Abstract
Individuals with chronic health conditions persist in smoking despite the presence of smoking-related illness. The aim of this study was to examine whether chronic health conditions moderate response to reduced nicotine content cigarettes (0.4, 2.4, 5.2, 15.8 mg/g of tobacco). This is a secondary analysis of a controlled clinical laboratory study that examined the acute effects of cigarettes varying in nicotine content among individuals especially vulnerable to smoking and tobacco dependence. Participants in the present study were categorized as having 0, 1–2, or ≥3 smoking-related chronic health conditions (i.e., chronic condition severity, CCS). Repeated-measures analysis of variance was used to examine whether CCS moderated response to cigarettes across measures of addiction potential (i.e., concurrent choice testing between nicotine dose pairs, Cigarette Purchase Task (CPT) performance, positive subjective effects), tobacco withdrawal, cigarette craving, and smoking topography. No main effects of CCS or interactions of CCS and nicotine dose were observed for concurrent choice testing, positive subjective effects, tobacco withdrawal, or smoking topography. Main effects of CCS were noted on the CPT with greater CCS being associated with less persistent demand. There was an interaction of CCS and nicotine dose on Factor 1 of the Questionnaire on Smoking Urges with the effects of dose significant only among those with 1–2 chronic conditions. Overall, we see minimal evidence that chronic condition severity affects response to reduced nicotine content cigarettes. A policy that reduces the nicotine content of cigarettes to minimally addictive levels may benefit smokers already experiencing smoking-related chronic conditions.
Keywords: Reduced nicotine content cigarettes, Addiction potential, Abuse liability, Chronic health conditions, Medical comorbidities, Chronic conditions, Vulnerable populations
1. Introduction
Chronic health conditions represent the leading causes of death in the U.S. and a substantial economic burden in terms of direct medical costs and lost productivity (CDC, 2013; Vogeli et al., 2007). Cigarette smoking is associated with the onset and progression of many chronic health conditions including site-specific cancers, coronary heart disease, diabetes, and respiratory diseases (U.S. Department of Health and Human Services, 2014). For example, there is evidence of a dose-response relationship between cigarette smoking rate and incidence of diabetes, and smoking has been linked with poorer asthma control among asthmatics (McLeish and Zvolensky, 2010; Willi et al., 2007).
Several recent studies have examined cigarette smoking and other tobacco use among those with and without chronic health conditions. Individuals with chronic conditions have higher prevalence of smoking and non-cigarette tobacco use, and have not exhibited the same declines in smoking prevalence over time as those without chronic conditions (Keith et al., 2017; Stanton et al., 2016). However, there is promising evidence to suggest that individuals with chronic health conditions attempt to quit smoking at higher rates than those without these conditions and may also have greater motivation to quit (Duffy et al., 2011; Gaalema et al., 2018; Kalkhoran et al., 2018). Regarding quit success, the literature is mixed. While some studies have suggested that smokers with chronic health conditions achieve higher rates of abstinence compared to those without chronic conditions (Holm et al., 2017; Lando et al., 2003; Streck et al., 2018; Yang et al., 2015), others have demonstrated poorer cessation outcomes in this group (Kalkhoran et al., 2018; Holm et al., 2017; Nair et al., 2018). There is some evidence to suggest that cigarette smokers with medical conditions may be more likely to concurrently use tobacco and electronic cigarettes (Kruse et al., 2017; Rigotti et al., 2015), which may lead to increased nicotine intake, exposure to additional toxins, and potentially more difficulty quitting, though more conclusive research is needed on this topic (National Academies of Sciences Engineering and Medicine, 2018).
Reducing the burden of cigarette smoking among smokers with chronic health conditions and other vulnerabilities will require tobacco control and regulatory policies that are more effective at changing behavior. In 2009, with the passage of the Family Smoking Prevention and Tobacco Control Act (FSPTCA), the FDA was granted regulatory authority over cigarettes, including authority to establish a standard to limit the maximal nicotine content of cigarettes if doing so benefits public health (111th Congress, 2009). In response, research on this topic is growing, including a recent multi-site clinical laboratory experiment that examined acute response to cigarettes varying in nicotine content among three vulnerable populations: individuals with affective disorders, opioid dependence, and economically disadvantaged women (Higgins et al., 2017). This study also collected information on the presence of co-morbid other chronic health conditions, which is the focus of the current report.
To our knowledge, no studies have reported examining potential moderating effects of chronic health conditions on response to cigarettes varying in nicotine content. This is a particularly important and timely question to examine as the U.S. FDA's Center for Tobacco Products deemed smokers with “mental health or medical-comorbidities” a specific vulnerable population on whom more research is needed to inform the regulation of tobacco products to protect public health (Department of Health and Human Services, 2017). Considering that individuals with chronic conditions persist in smoking despite the presence of serious smoking-related illness (Stanton et al., 2016), they could be at risk for attempting to sustain their usual nicotine exposure levels through compensatory smoking or for experiencing untoward levels of craving or withdrawal should those levels be reduced. Against this background, the aim of the present study was to examine whether the presence and severity of co-morbid chronic health conditions may moderate response to reduced nicotine content cigarettes using a battery of dependent measures designed to assess the addiction potential of smoking, craving and withdrawal, and compensatory smoking.
2. Methods
2.1. Sample
Participants were 169 adult daily smokers enrolled in a three-site (University of Vermont, Brown University, Johns Hopkins University School of Medicine) randomized controlled trial. Study methods have been described in detail previously (Higgins et al., 2017). Briefly, participants were recruited though advertisements placed on Facebook, community bulletin boards, buses and local newspapers from March 23, 2015 to April 25, 2016. Participants were recruited from three vulnerable populations: individuals with affective disorders (n = 56) as an exemplar of smokers with mental illness, individuals with opioid dependence as an exemplar of smokers with other substance use disorders (n = 60), and women of reproductive age with low educational attainment as an exemplar of smokers with socioeconomic disadvantage (n = 53). All participants provided written informed consent and procedures were approved by institutional review boards at each study site.
2.2. Chronic health conditions
Chronic health conditions were assessed at intake using an investigator-developed Medical History Questionnaire. Using an interview format, participants were asked, “Have you ever been diagnosed with or experienced symptoms of [insert chronic condition].”. Participants were also asked additional details about each self-reported medical condition including the start and end date of each condition if relevant, medications used for treatment, and whether the participant was seeing a doctor for the condition. The present study examines chronic health conditions associated with smoking, including high blood pressure, heart disease or stroke, cardiac arrhythmia, blood clots, ulcers, asthma or breathing problems, epilepsy/seizures/convulsions, other neurological problems, diabetes, hyperthyroidism, headaches, episodes of dizziness, overwhelming fatigue, chest pain, and kidney or bladder problems (U.S. Department of Health and Human Services, 2014). For the purposes of this secondary analysis, participants were categorized into three chronic condition severity (CCS) groups based on the number of chronic health conditions endorsed: 0, 1–2, or 3 or more chronic health conditions. This method of measuring the presence and number of chronic conditions is supported by prior publications on this topic (Stanton et al., 2016; Duffy et al., 2011; Kruse et al., 2017).
2.3. Research cigarettes
Spectrum research cigarettes manufactured by 22nd Century Group (Clarence, NY) were obtained from the National Institute on Drug Abuse for use in the trial. The study used four nicotine doses, 0.4, 2.4, 5.2, 15.8 mg of nicotine per gram of tobacco (mg/g), and cigarettes were available in assignment to menthol or non-menthol flavors based on participant's usual brand cigarette type. The 15.8 mg/g dose is similar to the nicotine content of commercially available cigarettes and functioned as a control condition. All experimental sessions using study cigarettes were conducted under double-blind conditions.
2.4. Procedure
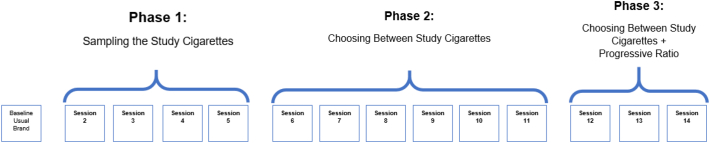
Participants presented for a study-intake assessment where we assessed demographic and smoking characteristics (i.e., age, gender, race, education level, employment status, annual income, marital status, cigarettes per day, menthol status, years smoked regularly, level of nicotine dependence) and determined study eligibility (Higgins et al., 2017). Eligible participants completed a three-phase study with 14 experimental sessions using a within-subjects design (Fig. 1). Prior to all experimental sessions, participants presented to the laboratory having abstained from smoking 6–8 h, operationalized as ≥50% reduction in baseline breath carbon monoxide (CO) levels. Session 1 served as a protocol-orientation session where participants smoked their usual brand cigarette according to Phase I procedures (described below).
Fig. 1.
Overview of study design.
In Phase 1 (Sessions 2–5), participants sampled each research cigarette dose (one cigarette per session) under double-blind conditions and in randomized order. Cigarettes were smoked ad libitum (i.e., cigarettes smoked as participants desired and as they would smoke their own brand cigarette) using a CReSS Smoking device (Clinical Research Support System, CReSS) (Lee et al., 2003) which recorded measures of smoking topography. Smoking topography measures how a participant smokes a cigarette and records indices such as number of puffs, duration of puffs etc. Before and every 15 min for an hour after smoking each cigarette, participants completed the Minnesota Tobacco Withdrawal Scale (MTWS) (Hughes and Hatsukami, 1986) and the Questionnaire of Smoking Urges-Brief (QSU-B) (Cox et al., 2001) to assess tobacco withdrawal and cigarette craving, respectively. Additionally, after smoking each research cigarette, participants completed the modified Cigarette Evaluation Questionnaire (mCEQ) (Arger et al., 2017; Cappelleri et al., 2007), which assesses the positive and negative subjective effects of smoking. They also completed the Cigarette Purchase Task (CPT) (Jacobs and Bickel, 1999; MacKillop et al., 2008) following smoking, which is a behavioral economic simulation task in which participants estimate the number of cigarettes they would purchase to smoke within a 24-hour period across a range of cigarette prices. In the CPT, the following indices are modeled: Intensity: daily cigarette smoking rate at no or minimal cost; Omax: maximum total expenditure on smoking in a 24-h period (e.g., $5.00); Pmax: the price at which maximum expenditure occurs and smoking rate begins decreasing corresponding to increasing price (e.g., $0.50/cigarette for a person smoking 10 cigarettes/day); Breakpoint: the price at which one would quit smoking rather than incur the cost (e.g., $1.00/cigarette); Elasticity: overall sensitivity of demand to increasing price.
Phase 2 (Sessions 6–11) and Phase 3 (Sessions 12–14) directly tested the reinforcing effects of all of the six possible cigarette dose pairs by giving participants the opportunity to choose which cigarette dose they preferred to smoke during 3-hour concurrent choice test sessions. In Phase 2, both cigarettes were always available at an equal response cost of 10 mouse clicks (Fixed-ratio 10). Specifically, participants had to click the computer mouse 10 times in order to receive two puffs of a cigarette. They could make as many choices as they wanted during this 3-hour period. In Phase 3, only the highest and lowest dose pair was compared with the highest dose cigarette available on a progressive ratio schedule. One dose pair was presented per session in Phases 2 and 3. Phase 3 results are not included in this report.
2.5. Statistical methods
We analyzed data from all participants reported in our parent study (N = 169; Higgins et al., 2017). The first step in creating the chronic condition severity (CCS) group variable was summing the chronic conditions for each participant. The distribution of the chronic condition sums was highly right-skewed and on an ordinal scale, thus CCS was treated as a categorical variable in all analyses. Categorizing participants with 0 and 1 or 2 conditions produced groups of similar size ensuring sufficient statistical power for comparisons between these two groups. The third group was comprised of the remaining participants with 3 or more conditions, which also provided sufficient statistical power for between-group comparisons.
Group comparisons (0, 1–2, ≥3 CCS) of demographic and other characteristics collected at baseline were conducted using Wilcoxon Rank Sum tests and Chi-Square tests. Analyses of Phase 1 results examined whether CCS moderated the effects of dose on CPT, mCEQ and smoking topography using repeated-measures analysis of covariance (ANCOVAs), with nicotine dose as the within-participant factor and CCS as a fixed effect with three levels. As there were differences between CCS groups in gender, and the proportion of participants from the three parent trial vulnerable populations (i.e., affective disorders, opioid-dependence, socioeconomically disadvantaged women), these variables were included as covariates. The MTWS and QSU-B were examined similarly using mixed-factor ANCOVAs with time added as an additional within-participant factor. Analyses also included fixed effects for session and the three parent study populations who were studied in independent parallel research protocols and combined for analysis in the original and this secondary study. Time-by-dose and CCS-by-dose interactions were included to test whether subjective effects before and after smoking differed by dose and to test for differential effects of CCS by dose; when not significant, interaction effects were dropped from models. An additional random effect was included to account for the three study sites.
In analyzing Phase 1 aggregate-level cigarette demand in the CPT, demand curves were fit to mean reported consumption at each price across participants, doses, and CCS groups. To quantify participant-level CPT demand elasticity, a demand curve was fit to individual consumption at each price for each dose. When fitting demand curves, we constrained demand intensity to the participants' reported consumption at $0.00 to leave elasticity as the only fitted parameter. Elasticity values >1.00 were winsorized to 1.00 prior to statistical analysis (22 of 845 cases). All other demand indices were empirically quantified from observed values. Omax, Pmax, Breakpoint, and Elasticity were log10 transformed to correct for skewness. We reviewed CPT results and found systematic patterns in 92.7% of demand curves. One data point for elasticity was an extremely small value (~1 E − 20) where the consumption values at all prices were the same. This data point was excluded from analyses. In cases where participants reported zero consumption across all prices (54 of 845 cases), curve fitting was not possible, so elasticity was not analyzed and other demand indices were quantified as 0.
Analyses of Phase 2 results on preference among all possible dose pairs were examined using repeated-measures analysis of variance, with each pairwise combination as the within-participant factor, CCS as a fixed effect, and population and gender as covariates.
Across all tests, statistical significance was defined a priori as p < 0.05 (2-tailed). Significant main or interaction effects were followed by post-hoc testing using Bonferroni corrections, dividing the critical value (p < 0.05) by the number of comparisons to derive a more conservative Type I error rate. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and GraphPad Prism (GraphPad Software, La Jolla, CA).
3. Results
3.1. Participant characteristics
Demographic and smoking characteristics across the three CCS categories are reported in Table 1, with 41% of the sample endorsing 0 chronic conditions, 44% 1–2 conditions, and 15% reporting ≥3 conditions. Only two significant differences in baseline characteristics were noted between CCS categories, with the proportion of males (χ2 (2) = 5.91, p = 0.05) and the proportion of participants with affective disorders in a severity category (χ2(4) = 36.79, p < 0.0001) increasing as CCS increased.
Table 1.
Participant characteristics by number of chronic health conditions.
| All (N = 169) |
Chronic condition severity (CSS) |
p value | |||
|---|---|---|---|---|---|
| 0 (n = 70) |
1–2 (n = 74) |
3+ (n = 25) |
|||
| Age (M ± SD) | 35.6 ± 11.4 | 33.1 ± 9.4 | 36.6 ± 11.4 | 39.6 ± 14.7 | 0.13 |
| Gender (% Female) | 120 (71.1) | 56 (80.0) | 50 (67.6) | 14 (56.0) | 0.05 |
| Study population | <0.0001 | ||||
| Affective disorders | 56 | 9 (12.9) | 30 (40.5) | 17 (68.0) | |
| Opioid dependent | 60 | 25 (35.7) | 28 (37.8) | 7 (28.0) | |
| Low SES women | 53 | 36 (51.4) | 16 (21.6) | 1 (4.0) | |
| Race/ethnicity | 0.84 | ||||
| White | 123 (72.8) | 49 (70.0) | 53 (71.6) | 21 (84.0) | |
| Native American/Alaskan Native | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Asian | 1 (0.6) | 1 (1.4) | 0 (0) | 0 (0) | |
| Black/African-American | 23 (13.6) | 12 (17.1) | 9 (12.2) | 2 (8.0) | |
| Native Hawaiian/Pacific-Islander | 1 (0.6) | 0 (0) | 1 (1.4) | 0 (0) | |
| Other or > 1 race | 15 (8.9) | 6 (8.6) | 8 (10.1) | 1 (4.0) | |
| Hispanic/Latino | 6 (3.5) | 2 (2.9) | 3 (4.1) | 1 (4.0) | |
| Education | 0.07 | ||||
| 8th grade or less | 4 (2.4) | 2 (2.9) | 2 (2.7) | 0 (0) | |
| Some high school | 23 (13.6) | 9 (12.9) | 10 (13.5) | 4 (16.0) | |
| High school graduate/equivalent | 58 (34.3) | 21 (30.0) | 26 (35.1) | 11 (44.0) | |
| Some college | 64 (37.9) | 36 (51.4) | 21 (28.4) | 7 (28.0) | |
| 2-Year Associate's Degree | 10 (5.9) | 1 (1.4) | 7 (9.5) | 2 (8.0) | |
| College graduate/4-year degree | 6 (3.5) | 0 (0) | 6 (8.1) | 0 (0) | |
| Graduate or professional degree | 4 (2.4) | 1 (1.4) | 2 (2.7) | 1 (4.0) | |
| Employment status | 0.10 | ||||
| Full-time | 41 (24.3) | 18 (25.7) | 19 (25.7) | 4 (16.0) | |
| Part-time | 36 (21.3) | 19 (27.1) | 14 (18.9) | 3 (12.0) | |
| Unemployed | 46 (27.2) | 20 (28.6) | 23 (31.1) | 3 (12.0) | |
| Disability | 30 (17.8) | 7 (10) | 13 (17.6) | 10 (40) | |
| Retired/other | 16 (9.5) | 6 (8.6) | 5 (6.8) | 5 (20.0) | |
| Annual Household Income (M ± SD) | 24,766.7 ± 20,711.1 | 25,305.2 ± 17,550.7 | 25,855.0 ± 25,907.4 | 20,481.3 ± 13,245.3 | 0.47 |
| Marital status | 0.36 | ||||
| Married | 27 (16.0) | 14 (20.0) | 8 (10.8) | 5 (20.0) | |
| Never married | 103 (60.9) | 44 (62.9) | 48 (64.9) | 11 (44.0) | |
| Divorced/separated | 35 (20.7) | 11 (15.7) | 16 (21.6) | 8 (32.0) | |
| Widowed | 4 (2.4) | 1 (1.4) | 2 (2.7) | 1 (4.0) | |
| Cigarettes per day (M ± SD) | 15.8 ± 7.5 | 15.3 ± 6.6 | 15.2 ± 6.7 | 19.1 ± 10.9 | 0.33 |
| Primary menthol smoker | 61 (36.1) | 26 (37.1) | 29 (39.2) | 6 (24.0) | 0.38 |
| Breath CO (ppm) (M ± SD) | 22.4 ± 12.0 | 21.2 ± 9.5 | 23.6 ± 14.8 | 22.3 ± 8.1 | 0.71 |
| Age started smoking regularly (M years ± SD) | 16.3 ± 4.3 | 16.3 ± 3.7 | 16.4 ± 4.4 | 15.8 ± 5.5 | 0.27 |
| Fagerström test for nicotine dependence (Fagerstrom and Schneider, 1989) (M ± SD) | 5.0 ± 2.2 | 4.9 ± 2.3 | 5.0 ± 2.0 | 5.4 ± 2.3 | 0.82 |
Note. Cells represent n (%) unless otherwise indicated.
Bolded values represent p ≤ 0.05.
3.2. Reinforcing effects of smoking via direct testing and simulation
3.2.1. Direct testing in phase 2 choice paradigm
No significant differences by CCS were observed in how participants chose between different dose pairs. Participants consistently chose the higher nicotine dose cigarette at greater than chance levels across all six dose pairs (F(5,825) = 4.86, p = 0.0002), and no interactions between CCS and nicotine dose were observed (Table 2).
Table 2.
Mean ± SEM proportion of choices for the higher dose in Phase 2 fixed ratio testing by nicotine content and chronic condition severity (CCS).
| Phase 2 - Fixed ratio 10 schedule |
||||||
|---|---|---|---|---|---|---|
| 15.8 v 0.4 mg/g | 15.8 v 2.4 mg/g | 15.8 v 5.2 mg/g | 5.2 v 0.4 mg/g | 5.2 v 2.4 mg/g | 2.4 v 0.4 mg/g | |
| Overall | 68.91 ± 3.33* | 65.58 ± 3.33* | 61.14 ± 3.33* | 59.86 ± 3.33* | 54.84 ± 3.33 | 55.70 ± 3.33 |
| 0 Chronic conditions | 73.77 ± 4.35 | 70.94 ± 4.35 | 63.51 ± 4.35 | 57.59 ± 4.35 | 61.37 ± 4.35 | 52.49 ± 4.36 |
| 1–2 Chronic conditions | 70.46 ± 4.44 | 64.29 ± 4.44 | 61.71 ± 4.45 | 64.49 ± 4.44 | 54.04 ± 4.44 | 63.64 ± 4.44 |
| 3+ Chronic conditions | 58.93 ± 6.89 | 62.57 ± 6.89 | 61.07 ± 6.89 | 60.81 ± 6.89 | 47.07 ± 6.88 | 49.38 ± 6.89 |
Note. Tabled values represent least square means ± SEM. Overall ratings collapsed across all subjects and by CCS are displayed by dose. Significant differences in choice for the higher dose over the lower dose are displayed for each dose pair in the overall data with an asterisk. There were no significant differences by CCS, so no post hoc testing between CCS groups were conducted.
3.2.2. Cigarette purchase task
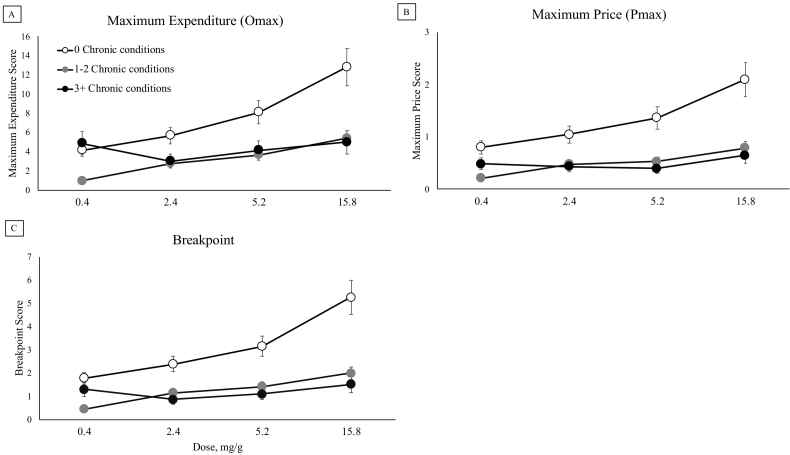
Main effects of CCS were observed on Omax (F(2,163) = 3.16, p = 0.04), Pmax (F(2,163) = 4.02, p = 0.02), and Breakpoint (F(2,163) = 3.48, p = 0.03), with individuals with no chronic health conditions showing more persistent demand (Fig. 2, Panels A, B, C). Stated another way, individuals with more chronic conditions generally evidenced lower maximum daily expenditure on smoking, began showing decreases in smoking rate at a lower price/cigarette, and ceased purchasing cigarettes (i.e., quit) rather than incurred the increasing cost of smoking at a lower price than those without chronic conditions. No main effects of medication use were noted on these three CPT indices when we replaced CCS with medication use in the models or included both (ps > 0.05). However, when examining medication use and CCS together in the models, CCS was no longer a significant predictor of Omax, Pmax, or Breakpoint (ps = 0.08, 0.08, and 0.16, respectively) demonstrating modest redundancy between these two independent variables. Across all five indices of demand, main effects of nicotine dose were noted, with more intense and persistent demand seen at higher nicotine doses (Intensity (F(3,495) = 3.26, p = 0.02); Omax (F(3,495) = 6.97, p = 0.0001); Pmax (F(3,495) = 7.41, p < 0.0001); Elasticity (F(3,447) = 4.13, p = 0.01); Breakpoint (F(3,495) = 8.39, p < 0.0001) (Fig. 2, Panels A, B, C).
Fig. 2.
Results from three indices of the Cigarette Purchase Task (CPT) simulating demand for each cigarette varying in nicotine content across escalating prices by chronic condition severity.
Note. White filled circles represent individuals with no chronic conditions, gray filled circles represent individuals with 1–2 conditions, and black filled circles represent those with 3 or more conditions. Omax: Maximum daily expenditure that one is willing to incur for daily smoking; Pmax: The price at which smoking rate becomes elastic and begins decreasing corresponding to increasing price, or in other words, the price at which Omax occurs; Breakpoint: The price at which one would quit smoking rather than incur the cost of cigarettes. There were main effects of chronic condition severity for Omax, Pmax, and Breakpoint. All means presented are least square means and error bars represent SEM.
3.3. Subjective effects
3.3.1. Modified cigarette evaluation questionnaire
There were no main effects of CCS on mCEQ ratings. Significant main effects of dose were observed across each of the five mCEQ subscales (Satisfaction (F(3,495) = 27.65, p < 0.0001); Psychological Reward (F(3,495) = 17.17, p < 0.0001); Aversion (F(3,495) = 5.13, p = 0.002); Enjoyment of Respiratory Tract Sensations (F(3,495) = 22.42, p < 0.0001); Craving Reduction (F(3,495) = 14.44, p < 0.0001), with generally higher positive ratings of smoking reported at higher doses. No significant interactions of CCS and dose were noted on the mCEQ (Table 3).
Table 3.
Mean ± SEM scores on subscales of the Modified Cigarette Evaluation Questionnaire by nicotine content and chronic condition severity (CCS).
| Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
|
|---|---|---|---|---|---|---|---|---|
| 0.4 mg/g | 2.4 mg/g | |||||||
| Satisfaction | 3.23 ± 0.15a | 3.47 ± 0.21 | 2.63 ± 0.20 | 3.61 ± 0.34 | 3.54 ± 0.15be | 3.64 ± 0.21 | 3.55 ± 0.20 | 3.45 ± 0.34 |
| Psychological reward | 2.69 ± 0.14a | 2.84 ± 0.19 | 2.18 ± 0.18 | 3.05 ± 0.30 | 2.75 ± 0.14ab | 2.86 ± 0.19 | 2.66 ± 0.18 | 2.72 ± 0.30 |
| Aversion | 1.48 ± 0.15a | 1.47 ± 0.16 | 1.53 ± 0.18 | 1.45 ± 0.23 | 1.52 ± 0.15a | 1.55 ± 0.16 | 1.53 ± 0.18 | 1.48 ± 0.23 |
| Enjoyment of Respiratory tract sensations | 2.85 ± 0.16a | 3.29 ± 0.22 | 2.41 ± 0.21 | 2.85 ± 0.37 | 2.99 ± 0.16b | 3.39 ± 0.22 | 2.98 ± 0.21 | 2.61 ± 0.36 |
| Craving reduction | 3.33 ± 0.29a | 3.47 ± 0.33 | 2.85 ± 0.34 | 3.67 ± 0.46 | 3.52 ± 0.29ab | 3.54 ± 0.33 | 3.55 ± 0.34 | 3.47 ± 0.46 |
| Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
|
|---|---|---|---|---|---|---|---|---|
| 5.2 mg/g | 15.8 mg/g | |||||||
| Satisfaction | 3.81 ± 0.15ce | 4.07 ± 0.21 | 3.59 ± 0.20 | 3.76 ± 0.34 | 4.55 ± 0.15 d | 4.83 ± 0.21 | 4.41 ± 0.20 | 4.43 ± 0.34 |
| Psychological reward | 3.02 ± 0.14b | 3.11 ± 0.19 | 2.77 ± 0.18 | 3.19 ± 0.30 | 3.44 ± 0.14c | 3.47 ± 0.19 | 3.09 ± 0.18 | 3.77 ± 0.30 |
| Aversion | 1.51 ± 0.15a | 1.46 ± 0.16 | 1.62 ± 0.18 | 1.44 ± 0.23 | 1.75 ± 0.15b | 1.77 ± 0.16 | 1.81 ± 0.18 | 1.66 ± 0.23 |
| Enjoyment of respiratory tract sensations | 3.49 ± 0.16c | 3.79 ± 0.22 | 3.23 ± 0.21 | 3.47 ± 0.37 | 4.07 ± 0.16d | 4.28 ± 0.22 | 3.90 ± 0.21 | 4.04 ± 0.37 |
| Craving reduction | 3.81 ± 0.29b | 3.92 ± 0.33 | 3.73 ± 0.34 | 3.79 ± 0.46 | 4.41 ± 0.29c | 4.41 ± 0.33 | 4.38 ± 0.34 | 4.43 ± 0.46 |
Note. Tabled values represent least square means ± SEM. Overall ratings collapsed across all subjects and by CCS are displayed by dose. Post-hoc testing is shown on subscales within the overall group where there were main effects of dose; data points not sharing a superscript letter differed significantly after Bonferroni correction. There were no significant differences by CCS, so no post hoc testing between CCS groups were conducted.
3.3.2. Minnesota tobacco withdrawal scale
No significant main effects of CCS were noted on the MTWS total scores or the individual ‘Desire to Smoke’ MTWS item. Significant interactions between nicotine dose and time were observed for total scores (F(12,1286) = 2.42, p = 0.004) and Desire to Smoke (F(12,1331) = 6.62, p < 0.0001); each of the doses decreased withdrawal below pre-smoking baseline levels, with the 15.8 mg/g dose producing longer duration effects (Table 4, only total scores shown to conserve space).
Table 4.
Mean ± SEM total scores on the Minnesota tobacco withdrawal scale by time and nicotine content and chronic condition severity (CCS).
| Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
|
|---|---|---|---|---|---|---|---|---|
| 0.4 mg/g | 2.4 mg/g | |||||||
| Pre-smoking baseline | 1.06 ± 0.12a⁎ | 0.90 ± 0.12 | 1.15 ± 0.13 | 1.11 ± 0.19 | 1.03 ± 0.11a⁎ | 0.83 ± 0.12 | 1.12 ± 0.13 | 1.19 ± 0.19 |
| 15 min | 0.70 ± 0.12b⁎ | 0.55 ± 0.12 | 0.79 ± 0.13 | 0.77 ± 0.19 | 0.66 ± 0.11b⁎ | 0.57 ± 0.12 | 0.67 ± 0.13 | 0.76 ± 0.19 |
| 30 min | 0.81 ± 0.12b⁎ | 0.66 ± 0.12 | 0.86 ± 0.13 | 0.95 ± 0.19 | 0.80 ± 0.11c⁎ | 0.73 ± 0.12 | 0.75 ± 0.13 | 1.00 ± 0.19 |
| 45 min | 0.95 ± 0.12a⁎ | 0.78 ± 0.12 | 1.01 ± 0.13 | 1.10 ± 0.19 | 0.93 ± 0.11a⁎ | 0.87 ± 0.12 | 0.88 ± 0.13 | 1.14 ± 0.19 |
| 60 min | 1.03 ± 0.12a⁎ | 0.92 ± 0.12 | 1.09 ± 0.13 | 1.09 ± 0.19 | 0.97 ± 0.11a⁎ | 0.89 ± 0.12 | 0.96 ± 0.13 | 1.13 ± 0.19 |
| Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
|
|---|---|---|---|---|---|---|---|---|
| 5.2 mg/g | 15.8 mg/g | |||||||
| Pre-smoking baseline | 1.08 ± 0.11ab⁎ | 0.86 ± 0.12 | 1.15 ± 0.13 | 1.34 ± 0.19 | 1.08 ± 0.12 a⁎ | 0.94 ± 0.12 | 1.06 ± 0.13 | 1.41 ± 0.19 |
| 15 min | 0.67 ± 0.11c⁎ | 0.58 ± 0.12 | 0.64 ± 0.13 | 0.86 ± 0.19 | 0.59 ± 0.12 b⁎ | 0.44 ± 0.12 | 0.61 ± 0.13 | 0.81 ± 0.19 |
| 30 min | 0.83 ± 0.11d⁎ | 0.70 ± 0.12 | 0.82 ± 0.13 | 1.09 ± 0.19 | 0.69 ± 0.12 c⁎ | 0.58 ± 0.12 | 0.68 ± 0.13 | 0.90 ± 0.19 |
| 45 min | 0.95 ± 0.11b⁎ | 0.85 ± 0.12 | 0.96 ± 0.13 | 1.11 ± 0.19 | 0.83 ± 0.12 a⁎ | 0.72 ± 0.12 | 0.81 ± 0.13 | 1.08 ± 0.19 |
| 60 min | 1.07 ± 0.11a⁎ | 0.97 ± 0.12 | 1.07 ± 0.13 | 1.21 ± 0.19 | 0.92 ± 0.12 a⁎ | 0.85 ± 0.12 | 0.89 ± 0.13 | 1.11 ± 0.19 |
Note. Tabled values represent least square means ± SEM. Overall ratings collapsed across all subjects and by CCS are displayed by dose. Post-hoc testing is shown on subscales within the overall group for the dose by time interaction; data points not sharing a superscript letter differed significantly after Bonferroni correction within each dose pair; data points not sharing a superscript symbol within each time point also differed significantly after Bonferroni correction. There were no significant differences by CCS, so no post hoc testing between CCS groups were conducted.
3.3.3. Questionnaire on smoking urges-brief
No main effects of CCS were noted on QSU Factor 1 or 2 subscales. Significant nicotine dose by time interactions were noted on the QSU Factor 1 (F(3,2014) = 9.04, p < 0.0001) and Factor 2 (F(12,2014) = 5.22, p < 0.0001) subscales, where the effect of the 15.8 mg/g dose produced longer duration effects (Table 5, only QSU Factor 2 shown to conserve space). There was a significant interaction of CCS and nicotine dose on QSU Factor 1 (F(6,495) = 2.24, p = 0.04), with the effect of dose significant among those with 1–2 chronic conditions (p < 0.0001) but not in the other two CCS categories (Fig. 3). This interaction remained significant when medication use was added to the model with CCS (p = 0.04). There was no main effect of medication use on QSU Factor 1 when it was examined in the model in place of CCS or when both were included (p > 0.05).
Table 5.
Mean ± SEM Factor 2 scores on the Questionnaire on Smoking Urges-Brief by time and nicotine content and chronic condition severity (CCS).
| Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
|
|---|---|---|---|---|---|---|---|---|
| 0.4 mg/g | 2.4 mg/g | |||||||
| Pre-smoking baseline | 3.84 ± 0.24a⁎ | 3.62 ± 0.27 | 3.70 ± 0.29 | 4.13 ± 0.40 | 3.81 ± 0.24a⁎ | 3.64 ± 0.27 | 3.54 ± 0.29 | 4.41 ± 0.40 |
| 15 min | 2.97 ± 0.24b⁎ | 2.73 ± 0.27 | 2.82 ± 0.29 | 3.34 ± 0.40 | 2.91 ± 0.24b⁎ | 2.64 ± 0.27 | 2.77 ± 0.29 | 3.35 ± 0.40 |
| 30 min | 3.15 ± 0.24bc⁎ | 2.98 ± 0.27 | 3.03 ± 0.29 | 3.26 ± 0.40 | 3.1 ± 0.24bc⁎† | 2.91 ± 0.27 | 2.87 ± 0.29 | 3.57 ± 0.40 |
| 45 min | 3.38 ± 0.24cd⁎ | 3.27 ± 0.27 | 3.24 ± 0.29 | 3.39 ± 0.40 | 3.32 ± 0.24cd⁎ | 3.20 ± 0.27 | 3.03 ± 0.29 | 3.82 ± 0.40 |
| 60 min | 3.56 ± 0.24d⁎ | 3.39 ± 0.27 | 3.40 ± 0.29 | 3.78 ± 0.40 | 3.55 ± 0.24ad⁎ | 3.40 ± 0.27 | 3.32 ± 0.29 | 3.97 ± 0.40 |
| Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
|
|---|---|---|---|---|---|---|---|---|
| 5.2 mg/g | 15.8 mg/g | |||||||
| Pre-smoking baseline | 3.85 ± 0.24a⁎ | 3.58 ± 0.27 | 3.60 ± 0.29 | 4.62 ± 0.40 | 3.97 ± 0.24a⁎ | 3.82 ± 0.27 | 3.67 ± 0.29 | 4.54 ± 0.40 |
| 15 min | 2.79 ± 0.24b⁎† | 2.55 ± 0.27 | 2.67 ± 0.29 | 3.09 ± 0.40 | 2.56 ± 0.24b† | 2.37 ± 0.27 | 2.33 ± 0.29 | 3.09 ± 0.40 |
| 30 min | 3.03 ± 0.24b⁎† | 2.90 ± 0.27 | 2.81 ± 0.29 | 3.33 ± 0.40 | 2.85 ± 0.24c† | 2.72 ± 0.27 | 2.59 ± 0.29 | 3.26 ± 0.40 |
| 45 min | 3.33 ± 0.24ce⁎ | 3.15 ± 0.27 | 3.16 ± 0.29 | 3.59 ± 0.40 | 3.16 ± 0.24df⁎ | 3.05 ± 0.27 | 2.89 ± 0.29 | 3.58 ± 0.40 |
| 60 min | 3.54 ± 0.24de⁎ | 3.40 ± 0.27 | 3.33 ± 0.29 | 3.82 ± 0.40 | 3.36 ± 0.24ef⁎ | 3.27 ± 0.27 | 3.08 ± 0.29 | 3.74 ± 0.40 |
Note. Tabled values represent least square means ± SEM. Overall ratings collapsed across all subjects and by CCS are displayed by dose. Post-hoc testing is shown on subscales within the overall group for the dose by time interaction; data points not sharing a superscript letter differed significantly after Bonferroni correction within each dose pair; data points not sharing a superscript symbol within each time point also differed significantly after Bonferroni correction. There were no significant differences by CCS, so no post hoc testing between CCS groups were conducted.
Fig. 3.
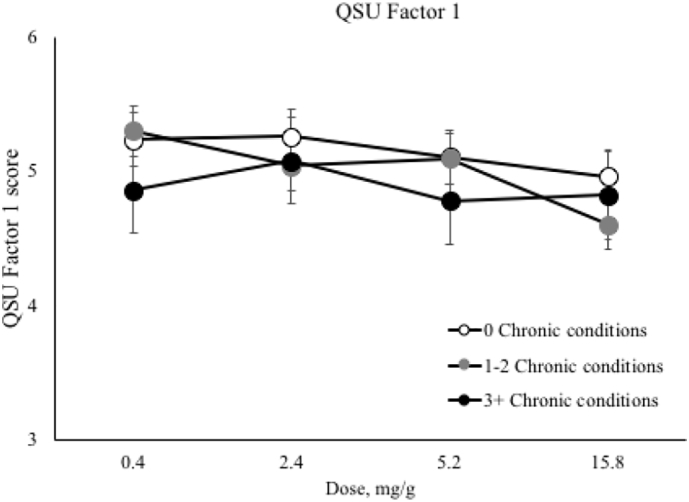
Results from the Questionnaire on Smoking Urges-Brief (QSU-B) Factor 1 depicting the interaction between chronic condition severity and nicotine dose.
Note. White filled circles represent individuals with no chronic conditions, gray filled circles represent individuals with 1–2 conditions, and black filled circles represent those with 3 or more conditions. QSU Factor 1 average scores range from 1 to 7 with higher scores indicating higher levels of craving. The y axis has been restricted to allow for ease of interpretation of the interaction. There was a significant interaction of chronic condition severity and nicotine dose on QSU Factor 1. Means presented are least square means and error bars represent SEM.
3.4. Smoking topography
There were no significant main effects of CCS on smoking topography. Main effects of nicotine dose were noted for three smoking topography indices (Total Puff Volume (F(3,481) = 3.87, p = 0.01); Mean Maximum Flow Rate (F(3,482) = 2.73, p = 0.04); Maximum Number of Puffs (F(3,480) = 11.86, p < 0.0001), with more intense rates of smoking observed at the higher vs. lower dose cigarettes—effects opposite of those associated with compensatory smoking. No significant interactions of CCS and nicotine dose were observed across any of the smoking topography measures (Table 6).
Table 6.
Mean ± SEM smoking topography measures by nicotine content and chronic condition severity (CCS).
| Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
|
|---|---|---|---|---|---|---|---|---|
| 0.4 mg/g | 2.4 mg/g | |||||||
| Total puff volume | 552.75 ± 58.24a | 570.39 ± 66.52 | 569.24 ± 68.26 | 518.61 ± 94.06 | 596.65 ± 58.27 a | 593.86 ± 66.64 | 557.05 ± 68.43 | 639.05 ± 93.99 |
| Mean puff volume | 46.06 ± 2.16 | 46.92 ± 2.97 | 46.76 ± 2.86 | 44.48 ± 4.85 | 47.96 ± 2.17 | 49.31 ± 2.98 | 45.76 ± 2.88 | 48.82 ± 4.85 |
| Mean puff duration | 1.43 ± 0.06 | 1.50 ± 0.07 | 1.41 ± 0.08 | 1.39 ± 0.11 | 1.42 ± 0.06 | 1.44 ± 0.07 | 1.40 ± 0.08 | 1.42 ± 0.11 |
| Mean inter-puff interval | 21.81 ± 0.99 | 20.32 ± 1.37 | 23.80 ± 1.31 | 21.31 ± 2.22 | 21.63 ± 0.99 | 20.44 ± 1.37 | 23.03 ± 1.31 | 21.42 ± 2.22 |
| Mean maximum flow rate | 38.71 ± 6.63a | 38.77 ± 6.74 | 38.84 ± 6.77 | 38.52 ± 7.19 | 40.28 ± 6.63 ab | 40.25 ± 6.74 | 39.98 ± 6.77 | 40.61 ± 7.19 |
| Puff number | 11.98 ± 0.75a | 11.83 ± 0.85 | 11.76 ± 0.88 | 12.36 ± 1.20 | 12.49 ± 0.75 a | 12.24 ± 0.85 | 12.06 ± 0.88 | 13.16 ± 1.20 |
| Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
Overall |
0 Chronic conditions |
1–2 Chronic conditions |
3+ Chronic conditions |
|
|---|---|---|---|---|---|---|---|---|
| 5.2 mg/g | 15.8 mg/g | |||||||
| Total puff volume | 581.01 ± 58.23a | 608.00 ± 66.69 | 579.60 ± 68.13 | 555.44 ± 94.06 | 675.66 ± 58.29 b | 747.24 ± 67.05 | 625.06 ± 68.27 | 654.69 ± 94.06 |
| Mean puff volume | 47.91 ± 2.16 | 49.37 ± 2.99 | 50.53 ± 2.85 | 43.84 ± 4.85 | 48.95 ± 2.17 | 51.23 ± 3.02 | 46.21 ± 2.85 | 49.41 ± 4.85 |
| Mean puff duration | 1.43 ± 0.06 | 1.48 ± 0.07 | 1.46 ± 0.08 | 1.36 ± 0.11 | 1.42 ± 0.06 | 1.44 ± 0.07 | 1.39 ± 0.08 | 1.43 ± 0.11 |
| Mean inter-puff interval | 22.46 ± 0.99 | 20.69 ± 1.37 | 23.34 ± 1.31 | 23.34 ± 2.22 | 22.87 ± 0.99 | 22.15 ± 1.38 | 22.98 ± 1.30 | 23.49 ± 2.22 |
| Mean maximum flow rate | 40.81 ± 6.63 ab | 40.76 ± 6.74 | 42.46 ± 6.77 | 39.21 ± 7.19 | 41.87 ± 6.63b | 42.77 ± 6.74 | 40.78 ± 6.77 | 42.06 ± 7.19 |
| Puff number | 12.24 ± 0.75 a | 12.44 ± 0.85 | 11.87 ± 0.88 | 12.40 ± 1.20 | 13.93 ± 0.75 b | 14.18 ± 0.85 | 14.03 ± 0.88 | 13.59 ± 1.20 |
Note. Tabled values represent least square means ±SEM. Overall ratings collapsed across all subjects and by CCS are displayed by dose. Post-hoc testing is shown on indices within the overall group where there were main effects of dose; data points not sharing a superscript letter differed significantly after Bonferroni correction within each subscale. There were no significant differences by CCS, so no post hoc testing between CCS groups were conducted.
4. Discussion
To our knowledge, this is the first study to directly examine response to reduced-nicotine content cigarettes among individuals already suffering with smoking-related chronic health conditions. This is a timely and important topic to examine in light of the FDA's Center for Tobacco Products having (a) identified smokers with medical comorbidities as a population on whom more research is needed to inform the regulation of tobacco products (Department of Health and Human Services, 2017) and, (b) recently announced a comprehensive tobacco regulatory plan that includes a potential national policy to lower the nicotine content of cigarettes to minimally addictive levels (Gottlieb and Zeller, 2017).
Overall, we found scant evidence that CCS has a substantive influence on acute response to reduced nicotine content cigarettes. More specifically, no main effects of CCS nor interactions between CCS and nicotine dose were observed on relative reinforcing effects measured by concurrent choice testing, positive subjective effects, measures of tobacco withdrawal, and smoking topography. Main effects of CCS were noted on CPT indices assessing hypothetical demand for the varying dose cigarettes, but those effects suggested an inverse relationship where greater CCS was associated with less persistent demand. A modest degree of that inverse relationship between CCS and demand appears to be attributable to greater medication use among those with greater CCS. The only interaction of CCS and nicotine dose was on craving for the positive reinforcing effects of smoking (QSU-B Factor 1), with significant dose effects being limited to those with 1–2 conditions. That association was independent of medication use. None of these effects suggest that individuals with greater CCS would respond unfavorably to reduced nicotine content cigarettes. If anything, they suggest that those with greater CCS may benefit more, which is important as the number of people with multiple chronic conditions in the U.S. is large and increasing over time (Vogeli et al., 2007; Ward and Schiller, 2013).
It is interesting that smokers without chronic conditions evidenced higher demand for cigarettes across all nicotine doses on the CPT. As cigarette demand is considered to be a measure of the reinforcing effects of smoking, this may suggest that individuals with more chronic health conditions find cigarettes less reinforcing under escalating constraint, and thus may be more apt to reduce or quit smoking than other smokers. Indeed, as was mentioned above, there is a small body of literature suggesting that individuals with smoking-related chronic conditions may be more motivated to quit and more likely to quit and remain abstinent from smoking (Duffy et al., 2011; Holm et al., 2017; Lando et al., 2003; Streck et al., 2018; Yang et al., 2015). This pattern of results is consistent with chronic health conditions increasing the salience of the potential adverse health consequences of smoking or, in economic terms, increasing the cost of smoking. Additional studies examining the reliability of this observation would be useful, especially in light of the growing prevalence of chronic health conditions.
Several limitations of this study merit mention. First, our questionnaire on chronic conditions was not exhaustive and there were certain health conditions of interest that we did not assess (e.g., cancer). Future study of additional chronic conditions as well as the independent contributions of specific conditions on response to reduced nicotine cigarettes may be warranted. Additionally, our questionnaire has not yet been tested for reliability and validity. Second, our assessment of chronic health conditions was based on participant self-report and not confirmed by a physician or medical record and thus may have been influenced by recall bias or inaccurate reporting. Third, we only assessed acute exposure to reduced nicotine content cigarettes and cannot rule out that CCS may be more influential during chronic exposure. An extended-exposure trial currently underway in these same vulnerable populations should allow examination of this research question through 12 weeks of exposure to reduced nicotine content cigarettes.
Overall, the present findings suggest that a policy that reduces the nicotine content of cigarettes to minimally addictive levels has the potential to benefit smokers who are already experiencing smoking-related chronic health conditions.
Funding
This project was supported by Tobacco Centers of Regulatory Science award P50DA036114 from the National Institute on Drug Abuse (NIDA) and Food and Drug Administration (FDA), Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences (NIGMS), and NIDA Institutional Training Grant T32DA007242. Support for JWT was also provided by U54DA031659. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, FDA, or NIGMS.
Disclosures
JRH has received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction and from several non-profit organizations that promote tobacco control. He also consults (without payment) for Swedish Match. All other authors have nothing to declare.
References
- 111th Congress . 2009. Family Smoking Prevention and Tobacco Control Act. HR 1256. 2011. [Google Scholar]
- Arger C.A., Heil S.H., Sigmon S.C. Preliminary validity of the modified cigarette evaluation questionnaire in predicting the reinforcing effects of cigarettes that vary in nicotine content. Exp. Clin. Psychopharmacol. 2017;25(6):473–478. doi: 10.1037/pha0000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelleri J.C., Bushmakin A.G., Baker C.L., Merikle E., Olufade A.O., Gilbert D.G. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict. Behav. 2007;32(5):912–923. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- CDC Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm (Published 2013)
- Cox L.S., Tiffany S.T., Christen A.G. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services Tobacco Centers of Regulatory Science for Research Relevant to the Family Prevention and Tobacco Control Act (U54), RFA-OD-17-003. 2017. https://grants.nih.gov/grants/guide/rfa-files/RFA-OD-17-003.html
- Duffy S.A., Biotti J.K., Karvonen-Gutierrez C.A., Essenmacher C.A. Medical comorbidities increase motivation to quit smoking among veterans being treated by a psychiatric facility. Perspect. Psychiatr. Care. 2011;47(2):74–83. doi: 10.1111/j.1744-6163.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K.O., Schneider N.G. Measuring nicotine dependence: a review of the Fagerstrom tolerance questionnaire. J. Behav. Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Gaalema D.E., Pericot-Valverde I., Bunn J.Y. Tobacco use in cardiac patients: perceptions, use, and changes after a recent myocardial infarction among US adults in the PATH study (2013–2015) Prev. Med. May 2018 doi: 10.1016/j.ypmed.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S., Zeller M. A nicotine-focused framework for public health. N. Engl. J. Med. August 2017 doi: 10.1056/NEJMp1707409. [DOI] [PubMed] [Google Scholar]
- Higgins S.T., Heil S.H., Sigmon S.C. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiat. 2017;74(10):1056–1064. doi: 10.1001/jamapsychiatry.2017.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M., Schiöler L., Andersson E. Predictors of smoking cessation: a longitudinal study in a large cohort of smokers. Respir. Med. 2017;132:164–169. doi: 10.1016/j.rmed.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Hughes J.R., Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jacobs E.A., Bickel W.K. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp. Clin. Psychopharmacol. 1999;7(4):412–426. doi: 10.1037//1064-1297.7.4.412. [DOI] [PubMed] [Google Scholar]
- Kalkhoran S., Kruse G.R., Chang Y., Rigotti N.A. Smoking-cessation efforts by US adult smokers with medical comorbidities. Am. J. Med. 2018;131(3):318.e1–318.e8. doi: 10.1016/j.amjmed.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Keith D.R., Stanton C.A., Gaalema D.E. Disparities in US healthcare provider screening and advice for cessation across chronic medical conditions and tobacco products. J. Gen. Intern. Med. 2017;32(9):974–980. doi: 10.1007/s11606-017-4062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse G.R., Kalkhoran S., Rigotti N.A. Use of electronic cigarettes among U.S. adults with medical comorbidities. Am. J. Prev. Med. 2017;52(6):798–804. doi: 10.1016/j.amepre.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando H., Hennrikus D., McCarty M., Vessey J. Predictors of quitting in hospitalized smokers. Nicotine Tob. Res. 2003;5(2):215–222. doi: 10.1080/0955300031000083436. [DOI] [PubMed] [Google Scholar]
- Lee E.M., Malson J.L., Waters A.J., Moolchan E.T., Pickworth W.B. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob. Res. 2003;5(5):673–679. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- MacKillop J., Murphy J.G., Ray L.A. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp. Clin. Psychopharmacol. 2008;16(1):57–65. doi: 10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- McLeish A.C., Zvolensky M.J. Asthma and cigarette smoking: a review of the empirical literature. J. Asthma. 2010;47(4):345–361. doi: 10.3109/02770900903556413. [DOI] [PubMed] [Google Scholar]
- Nair U.S., Bell M.L., Yuan N.P., Wertheim B.C., Thomson C.A. Associations between comorbid health conditions and quit outcomes among smokers enrolled in a State Quitline, Arizona, 2011–2016. Public Health Rep. 2018;133(2):200–206. doi: 10.1177/0033354918764903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering and Medicine . National Academies Press; Washington, DC: 2018. Public Health Consequences of E-Cigarettes. [PubMed] [Google Scholar]
- Rigotti N.A., Harrington K.F., Richter K. Increasing prevalence of electronic cigarette use among smokers hospitalized in 5 US cities, 2010–2013. Nicotine Tob. Res. 2015;17(2):236–244. doi: 10.1093/ntr/ntu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton C.A., Keith D.R., Gaalema D.E. Trends in tobacco use among US adults with chronic health conditions: National Survey on Drug Use and Health 2005–2013. Prev. Med. 2016;92:160–168. doi: 10.1016/j.ypmed.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streck J.M., Chang Y., Tindle H.A. Smoking cessation after hospital discharge: factors associated with abstinence. J. Hosp. Med. 2018 doi: 10.12788/jhm.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Surgeon General. Gov. Office on Smoking and Health: Centers for Disease Control and Prevention; 2014. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014; p. 944.http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html [Google Scholar]
- Vogeli C., Shields A.E., Lee T.A. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J. Gen. Intern. Med. 2007;22(Suppl. 3):391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.W., Schiller J.S. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Prev. Chronic Dis. 2013;10 doi: 10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi C., Bodenmann P., Ghali W.A., Faris P.D., Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- Yang J.J., Song M., Yoon H.-S. What are the major determinants in the success of smoking cessation: results from the health examinees study. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0143303. [DOI] [PMC free article] [PubMed] [Google Scholar]