Abstract
Purpose
The purpose of this study is to explore the differences in ocular symptoms and signs between Sjögren’s syndrome (SS) and non-SS aqueous-deficient dry eye (ADDE) patients.
Methods
Twenty-two eyes of 22 SS patients (Group 1) and 22 eyes of 22 non-SS ADDE patients (Group 2) were enrolled. The evaluated variables included the Standard Patient Evaluation of Eye Dryness (SPEED), the Ocular Surface Disease Index (OSDI), tear meniscus height, first and average non-invasive keratographic breakup time (fNIKBUT and avNIKBUT), Schirmer I test, lipid layer thickness (LLT), meibomian gland expressibility, Marx line, corneal staining, conjunctival congestion, incomplete blinking, and meibomian gland dropout using two novel, non-invasive instruments, the Keratograph and LipiView II.
Results
Ocular signs of the NIKBUT (fNIKBUT: 3.8 (2.7, 5.2)s and 6.3 (3.7, 8.9)s, P = 0.024; avNIKBUT: 5.4 (4.5, 8.9)s and 7.6 (5.8, 13.7)s, P = 0.041), meibomian gland dropout of the upper eyelid (35.5% (29.1%, 54.8%) and 21.9% (16.7%, 24.9%), P = 0.000), and corneal staining (P = 0.050) were more severe but were associated with less severe symptoms, i.e., a lower SPEED score (P = 0.001), in SS subjects than in non-SS subjects.
Conclusion
SS patients exhibit more severe meibomian gland destruction of the upper eyelid than non-SS patients. Meibomian gland dysfunction is another key cause of SS-associated dry eye.
Introduction
Dry eye disease was defined by the 2017 International Dry Eye Workshop as the following: “Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.” It is usually classified into two categories: evaporative dry eye, which primarily occurs because of a lack of the lipid layer of the tear film, and aqueous-deficient dry eye (ADDE), in which tear production is reduced, and Sjögren’s syndrome (SS) is a major subgroup [1]. SS is a chronic inflammatory autoimmune disease that is characterized by chronic infiltration of the exocrine glands, including the salivary and lacrimal glands [2]. The mechanism underlying SS dry eye currently remains unclear, but it is generally considered to be caused by activated T-cell infiltration of the lacrimal gland and the accompanying lacrimal hyposecretion [3].
Historically, the lack of aqueous tear production resulting from lacrimal changes in SS has been the focus of many research studies. However, meibomian gland dysfunction (MGD) is also associated with SS [4] and meibomian gland dropout in SS is more severe than in healthy individuals or non-SS dry eye patients [5, 6]. Various novel non-invasive instruments have recently been introduced to evaluate dry eye disease, including the Keratograph and the LipiView interferometer. Keratograph 5 M uses infrared light (IR), eliminating the fluorescein instillation that may disturb the ocular surface and shorten the result of breakup time (BUT), and can provide a quantitative assessment of tear meniscus height (TMH) and non-invasive keratographic BUT (NIKBUT) [7]. LipiView interferometry illuminates the patient’s eye with white light passing through the tear film and creates IR and transillumination images through dynamic illumination and adaptive transillumination to provide meibography [8], which provides a multidimensional and high-definition view of the meibomian gland structure. It can also objectively and quantitatively measure the lipid layer thickness (LLT) of a defined area of tear film and capture the blink profile within 20 s [9].
To date, few studies have focused on the meibography of SS patients [7, 10]. Thus, in our study, a thorough assessment of dry eye symptoms and signs, especially MGD problems in such patients, was performed non-invasively using a Keratograph and LipiView interferometry, and the differences in ocular symptoms and signs between SS and non-SS ADDE patients were explored.
Subjects and methods
Subjects
In this prospective case–control study, patients were recruited from the Department of Ophthalmology and Rheumatology of Guangdong General Hospital between March 2016 and June 2016. Twenty-two eyes of 22 patients with SS were examined (SS group: 1 male and 21 females; mean age: 50.1 ± 11.8 years). The diagnosis of SS was made according to the American–European Consensus Group [11]. Twenty-two ADDE eyes of 22 age- and gender-matched patients without SS were studied as control subjects (non-SS ADDE group: 1 male and 21 females; mean age: 49.6 ± 11.7 years). All enrolled eyes had decreased tear production ( ≤ 5 mm in the Schirmer I test), which was regarded to be ADDE. Subjects were excluded if they were pregnant or lactating or if they had a recent ocular infection, worn contact lenses, or a history of ocular surgery, lid abnormalities, use of sex-steroid medicine within the past 2 years, or other diseases that may induce dry eye, such as Steven–Johnson syndrome, vitamin A deficiency, Wegener’s granulomatosis, or any type of cancer. All the subjects were required to refrain from using any eye drops for at least 1 week before the examination. None of the subjects had received punctual plug insertion or any surgical procedures of the eye. This study was approved by the Research Ethics Committee of Guangdong General Hospital, and informed consent was obtained from each subject according to the tenets of the Declaration of Helsinki. All assessments were performed by the same ophthalmologist.
Questionnaires for ocular symptoms
The Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire [12] and the Ocular Surface Disease Index (OSDI) [13] were used to identify eye discomfort in the patients. The SPEED questionnaire was designed to explore the frequency and severity of ocular symptoms, including eye dryness, grittiness or scratchiness, soreness or irritation, burning or watering, and eye fatigue, within the past 3 months. The OSDI questionnaire provides a method to assess dry eye symptoms, vision-related functions, and environmental triggers during the past week. The SPEED score ranged from 0 to 28, whereas the OSDI score ranged from 0 to 100. Higher scores for either of the two questionnaires indicated more severe symptoms.
TMH, NIKBUT, and bulbar redness
A Keratograph 5 M (OCULUS, Wetzlar, Germany) was used to non-invasively quantify some signs of the ocular surface, including the TMH (temporal, central, and nasal); the NIKBUT, comprising the first NIKBUT (fNIKBUT), the average NIKBUT (avNIKBUT), and the NIKBUT level; and the level of bulbar redness. The NIKBUT level was classified as follows: Normal 0 (fNIKBUT not < 10 s and avNIKBUT not < 14 s); Borderline 1 (between levels 0 and 2); or Dry eye 2 (fNIKBUT not > 5 s and avNIKBUT not > 6 s). The score for bulbar redness ranged from 0 to 4, which is provided by the instrument itself through comparing the photos captured with those standard pictures stored in the programme. Higher scores indicate greater severity of conjunctive congestion.
LLT and blinking pattern
LipiView II (TearScience, Inc., Morrisville, NC), an interferometer that was introduced in 2011, was used to non-invasively quantify the signs of MGD. LipiView interferometry was applied to evaluate the tear film LLT and to monitor blinking patterns by capturing 20 s videos. The LLT results were converted from interferometric colour units into nanometres and the numbers of incomplete blinks and total blinks were calculated [14].
Sodium fluorescein dye: corneal staining, Marx line
Corneal fluorescein staining and Marx line evaluation were performed by placing a fluorescent dye-impregnated strip (Jingming, Tianjin, China) that was wet with a drop of preservative-free normal saline in the lower conjunctiva sac and then observing the dye with the aid of blue light illumination from a slit-lamp. The corneal fluorescein staining grade was classified as follows: 0 = no staining; 1 = less than 5 dots; 2 = between 1 and 3; and 3 = bulk or strip staining. The corneas were divided into four quadrants (superior temporal, inferior temporal, superior nasal, and inferior nasal), and each quadrant was scored separately and summed to a final score for each eye (total scores, 0–12) [15]. The Marx line is the mucocutaneous junction of the lid that can also be stained by fluorescein [16].The location of the Marx line was described in relation to the orifices of the meibomian glands in the lower lid as completely posterior to the glands (grade 0), less than half of the parts touching the glands (grade 1), most parts running though the glands (grade 2), or completely anterior to the glands (grade 3), as described by Yamaguchi et al. [17].
Schirmer I test
The patients were asked to rest for 10 min. Then, a Schirmer I test strip (30 mm; Jingming) was placed into in the midlateral portion of the lower fornix without the use of anesthesia for 5 min. During this procedure, the patients were required to close their eyes. The wetting length of the strip was recorded.
Meibomian gland expressibility and secretion quality
The Meibomian Gland Evaluator (MGE; TearScience, Inc.) was used to evaluate meibomian gland secretions behind the slit-lamp by using the device to apply consistent, gentle pressure for 10–15 s, similar to the pressure of a normal blink. Five glands were evaluated in the temporal, central, and nasal regions of the lower eyelid, as described by Korb and Blackie [18]. Meibum secreted from each gland was scored from 0 to 3, corresponding to no secretion (grade 0), inspissated (i.e., toothpaste-like) secretion (grade 1), cloudy secretion (grade 2), or clear secretion (grade 3), and the total scores (0 to 45) were calculated.
Meibography
Dynamic Meibomian Imaging of the upper and lower eyelids was performed using a LipiView II interferometer (http://www.tearscience.com). Dynamic illumination and adaptive transillumination were also performed for the lower eyelid to obtain a multidimensional view of the meibomian gland structure. Meibomian gland dropout rate was measured by using ImageJ as described by Pult and Nichols [19].
Statistics
All data are described as the median (25% interquartile, 75% interquartile). The Mann–Whitney non-parametric test was applied to compare examinations between the SS and non-SS ADDE groups. The Statistical Package for the Social Sciences software package (SPSS, version 19.0, Inc., USA) was used for the statistical analysis. A P-value of ≤ 0.05 was accepted as statistically significant.
Results
In this study, 22 eyes of each group were enrolled, and all the participants completed the whole examination. None of these patients experienced any discomfort or pain during the examination.
Ocular symptoms were milder in SS patients than in non-SS patients
The SPEED scores were significantly lower in the SS group than in the non-SS group (5.0 (0.5, 7.3) and 9.5 (7.8, 12.0), P = 0.001), whereas the OSDI scores were not significantly different between the two groups (P = 0.83) (Table 1).
Table 1.
Comparison of ocular symptoms and signs associated with dry eye between the SS and non-SS ADDE groups
| SS (n = 22) | Non-SS ADDE (n = 22) | P-value | |
|---|---|---|---|
| SPEED score | 5.0 (0.5, 7.3) | 9.5 (7.8, 12.0) | 0.001a |
| OSDI score | 30.0 (0, 59.75) | 31.5 (25.0, 50.0) | 0.83 |
| Schirmer I test (mm/5 min) | 5.0 (2.8, 5.0) | 5.0 (3.0, 5.0) | 0.82 |
| Temporal TMH (mm) | 0.25 (0.18, 0.33) | 0.26 (0.19, 0.33) | 0.74 |
| Central TMH (mm) | 0.20 (0.16, 0.23) | 0.20 (0.18, 0.24) | 0.90 |
| Nasal TMH (mm) | 0.24 (0.17, 0.30) | 0.26 (0.21, 0.31) | 0.47 |
| First NIKBUT (s) | 3.8 (2.7, 5.2) | 6.3 (3.7, 8.9) | 0.024a |
| Average NIKBUT (s) | 5.4 (4.5, 8.9) | 7.6 (5.8, 13.7) | 0.041a |
| NIKBUT level | 2.0 (1.0, 2.0) | 1.0 (0.8, 2.0) | 0.03a |
| MG dropout of upper eyelid (%) | 35.5 (29.1, 54.8) | 21.9 (16.7, 24.9) | 0.000a |
| MG dropout of lower eyelid (%) | 39.3 (22.9, 54.9) | 30.3 (18.6, 48.3) | 0.14 |
| LLT (nm) | 68.5 (46.8, 98.3) | 68.0 (55.0, 86.5) | 0.94 |
| MGE score | 10.5 (6.0, 15.8) | 6.0 (2.0, 12.0) | 0.053 |
| Marx line | 1.0 (0, 2.0) | 0.5 (0, 1.0) | 0.22 |
| Conjunctival congestion | 1.3 (1.0, 1.5) | 1.0 (0.9, 1.4) | 0.14 |
| Fluorescein staining | 1.0 (0,2.5) | 0 (0, 0.5) | 0.050a |
| Incomplete blinking (%) | 25.0 (3.9, 59.5) | 43.1 (25.0, 83.9) | 0.13 |
ADDE, aqueous-deficient dry eye; LLT, lipid layer thickness; MGE, Meibomian gland evaluator; NIKBUT, non-invasive breakup time; SS, Sjögren’s syndrome; TMH, tear meniscus height.
a Statistically significant.
Secretion of tears
Tear production by all enrolled eyes was less than 5 mm in 5 min according to the Schirmer I test, and values were similar between the two groups (P = 0.82) (Table 1). TMH was not significantly different between the groups (Table 1), either.
Evaluation of meibomian glands
The NIKBUT and meibomian gland dropout of the upper eyelid significantly differed between the two groups. In the SS group, both fNIKBUT and avNIKBUT were significantly shorter and the level of NIKBUT was higher than in the non-SS group (3.8 (2.7, 5.2) s and 6.3 (3.7, 8.9) s, P = 0.024; 5.4 (4.5, 8.9) s and 7.6 (5.8, 13.7) s, P = 0.041; 2.0 (1.0, 2.0) and 1.0 (0.8, 2.0), P = 0.03, respectively) (Table 1). Meibomian gland dropout of the upper eyelid was more severe in the SS patients than in the non-SS patients (35.5% (29.1%, 54.8%) and 21.9% (16.7%, 24.9%), P = 0.000), whereas that of the lower eyelid was much more severe but with no significant difference (P = 0.14) (Table 1). The LLT, MGE score, and grade of the Marx line exhibited no significant differences between the two groups (P = 0.94; P = 0.053; P = 0.22, respectively) (Table 1).
Meibomian gland dropout
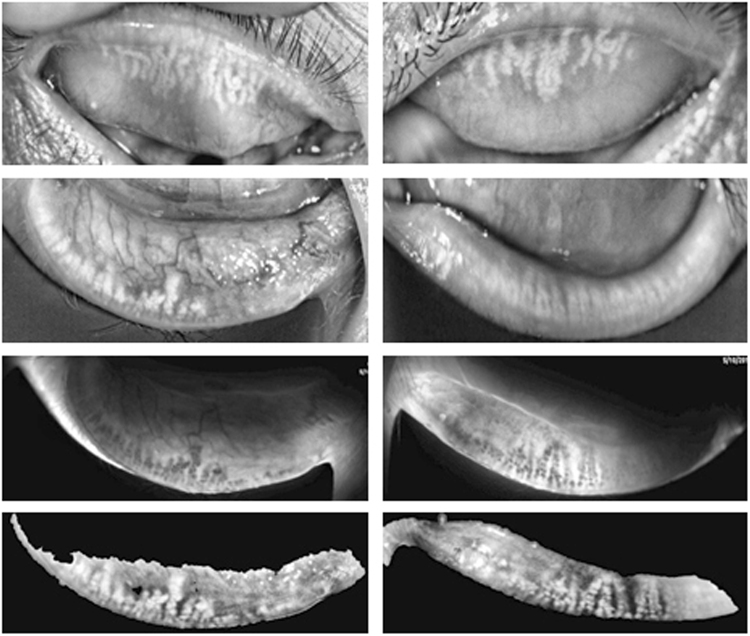
As described by Arita et al. [20], the grade of meibomian gland dropout was divided as 0% (grade 0), < 33% (grade 1), 33–67% (grade 2), or > 67% (grade 3). Meibomian gland dropout grading of the upper and lower eyelids is described in detail in Table 2 and Table 3. Two (9.1%) upper eyelids and 4 (18.2%) lower eyelids among the 22 eyes exhibited gland dropout, accounting for more than 2/3 of the tarsus in the SS group; this was not observed in the non-SS group. The meibography of a SS patient with severe meibomian gland dropout is shown in Fig. 1.
Table 2.
Meibomian gland dropout of the upper eyelid in SS and non-SS ADDE subjects
| Degree | SS | Non-SS |
|---|---|---|
| 0 | 1 (4.5%) | 3(13.6%) |
| 1 | 7 (31.9%) | 17 (77.3%) |
| 2 | 12 (54.5%) | 2 (9.1%) |
| 3 | 2 (9.1%) | 0 |
Table 3.
Meibomian gland dropout of the lower eyelid in SS and non-SS ADDE subjects
| Degree | SS | Non-SS |
|---|---|---|
| 0 | 2 (9.1%) | 2 (9.1%) |
| 1 | 7 (31.8%) | 12 (54.5%) |
| 2 | 9 (40.9%) | 8 (36.4%) |
| 3 | 4 (18.2%) | 0 |
Fig. 1.
Meibography of a SS patient with severe meibomian gland dropout
Epithelial injury of the ocular surface
The corneal fluorescein staining scores were significantly greater in the SS group than in the non-SS group (1.0 (0, 2.5) and 0 (0, 0.5), respectively, P = 0.05), whereas the severity of conjunctival congestion was similar between the two groups (P = 0.14) (Table 1).
Blinking pattern
The ratio of incomplete blinking exhibited no significant difference between the two groups (P = 0.13) (Table 1).
Discussion
This study focused on meibomian gland function in SS as a potential additional cause of SS dry eye. To our knowledge, this is the first report to demonstrate that meibomian gland dropout of the upper eyelid was vastly greater in SS patients than in other dry eye patients. To date, few studies have reported meibography of SS patients. In 1998, Shimazaki et al. [10] first reported that meibomian gland dropout of the lower eyelid was greater in SS patients than in non-SS dry eye patients, and 11 (57.9%) of 19 eyes exhibited gland dropout in more than half of the tarsus, but they neglected gland dropout of the upper eyelid. Menzies et al. [6] and Kang et al. [21] demonstrated that meibomian gland dropout was greater in SS patients’ eyes than that in normal eyes overall (upper + lower), but this result was not demonstrated for separate areas. In conventional methods, meibomian gland expressibility is assessed by applying digital pressure on the upper tarsus, and in previous studies using this method, positive results were obtained [6, 11]. In agreement with the previous results, destruction of the meibomian gland in the upper tarsus was significantly more severe in SS patients than in non-SS ADDE patients. In this study, the varieties of lower eyelid measurements, such as gland dropout, MGE score and Marx line, exhibited no significant differences between SS patients and non-SS control patients; this finding is similar to that for LLT. Pult et al. [22] found significantly less meibomian gland dropout in the upper lids than in the lower lids in MGD patients. In addition, undeniably in this study, another main reason for dry eye in non-SS patients was MGD, which also had gland destruction in the lower eyelid. Thus, we suspect that destruction of the meibomian gland in SS involves both of the upper and lower eyelids, which might explain the obvious meibomian gland dropout observed in the upper eyelid. The difference of meibomian gland expressibility and secretion quality of the upper eyelid between the SS and non-SS dry eye groups still need to be explored in the future. fNIKBUT and avNIKBUT were obviously reduced in SS and this result has been reported in several studies [6, 24]. Wang et al. [23] examined 71 Chinese SS cases and found that BUT reduction occurred in all SS dry eyes. Moreover, in this study, it was shown that more severe meibomian gland dropout and shorter NIKBUT in SS patients than in non-SS patients but similar LLT. This might imply that the remaining meibomian glands in SS patients could supply sufficient LLT but that the lipid layer was of poor quality. Therefore, this lipid layer in SS patients might evaporate more easily.
There has been minimal research regarding the mechanism of MGD in SS dry eye. However, there are two possible theories. First, the presence of lymphocyte infiltration around the meibomian gland contributing to the hyperkeratinization of ductal epithelium at meibomian gland orifices may be responsible for the obstructive MGD in SS patients. Villani et al. [24] reported that SS subjects exhibited more periglandular inflammation and higher secretion reflectivity of the meibomian glands than non-SS MGD subjects. Meibomian glands in SS observed under in vivo confocal laser microscopy exhibited higher acinar density, smaller diameters, greater density of periglandular inflammatory cells, and lower secretion reflectivity than those in non-SS MGD patients. Second, oestrogen and androgen receptors on the meibomian glands are key regulators of secretion by these glands. Reduced androgen and increased oestrogen in the serum of SS patients are believed to contribute to hyposecretory MGD [25, 26].
In addition, the results of this study indicate that SS patients have a higher degree of MGD with an inconsistent lesser symptom of ocular dryness than non-SS ADDE patients. First, the patients in the non-SS group who were from the ophthalmological clinic were inherently experiencing dry eye symptoms, while dry eye symptoms might not be a severe issue for the patients in SS group who were from the Department of Rheumatism. A second reason may be corneal nerve destruction in the SS patients. McNamara et al. [27] and Bianciardi et al. [28] recently proposed that corneal nerve fibre density and length were significantly decreased in SS patients and Labbe et al. [29] also obtained similar results for non-SS dry eye patients compared with healthy individuals, but no studies have reported those items for SS and non-SS patients. We hypothesized that the corneal nerve fibre density might be lower in SS patients, such that corneal sensitivity decreased and less symptoms occurred than in non-SS patients.
Given that SS is a subgroup of aqueous-deficient dry eye, the eyes in this study were enrolled based on tear production measuring 5 mm or less according to the Schirmer I test without anaesthesia to reduce the effect of aqueous tears between the two groups. Therefore, the Schirmer I test and the TMH between these two groups were almost the same.
Corneal staining was more severe in the SS patients and this finding is consistent with the results of Shimazaki et al. [10] and Goto et al. [5]. However, the conjunctival congestion did not differ between the two groups. We suspect that inflammatory injury in SS might concentrate on the epithelium and have little effect on angiectasis, which should be confirmed in a further study that includes additional cases.
The findings in this study should be viewed with several limitations in mind. First, meibography can be affected by several factors, such as the patient’s cooperation, the tightness of the eyelids, oedema or relaxation of the conjunctiva, and orbital fat prolapse. In addition, this study did not assess the expressibility or secretion quality of the meibomian gland of the upper eyelid. Furthermore, the sample size was limited. More cases and further studies are necessary to confirm the results.
In summary, two novel non-invasive quantitative instruments, the Keratograph and LipiView II, were applied in this study to accurately assess the meibomian gland function of SS patients. In this prospective age- and gender-matched case–control study, meibomian gland dropout of the upper eyelid was more obvious in SS subjects than in non-SS subjects, and we propose that the changes in the upper eyelid are responsible for the differences between SS and non-SS subjects.
Summary
What was known before
• Although meibomian gland dysfunction in Sjögren’s syndrome (SS) patients has been previously reported, the severity of MGD in SS patients compared with that in non-SS aqueous tear deficiency patients was investigated in this study.
What this study adds
• To our knowledge, no previous studies have compared lipid layer thickness (LLT) in aqueous tear-deficient patients with or without SS using LipiView II. Although LLT did not differ between the groups, other evaluations showed that MGD was worse in SS.
Acknowledgements
This study was supported by a grant from the Development Center for Medical Science and Technology National Health and the Family Planning Commission of the People’s Republic of China, Diagnosis and Treatment of Meibomian Gland Dysfunction Multicenter Research Project (No. 201542), and Guangdong Medical Research Funded Project, Guangzhou, China (B2018005). The funding organizations had no role in the design or conduct of this research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Craig JP, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Barone F, Colafrancesco S. Sjogren’s syndrome: from pathogenesis to novel therapeutic targets. Clin Exp Rheumatol. 2016;34:58–62. [PubMed] [Google Scholar]
- 3.Bron AJ, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Foulks GN, et al. Clinical guidelines for management of dry eye associated with Sjogren disease. Ocul Surf. 2015;13:118–32. doi: 10.1016/j.jtos.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Goto E, et al. Tear evaporation rates in Sjogren syndrome and non-Sjogren dry eye patients. Am J Ophthalmol. 2007;144:81–85. doi: 10.1016/j.ajo.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 6.Menzies KL, Srinivasan S, Prokopich CL, Jones L. Infrared imaging of meibomian glands and evaluation of the lipid layer in Sjogren’s syndrome patients and nondry eye controls. Invest Ophthalmol Vis Sci. 2015;56:836–41. doi: 10.1167/iovs.14-13864. [DOI] [PubMed] [Google Scholar]
- 7.Abdelfattah NS, Dastiridou A, Sadda SR, Lee OL. Noninvasive imaging of tear film dynamics in eyes with ocular surface disease. Cornea. 2015;34(Suppl 10):S48–52. doi: 10.1097/ICO.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 8.Gulati S, Jain S. Ocular pharmacology of tear film, dry eye, and allergic conjunctivitis. Handb Exp Pharmacol. 2017;242:97–118. doi: 10.1007/164_2016_73. [DOI] [PubMed] [Google Scholar]
- 9.Geerling G, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: proceedings of the OCEAN group meeting. Ocul Surf. 2017;15:179–92. doi: 10.1016/j.jtos.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Shimazaki J, Goto E, Ono M, Shimmura S, Tsubota K. Meibomian gland dysfunction in patients with Sjogren syndrome. Ophthalmology. 1998;105:1485–8. doi: 10.1016/S0161-6420(98)98033-2. [DOI] [PubMed] [Google Scholar]
- 11.Vitali C, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngo W, et al. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32:1204–10. doi: 10.1097/ICO.0b013e318294b0c0. [DOI] [PubMed] [Google Scholar]
- 13.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, et al. Assessment of the tear film lipid layer thickness after cataract surgery. Semin Ophthalmol. 2018;33:231–6. doi: 10.1080/08820538.2016.1208764. [DOI] [PubMed] [Google Scholar]
- 15.Qiu W, et al. Evaluation of the effects of conjunctivochalasis excision on tear stability and contrast sensitivity. Sci Rep. 2016;6:37570. doi: 10.1038/srep37570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bron AJ, Yokoi N, Gaffney EA, Tiffany JM. A solute gradient in the tear meniscus. I. A hypothesis to explain Marx’s line. Ocul Surf. 2011;9:70–91. doi: 10.1016/S1542-0124(11)70014-3. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi M, et al. Marx line: fluorescein staining line on the inner lid as indicator of meibomian gland function. Am J Ophthalmol. 2006;141:669–75. doi: 10.1016/j.ajo.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27:1142–7. doi: 10.1097/ICO.0b013e3181814cff. [DOI] [PubMed] [Google Scholar]
- 19.Pult H, Nichols JJ. A review of meibography. Optom Vis Sci. 2012;89:E760–69. doi: 10.1097/OPX.0b013e3182512ac1. [DOI] [PubMed] [Google Scholar]
- 20.Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–5. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Kang YS, Lee HS, Li Y, Choi W, Yoon KC. Manifestation of meibomian gland dysfunction in patients with Sjogren’s syndrome, non-Sjogren’s dry eye, and non-dry eye controls. Int Ophthalmol. 2018;38:1161–7. doi: 10.1007/s10792-017-0577-4. [DOI] [PubMed] [Google Scholar]
- 22.Pult H, Riede-Pult BH, Nichols JJ. Relation between upper and lower lids’ meibomian gland morphology, tear film, and dry eye. Optom Vis Sci. 2012;89:E310–5. doi: 10.1097/OPX.0b013e318244e487. [DOI] [PubMed] [Google Scholar]
- 23.Wang YX, Wang XC, Chen Y. [Observation of meibomian gland disease in 71 Sjogren syndrome dry eye cases.] Zhe Jiang Yi Xue. 2013;12:1188–9. [Google Scholar]
- 24.Villani E, et al. In vivo confocal microscopy of meibomian glands in Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2011;52:933–9. doi: 10.1167/iovs.10-5995. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan DA, et al. Androgens and dry eye in Sjogren’s syndrome. Ann N Y Acad Sci. 1999;876:312–24. doi: 10.1111/j.1749-6632.1999.tb07656.x. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan DA, et al. Sex steroids, meibomian gland dysfunction and evaporative dry eye in Sjogren’s syndrome. Lupus. 2002;11:667. doi: 10.1191/0961203302lu275oa. [DOI] [PubMed] [Google Scholar]
- 27.McNamara NA, et al. Reduced levels of tear lacritin are associated with corneal neuropathy in patients with the ocular component of Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2016;57:5237–43. doi: 10.1167/iovs.16-19309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianciardi G, Latronico ME, Traversi C. Entropy of corneal nerve fibers distribution observed by laser scanning confocal microscopy: a noninvasive quantitative method to characterize the corneal innervation in Sjogren’s syndrome patients. Microsc Res Tech. 2015;78:1069–74. doi: 10.1002/jemt.22586. [DOI] [PubMed] [Google Scholar]
- 29.Labbe A, et al. Corneal nerve structure and function in patients with non-sjogren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2013;54:5144–50. doi: 10.1167/iovs.13-12370. [DOI] [PubMed] [Google Scholar]