Release 14.2 (College Station, TX). Two-sided p-values were considered significant. The study was approved by the Yale Human Investigation Committee.
Results
Epidemiologic data
A total of 293,967 patients were included. The weighted data represented 1,408,117 patients nationwide (Figure 1). The most prevalent group underwent colonic resections (n=217,939, 74.1%), followed by gastric (n=25,552, 8.7%), rectal (n=17,503, 6.0%), and pancreatic (n=16,338, 5.6%) resections. A total of 8,803 patients (3.0%) underwent hepatectomy, and 7,832 (2.7%) underwent esophagectomy. Overall, a risk-adjusted 26.1% (95%CI: 25.7-26.4%) of patients experienced ≥1 pre-discharge complication(s) (Table 1). Documented complications most frequently included gastrointestinal (11.6%[11.3-11.8%]]), infectious (6.7%[6.6-6.9%]), and pulmonary (5.0%[4.8-5.2%]) manifestations. Complications were most prevalent among esophageal (45.3%[43.8-46.9%]), gastric (33.6%[32.8-34.5%]), and pancreatic (34.0%[32.7-35.3%]) cancer cases. Complete risk-adjusted distributions of complication prevalences for each cancer/resection type and complication group are presented in Table 1.
Table 1.
| Probability of Complications in Major Oncologic Resections for Malignant Diagnoses | |||||
|---|---|---|---|---|---|
| Any complication | Mechanical wound | Infection | Urinary | ||
| Esophagus | 45.3% (43.8-46.9%) |
4.4% (3.9-4.4%) |
12.9% (12.0-13.7%) |
1.3% (1.0-1.6%) |
|
| Stomach | 33.6% (32.8-34.5%) |
3.0% (2.7-3.2%) |
10.8% (10.4-11.2%) |
1.4% (1.2-1.6%) |
|
| Colon | 24.4% (24.0-24.8%) |
1.7% (1.7-1.8%) |
5.9% (5.8-6.1%) |
1.4% (1.3-1.4%) |
|
| Liver | 25.6% (24.5-26.7%) |
2.4% (2.1-2.7%) |
7.1% (6.5-7.7%) |
1.6% (1.3-1.8%) |
|
| Pancreas | 34.0% (32.7-35.3%) |
3.9% (3.6-4.3%) |
12.5% (11.7-13.2%) |
1.5% (1.3-1.7%) |
|
| Rectum | 24.0% (22.3-25.7%) |
2.8% (2.1-3.4%) |
5.2% (4.4-6.1%) |
2.3% (1.7-2.9%) |
|
| All operations | 26.1% (25.7-26.4%) |
2.0% (1.9-2.1%) |
6.7% (6.6-6.9%) |
1.4% (1.3-1.5%) |
|
| Pulmonary | Gastrointestinal | Cardiovascular | Systemic | Surgical | |
| Esophagus | 21.2% (19.6-22.8%) |
11.2% (710.3-12.1%) |
9.7% (9.0-10.6%) |
1.9% (1.6-2.3%) |
6.3% (5.7-6.9%) |
| Stomach | 11.0% (10.3-11.7%) |
10.3% (9.8-10.8%) |
5.9% (5.5-6.3%) |
1.7% (1.5-1.9%) |
4.5% (4.2-4.7%) |
| Colon | 3.7% (3.6-3.9%) |
12.0% (11.7-12.3%) |
3.0% (2.9-3.1%) |
1.0% (1.0-1.1%) |
3.1% (3.0-3.2%) |
| Liver | 6.3% (5.7-7.0%) |
9.3% (8.5-10.1%) |
3.3% (3.0-3.7%) |
1.2% (1.0-1.4%) |
4.4% (4.0-4.8%) |
| Pancreas | 7.6% (7.0-8.3%) |
11.5% (10.6-12.4%) |
4.1% (3.6-4.5%) |
1.7% (1.4-1.9%) |
6.0% (5.6-6.5%) |
| Rectum | 2.5% (1.8-3.1%) |
11.1% (9.9-12.4%) |
2.3% (1.8-2.9%) |
0.3% (0.1-0.5%) |
4.7% (3.9-5.5%) |
| All operations | 5.0% (4.8-5.2%) |
11.6% (11.3-11.8%) |
3.5% (3.3-3.6%) |
1.1% (1.1-1.2%) |
3.5% (3.4-3.6%) |
Models accounted for potential confounding due to patient characteristics (age, sex, race/ethnicity, insurance type, median income quartile, indication, primary diagnosis, CCI, and year) and hospital-level factors (hospital volume, teaching-location, and geographical region).
Modeling used NIS-provided population weights generalized with STATA’s “svy” command to account for patient clustering within hospital-level variables and to extrapolate the sample to a nationally representative version of the US population
Overall, the occurrence of complications became more prevalent as patients aged, rising from 20.7% among patients aged 18-45y to 30.1% among patients aged >85y (Table 2). Complications were more likely among males versus females, 28.8% versus 23.2%, (a trend which persisted for each complication group); patients with a higher CCI, ≥7: 28.9% versus ≤2: 24.3%; and patients with an emergent/urgent versus elective indication, 30.4% versus 24.4%. Limited differences were noted based on patient race/ethnicity, income, or insurance type. Relative to privately-insured patients, however, uninsured patients were 37.3% more likely to experience an infectious complication (8.1% versus 5.9%). Differences in hospital characteristics, while statistically significant, were largely comparable across groups.
Table 2.
Patient demographic and hospital-level characteristics (all operations; nationally-weighted percentages)
| Type of complication | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient Characteristic |
Any complication | Mechanical wound | Infection | Urinary | Pulmonary | Gastrointestinal | Cardiovascular | Systemic | Surgical |
| Age (years) | |||||||||
| 18-45 | 20.7 | 1.9 | 6.0 | 0.8 | 3.0 | 9.6 | 1.5 | 1.2 | 3.1 |
| 46-55 | 22.1 | 2.1 | 6.5 | 0.9 | 3.9 | 10.0 | 1.8 | 1.2 | 3.1 |
| 56-65 | 24.2 | 2.2 | 6.9 | 1.0 | 4.6 | 10.6 | 2.5 | 1.2 | 3.3 |
| 66-75 | 26.9 | 2.1 | 7.0 | 1.5 | 5.2 | 12.0 | 3.8 | 1.1 | 3.7 |
| 76-85 | 28.3 | 1.9 | 6.6 | 1.8 | 5.6 | 12.5 | 4.5 | 1.1 | 3.6 |
| >85 | 30.1 | 1.8 | 7.2 | 2.0 | 6.4 | 13.0 | 5.1 | 1.0 | 3.5 |
| Sex | |||||||||
| Male | 28.8 | 2.5 | 7.9 | 1.7 | 5.7 | 12.8 | 3.9 | 1.2 | 3.6 |
| Female | 23.2 | 1.5 | 5.6 | 1.1 | 4.3 | 10.3 | 3.1 | 1.0 | 3.3 |
| Race/Ethnicity | |||||||||
| Non-Hispanic White | 26.1 | 2.1 | 6.7 | 1.4 | 5.3 | 11.5 | 3.6 | 1.0 | 3.4 |
| Non-Hispanic Black | 26.2 | 2.0 | 7.6 | 1.2 | 4.5 | 11.7 | 2.9 | 1.4 | 3.5 |
| Hispanic | 25.6 | 2.2 | 7.7 | 1.2 | 5.2 | 10.8 | 2.6 | 1.2 | 3.4 |
| Non-Hispanic other | 25.0 | 2.0 | 7.0 | 1.6 | 4.2 | 10.8 | 2.9 | 1.5 | 3.9 |
| Unreported | 26.4 | 1.8 | 6.2 | 1.3 | 4.6 | 12.0 | 3.5 | 1.4 | 3.6 |
| Insurance | |||||||||
| Public | 27.8 | 2.1 | 7.1 | 1.6 | 5.5 | 12.2 | 4.0 | 1.1 | 3.6 |
| Private | 22.8 | 1.9 | 5.9 | 1.0 | 4.1 | 10.5 | 2.5 | 1.2 | 3.2 |
| Uninsured | 23.3 | 1.8 | 8.1 | 0.8 | 3.6 | 10.4 | 2.0 | 1.2 | 3.2 |
| Other | 23.7 | 2.3 | 6.8 | 0.9 | 4.3 | 10.6 | 2.2 | 1.3 | 3.3 |
| Income quartile (USD)A | |||||||||
| 1-38,999 | 26.7 | 2.3 | 7.5 | 1.3 | 5.2 | 11.6 | 3.1 | 1.1 | 3.7 |
| 39,000-47,999 | 25.7 | 2.1 | 6.6 | 1.4 | 4.9 | 11.4 | 3.3 | 1.0 | 3.4 |
| 48,000-63,999 | 26.2 | 2.0 | 6.7 | 1.4 | 5.1 | 11.8 | 3.5 | 1.2 | 3.6 |
| ≥ 64,000 | 25.8 | 1.8 | 6.4 | 1.5 | 4.9 | 11.5 | 3.8 | 1.3 | 3.3 |
| CCI | |||||||||
| ≤ 2 | 24.3 | 1.8 | 5.9 | 1.3 | 4.0 | 11.6 | 3.2 | 1.2 | 3.4 |
| 3-4 | 26.4 | 2.1 | 6.2 | 1.4 | 4.9 | 11.1 | 3.8 | 1.1 | 3.4 |
| 5-6 | 27.0 | 2.2 | 7.8 | 1.4 | 5.8 | 12.1 | 3.2 | 1.2 | 3.6 |
| ≥ 7 | 28.9 | 2.3 | 8.2 | 1.6 | 6.7 | 13.4 | 4.0 | 1.1 | 3.5 |
| Indication | |||||||||
| Elective | 24.4 | 1.8 | 5.5 | 1.4 | 4.5 | 11.1 | 3.5 | 1.1 | 3.5 |
| Emergent/Urgent | 30.4 | 2.5 | 9.9 | 1.3 | 6.3 | 12.7 | 3.5 | 1.3 | 3.5 |
| Type of complication | |||||||||
| Hospital Characteristic | Any complication | Mechanical wound | Infection | Urinary | Pulmonary | Gastrointestinal | Cardiovascular | Systemic | Surgical |
| Hospital volume | |||||||||
| High volume | 26.4 | 2.1 | 6.9 | 1.4 | 5.3 | 11.5 | 3.5 | 1.2 | 3.5 |
| Low volume | 25.5 | 1.9 | 6.4 | 1.4 | 4.5 | 11.8 | 3.3 | 1.1 | 3.3 |
| Teaching-Location | |||||||||
| Rural | 24.4 | 1.8 | 5.2 | 1.3 | 3.3 | 12.3 | 3.1 | 1.1 | 3.2 |
| Urban teaching | 25.9 | 1.7 | 6.4 | 1.3 | 5.1 | 12.3 | 3.1 | 1.2 | 3.2 |
| Urban non-teaching | 26.6 | 2.3 | 7.4 | 1.5 | 5.4 | 10.9 | 3.8 | 1.1 | 3.7 |
| Region | |||||||||
| Northeast | 26.2 | 2.0 | 7.0 | 2.0 | 5.3 | 10.1 | 4.9 | 1.3 | 3.2 |
| Midwest | 25.8 | 2.0 | 6.3 | 1.3 | 4.9 | 11.7 | 3.3 | 1.1 | 3.5 |
| South | 25.4 | 2.0 | 6.7 | 1.1 | 5.0 | 11.4 | 2.8 | 1.1 | 3.3 |
| West | 27.6 | 2.2 | 7.2 | 1.3 | 4.9 | 13.4 | 3.4 | 1.1 | 3.5 |
Bonferroni corrected p-values taken from two-sided Chi-squared tests were significant for all considered covariates, comparing patients without a complication to (1) patients with one or more complications overall as well as to (2) patients with one or more complications corresponding to each system-based complication group.
Abbreviations: CCI = Charlson Comorbidity Index
Values shown reflect the year 2011 (2011 USD), some variation each year with values coded by NIS
Differences in hospital costs by complication type and primary organ resected
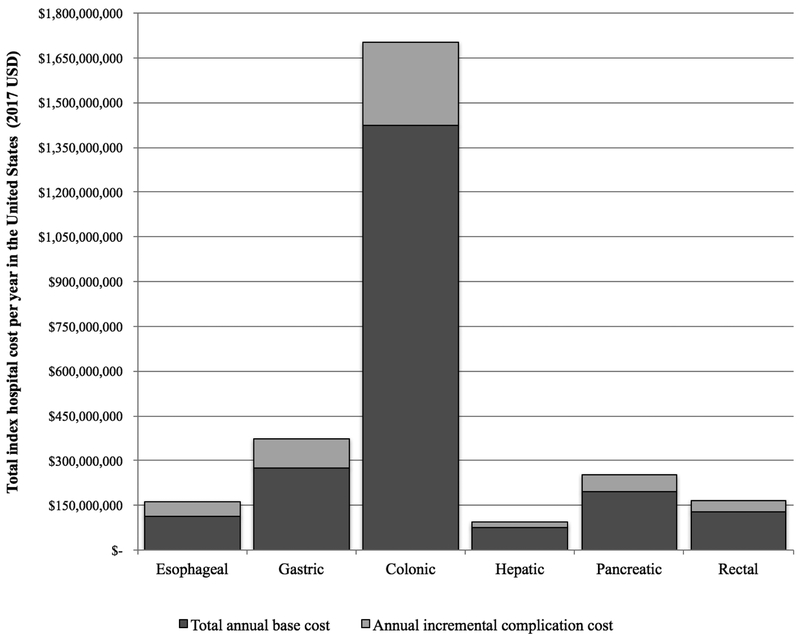
In 2017 USD, total annual index hospital costs for major oncologic GI malignant resections in the US totaled an average of $2.75 billion (Figure 2A). Complications accounted for 19.5%—an average of $540 million nationwide per year. By cancer/resection type, complications accounted for 30.1% of esophageal, 26.5% of gastric, 16.3% of colonic, 21.2% of hepatic, 22.1% of pancreatic, and 21.6% of rectal cancer costs.
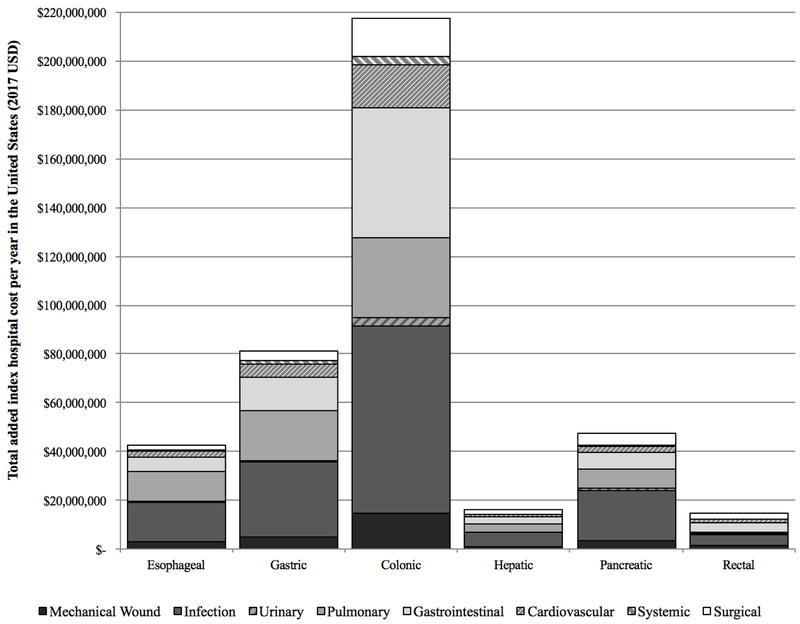
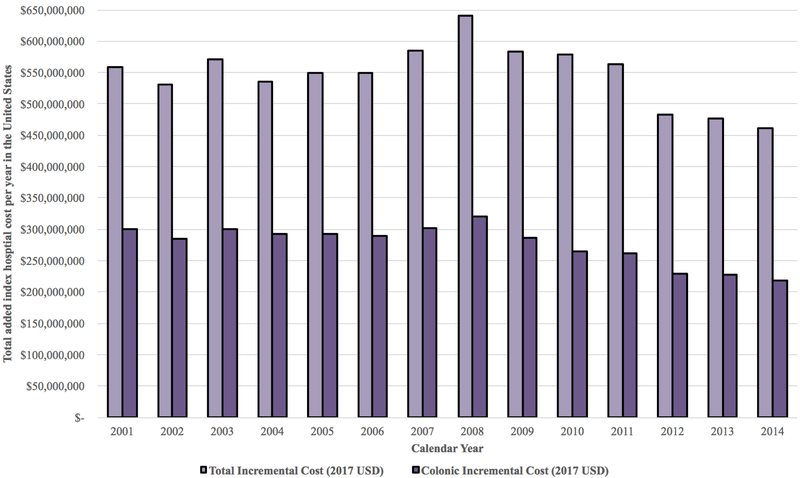
Figure 2.
A. Total annual index hospital cost for major oncologic malignant resections in the United States, 2001–2014. B. Breakdown of annual incremental costs by system-based complication group. C. Changes in annual incremental costs overall and for colonic resection, 2001-2014
Individual risk-adjusted incremental complication costs on a per-patient level are presented in Table 3. Overall, the occurrence of any isolated complication increased index hospital costs by an average of $20,900 (95%CI: $20,300-21,500)—a value which ranged from $12,700 ($10,700-14,700) among rectal resections to $40,400 ($37,400-43,400) for esophageal resections. Infectious complications were the single-most expensive complication with an average of $21,600 (95%CI: $21,000-22,200; range: rectal-$ 14,200 to esophageal-$46,600) in added total hospital costs. This was followed by pulmonary complications with average costs of $16,800 (95%CI: $16,200-17,400; range: rectal-$4,900 to esophageal-$21,700) and mechanical wounds with average costs of $13,600 (95%CI: $12,900-14,300; range: rectal-$ 10,500 to esophageal-$24,600).
Table 3.
Risk-adjusted predicted mean incremental complication costs (95%CI) in 2017 USDA,B (independent of other included complications)
| Cost of Major Oncologic Resections for Corresponding Malignant Diagnosis | |||||
|---|---|---|---|---|---|
| No complication | Any complication | Mechanical wound | Infection | Urinary | |
| (base value) | (incremental cost) | (incremental cost) | (incremental cost) | (incremental cost) | |
| Esophagus | $41,965.52 ($39,600-44,400) |
$40,373.00 ($37,400-43,400) p<0.001 |
$24,567.81 ($19,900-29,300) p<0.001 |
$46,583.13 ($42,100-51,100) p<0.001 |
$10,137.61 ($0-23,600) p=0.140 |
| Stomach | $31,294.51 ($30,400-32,200) |
$34,327.54 ($32,400-36,300) p<0.001 |
$18,974.45 ($15,700-22,300) p<0.001 |
$32,655.44 ($30,600-34,700) p<0.001 |
$3,270.68 ($0-7,000) p=0.087 |
| Colon | $19,134.92 ($18,900-19,400) |
$15,486.34 ($15,100-15,900) p<0.001 |
$11,810.92 ($11,100-12,500) p<0.001 |
$17,452.93 ($17,000-17,900) p<0.001 |
$3,124.35 ($2,400-3,800) p<0.001 |
| Liver | $24,545.76 ($23,600-25,500) |
$28,084.65 ($25,800-30,400) p<0.001 |
$14,039.63 ($10,700-17,400) p<0.001 |
$26,998.96 ($24,600-29,400) p<0.001 |
$8,028.56 ($3,500-12,500) p<0.001 |
| Pancreas | $34,948.59 ($33,700-36,100) |
$29,955.04 $28,100-31,800) p<0.001 |
$16,828.88 ($13,700-20,000) p<0.001 |
$29,338.55 ($27,100-31,600) p<0.001 |
$8,630.08 ($3,600-13,600) p=0.001 |
| Rectum | $22,389.27 ($21,700-23,000) |
$12,738.36 ($10,700-14,700) p<0.001 |
$10.458.97 ($6,900-14,100) p<0.001 |
$14,209.97 ($11,400-17,000) p<0.001 |
$1,219.58 ($0-4,600) p=0.474 |
|
All operations |
$21,438.63 ($21,000-21,700) |
$20,889.63 ($20,300-21,500) p<0.001 |
$13,632.87 ($12,900-14,300) p<0.001 |
$21,640.12 ($21,000-22,200) p<0.001 |
$3,514.51 ($2,700-4,400) p<0.001 |
| Pulmonary | Gastrointestinal | Cardiovascular | Systemic | Surgical | |
| (incremental cost) | (incremental cost) | (incremental cost) | (incremental cost) | (incremental cost) | |
| Esophagus | $21,744.40 ($18,600-24,900) p<0.001 |
$18,521.98 ($14,800-22,300) p<0.001 |
$9,376.70 ($6,000-12,700) p=0.001 |
$13,293.32 ($3,400-23,200) p=0.009 |
$10,733.90 ($6,500-15,000) p<0.001 |
| Stomach | $21,393.14 ($19,700-23,100) p<0.001 |
$14,798.45 ($13,100-16,500) p<0.001 |
$11,201.20 ($9,100-13,300) p<0.001 |
$8,730.07 ($5,500-11,900) p<0.001 |
$9,410.60 ($7,200-11,600) p<0.001 |
| Colon | $11,890.64 ($11,400-12,400) p<0.001 |
$5,958.92 ($5,700-6,300) p<0.001 |
$7,778.60 ($7,300-8,300) p<0.001 |
$4,699.68 ($3,800-5,600) p<0.001 |
$6,823.41 ($6,300-7,300) p<0.001 |
| Liver | $17,749.46 ($15,100-20.400) p<0.001 |
$9,207.44 ($7,600-10,800) p<0.001 |
$9,334.82 ($7,100-11,600) p<0.001 |
$3,805.61 ($100-7,600) p=0.048 |
$14,062.81 ($11,500-16,600) p<0.001 |
| Pancreas | $18,494.55 ($16,100-20,900) p<0.001 |
$10,733.72 ($8,900-12,600) p<0.001 |
$9,509.78 ($6,900-12,100) p<0.001 |
$3,858.17 ($0-8,000) p=0.068 |
$15,148.29 ($12,800-17,500) p<0.001 |
| Rectum | $4,870.77 ($1,100-8,700) p=0.012 |
$5,575.90 ($3,900-7,300) p<0.001 |
$10,375.04 ($4,100-16,600) p=0.001 |
$2,878.68 ($0-11,600) p=0.517 |
$8.094.11 ($4,700-11,500) p<0.001 |
|
All operations |
$16,809.14 ($16,200-17,400) p<0.001 |
$6,713.84 ($6,300-10,300) p<0.001 |
$9,733.42 ($9,200-10,300) p<0.001 |
$6,326.87 ($5,400-7,300) p<0.001 |
$9,009.16 (8,500-9,500) p<0.001 |
Models accounted for potential confounding due to patient characteristics (age, sex, race/ethnicity, insurance type, median income quartile, indication, primary diagnosis, CCI, and year) and hospital-level factors (hospital volume, teaching-location, and geographical region). Calculation of incremental additions also accounted for the potential influence of multiple complications by adjusting for the occurrence of all other complications to yield isolated, complication-specific effects.
Modeling used NIS-provided population weights generalized with STATA’s “svy” command to account for patient clustering within hospital-level variables and to extrapolate the sample to a nationally representative version of the US population. Predicted mean differences were calculated using post-estimation marginal effects following GLM with link log, family gamma, to account for the highly-skewed nature of the data.
When national prevalences were taken into account (Figure 2B), infectious complications again contributed the largest total amount, costing hospitals and payers an additional $154.7 million nationwide per year (36.9% of the total incremental complication cost). GI complications contributed an additional $85.5 million per year (20.4% of the total incremental complication cost). Mechanical wounds cost an average of $29.3 million (7.0%), urinary $5.3 million (1.3%), pulmonary $77.9 million (18.6%), cardiovascular $30.2 million (7.2%), systemic $6.0 million (1.4%), and surgical $30.6 million (7.3%). By resection type, the impact of complications ranged from $76.7 million per year for colonic resections with infectious complications to a more modest $0.2 million for rectal resections with urinary complications. For each resection type, infectious, GI, and pulmonary complications were among the most significant drivers of overall increased total index hospital costs.
Changes over time
Annual incremental complication costs from 2001-2014 are presented in Figure 2C. From 2001-2006, costs ranged from approximately $530 million to $570 million nationwide per year. They increased slightly between 2007-2011, $560 million to $640 million, before beginning to decrease from 2012-2014, eventually reaching an annual incremental index hospital cost of $460 million in 2014. Overall trends closely paralleled changes in colectomies, which dropped from an average of $280 million to $320 million per year between 2001-2009 to approximately $220 million per year in 2014 (Figure 2C).
Surgical outcome regression results
Risk-adjusted differences in surgical outcomes are presented in Table 4. Relative to patients without a complication, patients with any complication (OR[95%CI]: 2.65[2.58-2.72]) and with each complication type (range: infectious-5.18[4.91-5.46] to urinary 1.51[1.40-1.63]) were significantly more likely to require non-routine discharge. In addition, patients who experienced a complication had 6.08 times higher risk-adjusted odds of death (any complication OR[95%CI]: 6.08[5.79-6.39]; infectious: 8.60[8.13-9.11]) and index LOS that were longer by an average of 5.5 days (any complication predicted mean difference [95%CI]: 5.5 [5.4-5.6] days; infectious: 6.5 [6.3-6.6] days).
Table 4.
Risk-adjusted surgical outcomes (all operations)
| Risk-adjusted OR1/predicted mean difference (95% CI)B | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Any complication | Mechanical wound | Infection | Urinary | Pulmonary | Gastrointestinal | Cardiovascular | Systemic | Surgical |
| Non-routine | 2.65 (2.58-2.72) | 3.03 (2.81-3.27) | 5.18 (4.91-5.46) | 1.51 (1.40-1.63) | 2.44 (2.31-2.57) | 1.51 (1.46-1.56) | 1.87 (1.77-1.99) | 1.87 (1.77-1.99) | 1.52 (1.44-1.59) |
| discharge | |||||||||
| In-hospital | 6.08 (5.79-6.39) | 0.95 (0.84-1.07) | 8.60 (8.13-9.11) | 1.36 (1.17-1.58) | 2.93 (2.73-3.15) | 1.01 (0.95-1.08) | 2.55 (2.34-2.78) | 2.45 (2.14-2.82) | 2.08 (1.90-2.27) |
| mortality | |||||||||
| LOSA (days) | 5.54 (5.45-5.64) | 4.80 (4.61-4.99) | 6.47 (6.35-6.61) | 1.31 (1.10-1.52) | 3.41 (3.27-3.54) | 3.64 (3.55-3.74) | 2.01 (1.87-2.15) | 1.25 (1.00-1.51) | 1.83 (1.69-1.98) |
Abbreviation: LOS - length of stay
Modeling used NIS-provided population weights generalized with STATA’s “svy” command to account for patient clustering within hospital-level variables and to extrapolate the sample to a nationally representative version of the US population.
Interpretation: Risk-adjusted odds of non-routine discharge (in-hospital mortality) are x times higher among patients with a given type of complication relative to patients without that type of complication; Risk-adjusted predicted mean length of stay is x amount higher among patients with a given type of complication relative to patients without that type of complication.
To account for non-nonnally distributed data, modified Park tests were used to determine selection of a gamma distribution (lambda not significantly different from 2). Predicted mean differences were calculated using post-estimation average marginal effects following GLM with link log, family gamma.
Risk-adjusted models accounted for potential confounding due to other complications, patient characteristics (age, sex, race/ethnicity, insurance type, median income quartile, indication, primary diagnosis, CCI, and year), and hospital-level factors (hospital volume, teaching-location, and geographical region).
Discussion
The study investigated incremental costs of complications among organ-specific sites of major GI resection for malignant neoplasia, demonstrating a significant association between complications and increased costs of care. Each year, complications within this population cost hospitals and payers an average of $540 million, raising index hospital costs by a risk-adjusted average of 19.5% (range: 16.3% colectomy to 30.1% esophagectomy). Complications were associated with 3-times higher risk-adjusted odds of non-routine discharge, 6-times higher odds of in-hospital death, and LOS that were longer by an average of >5 days.
While significant contributors to increased hospital costs and poor patient outcomes in each type of resection considered, not all complications were equal. Important differences based on the type of complication and type of operation were found. For example, among patients with esophageal malignancies undergoing esophagectomy, 45.3% experienced ≥1 pre-discharge complication (21.2% pulmonary, 12.9% infection, 11.2% GI, etc.). Complication-specific incremental costs for esophagectomy ranged from $9,400 (cardiovascular) to $46,600 (infection) per patient. Among patients with colonic malignancies undergoing colectomy, a more modest 24.4% of patients experienced ≥1 pre-discharge complication (12.0% GI, 5.9% infection). Complication-specific incremental costs in this group ranged from $3,100 (urinary) to $17,500 (infection) per patient.
Such a wide range of costs underscores the need for value-maximization approaches that reflect the spectrum of adverse outcomes associated with surgical oncology care.27-32 Lacking this direction, well-intended interventions can easily go astray as has been demonstrated by the early surgical pay-for-performance complication-tracking established by Medicare and other partner organizations in 2006. The resultant program which targeted surgical site infections, venous thromboembolism, myocardial infarction, pneumonia, and catheter-associated urinary tract infections has been widely-criticized for overlooking important complications relevant to both elective10,34 and emergent8 indications and for focusing too much on urinary complications which minimally contributed to the overall cost of care.10 In the current study, urinary complications accounted for only 1.3% of incremental increases in index hospital costs. Where they did occur, they either contributed no significant additional incremental cost or a comparatively small additional amount that ranged from approximately $3,000 per patient for patients undergoing a colonic resection (and, correspondingly, any operation overall) to $8,000 for liver and pancreatic resections. The difference in magnitude of the effect of urinary complications between these three groups likely reflects underlying differences in the fragility and physiologic reserves of the corresponding patients. Across all complication types, hepatic-pancreatic-biliary (HPB) patients were more prone than other cancer patients to expensive increases in incremental index hospital costs when complications occurred. More recent initiatives targeting an array of complications among THA/TKA patients under Medicare’s Hospital Value Based Purchasing Program35, 36 and surgical site infections among all surgical patients under the Hospital Acquired Condition Reduction Program37 have met with more promising albeit still mixed success.
Declining trends in annual costs of complications for colon cancer patients suggest the potential early success of some value-maximization approaches. Drops since 2008 coincide with the timing of bundled-payment introduction and a widespread national change in the discourse surrounding the quality of the procedure. The extent to which this change can be attributed to either factor remains deeply contested 19,38,39 and is likely to be further confounded by parallel efforts to promote reductions in infections among elective colectomy patients.10 The results of this study noted that for all GI-related surgical oncology patients, infection was the highest single contributor to individual patient costs (average incremental cost $21,600), followed closely by pulmonary complications ($16,800) and mechanical wounds ($13,600). When national prevalences were taken into account, infections again contributed the largest total amount, costing an additional $155 million per year (36.9% of the total incremental amount). They increased patients’ odds of death 8.60-fold.
The mixed success of prior value-maximization endeavors raises the question, “Where do we go from here?” The results of this study build on prior initiatives and a growing body of literature criticizing prior initiatives by establishing an evidence-based framework that defines meaningful complication-based targets for value-maximization and assessment of surgical oncology patients. As efforts to promote value-maximization continue to increase, it will be essential to recognize the intricacies of outcomes and care encountered when dealing with complex cancer patients. Quality-improvement and cost-reduction approaches must be informed in order to account for the challenges of cancer patients’ needs. Related health policy ought to reflect similar considerations in order to target finite improvement efforts and resources toward areas with the greatest need. In looking at increases in index hospital costs upwards of 16 to 30%, the results of this study underscore the importance of including complications in ongoing development of quality-improvement assessments and value-maximization intervention approaches.7-10 Moving forward, as Medicare, professional organizations, and individual hospitals strive to address these concerns and increase their consideration of outcomes for complex surgical patients, there is a need to focus on both a broad array of complications in order to avoid the pitfalls of the 2006 pay-for-performance program as well as to hone in on meaningful complications for a given population. Among surgical GI oncology patients, one thing is abundantly clear: infections must be controlled.
The study is not without limitations, most of which come from its reliance on national administrative claims that can be subject to absent or misreporting of some data. Use of NIS allowed for a large, multiyear assessment of complications and costs on a national scale. It did not allow for assessment of clinical margins or stage. To help mitigate this concern, inclusion criteria were limited to patients with documented malignant neoplasia undergoing a major surgical resection of the corresponding organ. Data on costs in NIS are reported as charges and converted to costs using hospital-specific cost-to-charge ratios—an imperfect measure of individual patient costs and one that precludes a more nuanced assessment of the specific components of direct hospital costs. Data on readmissions and indirect hospital costs were not able to be assessed.
In conclusion, although value-maximization initiatives have started to account for the economic impact of complications, few have directly addressed the range of costs and prioritization of appropriate targets in complex surgical fields, leading to a dearth of information on the interplay between complications, costs, and outcomes on a national scale and a growing history of heavily-criticized interventions and measures. Quantifying and comparing the impact of complications on an indication-specific level in more complex patients offers an important step toward allowing providers and payers to meaningfully prioritize the design of novel and adaptation of existing value-maximization approaches in surgical fields.
Supplementary Material
Acknowledgments
Financial disclosure: No funding specifically for this work was provided. Cheryl K Zogg, MSPH, MHS, is supported by NIH Medical Scientist Training Program Training Grant T32GM007205. She is the Primary Investigator of a grant from the Emergency Medical Foundation and American College of Emergency Physicians entitled, “Understanding Emergency Medicine Providers’ Perceptions of the ACA in a Renewed Era of Healthcare Reform: National Survey and Qualitative Mixed-Methods Approach.” Adil H. Haider, MD, MPH, FACS, is the Primary Investigator of a contract (AD-1306-03980) with PCORI entitled “Patient-Centered Approaches to Collect Sexual Orientation/Gender Identity in the ED,” a Harvard Surgery Affinity Research Collaborative (ARC) Program Grant entitled “Mitigating Disparities Through Enhancing Surgeons’ Ability To Provide Culturally Relevant Care,” and a collaborative research grant from the Henry M. Jackson Foundation for the Advancement of Military Medicine in conjunction with the Uniformed Services University of the Health Sciences entitled “The Comparative Effectiveness and Provider Induced Demand Collaboration.” Haider is also a co-founder and equity-shareholder of the company Patient Doctor Technologies, Inc., which owns and operates the website www.doctella.com.
Footnotes
Conflict of interest: None
A portion of this work was previously presented at the Society of Surgical Oncology Annual Cancer Symposium March 2-5, 2016, in Boston, MA.
Author contributions: Zogg, Murthy, Changoor, Zogg, Pawlik, and Haider made substantial contributions to the conception or design of the work, Zogg, Ottesen, and Changoor participated in the acquisition and analysis of the data, Zogg, Ottesen, Kebaish, Galivanche, Murthy, Changoor, Zogg, Pawlik, and Haider contributed toward the interpretation of data for the work, Zogg, Ottesen, Kebaish, and Galivanche drafted the manuscript, and Murthy, Changoor, Zogg, Pawlik, and Haider critically revised the manuscript for intellectual content. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Health Expenditures Data for the U.S. May 2017, Centers for Disease Control and Prevention, National Center for Health Statistics. Available at: https://www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed January 1, 2018.
- 2.National Health Expenditure Projections 2015-2025 Centers for Medicare and Medicaid Services (CMS). Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/proj2015.pdf. Accessed January 1, 2018.
- 3.GDP per capita growth (annual %): United States 2017, The World Bank Group. Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.KD.ZG?locations=US. Accessed January 1, 2018.
- 4.Porter ME. What is value in health care? N Engl J Med 2010; 363(26):2477–81. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Gust C, Baser O, et al. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res 2010; 45(6 Pt 1):1783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimick JB, Weeks WB, Karia RJ, et al. Who pays for poor surgical quality? Building a business case for quality improvement. J Am Coll Surg 2006; 202(6):933–7. [DOI] [PubMed] [Google Scholar]
- 7.Vonlanthen R, Slankamenac K, Breitenstein S, et al. The impact of complications on costs of major surgical procedures: A cost analysis of 1200 patients. Ann Surg 2011; 254(6):907–13. [DOI] [PubMed] [Google Scholar]
- 8.Haider AH, Gupta S, Zogg CK, et al. Beyond incidence: Costs of complications in trauma and what it means for those who pay. Surgery 2015; 158(1):96–103. [DOI] [PubMed] [Google Scholar]
- 9.Mehrotra A, Hussey P. Including physicians in bundled hospital care payments: Time to revisit an old idea? Jama 2015; 313(19):1907–8. [DOI] [PubMed] [Google Scholar]
- 10.Zogg CK, Najjar P, Diaz AJ, et al. Rethinking priorities: Cost of complications after elective colectomy. Ann Surg 2016; 264(2):312–22. [DOI] [PubMed] [Google Scholar]
- 11.Shih T, Nicholas LH, Thumma JR, et al. Does pay-for-performance improve surgical outcomes? An evaluation of phase 2 of the Premier Hospital Quality Incentive Demonstration. Ann Surg 2014; 259(4):677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein AM, Joynt KE, Jha AK, et al. Access to coronary artery bypass graft surgery under pay for performance: Evidence from the premier hospital quality incentive demonstration. Circ Cardiovasc Qual Outcomes 2014; 7(5):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Value Based Programs [CMS.gov Centers for Medicare & Medicaid Services web site]. March 24, 2017. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.html. Accessed January 1, 2018.
- 14.Ryan AM, Krinsky S, Maurer KA, et al. Changes in hospital quality associated with hospital value-based purchasing. N Engl J Med 2017; 376(24):2358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim AM, Nathan H, Thumma JR, et al. Impact of the Hospital Readmission Reduction Program on surgical readmissions among Medicare beneficiaries. Ann Surg 2017; 266(4):617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih T, Ryan AM, Gonzalez AA, et al. Medicare’s Hospital Readmissions Reduction Program in surgery may disporportionately affect minority-serving hospitals. Ann Surg 2015; 261(6):1027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtin BM, Russell RD, Odum SM. Bundled payments for care improvement: Boom or bust? J Arthroplasty 2017; 32(10):2931–4. [DOI] [PubMed] [Google Scholar]
- 18.Courtney PM, Ashley BS, Hume EL, et al. Are bundled payments a viable reimbursement model for revision of total joint arthroplasty? Clin Orthop Relat Res 2016; 474(12):2714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gani F, Makary MA, Wick EC, et al. Bundled payments for surgical colectomy among Medicare enrollees: Potential savings vs the need for further reform. JAMA Surg 2016; 151(5):e160202. [DOI] [PubMed] [Google Scholar]
- 20.Hawken SR, Ryan AM, Miller DC. Surgery and Medicare Shared Savings Program accountable care organizations. JAMA Surg 2016; 151(1):5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehaffey JH, Hawkins RB, Mullen MG, et al. Access to quaternary care surgery: Implications for accountable care organizations. J Am Coll Surg 2017; 224(4):525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resnick MJ, Graves AJ, Buntin MB, et al. Surgeon participation in early accountable care organizations. Ann Surg 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011; 103(2):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan H, Atoria CL, Bach PB, et al. Hospital volume, complications, and cost of cancer surgery in the elderly. J Clin Oncol 2015; 33(1):107–14. [DOI] [PubMed] [Google Scholar]
- 25.Medicare Program: Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems and Quality Reporting Programs [CMS.gov Centers for Medicare & Medicaid Services web site]. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices-Items/CMS-1678-P.html. Accessed January 1, 2018.
- 26.2015 Measure Information About the 30-Day All-Cause Hospital Readmission Measure, Calculated for the Value-based Payment Modifier Program [CMS.gov Centers for Medicare & Medicaid Services web site]. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/Downloads/2015-ACR-MIF.pdf. Accessed January 1, 2018.
- 27.Gani F, Johnston FM, Nelson-Williams H, et al. Hospital volume and the costs associated with surgery for pancreatic cancer. J Gastrointest Surg 2017;21(9):1411–9. [DOI] [PubMed] [Google Scholar]
- 28.Ejaz A, Gonzalez AA, Gani F, et al. Effect of index hospitalization costs on readmission among patients undergoing major abdominal surgery. JAMA Surg 2016; 151(8):718–24. [DOI] [PubMed] [Google Scholar]
- 29.Cerullo M, Gani F, Chen SY, et al. Assessing the financial burden associated with treatment opioids for resectable pancreatic cancer. Ann Surg 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selby LV, Gennarelli RL, Schnorr GC, et al. Association of hospital costs with complications following total gastrectomy for gastric adenocarcinoma. JAMA Surg 2017;152(10):953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Short MN, Ho V, Aloia TA. Impact of processes of care aimed at complication reduction on the cost of complex cancer surgery. J Surg Oncol 2015; 112(6):610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu SJ, Ho VP, Ginsberg J, et al. Complications, not minimally invasive surgical technique, are associated with increased cost after esophagectomy. Minim Invasive Surg 2016; 2016:7690632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HCUP-US NIS Overview [HCUP Databases. Healthcare Cost and Utilization Project (HCUP) web site]. Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed January 1, 2018.
- 34.Scarborough JE, Schumacher J, Kent KC, et al. Associations of specific postoperative complications with outcomes after elective colon resection: A procedure-targeted approach toward surgical quality improvement. JAMA Surg 2017; 152(2):e164681. [DOI] [PubMed] [Google Scholar]
- 35.Minami CA, Dahlke AR, Barnard C, et al. Association between hospital characteristics and performance on the new Hospital-Acquired Condition Reduction Program’s surgical site infection measures. JAMA Surg 2016; 151(8):777–9. [DOI] [PubMed] [Google Scholar]
- 36.Bozic KJ, Grosso LM, Lin Z, et al. Variation in hospital-level risk-standardized complication rates following elective primary total hip and knee arthroplasty. J Bone Joint Surg Am 2014; 96(8):640–7. [DOI] [PubMed] [Google Scholar]
- 37.Rajaram R, Chung JW, Kinnier CV, et al. Hospital characteristics associated with penalities in the Centers for Medicare & Medicaid Services Hospital-Acquired Condition Reduction Program. JAMA 2015; 314(4):375–83. [DOI] [PubMed] [Google Scholar]
- 38.Pradarelli JC, Healy MA, Osborne NH, et al. Variation in Medicare expenditures for treating perioperative complications: The cost of rescue. JAMA Surg 2016; 151(12):e163340. [DOI] [PubMed] [Google Scholar]
- 39.Gani F, Cerullo M, Canner JK, et al. Defining payments associated with the treatment of colorectal cancer. J Surg Res 2017; 220:284–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.