Abstract
The selective expression of non-human genes in tumor tissue to activate non-toxic compounds (Gene Directed Prodrug Enzyme Therapy, GDEPT) is a novel strategy designed for killing tumor cells in patients with little or no systemic toxicity. Numerous non-human genes have been evaluated, but none have yet been successful in the clinic. We and others have demonstrated excellent in vitro and in vivo anti-tumor activity with various GDEPT strategies utilizing E. coli purine nucleoside phosphorylase (PNP) to activate purine nucleoside analogs. Unlike human PNP, the E. coli PNP enzyme accepts adenine containing nucleoside analogs as substrates, and is therefore able to selectively activate compounds such as fludarabine phosphate (F-araAMP) in tumor tissue. A phase I clinical trial utilizing recombinant adenoviral vector for delivery of E. coli PNP to solid tumors followed by systemic administration of F-araAMP (NCT01310179; IND# 14271) has recently been completed. In this trial, significant anti-tumor activity was demonstrated with negligible toxicity related to the therapy. The mechanism of cell kill (inhibition of RNA and protein synthesis) is distinct from all currently used anticancer drugs and all experimental compounds under development. The approach has demonstrated excellent ability to kill neighboring tumor cells that do not express E. coli PNP, is active against non-proliferating and proliferating tumors cells (as well as tumor stem cells, stroma), and is therefore very effective against solid tumors with a low growth fraction. These unique attributes distinguish this approach from other GDEPT strategies and are precisely those required to mediate significant improvements in antitumor therapy.
Keywords: Purine Nucleoside Phosphorylase, Fludarabine, 2-Fluoroadenine, 6-Methylpurine, GDEPT, Gene Therapy, Anticancer drugs
1. INTRODUCTION
Gene therapy is a relatively new approach for the treatment of cancer. Various strategies have been proposed to selectively introduce genes into tumor cells that will affect tumor progression. Three primary approaches have been evaluated: 1) correction of tumor phenotype, 2) enhancement of tumor immunogenicity, or 3) activation of nontoxic prodrugs. If successful, gene therapy promises the control of tumor growth with little to no systemic toxicity such as that seen with current chemotherapy. The primary problem with gene therapy is the difficulty in delivering genes to all tumor cells that express sufficient levels of the desired protein. Even in tumors that are directly injected with a gene transfer vector, only a small fraction of the cells express the therapeutic gene. Therefore, the ability of gene therapy approaches that can kill tumor cells that are not transfected or transduced is of critical importance (bystander activity). Gene therapy strategies to correct the tumor phenotype by expressing tumor suppressor genes, such as p53 in tumor cells, are limited, because the proteins generated from these vectors do not affect tumor phenotype in bystander cells. Strategies to enhance the immunogenicity of the tumor or selectively activate nontoxic prodrugs in tumor cells (GDEPT, gene-directed enzyme prodrug therapy) are appealing, because every tumor cell is not required to express the gene while still allowing total eradication of the tumor mass.
In GDEPT a vector is used to selectively transduce tumor cells with a nonhuman gene, which expresses an enzyme that can convert a nontoxic prodrug into a very toxic antitumor compound [1, 2, 3]. The vector is usually a virus that has been modified to reduce its pathogenic activity, and numerous viruses (retrovirus, replication defective adenovirus, replication competent adenovirus, etc) have been developed that can safely deliver a variety of therapeutic genes to tumor cells. Because the nonhuman gene is only expressed in tumor tissue, the nontoxic prodrug is only activated in tumor tissue. Therefore, unlike conventional chemotherapy, GDEPT should result in selective killing of tumor cells with little or no systemic toxicity. Theoretically, GDEPT could be as effective in the treatment of cancer as antibacterial therapy is in the treatment of bacterial infections.
Because most solid tumors are composed of many non-proliferating cells (low growth fraction), they are resistant to anticancer agents that primarily target DNA replication. Compounds toxic to non-proliferating cells have not been used in treatment of cancer, because most of the cells in a patient are not proliferating and such compounds are very toxic and have no selectivity when administered systemically. However, if the therapeutic gene is only expressed in tumor cells, then new agents with novel mechanisms of action that target non-proliferating tumor cells can be considered for use in the treatment of cancer. We believe that GDEPT strategies that produced potent cytotoxic agents (active against non-proliferating and proliferating tumors cells) that readily diffuse between tumor cells (high bystander activity) could have dramatic effects on the treatment of solid tumors.
GDEPT was first seriously evaluated in the early 1990’s where the herpes simplex virus thymidine kinase (HSV-TK) gene was used to sensitize tumor cells to ganciclovir (GCV). The enzyme coded by this gene is naturally expressed by the virus and is the basis for the selective antiviral activity of drugs used to treat herpes infections. Although human cells express many nucleoside kinases, the substrate preference of HSV-TK is sufficiently different from these enzymes so that anti-HSV nucleoside analogs are readily activated in infected cells, but are not in non-infected cells. GCV is the primary agent used in GDEPT strategies using HSV-TK, because its active metabolite (GCV triphosphate, GCV-TP) is a more potent inhibitor of DNA replication in human cells than the active metabolite of acyclovir (acyclovir triphosphate), the primary drug used to treat herpes infections. Once phosphorylated in a cell by HSV-TK, ganciclovir monophosphate is further activated by human monophosphate and diphosphate kinases to GCV-TP, which is incorporated into DNA resulting in disruption of replication and cell death. Although nucleotide analogs, such as GCV-TP, do not easily transfer across cell membranes, GDEPT strategies utilizing HSV-TK have been shown to have modest bystander activity due to the ability of cells to transfer nucleotides to neighboring cells through gap junctions [3]. Regardless, one of the primary reasons for the lack of clinical activity of GDEPT based on HSV-TK is the poor bystander activity associated with GCV-TP. Another deficiency of HSV-TK is that once activated GCV is primarily toxic to proliferating tumor cells and has very little activity against non-proliferating tumors cells, which is similar to nucleoside analogs that are currently approved for use in the treatment of cancer (fludarabine, cytarabine, clofarabine [4]). The HSV-TK GDEPT strategy has been evaluated in many clinical trials with little success [3].
Unfortunately, none of the other GDEPT strategies have been translated into effective anticancer treatment in patients, which we believe is due to a combination of two factors: poor bystander activity and/or lack of activity against non-proliferating tumor cells. In this review we describe the development of a potent GDEPT strategy, which addresses both of these issues and has demonstrated excellent in vivo antitumor activity in preclinical models of cancer and a phase I clinical trial.
2. IN VITRO STUDIES DEMONSTRATING E. COLI PNP GDEPT
In the early 1990’s it was widely recognized that the primary problem with HSV-TK GDEPT was poor bystander activity due to addition of a phosphate group to ganciclovir, which severely limits its ability to cross cell membranes. In an effort to develop a GDEPT treatment with enhanced bystander activity, we initiated a program using E. coli purine nucleoside phosphorylase (PNP) to activate nontoxic purine nucleoside analogs to very potent adenine analogs [5, 6, 7]. PNPs reversibly catalyze the phosphorolytic cleavage of the glycosidic bond of purine nucleosides to generate ribose-1-phosphate (or deoxyribose-1-phosphate) and a purine base. E. coli PNP, unlike human PNP, accepts adenosine and certain adenosine analogs as substrates [8, 9]. A single amino acid in the active site of PNP dictates whether or not PNP can cleave adenosine and its analogs. In E. coli PNP an aspartic acid at position 204 forms a hydrogen bond with N6 of adenosine allowing adenosine to bind in the active site and be efficiently cleaved, whereas in human PNP an asparagine residue at a comparable position prevents adenosine from binding. We sought to use this difference in substrate preference to selectively activate nontoxic purine nucleoside analogs in tumor cells. Unlike nucleoside monophosphate analogs generated by HSV-TK, purine bases freely diffuse across cell membranes, and it was anticipated that once produced in a cell, the anticancer purine analog would not only kill the tumor cell in which it was generated, but that the analog would also diffuse out of the cell and kill many surrounding tumor cells.
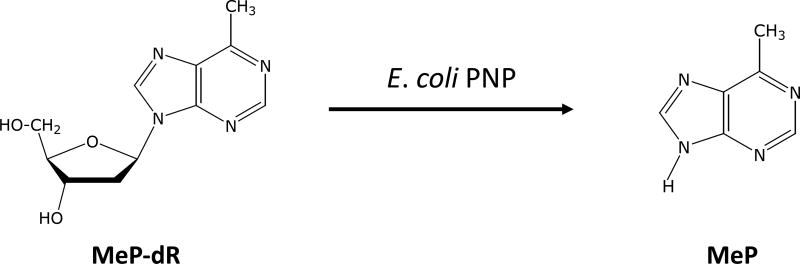
The first prodrug considered for use with E. coli PNP was 9-β-D-[2–deoxyribofuranosyl]-6-methylpurine (MeP-dR), an analog of 2’-deoxyadenosine (Figure 1). MeP-dR is not cytotoxic to human cells, because it is at best a very poor substrate for human purine salvage enzymes, such as deoxycytidine kinase and PNP. However, MeP-dR is an excellent substrate for bacterial PNPs, and 6-methylpurine (MeP) is very toxic to human tumor cells by virtue of its activation to cytotoxic nucleotides by adenine phosphoribosyltransferase (APRT). This knowledge has been used to detect mammalian cell cultures that are infected with mycoplasma, which express a PNP that cleaves adenosine and its analogs [10], because only infected cell cultures are sensitive to MeP-dR. Non-infected cultures can tolerate MeP-dR at concentrations as high as 100 µM.
Figure 1.
Conversion of MeP-dR by E. coli PNP to MeP
In our first experiments [11], human colon carcinoma cells (T84) were transfected with E. coli PNP using a relatively inefficient DNA delivery vehicle (cationic liposome mediated gene transfer), which resulted in transgene expression in less than 1% of the cultured tumor cells. These transfected cell cultures (but not parental non-transfected cultures) were very sensitive to treatment with MeP-dR, demonstrating robust bystander activity. E. coli PNP enzyme activity was detected in crude cell extracts of transfected T84 cells, and MeP was detected in the overlying culture medium [11]. Similar results were obtained in human melanoma cells following transfection of E. coli PNP with cationic liposomes [12]. These results suggested that E. coli PNP could be a very effective enzyme in GDEPT strategies for treatment of solid tumors with an extremely large bystander activity. Although E. coli PNP was expressed in only 1% of the tumor cells, sufficient amounts of MeP were produced to diffuse out of the cells expressing E. coli PNP and kill neighboring cells that did not express the transgene.
The potent activity of E. coli PNP plus MeP-dR was confirmed by establishing additional cell lines (murine melanoma, B16, and murine breast carcinoma (16/C) that stably express E. coli PNP using a retroviral vector with transgene expression under the control of the SV40 early promoter [13]. Transduced and parental (non-transduced) cells were mixed to generate cell cultures with precisely defined percentages of E. coli PNP expression. Growth was completely inhibited in cell cultures treated with MeP-dR when as few as 2% of the cells expressed E. coli PNP. In addition, plasmids were prepared in which E. coli PNP expression was controlled by the human tyrosinase promoter, and it was shown that MeP-dR was only active in transfected melanoma cells (Mel-1) in which the tyrosine promoter was active [12, 13]. In subsequent studies using D54 tumor cells that had been transduced with E. coli PNP more than 50% of MeP-dR (100 µM) was cleaved to MeP during an 8-hour incubation period, and almost all MeP generated was detected in the culture medium surrounding the cells at concentrations that mediated bystander killing [14]. These results demonstrated that MeP readily diffuses out of cells expressing E. coli PNP and was available to kill any surrounding tumor cells that do not express E. coli PNP.
A dramatic demonstration of the excellent bystander activity mediated by E. coli PNP GDEPT was published in 1998 [15]. In this work cell cultures were established in which the cells in the center of the culture, which expressed E. coli PNP, were physically separated from non-transduced cells in the outer ring. The E. coli PNP expressing cells were approximately 10% of the total number of cells in the culture well. Treatment with MeP-dR resulted in the death of all cells in the well even though there was no contact between cells expressing E. coli PNP and non-transduced cells. The results established that the bystander activity did not require cell to cell contact, and that the E. coli PNP expressing cells produced sufficient quantities of MeP, which was able to diffuse out of the cells and destroy non-expressing cells that were many millimeters distant.
Laboratories around the world have used a variety of methods to deliver E. coli PNP to tumor cells in vitro, and have confirmed the effectiveness of the approach with MeP-dR in various tumor cell lines [16 – 25]. For example, Vogelstein’s group designed a GDEPT strategy [16] using E. coli PNP and MeP-dR that was active only in cells that expressed p53. Because mutant p53 is expressed at high levels in tumor cells but neither mutant p53 nor wild-type p53 is detectable in most normal cells, such a strategy could be effective against most solid tumor types, if vectors could be developed to effectively deliver genes to tumor cells in vivo. The experiments demonstrated that all H1299 cells were destroyed by MeP-dR when less than 3% of the cells were transduced, confirming high bystander activity of this approach.
A few studies have directly compared GDEPT using E. coli PNP and MeP-dR with HSV-TK or E. coli cytosine deaminase (CD), which is another major enzyme used in GDEPT strategies [1, 2, 3]. E. coli CD activates 5-fluorocyotosine to produce 5-fluorouracil (FUra), a drug currently approved for use in the treatment of cancer. Like MeP, FUra easily diffuses across cell membranes and has high bystander activity, but is primarily toxic to proliferating tumor cells due to its inhibition of thymidylate synthase [26]. Nestler et al. [18] tested foamy virus vectors expressing HSV-TK, E. coli CD, or E. coli PNP and concluded that in terms of vector stability and specific cell killing, a virus transducing the PNP gene (used with MeP-dR) was superior to HSV-TK or E. coli CD. Likewise, Locket et al. [17] created identical replication-deficient adenoviral vectors expressing either HSV-TK or E. coli PNP and studied the relative efficacy of cell killing in head-to-head experiments. They concluded that E. coli PNP with MeP-dR was clearly superior in its ability to elicit cell death. Cells were killed more rapidly and less input virus was required with E. coli PNP. Puhlmann et al. [19] compared vaccinia viruses expressing either E. coli PNP or E. coli CD and concluded that the cytotoxic efficacy of E. coli PNP with MeP-dR was much more rapid and complete than that of E. coli CD.
These studies demonstrated that E. coli PNP is a very effective enzyme for use with GDEPT strategies, and that high bystander activity of the approach is a major distinguishing characteristic with respect to GDEPT using HSV-TK.
3. IN VITRO ACTIVITY OF E. COLI PNP WITH F-dAdo AND F-araAMP
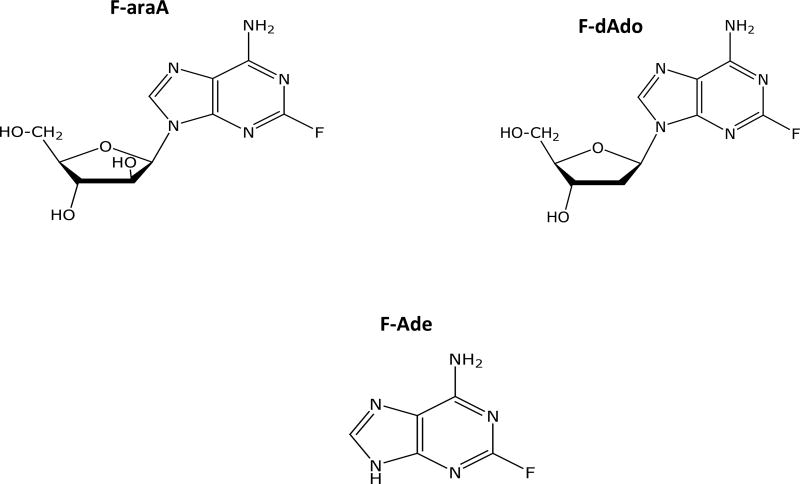
Two other deoxyadenosine analogs (2-fluoro-2’-deoxyadenosine, F-dAdo, and 9-β-D-arabinofuranosyl-2-fluoroadenine, F-araA) have also received considerable attention in conjunction with E. coli PNP (Figure 2). These two compounds are substrates of E. coli PNP and liberate 2-fluoroadenine (F-Ade), a very cytotoxic adenine analog that is approximately 100-fold more potent than MeP in in vitro cytotoxicity assays [27, 28]. F-dAdo and F-araA had been extensively studied as anti-cancer agents, but had initially not been considered for use with E. coli PNP, because they were known to be much more toxic than MeP-dR. In the ideal case the prodrug used in GDEPT strategies should have no toxicity. F-araA is approved for treatment of chronic lymphocytic leukemia and has well known toxicities when given to patients at effective doses. In the absence of E. coli PNP, these two compounds are toxic to proliferating tumor cells due to their activation (phosphorylation) by deoxycytidine kinase (Figure 3) and disruption of DNA synthesis [2, 29, 30], much like GCV. However, despite the well-known mechanism of tumor cell kill, these two nucleoside analogs have also been shown to serve as excellent prodrugs for use with E. coli PNP in vitro, including studies with numerous tumor cell types [24, 31–37].
Figure 2.
Structures of F-araA, F-dAdo, and F-Ade
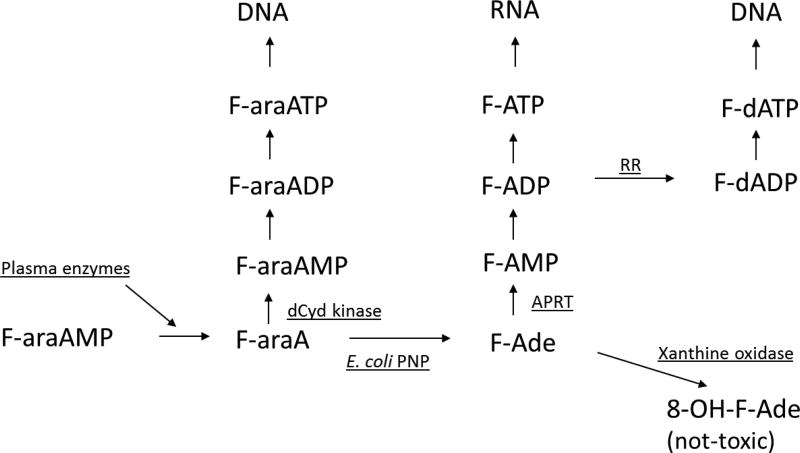
Figure 3.
Metabolism of F-araAMP in cell expressing E. coli PNP
In vitro GDEPT cytotoxicity studies with these two compounds (F-dAdo and F-araA) can be difficult to interpret because of their inherent cytotoxic activity. In other words, it is difficult to determine whether any in vitro cytotoxicity with these prodrugs is due to the generation of F-Ade. In addition, F-araA is an insoluble compound and it is administered to patients as F-araA-5’-monophosphate (F-araAMP), which must first be dephosphorylated to F-araA before it can enter tumor cells. In mice and in humans, the phosphate group is rapidly removed by plasma phosphatases to generate the primary circulating metabolite of F-araAMP, F-araA [30]. Because transport of F-araAMP into cells in culture is very limited and the generation of F-araA from F-araAMP is not well characterized in cell culture systems, the preferred compound to use in vitro experiments is F-araA.
Mohr et al. [31, 32] compared adenoviral vectors expressing either E. coli PNP or HSV-TK in various hepatocellular carcinoma cell lines, and using F-araAMP as a prodrug, they demonstrated efficient tumor cell killing at F-araAMP concentrations within the therapeutic range for humans. Cell death mediated by E. coli PNP occurred at much lower MOIs than HSV-TK, which was only effective at very high MOIs. Although the stability of F-araAMP was not determined in these experiments [31], F-araAMP had very little toxicity to cells that did not express E. coli PNP, suggesting that very little F-araAMP was dephosphorylated in these cell cultures and that the E. coli PNP system is even more potent than Mohr et al. concluded in their work. Xie et al. [38] compared adenoviral vectors expressing E. coli PNP or HSV-TK and concluded that E. coli PNP with F-araAMP was superior to HSV-TK with GCV in prostate cancer cells. Other research groups have supported these results by demonstrating the robust effectiveness of E. coli PNP and F-araAMP [24, 33–37]. Although it appears as though F-araAMP (not F-araA) was also used as prodrug in these studies, it is clear from these in vitro studies that F-araAMP could also serve as an effective prodrug in combination with E. coli PNP in numerous malignant cell types (thyroid carcinoma, neuroblastoma, kidney fibroblasts, melanoma, breast cancer, prostate cancer, and hepatocellular cancer).
4. IN VIVO ACTIVITY AGAINST TUMOR XENOGRAFTS THAT STABLY EXPRESS E. coli PNP
Based on the impressive in vitro activity observed with MeP-dR, we evaluated the antitumor activity against human tumor glioma xenografts (D54MG) that stably express the E. coli PNP gene [39]. Crude extracts from these cells were able to cleave 100 µM MeP-dR at a rate of 173 nmoles of MeP-dR cleaved per mg protein per hour. Parental D54 cell extracts are not able to cleave MeP-dR. Cells expressing E. coli PNP were implanted subcutaneously onto the flanks of mice and when tumors were approximately 150 mg, the mice were injected (intraperitoneally) with 67 mg/kg of MeP-dR daily for 3 consecutive days (total dose of 200 mg/kg). All of the E. coli PNP expressing D54 tumors rapidly regressed and all were tumor-free 50 days after cessation of MeP-dR treatment, which indicated that MeP-dR was very active against tumor xenografts expressing E. coli PNP [39]. Treatment with MeP-dR had no effect on D54 tumor xenografts that did not express E. coli PNP.
Although there was no significant weight loss in mice treated with MeP-dR in these experiments [39], previous studies had determined that this dose and schedule of MeP-dR was the maximally tolerated dose (MTD). Because of the experience at Southern Research Institute with development of anticancer nucleoside analogs, we were aware of the preclinical pharmacology and toxicology of F-dAdo and F-araA. When we observed the excellent activity of MeP-dR in human tumor xenografts expressing E. coli PNP and realized that the MTD of MeP-dR was less than that of either F-dAdo or F-araA (Table 1), we also evaluated these two compounds against D54 xenografts expressing E. coli PNP in mice. Mice can tolerate up to 20 mg/kg F-dAdo given 5 times daily for 3 days (total dose of 300 mg/kg) or 100 mg/kg F-araAMP given 5 times daily for 3 days (total dose of 1,500 mg/kg). As indicated above, F-Ade generated from these compounds by E. coli PNP is a very cytotoxic adenine analog that is approximately 100-fold more potent than MeP [27].
Table 1.
In vitro and In vivo toxicity of MeP-dR, F-dAdo, and F-araA
|
In vitro IC50 (µM) |
In vivo (maximally tolerated dose) |
Total dose (mg/kg) |
|
|---|---|---|---|
| MeP-dR | >100 | 67 mg/kg (3 doses) | 201 |
| F-dAdo | 0.1 to 1 | 20 mg/kg (15 doses) | 300 |
| F-araA/F-araAMP* | 1 to 10 | 100 mg/kg (15 doses) | 1,500 |
In vitro, F-araA; In vivo F-araAMP
Treatment of mice bearing E. coli PNP expressing tumors with F-dAdo resulted in excellent antitumor activity [14] that was as good as that seen with MeP-dR (sustained inhibition of tumor growth many days after cessation of F-dAdo treatment). The antitumor activity of F-dAdo was schedule dependent. F-dAdo resulted in only modest antitumor activity when administered with a dose and schedule similar to the schedule used with MeP-dR (100 mg/kg given daily for 3 days). However, when the dose was decreased to 20 mg/kg and given 5 times daily for 3 days, the antitumor activity of F-dAdo was as good as that seen with MeP-dR. Impressive antitumor activity was also observed with F-araAMP [39]. A complete response was achieved in all mice treated with F-araAMP, although all tumors eventually recurred 25 days following cessation of F-araAMP treatment. Neither compound was active against parental tumors that did not express E. coli PNP.
E. coli PNP expressing D54 cells were next mixed with parental D54 cells so that approximately 20% of the cells implanted in mice expressed E. coli PNP. When the tumors were approximately 150 mg, the mice were treated with MeP-dR, F-dAdo, or F-araAMP at their respective MTDs. Treatment with either MeP-dR or F-dAdo resulted in strong antitumor activity [14, 39, 40], which indicated good in vivo bystander activity. However, no activity was observed in mice treated with F-araAMP.
MeP-dR and F-dAdo are excellent substrates for E. coli PNP [14, 39]. The catalytic efficiency of E. coli PNP with these two compounds is very similar to that of adenosine, the natural substrate. However, F-araA is a very poor substrate for E. coli PNP, with a catalytic efficiency only 0.04% that of F-dAdo, which helps explain the relatively weak antitumor activity observed against these D54 xenografts.
In these experiments studies were done with radiolabeled compounds to determine how much of each prodrug was activated and retained in the tumors. Because the plasma half-life of both agents is very short (20 and 7 minutes, respectively [14]), there would be very little circulating prodrug 4 hours after administration, and the activation of either MeP-dR or F-Ado in tumor tissue would primarily occur soon after injection. Very little MeP or F-Ade would be formed and captured by the tumor cells 4 hours after injection of either compound. We found that there was a similar amount of MeP and F-Ade metabolites in tumor tissue 4 and 24 hours after injection of either 67 mg/kg MeP-dR or 20 mg/kg F-dAdo [14]. This result indicated that MeP and F-Ade metabolites are retained a very long time in tumor tissue. In these experiments less F-Ade was produced and trapped in the tumors after each injection of 100 mg/kg F-araA (0.27 mmoles injected) than after each injection of 20 mg/kg F-dAdo (0.07 mmoles injected), even though F-araA has 5-fold a longer plasma half-life than F-dAdo [14]. This result was consistent with the relative antitumor activity of the two prodrugs.
4.1 Activity in tumors expressing high levels of E. coli PNP activity
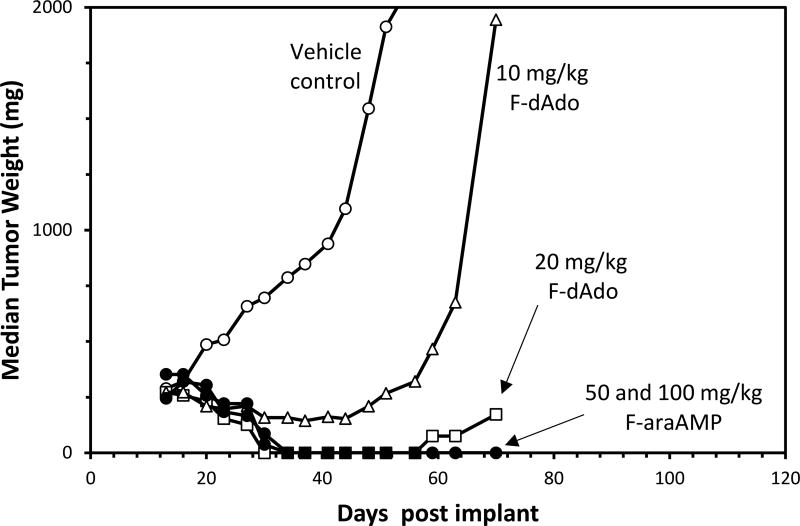
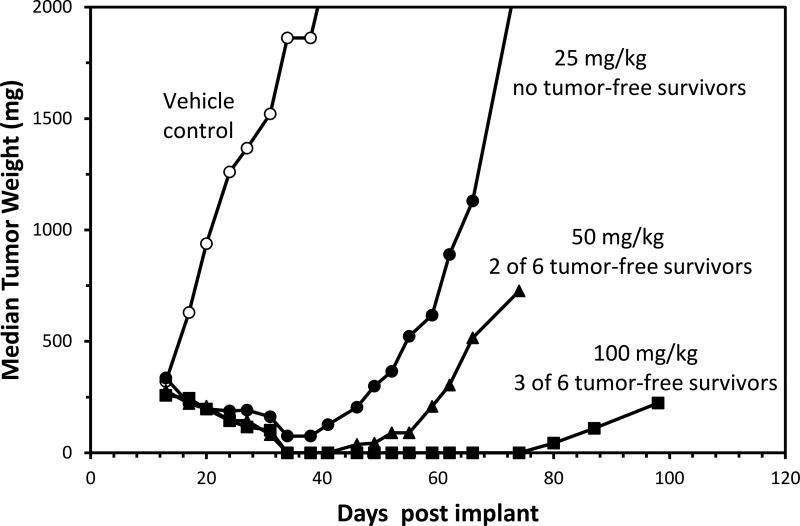
Although impressive antitumor activity was detected in the above studies, we wondered what would happen if the expression of E. coli PNP in the D54 tumor cells was increased. Therefore, a D54 tumor cell line was created that expressed much higher levels of E. coli PNP [41]. Crude extracts from this tumor cell line cleaved 100 µM MeP-dR at a rate of 126,000 nmoles/mg-hr, which was 700-fold higher than E. coli PNP activity in crude cell extracts used in the previous studies (173 nmoles/mg-hr). We were surprised to learn that the best prodrug against these D54 tumors was F-araAMP (Figure 4). Treatment with 50 or 100 mg/kg F-araAMP (q2hx5, q1dx3) resulted in 100% tumor-free animals more than 65 days following cessation of therapy, whereas treatment with 20 mg/kg F-dAdo (q2hx5, q1dx3) resulted in 100% tumor regressions and prolonged tumor suppression, but 3 of the 6 tumors subsequently regrew. Treatment with 10 mg/kg of F-dAdo resulted in excellent inhibition of tumor growth, but all tumors subsequently recurred. This result was puzzling, because F-dAdo is much better than F-araA as a substrate for E. coli PNP [14, 39] and there was little regrowth of F-dAdo treated tumors that expressed much lower levels of E. coli PNP [14]. Treatment with MeP-dR in this model was less effective than either F-dAdo or F-araAMP (unpublished observation), which could partially be explained by the increase in toxicity of MeP-dR observed in mice bearing E. coli PNP tumors. The dose of MeP-dR had to be reduced from 67 mg/kg (x3) to 17 mg/kg (x3) in mice bearing high expressing D54 tumors in order to avoid toxicity. Even so, treatment with MeP-dR at these lower doses resulted in excellent antitumor activity with sustained inhibition of growth many days after cessation of therapy. It is important to emphasize that the toxicity of MeP-dR, but not F-dAdo nor F-araAMP, was increased in mice bearing tumors expressing high levels of E. coli PNP [42]. None of these prodrugs were active against D54 tumors expressing a control gene (EGFP).
Figure 4.
Effect of F-araAMP and F-dAdo on tumors expressing high levels of E. coli PNP
Mice bearing subcutaneous D54 xenografts expressing E. coli PNP (126,000 nmoles/mg-hr) were treated with intraperitoneal F-dAdo or F-araAMP at the doses shown five times daily for 3 consecutive days beginning on Day 13. Six mice were treated per. Tumor size was measured using calipers, and the median tumor weight was plotted.
Curative activity was observed in mice bearing D54 tumors (100% of cells expressing E. coli PNP) with doses of F-araAMP as low as 15 mg/kg (q4hx3, q1dx3). This dose of F-araAMP is equivalent to 45 mg/m2 (total dose of 405 mg/m2), which is similar to the dose that can be achieved in humans (25 mg/m2 × 5, for a total dose of 125 mg/m2). Treatment of these tumors with 5 mg/kg F-araAMP (× 9, for a total dose of 135 mg/m2) resulted in significant tumor regressions and sustained inhibition of tumor growth. These results indicate that doses of F-araAMP that can be achieved in humans can dramatically affect all tumor cells expressing E. coli PNP in a body. It must be remembered that these doses of F-araAMP are very well tolerated in mice (MTD of F-araAMP is approximately 4500 mg/m2) and that these tumor responses occurred with little evidence of toxicity.
These impressive antitumor results with F-araAMP have been demonstrated in mice bearing large, slow growing human tumor xenografts (150 to 300 mg) that are resistant to standard chemotherapies. It is well known that established tumor xenografts are very difficult to treat and that larger masses are more resistant to cytotoxic chemotherapies than smaller ones. Therefore, the activity of E. coli PNP and F-araAMP against tumor masses that are approximately 1% of the total mouse weight provides a very stringent test for a cytotoxic chemotherapy, such as F-Ade. The fact that the therapy is very effective in causing these tumors to totally regress and not return (with negligible host toxicity) demonstrates that this strategy is very powerful in killing large slow growing tumor mass.
Based on the excellent results in tumors where 100% of the cells expressed E. coli PNP, studies were conducted with MeP-dR, F-dAdo, or F-araAMP using mixtures of tumors cells (10% expressing E. coli PNP) to evaluate in vivo bystander activity, and F-araAMP was once again shown to be the superior prodrug [42]. Treatment with F-araAMP completely cured mice when only 10% of the cells in a human tumor xenograft expressed E. coli PNP (all mice were tumor-free more than 60 days after cessation of F-araAMP therapy) [41–43]. Excellent antitumor activity was also observed in mice treated with F-araAMP when as few as 2.5% of the tumor cells expressed E. coli PNP [41], although there were no tumor-free survivors in mice at this dose. These results indicate that F-Ade generated by E. coli PNP in a small percentage of tumor cells is capable of reaching the vast majority (97.5%) of tumor tissue, including cells that do not express E. coli PNP. To our knowledge, no report has shown bystander killing with other prodrug activation strategies of the magnitude described here. An example of the excellent in vivo bystander activity observed with F-araAMP is shown in Figure 5 where excellent antitumor activity was observed against D54 tumor xenografts in which 10% of the tumor cells expressed E. coli PNP. The results in this figure also demonstrate that antitumor activity is dependent on the dose of F-araAMP administered. Treatment with F-araAMP resulted in tumor regressions and prolonged tumor suppression even at a dose (25 mg/kg) that was far below the MTD. The impressive in vivo results with F-araAMP demonstrate excellent in vivo bystander activity, and have been replicated in more than 10 separate in vivo experiments. A dose response has also been observed with MeP-dR [unpublished observation] and F-dAdo [14].
Figure 5.
Demonstration of dose dependence of F-araAMP antitumor activity and in vivo bystander activity against D54 xenografts
Mice bearing subcutaneous D54 xenografts in which 10% of the cells express E. coli PNP (14,000 nmoles/mg-hr) were treated with intraperitoneal F-araAMP at the doses shown five times daily for 3 consecutive days beginning on Day 13. Six mice were treated per group. Tumor size was measured using calipers, and the median tumor weight was plotted.
In one experiment, treatment with 100 mg/kg F-araAMP (q1dx3, q2hx5) resulted in excellent antitumor activity against D54 xenografts in which 5% of the tumor cells expressed E. coli PNP activity (7,600 nmoles MeP-dR cleaved/mg-hr at the time of treatment). There were 2 of 9 tumor free survivors and an excellent antitumor result in the other 7 mice (Significant regressions of these 7 tumors lasted for 40 days with eventual regrowth). Sixty days following cessation of F-araAMP treatment, the tumors were removed and E. coli PNP activity was found to be very low (ranging from no activity to less than 0.5%) of original values). This result provides further evidence that the therapy had killed all (or most) tumor cells expressing E. coli PNP and that tumor recurrence following treatment originates from bystander tumor cells.
Interestingly, unlike F-dAdo administration to low expressing tumor cells [14], the activity of F-araAMP was highly effective and independent of schedule [43]. F-araAMP was equally effective when administered once, three, or five times per day. This result can be explained by the Km values for F-dAdo and F-araA; 22 and 960 µM, respectively [14]. Since the peak plasma concentration of 20 mg/kg F-dAdo (approximately 20 µM) is near the Km value, increasing the dose 5-fold would have only a small effect on its activation. However, because the peak plasma concentration of 100 mg/kg F-araA (150 µM) is far below the Km value, increasing the dose by 5-fold would result in roughly a 5-fold increase in activation. Therefore, similar amounts of F-Ade would be generated in tumor tissue after administration of 15 doses of 100 mg/kg F-araAMP or 3 doses of 500 mg/kg F-araAMP.
It is important to note that excellent antitumor activity is routinely achieved in these experiments after only 3 days of therapy. This result has significance, since in GDEPT strategies when only a small percentage of tumor cells express the transgene, it is important for the therapy to be immediately active against the entire tumor mass. If therapy does not immediately destroy the bystander cells, then cells expressing the activating gene would be killed early in the treatment cycle and later dosing would no longer generate the active agent. In addition, the effectiveness of these prodrugs after 3 days of therapy, which is a small percentage of the tumor doubling time, indicates that the therapy is effective against non-cycling tumor cells, which supports our in vitro observations [11–13, 15]. This is an important attribute for drugs designed to treat solid tumors that typically exhibit a low growth fraction.
These in vivo results with cell lines that express high levels of E. coli PNP indicate that F-araAMP is the preferred prodrug for use with E. coli PNP. The level of expression of E. coli PNP in tumors derived from the D54 tumor line was similar to that observed following injection of tumors with an E1a, E3 deleted adenoviral vector expressing E. coli PNP [41, 43], which indicated that these studies with artificial tumor cell lines expressing E. coli PNP at these levels could be predictive of the clinical situation at least with vectors that expressed high levels of E. coli PNP.
The fact that F-araAMP was identified as the best prodrug is of practical importance towards clinical development of this strategy, because F-araAMP (an approved agent for treatment of hematological malignancy but without activity against solid tumors) is well known to oncologists, as well as the FDA, and preclinical toxicity studies with this prodrug itself, were not necessary prior to initiating clinical trials. The established dose of F-araAMP used in patients with chronic lymphocytic leukemia is 25 mg/m2 daily for 5 consecutive days. In the experiment shown in Figure 5, we demonstrate excellent antitumor activity against tumors in which 10% of the cells express E. coli PNP at a dose of 25 mg/kg F-araAMP (q2hx5, q1dx3), which is equivalent to approximately 75 mg/m2 in a mouse. F-araAMP is very effective therefore in mice at a total dose 9-fold greater than that well tolerated in humans. Because the plasma half-life of F-araAMP in humans is 10-fold longer than that in mice [14, 30], it is reasonable to expect that excellent bystander activity will also be observed in the treatment of human disease. Unpublished studies in which all tumor cells express E. coli PNP activity demonstrated excellent antitumor activity (100% tumor regressions with subsequent regrowth delayed by 40 days) at a dose of 5 mg/kg (15 mg/m2) given 3 times a day for 3 days, for a total dose of 135 mg/m2, which is near the total human dose (125 mg/m2). This result indicates that doses of F-araAMP can be achieved in humans that should destroy any cell that expresses E. coli PNP activity and should eliminate numerous bystander cells, as well. F-araAMP without E. coli PNP is routinely repeated every 28 days in human patients. These considerations indicate that the therapy could be repeated in patients after a suitable recovery period in order to address any remaining tumor burden.
4.2 Comparison of antitumor activity of F-araAMP with F-dAdo
Our results with tumors that express high levels of E. coli PNP indicate that F-araAMP is the preferred prodrug for use with E. coli PNP. It is not clear why this should be the case, and F-araAMP was not initially considered as a prodrug of choice, because it is a very poor substrate for E. coli PNP and is cytotoxic. As noted above the catalytic activity of E. coli PNP with F-araA is 0.04% that of F-dAdo [14]. The Km for F-araA is 44-fold higher than that of F-dAdo (960 vs 22 µM) and the Vmax of F-dAdo is 71-fold greater than that of F-araA (426 vs 6 µmole/mg-hr). It is doubtful, therefore, that this compound would have been evaluated as a potential prodrug with E. coli PNP, except for the fact that F-araAMP was readily available in our laboratory in sufficient quantities for in vivo testing (The antitumor activity of F-araA was discovered at Southern Research Institute and much of its preclinical testing occurred there). In addition, F-araAMP is commercially available and is therefore readily available to other investigators. It is important to note that treatment of just one group of six mice with 100 mg/kg F-araAMP (q2hx5, q1dx3) required 225 mg of compound, and that numerous in vivo studies have been conducted by our laboratory with this agent over a period of more than 15 years. Such quantities are not easily obtained by an academic drug discovery program unless there is reasonable rationale to support its synthesis. Because of the relatively poor cleavage of F-araA by E. coli PNP, there would not likely have been sufficient rationale to spend the considerable effort to synthesize compound needed for in vivo efficacy studies. We are indebted to Schering in Berlin Germany for providing much of the drug used in our preclinical studies.
The superior antitumor activity in tumors expressing high levels of E. coli PNP observed with F-araAMP with respect to F-dAdo correlated with the amount of F-Ade metabolites generated in tumor cells in vivo [42]. Four hours after intraperitoneal injection of 100 mg/kg F-araAMP, there were 122 nmoles of F-Ade metabolites (per gram of tissue) in D54 tumors in which 10% of the cells expressed E. coli PNP, whereas there were only 10 nmoles of F-Ade metabolites per gram of tumor tissue 4 hours after administration of 20 mg/kg F-dAdo. The first factor in explaining the superior anti-tumor activity of F-araAMP is that mice tolerate 5-fold more F-araAMP than F-dAdo. Secondly we have shown that the plasma half-life of F-araAMP in mice is 7-fold that of F-dAdo (50 vs 7.4 minutes [14]). Although differences in these two parameters suggest that tumor cells will be exposed to 35-fold more F-araAMP than F-dAdo at their respective MTDs in mice, tumor cells will be exposed to peak concentrations of F-araA (approximately 150 µM) that are only 16% of its Km value (960 µM), whereas tumor cells will be exposed to peak concentrations of F-dAdo (approximately 20 µM) that are 100% of its Km value. Using the Michaelis-Menten equation the rate of activation of F-dAdo at 20 mg/kg would be approximately 213 µmoles/mg-hr and for F-araA at a dose of 100 mg/kg it would be approximately 1 µmole/mg-hr. Therefore, these differences in plasma peak concentrations and half-life cannot explain the enhanced metabolism of F-araA in tumor cells.
Recently, in vitro studies in cell culture have identified another reason for the enhanced activity of F-araA [42]. Despite differences in catalytic efficiency between these two compounds, similar amounts of F-Ade are produced and activated in D54 tumor cells expressing E. coli PNP (126,000 nmoles/mg-hr) during a one-hour incubation regardless of whether cells are treated with 10 µM F-dAdo or F-araA. The reason for this better than expected cleavage of F-araA relative to F-dAdo is not understood, but these results indicate that E. coli PNP reaction in these D54 tumor cells does not obey classical Michaelis-Menten kinetics. Perhaps this observation will ultimately provide some insight regarding the ways in which enzymes operate when over-expressed in the cellular environment. Petsko [44] has pointed out that studies characterizing enzymatic activity are conducted in dilute aqueous solution (where the substrates are vastly in excess of the protein) and therefore may not reflect the situation inside a cell that is almost certainly the opposite. This result suggests that if a molecule of F-araA or F-dAdo enters a cell expressing E. coli PNP, then it will be efficiently cleaved regardless of its Michaelis-Menten parameters.
Interestingly, for tumors in which 100% of the cells express low amounts of E. coli PNP (173 nmoles/mg-hr, [14]) the levels of F-Ade metabolites in tumor tissue 4 hours following administration of 20 mg/kg F-dAdo was greater (57 nmoles per gram of tissue) than the amount of F-Ade metabolites after 100 mg/kg F-araAMP (34 nmoles per gram of tissue). Assuming that the metabolism of F-dAdo and F-araAMP would be reduced by 90% in tumors composed of 10% E. coli PNP expressing tumor cells, this finding indicates that administration of F-dAdo would result in 5.7 nmoles F-Ade metabolites per gram of tissue compared with 3.4 nmoles of F-Ade metabolites per gram of tumor tissue following F-araAMP. Comparing results in tumors expressing high [42] versus low [14] levels of E. coli PNP indicate that increasing E. coli PNP activity by 700-fold in tumor tissue has a small impact on activation of F-dAdo (2-fold) in tumor tissue, but a very large impact on the activation of F-araAMP (36-fold). This result suggests that the activation of F-dAdo is saturated at relatively low levels of enzyme expression, whereas increasing the level of E. coli PNP in tumor tissue results in increased activation of F-araAMP.
4.3 Adenine phosphoribosyltransferase (APRT)
The enzyme responsible for activating both MeP and F-Ade to cytotoxic nucleotides is APRT. Therefore, E. coli PNP expressing D54 cells (126,000 nmoles/mg-hr) were transduced with mammalian APRT to create a cell line that expresses levels of APRT approximately 60-fold greater than that in wild-type D54 cells, and in vivo studies were conducted to determine the effect of increased APRT activity on efficacy of GDEPT using E. coli PNP. D54 tumors expressing both E. coli PNP and enhanced APRT in 10% of the tumor cells were as sensitive to the three prodrugs (MeP-dR, F-dAdo, F-araAMP) as tumors that only expressed E. coli PNP activity [42], which indicated that activation of either MeP or F-Ade was not rate limiting for antitumor activity of these prodrugs. This conclusion is supported by an observation that similar amounts of MeP or F-Ade nucleotides are generated in tumor xenografts expressing E. coli PNP alone or E. coli PNP plus APRT following intraperitoneal administration of MeP-dR, F-dAdo, or F-araAMP.
A D54 cell line was also generated from wild-type cells that expressed increased APRT activity (170-fold above baseline), but not E. coli PNP. When mixed with cells expressing E. coli PNP, such that 10% of the cells expressed E. coli PNP and 90% expressed excess APRT, we noted a surprising result. Whereas the antitumor activity of MeP-dR was greatly enhanced, the efficacy of F-araAMP was greatly diminished, and the antitumor activity of F-dAdo was unchanged. The increased antitumor activity of MeP-dR was associated with 2.5-fold increase in MeP nucleotides in tumor xenografts, which suggested that increased APRT in bystander cells resulted in increased activation of MeP and augmented bystander cell killing. However, the F-Ade nucleotides in tumor xenografts after administration of either F-dAdo or F-araAMP were not changed in D54 tumors with increased APRT activity in bystander cells. The reason that the antitumor activity of F-araAMP was decreased in these tumors is not clear. Although these results are interesting, they do not have practical significance since it is not possible to reproduce this artificial situation in clinical disease.
4.4 Toxicity considerations of using GDEPT to generate MeP or F-Ade in tumor tissue
We and others were initially concerned about the potential for toxicity of GDEPT strategies utilizing E. coli PNP, because of the potent cytotoxicity of MeP and F-Ade, their ability to kill non-proliferating host cells, and their high bystander activity. It was not known whether significant amounts of either MeP or F-Ade formed in tumor tissue would escape into the systemic circulation resulting in toxicity to surrounding tissues and elsewhere. However, the initial in vivo studies in tumor xenografts with stable E. coli PNP expression were very encouraging, because they clearly demonstrated that very toxic compounds (MeP and F-Ade) could be selectively created in tumor tissue by E. coli PNP, resulting in complete regression of tumor masses with little or no toxicity to the host. The MTDs of F-araAMP and F-dAdo were the same in mice bearing parental tumors and E. coli PNP expressing tumors, which suggested that very little F-Ade produced in tumor tissue diffused out of the tumor mass during treatment. This was not the case with MeP-dR, where its dose had to be reduced in mice bearing tumors expressing E. coli PNP to avoid serious toxicity.
Because MeP-dR is not cytotoxic to cells in culture, we were surprised to find that of the three prodrugs tested (MeP-dR, F-dAdo, and F-araAMP), it was the most toxic. MeP-dR is inert in cell cultures, because it is not activated by any human enzyme. We hypothesized that its in vivo toxicity must be due to bacterial enzymes associated with mice. To test this hypothesis, MeP-dR was administered to normal and gnotobiotic (germfree) mice, and it was determined that gnotobiotic mice could tolerate much higher amounts of MeP-dR than normal mice [40]. In addition, pretreatment of mice with oral non-absorbable antibiotics allowed for higher doses of MeP-dR to be administered. It is of interest to note that increased administration of MeP-dR also resulted in enhanced antitumor activity, indicating that strategies to increase prodrug administration could be used to improve antitumor activity. These results indicate that gastrointestinal bacteria are responsible at least in part for generating MeP and that the toxicity of MeP-dR in non-tumor bearing mice is due to circulating MeP.
It is of interest that the MTDs of both F-dAdo and F-araAMP were not affected by treatment with non-absorbable antibiotics (unpublished observation), which indicates that any F-Ade generated in the gastrointestinal tract does not contribute to the toxicity of either agent. F-araA is a very poor substrate for bacterial PNP, but F-dAdo is as good as MeP-dR as a substrate for this enzyme. Therefore, it could be expected that as much F-Ade is generated from F-dAdo by intestinal bacteria as MeP is generated from MeP-dR. MeP is detected in plasma of mice treated with MeP-dR, but no plasma F-Ade was detected following treatment with either F-dAdo or F-araAMP [14]. Unpublished studies from our laboratory indicate that F-Ade is an excellent substrate for xanthine oxidase, and that MeP is not a substrate for this important catabolic enzyme. Xanthine oxidase is a ubiquitous enzyme in human tissues that oxidizes hypoxanthine and adenine at the 2 and 8 positions as the first step towards their excretion from the body. Because the fluorine atom at the 2 position in F-Ade prevents oxidation of this carbon, the product of the reaction of xanthine oxidase with F-Ade is 8-hydroxy-2-F-adenine, which is not toxic to human cells. Therefore, our results suggest that any F-Ade produced by gastrointestinal bacteria or tumor cells expressing E. coli PNP is rapidly deactivated to 8-hydroxy-2-F-adenine. Since MeP is not a substrate for xanthine oxidase, it is not deactivated and instead is absorbed from the intestine and/or released from tumor cells.
It is clear from our studies that MeP diffusing from tumor xenografts that express E. coli PNP significantly contributes to the toxicity of GDEPT with MeP-dR. It is possible that 9-β-ribofuranosyl-6-methylpurine (MeP-R) could also be released from dying tumor cells and that this compound could also contribute to the toxicity of this GDEPT strategy. As dead tumor cells are reabsorbed into the body, nucleotides and nucleic acids (DNA or RNA) containing MeP from these cells would be degraded by nucleases and phosphatases to MeP-R, which is not a substrate for human PNP and (in the absence of E. coli PNP) would be released into the systemic circulation. MeP-R is a much more potent cytotoxic agent than MeP (about 100-fold) [28], because MeP-R is an excellent substrate for adenosine kinase and is activated much more efficiently in human cells than MeP. After the first step in their respective activation the mechanism of toxicity of MeP-R and MeP is identical. Therefore, in the case of MeP, it is possible that a much more potent cytotoxic agent than MeP is released from dying tumor tissue and that this agent significantly contributes to the toxicity of any MeP prodrug used with E. coli PNP. Of course, if sufficient amounts of E. coli PNP are still present in the dying tumor tissue, then MeP-R would be converted to MeP, which would be released from the tumor mass. Since the toxicity of MeP-dR in mice with or without tumors is due to circulating MeP, any MeP (or MeP-R) that was released from the tumor tissue would increase circulating MeP levels (or worse add MeP-R to maximally tolerated dose of MeP) and therefore increase systemic toxicity.
Release of F-Ade from tumor tissue expressing E. coli PNP does not appear to contribute to the toxicity of either F-dAdo or F-araAMP. 2-F-adenosine (F-Ado) generated from F-Ade containing nucleotides could also be released from dying tumor cells. However the consequence of this possibility is much less serious than that of MeP-R, because the potency of F-Ado is similar to that of F-Ade (although F-Ade, but not F-Ado, would be deactivated by xanthine oxidase). No F-Ade was detected in plasma of mice treated with F-dAdo or F-araA [14]. Experiments have not been conducted to evaluate whether or not F-Ado is released from dying tumor cells.
There are at least three reasons that contribute to the lack of toxicity of F-Ade in GDEPT strategies using F-araAMP or F-dAdo and our inability to detect F-Ade in plasma of mice treated with either agent. First, F-Ade or F-Ado would be slowly released from tumor cells over a long period of time so that plasma levels would be very low. We have shown that the half-life of F-Ade metabolites in tumor tissue is quite long (>24 hours [10]). Second, any F-Ade or F-Ado that does escape tumor tissue would be diluted into the comparatively enormous plasma volume, also resulting in low plasma concentrations. Third, and possibly most importantly, F-Ade would be quickly detoxified by xanthine oxidase. Therefore, our results indicate that toxicity of GDEPT strategies using F-Ade prodrugs is only due to the well-known mechanisms of action associated with F-dAdo and F-araA (i.e., disruption of DNA replication, [2, 29]) and does not result from the generation of F-Ade either in the tumor or other tissues, such as the intestine.
4.5 In vivo activity against various tumors and with various vectors
The in vivo studies discussed above with tumors that stably express E. coli PNP were important in demonstrating proof-of-concept for the overall GDEPT strategy. The studies have also increased our knowledge regarding important mechanistic aspects of E. coli PNP based GDEPT, which helps differentiate this strategy from others such as HSV-TK or E. coli CD. However, these studies do not reveal a practical method for treating patients with an existing tumor. Therefore, we prepared an adenoviral vector (E1a, E3 deleted) expressing a modified E. coli PNP gene (Ad/PNP, Gedeptin™) and have evaluated it as a delivery vehicle to human tumor xenografts in mice [41, 43].
In studies using Ad/PNP, large subcutaneous tumors (approximately 300 mg) were injected with virus (2 × 109 PFU or ~4 × 1010 VP) suspended in 150 µl saline. The vector was injected into various locations within the tumor (approximately 25 µl each injection) along separate needle tracks in an effort to evenly distribute virus throughout the tumor mass. Two days following vector injection mice were treated systemically with prodrugs as described above. Studies have been conducted with MeP-dR, F-dAdo, and F-araAMP (unpublished observations) and consistent with the stably transfected tumor studies, the best results were observed with F-araAMP. Treatment with Ad/PNP plus F-araAMP resulted in significant inhibition of D54 tumor growth [41], although there were no tumor free survivors at the conclusion of the study. The expression of E. coli PNP in a tumor mass following injection of Ad/PNP [41, 43] was in the same range as expression of E. coli PNP in stably transfected D54 xenografts where 5 to 10% of cells expressed E. coli PNP (5,000 to 10,000 nmoles/mg-hr).
An adenoviral vector identical to Ad/PNP (except that EGFP replaced E. coli PNP) was used to determine transduction efficiency following injection, and it was found that vector was concentrated in regions surrounding the needle tracks where more than 50% of the cells expressed the protein. Overall considerably less than 10% of the cells in the tumor mass expressed the reporter gene. These results indicate that E. coli PNP delivery by adenoviral vectors is not evenly distributed within human tumor xenografts (in contrast to mixtures of tumor cells injected into the flanks of mice). We believe that this difference may help explain the difference in efficacy observed between experiments using stable cell lines and recombinant adenoviral vectors [41]. In support of this conclusion, formation of F-Ade nucleotides in tumor xenografts following injection of F-araAMP into Ad/PNP-treated tumors was considerably less than that seen in experiments using tumor xenografts created from mixtures of E. coli PNP expressing cells.
These results indicate that Ad/PNP plus F-araAMP can destroy sections of a tumor mass where the vector has been injected, but may not be able to reach areas far from the injection site; i.e., emphasizing the importance of vector delivery to the efficacy of this overall GDEPT strategy. Although E. coli PNP activity was also detected in the liver following intratumoral injection of Ad/PNP, the dose of F-araAMP did not need to be reduced, suggesting that ectopic expression of E. coli PNP in host tissues did not enhance F-araAMP toxicity. This was not the case for MeP-dR: As in previous studies the amount of MeP-dR that could be tolerated in mice was decreased following intratumoral injection of Ad/PNP. Other research groups have prepared adenoviral vectors expressing E. coli PNP and have demonstrate strong antitumor activity against hepatocellular and prostate xenografts in mice in conjunction with MeP-dR [45, 46] or F-araAMP [31, 38, 47–51].
In an effort to improve antitumor activity of this approach, we evaluated the antitumor activity when both Ad/PNP and F-araAMP were injected into the tumor tissue. Excellent antitumor results were observed [43] in three tumor types (glioma, prostate, nonsmall cell lung) following intratumoral injection of F-araAMP two days after intratumoral injection of Ad/PNP, which was substantially superior to that seen after intraperitoneal injection of F-araAMP after intratumoral Ad/PNP [41]. In these experiments, tumors were injected with 18 mg of F-araAMP daily for three days, 2 days after intratumoral injection of Ad/PNP. Intratumoral injection of maximally tolerated doses of F-Ade (1.26 mg daily for 3 days) had little, if any, antitumor activity, which indicated that simply injecting F-Ade into a tumor mass would not be an effective antitumor strategy. Administration of 18 mg F-araAMP into a human subject (surface area of 1.6 meter2) is equivalent to 11.25 mg/m2, which is less than that used in the treatment of chronic lymphocytic leukemia (25 mg/m2 daily for 5 days). This result suggests that intratumoral Ad/PNP followed by intratumoral F-araAMP could be very effective in control local tumor masses in humans at doses of F-araAMP known to be well tolerated in humans.
Our in vivo results with E. coli PNP have been confirmed by numerous research groups, and all published in vivo studies that evaluate the effectiveness of GDEPT using E. coli PNP are presented in Table 2. This body of work establishes that E. coli PNP is effective with numerous viral vectors (adenovirus, measles virus, retrovirus, vaccinia virus, foamy virus, herpes simplex virus), as well as bacterial delivery vehicles (S. Typhimurium) and mechanical means such as electrogene transfer and cationic liposomes. E. coli PNP plus F-araAMP has been shown to be effective in treatment of numerous cancer histotypes, including pancreas, glioma, hepatocellular, prostate, lung, mammary, melanoma, lymphoma, colon, bladder, and ovarian. In addition, we showed that combinations of F-araAMP with radiation resulted in improved antitumor activity [43]. Since radiation therapy is often used to control local tumor growth, this result indicates that Ad/PNP plus F-araAMP could be combined with radiation therapy in the treatment of numerous tumor types.
Table 2.
In vivo antitumor studies demonstrating efficacy of E. coli PNP against numerous human tumor xenografts in mice
| Vector | Prodrug | Histotype | Reference |
|---|---|---|---|
| Constitutively expressed | MeP-dR | Pancreas | 116, 117 |
| Constitutively expressed | MeP-dR/F-dAdo/F-araAMP | Glioma | 39 |
| Constitutively expressed | MeP-dR/F-dAdo/F-araAMP | Glioma | 14 |
| Constitutively expressed | MeP-dR | Glioma | 69 |
| Constitutively expressed | MeP-dR/F-dAdo/F-araAMP | Glioma | 42 |
| Constitutively expressed | 5’-methyl(talo)-MeP-R | Glioma | 104 |
| Adenovirus | F-araAMP | Hepatocellular | 31 |
| Adenovirus | MeP-dR | Prostate | 45, 46 |
| Adenovirus | F-araAMP | Prostate | 47 |
| Adenovirus | F-araAMP | Prostate | 38 |
| Adenovirus | F-araAMP | Glioma | 41 |
| Adenovirus | F-araAMP | Glioma/Prostate/Lung | 43 |
| Ovine Adenovirus | F-araAMP | Prostate | 48–51 |
| S. typhimurium | MoP-dR/MeP-dR | Mammory | 25*, 103 |
| S. typhimurium | MeP-dR | Melanoma | 118 |
| S. typhimurium | F-dAdo | Unknown | 119 |
| Invasive E. coli | MeP-dR | Pancreas | 120 |
| Measles Virus | F-araAMP | Lymphoma | 121 |
| Measles Virus | F-araAMP | Pancreas | 122 |
| Measles Virus | MeP-dR | Colon | 123 |
| Measles Virus | F-araAMP | Lymphoma | 124 |
| Retrovirus | F-araAMP | Glioma | 125 |
| Retrovirus | F-araAMP | Bladder | 126 |
| Vaccinia | MeP-dR | Colon | 19 |
| Electrogene transfer | MeP-dR/F-araAMP | Pancreas | 127 |
| Foamy virus | MeP-dR | Glioma | 128 |
| Cationic liposomes | MeP-dR | Ovarian | 129 |
| Herpes Simplex Virus | MeP-dR | Glioma | 40 |
MeP-dR was very active in tumors treated with the control vector that did not express E. coli PNP activity. This could be explained by the fact that S. typhimurium naturally express a PNP that cleaves MeP-dR. Others have shown that cleavage of purine analogs in bacterial cells is linked to the transport of purine nucleosides into the cells [130]. Therefore, purine nucleosides are cleaved as they enter bacterial cells, and if PNP activity is increased intracellularly this will not increase production of MeP. Our unpublished work agrees with the prior observations [130] that increased expression of E. coli PNP in S. typhimurium does not result in enhanced cleavage of MeP-dR in S. typhimurium cell culture.
5. PHASE I CLINICAL TRIAL WITH E. coli PNP AND F-araAMP
The preclinical studies described above suggest that Ad/PNP plus F-araAMP could be very effective as a treatment for solid tumors. Because Ad/PNP is not a targeted vector, it must be delivered to tumor tissue using a syringe, and therapies based on Ad/PNP are therefore limited to treatment of locally invasive solid tumor masses for which other treatment options do not exist. Because of the anatomic complexity in the head and neck region, cancers that develop here are prime candidates for treatment with Ad/PNP plus F-araAMP. Surgery, radiation, and chemotherapy are often used within this setting to eradicate or control local disease, but these therapies are not curative in 60% of patients and can result in significant morbidity [52]. Because Ad/PNP plus F-araAMP is effective at reducing tumor mass with little or no significant toxicity, this approach could be used to control tumor growth, maintain quality of life during therapy, and enhance or perhaps replace existing treatments. We estimate that as many as 30,000 head and neck cancer patients per year in the US could benefit from this type of intervention. In addition, preclinical studies indicate that F-Ade is active against all tumor cell lines tested to date. Therefore, Ad/PNP treatment could be effective against local tumor masses of any histotype (prostate, glioma, vulvar, cervical, etc). Although Ad/PNP is not designed to treat disseminated metastatic disease, GDEPT based on E. coli PNP could also be effective in the treatment of metastatic disease, if vectors were developed that selectively express significant amounts of transgene in metastatic lesions.
A small company (PNP Therapeutics) was formed to commercially develop Ad/PNP plus F-araAMP for treatment of solid tumors. Funds were acquired to produce clinical grade Ad/PNP, to conduct IND enabling toxicology studies, and to conduct a phase I clinical trial.
5.1 Head and neck cancer
Because of the unmet clinical need, the initial target patient population for Ad/PNP is head and neck cancer, which refers to a group of similar cancers originating from a variety of sites in the upper aero-digestive tract including the lip, oral cavity, nasal cavity, paranasal sinuses, pharynx and larynx. Ad/PNP plus F-araAMP obtained orphan drug status from the FDA in 2015 for “treatment of anatomically accessible oral and pharyngeal cancers (lip, tongue, gum, floor of mouth, salivary gland, and other oral cavity)”. The most common head and neck cancers (~90%) are squamous cell carcinomas (HNSCC) that originate from the mucosal lining of these regions. Cancers of this type often spread to the lymph nodes of the neck, representing the sentinel sign of disease, and leading to diagnosis. HNSCC is the 6th leading cancer by incidence worldwide, with approximately 550,000 cases/year globally, and 55,000/year in the US. These cancers are usually treated with surgery, radiation, and/or chemotherapy. Approximately 70% of head and neck cancers are not discovered until they are in advanced stages, with 60% of tumors at stage III and approximately 10% of tumors at stage IV, per year.
First-line therapy in locally advanced patients includes surgery, radiotherapy and concurrent chemotherapy (typically with cisplatin or carboplatin). Second-line interventions for patients with recurrent tumors is limited. Cetuximab (trade name Erbitux™) and chemotherapy are widely utilized in this clinical setting. The survival benefit associated with adding cetuximab to standard chemotherapy was found to be almost three months, increasing median overall survival from 7.4 to 10.1 months [52]. Despite these treatment options, numerous patients experience progression and/or further recurrent disease. A modest benefit in head and neck cancer has recently been reported with emerging immune activating agents, Opdivo and Keytruda [53, 54]. In a phase II trial of Keytruda for head and neck cancer patients, there were 28 overall responses among 171 patients, or a 16% overall response rate. Median progression-free survival was 2.1 months and median overall survival was 8 months. Opdivo was recently approved by the FDA for the treatment of patients with recurrent or metastatic HNSCC due to superior overall survival with Opdivo (7.5 months versus 5.1 months) with a response rate of 13%. Although these successes with check point inhibitors are significant, it is clear that new and more active agents need to be developed to treat this disease. Moreover, all current therapies, including surgery and radiation therapy, result in significant morbidity or toxicity, and there is need to identify effective and less toxic approaches for patients with HNSCC.
5.2 Preclinical toxicology
The IND-enabling toxicology study was performed with D54 tumor bearing mice. This study was designed to assess toxicities associated with Ad/PNP alone and in combination with F-araAMP. The protocol also included assessment of Ad/PNP tissue distribution and persistence following intratumoral administration of Ad/PNP to identify any secondary tissues where transduction by Ad/PNP followed by F-araAMP in non-target tissue could produce unanticipated amounts of F-Ade.
In this study only mild toxicities were observed in mice treated with Ad/PNP alone, F-araAMP alone, or with the Ad/PNP-F-araAMP combination. The no-observed-adverse-effect-level (NOAEL) of Ad/PNP alone was 0.71 × 1012 vp/m2, which was the dose equivalent to the maximum clinical dose used in the phase I clinical trial described below. The only toxicities observed with Ad/PNP alone were at a dose 10-fold greater, and these toxicities were quite mild. The side effects of F-araAMP alone were also evaluated at a dose that was 10-fold greater than that used in the highest dose in our phase I clinical trial. In this treatment group only mild toxicities were observed. Importantly, no new toxicities were observed in mice treated with the Ad/PNP plus F-araAMP combination, even though each part of the treatment was given at a dose that was 10-fold greater than that used in the phase I clinical trial. Consistent with our preclinical efficacy studies in mice, these results indicated that the generation of F-Ade in tumor tissue (and/or elsewhere) did not cause toxicity beyond that observed with the vector alone or F-araAMP alone. Overall the approach appeared to be very safe.
As expected the Ad/PNP DNA was detected in tumor tissue 6 days following administration of vector, but it was also detected at much lower levels in all tissues examined. In host tissues the liver had most of the Ad/PNP, but at levels (0.22 million copies/µg DNA) more than 100-fold below that in tumor tissue (67 million copies/µg DNA), which confirmed selective delivery of Ad/PNP. E. coli PNP enzyme activity was also detected in the liver. Even though Ad/PNP DNA was detected in host tissues, no toxicities were observed in the combination group that were not also observed in the groups treated with Ad/PNP alone or F-araAMP alone, which indicated that ectopic expression of Ad/PNP DNA in host tissues at these low levels was well tolerated. By the end of the study (28 days after the last Ad/PNP injection), levels of Ad/PNP DNA were significantly lower in all tissues, indicating progressive clearance of the adenovirus.
To supplement the IND-enabling Ad/PNP-F-araAMP toxicology study described above, a repeat dose, intravenous toxicology study in rats was performed with F-Ade. Toxicities observed after 7 consecutive days of IV administration were, for the most part, as expected for other chemotherapeutic agents of this type and included, but are not limited to, alopecia, reduction in food consumption, body weights and reticulocyte count, and increase in liver enzymes. Microscopically, the most significant findings were subacute inflammation and epithelial degeneration and/or regeneration of the cecum, colon, and rectum; mononuclear infiltrate in the endocardium; single cell necrosis in acinar cells of Harderian and mandibular salivary glands; and hypocellular bone marrow of the femur and sternum. By the end of the 14-day recovery phase, most of these findings had resolved. One unexpected clinical observation was transient ventral neck swelling that occurred in some of the animals during the recovery phase. Although the origin and toxicological significance of this finding are unknown, it is perceived that the occurrence is associated with an animal recovery mechanism, as the swelling was prominent for 4–6 days and then completely resolved; i.e., no microscopic correlates were found. The NOAEL for F-Ade in this study was determined to be 1 mg/kg (6 mg/m2) when given in 7 daily doses.
5.3 Design of phase I clinical trial
PNP Therapeutics initiated a clinical trial [55, 56], entitled “Phase I, open-label study evaluating the safety of escalating doses of PNP adenovirus injected intratumorally with co-administration of fludarabine in subjects with advanced solid tumors” (IND 14271). In this trial Ad/PNP was injected into tumor tissue followed by intravenous (IV) administration of F-araAMP. The clinical trial was designed in a conventional 3+3 format with escalating doses of F-araAMP (5, 15, or 25 mg/m2) in the first three cohorts and escalating Ad/PNP in the fourth (Table 3). Five hundred microliters (500 µl) of Ad/PNP were injected into tumor tissue twice on day 1 (separated by about 6 hours) and once on day 2 of the treatment. F-araAMP was administered IV three times in a manner similar to its use in the treatment of chronic lymphocytic leukemia (CLL); i.e., once daily over a 30 minute period on days 3, 4, and 5 of treatment. As noted above one cycle of F-araAMP therapy in the treatment of CLL is 25 mg/m2 (IV) once daily for 5 consecutive days. Therefore, 60% of the standard dose of F-araAMP used in the treatment of CLL was administered to patients in the highest cohort in this trial.
Table 3.
Ad/PNP plus F-araAMP phase I clinical trial
| Cohort | Total Ad/PNP | Total F-araAMP (regimen) |
|---|---|---|
| 1 | 3 × 1011 VP (1 × 1011 VP × 3 inj) | 15 mg/m2 (5 mg/m2 for 3 days IV) |
| 2 | 3 × 1011 VP (1 × 1011 VP × 3 inj) | 45 mg/m2 (15 mg/m2 for 3 days IV) |
| 3 | 3 × 1011 VP (1 × 1011 VP × 3 inj) | 75 mg/m2 (25 mg/m2 for 3 days IV) |
| 4 | 3 × 1012 VP (1 × 1012 VP × 3 inj) | 75 mg/m2 (25 mg/m2 for 3 days IV) |
5.4 Summary of toxicity data
All twelve patients successfully completed therapy without serious adverse events attributable to the treatment (Table 4). Ad/PNP plus F-araAMP was well tolerated: No subject experienced a dose-limiting toxicity or a treatment-related serious adverse event (SAE). There were two grade 3 treatment-related adverse events associated with the therapeutic intervention. One subject in cohort 2 experienced grade 3 decreased lymphocyte count, which resolved spontaneously, and one subject in cohort 4 experienced Grade 3 tumor injection pain, which was relieved by Tylenol. Pain with subsequent Ad/PNP injection in this subject was managed with local lidocaine and did not lead to discontinuation of study treatment. There was no evidence for an increased incidence of treatment emergent adverse events (TEAE), treatment-related adverse events, or Grade 3/4 TEAEs across dose cohorts. The most frequently reported TEAE was injection site pain immediately following viral injection (in 11 of the 12 patients, 91.7%). The incidence of these events did not increase with dose, and most were mild or moderate in intensity. Other injection site reactions included injection site discharge (4 subjects receiving either 1011 or 1012 VP/dose, 33.3%); injection site erythema, hemorrhage, and pruritus (2 subjects each receiving 1011 VP/dose, 16.7%), and injection site paresthesia (1 subject receiving 1012 VP/dose, 8.3%). Prior to initiation of the study, there were concerns regarding the possibility of superficial or deep ulcerations in the tumor resulting from direct injection of the tumor. Although some necrosis was observed in patient 7, the lesion in that patient was previously ulcerated. No ulceration was observed in the other patients undergoing treatment.
Table 4.
Toxicity results of phase I clinical trial
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total | |
|---|---|---|---|---|---|
| TEAEs | 3 | 3 | 3 | 3 | 12 |
| TEAEs ≥ grade 3 | 2 | 3 | 1 | 1 | 7 |
| TEAEs leading to hospitalization | 1 | 0 | 1 | 1 | 3 |
| TEAEs leading to treatment termination | 0 | 0 | 0 | 0 | 0 |
| TEAEs leading to death | 0 | 0 | 0 | 0 | 0 |
| Treatment-emergent SAEs | 2 | 1 | 1 | 1 | 5 |
| Treatment-related AEs | 3 | 3 | 3 | 3 | 12 |
| Treatment-related AEs ≥ grade 3 | 0 | 1 | 0 | 1 | 2 |
| Treatment-related SAEs | 0 | 0 | 0 | 0 | 0 |
| Dose limiting toxicities | 0 | 0 | 0 | 0 | 0 |
TEAEs, Treatment-Emergent Adverse Events
AEs, Adverse Events
SAEs, Serious Adverse Events
5.5. Summary of efficacy data
Clinical monitoring of the tumors was performed using calipers to evaluate effect of treatment on injected tumor tissue (Table 5). The target lesions in 4 of the 6 patients that received low doses of F-araAMP (5 and 15 mg/m2 daily for 3 days, cohorts 1 and 2) did not grow during the 2 month follow-up period (stable disease). A strong anti-tumor response was observed in the 6 patients that were treated with the highest dose of F-araAMP (25 mg/m2 daily for 3 days). Two of the 6 patients experienced a complete response in the target lesion during the observation period, although the tumor in patient 8 subsequently returned and was 40% of the original mass on day 56. In the other patient that experienced a complete response, the tumor mass rapidly decreased to undetectable levels by day 13 and was less than 5% of the original mass on Day 28. This patient required additional tumor related treatment with surgical resection on day 54, and additional tumor measurements were not determined after day 28 because of this intervention. In all, 5 of the 6 patients in cohorts 3 and 4 experienced a partial response in the target lesions as determined on the last day of the observation period (day 56). In the 4 patients with a partial response who completed the 56 day observation period, the target lesion mass was decreased by 30, 60, 60, and 60%. Although one patient in the last cohort did not have an objective response, this patient’s tumor did not grow and it was only 80% of its original mass on day 56 and was therefore graded as “stable” disease. In contrast, 4 of 6 non-target tumor lesions in patients in cohorts 3 and 4 increased in volume by 33, 52, 105 and 108%. One non-target lesion did not grow and one non-target lesion decreased in volume by 38%. An important observation is that the antitumor effect seen in cohort 3 was similar to that in cohort 4. The only difference in the treatment between these two cohorts was that 10-fold more virus was injected into the tumor mass in cohort 4. These results suggest that E. coli PNP expression and/or F-araAMP activation may have been saturated at the low dose of vector.
Table 5.
Efficacy results of phase I clinical trial
| Best response observed at any time during the study. | |||||
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total | |
| Complete response | 0 | 0 | 1 | 1 | 2 |
| Partial response | 1 | 0 | 2 | 1 | 3 |
| Stable disease | 2 | 3 | 0 | 1 | 7 |
| Response observed at the end of the study. | |||||
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total | |
| Partial response | 0 | 0 | 3 | 2 | 5 |
| Stable disease | 1 | 3 | 0 | 1 | 5 |
| Progression | 2 | 0 | 0 | 0 | 2 |
Findings of the clinical trial indicate that generation of F-Ade in tumor tissues using Ad/PNP plus F-araAMP is safe, and although only a small group of patients have been treated, the results strongly suggest that this therapy is effective at treating local tumor disease. The effect on tumor volume occurred with much less morbidity than typically associated with surgery, radiation, or chemotherapy, suggesting that this treatment could easily combine with (or in some cases replace) conventional therapies for this disease. The pronounced effect on tumor volume after a single treatment cycle suggests that repeat administration of the therapy could be very useful against large tumor masses. Future clinical studies will evaluate repeat administration of Ad/PNP plus F-araAMP every 28 days and the effect of injecting Ad/PNP in larger volumes (5 vs 0.5 ml) to deliver vector to more of the tumor mass. We believe that these two changes will significantly improve tumor response without increasing toxicity.
6. MECHANISM OF ACTION OF MeP AND F-Ade
Although there are numerous adenine analogs that could be liberated by E. coli PNP, two compounds have received the most attention, MeP and F-Ade, because of their potent cell killing activity. The concentration of F-Ade necessary to inhibit CEM-CCRF cell growth by 50% (IC50) was 0.15 µM when the incubation period was just 4 hours [27]. MeP was much less potent than F-Ade (IC50 of 9 µM, 4 hour incubation). If MeP or F-Ade are not removed from cell culture during the 72 hour incubation period, then the IC50 of F-Ade decreased a small amount (to 0.1 µM), but that of MeP decreased 9-fold to 1.2 µM. These results indicate that significant quantities of MeP or F-Ade cytotoxic nucleotides accumulate in CEM cells during short incubation periods. Importantly, both compounds were much more potent that FUra, which had an IC50 value of 120 µM following 4 hours of incubation, and 7.2 µM when present for the 72 hour incubation period. MeP and F-Ade were first synthesized many decades ago and tested as potential anticancer agents, and both compounds were found to have no selectivity for tumor versus normal cells in animals when administered systemically [57].
The mechanism of action of these two agents are very different from all currently used anticancer drugs and all compounds under clinical development. These compounds represent an entirely novel mechanistic approach to killing tumor cells. Generally speaking, MeP and F-Ade are metabolized by the enzymes in the purine metabolic pathway to nucleotide analogs, which inhibit important metabolic functions in the cell resulting in cell death. These molecules disrupt basic cellular metabolism in all phases of the cell cycle and are therefore effective against tumor cells regardless of their proliferative state. These compounds should also destroy tumor stem cells, tumor vascularity, and tumor stroma because of their fundamental need for RNA and protein biosynthesis to remain viable. This unique mechanism of cell kill is a major reason for the substantial in vivo antitumor activity that has been described in the previous pages and distinguishes GDEPT using E. coli PNP from all other GDEPT strategies. We believe that the unique attributes of MeP and F-Ade (high potency, activity against non-proliferating cells, high bystander activity) are precisely those required to treat solid tumors, and that significant improvement in antitumor therapy could be achieved using existing vectors to deliver E. coli PNP.
Most MeP or F-Ade formed in tumor cells expressing E. coli PNP (at least 50%) readily diffuse into the cell culture medium rather than being activated to nucleotides [14], demonstrating that significant amounts of these compounds are accessible to neighboring tumor cells not expressing E. coli PNP after they are generated. This ability to readily cross cell membranes accounts for the high bystander activity that has been observed with GDEPT approaches utilizing E. coli PNP and is a major distinguishing characteristic with respect to GDEPT approaches utilizing HSV-TK. Although no formal transport studies have been conducted with either MeP or F-Ade, purine and pyrimidine bases are known to freely diffuse across cell membranes via es and ei equilibrative nucleoside transporters present in most, if not all, cell types [58, 59], and it is likely that these transporters mediate the transport of both MeP and F-Ade.
6.1 Metabolism and mechanism of action of F-Ade
The metabolism of F-araA in a human cell expressing E. coli PNP is summarized in Figure 3. In vitro studies have been conducted with F-Ade indicating that it is readily converted to 2-F-adenosine triphosphate (F-ATP) [27], which is the primary acid-soluble metabolite in cells. These results indicate that F-Ade is a good substrate for APRT and that its metabolites (F-AMP and F-ADP) are good substrates for the subsequent steps in metabolism: AMP kinase, and nucleoside diphosphate kinase, respectively. APRT is the rate limiting enzyme in the activation of F-Ade. Cells lacking APRT activity are resistant to F-Ade [28], indicating that its conversion to its nucleotide metabolites is required for its cell killing action. Significant cytotoxicity was observed when a relatively small amount of F-ATP was generated in cells (13% of the ATP pool). Numerous research groups have shown that F-Ade is converted to F-ATP in various other biological systems [28, 60–68]. 2-F-S-adenosylmethionine and 2-F-cyclicAMP have been detected in cells treated with 2-F-adenosine (F-Ado) [61–63]. Because the metabolism of F-Ade and F-Ado after the first metabolic step (APRT or adenosine kinase) is identical, these metabolites would also be expected in cells treated with F-Ade. Although these metabolites were not detected in our experiments [27], more than 90% of the radioactivity in the acid-soluble pool of CEM cells treated with [3H]F-Ade was detected in the triphosphate fraction, which indicated that 2-F-S-adenosylmethionine and 2-F-cyclicAMP are at best relatively minor metabolites of F-Ade in CEM cells. F-Ade is readily incorporated into both RNA and DNA in human tumor cells [27], with approximately 5 times more in RNA than in DNA. F-Ade has also been shown to be incorporated into a fraction that was insoluble in alcohol (mixture of RNA and DNA) in Tetrahymena pyriformis [69]. The fact that F-Ade is detected in DNA indicates that F-ADP is a substrate for ribonucleotide reductase, and that deoxynucleotide metabolites are also formed in cells and used for DNA synthesis. Although incorporation of F-Ade into DNA can result in toxicity [70], we believe that this effect of F-Ade is not the primary target responsible for the anticancer activity of this agent.
Treatment of cells with F-Ade resulted in the inhibition of protein, RNA, and DNA synthesis [27]. Protein synthesis was the predominant effect and the pattern of inhibition was similar to that seen with cycloheximide, a known inhibitor of protein synthesis. RNA and DNA synthesis are also inhibited by specific inhibitors of protein synthesis, such as cycloheximide, due to the decrease of critical enzymes with short half-lives. Therefore, the inhibition of RNA and DNA synthesis by F-Ade is likely the result of inhibition of protein synthesis (as is the case with cycloheximide). Studies have shown that F-dATP is a good substrate for DNA polymerase α [70]. It is readily incorporated into DNA by this enzyme, and unlike most anticancer nucleoside analogs incorporation of F-dAdo into DNA does not cause DNA chain termination. Because RNA and protein synthesis are two metabolic processes important to all cells, inhibition of these activities is consistent with the ability of F-Ade to kill all cells regardless of their proliferative state [27]. Inhibition of protein synthesis by cycloheximide is reversible and cells are not killed by brief exposures to this agent. However, this is not the case with F-Ade, because the inhibition of protein synthesis by these molecules is due to the production of nucleotide metabolites, which do not rapidly disappear from cells when the primary agent is removed. We have shown that F-ATP has a half-life of approximately 7 hours in CEM cells [27] and that F-Ade metabolites (F-ATP, F-Ade in RNA, and F-Ade in DNA) are retained in human tumor xenografts in mice for much longer than 24 hours [14]. Therefore, once F-Ade has been removed from the tumor cell by natural processes, the active metabolites within the cell continue to inhibit cellular targets responsible for cell death.
In contrast to these results with F-Ade, DNA synthesis was specifically inhibited in cells treated with FUra (the product of E. coli CD based GDEPT strategies), and FUra had little or no effect on protein or RNA synthesis [27]. These results are consistent with the large literature on the mechanism of action FUra [71, 72], which is converted in human cells to three primary metabolites (F-UTP, F-dUMP, and F-dUTP) that are responsible for its cytotoxicity. Although most of the FUra activated in a tumor cell is incorporated into RNA, the inhibition of thymidylate synthetase by F-dUMP, the incorporation of FUra into DNA, and the subsequent disruption of DNA synthesis is believed to be the primary effect of this agent that is responsible for its anticancer activity [72]. Inhibition of DNA synthesis is also the primary mechanism of action for GCV, the prodrug used with HSV-TK [73–76]. Once GCV is phosphorylated by HSV-TK, GCV-MP is then further phosphorylated by human nucleotide kinases to GCV-TP, which is a substrate for human DNA polymerases resulting in the inhibition of DNA chain elongation, inhibition of DNA replication, and cell death. One of the reasons that antitumor therapy with conventional agents has only modest activity against solid tumors is that most (if not all) conventional antitumor agents primarily target proliferating cells, whereas most solid tumors have a low fraction of actively dividing cells [77–79]. Our results demonstrate that the mechanism of cell kill by F-Ade is significantly different from that of both FUra and GCV-MP, in that it targets one or more enzymes not related to DNA synthesis. The mechanism of action of F-Ade is also quite different from all of the drugs currently used to treat cancer and those in clinical development.
The precise target of F-Ade is not known and may be difficult to determine, because of the numerous enzymes that accept adenosine nucleotides as substrates. It is possible that F-Ade metabolites could inhibit any one of these enzymes and that more than one of these enzymes could be inhibited. That being said, specific studies evaluating F-Ade nucleotide metabolites as substrates for various enzymes have found these metabolites to be good substrates for their respective enzymes: RNA polymerase isolated from Micrococcus lysodeikticus [60]; catechol-O-methyltransferase [61]; activation of protein kinase isolated from rat brain [61]; adenylate cyclase [62, 63] and DNA polymerase α [70]. In cells treated with high concentrations of F-Ado, the F-ATP pool can replace the ATP pool [61–63, 64, 65], which suggests that F-Ade and its metabolites are recognized as adenine and its metabolites by all of the enzymes involved in purine metabolism in human cells [80]. There are numerous protein kinases in tumor cells that utilize ATP as a phosphate donor, and many of these play an important role in tumor cell biology. Because of the small structural difference between ATP and F-ATP (replacement of a hydrogen atom with a fluorine at the 2 position of the purine ring) and the fact that the 2 position of the purine ring is far from the β and γ phosphates, we believe that most, if not all, enzymes that utilize ATP as a phosphate donor are unlikely to be inhibited by F-ATP and that these enzymes would likely interact with F-ATP as a phosphate donor as if it was ATP. We believe that it is likely that inhibition of protein synthesis by F-Ade is due to its incorporation into RNA and subsequent disruption of RNA function.
An important enzyme in RNA metabolism is adenosine deaminase acting on RNA (ADAR), which mediates the post-transcriptional deamination of adenosine residues in RNA to form inosine, known as A to I editing. This enzyme was first identified as “RNA unwindase” due to its ability to deaminate adenosine in double stranded regions in the RNA. Because inosine does not hydrogen bond with uridine, the double stranded regions within RNA become single stranded. ADAR is involved in many important processes and is and essential enzyme for normal development [81]. It is well known that F-Ado is a very poor substrate for adenosine deaminase, and if ADAR is similar to adenosine deaminase and is unable to deaminate F-Ade incorporated into double stranded regions of RNA, it is reasonable to imagine that incorporation of F-Ade into RNA could prevent unwinding of double stranded RNA and disrupt numerous important control mechanisms in the cell. We believe that this enzyme could be the primary target of F-Ade responsible for its potent cytotoxicity. Further studies are needed to test this hypothesis.
Krohne et al. [32] demonstrated that the induction of apoptosis by an adenoviral vector expressing E. coli PNP plus F-araAMP was independent of Fas/FasL signaling pathway and was similar in both p53-positive and p53-negative cells. They concluded that E. coli PNP GDEPT using F-araAMP may be superior to HSV-TK plus ganciclovir, because of its independence from p53 and the Fas/FasL signaling systems. Proteomic analyses of ovarian tumor cells treated with E. coli PNP plus F-araAMP indicated down regulation of proteins involved in oncogenesis/cancer drug resistance and up regulation of apoptotic and tumor suppressor proteins [82]. Cell cycle analysis confirmed induction of apoptosis by E. coli PNP plus F-araAMP. In addition, combination of E. coli PNP pus F-araAMP with standard chemotherapy of ovarian tumor cells (docetaxel and carboplatin) significantly improved cell kill in vitro and suggested that the doses of docetaxel and carboplatin could be reduced by 10 to 50-fold [82].
6.2 Metabolism and mechanism of action of MeP
MeP was first synthesized and evaluated in the 1950’s and was found to be a very toxic compound in several biological organisms. Although some biochemical studies were performed, very few were conducted using human or mammalian enzymes. We have evaluated the cytotoxicity of MeP in CEM cells and have shown that both the metabolism and mechanism of action of MeP are similar to F-Ade [27]. The primary intracellular metabolite of MeP is 6-methylpurine riboside 5’-triphosphate (MeP-R-TP), and MeP is incorporated into both RNA and DNA. Furthermore, like F-Ade, MeP is active against both non-proliferating and proliferating tumor cells due to its inhibition of RNA, protein, and DNA synthesis [27, 84]. However, there are significant quantitative differences in the utilization of MeP by two enzymes involved in purine metabolism that affect its potency. Most importantly, F-Ade is a much better substrate for APRT than MeP (approximately 100-fold) [27], which provides an explanation for the potent in vitro cytotoxicity of F-Ade with respect to MeP. In addition, MeP is a very poor substrate for xanthine oxidase [83] and therefore, unlike F-Ade, it is not rapidly deactivated in mice, which helps explain the much higher in vivo toxicity of GDEPT strategies using MeP-dR versus F-dAdo or F-araA.
As with F-Ade the actual target (or targets) responsible for inhibition of protein, RNA, and DNA synthesis by MeP metabolites is not known. It is possible, and perhaps likely, that MeP metabolites disrupt macromolecular synthesis in a manner distinct from F-Ade. The substitution of the 6 amino group of adenosine nucleotides by a methyl group could interfere with numerous enzymes that would not be affected by addition of a fluorine atom at the 2 position of the purine ring. In this respect, it is worth noting a series of enzymatic studies conducted many years ago with MeP nucleotides. This work has only been presented in an abstract [85], but indicated that 1) MeP-R-5’diphosphate (MeP-R-DP) was a good substrate for pyruvate kinase, 2) MeP-R-TP was a good substrate for hexokinase, and 3) MeP-R-TP was capable of replacing ATP in cell-free protein synthesis reactions and muscle contraction experiments. However, MeP-R-TP was not able to reverse thyroxin-induced mitochondrial swelling and MeP-R-DP was not used as a substrate for phosphoglycerate kinase. Although MeP nucleotides are not substrates for these two enzymes, it unclear whether these enzymes were inhibited in the presence of either MeP-R-TP or MeP-R-DP, respectively. Other studies have been conducted with MeP, but no clear mechanistic details were elucidated [86–88].
We believe it is likely that incorporation of MeP into RNA and the subsequent disruption of its function is also the primary activity of MeP that is responsible for its cytotoxicity. Because the carbon-carbon bond of MeP-R is quite different from that of a carbon-nitrogen bond of adenosine, incorporation of MeP-R into double stranded regions of RNA in place of adenosine would result in double stranded regions that could not be unfolded by ADAR. Conversely, since the 6- amino group of adenosine participates in one of the two hydrogen bonds formed when adenine undergoes Watson-Crick base pairing, it is possible that the 6 methyl group of MeP-R does not form hydrogen bonds with uracil, preventing formation of double stranded regions in the RNA in the first place [89, 90]. Either of these consequences related to incorporation of MeP into RNA could disrupt important metabolic processes and would be quite cytotoxic to the cell [81]. Further studies are needed to test this hypothesis.
6.3 Toxicity considerations of different GDEPT strategies
An initial concern with selectively generating compounds such as F-Ade or MeP in tumor tissues – particularly those agents that target nonproliferating as well as proliferating cells - was that the bystander activity would be unmanageable, and that normal, nonproliferating host cells would also be killed by these agents once F-Ade or MeP had been generated in tumor tissue and released into the general circulation. Although our in vivo studies indicate that this concern is valid with prodrugs that liberate MeP, numerous in vivo studies by our group and others have shown that this is not an issue with F-Ade prodrugs such as F-araAMP, which release F-Ade. Lack of systemic toxicity is likely due to the rapid de-activation F-Ade by xanthine oxidase. Toxicity is not a concern with the HSV-TK GDEPT strategies, because any GCV nucleotide from tumor tissue would be rapidly inactivated by serum phosphatases back to GCV, which is not toxic. The inability of cells to transport nucleotides coupled with rapid deactivation of GCV nucleotides represents a serious disadvantage of HSV-TK dependent strategies, since these features dramatically limit the ability of this approach to kill neighboring tumor cells that do not express HSV-TK. Although GDEPT strategies using E. coli CD would result in the release of FUra from a tumor mass, similar to that seen with MeP and F-Ade, it is unlikely to cause systemic toxicity because FUra is also rapidly deactivated by uracil dehydrogenase, it is much less potent than both MeP and F-Ade, and it is primarily toxic to proliferating cells.
7. DESIGN OF NEW PRODRUGS
Although excellent in vivo antitumor activity has been observed with MeP-dR, F-dAdo, and particularly F-araAMP, these agents have considerable toxicities that limit the amount of compound that can be administered to patients, and identification of less toxic prodrugs could lead to superior agents for use with E. coli PNP. The ideal drug would be inert when administered in vivo patients, but would be readily cleaved to a potent cytotoxic agent, such as MeP or F-Ade. Our results with F-araA indicate that a prodrug does not need to be a particularly good substrate for E. coli PNP in order to be effective in the treatment of large human tumor xenografts expressing E. coli PNP in mice. Low toxicity and relatively long plasma half-life seem to be more important characteristics in terms of determining in vivo anticancer activity.
In an effort to better understand the catalytic activity of E. coli PNP, numerous purine analogs have been synthesized [5, 91–98] and evaluated for activity with E. coli PNP. This information could be helpful in design of novel nucleosides containing either MeP or F-Ade with modifications in the sugar moiety that would allow cleavage by E. coli PNP, but prevent activation by human enzymes to cytotoxic nucleotides. Although a considerable amount of information regarding the substrate preferences of E. coli PNP has been learned from these studies [5, 97, 98], new prodrugs have not yet been identified.
We believe that the characteristics of F-Ade (high potency, high bystander activity, targeting of non-proliferating tumor cells, rapid inactivation) are responsible for the excellent in vivo antitumor activity seen with F-araAMP and E. coli PNP. F-Ade therefore represents a preferred adenine analog to be incorporated into prodrugs for use with GDEPT strategies based on cleavage of purine nucleoside prodrugs. Design of new compounds is not a trivial task due to the many endogenous enzymes that must be considered. A candidate prodrug must, of course, be a substrate for the nonhuman activating enzyme, but must not be activated by any human enzyme. By design all adenosine analogs are not substrates for human PNP [8]. However, there are three primary enzymes expressed in human cells that could potentially activate purine nucleosides to toxic metabolites in human cells: deoxycytidine kinase, the enzyme responsible for activation of F-araAMP to cytotoxic nucleotides [4], adenosine kinase, and methylthioadenosine phosphorylase (MTAP). Our studies have shown that activation by bacterial PNPs must also be avoided when considering prodrugs containing MeP [40].
7.1 Prodrugs containing an alternative cytotoxic base
Several purine bases are known that are toxic to human cells. Although these compounds share many mechanistic characteristics with F-Ade, it is likely that their mechanism of cell kill is distinct and could therefore be useful in GDEPT strategies using E. coli PNP. For example, 7-β-D-ribosyl-3-deazaguanine is a nontoxic nucleoside analog that has been shown to be active against bacteria due to its cleavage by bacterial purine phosphorylases [99]. 3-deazaguanine is a potent guanine analog [100], and when attached to the ribose or deoxyribose at the 7 position of a purine ring (rather than the 9 position), the 2 amino group is “up” relative to the ribose and the compound resembles adenosine. Therefore, 7-β-D-ribosyl-3-deazaguanine is cleaved by E. coli, but not human PNP. 2-amino-6-chloro-1-deazapurine is also a toxic purine base [101], and the riboside containing this base, which is not cytotoxic, is a reasonable substrate for E. coli PNP (Activity about 10% that of MeP-dR or F-dAdo [5]). These compounds have not been evaluated in GDEPT with E. coli PNP.
Cladribine (2-Cl-2’-deoxyadenosine, Cl-dAdo) is a dAdo analog that is currently approved for use in the treatment of hairy cell leukemia and could also be a candidate for GDEPT using purine cleavage enzymes. It is structurally similar to F-dAdo (2-F-2’-deoxyadenosine) and it is a good substrate for E. coli PNP (~10% of the activity seen with F-dAdo [5]). 2-Chloroadenine (Cl-Ade) is not a potent cytotoxic agent, but its IC50 of 5 to 15 µM [28, 102] is similar to that of FUra and MeP (approximately 2 and 10-fold less potent, respectively). Because Cl-dAdo is an approved clinical agent, we evaluated its antitumor activity against D54 tumors in which 10% of the cells expressed E. coli PNP. However, no antitumor activity was seen with Cl-dAdo [unpublished observation], mostly likely due to relatively low activation by E. coli PNP and the less potent cell killing activity of Cl-Ade.
Fu et al. [25, 103] have demonstrated good antitumor activity with both 6-methoxypurine-2’-deoxyriboside (MoP-dR) and MeP-dR against mammary cells infected with S. typhimurium expressing E. coli PNP. However, MoP-dR was more toxic than MeP-dR [25], suggesting that it would not serve as a useful prodrug.
7.2 Alternative enzymes
There are numerous adenosine cleavage enzymes (hydrolases and phosphorylases) expressed in nature that could be used to selectively activate purine nucleoside analogs in tumor tissues using GDEPT. We and others have evaluated many of these enzymes, including attempts to design new enzymes. Examples of these studies are summarized below.
7.2.1 Design of mutant E. coli PNP/prodrug combinations
Our previous findings establish that generation of MeP from MeP-dR by intestinal bacteria is responsible for the toxicity of MeP-dR in mice [40]. Since both the toxicity and efficacy of MeP-dR are linked to the activity of E. coli PNP, a research program was initiated to design modified E. coli PNP enzymes that could activate MeP prodrugs but would not be activated by wild-type E. coli PNP [104]. Previous studies had determined that 5’-methyl(talo)-MeP-R (9-(6-deoxy-β-D-talofuranosyl)-6-methylpurine) was a very poor substrate for E. coli PNP (its rate of cleavage was 0.2% that of MeP-dR, [5]). E. coli PNP mutants were therefore designed based on structural information regarding the catalytic site of E. coli PNP bound to MeP-dR [97, 98, 105] in an attempt to create an enzyme that would efficiently cleave 5’-methyl(talo)-MeP-R to MeP. Each mutant enzyme was evaluated against a panel of 17 purine analogs with structural modifications in the deoxyribose portion of the molecule. Using this strategy, a mutant enzyme (M64V, replacement of methionine at position 64 in E. coli PNP by valine) was identified that cleaved 5’-methyl(talo)-MeP-R with a catalytic efficiency 130-fold greater than the wild-type enzyme. The Km for this enzyme with 5’-methyl(talo)-MeP-R was decreased by approximately 10-fold and its Vmax was increased by approximately 13-fold. Numerous other nucleosides containing MeP with structural modifications involving the 5 position of the sugar were also better substrates for M64V than the wild-type enzyme (Table 6). Interestingly, M64V PNP cleaved MeP-R, MeP-dR, and F-araA at rates that were similar to the wild-type enzyme.
Table 6.
Substrate activity of various MeP prodrugs with M64V PNP
| Compound | wild-type | M64V | M64V/wt |
|---|---|---|---|
| nmoles/mg-hr | |||
| 9-β-D-ribofuranosyl-6-methylpurine (MeP-R) | 84,000 | 176,000 | 2 |
| 9-(2-deoxy-β-D-ribofuranosyl)-6-methylpurine (MeP-dR) | 528,000 | 593,000 | 1 |
| 9-(α-L-lyxofuranosyl)-MeP | 218 | 10,200 | 47 |
| 9-(5-deoxy-α-L-lyxofuranosyl)-MeP | 20 | 3,500 | 175 |
| 9-(6-deoxy-β-D-allofuranosyl)-6-methylpurine | 47 | 316 | 7 |
| 9-(6-deoxy-α-L-talofuranosyl)-6-methylpurine (5’-methyl (talo) MeP-R) | 1,440 | 86,000 | 60 |
| 9-(2,6-dideoxy-β-D-talofuranosyl)-6-methylpurine | 3,600 | 264,000 | 73 |
| 9-(5,5-dimethyl-β-D-ribofuranosyl)-6-methylpurine | - | 280 | >280 |
| F-dAdo | 435,000 | 1,595,000 | 4 |
| 9-β-D-arabinofuranosyl-2-F-adenine (F-araA) | 1,250 | 1,000 | 1 |
| 9-α-L-lyxofuranosyl-2-F-adenine | 7,800 | 244,000 | 31 |
| 9-(6-deoxy-β-D-allofuranosyl)-2-F-adenine | 2,700 | 92,000 | 34 |
| 9-(6-deoxy-β-D-talofuranosyl)-2-F-adenine (5’-methyl (talo) F-Ado) | 34,000 | 1,360,000 | 40 |
Enzymes were incubated with 100 µM of each substrate and the rate of cleavage was determined by HPLC separation of the base form the nucleoside. Each value is the average of at least two experiments that were in good agreement.
Addition of a methyl group to the 5’-carbon of MeP-R (5’-methyl(talo)-MeP-R) dramatically reduced the toxicity of MeP-R to cells in culture (IC50 greater than 210 µM; [100]), which suggested that this structural modification prevented phosphorylation by adenosine kinase, a result that was confirmed with radiolabeled compound. The lack of in vitro toxicity also indicated that 5’-methy(talo)-MeP-R was not cleaved by MTAP to generate MeP. However, despite low activity of 5’-methyl(talo)-MeP-R with wild type E. coli PNP, toxicity in mice was only modestly improved in comparison with MeP-dR [105], and further studies indicated that 5’-methyl(talo)-MeP-R was also activated by intestinal bacteria (The maximally tolerated dose was approximately 400 mg/kg daily for 3 consecutive days, which was only about 4 to 5-fold higher than that of MeP-dR). Although this compound was active in vivo against tumor xenografts composed of D54 tumor cells that stably expressed M64V E. coli PNP, its antitumor activity was not superior to that seen with MeP-dR in combination with the wild-type enzyme.
Based on the activity of 5’-modified MeP containing nucleoside analogs, we also synthesized 9-(6-deoxy-β-D-talofuranosyl)-2-F-adenine (5’-methyl (talo)-F-Ado) and 9-α-L-lyxofuranosyl-2-F-adenine (lyxo-F-Ade). Both of these compounds are excellent substrates with M64V E. coli PNP with activities that are similar to that of F-dAdo (Table 6). Unfortunately, both compounds are cytotoxic to cells in culture due to cleavage of the glycosidic bond by human MTAP [106]. Because of its unique structure, relatively low in vitro toxicity (average IC50 of 23 µM; N=8), and cleavage activity with M64V E. coli PNP similar to that of F-dAdo with the wild-type enzyme, larger quantities of lyxo-F-Ade were synthesized and evaluated in vivo against D54 tumors that stably expressed M64V E. coli PNP. Although good in vivo antitumor activity was detected with lyxo-F-Ade against in these xenografts [unpublished observation], it was not superior to the combination of F-dAdo with the wild-type enzyme. In addition, the maximally tolerated dose of lyxo-F-Ade was similar to that of F-dAdo. Therefore, the activity/toxicity profiles for these compounds (5’-methyl(talo)-MeP-R and lyxo-F-Ade) were not sufficiently different from either MeP-dR or F-dAdo, and these compounds have not been pursued further.
7.2.2 Trichomonas vaginalis PNP
Because of the relatively poor activity of E. coli PNP with F-araA, we have evaluated numerous enzymes from other organisms that can cleave purine analogs in order to identify enzymes that more effectively cleave F-araA. It is hoped that utilization of enzymes that could cleave F-araA better than E. coli PNP in GDEPT would lead to enhanced cleavage of F-araA in tumor tissue and improved anti-tumor activity. Although in most cases (E. aerogenes PNP, A. Laidlawii PNP, Klebsiella sp PNP, Salmonella typhimurium PNP, B. cereus PNP, Tularemia PNP, T. bruceii hydrolase, Sulfolobus solfataricus MTAP, E. coli SAH/MTA hydrolase, grouper iridovirus PNP), F-araA cleavage by other enzymes was no different than E. coli PNP, we discovered that Trichomonas vaginalis PNP (Tv-PNP) was able to hydrolyze F-araA with a catalytical efficiency 25-fold greater than the E. coli enzyme [107]. The Km value for F-araA with Tv-PNP was approximately 3-fold lower, and the Vmax value of F-araA with Tv-PNP was approximately 10-fold greater than that of E. coli PNP. 9-β-D-arabinofuranosyl adenine and 9-β-D-arabinofuranosyl-6-methylpurine at 100 µM substrate concentrations were also cleaved much more efficiently by Tv-PNP than E. coli PNP (62- and 41-fold, respectively). In contrast, cleavage for adenosine, MeP-R, MeP-dR, F-Ado, and F-dAdo by Tv-PNP was similar to that of E. coli PNP. The average ratio of Tv-PNP/E. coli PNP cleavage rates with these substrates each at a concentration of 100 µM was 1.12.
D54 tumor cells were prepared that stably expressed Tv-PNP at enzyme rate of 4,270 nmoles of MeP-dR cleaved/mg-hr which was 25-fold greater than the level of expression of E. coli PNP in our first in vivo experiments (173 nmoles of MeP-dR cleaved/mg-hr [39]) but 30-fold less than the level of expression of E. coli PNP in our most recent experiments (126,000 nmoles of MeP-dR cleaved/mg-hr [41–43]). F-araA cleavage rate in these D54 tumor cells were 0.41 [39], 16 [107], and 298 [41–43] nmoles of F-araA cleaved/mg/hr, respectively. F-araAMP treatment of mice bearing these D54 tumors in which 10% of the cells expressed the Tv-PNP gene resulted in excellent in vivo bystander activity [107], which was significantly greater than that seen in low expressing tumors [39], but not as good as that seen in high expressing tumor cells [28, 60, 65]. These studies suggest that Tv-PNP may be a superior enzyme for use in GDEPT strategies designed to activate F-araAMP in tumor cells.
Subsequent studies with an adenoviral vector expressing Tv-PNP have also demonstrated good in vivo antitumor activity, but the activity was not significantly different than that seen with Ad/PNP (unpublished observation), which suggested that F-araA activation may be saturated at high expression levels of enzyme. These results suggest that Tv-PNP may be of particular use with vectors that express low levels of transgene. As described above for E. coli PNP, F-araAMP is the preferred prodrug when high levels of the enzyme are established in tumor cells [41–43]. However, when cells express low levels of E. coli PNP, F-araA is significantly less active than either MeP-dR or F-dAdo [39]. When F-araAMP is given in combination with GDEPT systems that do not result in high expression of the transgene, such as with herpes viral constructs [40], it is expected that Tv-PNP would result in much better activation of F-araAMP than E. coli PNP.
Tv-PNP may be the preferred enzyme for use in humans versus mice, because of the different levels of toxicity, peak plasma F-araA concentrations, and plasma F-araA half-lives. Humans tolerate much less F-araAMP than mice (25 mg/m2, administered daily for 5 consecutive days versus vs 300 mg/m2, administered 5 times daily for 3 consecutive days). The peak plasma concentration of F-araA following systemic administration of F-araAMP to humans (1 to 2 µM [30]) is much lower than plasma F-araA following intraperitoneal injection of F-araAMP in mice (approximately 100 µM [14]). The plasma half-life of F-araA in humans (10 to 20 hours [26]) is much longer than mice (50 minutes [14]), a feature that compensates for low plasma concentrations in humans. Based on a markedly prolonged circulation half-life of F-araA in humans versus mice and lower serum levels of the compound, the use of an enzyme with a lower Km and higher Vmax should lead to greater amounts of F-Ade generated in tumor cells expressing PNP.
7.2.3 Sulfolobus solfataricus MTAP
Cacciapuoti et al. [108] have evaluated cleavage of F-araA by MTAP and MTAP-II isolated form the hyperthermophilic archaeon Sulfolobus solfataricus. These investigators found that the Km values were similar to that seen with E. coli PNP (1,836 vs 938 µM) but that the Kcat values were 12 to 16 fold greater, suggesting that both of these enzymes would be better for the activation of F-araA than E. coli PNP. The kinetic parameters for these S. solfactaricus enzymes were determined at 70°C and were compared to E. coli PNP kinetic parameters determined at 25°C. Although the investigators report that Kcat/Km is insensitive to temperature, the cleavage parameters for both enzymes should be determined at 37°C, which is the temperature in tumor tissue.
7.2.4 Mycoplasma
Mycoplasmas are small, wall-less prokaryotes usually regarded as commensals that firmly adhere to the surface of eukaryotic cells and are known to often contaminate cell cultures. These organisms express many purine and pyrimidine enzymes that can affect the metabolism of nucleoside analogs [109], and as indicated above [10] they express a PNP that can efficiently cleave adenosine analogs. It has been suggested [110] that implantation of mycoplasma in the vicinity of a tumor mass could be used as an alternative to recombinant viral vectors as a means to deliver a phosphorylase that could activate MeP-dR or other prodrugs.
8. ANTIBODY-DIRECTED ENZYME PRODRUG THERAPY (ADEPT)
Antibody-directed enzyme prodrug therapy (ADEPT) is a similar approach to selectively generate cytotoxic agents in tumor cells. Unlike GDEPT, in which a vector of some sort delivers a non-human gene to tumor cells, ADEPT uses an antibody to deliver a non-human enzyme. Because antibodies often deliver enzymes to the cell surface, the use of a PNP to activate purine prodrugs is particularly well suited for ADEPT because the reaction product (F-Ade) can diffuse into surrounding tumor cells, even if it is created extracellularly. However, the use of non-human enzymes in ADEPT could limit the number of times that it could be administered due to immunogenicity of the non-human protein. It has long been known that human PNP does not recognize adenosine as a substrate due to an asparagine at position 243, which interferes with the hydrogen bonding associated with the N6 amino group on adenosine [8]. Replacing this amino acid with aspartic acid results in an enzyme that efficiently cleaves adenosine and its analogs [8, 111]. A modified human PNP (N243D) was prepared and fused to an anti-HER-2/neu peptide mimetic, and this enzyme was able to selectively activate F-dAdo to F-Ade in HER-2/neu-expressing tumor cells in vitro [112, 113]. Because this enzyme is derived from human PNP, ADEPT strategies utilizing this enzyme should have minimal immunogenicity. In addition, Asai et. al. have prepared two antibodies conjugated to E. coli PNP that were effective in activating F-dAdo in cell culture [114, 115].
9. CONCLUSIONS
GDEPT using E. coli PNP is a novel strategy for the treatment of solid tumors. We and others have demonstrated impressive in vivo antitumor activity in numerous in vitro and in vivo models of cancer, and a phase I trial has confirmed low toxicity of the approach with strong evidence of antitumor activity. The characteristics of this approach as described in this review are summarized in Table 7. Possibly the most important aspect of E. coli PNP-based tumor cell killing is the mechanism of tumor cell kill of F-Ade, which is fundamentally different from all drugs currently in use for the treatment of cancer as well as all newer agents under development. Because of this unique mechanism of action, GDEPT with E. coli PNP is expected to be effective against all tumor cell types regardless of their tissue derivation and proliferative state. Activity against non-proliferating cells is of particular importance to the treatment of solid tumors (malignancies such as glioma, prostate, breast, lung, colon, and others), because the inability to kill non-proliferating tumor cells represents a major obstacle to the current therapies, which primarily target the proliferating component of solid tumors.
Table 7.
Summary of Ad/PNP characteristics
|
Because the intracellular target of F-Ade mediates a critical function essential to all cells (protein and RNA synthesis), the PNP approach should be effective against any histotype of cancer and, unlike many of the new “targeted” antineoplastic agents, should not be subject to barriers encountered as part of “personalized” or “precision” cancer treatments. In other words, the therapy is not dependent on any specific trait associated with tumor cell biology. The seminal requirement is selective expression of E. coli PNP in tumor tissue. F-Ade should also be active against the putative tumor stem cell compartment, which is composed of cells that spend a substantial amount of time in a quiescent state. Targeting of RNA and protein synthesis for the treatment of cancer is only feasible in conjunction with a gene delivery strategy, since there is no selectivity otherwise inherent in the cell killing action of F-Ade; i.e., the specificity of this approach is due to the selective generation of F-Ade in a tumor mass. Although the current clinical product (Ad/PNP) is limited to local disease that can be injected with a needle, this approach would work well with any vector that can selectively deliver genes to metastatic disease.
The mechanistic aspects associated with the generation of F-Ade by E. coli PNP described above are a major distinguishing characteristic with regard to the numerous other GDEPT strategies (HSV-TK, E. coli CD, nitroreductase, APRT, cytochrome P40, others) that have been evaluated previously. The attributes of E. coli PNP described here indicate that E. coli PNP is the preferred enzyme to use with GDEPT. Most, if not all, other GDEPT approaches seek to activate compounds that are targeted to proliferating cells and therefore suffer from the same problem as conventional cancer treatments. Although selective generation in tumor tissue may reduce toxicity, these agents will still not be effective against the non-proliferating component of a tumor mass. Moreover, many of the earlier approaches seek to produce approved anticancer drugs (such as FUra) selectively in tumor cells. Although, this seems like a reasonable strategy, such agents were designed to be effective after systemic administration and are therefore much less potent than they could be, because the drugs were optimized to be effective when administered systemically and toxicity limits their efficacious use.
An important characteristic that distinguishes E. coli PNP based GDEPT from all others is the high in vivo bystander activity. Because of the difficultly delivering and expressing genes to tumors in patients, it is critical that any GDEPT strategy be effective against numerous tumor cells that are near the transduced cells but do not express the transgene. To our knowledge, no report has shown bystander killing with any other prodrug activation strategy of the magnitude shown with E. coli PNP.
Another characteristic that distinguishes F-Ade is its potent cytotoxicity: F-Ade is 100 to 1000-fold more cytotoxic than FUra. It is possible that F-Ade could cause tumor regressions in animals under conditions (equal expression of activating enzyme) where FUra would have little effect. Given the difficulty of selectively delivering genes to tumor cells in an intact animal, the potency of F-Ade can greatly augment GDEPT strategies when only small amount of gene expression in the tumor is achieved. The high potency of F-Ade also contributes to the high bystander activity of this approach, because only a small amount of F-Ade needs to reach surrounding tumor cells that do not express E. coli PNP to be effective.
F-Ade is very effective in vivo following a relatively short treatment period (48 hours), which has particular importance in the context of GDEPT. Under circumstances when only a small percentage of cells are transduced, it is important that the anti-cancer agent is immediately toxic to the entire tumor (transduced and nontransduced cells). Otherwise, if prolonged therapy is necessary to destroy the tumor (as with many current chemotherapies), transduced cells would be eliminated prior to achieving a significant antitumor effect. Furthermore, the duration of enzyme expression is often limited to only a few days. Obviously, it is necessary to treat with prodrug when the activating enzyme is being expressed.
A problem with GDEPT based on HSV-TK is that once the activated form of the drug leaves the cells it is rapidly deactivated by ubiquitous phosphatases to the inactive prodrug. Although this rapid inactivation makes for a very safe treatment with little toxicity, it severely limits the ability of this strategy to kill tumor cells. This is not the case with F-Ade, which is regenerated in the form of F-Ado or F-Ade from dying cells and is able to diffuse to neighboring tumor cells.
In conclusion, results from numerous laboratories indicate that GDEPT strategies using E. coli PNP plus F-araAMP are an effective approach to the treatment of refractory solid tumors. The approach realizes the promise of gene therapy for cancer: namely potent antitumor activity with negligible toxicity. The use of E. coli PNP with GDEPT allows for safe production of novel antitumor drugs (such as F-Ade) in tumor cells that could not be used as part of systemic therapy for cancer. It is our opinion that the attributes of F-Ade are precisely those that are required to kill refractory tumor cells in patients (high potency, high diffusion rate, and activity against non-proliferating cells). Although impressive antitumor activity has been observed with F-araAMP at nontoxic doses, this compound has known toxicities that limit the amount of drug that can be administered to a patient, which potentially limits the efficacy of this approach. Therefore, identification of less toxic prodrugs that are activated by wild-type or mutant purine cleavage enzymes is an important goal for the future, which could further enhance efficacy of this approach to cancer treatment.
Footnotes
Conflict of Interest
Drs. Parker and Sorscher have a significant equity position in PNP Therapeutics, which has licensed the patents concerning the use of E. coli PNP for the treatment of cancer.
References
- 1.Hébrard C, Dumontet C, Jordheim LP. Development of gene therapy in association with clinically used cytotoxic deoxynucleoside analogues. Cancer Gene Ther. 2009;16:541–550. doi: 10.1038/cgt.2009.25. [DOI] [PubMed] [Google Scholar]
- 2.Ardiani A, Johnson AJ, Ruan H, Sanchez-Bonilla M, Serve K, Black ME. Enzymes to die for: Exploiting nucleotide metabolizing enzymes for cancer gene therapy. Current Gene Therapy. 2012;12:77–91. doi: 10.2174/156652312800099571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karjoo Z, Chen X, Hatefi A. Progress and problems with the use of suicide genes for targeted cancer therapy. Advanced Drug Delivery Reviews. 2016;99:113–128. doi: 10.1016/j.addr.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker WB. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chemical Reviews. 2009;109:2880–2893. doi: 10.1021/cr900028p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Secrist JA, III, Parker WB, Allan PW, Bennett LL, Jr, Waud WR, Truss JW, Fowler AT, Montgomery JA, Ealick SE, Wells AH, Gillespie GY, Gadi VK, Sorscher EJ. Gene therapy of cancer: Activation of nucleoside prodrugs with E. coli purine nucleoside phosphorylase. Nucleosides Nucleotides. 1999;18:745–757. doi: 10.1080/15257779908041562. [DOI] [PubMed] [Google Scholar]
- 6.Curlee KV, Parker WB, Sorscher EJ. Tumor sensitization to purine analogs by E. coli PNP. Methods Mol Med. 2004;90:223–246. doi: 10.1385/1-59259-429-8:223. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Parker WB, Sorscher EJ, Ealick SE. PNP anticancer gene therapy. Current Topics in Medicinal Chemistry. 2005;5:1259–1274. doi: 10.2174/156802605774463105. [DOI] [PubMed] [Google Scholar]
- 8.Stoeckler JD, Poirot AF, Smith RM, Parks RE, Jr, Ealick SE, Takabayashi K, Erion MD. Purine nucleoside phosphorylase. 3. Reversal of purine base specificity by site-directed mutagenesis. Biochemistry. 1997;36:11749–11756. doi: 10.1021/bi961971n. [DOI] [PubMed] [Google Scholar]
- 9.Bzowska A, Kulikowska E, Shugar D. Purine nucleoside phosphorylases: properties, functions, and clinical aspects. Pharmacol. Ther. 2000;88:349–425. doi: 10.1016/s0163-7258(00)00097-8. [DOI] [PubMed] [Google Scholar]
- 10.McGarrity GJ, Carson DA. Adenosine phosphorylase-mediated nucleoside toxicity. Application towards the detection of mycoplasmal infection in mammalian cell cultures. Exp Cell Res. 1982;139:199–205. doi: 10.1016/0014-4827(82)90333-0. [DOI] [PubMed] [Google Scholar]
- 11.Sorscher EJ, Peng S, Bebok Z, Allan PW, Bennett LL, Jr, Parker WB. Tumor cell bystander killing in colonic carcinoma utilizing the E. coli Deo D gene to generate toxic purines. Gene Therapy. 1994;1:233–238. [PubMed] [Google Scholar]
- 12.Park BJ, Brown CK, Hu Y, Alexander HR, Horti J, Raje S, Figg WD, Bartlett DL. Augmentation of melanoma-specific gene expression using a tandem melanocyte-specific enhancer results in increased cytotoxicity of the purine nucleoside phosphorylase gene in melanoma. Human Gene Therapy. 1999;10:889–898. doi: 10.1089/10430349950018292. [DOI] [PubMed] [Google Scholar]
- 13.Hughes BW, Wells AH, Bebok Z, Gadi VK, Garver RI, Jr, Parker WB, Sorscher EJ. Bystander killing of melanoma cells using the human tyrosinase promoter to express the Escherichia coli purine nucleoside phosphorylase gene. Cancer Res. 1995;55:3339–3345. [PubMed] [Google Scholar]
- 14.Parker WB, Allan PW, Hassan AEA, Secrist JA, III, Sorscher EJ, Waud WR. Antitumor activity of 2-fluoro-2'-deoxyadenosine against tumors that express Escherichia coli purine nucleoside phosphorylase. Cancer Gene Therapy. 2003;10:23–29. doi: 10.1038/sj.cgt.7700520. [DOI] [PubMed] [Google Scholar]
- 15.Hughes BW, King SA, Allan PW, Parker WB, Sorscher EJ. Cell to cell contact is not required for bystander cell killing by E. coli purine nucleoside phosphorylase. J Biol Chem. 1998;273:2322–2328. doi: 10.1074/jbc.273.4.2322. [DOI] [PubMed] [Google Scholar]
- 16.Da Costa LT, Jen J, He TC, Chan TA, Kinzler KW, Vogelstein B. Converting cancer genes into killer genes. Proc Natl Acad Sci USA. 1996;93:4192–4196. doi: 10.1073/pnas.93.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockett LJ, Molloy PL, Russell PJ, Both GW. Relative efficiency of tumor cell killing in vitro by two enzyme-prodrug systems delivered by identical adenovirus vectors. Clin Cancer Res. 1997;3:2075–2080. [PubMed] [Google Scholar]
- 18.Nestler U, Heinkelein M, Lucke M, Meixensberger J, Scheurlen W, Kretschmer A, Rethwilm A. Foamy virus vectors for suicide gene therapy. Gene Therapy. 1997;4:1270–1277. doi: 10.1038/sj.gt.3300561. [DOI] [PubMed] [Google Scholar]
- 19.Puhlmann M, Gnant M, Brown CK, Alexander HR, Bartlett DL. Thymidine kinase-deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor-directed gene therapy. Human Gene Therapy. 1999;10:649–657. doi: 10.1089/10430349950018724. [DOI] [PubMed] [Google Scholar]
- 20.Zhou JH, Tang B, Liu XL, He DW, Yang DT. hTERT-targeted E. coli purine nucleoside phosphorylase gene/6-methylpurine deoxyribose therapy for pancreatic cancer. Chin Med J Aug. 2007;120:1348–1352. [PubMed] [Google Scholar]
- 21.Cai X, Zhou J, Lin J, Sun X, Xue X, Li C. Experimental studies on PNP suicide gene therapy of hepatoma. J Huazhong Univ Sci Technolog Med Sci. 2005;25:178–181. doi: 10.1007/BF02873570. [DOI] [PubMed] [Google Scholar]
- 22.Cai XK, Zhou JL, Zhou HJ, Zhang L, Wu JH, Lin JS. Killing effect of PNP/MeP-dR suicide gene system driven by an AFP promoter AF0.3 on AFP-positive hepatoma cells. Ai Zheng. 2006;25:1334–1339. [PubMed] [Google Scholar]
- 23.Cai X, Zhou J, Chang Y, Sun X, Li P, Lin J. Targeting gene therapy for hepatocarcinoma cells with the E. coli purine nucleoside phosphorylase suicide gene system directed by a chimeric alpha-fetoprotein promoter. Cancer Lett. 2008;264:71–82. doi: 10.1016/j.canlet.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Stritzker J, Pilgrim S, Szalay AA, Goebel W. Prodrug converting enzyme gene delivery by L. monocytogenes. BMC Cancer. 2008;8:94–103. doi: 10.1186/1471-2407-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu W, Lan H, Li S, Han X, Gao T, Ren D. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2'-deoxyriboside via Salmonella against murine tumors. Cancer Gene Ther. 2008;15:474–484. doi: 10.1038/cgt.2008.19. [DOI] [PubMed] [Google Scholar]
- 26.Parker WB, Cheng YC. Metabolism and mechanism of action of 5-fluorouracil. Pharmac Ther. 1990;48:381–395. doi: 10.1016/0163-7258(90)90056-8. [DOI] [PubMed] [Google Scholar]
- 27.Parker WB, Allan PW, Shaddix SC, Rose LM, Speegle HF, Gillespie GY, Bennett LL., Jr Metabolism and metabolic actions of 6-methylpurine and 2-fluoroadenine in human cells. Biochem Pharmacol. 1998;55:1673–1681. doi: 10.1016/s0006-2952(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 28.Bennett LL, Jr, Vail MH, Chumley S, Montgomery JA. Activity of adenosine analogs against a cell culture, line resistant to 2-fluoroadenine. Biochem Pharmacol. 1966;15:1719–1728. [Google Scholar]
- 29.Parker WB, Bapat AR, Shen JX, Townsend AJ, Cheng YC. Interaction of 2-halogenated dATP analogs (F, Cl, and Br) with human DNA polymerases, DNA primase, and ribonucleotide reductase. Mol. Pharmacol. 1998;34:485–491. [PubMed] [Google Scholar]
- 30.Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41:93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Mohr L, Shankara S, Yoon SK, Krohne TU, Geissler M, Roberts B, Blum HE, Wands JR. Gene therapy of hepatocellular carcinoma in vitro and in vivo in nude mice by adenoviral transfer of the Escherichia coli purine nucleoside phosphorylase gene. Hepatology. 2000;31:606–614. doi: 10.1002/hep.510310310. [DOI] [PubMed] [Google Scholar]
- 32.Krohne TU, Shankara S, Geissler M, Roberts BL, Wands JR, Blum HE, Mohr L. Mechanisms of cell death induced by suicide genes encoding purine nucleoside phosphorylase and thymidine kinase in human hepatocellular carcinoma cells in vitro. Hepatology. 2001;34:511–518. doi: 10.1053/jhep.2001.26749. [DOI] [PubMed] [Google Scholar]
- 33.Messina M, Yu DM, Both GW, Molloy PL, Robinson BG. Calcitonin-specific transcription and splicing targets gene-directed enzyme prodrug therapy to medullary thyroid carcinoma cells. J Clin Endocrinol Metab. 2003;88:1310–1318. doi: 10.1210/jc.2002-021501. [DOI] [PubMed] [Google Scholar]
- 34.Arvidsson Y, Sumantran V, Watt F, Uramoto H, Funa K. Neuroblastoma-specific cytotoxicity mediated by the Mash1-promoter and E. coli purine nucleoside phosphorylase. Pediatr Blood Cancer. 2005;44:77–84. doi: 10.1002/pbc.20163. [DOI] [PubMed] [Google Scholar]
- 35.Krais JJ, De Crescenzo O, Harrison RG. Purine nucleoside phosphorylase targeted by annexin v to breast cancer vasculature for enzyme prodrug therapy. PLoS One. 2013;8(10):e76403. doi: 10.1371/journal.pone.0076403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillen K, Kurkjian C, Harrison R. Targeted enzyme prodrug therapy for metastatic prostate cancer – A comparative study of L-methionase, purine nucleoside phosphorylase, and cytosine deaminase. Journal of Biomedical Science. 2014;21:65. doi: 10.1186/s12929-014-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai Y-H, Lin C-C, Chen S-H, Tai C-K. Tumor-specific suicide gene therapy for hepatocellular carcinoma by transcriptionally targeted retroviral replicating vectors. Gene Therapy. 2015;22:155–162. doi: 10.1038/gt.2014.98. [DOI] [PubMed] [Google Scholar]
- 38.Xie X, Guo J, Kong Y, Xie GX, Li L, Lv N, Xiao X, Tang J, Wang X, Liu P, Yang M, Xie Z, Wei W, Xie X. Targeted expression of E. coli purine nucleoside phosphorylase and Fludara for prostate cancer therapy. J Gene Med. 2011;13:680–691. doi: 10.1002/jgm.1620. [DOI] [PubMed] [Google Scholar]
- 39.Parker WB, King SA, Allan PW, Bennett LL, Jr, Secrist JA, III, Montgomery JA, Gilbert KS, Waud WR, Wells AH, Gillespie GY, Sorscher EJ. In vivo gene therapy of cancer with E. coli purine nucleoside phosphorylase. Human Gene Therapy. 1997;8:1637–1644. doi: 10.1089/hum.1997.8.14-1637. [DOI] [PubMed] [Google Scholar]
- 40.Bharara S, Sorscher EJ, Gillespie GY, Lindsey JR, Hong JS, Curlee KV, Allan PW, Gadi VK, Alexander SA, Secrist JA, 3rd, Parker WB, Waud WR. Antibiotic-mediated chemoprotection enhances adaptation of E. coli PNP for herpes simplex virus-based glioma therapy. Hum Gene Ther. 2005;16:339–47. doi: 10.1089/hum.2005.16.339. [DOI] [PubMed] [Google Scholar]
- 41.Hong JS, Waud WR, Levasseur DN, Townes TM, Wen H, McPherson SA, Moore BA, Bebok Z, Allan PW, Secrist JA, 3rd, Parker WB, Sorscher EJ. Excellent In vivo Bystander Activity of Fludarabine Phosphate against Human Glioma Xenografts that Express the Escherichia coli Purine Nucleoside Phosphorylase Gene. Cancer Res. 2004;64:6610–6615. doi: 10.1158/0008-5472.CAN-04-0012. [DOI] [PubMed] [Google Scholar]
- 42.Parker WB, Allan PW, Waud WR, Hong JS, Sorscher EJ. Effect of expression of adenine phosphoribosyltransferase on the in vivo anti-tumor activity of prodrugs activated by E. coli purine nucleoside phosphorylase. Cancer Gene Therapy. 2011;18:390–398. doi: 10.1038/cgt.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorscher EJ, Hong JS, Allan PW, Waud WR, Parker WB. In vivo antitumor activity of intratumoral fludarabine phosphate in refractory tumors expressing E. coli purine nucleoside phosphorylase. Cancer Chemotherapy and Pharmacology. 2012;70:321–329. doi: 10.1007/s00280-012-1908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petsko GA. Open questions: Zombie projects, translational research, and the real secret of the inside of the cell. BMC Biology. 2013;11:97. doi: 10.1186/1741-7007-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martiniello-Wilks R, Garcia-Aragon J, Daja MM, Russell P, Both GW, Molloy PL, Lockett LJ, Russell PJ. In vivo gene therapy for prostate cancer: Preclinical evaluation of two different enzyme-directed prodrug therapy systems delivered by identical adenovirus vectors. Human Gene Therapy. 1998;9:1617–1626. doi: 10.1089/hum.1998.9.11-1617. [DOI] [PubMed] [Google Scholar]
- 46.Martiniello-Wilks R, Tsatralis T, Russell P, Brookes DE, Zandvliet D, Lockett LJ, Both GW, Molloy PL, Russell PJ. Transcription-targeted gene therapy for androgen-independent prostate cancer. Cancer Gene Therapy. 2002;9:443–452. doi: 10.1038/sj.cgt.7700451. [DOI] [PubMed] [Google Scholar]
- 47.Singh PP, Joshi S, Russell PJ, Verma ND, Wang X, Khatri A. Molecular chemotherapy and chemotherapy: a new front against late-stage hormone-refractory prostate cancer. Clin Cancer Res. 2011;17:4006–4018. doi: 10.1158/1078-0432.CCR-11-0248. [DOI] [PubMed] [Google Scholar]
- 48.Voeks D, Martiniello-Wilks R, Madden V, Smith K, Bennetts E, Both GW, Russell PJ. Gene therapy for prostate cancer delivered by ovine adenovirus and mediated by purine nucleoside phosphorylase and fludarabine in mouse models. Gene Therapy. 2002;9:759–768. doi: 10.1038/sj.gt.3301698. [DOI] [PubMed] [Google Scholar]
- 49.Martiniello-Wilks R, Dane A, Voeks DJ, Jeyakumar G, Mortensen E, Shaw JM, Wang XY, Both GW, Russell PJ. Gene-directed enzyme prodrug therapy for prostate cancer in a mouse model that imitates the development of human disease. J Gene Med. 2004;6:43–54. doi: 10.1002/jgm.474. [DOI] [PubMed] [Google Scholar]
- 50.Wang XY, Martiniello-Wilks R, Shaw JM, Ho T, Coulston N, Cooke-Yarborough C, Molloy PL, Cameron F, Moghaddam M, Lockett TJ, Webster LK, Smith IK, Both GW, Russell PJ. Preclinical evaluation of a prostate-targeted gene-directed enzyme prodrug therapy delivered by ovine atadenovirus. Gene Therapy. 2004;11:1559–1567. doi: 10.1038/sj.gt.3302308. [DOI] [PubMed] [Google Scholar]
- 51.Martiniello-Wilks R, Wang XY, Voeks DJ, Dane A, Shaw JM, Mortensen E, Both GW, Russell PJ. Purine nucleoside phosphorylase and fludarabine phosphate gene-directed enzyme prodrug therapy suppresses primary tumour growth and pseudo-metastases in a mouse model of prostate cancer. J Gene Med. 2004;6:1343–1357. doi: 10.1002/jgm.629. [DOI] [PubMed] [Google Scholar]
- 52.Specenier P, Vermorken JB. Cetuximab: its unique place in head and neck cancer treatment. Biologics: Targets and Therapy. 2013;7:77–90. doi: 10.2147/BTT.S43628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, Kang H, Gibson MK, Massarelli E, Powell S, Meister A, Shu X, Cheng JD, Haddad R. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, MOnga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenthal EL, Chung TK, Parker WB, Allan PW, Clemons L, Lowman D, Hong J, Hunt FR, Richman J, Conry RM, Mannion K, Carroll WR, Nabell L, Sorscher EJ. Phase 1 dose-escalating trial of Escherichia coli purine nucleoside phosphorylase and fludarabine gene therapy for advanced solid tumors. Annals of Oncology. 2015;26:1481–1487. doi: 10.1093/annonc/mdv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorscher EJ, Hong JS, Parker WB. Preclinical and clinical validation of an anti-cancer modality that ablates refractory, low growth fraction tumors. Transactions of the American Clinical and Climatological Association. 2016;127:59–70. [PMC free article] [PubMed] [Google Scholar]
- 57.Skipper HE, Montgomery JA, Thomson JR, Schabel FM., Jr Structure-activity relationships and cross-resistance observed on evaluation of a series of purine analogs against experimental neoplasms. Cancer Res. 1959;19:425–437. [PubMed] [Google Scholar]
- 58.Yao SY, Ng AM, Cass CE, Baldwin SA, Young JD. Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1) J Biol Chem. 2011;286:32552–32562. doi: 10.1074/jbc.M111.236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J, Visser F, King KM, Baldwin SA, Young JD, Cass CE. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev. 2007;26:85–110. doi: 10.1007/s10555-007-9044-4. [DOI] [PubMed] [Google Scholar]
- 60.Shigeura HT, Boxer GE, Sampson SD, Meloni ML. Metabolism of 2-fluoroadenosine by Ehrlich ascites cells. Arch. Biochem. Biophys. 1965;111:713–719. doi: 10.1016/0003-9861(65)90254-7. [DOI] [PubMed] [Google Scholar]
- 61.Zimmerman TP, Deeprose RD, Wolberg G, Duncan GS. Metabolic formation of nucleoside-modified analogues of S-adenosylmethionine. Biochem Biophys Res Commun. 1979;91:997–1004. doi: 10.1016/0006-291x(79)91978-8. [DOI] [PubMed] [Google Scholar]
- 62.Zimmerman TP, Rideout JL, Wolberg G, Duncan GS, Elion GB. 2-Fluoroadenosine 3′:5′-monophosphate. A metabolite of 2-fluoroadenosine in mouse cytotoxic lymphocytes. J Biol Chem. 1976;251:6757–6766. [PubMed] [Google Scholar]
- 63.Zimmerman TP, Wolberg G, Duncan GS, Rideout JL, Beachman LM, III, Krenitsky TA, Elion GB. Inhibition of lymphocyte-mediated cytolysis by 2-fluoroadenosine-Evidence for two discrete mechanisms of drug action. Biochem Pharmacol. 1978;27:1731–1737. doi: 10.1016/0006-2952(78)90549-x. [DOI] [PubMed] [Google Scholar]
- 64.Parks RE, Jr, Brown PR. Incorporation of nucleosides into the nucleotide pools of human erythrocytes and its analogs. Biochem. 1973;12:3294–3302. doi: 10.1021/bi00741a022. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal KC, Parks RE. Adenosine analogs and human platelets. Effects on nucleotide pools and the aggregation phenomenon. Biochem Pharmacol. 1975;24:2239–2248. doi: 10.1016/0006-2952(75)90261-0. [DOI] [PubMed] [Google Scholar]
- 66.Hill DL, Straight S, Allan PW. Use of Tetrahymena pyriformis to evaluate the effects of purine and pyrimidine analogs. J Protozool. 1970;17:619–623. doi: 10.1111/j.1550-7408.1970.tb04739.x. [DOI] [PubMed] [Google Scholar]
- 67.Parks RE, Jr, Crabtree GW, Kong CM, Agarwal RP, Agarwal KC, Scholar EM. Incorporation of analog purine nucleosides into the formed elements of human blood, erythrocytes, platelets, and lymphocytes. Annal NY Acad Sci. 1975;255:412–434. doi: 10.1111/j.1749-6632.1975.tb29249.x. [DOI] [PubMed] [Google Scholar]
- 68.Avramis VI, Plunkett W. 2-Fluoro-ATP: A toxic metabolite of 9-β-D-arabinosyl-2-fluoroadenine. Biochem. Biophys Res Commun. 1983;113:35–43. doi: 10.1016/0006-291x(83)90428-x. [DOI] [PubMed] [Google Scholar]
- 69.Gadi VK, Alexander SD, Waud WR, Allan PW, Parker WB, Sorscher EJ. A long-acting suicide gene toxin, 6-methylpurine, inhibits slow growing tumors after a single administration. J Pharmacol Exp Ther. 2003;304:1280–1284. doi: 10.1124/jpet.102.044743. [DOI] [PubMed] [Google Scholar]
- 70.Parker WB, Bapat AR, Shen J-X, Townsend AJ, Cheng Y-C. Interaction of 2-halogenated dATP analogs (F, Cl, and Br) with human DNA polymerases, DNA primase, and ribonucleotide reductase. Mol Pharmacol. 1988;34:485–491. [PubMed] [Google Scholar]
- 71.Myers CE. The pharmacology of the fluoropyrimidines. Pharmacological Reviews. 1981;33:1–15. [PubMed] [Google Scholar]
- 72.Parker WB, Cheng Y-C. Metabolism and mechanism of action of 5-fluorouracil. Pharmac Ther. 1990;48:381–395. doi: 10.1016/0163-7258(90)90056-8. [DOI] [PubMed] [Google Scholar]
- 73.Cheng YC, Grill SP, Dutschman GE, Nakayama K, Bastow KF. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983;258:12460–12464. [PubMed] [Google Scholar]
- 74.Reid R, Mar EC, Huang ES, Topal MD. Insertion and extension of acyclic, dideoxy, and ara nucleotides by herpesviridae, Human α and human β polymerases. A unique inhibition mechanism for 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1988;263:3898–3904. [PubMed] [Google Scholar]
- 75.Balzarini J, Bohman C, DeClercq E. Differential mechanism of cytostatic effect of (E)-5-2-bromovinyl)-2′-deoxyuridine, 9-1(1,3-dihydroxy-2-propoxymethyl)guanine, and other antiherpetic drugs on tumor cells transfected by the thymidine kinase gene of herpes simplex virus type 1 or type 2. J Biol Chem. 1993;268:6332–6337. [PubMed] [Google Scholar]
- 76.Ilsley DD, Lee SH, Miller WH, Kutcha RD. Acyclic guanosine analogs inhibit DNA polymerases α, δ, and ε with very different potencies and have unique mechanisms of action. Biochem. 1995;34:2504–2510. doi: 10.1021/bi00008a014. [DOI] [PubMed] [Google Scholar]
- 77.Tannock IF. Principles of cell proliferation: Cell kinetics in cancer. In: Devita VJ, Hellman S, Rosenberg S, editors. Principles and practice of oncology. Philadelphia: L.B. Lippincott; 1989. pp. 3–13. [Google Scholar]
- 78.Bruce WR, Meeker BE. Comparison of the sensitivity of hematopoietic colony-forming cells in different proliferative states to 5-fluorouracil. J Natl Cancer Inst. 1967;38:401–405. [PubMed] [Google Scholar]
- 79.Komlodi-Pasztor E, Sackett DL, Fojo AT. Inhibitors targeting mitosis: Tales of how great drugs against a promising target were brought down by a flawed rationale. Clinical Cancer Research. 2012;18:51–63. doi: 10.1158/1078-0432.CCR-11-0999. [DOI] [PubMed] [Google Scholar]
- 80.Bennett LL, Jr, Smithers D. Feedback inhibition of purine biosynthesis in H. EP. #2 cells by adenine analogs. Biochem Pharmacol. 1964;13:1331–1339. doi: 10.1016/0006-2952(64)90233-3. [DOI] [PubMed] [Google Scholar]
- 81.Song C, Sakurai M, Shiromoto Y, Nishikura K. Functions of the RNA editing enzyme ADAR1 and their relevance to human diseases. Genes. 2016;7:129. doi: 10.3390/genes7120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh PP, Joshi S, Russell PJ, Nair S, Khatri A. Purine Nucleoside Phosphorylase mediated molecular chemotherapy and conventional chemotherapy: a tangible union against chemoresistant cancer. BMC Cancer. 2011;11:368. doi: 10.1186/1471-2407-11-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bergmann F, Ungar-Waron H, Goldberg H, Kalmus A. The enzymatic oxidation of 6-methylpure. Archives Biochem Biophys. 1961;94:94–98. [Google Scholar]
- 84.Leonhardt K, Anke T. 6-Methylpurine, 6-methyl-9-β-D-ribofuranosylpurine, and 6-hydroxymethyl-9-β-D-ribofuranosylpurine as antiviral metabolites of Collybia maculata (Basidiomycetes) Z. Naturforsch. 1987;42c:420–424. doi: 10.1515/znc-1987-0415. [DOI] [PubMed] [Google Scholar]
- 85.Davidson IWF, Fellig J. Cellular conversion of methylpurine to an active analog of ATP. Fed Proc. 1962;21:160. [Google Scholar]
- 86.Benson CE, Love SH, Remy CN. Inhibition of de novo purine biosynthesis and interconversion by 6-methylpurine in Escherichia coli. J. Bacteriol. 1970;101:872–880. doi: 10.1128/jb.101.3.872-880.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller JH, Kempner ES. Effects of an adenine analog on yeast metabolism. Biochim Biophys Acta. 1963;76:333–340. [PubMed] [Google Scholar]
- 88.Dewey VC, Heinrich MR, Markees DG, Kidder GW. Multiple inhibition by 6-methylpurine. Biochem Pharmacol. 1960;3:173–180. doi: 10.1016/0006-2952(60)90104-0. [DOI] [PubMed] [Google Scholar]
- 89.Lambe CA, John PCL. Action of 6-methyl purine on RNA synthesis: Incorporation of the adenine analogue into coding sequences and polyadenylate tracts of messenger RNA in Chlorella. New Phytol. 1979;83:321–341. [Google Scholar]
- 90.Kohlschein J, Hagenberg L, Gassen HG. Synthesis and properties of poly(6-methylpurinylic acid), poly(6-methoxypurinylic acid), and poly(6-methylthiopurinylic acid) Biochem Biophys Acta. 1974;374:407–416. doi: 10.1016/0005-2787(74)90262-7. [DOI] [PubMed] [Google Scholar]
- 91.Silamkoti AV, Allan PW, Hassan AE, Fowler AT, Sorscher EJ, Parker WB, Secrist JA., 3rd Synthesis and biological activity of 2-fluoro adenine and 6-methyl purine nucleoside analogs as prodrugs for suicide gene therapy of cancer. Nucleosides, Nucleotides, and Nucleic Acids. 2005;24:881–885. doi: 10.1081/ncn-200059237. [DOI] [PubMed] [Google Scholar]
- 92.Hassan AE, Abou-Elkhair RA, Parker WB, Allan PW, Secrist JA., 3rd 6-Methylpurine derived sugar modified nucleosides: Synthesis and evaluation of their substrate activity with purine nucleoside phosphorylases. 2015;65:9–16. doi: 10.1016/j.bioorg.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 93.Hassan AEA, Abou-Elkhair RAI, Parker WB, Allan PW, Secrist JA., III 6-Methylpurine derived sugar modified nucleosides: Synthesis and in vivo antitumor activity in D54 tumor expressing M64V-Escherichia coli purine nucleoside phosphorylase. European Journal of Medicinal Chemistry. 2015;108:616–622. doi: 10.1016/j.ejmech.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 94.Hassan AEA, Abou-Elkhair RAI, Riordan JM, Allan PW, Parker WB, Khare R, Waud WR, Montgomery JA, Secrist JA., III Synthesis and evaluation of the substrate activity of C-6 substituted purine ribosides with E. coli purine nucleoside phosphorylase: Palladium mediated cross-coupling of organozinc halides with 6-chloropurine nucleosides. Eur J Med Chem. 2012;47:167–174. doi: 10.1016/j.ejmech.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hassan AEA, Parker WB, Allan PW, Secrist JA., III Regioselective metalation of 6-methylpurines: synthesis of fluoromethyl purines and related nucleosides for suicide gene therapy of cancer. Nucleosides, Nucleotides and Nucleic Acids. 2009;28:642–656. doi: 10.1080/15257770903091938. [DOI] [PubMed] [Google Scholar]
- 96.Hassan AE, Parker WB, Allan PW, Montgomery JA, Secrist JA., III Selective metalation of 6-methylpurines: synthesis of 6-fluoromethylpurines and related nucleosides. Nucleosides Nucleotides Nucleic Acids. 2003;22:747–749. doi: 10.1081/NCN-120022625. [DOI] [PubMed] [Google Scholar]
- 97.Ealick EA, Parker WB, Secrist JA, III, Sorscher EJ. Mutant purine nucleoside phosphorylase proteins and cellular delivery thereof. 2003/0228576 A1. US Patent application number. 2003
- 98.Ealick EA, Parker WB, Secrist JA, III, Sorscher EJ. Mutant purine nucleoside phosphorylase proteins and cellular delivery thereof. 2005/0214901 A1. US Patent application number. 2005
- 99.Streeter DG, Miller M, Mathews TR, Robins RK, Miller JP. 7-Ribosyl-3-deazaguanine - mechanism of antibacterial action. Biochem Pharm. 1980;29:1971–1797. doi: 10.1016/0006-2952(80)90141-0. [DOI] [PubMed] [Google Scholar]
- 100.Plunkett W, Saunders PP. Metabolism and action of purine nucleoside analogs. Pharmacol. Ther. 1991;49:239–268. doi: 10.1016/0163-7258(91)90057-s. [DOI] [PubMed] [Google Scholar]
- 101.Bennett LL, Jr, Smithers D, Rose LM, Adamson DJ, Brockman RW. Mode of action of 2-amino-6-chloro-1-deazapurine. Biochem Pharm. 1984;33:261–271. doi: 10.1016/0006-2952(84)90484-2. [DOI] [PubMed] [Google Scholar]
- 102.Bontemps F, Delacauw A, Cardoen S, Van Den Neste E, Van Den Berghe G. Metabolism and cytotoxic effects of 2-chloroadenine, the major catabolite of 2-chloro-2’-deoxyadenosine. Biochemical Pharmacology. 2000;59:1237–1243. doi: 10.1016/s0006-2952(00)00258-6. [DOI] [PubMed] [Google Scholar]
- 103.Fu W, Lan H, Liang S, Gao T, Ren D. Suicide gene/prodrug therapy using salmonella-mediated delivery of Escherichia coli purine nucleoside phosphorylase gene and 6-methoxypurine 2-deoxyriboside in murine mammary carcinoma 4T1 model. Cancer Sci. 2008;99:1172–1179. doi: 10.1111/j.1349-7006.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bennett EM, Anand R, Allan PW, Hassan AE, Hong JS, Levasseur DN, McPherson DT, Parker WB, Secrist JA, 3rd, Sorscher EJ, Townes TM, Waud WR, Ealick SE. Designer gene therapy using an Escherichia coli purine nucleoside phosphorylase/prodrug system. Chem Biol. 2003;10:1173–1181. doi: 10.1016/j.chembiol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 105.Bennett EM, Li C, Allan PW, Parker WB, Ealick SE. Structural basis for substrate specificity of Escherichia coli purine nucleoside phosphorylase. J Biol Chem. 2003;278:47110–47118. doi: 10.1074/jbc.M304622200. [DOI] [PubMed] [Google Scholar]
- 106.Parker WB, Allan PW, Ealick SE, Sorscher EJ, Hassan AEA, Silamkoti AV, Fowler AT, Waud WR, Secrist JA., III Design and evaluation of 5’-modified nucleoside analogs as prodrugs for an E. coli purine nucleoside phosphorylase mutant. Nucleosides, Nucleotides, and Nucleic Acids. 2005;24:387–392. doi: 10.1081/ncn-200059807. [DOI] [PubMed] [Google Scholar]
- 107.Parker WB, Sorscher EJ. Purine nucleoside phosphorylase as enzymatic activator of nucleoside prodrugs. 2011/0212073 A1. US Patent application number. 2011
- 108.Cacciapuoti G, Bagarolo ML, Martino E, Scafuri B, Marabotti A, Porcelli M. Efficient Fludarabine-Activating PNP From Archaea as a Guidance for Redesign the Active Site of E. coli PNP. J Cell Biochem. 2016;117:1126–1135. doi: 10.1002/jcb.25396. [DOI] [PubMed] [Google Scholar]
- 109.Voorde JV, Liekens S, Balzarini J. Mycoplasma Hyorhinis-encoded purine nucleoside phosphorylase: Kinetic properties and its effect on the cytostatic potential of purine-based anticancer drugs. Mol Pharmacol. 2013;84:865–875. doi: 10.1124/mol.113.088625. [DOI] [PubMed] [Google Scholar]
- 110.Berger F, Canova C, Vicat JM, Benabid AL, Wion D. Mycoplasma: a new potential vector for gene therapy? Medical hypotheses. 1999;52:605–607. doi: 10.1054/mehy.1998.0816. [DOI] [PubMed] [Google Scholar]
- 111.Afshar S, Sawaya MR, Morrison SL. Structure of a mutant human purine nucleoside phosphorylase with the prodrug, 2-fluoro-2'-deoxyadenosine and the cytotoxic drug, 2-fluoroadenine. Protein Sci. 2009;18:1107–1114. doi: 10.1002/pro.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Afshar S, Olafsen T, Wu AM, Morrison SL. Characterization of an engineered human purine nucleoside phosphorylase fused to an anti-her2/neu single chain Fv for use in ADEPT. J Exp Clin Cancer Res. 2009;28:147. doi: 10.1186/1756-9966-28-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Afshar S, Asai T, Morrison SL. Humanized ADEPT comprised of an engineered human purine nucleoside phosphorylase and a tumor targeting peptide for treatment of cancer. Mol Cancer Ther. 2009;8:185–193. doi: 10.1158/1535-7163.MCT-08-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Asai T, Wims LA, Morrison SL. An interaction between S*tag and S*protein derived from human ribonuclease 1 allows site-specific conjugation of an enzyme to an antibody for targeted drug delivery. J Immunol Methods. 2005;299:63–76. doi: 10.1016/j.jim.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 115.Asai T, Trinh R, Ng PP, Penichet ML, Wims LA, Morrison SL. A human biotin acceptor domain allows site-specific conjugation of an enzyme to an antibody-avidin fusion protein for targeted drug delivery. Biomol Eng. 2005;21:145–155. doi: 10.1016/j.bioeng.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Deharvengt S, Wack S, Uhring M, Aprahamian M, Hajri A. Suicide gene/prodrug therapy for pancreatic adenocarcinoma by E. coli purine nucleoside phosphorylase and 6-methylpurine 2'-deoxyriboside. Pancreas. 2004;28:E54–64. doi: 10.1097/00006676-200403000-00020. [DOI] [PubMed] [Google Scholar]
- 117.Deharvengt S, Wack S, Aprahamian M, Hajri A. Transcriptional tumor-selective promoter targeting of E. coli purine nucleoside phosphorylase for pancreatic cancer suicide gene therapy. J Gene Med. 2005;7:672–680. doi: 10.1002/jgm.701. [DOI] [PubMed] [Google Scholar]
- 118.Chen G, Tang B, Yang BY, Chen JX, Zhou JH, Li JH, Hua ZC. Tumor-targeting Salmonella typhimurium, a natural tool for activation of prodrug 6MePdR and their combination therapy in murine melanoma model. Appl Microbiol Biotechnol. 2013;97:4393–4401. doi: 10.1007/s00253-012-4321-8. [DOI] [PubMed] [Google Scholar]
- 119.Chen ZH, Huang GL, Tu YQ, Jiang Y, Dai WX. Dual specific antitumor effects of Semliki Forest virus-based DNA vector carrying suicide Escherichia coli purine nucleoside phosphorylase gene via Salmonella. Int J Oncol. 2013;42:2009–2018. doi: 10.3892/ijo.2013.1900. [DOI] [PubMed] [Google Scholar]
- 120.Critchley RJ, Jezzard S, Radford KJ, Goussard S, Lemoine NR, Grillot-Courvalin C, Vassaux G. Potential therapeutic applications of recombinant, invasive E. coli. Gene Therapy. 2004;11:1224–1233. doi: 10.1038/sj.gt.3302281. [DOI] [PubMed] [Google Scholar]
- 121.Ungerechts G, Frenzke ME, Yaiw KC, Miest T, Johnston PB, Cattaneo R. Mantle cell lymphoma salvage regimen: synergy between a reprogrammed oncolytic virus and two chemotherapics. Gene Therapy. 2010;17:1506–1516. doi: 10.1038/gt.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bossow S, Grossardt C, Temme A, Leber MF, Sawall S, Rieber EP, Cattaneo R, von Kalle C, Ungerechts G. Armed and targeted measles virus for chemovirotherapy of pancreatic cancer. Cancer Gene Therapy. 2011;18:598–608. doi: 10.1038/cgt.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ungerechts G, Springfield C, Frenzke ME, Lampe J, Parker WB, Sorscher EJ, Cattaneo R. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Molecular Therapy. 2007;15:1991–1997. doi: 10.1038/sj.mt.6300291. [DOI] [PubMed] [Google Scholar]
- 124.Ungerechts G, Springfield C, Frenzke ME, Lampe J, Johnston P, Parker WB, Sorscher EJ, Cattaneo R. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007;67:10939–10947. doi: 10.1158/0008-5472.CAN-07-1252. [DOI] [PubMed] [Google Scholar]
- 125.Tai CK, Wang W, Lai YH, Logg CR, Parker WB, Li YF, Hong JS, Sorscher EJ, Chen TC, Kasahara N. Enhanced efficiency of prodrug activation therapy by tumor-selectively replicating retrovirus vectors armed with the Escherichia coli purine nucleoside phosphorylase. Cancer Gene Therapy. 2010;17:614–623. doi: 10.1038/cgt.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kikuchi E, Menendez S, Ozu C, Ohori M, Cordon-Cardo C, Logg CR, Kasahara N, Bochner BH. Delivery of replication-competent retrovirus expressing Escherichia coli purine nucleoside phosphorylase increases the metabolism of the prodrug, fludarabine phosphate and suppresses the growth of bladder tumor xenografts. Cancer Gene Ther. 2007;14:279–86. doi: 10.1038/sj.cgt.7701013. [DOI] [PubMed] [Google Scholar]
- 127.Deharvengt S, Rejiba S, Wack S, Aprahamian M, Hajri A. Efficient electrogene therapy for pancreatic adenocarcinoma treatment using the bacterial purine nucleoside phosphorylase suicide gene with fludarabine. Int J Oncol. 2007;30:1397–1406. [PubMed] [Google Scholar]
- 128.Heinkelein M, Hoffmann U, Lucke M, Imrich H, Muller JG, Meixensberger J, Westphahl M, Kretschmer A, Rethwilm A. Experimental therapy of allogeneic solid tumors induced in athymic mice with suicide gene-transducing replication-competent foamy virus vectors. Cancer Gene Ther. 2005;12:947–953. doi: 10.1038/sj.cgt.7700855. [DOI] [PubMed] [Google Scholar]
- 129.Gadi VK, Alexander SD, Kudlow JE, Allan P, Parker WB, Sorscher EJ. In vivo sensitization of ovarian tumors to chemotherapy by expression of E. coli purine nucleoside phosphorylase in a small fraction of tumor cells. Gene Therapy. 2000;7:1738–1743. doi: 10.1038/sj.gt.3301286. [DOI] [PubMed] [Google Scholar]
- 130.Munch-Peterson A, Mygind B. Transport of nucleic acid precursors. In: Munch-Peterson A, editor. Metabolism of nucleotides nucleosides and nucleobases in microorganisms. London, New York, Paris, San Diego, San Francisco, Sao Paulo, Sydney Tokyo, Toronto: Academic Press; 1983. pp. 260–264. [Google Scholar]