Abstract
Delayed brain development in perinatally HIV-infected children may affect the functional brain activity and subsequently cognitive function. The current study evaluated the functional brain activity in HIV-infected children by quantifying the amplitude of low frequency fluctuations (ALFF) and functional connectivity (FC). Additionally, correlation of ALFF and FC with cognitive measures was performed. Twenty-six HIV-infected children and 20 control children underwent neuropsychological (NP) assessment and resting-state functional magnetic resonance imaging (rs-fMRI). ALFF and FC maps were generated and group differences were analyzed using two-sample t-test. Furthermore, ALFF and FC showing significant group differences were correlated with NP scores using Pearson's correlation. Significantly lower ALFF in the left middle temporal gyrus, precentral and post central gyrus was observed in HIV-infected children compared to controls. FC was significantly reduced in the right inferior parietal, vermis, middle temporal and left postcentral regions, and significantly increased in the right precuneus, superior parietal and left middle frontal regions in HIV-infected children as compared to control. HIV-infected children showed significantly lower NP scores in various domains including closure, exclusion, memory, verbal meaning, quantity and hidden figure than controls. These waning cognitive functions were significantly associated with changes in ALFF and FC in HIV-infected children. The findings suggest that abnormal ALFF and FC may responsible for cognitive deficits in HIV-infected children. ALFF and FC in association with cognitive evaluation may provide a clinical biomarker to evaluate functional brain activity and to plan neurocognitive intervention in HIV-infected children undergoing standard treatment.
Keywords: Human immunodeficiency virus, Resting-state functional imaging, Magnetic resonance imaging, Amplitude of low frequency fluctuations, Functional connectivity
Abbreviations: HIV, Human immunodeficiency virus; ALFF, Amplitude of Low frequency fluctuations; FC, Functional connectivity; rs-fMRI, Resting-state functional magnetic resonance imaging; NPT, Neuropsychological test; CNS, Central nervous system; HAART, Highly-active-antiretroviral-therapy; MRI, Magnetic resonance imaging; 1H-MRS, Proton magnetic resonance spectroscopy; SNA, Spontaneous neural activity; ELISA, Enzyme-linked-immunosorbent-assay; RAKIT, Revisie Amsterdamse Kinder Intelligentie Test; BRAVO, Brain volume imaging; TR, Repetition time; TE, Echo time; FOV, Field of view; TI, Inversion time; FA, Flip angle; FLAIR, Fluid-attenuated-inversion recovery; FWHM, Full-width at half-maximum; cART, Combination antiretroviral therapy
Highlights
-
•
Resting-state fMRI and cognitive assessment were performed in HIV-infected children.
-
•
HIV-infected children showed abnormal spontaneous neural activity (SNA) and functional connectivity (FC).
-
•
Lower cognitive scores were significantly associated with SNA and FC in HIV-infected children.
-
•
HIV infection may have significant impact on neurodevelopment and cognitive performance.
1. Introduction
Transfer of human immunodeficiency virus (HIV) from infected mothers to infants during pregnancy, delivery, or breast-feeding is the major route of infection (Rossi and Moschese, 1991). By the time HIV clinical symptoms appear, viruses reach different parts of the body including the central nervous system (CNS) (An et al., 1999; Valcour et al., 2011). Inability of the current treatment to effectively act on the virus in the CNS may be responsible for the neural damage and delayed neurodevelopment in pediatric patients (Eggers et al., 2003; Laughton et al., 2013; Patel et al., 2009; Smith et al., 2006). These damages tend to be more intense in the brain of HIV infected infants and children due to their immature nervous and immune systems (Van Rie et al., 2009; Wachsler-Felder and Golden, 2002).
Structural and functional magnetic resonance imaging (MRI) methods are well established in studying several neurological disorders as they offer direct evidence of brain changes in association with clinical and behavioral outcomes. Structural and functional brain changes in adolescent and adult HIV patients have been examined non-invasively using MRI. These changes include lower brain volumes, gray matter loss, white matter hyper-intensity, altered brain metabolites, higher white matter diffusivity and lower functional brain activity (Chang et al., 2016; Hakkers et al., 2017; Janssen et al., 2016; Ragin et al., 2012; Sanford et al., 2017; Sarma et al., 2014; Thompson and Jahanshad, 2015; Wang et al., 2016). Few studies have reported the structural brain changes including lower cortical and total gray matter volume, lower white matter volume, and altered brain cortical thickness and structural connectivity (Cohen et al., 2016; Hoare et al., 2012; Yadav et al., 2017) in HIV-infected children. Another study based on proton magnetic resonance spectroscopy (1H MRS) observed that altered metabolite levels in the basal ganglia were associated with lower CD4+ and CD4/CD8+ count in HIV-infected children (Mbugua et al., 2016). To the best of our knowledge, there is no published study evaluating the functional brain changes [either by using task-based functional MRI (fMRI) or resting-state fMRI (rs-fMRI)] in HIV-infected children.
Neurocognitive and motor function deficits together with delayed language development suggest neurodevelopmental delays in HIV infected pediatric patients (Laughton et al., 2013; Koekkoek et al., 2008; Le Doare et al., 2012; Lindsey et al., 2007; Van Rie et al., 2008). The rs-fMRI technique can be used for the assessment of neurocognitive functions and neurodevelopmental delays as it offers functional activity outcomes within a short period of time without any confounding factors such as task performance inside the magnet, attention and language, making it ideal for pediatric studies. Rs-fMRI derived matrices such as amplitude of low frequency fluctuations (ALFF) are used to measure regional spontaneous neural activity (SNA), while functional connectivity (FC) is used to evaluate the functional changes in the brain by focusing on long distance patterns of connectivity (Fox and Raichle, 2007; Liu et al., 2014). It has been shown that neuronal changes occur earlier than cognitive or clinical symptoms. Rs-fmri matrices have the potential to improve the early detection of neuronal injury and can be used as a potential biomarker for neurocognitive interventions (Chang et al., 2016).
In the current study, we evaluated the functional brain activity by quantifying ALFF and FC in HIV-infected children. Additionally, we investigated the relationship between cognitive measures and rs-fMRI derived matrices to examine the impact of HIV infection on brain functional outcomes.
2. Materials and methods
The study protocol was approved by the Institutional Regulatory Board and Ethics Committee. Informed consent was obtained from all the participants or their next of kin prior to the study. We prospectively enrolled 49 HIV-infected children (mean ± SD, 9.27 ± 2.50 years) and 23 age/sex matched control children (mean ± SD, 8.66 ± 2.68 years) of the same socioeconomic status and level of education. Diagnosis of HIV was performed according to national HIV testing protocols that include screening by HIV enzyme-linked-immunosorbent-assay (ELISA)/Rapid test followed by confirmation with 2 further HIV rapid tests of higher specificity. All HIV-infected children were treated with standard combination antiretroviral therapy (cART) under the national HIV program. The standard regimen for first-line cART consists of 2 nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) with 1 non-nucleoside reverse-transcriptase inhibitors (NNRTI). NRTIs include thymidine analogue Zidovudine (AZT) or Stavudine (d4T) which are guanosine analogue Abacavir (ABC) in combination with a cytidine analogue Lamivudine (3TC). NNRTIs include Nevirapine (NVP) or Efavirenz (EFV).
All participants underwent clinical assessment including neuropsychological test and brain MRI. None of the subjects were sedated during the MRI examination.
2.1. Neuropsychological assessment
Each subject completed the Revisie Amsterdamse Kinder Intelligentie Test (RAKIT) battery, designed especially for children to detect abnormalities in NP functions (Khire et al., 1992). It contains a battery of nine subsets (closure, exclusion, memory span, verbal meaning, mazes, learning names, quantity, discs, and hidden figure) that assess various domains of cognitive functions such as attention, language, memory, learning, visual, and motor coordination (Khire et al., 1992). All raw test scores were converted to standard scores. The entire test battery required approximately 90–120 min for completion per subject.
2.2. Magnetic resonance imaging
Brain MRI was performed on a 3-T clinical MRI system (Signa Hdxt; GE Healthcare, Milwaukee, Wisconsin) using a vendor supplied 8-channel head coil. 3D high-resolution T1-weighted brain volume imaging (BRAVO) was performed using a IR-prepared fast spoiled gradient echo (FSPGR) pulse sequence with following parameters: repetition time (TR) = 8.4 ms, echo time (TE) = 3.32 ms, inversion time (TI) = 400 ms, flip angle (FA) = 13°, matrix size = 512 × 512, field of view (FOV) = 240 × 240 mm2, slice-thickness = 1.0 mm. T2-weighted images covering the whole-brain in the axial plane were acquired using a dual-echo turbo spin-echo pulse sequence (TR = 5660 ms; TE = 98 ms; FA = 90°; matrix size = 256 × 256; FOV = 240 × 240 mm2; slice-thickness = 3.0 mm). Fluid-attenuated-inversion recovery (FLAIR) imaging was performed using parameters: TR = 9000 ms, TE = 128 ms, TI = 2400 ms, section thickness = 3 mm, FA = 90°, matrix = 320 × 256, FOV = 240 × 240 mm2. The rs-fMRI images were acquired using an echo-planar imaging sequence with following parameters: TR = 2500 ms, TE =30 ms, FA = 90°, number of slices = 46, slice thickness = 3 mm, inter-slice gap = 0 mm with interleaved mode of slice acquisition, and volumes per acquisition = 120. In order to securely support the head and minimize the head movement, vacuum molded cushions and soft pads were used.
Conventional T2-weighted, T1-weighted, and FLAIR images were used to delineate any gross changes in the brain such as hyper-intensity, ventricular enlargements and/or other lesions. Sixteen HIV infected children showed visual MRI abnormalities such as presence of hyper-intensity on FLAIR or brain lesions. While 7 HIV-infected and 3 control children did not meet the motion criteria (2.5 mm translational or 2.5° rotational) and therefore were excluded from the study. Final data analysis was performed on 26 HIV-infected children and 20 control (demographic information is given in Table 1).
Table 1.
Demographic, clinical and neuropsychological test variables of HIV-infected children and control subjects.
| Demographic, clinical and neuropsychological tests | Control (Mean ± SD) | HIV (Mean ± SD) | P value |
|---|---|---|---|
| Age (years) | 8.78 ± 2.64 | 9.92 ± 2.12 | 0.12 |
| Male/Female | 11/9 | 14/12 | 0.94 |
| Education (years) | 4.10 ± 2.23 | 3.87 ± 2.96 | 0.827 |
| Ethnicity | Asian Indian | Asian Indian | NA |
| Peak HIV viral load (log copy/ml) | – | 4.02 ± 1.17 | NA |
| CD4+ T-cell count at MRI (×106/L) | – | 490 ± 260 | NA |
| Closure | 19.6 ± 4.5 | 13.4 ± 5.4 | 0.001 |
| Exclusion | 16.2 ± 7.2 | 9.42 ± 5.7 | 0.002 |
| Memory-span | 11.7 ± 5.4 | 5.58 ± 5.5 | 0.002 |
| Verbal meaning | 19.8 ± 5.9 | 15.5 ± 6.3 | 0.044 |
| Mazes | 14.3 ± 7.3 | 11.8 ± 7.9 | 0.330 |
| Learning names | 19.2 ± 3.7 | 15.6 ± 6.8 | 0.077 |
| Quantity | 19.0 ± 5.6 | 9.73 ± 7.3 | 0.001 |
| Discs | 16.0 ± 6.2 | 14.1 ± 8.4 | 0.468 |
| Hidden figure | 18.2 ± 6.3 | 12.9 ± 7.7 | 0.032 |
2.3. Resting-state f-MRI data preprocessing
The rs-fMRI brain images were processed using statistical parametric mapping software (SPM12, Wellcome Department of Imaging Neuro-Science, London, U.K.) implemented in the RESTplus (http://restfmri.net/) (Song et al., 2011). In brief, the processing pipeline includes following steps- conversion of digital imaging and communications in medicine (DICOM) to neuroimaging informatics technology initiative (NIFTI), removal of initial volumes (n = 10) to allow magnetization stabilization, slice timing correction, and motion correction (up to 2.5 mm of displacement with 2.5° rotation). This was followed by co-registration of high resolution T1-weighted structural images with the mean image of rs-fMRI series. The higher resolution structural images were normalized to the MNI standard space using dartel normalization and the obtained normalized parameters were applied to rs-fMRI images. These images were then resampled to 3 mm and smoothed with a 6 mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. Following that, linear trend discarding and temporal band pass filtering (0.01 Hz–0.08 Hz) were performed to reduce the effects of low and high frequency noise. For the removal of cofounding effect, linear regression analysis with six parameters obtained by rigid body correction of head motion, mean signals from cerebrospinal fluid, averaged white matter signals, and averaged signals across whole brain was used.
2.4. Computation of ALFF maps
ALFF maps of all individual subjects were calculated and used to measure the spontaneous brain activity. For standardizing variability across participants, ALFF map of each subject was normalized to the mean ALFF (mALFF) map and used for group comparison.
2.5. Computation of FC maps
We selected brain regions that showed significant group differences in ALFF as seed regions for FC analysis. In all subjects, three brain areas (left middle temporal gyrus, left precentral and left post central) were extracted as seed regions and used to define the reference time series for FC analysis with spherical seed regions as well as each voxel of the whole brain. Fisher r to z transformation was performed to improve the normality of FC maps and these FC maps were finally used for group comparison.
3. Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, USA). Demographic variables between HIV-infected children and control subjects were compared either by Chi-square test or independent student t-test. The NPT scores were assessed using an independent student t-test. Voxel-based comparison using two sample t-test was performed to investigate differences in ALFF and FC between HIV-infected children and control. AlphaSim method, which employs Monte Carlo simulation was used for multiple comparisons correction for both ALFF and FC by applying following thresholds- 10,000 iterations, p < .005 both at voxel and cluster level (minimum 35 contiguous voxels; age and gender as covariates). Finally, z scores of the brain regions that showed significant changes in ALFF and FC were extracted for further correlation analysis. Pearson's correlation was performed to see the association between rs-fMRI matrices (ALFF and FC: clusters showing significant group difference) and NPT scores.
4. Results
4.1. Demographic, neuropsychological and clinical characteristics
Demographic, neuropsychological and clinical information of subjects included in the final analysis are shown in Table 1. No significant difference in age (p = .11) and gender (p = .94) was observed between HIV-infected children and control. Overall, HIV-infected children showed lower neuropsychological test scores with significant decrease in closure, exclusion, memory, verbal meaning, quantity and hidden figure than control (Table 1). None of the participants had any history of receiving psychiatric treatment. None of the control children were positive for HIV test.
4.2. Resting state-fMRI matrices (ALFF and FC)
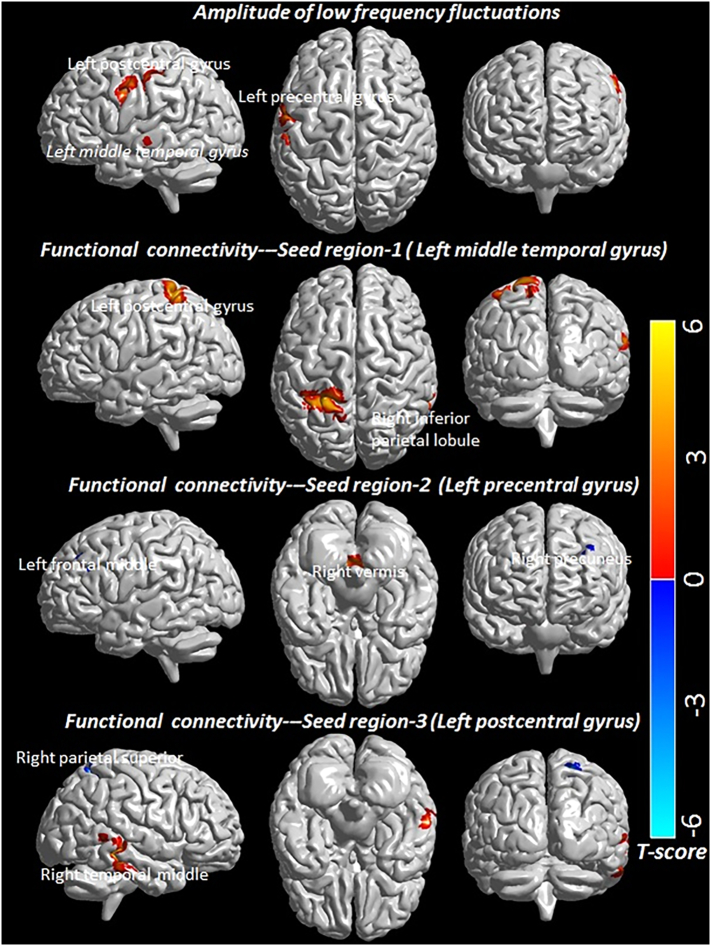
HIV-infected children showed significantly decreased ALFF in multiple brain regions including left middle temporal gyrus, left precentral and left post central gyrus as compared to controls (Fig. 1 and Table 2). Significantly reduced FC for the seed region in the left middle temporal gyrus with clusters in the right inferior parietal and left postcentral gyrus was observed in HIV-infected children. The seed region in the left precentral gyrus of the motor network showed significantly reduced FC with the cluster in the right vermis region and significantly increased FC with clusters in the right precuneus and left middle frontal regions, while the seed region in the left postcentral gyrus of the sensory network showed significantly decreased FC with the cluster in the right middle temporal region and significantly increased FC with the cluster in the right superior parietal region in HIV-infected children (Fig. 1 and Table 2).
Fig. 1.
T-statistics maps are showing changes in amplitude of low frequency fluctuations and functional connectivity in multiple brain areas of HIV-infected children as compared to control. AlphaSim method for multiple comparisons correction both for ALFF and FC was performed by applying following thresholds- 10,000 iterations, p < .005 both at voxel and cluster level (minimum 35 contiguous voxels).
Table 2.
Amplitude of low frequency fluctuations (ALFF) and functional connectivity (FC) in control and pediatric HIV. For each cluster, the coordinates of the peak difference as well as the anatomical location and size (in voxels) are shown.
| RS-fmri metric | Brain region | Cluster size | T-scores | Peak MNI coordinates |
Control Mean ± SD | HIV Mean ± SD | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| ALFF | L Middle temporal | 36 | 4.29 | −60 | −21 | −6 | 1.31 ± 0.25 | 1.03 ± 0.16 | <0.001 |
| L Precentral | 37 | 3.80 | −57 | 0 | 33 | 1.15 ± 0.20 | 0.91 ± 0.13 | <0.001 | |
| L Postcentral | 79 | 5.22 | −39 | −27 | 42 | 1.28 ± 0.27 | 0.99 ± 0.18 | <0.001 | |
| FC-Seed region-1 | R Inferior Parietal | 43 | 3.9 | 63 | −42 | 21 | 0.084 ± 0.184 | −0.138 ± 0.147 | <0.001 |
| L Postcentral | 236 | 4.8 | −21 | −45 | 69 | 0.003 ± 0.129 | −0.227 ± 0.163 | <0.001 | |
| FC-Seed region-2 | R Vermis | 105 | 4.1 | 0 | −51 | −33 | 0.046 ± 0.104 | −0.156 ± 0.181 | <0.001 |
| R Precuneus | 61 | −4.6 | 18 | −39 | 3 | −0.147 ± 0.113 | 0.041 ± 0.130 | <0.001 | |
| L Middle Frontal | 41 | −3.9 | −36 | 27 | 30 | −0.112 ± 0.180 | 0.124 ± 0.233 | 0.001 | |
| FC-Seed region-3 | R Middle Temporal | 137 | 4.9 | 60 | −33 | −9 | 0.012 ± 0.125 | −0.204 ± 0.134 | <0.001 |
| R Superior Parietal | 91 | −4.2 | 24 | −54 | 57 | 0.058 ± 0.201 | 0.299 ± 0.157 | <0.001 | |
L, Left; R, Right; ALFF, Amplitude of low frequency fluctuations; FC, functional connectivity.
4.3. Correlation of resting state-fMRI matrices (ALFF and FC) with NPT score
Reduced ALFF from the left middle temporal region correlated with mazes (p = .005), and reduced ALFF from the left post central region correlated with closure (p = .006) and memory (p = .005), (Table 3). FC of the seed region in the left precentral gyrus of the motor network and the right precuneus region correlated with closure (p = .003) and memory (p = .001), while FC of the left middle frontal region correlated with closure (p = .005), (Table 3). No significant association of age and CD4+ count with either ALFF or FC was observed.
Table 3.
Pearson correlation coefficients of amplitude of low frequency fluctuations and functional connectivity with neuropsychological tests.
| Brain regions | Closure | Exclusion | Memory | VM | Mazes | LN | Quantity | Discs | HF |
|---|---|---|---|---|---|---|---|---|---|
| Correlation of Amplitude of low frequency fluctuations with NPT scores | |||||||||
| L Middle Temporal | 0.32 | 0.41 | 0.32 | 0.13 | 0.43⁎ | 0.15 | 0.27 | 0.05 | 0.26 |
| L Postcentral | 0.43⁎ | 0.38 | 0.44⁎ | 0.20 | 0.34 | 0.32 | 0.30 | 0.21 | 0.36 |
| L Precentral | 0.42 | 0.11 | 0.32 | 0.10 | 0.15 | 0.23 | 0.23 | 0.26 | 0.14 |
| Correlation of Functional connectivity with NPT scores – Seed region-1(Left middle temporal) | |||||||||
| R Inferior Parietal | 0.27 | 0.40 | 0.27 | 0.13 | 0.17 | 0.09 | 0.14 | −0.04 | 0.21 |
| L Postcentral | 0.32 | 0.23 | 0.34 | 0.15 | −0.12 | 0.27 | 0.28 | −0.19 | 0.22 |
| Correlation of Functional connectivity with NPT scores – Seed region-2 (Left precentral) | |||||||||
| R Vermis | 0.14 | 0.03 | 0.14 | −0.04 | −0.16 | 0.14 | 0.05 | −0.13 | 0.14 |
| R Precuneus | −0.45⁎ | −0.34 | −0.52⁎ | −0.24 | −0.19 | −0.25 | −0.42 | −0.07 | −0.34 |
| L Frontal Middle | −0.44⁎ | −0.22 | −0.34 | −0.07 | −0.31 | −0.21 | −0.26 | −0.15 | −0.30 |
| Correlation of Functional connectivity with NPT scores – Seed region-3 (Left postcentral) | |||||||||
| R Temporal Middle | 0.23 | 0.27 | 0.24 | 0.11 | 0.08 | 0.05 | 0.37 | 0.25 | 0.22 |
| R Parietal Superior | −0.32 | −0.28 | −0.37 | −0.08 | −0.25 | −0.16 | −0.14 | −0.17 | −0.13 |
L, Left; R, Right; VM, verbal meaning, LN, learning name; HF, hidden figure; Neuropsychological tests, NPT.
Significant at 0.005 level.
5. Discussion
The current study investigated the effect of HIV on functional brain activity by evaluating ALFF and FC in HIV-infected children. The difference in ALFF and FC in multiple brain regions of HIV infected children suggests altered functional brain activity. Since these brain regions are associated with auditory, language, sensory and motor functional networks, any alteration in their functional activity may be responsible for cognitive deficits as observed in the current study.
It is reported that the middle temporal gyrus is responsible for face recognition, and accessing words while reading (Ischebeck et al., 2004). In the current study, decreased ALFF in the left middle temporal gyrus indicates alteration in auditory and language processes in HIV-infected children. Our findings are consistent with a previous study performed in HIV-infected adult patients, which showed that lower temporal cortex tissue volume was significantly correlated with impaired cognitive functions (Kuper et al., 2011).
Mazes, a subset of the cognitive test battery, evaluates visual-motor coordination. Lower mazes in HIV-infected children correlated with reduced ALFF from the left middle temporal lobe suggesting that HIV-infected children lack profound activity in auditory and visual coordination. It is well reported that left precentral gyrus is involved in motor activity (Yousry et al., 1997), and the presence of reduced ALFF in this brain regions hints towards decreased motor network functioning in HIV-infected children.
The postcentral gyrus is a prominent gyrus in the lateral parietal lobe of the human brain and regulates the primary somatosensory functions. The significant association of reduced ALFF from the postcentral gyrus with lower cognitive functions in HIV-infected children suggests problems in their sensory network. A previous study based on magnetoencephalography showed that significantly reduced theta response in the postcentral gyrus was correlated with neuropsychological performance in HIV-infected patients and suggested the involvement of postcentral gyrus in sensory activity (Wilson et al., 2015). Similarly, a task based fMRI study demonstrated increased activation of the right postcentral gyrus in adult HIV patients suggestive of altered functional connectivity (Melrose et al., 2008).
Previous studies showed significantly reduced cortical thickness in the temporal gyrus of both adult and pediatric patients with HIV (Yadav et al., 2017; Kallianpur et al., 2012) and suggested that neuronal degeneration secondary to viral infection in the brain is responsible for tissue loss. Similarly, loss in gray matter volume and microstructural integrity in precentral and postcentral brain areas has been shown in both adult and adolescent HIV patients. Decreased ALFF in these brain regions of HIV-infected children as observed in the current study may be due to neuronal damage secondary to viral infection in the brain (Yadav et al., 2017; Liu et al., 2014; Kallianpur et al., 2012; Everall et al., 1991; Ketzler et al., 1990; Smith et al., 2008). The functional brain changes observed in the current study did not correspond with the structural brain changes as shown in our previous study (Yadav et al., 2017). This suggests that the functional brain changes may be independent of the structural brain changes. The mutual relationship between structural and functional brain changes remains highly debated. The temporal relationship between structural and functional properties for a particular set of brain regions is not yet fully understood. Moreover, even if the functional connectivity changes precede the modulation of structural connectivity, it is still unclear whether the difference in functional connectivity and structural connectivity changes are constant across functionally heterogeneous human brain networks (Deco et al., 2014; Meier et al., 2016; Robinson et al., 2014). However, a recent study showed that the difference between functional and structural brain changes varies across various brain networks involved in different cognitive and perceptual functions (Takamitsu Watanabe, 2018).
Previous functional neuroimaging studies on various human pathologies have suggested that the inferior and superior parietal gyrus, middle temporal gyrus, middle frontal gyrus, precuneus, postcentral and vermis are involved in several neurocognitive processes including auditory, visual, language, attention, sensory and motor networks (Lowe et al., 2002; Remy et al., 2005; Waites et al., 2006; Wang et al., 2006; Yu et al., 2008; Zang et al., 2007; Zhou et al., 2007). In the current study, altered FC was observed in the right inferior parietal, left postcentral, right vermis, right middle temporal, right precuneus, left middle frontal and right superior parietal gyrus of auditory, visual, language, attention, sensory and motor networks in HIV-infected children. FC difference between multiple brain sites suggests that regions of various functional networks are implicated in reduced brain activity in HIV-infected children. Altered FC connectivity may be responsible for hindering the flow of information in the brain and subsequently for lower cognitive functioning in HIV-infected children.
Amplitude of low frequency fluctuations (ALFF) and regional homogeneity (ReHo) are RsfMRI derived matrices frequently used to assess the brain's neural activity. ALFF and ReHo provide different aspects of brain functions and abnormalities. ALFF reflects the amplitude of spontaneous low-frequency fluctuations or oscillations in the Rs-fMRI signal. The advantage of ALFF is the remarkably high temporal stability and long-term test-retest reliability, however it is prone to noise from physiological sources, particularly near the ventricles and large blood vessels (Zou et al., 2008; Zuo et al., 2010). ReHo is a voxel-based measure of the similarities between the time-series of a given voxel and its nearest neighbors, as calculated by the Kendall coefficient of concordance of the Rs-fMRI time-series. The test-retest reliability of the ReHo is high, although it has a few limitations such as relatively unclear biologic meaning in the different frequency bands and analyses vary significantly depending on both the number of neighbors in a cluster and the amount of spatial smoothing applied to the data (Zang et al., 2004).
In the current study, we did not compare the functional brain changes between treated and untreated HIV-infected children. However, previous studies observed improved functional connectivity in HIV patients that received medications (Ortega et al., 2015). Another study showed that cART improves cognitive performance and functional connectivity in ARV treated-naïve HIV-infected individuals (Zhuang et al., 2017). We are expecting similar impact of cART treatment on patients included in the current study.
A previous study in adolescent patients with HIV showed a significant association of CD4+ count with FC in the brain (Herting et al., 2015). In the current study, we did not observe any significant correlation of CD4 + count with ALFF or FC. We suggest that this may be due to the small sample size which might be considered a limitation of the current study. Future studies will explore the data in multiple dimensions measuring topological properties using graph theoretical methods (Abidin et al., 2018; Bell et al., 2018; Thomas et al., 2015) and evaluate the effect of working memory training on neurocognitive performance of HIV patients. The present study did not observe any significant correlations of age with ALFF and FC in either HIV+ or HIV- participants and there was no interaction effect between HIV and aging, suggesting that HIV may not have an age related neurodegeneration effect. However, it is worth evaluating the effect of aging in this population with a large sample size including a wide age range of ages from childhood to adolescence.
The current study used the RAKIT battery (adopted for demographic, ethnic background, education level, age and gender), which only assessed the neuropsychological profile and is not appropriate for the classification of HIV patients due to the absence of additional test components such as daily functioning, social functioning, status of dementia and pre-existing cause for mild neurocognitive disorder as incorporated in HAND literature criteria (Antinori et al., 2007). However, the RAKIT battery (Khire et al., 1992) has parallel neuropsychological tests (verbal/language; attention/working memory; abstraction/executive; memory; speed of information processing; sensory-perceptual, motor skills) as used in the standard established HAND literature criteria. Incorporating a large sample size, additional components of HAND literature criteria and a follow up study with working memory training (Chang et al., 2016) may provide a comprehensive understanding of the functional brain changes in these patients.
6. Conclusion
In summary, we observed significantly altered ALFF and FC in auditory, visual, language, motor and sensory networks in HIV-infected children. These changes were correlated with waning cognitive functions. The current findings suggest that HIV affects the brain's SNA and FC in pediatric patients, which may have a significant impact on the neurodevelopment and cognitive functioning.
Funding
This study was funded by Department of Science and Technology, New Delhi, India (Grant number: SR/CSI/02/2 0 10, G) and Sidra Medicine, Doha, Qatar, has provided the workstation for image processing.
Conflict of interest
All listed authors declare no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- Abidin A.Z., Am D.S., Nagarajan M.B., Wang L., Qiu X., Schifitto G. Alteration of brain network topology in HIV-associated neurocognitive disorder: a novel functional connectivity perspective. NeuroImage Clin. 2018;17:768–777. doi: 10.1016/j.nicl.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S.F., Groves M., Gray F., Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J. Neuropathol. Exp. Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Antinori A., Arendt G., Becker J.T., Brew B.J., Byrd D.A., Cherner M. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R.P., Barnes L.L., Towe S.L., Chen N.K., Song A.W., Meade C.S. Structural connectome differences in HIV infection: brain network segregation associated with nadir CD4 cell count. J. Neurovirol. 2018;24:454–463. doi: 10.1007/s13365-018-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Lohaugen G.C., Douet V., Miller E.N., Skranes J., Ernst T. Neural correlates of working memory training in HIV patients: study protocol for a randomized controlled trial. Trials. 2016;17:62. doi: 10.1186/s13063-016-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Caan M.W., Mutsaerts H.J., Scherpbier H.J., Kuijpers T.W., Reiss P. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology. 2016;86:19–27. doi: 10.1212/WNL.0000000000002209. [DOI] [PubMed] [Google Scholar]
- Deco G., McIntosh A.R., Shen K., Hutchison R.M., Menon R.S., Everling S. Identification of optimal structural connectivity using functional connectivity and neural modeling. J. Neurosci. 2014;34:7910–7916. doi: 10.1523/JNEUROSCI.4423-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers C., Hertogs K., Sturenburg H.J., van Lunzen J., Stellbrink H.J. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17:1897–1906. doi: 10.1097/00002030-200309050-00008. [DOI] [PubMed] [Google Scholar]
- Everall I.P., Luthert P.J., Lantos P.L. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Hakkers C.S., Arends J.E., Barth R.E., Du Plessis S., Hoepelman A.I., Vink M. Review of functional MRI in HIV: effects of aging and medication. J. Neurovirol. 2017;23:20–32. doi: 10.1007/s13365-016-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Uban K.A., Williams P.L., Gautam P., Huo Y., Malee K. Default mode connectivity in youth with perinatally acquired HIV. Medicine. 2015;94 doi: 10.1097/MD.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J., Fouche J.P., Spottiswoode B., Donald K., Philipps N., Bezuidenhout H. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naive "slow progressors". J. Neurovirol. 2012;18:205–212. doi: 10.1007/s13365-012-0099-9. [DOI] [PubMed] [Google Scholar]
- Ischebeck A., Indefrey P., Usui N., Nose I., Hellwig F., Taira M. Reading in a regular orthography: an FMRI study investigating the role of visual familiarity. J. Cogn. Neurosci. 2004;16:727–741. doi: 10.1162/089892904970708. [DOI] [PubMed] [Google Scholar]
- Janssen M.A., Hinne M., Janssen R.J., van Gerven M.A., Steens S.C., Goraj B. Resting-state subcortical functional connectivity in HIV-infected patients on long-term cART. Brain Imag. Behav. 2016;11:1555–1560. doi: 10.1007/s11682-016-9632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur K.J., Kirk G.R., Sailasuta N., Valcour V., Shiramizu B., Nakamoto B.K. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb. Cortex. 2012;22:2065–2075. doi: 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketzler S., Weis S., Haug H., Budka H. Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol. 1990;80:92–94. doi: 10.1007/BF00294228. [DOI] [PubMed] [Google Scholar]
- Khire U., Bn Hoksbergen, Rene A.C., Bharat S., Athavale U., Kher R. 1992. Indian Child Intelligence Test (ICIT), Technical Manual, Adaptation of Revised Amsterdamse Kinder Intelligentie Test. Jnana Prabodhini's Samshodhan Sanstha. [Google Scholar]
- Koekkoek S., de Sonneville L.M., Wolfs T.F., Licht R., Geelen S.P. Neurocognitive function profile in HIV-infected school-age children. Eur. J. Paediatr. Neurol. 2008;12:290–297. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kuper M., Rabe K., Esser S., Gizewski E.R., Husstedt I.W., Maschke M. Structural gray and white matter changes in patients with HIV. J. Neurol. 2011;258:1066–1075. doi: 10.1007/s00415-010-5883-y. [DOI] [PubMed] [Google Scholar]
- Laughton B., Cornell M., Boivin M., Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J. Int. AIDS Soc. 2013;16 doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doare K., Bland R., Newell M.L. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130:e1326–e1344. doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- Lindsey J.C., Malee K.M., Brouwers P., Hughes M.D., Team P.C.S. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119:e681–e693. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yu C., Zhang X., Liu J., Duan Y., Alexander-Bloch A.F. Impaired long distance functional connectivity and weighted network architecture in Alzheimer's disease. Cereb. Cortex. 2014;24:1422–1435. doi: 10.1093/cercor/bhs410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M.J., Phillips M.D., Lurito J.T., Mattson D., Dzemidzic M., Mathews V.P. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224:184–192. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Mbugua K.K., Holmes M.J., Cotton M.F., Ratai E.M., Little F., Hess A.T. HIV-associated CD4+/CD8+ depletion in infancy is associated with neurometabolic reductions in the basal ganglia at age 5 years despite early antiretroviral therapy. AIDS. 2016;30:1353–1362. doi: 10.1097/QAD.0000000000001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J., Tewarie P., Hillebrand A., Douw L., van Dijk B.W., Stufflebeam S.M. A mapping between structural and functional brain networks. Brain Connect. 2016;6:298–311. doi: 10.1089/brain.2015.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose R.J., Tinaz S., Castelo J.M., Courtney M.G., Stern C.E. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav. Brain Res. 2008;188:337–347. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Ortega M., Brier M.R., Ances B.M. Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. AIDS. 2015;29:703–712. doi: 10.1097/QAD.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K., Ming X., Williams P.L., Robertson K.R., Oleske J.M., Seage G.R., 3rd Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009;23:1893–1901. doi: 10.1097/QAD.0b013e32832dc041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin A.B., Du H., Ochs R., Wu Y., Sammet C.L., Shoukry A. Structural brain alterations can be detected early in HIV infection. Neurology. 2012;79:2328–2334. doi: 10.1212/WNL.0b013e318278b5b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy F., Mirrashed F., Campbell B., Richter W. Verbal episodic memory impairment in Alzheimer's disease: a combined structural and functional MRI study. NeuroImage. 2005;25:253–266. doi: 10.1016/j.neuroimage.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Robinson P.A., Sarkar S., Pandejee G.M., Henderson J.A. Determination of effective brain connectivity from functional connectivity with application to resting state connectivities. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2014;90:012707. doi: 10.1103/PhysRevE.90.012707. [DOI] [PubMed] [Google Scholar]
- Rossi P., Moschese V. Mother-to-child transmission of human immunodeficiency virus. FASEB J. 1991;5:2419–2426. doi: 10.1096/fasebj.5.10.2065890. [DOI] [PubMed] [Google Scholar]
- Sanford R., Fernandez Cruz A.L., Scott S.C., Mayo N.E., Fellows L.K., Ances B.M. Regionally specific brain volumetric and cortical thickness changes in HIV-infected patients in the HAART Era. J. Acquir. Immune Defic. Syndr. 2017;74:563–570. doi: 10.1097/QAI.0000000000001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma M.K., Nagarajan R., Keller M.A., Kumar R., Nielsen-Saines K., Michalik D.E. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. NeuroImage Clin. 2014;4:29–34. doi: 10.1016/j.nicl.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Malee K., Leighty R., Brouwers P., Mellins C., Hittelman J. Effects of perinatal HIV infection and associated risk factors on cognitive development among young children. Pediatrics. 2006;117:851–862. doi: 10.1542/peds.2005-0804. [DOI] [PubMed] [Google Scholar]
- Smith A.B., Smirniotopoulos J.G., Rushing E.J. From the archives of the AFIP: central nervous system infections associated with human immunodeficiency virus infection: radiologic-pathologic correlation. Radiographics. 2008;28:2033–2058. doi: 10.1148/rg.287085135. [DOI] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., Li S.F., Zuo X.N., Zhu C.Z. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamitsu Watanabe G.R. Comparing the temporal relationship of structural and functional connectivity changes in different adult human brain networks: a single-case study. Wellcome Open Res. 2018;3:50. [Google Scholar]
- Thomas J.B., Brier M.R., Ortega M., Benzinger T.L., Ances B.M. Weighted brain networks in disease: centrality and entropy in human immunodeficiency virus and aging. Neurobiol. Aging. 2015;36:401–412. doi: 10.1016/j.neurobiolaging.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Jahanshad N. Novel neuroimaging methods to understand how HIV affects the brain. Curr. HIV/AIDS Rep. 2015;12:289–298. doi: 10.1007/s11904-015-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V., Sithinamsuwan P., Letendre S., Ances B. Pathogenesis of HIV in the central nervous system. Curr. HIV/AIDS Rep. 2011;8:54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie A., Mupuala A., Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122:e123–e128. doi: 10.1542/peds.2007-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie A., Dow A., Mupuala A., Stewart P. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. J. Acquir. Immune Defic. Syndr. 2009;52:636–642. doi: 10.1097/QAI.0b013e3181b32646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsler-Felder J.L., Golden C.J. Neuropsychological consequences of HIV in children: a review of current literature. Clin. Psychol. Rev. 2002;22:443–464. doi: 10.1016/s0272-7358(01)00108-8. [DOI] [PubMed] [Google Scholar]
- Waites A.B., Briellmann R.S., Saling M.M., Abbott D.F., Jackson G.D. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann. Neurol. 2006;59:335–343. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Wang L., Zang Y., He Y., Liang M., Zhang X., Tian L. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. NeuroImage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wang B., Liu Z., Liu J., Tang Z., Li H., Tian J. Gray and white matter alterations in early HIV-infected patients: combined voxel-based morphometry and tract-based spatial statistics. J. Magn. Resonance Imag. 2016;43:1474–1483. doi: 10.1002/jmri.25100. [DOI] [PubMed] [Google Scholar]
- Wilson T.W., Heinrichs-Graham E., Becker K.M., Aloi J., Robertson K.R., Sandkovsky U. Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV-infected older adults. Hum. Brain Mapp. 2015;36:897–910. doi: 10.1002/hbm.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S.K., Gupta R.K., Garg R.K., Venkatesh V., Gupta P.K., Singh A.K. Altered structural brain changes and neurocognitive performance in pediatric HIV. NeuroImage Clin. 2017;14:316–322. doi: 10.1016/j.nicl.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry T.A., Schmid U.D., Alkadhi H., Schmidt D., Peraud A., Buettner A. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain J. Neurol. 1997;120:141–157. doi: 10.1093/brain/120.1.141. Pt 1. [DOI] [PubMed] [Google Scholar]
- Yu C., Liu Y., Li J., Zhou Y., Wang K., Tian L. Altered functional connectivity of primary visual cortex in early blindness. Hum. Brain Mapp. 2008;29:533–543. doi: 10.1002/hbm.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y., Jiang T., Lu Y., He Y., Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Zang Y.F., He Y., Zhu C.Z., Cao Q.J., Sui M.Q., Liang M. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Liang M., Jiang T., Tian L., Liu Y., Liu Z. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci. Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Qiu X., Wang L., Ma Q., Mapstone M., Luque A. Combination antiretroviral therapy improves cognitive performance and functional connectivity in treatment-naive HIV-infected individuals. J. Neurovirol. 2017;23:704–712. doi: 10.1007/s13365-017-0553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q.H., Zhu C.Z., Yang Y., Zuo X.N., Long X.Y., Cao Q.J. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Di Martino A., Kelly C., Shehzad Z.E., Gee D.G., Klein D.F. The oscillating brain: complex and reliable. NeuroImage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]