Abstract
Background
The incidence of penile cancer in Europe lies in the range of 0.9 to 2.1 cases per 100 000 persons per year. Carcinogenesis is associated with human papilloma virus (HPV) infection and with chronic inflammation.
Methods
This review is based on publications (2010–2017) retrieved by a selective search in PubMed and EMBASE and on the guidelines of the European Association of Urology, the European Society of Medical Oncology, the National Comprehensive Cancer Network, and the National Institute for Health and Care Excellence (NICE).
Results
95% of cases of penile cancer are accounted for by squamous cell carcinoma, whose numerous subtypes have different clinical courses. Chronic preputial inflammation due to phimosis or lichen sclerosus is often associated with penile cancer. Circumcision lowers the risk of penile cancer (hazard ratio: 0.33). Maximally organ-preserving surgery with safety margins of no more than a few millimeters is the current therapeutic standard, because a local recurrence, if it arises, can still be treated locally with curative intent. Local radiotherapy can be performed in early stages. Lymphogenic metastasis must be treated with radical lymphadenectomy and adjuvant chemotherapy. Patients with clinically unremarkable inguinal lymph nodes nonetheless need invasive lymph node staging because of the high rate of lymphogenic micrometastasis.
Conclusion
Penile cancer is curable in all early stages with the appropriate treatment, but its prognosis depends crucially on the proper management of the regional (i.e., inguinal) lymph nodes. In many countries, the treatment of this rare disease entity has been centralized.
Penile cancer is an aggressive squamous cell carcinoma of the skin of the glans or of the inner layer of the prepuce, characterized by invasive growth and early metastatic spread to lymph nodes. While penile cancer is uncommon in Europe, incidence rates are very high in parts of South America and Africa. Since its treatment is often associated with significant cosmetic and functional defects, the disease is of critical importance to the affected men. Early metastatic spread to regional lymph nodes can be life-threatening. It is not uncommon that factors, both from the patient and the treating physician, are causing delays in diagnosis and start of treatment.
With penile cancer being a comparatively rare disease, many physicians are unfamiliar with its management. Thus, several countries have centralized the treatment of this rare tumor. Penile cancer is an orphan disease. Due the low numbers of patients, no prospective randomized studies have become available. Most of the available data is from small retrospective studies; larger studies result from retrospective multicenter data collections. Thus, the level of evidence reached for penile cancer based on the literature is consistently low. For the same reasons, pharmaceutical companies which sponsor studies are not interested in penile cancer.
This article summarizes the current knowledge of the disease and the management strategies for penile cancer. The aim of this paper is to highlight the complexity of this cancer and to show that delayed or incorrect treatment can be life-threatening. For this end, the current versions of all available guidelines on penile cancer (European Association of Urology [EAU] (9), European Society of Medical Oncology [ESMO], National Comprehensive Cancer Network [NCCN], National Institute for Health and Care Excellence [NICE], and existing Cochrane reviews as well as the respective cited literature were included in our review. In addition, an updated search of the literature was performed in the databases PubMed, EMBASE and Cochrane Database of Systematic Reviews for the period from 2010 to 2017, using the search terms “penile or penis, penile neoplasms, penile cancer“.
Pathology and pathogenesis of penile cancer
Of all penile malignancies, 95% are squamous cell carcinomas; about half of these originate from non-keratinized epithelium of the glans or the inner layer of the prepuce. Other malignancies (melanoma, sarcoma) or metastases are extremely rare at the penis.
In penile squamous cell carcinoma, various histologic subtypes are distinguished based on the classification of the Union Internationale Contre le Cancer (UICC) (table 1) (1). These subtypes differ in terms of their histologic and molecular genetic characteristics, pathogenesis and prognosis.
Table 1. WHO classification of penile carcinomas, their relative frequency and mean cancer-specific mortality (1, 2, 32).
| Squamous cell carcinomas | Relative frequency | Tumor-specific mortality |
| Non-HPV-associated | ||
| Squamous cell carcinoma, common type | 70–75% | 30% |
| Pseudohyperplastic carcinoma | 0% | |
| Pseudoglandular carcinoma | >50% | |
| Verrucous carcinoma | 2–3% | low |
| Carcinoma cuniculatum | low | |
| Papillary carcinoma, NOS | 5–8% | low |
| Adenosquamous carcinoma | rare | low |
| Sarcomatoid carcinoma | 1–4% | 75% |
| HPV-associated | ||
| Basaloid carcinoma | 5–10% | >50% |
| Papillary basaloid carcinoma | rare | |
| Warty carcinoma | 5–10% | low |
| Warty basaloid carcinoma | 30% | |
| Clear-cell carcinoma | 20% | |
| Lymphoepithelioma-like carcinoma | not known | |
There are two different pathogenic pathways involved in the development of penile carcinomas. About one third of cases is associated with human papilloma virus (HPV) infection. Immunohistochemical detection of p16 is used as a surrogate parameter for HPV association (2). The most commonly identified HPV serotypes are HPV 16, 18, 31, 33, 45, 56, and 65. HPV-associated penile cancer can be distinguished from non-HPV-associated types by means of polymerase chain reaction (PCR) or immunohistochemistry.
The second pathogenic pathway is chronic inflammation, associated with, for example, lichen sclerosus or chronic inflammation of the foreskin related to phimosis. Because of these two different pathogenic pathways, the new UICC classification distinguishes between HPV-associated and non-HPV-associated penile carcinomas (1).
Thus, epidemiologically confirmed risk factors include phimosis and chronic inflammation (balanoposthitis; hazard ratio [HR] 9.5; phimosis HR 3.5). Circumcision in childhood significantly reduces the prevalence of the condition (HR 0.33) (3). However, the removal of the foreskin also reduces the exposed surface of the non-keratinized penile skin by 50%. In epidemiological studies, chronic cigarette smoking is described as another risk factor (HR 2.8) (4– 6).
Molecular biological changes may be relevant to prognosis. In penile cancer, loss of heterozygosity (LOH) adjacent to tumor suppressor genes (2q, 6p, 8q, 9p, 12q, 17p13) is commonly observed and occurs even more frequently in lymph nodes metastases (3p, 6p, 6q, 8q, 9p, 11q, 12q, 15q, 17p, 18q) (3). Particularly common are losses of alleles in the regions 9p21 und 17p, coding the tumor suppressor genes p16 and p53, respectively (3). Inactivation of p16 and p53 is also effected by the HPV oncogenes E6 and E7. P16 promoter hypermethylation and LOH as well as other changes of other tumor suppressor genes (KAI1, nm23H1) are associated with metastatic spread. However, whether p53 alterations are of prognostic relevance has not yet been conclusively established (4, 5).
The most prevalent histologic type, accounting for about 70 to 75% of cases, is the “common“ squamous cell carcinoma with or without keratinization (7). It is an aggressive tumor characterized by early metastatic spread. The second most common type, accounting for approximately 10% of cases, is the basaloid subtype which is very aggressive, as are the sarcomatoid and warty subtypes. By contrast, the verrucous and condylomatous subtypes only spread in exceptional cases and have a much better prognosis (table 1) (2).
Epidemiology
In Europe and North America, the incidence of penile cancer is approximately 1.0 new case per 100 000 population (7). In Sweden, the incidence is higher with comprehensive data collection (2,1). In Germany, altogether 940 new cases were recorded in 2014, with a mortality of 195 cases and a mean age of onset of 70 years (3). However, penile cancer also occurs in significantly younger men, starting age 30 years.
In some developing countries, penile cancer represents a serious public health problem. The incidence rates in Central and South America, parts of Asia and Africa are significantly higher (Brazil 6–8/100 000). In rural regions in India, penile cancer accounts for up to 6% of all male cancer cases; in Uganda, the cumulative morbidity among men up to age 75 years is 1% (5). These peculiarities have been attributed to shortcomings in the healthcare system and lack of hygiene, high rates of sexually transmitted infections and a high rate of phimosis. Patient-related delays in diagnosis and treatment are not rare and associated with low socioeconomic status and low level of education (5).
Population-based analyses from Europe and the United States have shown that, in contrast to other types of cancer, the tumor-specific survival rates for penile cancer have not shown any improvement since 1990 (6).
Diagnosis
Patients note changes of the glans or foreskin, but experience no pain. In many cases, the diagnosis of exophytic penile cancer is established by inspection. Superficial stages of penile cancer (pTis, pTa [Table 2]) are often limited to surface changes. Early suspicion and biopsy are necessary to prevent delays in treatment initiation.
Pathological processing, grading and staging
Confirmation of the diagnosis by biopsy and tumor staging are both required for treatment planning. Invasive penile cancer typically shows exophytic growth. Histologic subtype and tumor grade are key determinants of prognosis. The UICC classification categorizes the grades I to III and the sarcomatoid, dedifferentiated type (1).
Grading is more difficult with squamous cell carcinomas compared to adenocarcinomas. This explains the high interobserver variability in the grading of penile cancer (8). Consequently, there is no definitive prognostic difference between G1 and G2 in penile cancer, especially since highly differentiated penile squamous cell carcinomas can show invasive growth and metastatic spread, too.
Pathological processing of the specimen should be undertaken with great care. It is important to distinguish between stage T1a and stage T1b. This requires special expertise for discriminating between the two can be challenging due to lymphovascular invasion or poor degree of differentiation (G3). Lymph node evaluation is of great prognostic significance because extracapsular spread of lymph node metastases—which is classified as pN3 even with only 1 lymph node—ultimately requires adjuvant chemotherapy (table 2) (2).
Table 2. TNM classification of penile carcinomas of the Union Internationale Contre le Cancer (UICC) (32).
| Primary tumor (T) | Tis | Carcinoma in situ |
| Ta | Non-invasive localized verrucous carcinoma | |
| T1 | Tumor invades subepithelial connective tissue | |
| T1a | Without lymphovascular invasion and well differentiated | |
| T1b | With lymphovascular invasion or poorly differentiated | |
| T2 | Tumor invades corpus spongiosum with or without invasion of the urethra | |
| T3 | Tumor invades corpus cavernosum without or with invasion of the urethra | |
| T4 | Tumor invades other adjacent structures | |
| Regional lymph nodes (N) (clinical) | N0 | No palpable or visibly enlarged inguinal lymph nodes |
| N1 | Palpable mobile unilateral lymph node | |
| N2 | Palpable mobile multiple or bilateral inguinal lymph nodes | |
| N3 | Fixed inguinal nodal mass or pelvic lymphadenopathy, unilateral or bilateral | |
| Regional lymph nodes (pN) (pathological) | pN0 | No regional lymph node metastasis |
| pN1 | Metastasis in up to two regional lymph nodes | |
| pN2 | Metastases in three or more unilateral lymph nodes or bilateral inguinal lymph nodes | |
| pN3 | Metastasis in pelvic lymph nodes, unilateral or bilateral, or extranodal extension of any regional lymph node metastasis | |
| Distant metastasis (M) | M0 | No distant metastasis |
| M1 | Distant metastasis | |
Management
For the management of penile cancer, interdisciplinary guidelines of varying quality are available (EAU [9], ESMO, NCCN, NICE [10, 11]). Several European countries (United Kingdom, Sweden, Denmark, the Netherlands) have centralized the management of penile cancer. With this approach, the interval between diagnosis and treatment was significantly shortened in Denmark, and in Sweden it led to improvements in guideline adherence (12, 13). Having established national penile cancer registries, these countries are in the position to collect data which can be used to improve treatment strategies.
Stage-adapted treatment
Especially for early stages limited to the foreskin or glans, treatment alternatives, with equal effectiveness, are available. The patient should receive in-depth advice and detailed information. However, ultimately treatment decisions should be based on the wishes of the patient.
Surgical treatment of penile cancer is guided by the following principle: as much organ preservation as possible and as much radicality as necessary. Penile cancer limited to the inner layer of the prepuce is treated by “radical circumcision“. In case of superficial tumors of the glans which are limited to the epithelium (pTis, pTa), the glans should be spared. This can be achieved using focal chemotherapy or immunotherapy, laser ablation, radiation therapy or surgery. Carcinoma in situ (pTis) can be treated successfully in half of the cases using topical immunotherapy or chemotherapy (imiquimod, 5-fluorouracil, applied as an aqueous solution) (14). Alternative treatment options—also for recurrence or persistence of the lesion— to be considered include laser (CO2, Neodym:YAG) ablation, complete removal of the epithelium of the glans (“glans resurfacing“) or radiation therapy. Here, it is important to keep in mind that superficial tumors often show invasive growth only at isolated points of the lesion (15). The success of focal treatment should be confirmed by a follow-up biopsy. In most cases, local recurrences occur within 1 to 2 years after the initial treatment, most frequently after laser ablation (10–48%), less frequently after glans resurfacing (0–6%) and very rarely after glansectomy (0–2%) (16– 18).
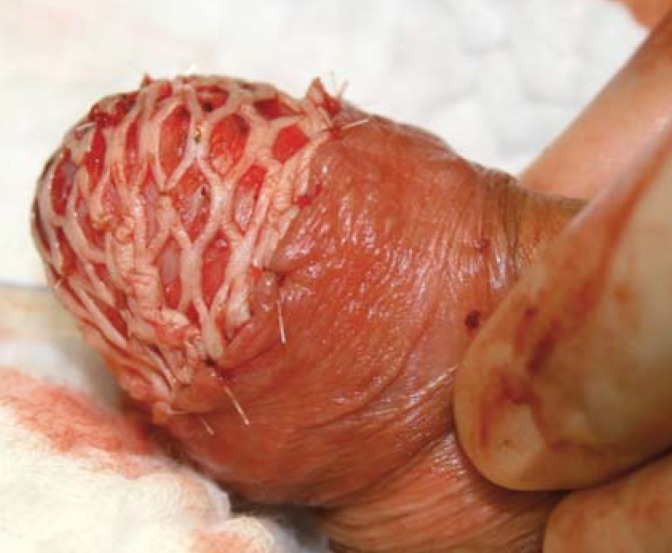
Current recommendations for surgical treatment allow very narrow tumor-negative margins as long as a complete excision of the tumor is achieved (19). Today, the wide negative margins of up to 2 cm at the penis shaft recommended in the past are considered completely obsolete. Since a local recurrence as such is not a threat to the life of the patient, as it is curable by renewed local treatment (20), the most recent strategy is to try to achieve narrow, but clearly tumor-negative margins to ensure optimum quality of life is maintained. Guidelines recommend to decide the required width of the tumor-negative margin based on the grading (1 mm for G1, 5 mm for G3) (9). Diverse surgical techniques are available to treat the various stages of invasive penile cancer. Invasive tumors of the glans (pT1, pT2) are treated by local excision, partial resection of the glans or amputation of the glans. While it is possible to achieve satisfactory cosmetic results by glans repair using a split-thickness skin graft or a buccal mucosa graft (figure 1), the repaired glans will have no sensory innervation.
Figure 1.
Glans resection with split-thickness skin graft closure
Larger tumors with invasion of the corpus spongiosum (pT2) or the corpora cavernosa (pT3) require glans amputation, in some cases including removal of the tips of the corpora. In these cases, plastic reconstruction should also be attempted.
Extensive tumors (pT4) require extensive amputation (partial amputation of the penis) or radical penectomy with complete removal of the corpora cavernosa (up to the insertion on the pelvic bones). In these cases, reconstructive techniques are challenging and should only be attempted in the interval if curative treatment is possible.
Role of radiation therapy
Squamous cell carcinomas are generally radiosensitive tumors. Thus, penile carcinomas can in principle be treated by percutaneous radiotherapy or focal brachytherapy. However, due to the lack of data on the radiosensitivity of the various types of penile squamous cell carcinomas, no differentiated treatment with radiation therapy can be administered.
While radiation therapy offers the potential advantage of sparing the morphological integrity of the organ, radiation-related functional impairments of the corpora cavernosa and, with meatus stenosis being a common complication (10–35%), of urination are unavoidable (21). Local radiation therapy is a recommended option for tumors up to a maximum size of 4 cm in the stages T1 and T2 (4). Local brachytherapy achieves lower local control rates compared to surgical treatment (70–90% versus 90–92% and 94–96% for glansectomy and glans resurfacing, respectively) (14, 15, 22– 25). With the majority of cases of penile cancer being treated surgically, data on radiation therapy are scarce.
Regional lymph nodes
Due to the tendency for early lymphatic metastasis, treatment of regional (inguinal and pelvic) lymph nodes is critical for prognosis. Approximately 20% of patients have palpable inguinal lymph nodes at the time of diagnosis (26). Diagnostic assessment of regional lymph nodes is usually limited to clinical examination (finding of palpable inguinal lymph nodes). However, in obese patients clinical differentiation can be challenging; in this situation, ultrasound evaluation of inguinal lymph nodes can be advantageous (20).
Non-enlarged (non-palpable) inguinal lymph nodes
The management of patients with unremarkable inguinal lymph nodes on physical examination is particularly challenging because in up to 20 to 25% of cases— depending on local stage and degree of differentiation of the tumor—inguinal lymphatic micrometastases (0.2 to 2 mm in diameter) are present (27). If left untreated, a regional lymph node recurrence will occur within a period of 1 to 2 years which has a detrimental effect on prognosis (long-term survival <40%).
In patients with clinically unremarkable inguinal lymph node status, diagnostic imaging does not improve the detection of lymph node metastases measuring less than 1 cm in diameter. Consequently, in patients with clinically unremarkable inguinal lymph nodes, invasive diagnostic investigations are performed starting from stage pT1 and grade G2–3 The two methods used for this end are:
dynamic sentinel lymph node biopsy (DSNB) using technetium-labeled nanocolloid and patent blue dye (PBD) or
(diagnostic) modified inguinal lymph node dissection.
For DSNB, high micrometastasis detection rates (sensitivity approximately 90–95%) along with rates of false-negative results of 5 to 10% as well as low morbidity have been described (28).
Enlarged inguinal lymph nodes
In patients with inguinal lymph nodes suspicious on palpation, surgical removal, histologic confirmation by means of intraoperative frozen-section analysis, and, in case of positive findings, radical inguinal lymphadenectomy are indicated. Radical inguinal lymph node dissection is mandatory since it is not sufficient to only remove lymph nodes with macroscopic involvement. Prolonged attempts of antibiotic treatment for presumed inflammatory enlargement of inguinal lymph nodes are contraindicated (5). If involvement of two or more lymph nodes is detected on one inguinal side, it is necessary to perform additional ipsilateral pelvic lymphadenectomy (9). While penile cancer is characterized by bilateral lymphatic spread to the groins, metastasis from inguinal to pelvic lymph nodes is strictly ipsilateral; thus, unilateral radical pelvic lymph node dissection is sufficient in these cases (27).
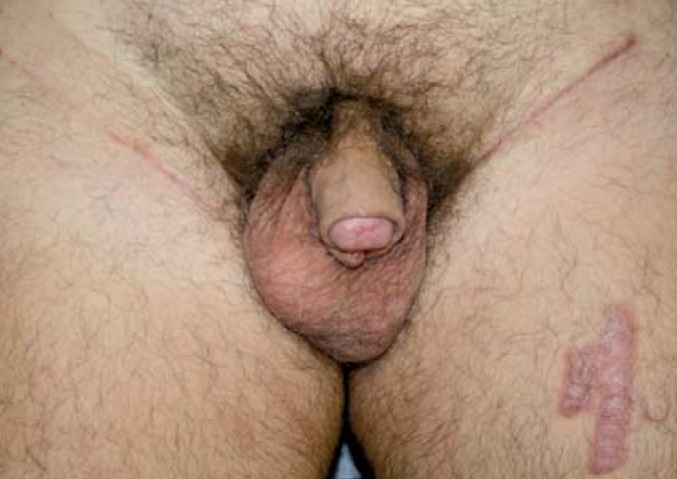
Inguinal lymph node dissection is associated with considerable morbidity in the form of lymphedema, lymphoceles and complications of wound healing. In older series, this morbidity reached levels of up to 50%, but advances in surgical technique have led to a reduction to a rate of approximately 25% (figure 2) (17, 18). Minimally invasive surgical techniques for inguinal lymph node dissection (laparoscopic, robot-assisted) are associated with lower morbidity (19).
Figure 2.
One year after glans resection for pT2G3 penile cancer with split-thickness skin graft repair and bilateral inguinal lymphadenectomy (pN1 bilaterally) and adjuvant chemotherapy
Adjuvant therapy for lymph node metastasis
After radical lymphadenectomy, adjuvant chemotherapy improves tumor-specific survival (29– 31). Depending on the extent of lymph node metastasis and the patient’s comorbidities, 4 to 6 cycles of adjuvant chemotherapy are required to achieve this survival benefit.
Adjuvant inguinal lymph node irradiation is not recommended by any of the guidelines due to the lack of relevant data (9– 11). Only for adjuvant irradiation of the pelvis after surgical removal of pelvic lymph node metastases, a minor survival benefit was reported.
Management of advanced stages
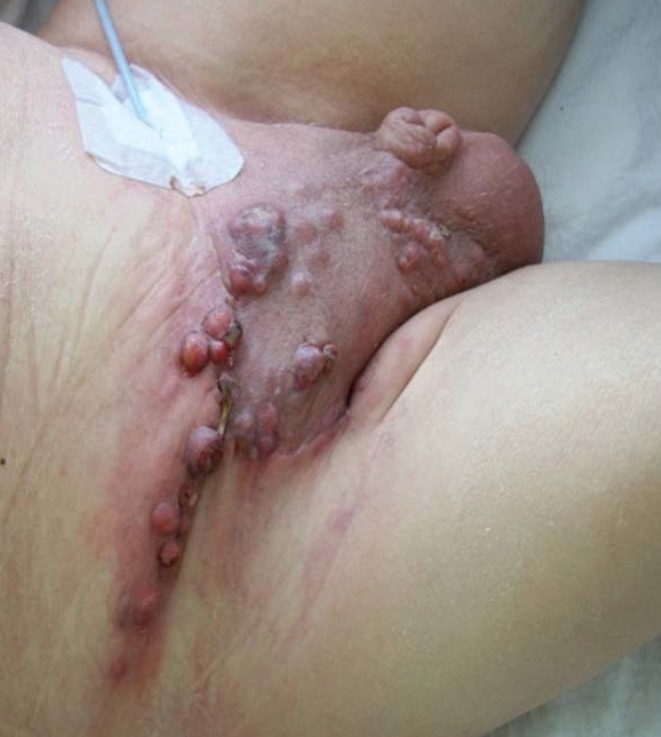
Locally advanced tumor stages can be treated with palliative intention either by radical surgery or radiation therapy. In patients with large inguinal nodal masses (cN3), operability can be achieved if they respond to neoadjuvant chemotherapy, ultimately resulting in long-term survival rates of up to 50% (figure 3) (32).
Figure 3.
Extensive fixed inguinal lymph node metastases in penile cancer (N3)
In patients with systemic metastasis (most commonly to the lungs, liver and brain), palliative chemotherapy can achieve a limited survival benefit. Second-line therapies are available, but have not been sufficiently evaluated yet. In principle, several chemotherapeutic agents are effective in penile cancer. First, the comparatively toxic Dexeus regimen was established, combining cisplatin, methotrexate and bleomycin (33). Improved tolerability was then offered by the Pizzocaro regimen (vinblastine, methotrexate, bleomycin) (34). Today, taxane-containing regimens have been established. In Europe, the combination of paclitaxel, cisplatin and 5-fluoruracil is preferred (35), in the United States the TIP regimen (paclitaxel, ifosfamide, cisplatin) (36).
New systemic therapies have shown little success in the treatment of penile cancer. Sorafenib and sunitinib are without effect (23); for some PD-1 inhibitors, data are available, showing a limited response (24).
Outcomes and prognosis
The overall 5-year relative survival rates were 97% for pTis/pTa tumors, 90% for pT1, 66% for pT2, 55% for pT3, and 46% for patients with positive lymph node status (pN1–3) (13). If limited lymphatic metastasis is properly treated, the prognosis remains good (37). In most cases, local recurrences respond well to treatment and have little negative impact on prognosis for survival (20). In case of a lymph node recurrence, tumor-specific survival deteriorates to levels below 40% (20). Extensive lymph node metastasis can only be cured if the patients responds well to neoadjuvant chemotherapy (38). The prognosis for patients with systemic metastasis remains extremely poor.
HPV carcinogenesis and vaccination
By analogy with the vaccination in girls, it is reasonable to assume that HPV vaccination in boys can prevent the development of a portion of penile cancer (as well as anal cancer), even though no related data have yet become available.
Key messages.
Penile squamous cell carcinoma includes numerous histologic subtypes, some of which are HPV-associated, and is frequently an aggressive tumor.
Surgical treatment is based upon the principle: as much radicality as necessary, as much organ preservation as possible.
The treatment of regional inguinal lymph nodes is decisive for the prognosis.
Non-enlarged inguinal lymph nodes contain micrometastases in up to 25% of cases and require, depending on their stage, invasive surgical evaluation.
In cases with limited lymph node metastasis, radical lymphadenectomy with adjuvant chemotherapy represents a potentially curative treatment option.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours Union for International Cancer Control; Oxford. John Wiley & Sons. 2017 [Google Scholar]
- 2.Erbersdobler A. [Pathology and histopathological evaluation of penile cancer] Urologe. 2018;57:391–397. doi: 10.1007/s00120-018-0592-8. [DOI] [PubMed] [Google Scholar]
- 3.Robert Koch-Institut und Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V Krebs in Deutschland für 2013/2014. www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2017/krebs_in_deutschland_2017.pdf?__blob=publicationFile (last accessed on 8 August 2018) Berlin: 2017. [Google Scholar]
- 4.de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attalla K, Paulucci DJ, Blum K, et al. Demographic and socioeconomic predictors of treatment delays, pathologic stage, and survival among patients with penile cancer: a report from the national cancer database. Urol Oncol. 2018;36:14 e17–14 e24. doi: 10.1016/j.urolonc.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhoeven RH, Janssen-Heijnen ML, Saum KU, et al. Population-based survival of penile cancer patients in Europe and the United States of America: no improvement since 1990. Eur J Cancer. 2013;49:1414–1421. doi: 10.1016/j.ejca.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Compérat E. Pathology of penile cancer. Eur Urol Suppl. 2018;17:132–137. [Google Scholar]
- 8.Kakies C, Lopez-Beltran A, Comperat E, et al. Reproducibility of histopathologic tumor grading in penile cancer-results of a European project. Virchows Arch. 2014;464:453–461. doi: 10.1007/s00428-014-1548-z. [DOI] [PubMed] [Google Scholar]
- 9.Hakenberg OW, Comperat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015;67:142–150. doi: 10.1016/j.eururo.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 10.van Poppel H, Watkin NA, Osanto S, et al. Penile cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;(6):115–124. doi: 10.1093/annonc/mdt286. [DOI] [PubMed] [Google Scholar]
- 11.Clark PE, Spiess PE, Agarwal N, et al. Penile cancer: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2013;11:594–615. doi: 10.6004/jnccn.2013.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakobsen JK, Jensen JB. DaPeCa-2: implementation of fast-track clinical pathways for penile cancer shortens waiting time and accelerates the diagnostic process—a comparative before-and-after study in a tertiary referral centre in Denmark. Scand J Urol. 2016;50:80–87. doi: 10.3109/21681805.2015.1077472. [DOI] [PubMed] [Google Scholar]
- 13.Kirrander P, Sherif A, Friedrich B, et al. Swedish National Penile Cancer Register: incidence, tumour characteristics, management and survival. BJU Int. 2016;117:287–292. doi: 10.1111/bju.12993. [DOI] [PubMed] [Google Scholar]
- 14.Manjunath A, Brenton T, Wylie S, et al. Topical therapy for non-invasive penile cancer (Tis)-updated results and toxicity. Transl Androl Urol. 2017;6:803–808. doi: 10.21037/tau.2017.06.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shabbir M, Muneer A, Kalsi J, et al. Glans resurfacing for the treatment of carcinoma in situ of the penis: surgical technique and outcomes. Eur Urol. 2011;59:142–147. doi: 10.1016/j.eururo.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Imamura M, MacLennan S, Lam TBL, et al. Surgical management for localised penile cancer. Cochrane Database Syst Rev. 2015;3 CD011533. [Google Scholar]
- 17.Chipollini J, Yan S, Ottenhof SR, et al. Surgical management of penile carcinoma in situ: results from an international collaborative study and review of the literature. BJU Int. 2018;121:393–398. doi: 10.1111/bju.14037. [DOI] [PubMed] [Google Scholar]
- 18.Tang DH, Yan S, Ottenhof SR, et al. Glansectomy as primary management of penile squamous cell carcinoma: an international study collaboration. Urology. 2017;109:1040–1043. doi: 10.1016/j.urology.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040–1043. doi: 10.1111/j.1464-410X.2005.05769.x. [DOI] [PubMed] [Google Scholar]
- 20.Leijte JA, Kirrander P, Antonini N, et al. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow-up based on a two-centre analysis of 700 patients. Eur Urol. 2008;54:161–168. doi: 10.1016/j.eururo.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Zouhair A, Coucke PA, Jeanneret W, et al. Radiation therapy alone or combined surgery and radiation therapy in squamous-cell carcinoma of the penis? Eur J Cancer. 2001;37:198–203. doi: 10.1016/s0959-8049(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 22.Crook J, Ma C, Grimard L. Radiation therapy in the management of the primary penile tumor: an update. World J Urol. 2009;27:189–196. doi: 10.1007/s00345-008-0309-5. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Zhu Y, Zhang SL, et al. Organ-sparing surgery for penile cancer: complications and outcomes. Urology. 2011;78:1121–1124. doi: 10.1016/j.urology.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Crook J, Grimard L, Pond G, et al. Penile brachytherapy: results for 60 patients. Urology. 2007;70:161–165. [Google Scholar]
- 25.Escande A, Haie-Meder C, Mazeron R, et al. Brachytherapy for conservative treatment of invasive penile carcinoma: prognostic factors and long-term analysis of outcome. Int J Radiat Oncol Biol Phys. 2017;99:563–570. doi: 10.1016/j.ijrobp.2017.02.090. [DOI] [PubMed] [Google Scholar]
- 26.Persson B, Sjödin JG, Holmberg L, et al. The national penile cancer register in Sweden 2000-2003. Scand J Urol Nephrol. 2007;41:278–282. doi: 10.1080/00365590601183709. [DOI] [PubMed] [Google Scholar]
- 27.O‘Brien JS, Perera M, Manning T, et al. Penile cancer: contemporary lymph node management. J Urol. 2017;197:1387–1395. doi: 10.1016/j.juro.2017.01.059. [DOI] [PubMed] [Google Scholar]
- 28.Zou ZJ, Liu ZH, Tang LY, et al. Radiocolloid-based dynamic sentinel lymph node biopsy in penile cancer with clinically negative inguinal lymph node: an updated systematic review and meta-analysis. Int Urol Nephrol. 2016;48:2001–2013. doi: 10.1007/s11255-016-1405-x. [DOI] [PubMed] [Google Scholar]
- 29.Necchi A, Pond GR, Raggi D, et al. Clinical outcomes of perioperative chemotherapy in patients with locally advanced penile squamous-cell carcinoma: results of a multicenter analysis. Clin Genitourin Cancer. 2017;15:548–555. doi: 10.1016/j.clgc.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Necchi A, Lo Vullo S, Nicolai N, et al. Prognostic factors of adjuvant taxane, cisplatin, and 5-fluorouracil chemotherapy for patients with penile squamous cell carcinoma after regional lymphadenectomy. Clin Genitourin Cancer. 2016;14:518–523. doi: 10.1016/j.clgc.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Nicolai N, Sangalli LM, Necchi A, et al. A combination of cisplatin and 5-fluorouracil with a taxane in patients who underwent lymph node dissection for nodal metastases from squamous cell carcinoma of the penis: treatment outcome and survival analyses in neoadjuvant and adjuvant settings. Clin Genitourin Cancer. 2016;14:323–330. doi: 10.1016/j.clgc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Dickstein RJ, Munsell MF, Pagliaro LC, et al. Prognostic factors influencing survival from regionally advanced squamous cell carcinoma of the penis after preoperative chemotherapy. BJU Int. 2016;117:118–125. doi: 10.1111/bju.12946. [DOI] [PubMed] [Google Scholar]
- 33.Hakenberg OW, Nippgen JB, Froehner M, et al. Cisplatin, methotrexate and bleomycin for treating advanced penile carcinoma. BJU Int. 2006;98:1225–1227. doi: 10.1111/j.1464-410X.2006.06496.x. [DOI] [PubMed] [Google Scholar]
- 34.Pizzocaro G, Nicolai N, Milani A. Taxanes in combination with cisplatin and fluorouracil for advanced penile cancer: preliminary results. Eur Urol. 2009;55:546–551. doi: 10.1016/j.eururo.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Pizzocaro G, Piva L. Adjuvant and neoadjuvant vincristine, bleomycin, and methotrexate for inguinal metastases from squamous cell carcinoma of the penis. Acta Oncol. 1988;27:823–824. doi: 10.3109/02841868809094366. [DOI] [PubMed] [Google Scholar]
- 36.Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol. 2010;28:3851–3857. doi: 10.1200/JCO.2010.29.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djajadiningrat RS, Graafland NM, van Werkhoven E, et al. Contemporary management of regional nodes in penile cancer-improvement of survival? J Urol. 2014;191:68–73. doi: 10.1016/j.juro.2013.07.088. [DOI] [PubMed] [Google Scholar]
- 38.Necchi A, Lo Vullo S, Perrone F, et al. First-line therapy with dacomitinib, an orally available pan-HER tyrosine kinase inhibitor, for locally advanced or metastatic penile squamous cell carcinoma: results of an open-label, single-arm, single-centre, phase 2 study. BJU Int. 2018;121:348–356. doi: 10.1111/bju.14013. [DOI] [PubMed] [Google Scholar]