Summary
Kinesin-1 transports numerous cellular cargoes along microtubules. The kinesin-1 light chain (KLC) mediates cargo binding and regulates kinesin-1 motility. To investigate the molecular basis for kinesin-1 recruitment and activation by cargoes, we solved the crystal structure of the KLC2 tetratricopeptide repeat (TPR) domain bound to the cargo JIP3. This, combined with biophysical and molecular evolutionary analyses, reveals a kinesin-1 cargo binding site, located on KLC TPR1, which is conserved in homologs from sponges to humans. In the complex, JIP3 crosslinks two KLC2 TPR domains via their TPR1s. We show that TPR1 forms a dimer interface that mimics JIP3 binding in all crystal structures of the unbound KLC TPR domain. We propose that cargo-induced dimerization of the KLC TPR domains via TPR1 is a general mechanism for activating kinesin-1. We relate this to activation by tryptophan-acidic cargoes, explaining how different cargoes activate kinesin-1 through related molecular mechanisms.
Keywords: kinesin, cargo, activation, molecular, motor, JIP3, ARF6, KLC, regulation, kinesin-1
Highlights
-
•
Crystal structure of the kinesin-1 KLC2 TPR domain bound to the cargo JIP3
-
•
JIP3 binding site is located on KLC TPR1 and is conserved from sponges to humans
-
•
Molecular mechanism by which ARF6 regulates kinesin-1 recruitment by JIP3/4 revealed
-
•
A unified framework explaining how unrelated cargoes activate kinesin-1
Cockburn et al. present the structure of the cargo binding TPR domain of kinesin light chain bound to the cargo molecule JIP3. From this they propose a mechanism by which multiple, unrelated cellular cargo molecules can “hotwire” the kinesin-1 molecule into motility.
Introduction
Kinesin-1 transports a wide variety of cellular cargoes toward the plus-ends of microtubules, including proteins, vesicles, mRNP complexes, and organelles, and is implicated in a number of diseases including neurodegeneration, viral and bacterial infections, and cancer (Dodding and Way, 2011, Hirokawa et al., 2009). The simplest form of kinesin-1 is the kinesin heavy chain (KHC) homodimer (Diefenbach et al., 2002, Glater et al., 2006, Su et al., 2004, Sun et al., 2011, Vale, 2003). In animal cells, kinesin-1 exists predominantly as a heterotetramer, which consists of the KHC homodimer bound to two copies of kinesin light chain (KLC) (Figure S1) (Gindhart et al., 1998, Johnson et al., 1990, Rahman et al., 1999). KHC comprises an N-terminal motor domain, a central coiled-coil stalk, and a C-terminal tail domain. KLC comprises an N-terminal heptad repeat region that oligomerizes with the KHC stalk, an acidic linker region, and a tetratricopeptide repeat (TPR) domain containing six TPRs (TPR1-6) (Figure 1A) (Gauger and Goldstein, 1993, Verhey et al., 1998, Wong and Rice, 2010, Zhu et al., 2012). The mammalian genome contains four KLC genes (KLC1-4). KLC2 is ubiquitously expressed, while other KLCs are enriched in certain tissues (Junco et al., 2001, Rahman et al., 1998).
Figure 1.
The Crystal Structure of the KLC2TPR:JIP3LZ Complex
(A and B) Schematics of the KLC2 (A) and JIP3 (B) molecules.
(C) The KLC2TPR:JIP3LZ complex. The KLC2TPRs are shown in teal (chain A) and cyan (chain B). The KLC2TPR α helices are shown as cylinders and labeled 1A through 6B. JIP3LZ subunits are shown in magenta (chain C) and pink (chain D) cartoon. Blue/red spheres are N- and C-terminal Cα atoms, respectively.
See also Figure S1.
The KHC motor domain hydrolyses ATP to power microtubule-based motility. At the other end of the molecule, the KHC tail domain and KLC harbor binding sites for cargoes (Blasius et al., 2007, Bowman et al., 2000, Byrd et al., 2001, Diefenbach et al., 2002, Fu and Holzbaur, 2013, Gindhart et al., 2003, Hammond et al., 2008, Nguyen et al., 2005, Pernigo et al., 2013, Seiler et al., 2000, Su et al., 2004, Sun et al., 2011, Verhey et al., 2001, Watt et al., 2015, Zhu et al., 2012). The ATPase activity of the motor domains is tightly regulated by cargo binding. When not bound to cargo, kinesin-1 adopts a folded-up conformation wherein the cargo binding regions inhibit the motor domains (Figure S1) (Cai et al., 2007, Coy et al., 1999, Hackney et al., 1992, Hackney et al., 2009, Hackney and Stock, 2000, Stock et al., 1999). The binding of cargoes to the KHC tail and/or KLC releases the motor domains, leading to activation of kinesin-1 motility (Blasius et al., 2007, Dodding et al., 2011, Friedman and Vale, 1999, Fu and Holzbaur, 2013, Sun et al., 2011, Watt et al., 2015). This regulatory autoinhibition mechanism prevents futile ATP hydrolysis and congestion of microtubule tracks by kinesin-1 motors that are not engaged in cargo transport.
The molecular mechanism of kinesin-1 autoinhibition is best understood for the KHC homodimer. Here, a conserved stretch of residues in one copy of the KHC tail (the IAK motif) locks the motor domains together, preventing their stable association with microtubules (Hackney et al., 2009, Hackney and Stock, 2000, Kaan et al., 2011, Stock et al., 1999). Autoinhibition of the kinesin-1 heterotetramer involves an additional, poorly understood role for KLC (Verhey et al., 1998). Fluorescence resonance energy transfer experiments on KHC homodimers and kinesin-1 heterotetramers in cells showed that the presence of KLC results in the motor domains being splayed apart, relative to their configuration in the KHC homodimer, which was confirmed by chemical crosslinking (Cai et al., 2007). In vitro binding studies using purified recombinant proteins also showed that KLC reduces the affinity for the KHC tail domains for both the motor domains and microtubules (Wong and Rice, 2010). Recent work has shown that the kinesin-1 molecule is regulated by an intramolecular interaction within the KLC molecule, in which the TPR domain binds to a conserved Leu-Phe-Pro (LFP) motif in the upstream acidic linker (Yip et al., 2016) (Figure 1A). Disrupting this interaction either through cargo binding or pharmacological inhibition destabilizes the autoinhibited state of the kinesin-1 molecule, with drastic effects on the organization of the microtubule cytoskeleton (Randall et al., 2017). Thus, KLC adds an additional layer of regulation to the kinesin-1 molecule, but its molecular basis is poorly understood. There is thus a major gap in our understanding of the predominant form of kinesin-1 found in animal cells.
The KLC TPR domain (KLCTPR) is unusual in binding to multiple ligands using distinct sites (Hammond et al., 2008, Zhu et al., 2012). How are these distinct molecular recognition events integrated into a common pathway for activating kinesin-1? The molecular mechanisms by which cargoes bind to kinesin-1 and activate its motility are not well understood. Only one crystal structure of a kinesin-1:cargo complex has been published (Pernigo et al., 2013). Certain cargo molecules bind to the KLCTPR using tryptophan-acidic motifs, which bind to the inner concave surface of the KLCTPR, spanning TPRs 2–4 (Araki et al., 2007, Dodding et al., 2011, Kawano et al., 2012, Pernigo et al., 2013, Zhu et al., 2012). This releases the KLC LFP motif from its binding site on the TPR domain, resulting in kinesin-1 activation; although how release of the LFP motif is coupled to kinesin-1 activation has not been demonstrated (Yip et al., 2016).
Other kinesin-1 cargoes do not contain tryptophan-acidic motifs, yet the molecular details of how these cargoes bind to and activate kinesin-1 remain to be described. The c-Jun N-terminal kinase interacting protein 3 (JIP3) (Figure 1B) and the related protein JIP4 are adaptor molecules that mediate bidirectional transport of a variety of cargoes by linking them to kinesin-1 and dynein (Bowman et al., 2000, Byrd et al., 2001, Cavalli et al., 2005, Huang et al., 2011, Kelkar et al., 2000, Kelkar et al., 2005, Schulman et al., 2014, Verhey et al., 2001). Kinesin-1 recruitment by JIP3/4 is regulated by the GTPase ARF6, which binds to the JIP3/4 leucine zipper (LZ) domain (JIP3/4LZ) and prevents KLC binding (Montagnac et al., 2009). Kinesin-1-driven motility of JIP3/4 is involved in numerous cellular processes and diseases including axonal outgrowth, transport and damage signaling; muscle development, endosomal trafficking, Huntington's disease, and cancer (Bowman et al., 2000, Byrd et al., 2001, Cavalli et al., 2005, Edwards et al., 2013, Marchesin et al., 2015, Montagnac et al., 2009, Morfini et al., 2009, Schulman et al., 2014, Watt et al., 2015). JIP3 activates kinesin-1 by relieving autoinhibition in two stages (Watt et al., 2015). The JIP3LZ (Figure 1B) binds to the KLCTPR, inducing an immotile microtubule-bound intermediate (Nguyen et al., 2005, Watt et al., 2015). Binding of the JIP3 N-terminal region to the KHC tails then triggers motility (Sun et al., 2017, Watt et al., 2015). JIP3 thus sequentially relieves KLC and KHC regulation of the kinesin-1 molecule, making it an ideal model cargo to study kinesin-1 activation.
Here, we describe the crystal structure of the KLC2 TPR domain (KLC2TPR) bound to the JIP3LZ. This reveals how ARF6 regulates kinesin-1 recruitment by JIP3/4. We show that JIP3 activates kinesin-1 by a different mechanism to tryptophan-acidic cargoes, and propose a unified framework explaining how unrelated cargoes activate kinesin-1 through related mechanisms.
Results
The Crystal Structure of the KLC2TPR:JIP3LZ Complex
To investigate the molecular mechanisms of kinesin-1 recruitment and activation by JIP3/4, and how this is regulated by ARF6, we determined the crystal structure of the murine KLC2 TPR domain (KLC2TPR) in complex with the murine JIP3LZ to a resolution of 3.2 Å (Figure 1C; Table 1). The crystallographic asymmetric unit contains a single copy of the complex, which comprises two copies of KLC2TPR bound to one copy of the JIP3LZ dimer, which adopts a parallel coiled-coil conformation. The structures of the two KLC2TPRs are essentially identical to each other and to those reported previously (Nguyen et al., 2017, Yip et al., 2016, Zhu et al., 2012). The six KLC TPRs stack into a solenoidal structure with a super-helical twist, generating an inner (concave) and outer (convex) surface (Figure 1C). The KLCTPR binds JIP3LZ end-on, via TPR1. This was unanticipated, since TPR domain ligands usually bind to the concave inner surface (Zeytuni and Zarivach, 2012). As a result, the TPR domains wind away from the JIP3LZ when the complex is viewed down the 2-fold axis. The complex contains two non-overlapping, identical KLC2TPR:JIP3LZ interfaces. At each interface, a single KLC2TPR (chain A or B in the PDB file) binds to both JIP3LZ subunits (chains C and D). The KLC2 TPR1 α helices pack flat against the side of the JIP3LZ, lying across the JIP3LZ helices at a ∼90° angle, and oriented with helix 1B proximal to the JIP3LZ N terminus (Figure 1C). This buries 571 Å2 of surface area on each KLC2TPR. Binding of KLC2TPR chains A/B buries 325 and 207 Å2 on JIP3LZ chains C/D and D/C, respectively.
Table 1.
X-Ray Data Collection and Refinement Statistics
| KLC2TPR:JIP3LZ | KLC2TPR:CSTN-WD2 | |
|---|---|---|
| Data collectiona | ||
| Space group | P 65 | P 3121 |
| Cell dimensions | ||
| a, b, c (Å) | 163.30, 163.30, 77.12 | 75.44, 75.44, 303.36 |
| α, β, γ (°) | 90.0, 90.0, 120.0 | 90.0, 90.0, 120.0 |
| Resolution (Å) | 81.65–3.20 (3.37–3.20)b | 44.46–3.99 (4.21–3.99) |
| Rsym | 0.105 (1.59) | 0.095 (0.838) |
| I/σI | 13.8 (1.6) | 6.28 (1.4) |
| Completeness (%) | 99.5 (99.8) | 94.9 (95.9) |
| Redundancy | 7.9 (8.0) | 2.8 (2.8) |
| Refinement | ||
| Resolution (Å) | 67.71–3.20 | 44.46–3.99 |
| No. of reflections | 19,378 | 8,641 |
| Rwork/Rfree | 0.191/0.211 | 0.253/0.273 |
| No. of atoms | ||
| Protein | 5,207 | 8,650 |
| Ligand/ion | – | – |
| Water | – | – |
| B factors | ||
| Protein | 127.23 | 209 |
| Ligand/ion | ||
| Water | ||
| RMSD | ||
| Bond lengths (Å) | 0.009 | 0.005 |
| Bond angles (°) | 1.12 | 0.976 |
RMSD, root-mean-square deviation.
Data collected from one crystal in each case.
Values in parentheses are for highest-resolution shell.
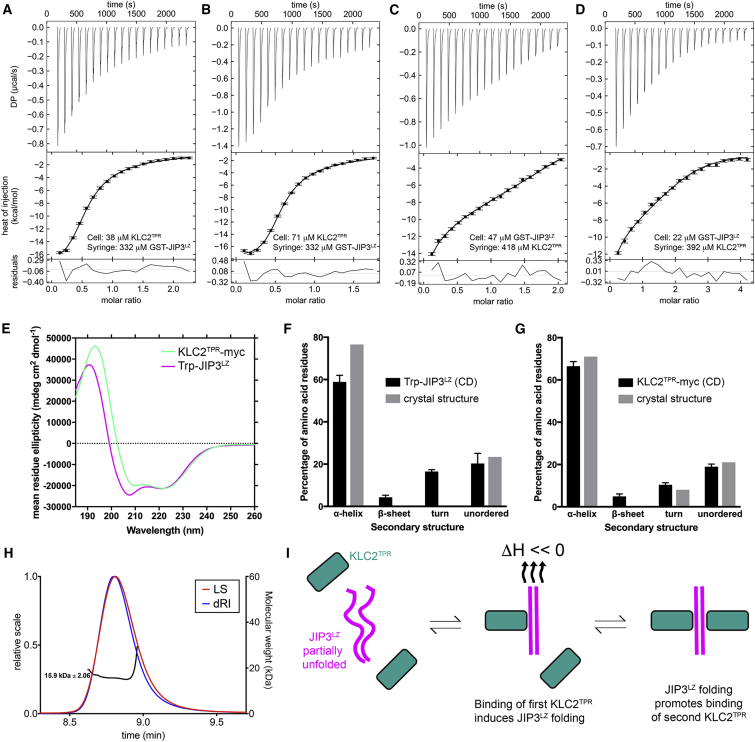
We studied binding of the KLC2TPR to the GST3C-JIP3LZ by isothermal titration calorimetry (ITC) (Figures 2A–2D). The data could not be globally fitted with a single-site binding model, showing that the two binding sites on the JIP3LZ dimer do not bind KLC2TPR independently. Therefore, we globally fitted a two-stage sequential binding model (A + B + B → BA + B → BAB, where A and B represent the GST3C-JIP3LZ dimer and KLC2TPR, respectively) to the ITC data, which gave a good fit across the entire dataset (Figures 2A–2D; Tables S1 and 2). The macroscopic association constants for binding of the first and second KLC2TPR subunits were very similar, suggesting that binding displays positive cooperativity. Binding of the first KLC2TPR is enthalpically driven and involves a large, favorable enthalpy change, and a large, unfavorable entropy change, whereas binding of the second KLC2TPR is entropically driven and involves much smaller energetic changes. These observations suggest that binding of the first KLC2TPR induces conformational changes in the GST3C-JIP3LZ. We therefore investigated the structure of the JIP3LZ using circular dichroism (CD) spectroscopy (Figure 2E). For these experiments, we used a JIP3LZ construct N-terminally fused to a tryptophan residue (Trp-JIP3LZ) to facilitate protein concentration determination since JIP3LZ does not contain any Try or Trp residues. Analysis of the Trp-JIP3LZ CD spectra revealed a mean α-helical content of only 59% ± 3%, compared with 77% calculated for JIP3LZ from the KLC2TPR:JIP3LZ crystal structure (Figure 2F). By comparison, CD spectra for KLC2TPR-myc revealed a secondary structure content very similar to that calculated from the KLC2TPR:JIP3LZ crystal structure (Figure 2G). The Trp-JIP3LZ CD spectra also displayed a deeper minimum at 208 nm relative to that at 222 nm (Figure 2E): the ratio of ellipticities at 222 and 208 nm was 0.88 ± 0.01 (n = 3), whereas coiled coils typically possess ratios greater than 1.0 (Zhou et al., 1994). Nonetheless, in size-exclusion coupled to multiple-angle laser light scattering (SEC-MALLS) experiments the JIP3LZ eluted as a single peak with a molecular weight of 15.9 kDa (Figure 2H), showing that the JIP3LZ is a stable dimer. The structure of the unbound Trp-JIP3LZ thus differs significantly from that observed in the KLC2TPR:JIP3LZ crystal structure. These observations suggest that binding of the first KLC2TPR induces the JIP3LZ dimer to adopt the coiled-coil conformation, resulting in large energetic changes and promoting binding of the second KLC2TPR (Figure 2H).
Figure 2.
Biophysical Studies of KLC2TPR Binding to the JIP3LZ
(A–D) ITC thermograms and isotherms for KLC2TPR binding to GST3C-JIP3LZ. The error bars in the isotherms show the errors associated with integration of the injection peaks in the corresponding thermograms. Molar ratios correspond to the GST3C-JIP3LZ dimer concentration. The curves show the sequential binding model that was globally fitted across all the isotherms in the dataset. The lower panel shows the residuals between the isotherm data points and the fitted model.
(E) CD spectra of the Trp-JIP3LZ and KLC2TPR-myc.
(F and G) Secondary structure composition of the Trp-JIP3LZ (F) and KLC2TPR-myc (G). The black bars show the secondary structure composition determined from deconvolution of the CD spectra (mean and SD from three experiments). The gray bars show the Trp-JIP3LZ and KLC2TPR-myc secondary structure compositions calculated from the KLC2TPR:JIP3LZ crystal structure.
(H) SEC-MALLS chromatogram for the JIP3LZ domain showing the light scattering (LS) and differential refractive index (dRI) traces and molecular weight (black curve).
(I) Schematic showing the sequential binding model for KLC2TPR binding to the JIP3LZ.
Table 2.
Globally Fitted Parameters for Sequential Binding of KLC2TPR to GST-JIP3LZ
| Stagea | K (μM−1) | ΔH (kCal/mol) | –TΔS (kCal/mol) | Active Fraction (A) | Active Fraction (B) |
|---|---|---|---|---|---|
| A + B + B → BA + B | 0.1317 | −18.756 | 11.772 | 0.973 | 0.965 |
| BA + B → BAB | 0.1315 | 0.952 | −7.935 |
JIP3LZ. See also Table S1.
A, GST-JIP3LZ dimer; B, KLC2TPR.
The JIP3LZ Binds to KLC2 TPR1
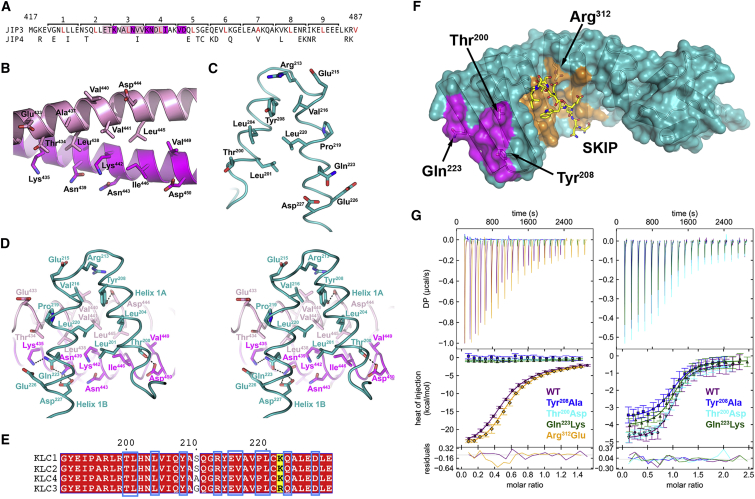
The KLC2TPR binding site spans heptad repeats 2–5 (residues 433–435, 437–442, 444–446, and 450) of the JIP3LZ (Figure 3A). The JIP3LZ coiled coil organizes these residues into a continuous KLC2 binding site on one side of the JIP3LZ (Figure 3B). The following describes the KLC2TPR:JIP3LZ interface involving chain A. The side chains of JIP3 residues Ala437, Leu438, Val441, and Leu445 (chain D), and Ile446 (chain C) form a hydrophobic patch, which is flanked by the hydrophilic residues Glu433, Thr434, and Asp450 (chain D) and Lys435, Asn439 Lys442, and Asp450 (chain C). The JIP3LZ hydrophobic patch is recognized by a number of hydrophobic side chains that line the inside of KLC2 TPR1 (Leu201, Leu204, Tyr208, Val216, and Leu220) (Figure 3C). Around the edge of the hydrophobic core of the KLC2TPR:JIP3LZ interface, the side chains of KLC2 residues Thr200 and Tyr208 (TPR1 helix A), and Gln223 and Asp227 (helix B), hydrogen bond to the side chains of JIP3 residues Asp450, Asp444, Lys435, and Asn439 (Figure 3D). The interacting residues in the complex are very highly conserved in KLC1 and KLC3-4 and in JIP4 (Figures 3A and 3E), consistent with previous studies (Bowman et al., 2000, Byrd et al., 2001, Nguyen et al., 2005).
Figure 3.
The JIP3LZ Binds to KLC2 TPR1
(A) Amino acid sequence of the murine JIP3LZ. Heptad repeats are numbered and the amino acid in the “d” position is written in red. Residues that bind to KLC2TPR chain A are highlighted in magenta (chain C) and pink (chain D). JIP4 residues not conserved with JIP3 are written beneath.
(B) The KLC2 binding site on the JIP3LZ domain (chain C, magenta; chain D, pink). The side chains of residues that interact with the KLC2TPR domain are shown as sticks (carbon, main chain color scheme; nitrogen, blue; oxygen, red).
(C) The JIP3 binding site on the KLC2 TPR1. The side chains of residues that interact with the JIP3LZ are shown as sticks.
(D) Stereo image of the KLC2TPR:JIP3LZ interface involving KLC2TPR chain A. Putative hydrogen bonds are shown as dashed lines.
(E) Amino acid sequence alignment for murine KLC1-4 over TPR1. Conserved and semi-conserved residues are highlighted in red and yellow, respectively. KLC2 residues that interact with JIP3 and their counterparts in KLC1/3/4 are outlined in blue. Numbering corresponds to KLC2.
(F) The KLC2TPR from the KLC2TPR:JIP3LZ crystal structure showing the JIP3 (magenta) and tryptophan-acidic (orange) binding sites. The tryptophan-acidic peptide from SKIP (sticks; carbon, yellow; nitrogen, blue; oxygen, red) was modeled based on PDB: 3ZFW (Pernigo et al., 2013). TPR1 residues that were mutated in the binding studies in (G) are labeled.
(G) Representative ITC thermograms and isotherms for titration of the Trp-JIP3LZ domain (left panel) or FITC-CSTN-WD2 peptide (right panel) into wild-type and mutant KLC2TPR-myc. Molar ratios for the Trp-JIP3LZ domain correspond to the Trp-JIP3LZ dimer concentration. The error bars in the isotherms show the errors associated with integration of the injection peaks in the corresponding thermograms.
See also Table S2.
To verify that the crystal structure of the KLC2TPR:JIP3LZ complex is the same as in solution, we probed the KLC2TPR:JIP3LZ interface using ITC binding experiments with wild-type and mutant KLC2TPR-myc. (Figure 3F). All three mutations in TPR1 completely abrogated binding of the Trp-JIP3LZ, but had only minor effects on binding of a tryptophan-acidic cargo peptide (Figure 3G; Table S2). In contrast, the Arg312Glu mutant, which is unable to bind to tryptophan-acidic cargoes (Pernigo et al., 2013), retained the ability to bind to the Trp-JIP3LZ. These results validate the crystal structure of the KLC2TPR:JIP3LZ complex and confirm the presence of a cargo binding site on the kinesin-1 molecule, separate from that of tryptophan-acidic cargoes, located on KLC TPR1 (Figure 3F).
JIP3LZ Binding to KLC2TPR Does Not Affect LFP Motif Binding
Kinesin-1 activation by JIP3 requires JIP3LZ binding to KLCTPR (Watt et al., 2015). How does JIP3LZ binding to the KLCTPR relieve KLC regulation of the kinesin-1 molecule? Tryptophan-acidic cargoes activate kinesin-1 by releasing the KLC LFP motif from the KLCTPR (Yip et al., 2016). A previous X-ray crystallographic study at 4 Å resolution suggested that the LFP motif binds to a site on the inner surface of the KLC2TPR located on TPR2, immediately adjacent to TPR1 (Figure 4A) (Yip et al., 2016). We therefore wondered whether JIP3LZ binding to the KLC2TPR allosterically regulates interactions between KLC2TPR and the LFP motif. We investigated this using an in vitro, fluorescence anisotropy-based binding competition assay with purified proteins and peptides (Yip et al., 2016). This showed that the Trp-JIP3LZ had no effect on the binding of the KLC2TPR-myc to a fluorescein isothiocyanate (FITC)-labeled LFP motif peptide (Figure 6B). In contrast, a tryptophan-acidic peptide (CSTN-WD1) blocked binding of the fluorescent peptide to the KLC2TPR-myc, with a KI of 11.3 μM, in line with previous results (Figure 4B) (Yip et al., 2016). This demonstrates that JIP3LZ binding would not release the LFP motif from its binding site on the KLCTPR. Thus, JIP3 activates kinesin-1 by a different mechanism to tryptophan-acidic cargoes.
Figure 4.
The JIP3LZ Does Not Disrupt LFP Motif Binding to the KLC2TPR
(A) View of the KLC2TPR from the KLC2TPR:JIP3LZ complex. The bound LFP motif (red cartoon) was modeled using PDB: 5FJY (Yip et al., 2016).
(B) Fluorescence anisotropy from the FITC-LFP peptide with a fixed concentration of KLC2TPR-myc (21 μM) and varying concentrations of Trp-JIP3LZ or CSTN-WD1 peptide as indicated. For the Trp-JIPLZ data, concentration values correspond to the total concentration of monomeric Trp-JIPLZ subunits. The calsyntenin-1 data are fitted with a competitive inhibition model (see the STAR Methods). The JIP3 data points are joined by straight lines. Data from a single experiment is shown in each case. Data points and error bars correspond to the mean and SD of triplicate measurements. Where error bars are not visible they are smaller than the marker.
Figure 6.
KLCTPR Dimerization via TPR1 Mimics JIP3 Binding
(A–C) Structural comparison of the KLC2TPR:JIP3LZ interface with the crystallographic TPR1:TPR1 dimer interface in crystals of the KLC2TPR (PDB: 5OJF). (A–C) show three views of the PDB: 5OJF dimer interface, with chain A in teal and the dimer mate in yellow. The JIP3LZ (pink and magenta) was positioned onto PDB: 5OJF chain A by superposing KLC2 chain A of the KLC2TPR:JIP3LZ complex onto subunit A of PDB: 5OJF via TPR1.
(D–E) TPR1 from KLC2 (D) and KLC1 (E) shown in gray loop representation (PDB: 5OJF and 3NF1, respectively). Interfacial residues are shown in stick representation with carbon atoms colored according to the interface(s) they participate in.
See also Figure S2.
Evolutionary Analysis Points to a Role for KLC TPR1 beyond JIP3 Binding
We analyzed the conservation of the amino acid residues at the KLC2TPR:JIP3LZ interface using used a recently published dataset containing a significantly enhanced number of non-Bilaterian genomes (Figure 5A; Tables S3 and S4) (Simion et al., 2017). This allowed us to examine the conservation of this binding interface across the full breadth of the animal kingdom. We found that the KLC2TPR binding site on JIP3 is specific to animals with bilateral body symmetry (Bilateria): the amino acids involved in KLC2TPR binding are totally conserved in almost all Bilaterian JIP3/4 homologs, but not those from any species in the other animal phyla. However, the JIP3LZ binding site on TPR1 is more widely conserved, being totally conserved in essentially all Bilateria, but also in over half of the non-Bilaterian species in our datasets, across all phyla (Figure 5B; Table S4). The two faces of the KLC2TPR:JIP3LZ interface are therefore conserved to very different extents throughout the animal kingdom. Thus, while JIP3 evolved to bind to the KLCTPR at or shortly after the divergence of Bilateria, the cognate region of the KLCTPR evolved much earlier, or evolved multiple times in the absence of JIP3LZ binding. For comparison, the KLC LFP motif was found to be specific to Bilateria, while the tryptophan-acidic cargo binding site was present in 31% of non-Bilaterian species in addition to being fully conserved in essentially all Bilateria (Figure 5B). We conclude that the JIP3LZ binding site on the KLCTPR is the most widely conserved binding interface on KLC mapped to date. This points to additional roles for KLC TPR1 in kinesin-1 function beyond serving as a binding site for the JIP3LZ, perhaps in binding to other, as-yet unknown cargoes, and/or as a regulator of kinesin-1 activity.
Figure 5.
Conservation of the KLC2TPR:JIP3LZ Interface across The Animal Kingdom
(A) Canonical animal species phylogeny complete with the evolutionary timeline. The timing of the emergence of Metazoa and Bilateria in the fossil record is indicated by orange bars. The number of species in our dataset for each phylum is given in parentheses.
(B) Bar graph showing the percentage of species in our datasets containing the indicated KLC or JIP3 motifs. Solid and hatched bars show data for Bilaterian and non-Bilaterian species, respectively. Bars are color-coded by motif as shown in the key.
TPR1 Forms a Conserved Crystallographic Interface that Resembles JIP3 Binding
Intriguingly, TPR1 forms a similar dimer interface in all crystal structures of the full-length KLCTPR (Figures S2A and S2B) (Nguyen et al., 2017). Notably, we also found the same interface in our previously unpublished, 4-Å crystal structure of the KLC2TPR bound to a tryptophan-acidic peptide derived from calsyntenin-1 (CSTN-WD2) (Figure S2C; Table 1) (Araki et al., 2007). The TPR1:TPR1 packing interaction is unique in being found in every copy of the KLC1TPR and KLC2TPR in all crystal structures (Figure S2D), suggesting that it is independent of crystallographic environment or crystallization conditions (Table S5). The dimer interfaces in PDB: 3NF1, 5FJY, and our KLC2TPR:CSTN-WD2 structure are very similar, with pairwise superposition of these dimers via TPR1 giving root-mean-square deviation (RMSD) values of 0.7–1.0 Å between 81 and 83 structurally equivalent Cα atoms (Figure S2E). The amino acid residues that form the crystallographic TPR1:TPR1 interfaces are totally conserved in essentially all Bilateria and over half of the non-Bilaterian species in our datasets, across all phyla (Figure 5B; Tables S3 and S4). These observations suggest that the TPR1:TPR1 interfaces observed in crystals of the KLCTPR are not simply crystal packing artifacts and may be functionally relevant.
We compared the KLC2TPR:JIP3LZ interface with the crystallographic TPR1:TPR1 packing interfaces observed in other KLCTPR crystal structures (Nguyen et al., 2017, Yip et al., 2016, Zhu et al., 2012). The conformation of TPR1 in complex with the JIP3LZ is essentially identical to that observed in crystals of the unbound, full-length KLC2TPR (PDB: 5OJF; Nguyen et al., 2017), with pairwise RMSD of 0.4–0.6 Å over 41 equivalent Cα atoms (Figure S2F). Remarkably, TPR1 dimerization in PDB: 5OJF is strikingly similar to JIP3LZ binding (Figures 6A–6C), with most of the residues in the JIP3 binding site engaged in the TPR1 dimer interface (Figure 6D). Comparison with the other KLCTPR structures, which possess a slightly different TPR1 conformation, showed similar results, with an even greater overlap between the JIP3 binding site and the TPR1 dimerization interface (Figure 6E). Thus, the KLCTPR possesses the propensity to self-associate via TPR1 in a manner that mimics JIP3LZ domain binding. Since JIP3 binding to KLC TPR1 is required for activation of the kinesin-1 heterotetramer, self-association of the two KLCTPRs inside the kinesin-1 molecule via TPR1 could also play a role in kinesin-1 activation.
Discussion
While most eukaryotes possess a single cytoplasmic dynein that functions in conjunction with a multitude of regulators, eukaryotic genomes typically encode multiple kinesins. Each kinesin contains a conserved motor domain, with variable flanking regions that regulate the activity of the motor domains and mediate binding to effectors, e.g., cargoes (Hirokawa et al., 2009). An emerging theme is that kinesin motor domains are regulated by intramolecular interactions with the flanking non-motor regions (Espeut et al., 2008, Hackney et al., 2009, Hackney and Stock, 2000, Hammond et al., 2010, Kaan et al., 2011, Stock et al., 1999, Talapatra et al., 2015). These interactions are relieved by cargo binding, post-translational modifications, or, in the case of microtubule depolymerizing kinesins, even microtubule ends themselves (Espeut et al., 2008, Friedman and Vale, 1999, Talapatra et al., 2015, Welburn, 2013). In the kinesin-1 heterotetramer, motor domain regulation involves a direct interaction between the KHC tail and motor domains, and an additional, poorly understood role for KLC. Here, we have solved the crystal structure of the KLC2TPR bound to the cognate region of a cargo molecule, the JIP3LZ. In the following we explore the implications of this structure for our molecular understanding of kinesin-1 recruitment and activation by cargo.
Molecular Mechanisms by which ARF6 Regulates Kinesin-1 Recruitment by JIP3/4
JIP3 and JIP4 are molecular adaptors that link kinesin-1 and dynein to a range of other cellular cargoes, expanding the repertoire of cellular cargoes that can be transported by these microtubule motors. The switch between kinesin-1 and dynein-driven motility is controlled by ARF6 (Montagnac et al., 2009). Comparison with the crystal structure of the ARF6:JIP4LZ complex shows that the KLC and ARF6 binding sites on the JIP3/4LZ overlap (Figure 7A), consistent with KLC and ARF6 competing directly for binding (Montagnac et al., 2009). However, only one molecule of ARF6 can bind to the JIP3/4LZ dimer, because active ARF6 is anchored into membranes via its N-terminal myristoyl group (Gillingham and Munro, 2007, Isabet et al., 2009). As a result, the other ARF6/KLC binding site on the JIP3/4LZ would be vacant. Comparison with our KLC2TPR:JIP3LZ structure shows that the vacant binding site would be inaccessible to the KLC2TPR due to its proximity to the membrane (Figure 7B). Thus, one molecule of activated ARF6 blocks KLCTPR binding to both sites on the JIP3/4LZ.
Figure 7.
Molecular Mechanisms by which ARF6 Regulates Kinesin-1 Recruitment by JIP3/4
(A) Molecular surface of the JIP4LZ (PDB: 2W83) showing the KLC and ARF6 binding sites (Isabet et al., 2009).
(B) Model of the JIP4LZ bound to the KLC2TPR and active, membrane-associated ARF6. GTP, sticks with carbon/oxygen/nitrogen/phosphorus, gray/red/blue/orange; N-terminal myristoyl group, black zigzag; N-terminal amphipathic helix, yellow cylinder.
Insights into Kinesin-1 Activation by Cargo
JIP3 binding to the KLCTPR relieves KLC regulation of the kinesin-1 molecule. TPR1 adopts two slightly different conformations in crystal structures of unbound KLCTPRs (Figures 6D and 6E) (Nguyen et al., 2017). Conformational changes in TPR1 induced by JIP3 binding could form part of an allosteric mechanism for relieving KLC regulation. However, we found that JIP3 does not disrupt interactions between the KLC LFP motif and the TPR domain (Figure 4B). Thus, JIP3 activates kinesin-1 through a different mechanism to tryptophan-acidic cargoes. Interestingly, we found that the KLC binding site on JIP3 and the KLC LFP motif co-evolved at an early stage of the Bilaterian split (Figure 6B), suggesting a functional relationship between the LFP motif and JIP3 binding to KLC. Structural information on the intact kinesin-1 molecule is scarce, and thus how KLC interacts with KHC in the autoinhibited state is poorly understood. Previous results are consistent with the KLC acidic linker and TPR domain forming interactions with the KHC motor and/or tail domains in the autoinhibited state (Wong and Rice, 2010). Fluorescence lifetime imaging experiments showed that the KLC N- and C-termini are in close spatial proximity inside the kinesin-1 molecule, and that this configuration requires LFP motif binding to the TPR domain (Yip et al., 2016). Thus, the LFP motif would naturally direct the JIP3 binding site on the KLCTPR toward the KHC motor and tail domains in the autoinhibited state (Figure 8A). This points to a role for the KLC LFP motif in scaffolding regulatory interactions between the JIP3 binding site (or adjoining regions of KLC) and the KHC motor domains/tails. Within this picture, cargoes could relieve these regulatory interactions either through binding to KLC TPR1, as for JIP3, or by dissociating the LFP motif from the TPR domain, as with tryptophan-acidic cargoes. Previously, it was proposed that the KLC acidic linker (Figure 1A) is an activator of kinesin-1 motility, and that binding of the LFP motif to the KLCTPR constitutes an autoinhibition mechanism (Yip et al., 2016). Our hypothesis above is entirely compatible with this model, but suggests additional functions for the LFP motif, allowing us to reconcile the activation pathways of different cargoes into a common molecular framework.
Figure 8.
A Unified Framework for Kinesin-1 Activation by Different Cargoes
(A) Model for the autoinhibited state of the kinesin-1 heterotetramer, showing how binding of the KLC LFP motif to the TPR domain would steer the JIP3 binding site on KLC toward the KHC motor domains and tails.
(B) Proposed pathway for kinesin-1 activation by JIP3 (LZ domain, oval; tail-binding region, square).
(C) Proposed pathway for kinesin-1 activation by a general dimeric cargo that induces TPR dimerization via TPR1.
(D) Proposed pathway for kinesin-1 activation by a tryptophan-acidic cargo (tryptophan-acidic motif, hexagon). This has been conceptually broken down into two steps to show relief of KLC and KHC tail regulation of the motor domains, but both steps could occur simultaneously (Yip et al., 2016).
TPR1 forms similar crystal packing interfaces in all crystal structures of the KLCTPR (Figure S2) (Nguyen et al., 2017). However, it was unclear whether these TPR1 conformations and the corresponding TPR1:TPR1 interfaces correspond to functionally relevant states of the protein or represent crystal packing artifacts. We have shown that TPR1 harbors the binding site for a cargo, JIP3, and that the JIP3-bound TPR1 conformation is virtually identical to that observed in crystals of the unbound KLC2TPR (PDB: 5OJF) (Figure S2F) (Nguyen et al., 2017). This shows that the TPR1:TPR1 dimer interface in PDB: 5OJF involves a functional TPR1 conformation. TPR1-mediated dimerization mimics JIP3 binding (Figures 6A–6C), and uses an almost identical set of residues that are conserved in KLC homologs from sponges to humans (Figures 6D and 6E; Tables S3 and S4). These observations argue strongly in favor of a functional role for TPR1-mediated dimerization of the KLCTPRs in the kinesin-1 molecule. Since JIP3 binding to TPR1 relieves KLC regulation (Watt et al., 2015), then so should dimerization of the KLCTPRs via TPR1. It thus appears that, following the emergence of LFP motif-mediated KLC regulation in Bilateria, JIP3 evolved to relieve this regulation by co-opting an ancestral TPR1-mediated dimerization mechanism. Purified, recombinant KLCTPRs are monomeric in solution, showing that this interaction is weak (Nguyen et al., 2017). Considering the two KLCTPRs inside the kinesin-1 molecule as confined to a sphere of maximum radius 150 Å (corresponding to a fully extended acidic linker) shows that the KLCTPR concentration inside the kinesin-1 molecule would be at least 100 μM. Nevertheless, we anticipate that the monomer-dimer equilibrium of KLCTPRs in the kinesin-1 molecule would favor the monomer in the autoinhibited state, since dimerization would mask the JIP3 binding site and lead to relief of KLC regulation. However, cargoes that tether the TPR domains together would promote dimerization via TPR1, leading to relief of KLC regulation. Indeed, KLC cargoes are usually dimeric or possess two KLC binding sites. This would allow cargoes binding to different regions of the KLCTPR to relieve KLC regulation by the same mechanism. For example, kinesin-1 activation by JIP1 (which is structurally unrelated to JIP3/4) follows a similar scheme to JIP3 despite engaging the KLCTPR in a completely different manner (Blasius et al., 2007, Fu and Holzbaur, 2013, Sun et al., 2011, Verhey et al., 2001, Watt et al., 2015, Zhu et al., 2012). Interestingly, JIP1 and JIP3 promote each other's transport inside cells, which requires binding of both cargoes to the KLCTPR (Hammond et al., 2008, Sun et al., 2017). Unrelated cargoes could facilitate each other's transport by cooperating with each other to promote KLCTPR dimerization.
An important functional requirement of molecular motors such as kinesin-1 is the ability to transport multiple, unrelated cellular cargoes. Indeed, the KLCTPR possesses separate binding sites for multiple cargoes, yet how the kinesin-1 molecule integrates these seemingly unrelated molecular recognition events into a common activation pathway is unknown. Based on our observations, we suggest the following model to explain this (Figures 8B–8D). Binding of the LFP motif to the KLCTPR promotes regulatory interactions between KLC and KHC that involve KLC TPR1 or nearby regions. Cargoes can therefore relieve KLC regulation by one of two mechanisms. The first is crosslinking the KLCTPRs together via their TPR1s. This could occur via direct binding to TPR1, as seen for JIP3 (Figure 8B), or by promoting dimerization of the TPR domains via TPR1 (Figure 8C). The second mechanism for relieving KLC regulation involves “pulling the rug out from underneath it,” i.e., dissociating the KLC LFP motif from the TPR domain, as seen for tryptophan-acidic cargoes (Figure 8D). Whichever way KLC regulation is disabled, it results in an intermediate that resembles the KHC homodimer, in which the motor domains are autoinhibited by the KHC tails alone. Binding of other factors to the KHC tail is then required to activate motility. This could be a cargo molecule (Figures 8B and 8C) (Blasius et al., 2007, Fu and Holzbaur, 2013, Sun et al., 2011, Watt et al., 2015), or, as suggested previously, the KLC acidic linker (Figure 8D) (Yip et al., 2016), although recent work shows that robust activation of kinesin-1 by tryptophan-acidic cargoes requires cargo binding to the KHC tails (Sanger et al., 2017). Detailed information on the structure of the intact kinesin-1 molecule and further mechanistic studies will be needed to investigate these issues further.
In summary, this work expands our knowledge of how cargoes recruit kinesin-1, how this is regulated by factors such as ARF6, and suggests how multiple, unrelated cargoes binding to different sites on the KLC TPR domain can activate kinesin-1 through related mechanisms.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| BL21 (DE3) Rosetta | Merck Millipore | Cat#70954 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| FITC-labeled CSTN(WD2) peptide (FITC-TRQLEWDDSTL-COOH) | Biomatik | N/A |
| FITC-labeled KLC2 LFP motif peptide (FITC-DSLDDLFPNEDEQS-COOH) | Biomatik | N/A |
| N-terminally acetylated CSTN-WD1 peptide (Ac-GKENEMDWDDSALTITVN-COOH) | Peptide Synthesis Laboratory, Francis Crick Institute, UK | N/A |
| N-terminally acetylated CSTN-WD2 peptide (Ac-NATRQLEWDDSTLSY-COOH) | Peptide Synthesis Laboratory, Francis Crick Institute, UK | N/A |
| KLC2TPR protein | This study | N/A |
| KLC2TPR-myc protein | This study | N/A |
| KLC2TPR-myc Tyr208Ala mutant protein | This study | N/A |
| KLC2TPR-myc Thr200Asp mutant protein | This study | N/A |
| KLC2TPR-myc Gln223Lys mutant protein | This study | N/A |
| GST3C-JIP3LZ protein | This study | N/A |
| JIP3LZ protein | This study | N/A |
| Trp-JIP3LZ protein | This study | N/A |
| cOmplete EDTA-free protease inhibitor cocktail | Roche | Cat#4693116001 |
| His-trap HP column (1 mL) | GE Life Sciences | Cat#17524701 |
| Glutathione sepharose 4B resin | GE Life Sciences | Cat#17075601 |
| Superdex 75 16 60 | GE Life Sciences | Cat#28989333 |
| Superdex 200 16 60 | GE Life Sciences | Cat#28989335 |
| HiTrap Q XL column | GE Life Sciences | Cat#17515801 |
| 50% (w/v) PEG 3350 solution | Rigaku | Cat#1008054 |
| 1M sodium thiocyante solution | Rigaku | Cat#1008268 |
| JCSG Core screens I-IV | Qiagen | Cat#130924-7 |
| Wizard Classic 3 & 4 HT96 screen | Molecular Dimensions | Cat#MD15-W34-B |
| Deposited Data | ||
| Atomic coordinates and structure factors | This study | PDB: 6EJN |
| Atomic coordinates and structure factors | This study | PDB: 6F9I |
| Bioinformatics sequence dataset 1 | OMA Orthology database (Altenhoff et al., 2015) | https://omabrowser.org/oma/home/ |
| Bioinformatics sequence dataset 2 | Simion et al., 2017 | N/A |
| Atomic coordinates | Zhu et al., 2012 | PDB: 3CEQ |
| Atomic coordinates | Zhu et al., 2012 | PDB: 3NF1 |
| Atomic coordinates | Isabet et al., 2009 | PDB: 2W83 |
| Atomic coordinates | Pernigo et al., 2013 | PDB:3ZFW |
| Atomic coordinates | Yip et al., 2016 | PDB: 5FJY |
| Atomic coordinates | Nguyen et al., 2017 | PDB: 5OJF |
| Recombinant DNA | ||
| pMW GST-3C vector | Boeda et al., 2007 | N/A |
| pMW His-Sumo vector | This study | N/A |
| murine KLC2 cDNA | Dodding et al., 2011 | N/A |
| murine JIP3 isoform A cDNA | GenomeCUBE | Cat#IRAVp968C03156D |
| Software and Algorithms | ||
| XDS | Kabsch, 2010 | http://xds.mpimf-heidelberg.mpg.de/ |
| CCP4 program suite | Winn et al., 2011 | http://www.ccp4.ac.uk |
| COOT | Emsley et al., 2010 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| BUSTER 2.10.3 | Global Phasing Ltd. | https://www.globalphasing.com |
| MOLPROBITY | Chen et al., 2010 | http://molprobity.biochem.duke.edu |
| PHENIX | Adams et al., 2010 | https://www.phenix-online.org/documentation/reference/refinement.html |
| NITPIC | Keller et al., 2012 | http://biophysics.swmed.edu/MBR/software.html |
| SEDPHAT | Zhao et al., 2015 | http://www.analyticalultracentrifugation.com/sedphat/ |
| GUSSI | University of Texas Southwestern Medical Center | http://biophysics.swmed.edu/MBR/software.html |
| Prism 5.0 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| BLASTp | Altschul et al., 1997 | https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastDocs&DOC_TYPE=Download |
| TBLASTN | Gertz et al., 2006 | https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastDocs&DOC_TYPE=Download |
| PYMOL | Schrodinger LLC | http://www.pymol.org |
| DICHROWEB | Whitmore and Wallace, 2008 | http://dichroweb.cryst.bbk.ac.uk/html/references.shtml |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Joseph Cockburn (j.j.b.cockburn@leeds.ac.uk)
Experimental Model and Subject Details
We used E.coli Rosetta (DE3) cells for production of all recombinant proteins used in this study. The cells were cultured using standard practices in LB media.
Method Details
Construction of Expression Vectors
Plasmids pMW his-SUMO and pMW GST3C (Boeda et al., 2007) are bacterial expression vectors from the Way Lab at the Francis Crick Institute, London, UK. These vectors contain a T7 promoter and encode an N-terminal hexahistidine-SUMO tag, or a GST tag followed and a 3C protease cleavage site, respectively.
The KLC2TPR construct used in this work comprised murine KLC2 residues 191-480, which is similar to those used in previous studies (Nguyen et al., 2017, Zhu et al., 2012) but with a slightly longer N-terminal region to include residues 191-194, which are very highly conserved in Bilaterian KLCs. To construct the pMW-His6-SUMO-KLC2TPR vector, the sequence encoding KLC2TPR (residues 191-480) was amplified by PCR from a murine template (Dodding et al., 2011) and cloned into the NotI/EcoRI sites of pMW his-SUMO. The His6-SUMO- KLC2TPR-myc vectors were constructed likewise, but using a reverse primer sequence encoding the KLCTPR C-terminal region up to residue 480, a BglII site, and an in-frame myc-tag sequence, thus fusing the amino acid sequence RSEQKLISEEDL to the KLC2TPR C-terminus. KLC2TPR-myc mutants (Thr200Asp, Tyr208Ala, Gln223Lys and Arg312Glu) were generated by overlap PCR.
The JIP3LZ domain construct used in this work (residues 417-487 of murine JIP3 isoform A) is equivalent to the JIP4LZ construct that was previously co-crystallised in complex with ARF6 (Isabet et al., 2009). To construct the pMW-GST3C-JIP3LZ vector, the sequence encoding JIP3LZ was amplified by PCR from a murine JIP3 template (GenomeCUBE) and cloned into the NotI/EcoRI sites of pMW-GST3C. For CD and binding studies, we used a JIP3LZ construct N-terminally fused to a tryptophan residue (Trp-JIP3LZ) for quantitation of protein concentration by UV-VIS. The pMW-GST3C-Trp-JIP3LZ vector was constructed as above but using a forward primer encoding an in-frame, N-terminal tryptophan residue. All inserts were sequence-verified.
Protein Production
The pMW plasmids do not contain the lac repressor gene or a lac operator sequence downstream from the T7 promoter. Proteins were produced by leaky expression in BL21(DE3) Rosetta cells. The relevant plasmid was transformed into BL21(DE3) Rosetta cells. 5 ml LB-ampicillin (100 μg/ml) cultures were inoculated from the resulting colonies and grown at 37°C for 8 hours. Each 5 ml culture was then used to inoculate a 1 L LB-ampicillin culture. The 1 L cultures were grown in 2 L baffled flasks overnight at 30°C and 185 rpm. Cultures were clarified by centrifugation and the pellets were re-suspended in cold binding buffer supplemented with cOmplete EDTA-free protease inhibitor cocktail (Roche). Re-suspended pellets were stored at -80°C until further use. Re-suspended bacteria were thawed and lysed by sonication on ice. Lysates were clarified by centrifugation.
Protein Purification
For Crystallographic Studies
The His-SUMO-KLC2TPR protein was purified by nickel-ion affinity chromatography. A 1 ml His-trap column (GE Healthcare) was equilibrated in binding buffer (0.5 M NaCl, 50 mM HEPES, 25 mM imidazole, 1 mM TCEP; final pH adjusted to 8.0). The clarified lysate was loaded onto the column, and unbound material washed out back to baseline with binding buffer. Bound protein was then eluted in a linear gradient of elution buffer (0.5 M NaCl, 50 mM HEPES, 500 mM imidazole, 1 mM TCEP; final pH adjusted to 8.0). Fractions were analysed by SDS-PAGE and pure fractions pooled. The his-SUMO tag was cleaved off overnight at 4°C with ULP1 protease. The KLC2TPR protein was separated from the his-SUMO tag by size-exclusion chromatography on a Superdex 75 16/600 column (GE Healthcare) equilibrated in 100 mM NaCl, 25 mM HEPES, 1 mM TCEP (final pH 7.5).
The GST3C-JIP3LZ protein was batch-purified using glutathione sepharose 4B resin (GE Healthcare). Resin was pre-equilibrated in binding buffer (0.25 M NaCl, 50 mM TRIS.HCl, 10 % glycerol, 0.1 % triton X100, 1 mM EDTA, 1 mM TCEP; final pH 7.5) and incubated in suspension with the clarified lysate for at least 4 hours at 4°C. Unbound material was removed by washing the resin 5 times in wash buffer (0.25 M NaCl, 50 mM TRIS.HCl, 1 mM TCEP; final pH 7.5). The JIP3LZ was obtained by cleaving the resin-bound GST3C-JIP3LZ protein with 3C protease overnight at 4°C, and purified by size-exclusion chromatography as described above. The pooled fractions were further purified to remove contaminating GST by anion exchange chromatography as follows. The protein was diluted 4-fold in 25 mM HEPES pH 7.0 to a final NaCl concentration of 25 mM. The diluted protein was applied to a 1 ml HiTrap Q XL column (GE Healthcare) pre-equilibrated in 25 mM HEPES pH 7.0. The pure JIP3LZ was collected as the flow through. The NaCl concentration in the pure protein was increased to 100 mM using a 5 M NaCl solution.
For Functional Studies
For ITC studies with the KLC2TPR and GST-JIP3LZ, the high KLC2TPR concentrations required to use this protein as titrant necessitated a higher salt concentration than that used for crystallographic studies. The KLC2TPR was prepared as above for crystallographic studies but the final size exclusion chromatography step was performed in 500 mM NaCl, 25 mM HEPES, 5mM MgCl2, 1 mM TCEP (final pH 7.5). The GST-JIP3LZ protein was bound to glutathione sepharose resin in batch as described above for the JIP3LZ. The GST-JIP3LZ protein was eluted in 0.25 M NaCl, 50 mM TRIS.HCl, 1 mM TCEP, 25 mM glutathione; final pH 7.5, and purified by size exclusion chromatography on a Superdex 200 16/600 column (GE Healthcare) equilibrated in 500 mM NaCl, 25 mM HEPES, 5mM MgCl2 and 1 mM TCEP (final pH 7.5).
The Trp-JIP3LZ protein used for CD and ITC experiments was prepared as described above for the JIP3LZ, with the following modifications. Following 3C cleavage, contaminating GST3C was removed by incubation of the eluate with fresh, pre-equilibrated glutathione sepharose 4B resin for 2 hours at room temperature. The Trp-JIP3LZ was then further purified by size exclusion chromatography using a Superdex 75 16/600 column equilibrated in 100 mM NaCl, 25 mM HEPES, 1 mM TCEP (final pH 7.5). Wild-type/mutant KLC2TPR-myc samples used for CD and ITC experiments were prepared as described above the KLC2TPR used in crystallographic studies. The Trp-JIP3LZ and KLC2TPR-myc samples used in the fluorescence anisotropy-based binding competition assays were prepared as for those used in the CD and ITC studies, but the final size-exclusion step was performed in 100 mM NaCl, 25 mM HEPES, 5mM MgCl2, 1 mM TCEP (final pH 7.5).
Crystal Structure Determination
The KLC2TPR:JIP3LZ Complex
The purified JIP3LZ protein was concentrated in a Vivaspin column (PES membrane, 2 kDa cut-off) to a concentration of 0.63 mg/ml as quantified by a Bradford assay, and mixed with pure KLC2TPR (at 4.1 mg/ml) to give a final KLC2TPR:JIP3LZ stoichiometry of 3:1. The complex was concentrated in a spin concentrator (Vivaspin, PES, 10 kDa cut-off) to a total protein concentration of 4.5 mg/ml, and incubated at 4°C overnight. Sitting drop crystallisation trials were set up the following day in SWISSCI MRC 3-well crystallisation plates (Jena Bioscience) using a Formulatrix NT8 crystallisation robot before storage and imaging in a Formulatrix Rock Imager at 19°C. Three-dimensional crystals grew in condition A10 of the Wizard Classic 3/4 HT96 screen (Molecular Dimensions) in a drop composed of 200 nl protein and 100 nl reservoir (20% PEG 3350, 0.2 M NaCSN); the drop ratio was further optimised to 200 nl protein plus 50 nl reservoir. Crystals were cryoprotected in 10% PEG 3350, 75 mM NaCSN, 150 mM NaCl, 37.5 mM HEPES pH 7.5 and 25% glycerol and flash frozen in liquid nitrogen. X-ray diffraction data were collected at beamline I24 of Diamond Light Source (Didcot, Oxfordshire, UK) at a wavelength of 0.96861 Å. Data were processed using XDS (Kabsch, 2010) and programs from the CCP4 program suite (Winn et al., 2011). Phases were obtained by molecular replacement with PHASER (McCoy et al., 2007), using the crystal structures of KLC2TPR (PDB: 3CEQ) and the JIP4LZ (chains C and D from PDB: 2W83) (Isabet et al., 2009, Zhu et al., 2012). The structure was rebuilt in COOT (Emsley et al., 2010) and refined in BUSTER 2.10.3 (Global Phasing) to 3.2 Å resolution, with each TPR domain and the LZ domain as separate TLS groups, LSSR NCS restraints, and LSSR restraints to the higher resolution structures of the JIP4LZ (PDB: 2W83) and KLC1TPR (PDB: 3NF1) (Isabet et al., 2009, Zhu et al., 2012). The model was validated using the MOLPROBITY server (http://molprobity.biochem.duke.edu) (Chen et al., 2010). The final model had a MOLPROBITY score of 1.91 (100th percentile), with 96.75/0% residues in the favoured/forbidden regions of the Ramachandran plot. Figures were prepared using PYMOL (Schrodinger).
The KLC2TPR:CSTN-WD2 Complex
CSTN-WD2 peptide was dissolved in KLC2TPR gel filtration buffer and mixed with purified KLC2TPR protein, at 2:1 (peptide:TPR) stoichiometry, giving a final KLC2TPR concentration of 5.5 mg/ml. The complex was incubated at room temperature for 1 hour prior to setting up robotic crystallisation trials at 19°C. Crystals were grown in condition D1 of the JCSG Core III screen (1M sodium potassium tartrate, 0.2 M NaCl, 0.1 M imidazole pH 8.0) in a drop composed of 100 nl protein and 100 nl reservoir. This was further optimized to 0.893 M sodium potassium tartrate, 0.2 M NaCl, 0.1 M imidazole pH 8.0. Crystals were cryoprotected in reservoir plus 25% glycerol and flash frozen in liquid nitrogen. X-ray diffraction data were collected at beamline I04-1 of Diamond Light Source (Didcot, Oxfordshire, UK) at a wavelength of 0.9200 Å. Data were processed as described above. Phases were obtained by molecular replacement with PHASER (McCoy et al., 2007) using PDB: 3ZFW (Pernigo et al., 2013) as the search model. Since the search model lacked TPR1, this was modelled from the structure of the KLC1TPR (Zhu et al., 2012) (PDB: 3NF1). The CSTN-WD2 peptide structure was rebuilt and the model refined in BUSTER 2.10.3 (Global Phasing) to 4.0 Å resolution with each TPR domain:peptide complex as a separate TLS group, LSSR NCS restraints, and LSSR restraints to the higher resolution structure of the KLC1 TPR domain (PDB: 3NF1) (Zhu et al., 2012). The structure was then refined in PHENIX (Adams et al., 2010) with NCS and secondary structure restraints applied. The final model had a MOLPROBITY score of 1.58, with 96.2/0% residues in the favoured/forbidden regions of the Ramachandran plot.
Isothermal Titration Calorimetry
To study the binding of the KLC2TPR to GST3C-JIP3LZ, the proteins were prepared by size exclusion chromatography in 0.5 M NaCl, 25 mM HEPES, 5 mM MgCl2, 1 mM TCEP, final pH 7.5. ITC measurements were performed on a MicroCal iTC200 calorimeter (Malvern) at 25°C, with a differential power of 5.0 μcal/s and stirring at 750 rpm. Experiments consisted of an initial sacrificial 0.5 μl injection, followed 120 s later by 19 injections of 2 μl spread over 4 s, spaced 120 s apart. Thermograms were integrated and corrected for heats of dilution using NITPIC (http://biophysics.swmed.edu/MBR/software.html). The resulting isotherms were analysed in SEDPHAT (http://www.analyticalultracentrifugation.com/sedphat/) (Keller et al., 2012, Zhao et al., 2015). Four experiments were performed which are shown in Figures 2A–2D. The isotherms from these experiments were globally fitted with the A + B + B → AB + B → ABB model. During the fitting, the inactive fractions of A and B were fitted globally, whilst the cell and syringe concentrations and baselines of each experiment were fitted locally. The locally and globally fitted parameters are listed in Tables S1 and 2, respectively. Figures were prepared using GUSSI (http://biophysics.swmed.edu/MBR/software.html).
To study binding of the Trp-JIP3LZ and FITC-CSTN-WD2 peptide to wild-type and mutant KLC2TPR-myc, the recombinant proteins were prepared by size exclusion chromatography in 0.1 M NaCl, 25 mM HEPES, 1 mM TCEP, final pH 7.5. Wild-type or mutant KLC2TPR-myc at 44-50 μM were placed in the cell. The Trp-JIP3LZ was used as the titrant at dimer concentrations of 297-330 μM. The FITC-CSTN-WD2 peptide was used as the titrant at a concentration of 380 μM. The peptide concentration was determined by measuring the absorbance at 483 nm and using a molar extinction coefficient of 68,000 M-1cm-1. ITC experiments were performed as described above with injections spaced 120-150 s apart. Thermograms were integrated and corrected for heats of dilution using NITPIC (http://biophysics.swmed.edu/MBR/software.html). The resulting isotherms were analysed in SEDPHAT. Data for the Trp-JIP3LZ as titrant were fitted with the A + B + B → AB + B → ABB model, whilst binding of the FITC-CSTN-WD2 peptide was analysed using the A + B → AB model. Between 2 and 3 experiments were performed for each titrand/titrant pair. For each titrand/titrant pair, the isotherms from the experiments were globally fitted with the relevant model. During the fitting, the inactive fractions of A and B were globally fitted, whilst the cell and syringe concentrations and baselines of each experiment were fitted locally. The results are listed in Table S2. For the experiments with the FITC-CSTN-WD2 peptide as titrant, Table S2 also lists the mean apparent binding stoichiometry value (Napp) for each titrand/titrant pair, defined as
where FKLC2,i and Flig,i are the locally-fitted correction factors for KLC2 and ligand concentrations for experiment i, and AKLC2 and Alig are the active fractions of KLC2 and ligand globally fitted over the n experiments. During fitting, the FITC-CSTN-WD2 peptide concentrations consistently refined to values around 30% greater than the measured value of 380 μM, with Napp values around 0.7 (Table S2). We ascribe this to an under-quantification of the FITC-CSTN-WD2 peptide concentration since the peptide contains a single tryptophan-acidic motif, and the concentration values for experiments with Trp-JIP3LZ as ligand refined close to their measured values.
CD Spectroscopy
All CD spectra were recorded on a Chirascan CD spectrometer (Applied Photophysics) in quartz cuvettes with a 0.1 cm path length. Data were recorded at 20°C between 260 - 180 nm in 1 nm increments and a 2 nm bandwidth over an average of two scans. The KLC2TPR-myc and Trp-JIP3LZ proteins were dialysed extensively into 20 mM potassium phosphate pH 7.5, 0.5 mM DTT. Spectra were collected from KLC2TPR-myc samples at concentrations of 0.29, 0.14 and 0.086 mg/ml. Trp-JIP3LZ spectra were collected at 0.15, 0.074 and 0.044 mg/ml. One spectrum was recorded for each sample at each concentration and background-corrected by subtraction of a buffer-only spectrum. Corrected spectra were converted to units of mean residue ellipticity and deconvolved using the Dichroweb server (Whitmore and Wallace, 2008). KLC2TPR-myc and Trp-JIP3LZ spectra were analysed using the SELCON3 and CONTIN algorithms, respectively, using reference dataset set 4 (Sreerama et al., 1999, van Stokkum et al., 1990). For each protein, the mean percentage of each secondary structure type, and the corresponding standard deviation, was calculated from the values obtained from the three concentrations. The secondary structure content for KLC2TPR-myc and Trp-JIP3LZ were calculated using the KLC2TPR:JIP3LZ crystal structure using the STRIDE server (http://webclu.bio.wzw.tum.de/cgi-bin/stride/stridecgi.py) (Frishman and Argos, 1995).
SEC-MALLS Experiments
Fifty microliters of JIP3LZ was injected onto a WTC-030S5 column, pre-equilibrated with buffer containing 25 mM HEPES pH 7.5, 0.1 M NaCl and 1 mM TCEP at a flow rate of 1 ml/min. Data were recorded using a DAWN 8+ multi-angle light scattering (LS) detector, an Optilab T-rEX differential refractive index (dRI) detector and UV absorbance (UV) detector (Wyatt Technology) and analyzed with the Astra 6.2 software package provided by the manufacturer (Wyatt Technology).
Binding Competition Experiments
The binding affinity between the FITC-labelled LFP-motif peptide and the KLC2TPR-myc was measured essentially as described previously (Yip et al., 2016). FITC-labelled LFP motif peptide (300 nM) was incubated with 2-fold dilutions of KLC2TPR-myc in assay buffer (0.1 M NaCl, 25 mM HEPES, 5 mM MgCl2, 1 mM TCEP, final pH 7.5) in triplicate in 384-well plates, for 1 hour at room temperature. Fluorescence measurements were recorded on a TecanSpark plate reader at excitation and emission wavelengths of 488 nm and 535 nm respectively. Fluorescence anisotropy values were calculated at each KLC2TPR-myc concentration and the data were fitted with a fixed-slope dose response curve in Prism (GraphPad). This gave an affinity of 37 μM, similar to that found previously (Yip et al., 2016). For competition experiments, 2-fold serial dilutions of unlabelled CSTN-WD1 peptide or Trp-JIP3LZ were incubated with the FITC-labelled LFP motif peptide (300 nM) and a fixed concentration of KLC2TPR-myc (21 μM). A KI value was obtained for the CSTN-WD1 peptide data by fitting the anisotropy values A with following equation using Prism (Graphpad):
where Amin and Amax are the minimum and maximum anisotropy values, [KLC] and [CSTN] are the total concentrations of KLC2TPR-myc and calsyntenin-1 peptide, respectively, and KLFP is the dissociation constant for the FITC-LFP motif peptide binding to the KLC2TPR-myc (37 μM; see above). Two independent experiments were performed with the Trp-JIP3LZ. One experiment was performed for the CSTN-WD1 peptide. Graphs in Figure 4B show data from a single representative experiment.
Bioinformatic Sequence Analyses
Two datasets were constructed from available genomic and transcriptomic data. Combined, these two datasets ensured dense sampling of bilaterian and non-bilaterian species. Dataset 1 was constructed using the OMA Orthology database (Altenhoff et al., 2015) and contains protein-coding sequences at the amino acid level from 59 Bilaterian genomes (42 Chordata, 10 Arthropoda, 3 Nematoda, 2 Annelida, 1 Playthelminthes and 1 Mollucsa) and 3 non-Bilaterian genomes (1 Porifera, 1 Ctenophora and 1 Cindaria). Dataset 2 was assembled from Simion et al. (Simion et al., 2017) and consisted of 3 Ichthyosporea, 14 Porifera, 15 Ctenophora and 10 Cnidaria, including 15 recently sequenced non-bilateria. Searches were carried out using BLASTp (Altschul et al., 1997) for Dataset 1 and TBLASTN (Gertz et al., 2006) for Dataset 2 with human JIP3 (NP_055948.2) and KLC2 (NP_001305663.1) as the query sequences. The KLC and JIP3 residues at the interfaces depicted in Figure 5B were identified as described above. The motifs were defined as follows. JIP3 binding site on KLC: residues 200-201, 204, 208, 213, 215-216, 219-20, 223 and 227. Tryptophan-acidic cargo binding site on KLC: residues 244-245, 248, 251, 263, 270, 283- 284, 286-287, 290-291, 294, 305, 312, 325, 329, 332-333, 335-336. TPR1 dimerisation site: 197, 200-201, 204, 207-208, 213, 215-216, 219-220, 223. KLC binding site on JIP3: residues 432-434, 436-445 and 448-449. The conservation of these residues, and the KLC2 LFP motif (residues 167-169), was assessed from the resulting pairwise alignments. In general, strict conservation of all residues was required for a motif to be classified as conserved in a given homolog. The exception to this was the KLC-binding region of JIP3. Here, sequence variation at residues 432-433, 439, 442 and 446-447 was allowed, which was based on comparison of human, D. melanogaster and C. elegans JIP3/4 homologs and prior knowledge of binding in these species (Bowman et al., 2000, Byrd et al., 2001, Nguyen et al., 2005). The full list of results is tabulated in Tables S3 and S4. The results were then compared with the current canonical species phylogeny for Metazoa (Simion et al., 2017).
Structural Analyses
Analysis of Protein:Protein Interfaces
Buried surface areas were calculated using program AREAIMOL from the CCP4 suite (Winn et al., 2011). Residues at protein:protein interfaces were identified using program CONTACT from the CCP4 suite with a 4.2 Å interatomic distance cut off. Any amino acid with at least one atom within the cut-off distance of an atom from the target protein was defined as being at the interface. Putative hydrogen bonds were assigned using a cut off distance of 3.4 Å between donor and acceptor N/O atoms across the interface.
KLC2TPR Binding to ARF6-bound JIP4LZ
The KLC2TPR:JIP3LZ complex was superposed onto the crystal structure of the ARF6:JIP4LZ complex (Isabet et al., 2009) (PDB: 2W83), via residues 433-450 from JIP3LZ chains C and D and the equivalent residues in the JIP4LZ using LSQKAB from the CCP4 suite (Winn et al., 2011) (r.m.s.d. of 0.4 Å between equivalent Cα atoms). Full length ARF6 contains an N-terminal myristoylation group on residue 2 and an amphipathic helix (residues 2-11), both of which were absent from the ARF6:JIP4LZ crystal structure. The thickness of the membrane and positioning of the ARF6 amphipathic helix along the bilayer normal in Figure 1E are taken from on neutron scattering experiments of DOPC bilayers in complex with an amphipathic helix (Hristova et al., 1999).
Crystal Packing Analysis
For each copy of the KLCTPR in the crystallographic asymmetric unit, the neighbouring TPR domains inside the crystal were identified in COOT (Emsley et al., 2010) using a cut-off of 10 Å between Cα atoms. The packing models for each independent TPR domain from the various crystal structures were superposed via the subunit of interest onto PDB: 3NF1, using SUPERPOSE (Krissinel and Henrick, 2004) from the CCP4 program suite (Winn et al., 2011). This gave pairwise r.m.s.d. values of 1.7-2.6 Å for 226-250 equivalent Cα atoms. The TPR1:TPR1 interfaces were compared by pairwise superposition of the dimers via TPR1 using SUPERPOSE from the CCP4 suite (Krissinel and Henrick, 2004, Winn et al., 2011). TPR1 was defined as residues 195-236 for KLC2 and residues 210-251 for the KLC1 TPR domain. The sequence register in the PDB: 5FJY TPR1s was shifted by one residue relative to that in the other structures. The TPR1s in PDB: 5FJY were modelled from a 4 Å resolution electron density map that showed very few features for amino acid side-chains (Yip et al., 2016). Comparison of PDB: 5FJY with the highest resolution structure available (the KLC1TPR, solved to 2.8 Å resolution (Zhu et al., 2012); PDB: 3NF1) showed that the TPR1 polypeptide chain conformations were very similar (r.m.s.d. values of 0.7-0.8 Å between 82 structurally equivalent TPR1 Cα atoms). Taken together with the fact that the KLC1 and KLC2 TPR1s are 100% identical in amino acid sequence, we interpreted this discrepancy as being due to difficulties in modelling the PDB: 5FJY TPR1s into the original electron density map, rather than a difference in structure of the TPR1:TPR1 interfaces between these two crystals.
Quantification and Statistical Analysis
Information on the number of experiments performed and definitions of errors and means can be found in the relevant sections of the methods and the table/figure legends.
Data and Software Availability
The accession numbers for the coordinates and structure factors for the KLC2TPR:JIP3LZ and KLC2TPR:CSTN-WD2 crystal structures reported in this paper are PDB: 6EJN and PDB: 6F9I respectively.
Acknowledgments
We gratefully acknowledge assistance from Dr. Iain Manfield, Dr. Chi Trinh, Dr. G. Nasir Khan, and Dr. Amy Barker (University of Leeds), and Dr. Nicola O'Reilly (Peptide Synthesis Laboratory, Francis Crick Institute). We also thank Dr. Roberto Steiner (King's College, University of London) and Dr. Mark Dodding (University of Bristol) for helpful discussions. This work was made possible by grant 094232MA 094232/Z/10/Z from The Wellcome Trust. S.J.H. was supported by a BBSRC White Rose Doctoral Training Fellowship. M.W. is supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001209), the UK Medical Research Council (FC001209), and the Wellcome Trust (FC001209). J.J.B.C. was part-funded by a Cancer Research UK post-doctoral fellowship from the London Research Institute (Way lab), start-up funding from the University of Leeds, Royal Society Research Grant RG150205, and a Wellcome Trust Seed Award (204576/Z/16/Z).
Author Contributions
J.J.B.C. and M.W. conceived the study. J.J.B.C. and S.J.H. solved the crystal structures. J.J.B.C. performed the binding experiments. M.T. performed SEC-MALLS experiments. P.M. and M.J.O’C. performed the molecular evolutionary analyses. J.J.B.C. wrote the paper with contributions from all authors. All authors read and commented on the final version of the manuscript.
Declaration of Interests
The authors declare that they have no competing financial interests.
Published: September 6, 2018
Footnotes
Supplemental Information includes two figures and five tables and can be found with this article online at https://doi.org/10.1016/j.str.2018.07.011.
Supplemental Information
References
- Adams P.D., Afonine P.V., Bunkoczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenhoff A.M., Skunca N., Glover N., Train C.M., Sueki A., Pilizota I., Gori K., Tomiczek B., Muller S., Redestig H. The OMA orthology database in 2015: function predictions, better plant support, synteny view and other improvements. Nucleic Acids Res. 2015;43:D240–D249. doi: 10.1093/nar/gku1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Kawano T., Taru H., Saito Y., Wada S., Miyamoto K., Kobayashi H., Ishikawa H.O., Ohsugi Y., Yamamoto T. The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport. EMBO J. 2007;26:1475–1486. doi: 10.1038/sj.emboj.7601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius T.L., Cai D., Jih G.T., Toret C.P., Verhey K.J. Two binding partners cooperate to activate the molecular motor Kinesin-1. J. Cell Biol. 2007;176:11–17. doi: 10.1083/jcb.200605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeda B., Briggs D.C., Higgins T., Garvalov B.K., Fadden A.J., McDonald N.Q., Way M. Tes, a specific Mena interacting partner, breaks the rules for EVH1 binding. Mol. Cell. 2007;28:1071–1082. doi: 10.1016/j.molcel.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Bowman A.B., Kamal A., Ritchings B.W., Philp A.V., McGrail M., Gindhart J.G., Goldstein L.S. Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell. 2000;103:583–594. doi: 10.1016/s0092-8674(00)00162-8. [DOI] [PubMed] [Google Scholar]
- Byrd D.T., Kawasaki M., Walcoff M., Hisamoto N., Matsumoto K., Jin Y. UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron. 2001;32:787–800. doi: 10.1016/s0896-6273(01)00532-3. [DOI] [PubMed] [Google Scholar]
- Cai D., Hoppe A.D., Swanson J.A., Verhey K.J. Kinesin-1 structural organization and conformational changes revealed by FRET stoichiometry in live cells. J. Cell Biol. 2007;176:51–63. doi: 10.1083/jcb.200605097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V., Kujala P., Klumperman J., Goldstein L.S. Sunday Driver links axonal transport to damage signaling. J. Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen V.B., Arendall W.B., 3rd, Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy D.L., Hancock W.O., Wagenbach M., Howard J. Kinesin's tail domain is an inhibitory regulator of the motor domain. Nat. Cell. Biol. 1999;1:288–292. doi: 10.1038/13001. [DOI] [PubMed] [Google Scholar]
- Diefenbach R.J., Diefenbach E., Douglas M.W., Cunningham A.L. The heavy chain of conventional kinesin interacts with the SNARE proteins SNAP25 and SNAP23. Biochemistry. 2002;41:14906–14915. doi: 10.1021/bi026417u. [DOI] [PubMed] [Google Scholar]
- Dodding M.P., Mitter R., Humphries A.C., Way M. A kinesin-1 binding motif in vaccinia virus that is widespread throughout the human genome. EMBO J. 2011;30:4523–4538. doi: 10.1038/emboj.2011.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodding M.P., Way M. Coupling viruses to dynein and kinesin-1. EMBO J. 2011;30:3527–3539. doi: 10.1038/emboj.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S.L., Yu S.C., Hoover C.M., Phillips B.C., Richmond J.E., Miller K.G. An organelle gatekeeper function for Caenorhabditis elegans UNC-16 (JIP3) at the axon initial segment. Genetics. 2013;194:143–161. doi: 10.1534/genetics.112.147348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeut J., Gaussen A., Bieling P., Morin V., Prieto S., Fesquet D., Surrey T., Abrieu A. Phosphorylation relieves autoinhibition of the kinetochore motor Cenp-E. Mol. Cell. 2008;29:637–643. doi: 10.1016/j.molcel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Friedman D.S., Vale R.D. Single-molecule analysis of kinesin motility reveals regulation by the cargo-binding tail domain. Nat. Cell. Biol. 1999;1:293–297. doi: 10.1038/13008. [DOI] [PubMed] [Google Scholar]
- Frishman D., Argos P. Knowledge-based protein secondary structure assignment. Proteins. 1995;23:566–579. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- Fu M.M., Holzbaur E.L. JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. J. Cell Biol. 2013;202:495–508. doi: 10.1083/jcb.201302078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauger A.K., Goldstein L.S. The Drosophila kinesin light chain. Primary structure and interaction with kinesin heavy chain. J. Biol. Chem. 1993;268:13657–13666. [PubMed] [Google Scholar]
- Gertz E.M., Yu Y.K., Agarwala R., Schaffer A.A., Altschul S.F. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol. 2006;4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham A.K., Munro S. The small G proteins of the Arf family and their regulators. Annu. Rev. Cell Dev. Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- Gindhart J.G., Chen J., Faulkner M., Gandhi R., Doerner K., Wisniewski T., Nandlestadt A. The kinesin-associated protein UNC-76 is required for axonal transport in the Drosophila nervous system. Mol. Biol. Cell. 2003;14:3356–3365. doi: 10.1091/mbc.E02-12-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart J.G., Jr., Desai C.J., Beushausen S., Zinn K., Goldstein L.S. Kinesin light chains are essential for axonal transport in Drosophila. J. Cell Biol. 1998;141:443–454. doi: 10.1083/jcb.141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glater E.E., Megeath L.J., Stowers R.S., Schwarz T.L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D.D., Baek N., Snyder A.C. Half-site inhibition of dimeric kinesin head domains by monomeric tail domains. Biochemistry. 2009;48:3448–3456. doi: 10.1021/bi8022575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D.D., Levitt J.D., Suhan J. Kinesin undergoes a 9 S to 6 S conformational transition. J. Biol. Chem. 1992;267:8696–8701. [PubMed] [Google Scholar]
- Hackney D.D., Stock M.F. Kinesin's IAK tail domain inhibits initial microtubule-stimulated ADP release. Nat. Cell. Biol. 2000;2:257–260. doi: 10.1038/35010525. [DOI] [PubMed] [Google Scholar]
- Hammond J.W., Blasius T.L., Soppina V., Cai D., Verhey K.J. Autoinhibition of the kinesin-2 motor KIF17 via dual intramolecular mechanisms. J. Cell Biol. 2010;189:1013–1025. doi: 10.1083/jcb.201001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J.W., Griffin K., Jih G.T., Stuckey J., Verhey K.J. Co-operative versus independent transport of different cargoes by Kinesin-1. Traffic. 2008;9:725–741. doi: 10.1111/j.1600-0854.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Noda Y., Tanaka Y., Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- Hristova K., Wimley W.C., Mishra V.K., Anantharamiah G.M., Segrest J.P., White S.H. An amphipathic alpha-helix at a membrane interface: a structural study using a novel X-ray diffraction method. J. Mol. Biol. 1999;290:99–117. doi: 10.1006/jmbi.1999.2840. [DOI] [PubMed] [Google Scholar]
- Huang S.H., Duan S., Sun T., Wang J., Zhao L., Geng Z., Yan J., Sun H.J., Chen Z.Y. JIP3 mediates TrkB axonal anterograde transport and enhances BDNF signaling by directly bridging TrkB with kinesin-1. J. Neurosci. 2011;31:10602–10614. doi: 10.1523/JNEUROSCI.0436-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabet T., Montagnac G., Regazzoni K., Raynal B., El Khadali F., England P., Franco M., Chavrier P., Houdusse A., Menetrey J. The structural basis of Arf effector specificity: the crystal structure of ARF6 in a complex with JIP4. EMBO J. 2009;28:2835–2845. doi: 10.1038/emboj.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.S., Buster D., Scholey J.M. Light chains of sea urchin kinesin identified by immunoadsorption. Cell. Motil. Cytoskeleton. 1990;16:204–213. doi: 10.1002/cm.970160307. [DOI] [PubMed] [Google Scholar]
- Junco A., Bhullar B., Tarnasky H.A., van der Hoorn F.A. Kinesin light-chain KLC3 expression in testis is restricted to spermatids. Biol. Reprod. 2001;64:1320–1330. doi: 10.1095/biolreprod64.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaan H.Y., Hackney D.D., Kozielski F. The structure of the kinesin-1 motor-tail complex reveals the mechanism of autoinhibition. Science. 2011;333:883–885. doi: 10.1126/science.1204824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T., Araseki M., Araki Y., Kinjo M., Yamamoto T., Suzuki T. A small peptide sequence is sufficient for initiating kinesin-1 activation through part of TPR region of KLC1. Traffic. 2012;13:834–848. doi: 10.1111/j.1600-0854.2012.01350.x. [DOI] [PubMed] [Google Scholar]
- Kelkar N., Gupta S., Dickens M., Davis R.J. Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Mol. Cell Biol. 2000;20:1030–1043. doi: 10.1128/mcb.20.3.1030-1043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar N., Standen C.L., Davis R.J. Role of the JIP4 scaffold protein in the regulation of mitogen-activated protein kinase signaling pathways. Mol. Cell Biol. 2005;25:2733–2743. doi: 10.1128/MCB.25.7.2733-2743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S., Vargas C., Zhao H., Piszczek G., Brautigam C.A., Schuck P. High-precision isothermal titration calorimetry with automated peak-shape analysis. Anal. Chem. 2012;84:5066–5073. doi: 10.1021/ac3007522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E., Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- Marchesin V., Castro-Castro A., Lodillinsky C., Castagnino A., Cyrta J., Bonsang-Kitzis H., Fuhrmann L., Irondelle M., Infante E., Montagnac G. ARF6-JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion. J. Cell Biol. 2015;211:339–358. doi: 10.1083/jcb.201506002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnac G., Sibarita J.B., Loubery S., Daviet L., Romao M., Raposo G., Chavrier P. ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr. Biol. 2009;19:184–195. doi: 10.1016/j.cub.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Morfini G.A., You Y.M., Pollema S.L., Kaminska A., Liu K., Yoshioka K., Bjorkblom B., Coffey E.T., Bagnato C., Han D. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat. Neurosci. 2009;12:864–871. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q., Lee C.M., Le A., Reddy E.P. JLP associates with kinesin light chain 1 through a novel leucine zipper-like domain. J. Biol. Chem. 2005;280:30185–30191. doi: 10.1074/jbc.M505499200. [DOI] [PubMed] [Google Scholar]
- Nguyen T.Q., Chenon M., Vilela F., Velours C., Aumont-Nicaise M., Andreani J., Varela P.F., Llinas P., Menetrey J. Structural plasticity of the N-terminal capping helix of the TPR domain of kinesin light chain. PLoS One. 2017;12:e0186354. doi: 10.1371/journal.pone.0186354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernigo S., Lamprecht A., Steiner R.A., Dodding M.P. Structural basis for kinesin-1:cargo recognition. Science. 2013;340:356–359. doi: 10.1126/science.1234264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A., Friedman D.S., Goldstein L.S. Two kinesin light chain genes in mice. Identification and characterization of the encoded proteins. J. Biol. Chem. 1998;273:15395–15403. doi: 10.1074/jbc.273.25.15395. [DOI] [PubMed] [Google Scholar]
- Rahman A., Kamal A., Roberts E.A., Goldstein L.S. Defective kinesin heavy chain behavior in mouse kinesin light chain mutants. J. Cell Biol. 1999;146:1277–1288. doi: 10.1083/jcb.146.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall T.S., Yip Y.Y., Wallock-Richards D.J., Pfisterer K., Sanger A., Ficek W., Steiner R.A., Beavil A.J., Parsons M., Dodding M.P. A small-molecule activator of kinesin-1 drives remodeling of the microtubule network. Proc. Natl. Acad. Sci. USA. 2017;114:13738–13743. doi: 10.1073/pnas.1715115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger A., Yip Y.Y., Randall T.S., Pernigo S., Steiner R.A., Dodding M.P. SKIP controls lysosome positioning using a composite kinesin-1 heavy and light chain-binding domain. J. Cell. Sci. 2017;130:1637–1651. doi: 10.1242/jcs.198267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman V.K., Folker E.S., Rosen J.N., Baylies M.K. Syd/JIP3 and JNK signaling are required for myonuclear positioning and muscle function. PLoS Genet. 2014;10:e1004880. doi: 10.1371/journal.pgen.1004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler S., Kirchner J., Horn C., Kallipolitou A., Woehlke G., Schliwa M. Cargo binding and regulatory sites in the tail of fungal conventional kinesin. Nat. Cell. Biol. 2000;2:333–338. doi: 10.1038/35014022. [DOI] [PubMed] [Google Scholar]
- Simion P., Philippe H., Baurain D., Jager M., Richter D.J., Di Franco A., Roure B., Satoh N., Queinnec E., Ereskovsky A. A Large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr. Biol. 2017;27:958–967. doi: 10.1016/j.cub.2017.02.031. [DOI] [PubMed] [Google Scholar]
- Sreerama N., Venyaminov S.Y., Woody R.W. Estimation of the number of alpha-helical and beta-strand segments in proteins using circular dichroism spectroscopy. Protein Sci. 1999;8:370–380. doi: 10.1110/ps.8.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock M.F., Guerrero J., Cobb B., Eggers C.T., Huang T.G., Li X., Hackney D.D. Formation of the compact confomer of kinesin requires a COOH-terminal heavy chain domain and inhibits microtubule-stimulated ATPase activity. J. Biol. Chem. 1999;274:14617–14623. doi: 10.1074/jbc.274.21.14617. [DOI] [PubMed] [Google Scholar]
- Su Q., Cai Q., Gerwin C., Smith C.L., Sheng Z.H. Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat. Cell. Biol. 2004;6:941–953. doi: 10.1038/ncb1169. [DOI] [PubMed] [Google Scholar]
- Sun F., Zhu C., Dixit R., Cavalli V. Sunday Driver/JIP3 binds kinesin heavy chain directly and enhances its motility. EMBO J. 2011;30:3416–3429. doi: 10.1038/emboj.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T., Li Y., Li T., Ma H., Guo Y., Jiang X., Hou M., Huang S., Chen Z. JIP1 and JIP3 cooperate to mediate TrkB anterograde axonal transport by activating kinesin-1. Cell Mol. Life Sci. 2017;74:4027–4044. doi: 10.1007/s00018-017-2568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talapatra S.K., Harker B., Welburn J.P. The C-terminal region of the motor protein MCAK controls its structure and activity through a conformational switch. Elife. 2015;4 doi: 10.7554/eLife.06421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R.D. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- van Stokkum I.H., Spoelder H.J., Bloemendal M., van Grondelle R., Groen F.C. Estimation of protein secondary structure and error analysis from circular dichroism spectra. Anal. Biochem. 1990;191:110–118. doi: 10.1016/0003-2697(90)90396-q. [DOI] [PubMed] [Google Scholar]
- Verhey K.J., Lizotte D.L., Abramson T., Barenboim L., Schnapp B.J., Rapoport T.A. Light chain-dependent regulation of Kinesin's interaction with microtubules. J. Cell Biol. 1998;143:1053–1066. doi: 10.1083/jcb.143.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey K.J., Meyer D., Deehan R., Blenis J., Schnapp B.J., Rapoport T.A., Margolis B. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt D., Dixit R., Cavalli V. JIP3 activates kinesin-1 motility to promote axon elongation. J. Biol. Chem. 2015;290:15512–15525. doi: 10.1074/jbc.M115.651885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn J.P. The molecular basis for kinesin functional specificity during mitosis. Cytoskeleton (Hoboken) 2013;70:476–493. doi: 10.1002/cm.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore L., Wallace B.A. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers. 2008;89:392–400. doi: 10.1002/bip.20853. [DOI] [PubMed] [Google Scholar]
- Winn M.D., Ballard C.C., Cowtan K.D., Dodson E.J., Emsley P., Evans P.R., Keegan R.M., Krissinel E.B., Leslie A.G., McCoy A. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y.L., Rice S.E. Kinesin's light chains inhibit the head- and microtubule-binding activity of its tail. Proc. Natl. Acad. Sci. USA. 2010;107:11781–11786. doi: 10.1073/pnas.1005854107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip Y.Y., Pernigo S., Sanger A., Xu M., Parsons M., Steiner R.A., Dodding M.P. The light chains of kinesin-1 are autoinhibited. Proc. Natl. Acad. Sci. USA. 2016;113:2418–2423. doi: 10.1073/pnas.1520817113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeytuni N., Zarivach R. Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure. 2012;20:397–405. doi: 10.1016/j.str.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Zhao H., Piszczek G., Schuck P. SEDPHAT – a platform for global ITC analysis and global multi-method analysis of molecular interactions. Methods. 2015;76:137–148. doi: 10.1016/j.ymeth.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N.E., Kay C.M., Hodges R.S. The net energetic contribution of interhelical electrostatic attractions to coiled-coil stability. Protein Eng. 1994;7:1365–1372. doi: 10.1093/protein/7.11.1365. [DOI] [PubMed] [Google Scholar]
- Zhu H., Lee H.Y., Tong Y., Hong B.S., Kim K.P., Shen Y., Lim K.J., Mackenzie F., Tempel W., Park H.W. Crystal structures of the tetratricopeptide repeat domains of kinesin light chains: insight into cargo recognition mechanisms. PLoS One. 2012;7:e33943. doi: 10.1371/journal.pone.0033943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.