Abstract
Gut microbiota have been implicated in the development of atherosclerosis and cardiovascular disease. Since the prebiotic inulin is thought to beneficially affect gut microbiota, we aimed to determine the effect of inulin supplementation on atherosclerosis development in APOE*3-Leiden.CETP (E3L.CETP) mice. Female E3L.CETP mice were fed a western-type diet containing 0.1% or 0.5% cholesterol with or without 10% inulin. The effects of inulin were determined on: microbiota composition, cecal short-chain fatty acid (SCFA) levels, plasma lipid levels, atherosclerosis development, hepatic morphology and hepatic inflammation. Inulin with 0.5% dietary cholesterol increased specific bacterial genera and elevated levels of cecal SCFAs, but did not affect plasma cholesterol levels or atherosclerosis development. Surprisingly, inulin resulted in mild hepatic inflammation as shown by increased expression of inflammation markers. However, these effects were not accompanied by increased hepatic macrophage number. Analogously, inulin induced mild steatosis and increased hepatocyte size, but did not affect hepatic triglyceride content. Inulin with 0.1% dietary cholesterol did not affect hepatic morphology, nor hepatic expression of inflammation markers. Overall, inulin did not reduce hypercholesterolemia or atherosclerosis development in E3L.CETP mice despite showing clear prebiotic activity, but resulted in manifestations of hepatic inflammation when combined with a high percentage of dietary cholesterol.
Introduction
Atherosclerosis is a narrowing of arteries due to accumulation of lipids and cells in the intima, leading to cardiovascular diseases (CVD) including heart attack and stroke. CVD are a leading cause of morbidity and mortality in Western Society1. Hypercholesterolemia is one of the main underlying risk factors of atherosclerosis development, and is routinely treated by prescription of statins. However, statin treatment lowers total plasma cholesterol levels by approximately 30%2 and only prevents 25–45% of all cardiovascular events3, indicating the demand for additional therapies. The gut microbiota have been discovered as an important player in the onset of atherosclerosis and CVD4. Disturbances in lipid metabolism, the precursor for the development of atherosclerosis and CVD, have also been associated with gut microbiota dysbiosis in both rodents5 and humans6. A well-known factor that modulates the gut microbiota composition and function are dietary fibers or prebiotics. Inulin is a widely studied prebiotic that has been shown to beneficially modify gut microbiota composition and function7. Inulin is fermented by specific bacteria in the gut, leading to outgrowth of these bacteria and short-chain fatty acid (SCFA) production8, which is thought to improve colonic and systemic health9. Inulin has been shown to exert multiple beneficial effects on the host, including lowering inflammation10,11, as well as decreasing hyperlipidemia12–15. Whether inulin affects the development of atherosclerosis is currently underexplored. Therefore, we set out to determine the effect of inulin on the development of atherosclerosis in hypercholesterolemic APOE*3-Leiden.CETP (E3L.CETP) mice, a model that is characterized by a human-like lipoprotein metabolism and that is susceptible to the development of atherosclerosis. Particularly female E3L.CETP mice are highly sensitive to dietary cholesterol and they respond in a human-like manner to lipid-lowering anti-atherogenic therapies16. We found that inulin drastically altered gut microbiota composition and function. However, inulin neither decreased hypercholesterolemia nor ameliorated atherosclerosis development in this mouse model. Notably, inulin in combination with a high percentage of dietary cholesterol resulted in manifestations of hepatic inflammation.
Results
Inulin modified gut microbiota composition and function
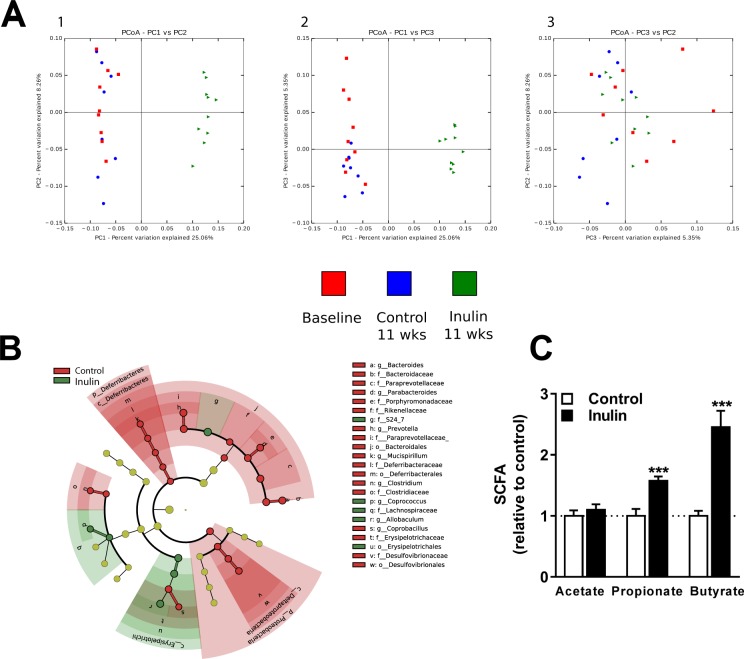
To determine the effects of inulin on gut microbiota in mice fed a WTD with 0.5% cholesterol, 16S rRNA gene sequencing was used to assess the effect of inulin on gut microbiota composition and relative abundance of specific gut microbial taxa. Cluster analysis based on unweighted UniFrac distances revealed a clear difference between the control group and the inulin group after 11 weeks of intervention, while the control group did not change after 11 weeks compared to the baseline measurement (Fig. 1A).
Figure 1.
Inulin modified gut microbiota composition and function. The effect of inulin supplementation on microbiota composition and function in mice fed a WTD containing 0.5% cholesterol ± inulin. (A) Principal coordinates analysis (PCoA) plot of unweighted UniFrac distances metric of 16S rRNA gene sequences at baseline (depicted in red) and for the subsequent control group (depicted in blue) and inulin group (depicted in green) after 11 weeks (wks) of treatment. To evaluate similarities between bacterial communities, graphs 1, 2, and 3 were generated using OTU’s based on the unweighted UniFrac distance metrics PC1 and PC2, PC1 and PC3, and PC3 and PC2, respectively, and are shown on multiple two-dimensional arrays. (B) The cladogram reports the taxa (highlighted by small circles and by shading) showing relative abundance values with a maximum depth to genus level (according to LEfSe). Red or green circles/shading represents taxa that are significantly higher in relative abundance, while yellow indicates taxa which did not differ in relative abundance between the two groups. The taxonomic level is indicated by letter preceding the underscore: o, order; f, family; g, genus. The central point in the cladogram represents the taxonomic domain ‘bacteria’ and each ring outward represents the next lower taxonomic level (phylum to genus). The darker the shading of the red or green colours, the higher the abundance (n = 8–10 mice per group). (C) Finally, the cecal SCFAs acetate, propionate, and butyrate were determined (n = 10 mice per group). Values are presented as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
Further analysis of the gut microbiota revealed clear differences in the composition of the microbial community between the control group and the inulin group. Figure 1B schematically depicts LEfSe’s results included in a cladogram showing the significant differences on each taxonomic level with a maximum depth to genus level. Table 1 shows the relative abundances (%) of genera in the control and the inulin group and the percentage difference between the two groups based on LEfSe and ANCOM analyses. Based on LEfSe analysis, inulin significantly increased the relative abundance of the genera Coprococcus (+409%), and Allobaculum (+833%), whereas the genera Bacteroides (−59%), Parabacteroides (−60%), Prevotella (−88%), Micispirillum (−100%), Clostridium (−100%), and Coprobacillus (−100%) were reduced compared to control mice (Table 1). Based on ANCOM analysis, inulin increased the relative abundance of the genera Coprococcus (+409%), Ruminococcus (+52%), Allobaculum (+833%), and Sutterella (+12%), whereas the genera Mucispirillum (−100%) and Coprobacillus (−100%) were decreased compared to control mice (Table 1). The overlapping genera that significantly increased after inulin supplementation compared to control mice according to both analyses were Allobaculum and Coprococcus.
Table 1.
Relative abundance and the percentage difference of the inulin group (n = 10) and the control group (n = 8) for each genera at 11 weeks of intervention. Data are presented as mean ± standard error of the mean (SEM).
| Phylum | Class | Order | Family | Genus | Control | Inulin | Inulin/Control |
|---|---|---|---|---|---|---|---|
| Abundance (%) | Abundance (%) | % difference | |||||
| Mean ± SEM | Mean ± SEM | Mean ± SEM | |||||
| Bacteroidetes | |||||||
| Bacteroidia | |||||||
| Bacteroidales | |||||||
| Bacteroidaceae | |||||||
| Bacteroides | 15.53 ± 1.87 | 6.33 ± 1.00 | −59%* | ||||
| Porphyromonadaceae | |||||||
| Parabacteroides | 8.88 ± 1.60 | 3.52 ± 0.71 | −60%* | ||||
| Paraprevotellaceae | |||||||
| Prevotella | 17.2 ± 6.29 | 2.1 ± 2.03 | −88% | ||||
| Rikenellaceae | |||||||
| Unidentified | 3.28 ± 0.90 | 0.06 ± 0.02 | −98% | ||||
| S24-7 | |||||||
| Unidentified | 5.54 ± 0.90 | 44.88 ± 2.76 | 710% | ||||
| Unidentified | |||||||
| Unidentified | 3.21 ± 0.68 | 0.03 ± 0.02 | −99% | ||||
| Deferribacteres | |||||||
| Deferribacteres | |||||||
| Deferribacterales | |||||||
| Deferribacteraceae | |||||||
| Mucispirillum | 1.83 ± 0.75 | 0 ± 0 | −100% | ||||
| Firmicutes | |||||||
| Bacilli | |||||||
| Lactobacillales | |||||||
| Lactobacillaceae | |||||||
| Lactobacillus | 5.69 ± 2.06 | 3.27 ± 0.76 | −43% | ||||
| Clostridia | |||||||
| Clostridiales | |||||||
| Clostridiaceae | |||||||
| Clostridium | 1.52 ± 0.70 | 0 ± 0 | −100%* | ||||
| Lachnospiraceae | |||||||
| Coprococcus | 0.38 ± 0.12 | 1.91 ± 0.45 | 403%*$ | ||||
| Ruminococcus | 1.08 ± 0.36 | 1.64 ± 0.44 | 52%$ | ||||
| Ruminococcaceae | |||||||
| Oscillospira | 2.08 ± 0.60 | 0.96 ± 0.21 | −54% | ||||
| Unidentified | 1.48 ± 0.85 | 0.48 ± 0.11 | −68% | ||||
| Unidentified | |||||||
| Unidentified | 14.65 ± 3.37 | 12.85 ± 2.46 | −12% | ||||
| Erysipelotrichi | |||||||
| Erysipelotrichales | |||||||
| Erysipelotrichaceae | |||||||
| Allobaculum | 1.88 ± 0.55 | 17.50 ± 2.30 | 831%*$ | ||||
| Coprobacillus | 1.17 ± 0.44 | 0 ± 0 | −100%*$ | ||||
| Unidentified | 5.86 ± 1.93 | 0 ± 0 | −100% | ||||
| Proteobacteria | |||||||
| Betaproteobacteria | |||||||
| Burkholderiales | |||||||
| Alcaligenaceae | |||||||
| Sutterella | 2.34 ± 0.80 | 2.63 ± 0.55 | 12%$ | ||||
| Deltaproteobacteria | |||||||
| Desulfovibrionale | |||||||
| Desulfovibrionaceae | |||||||
| Unidentified | 4.78 ± 1.54 | 1.52 ± 0.71 | −68% | ||||
| Gammaproteobacteri | |||||||
| Enterobacteriales | |||||||
| Enterobacteriaceae | |||||||
| Unidentified | 1.61 ± 0.75 | 0.32 ± 0.17 | −80% |
*P < 0.05 according to LEfSe analysis; $P < 0.05 according to ANCOM analysis.
Subsequently, we determined the effects of inulin on SCFA levels in the cecum. Inulin increased cecal levels of propionate (+57% vs. control; P = 0.0005) and butyrate (+146% vs. control; P = 0.0002), but not of acetate (Fig. 1C). These data indicate that dietary inulin supplementation modulated both microbial composition and function in E3L.CETP mice.
Inulin neither decreased hyperlipidemia nor ameliorated atherosclerosis development
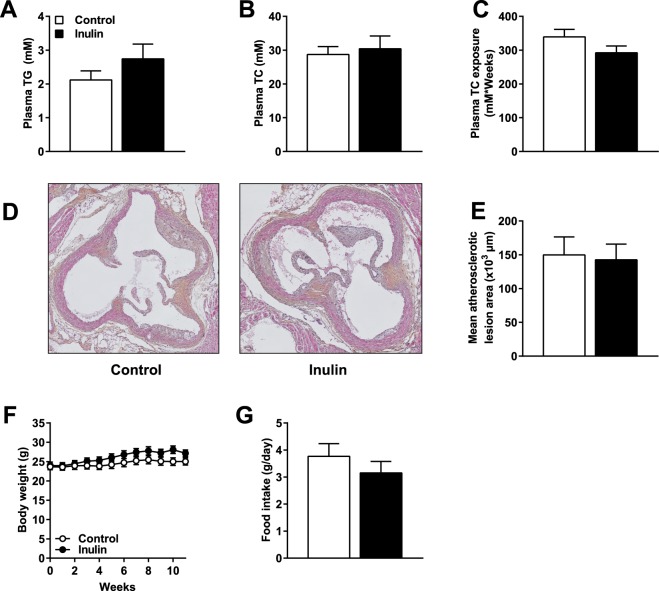
Given the potential beneficial effects of inulin on plasma lipid parameters, we determined the effect of inulin on plasma triglycerides (TG) and total cholesterol (TC) levels in E3L.CETP mice fed a Western-type diet (WTD) containing 0.5% cholesterol ± inulin. Surprisingly, inulin did not affect plasma TG levels (Fig. 2A), plasma TC levels (Fig. 2B), or TC exposure (Fig. 2C). In order to determine whether inulin affected atherosclerosis development, we measured atherosclerosis progression in the aortic root of the heart after 11 weeks of inulin supplementation. Representative images (Fig. 2D) illustrate that inulin did not affect mean atherosclerotic lesion area throughout the aortic root (Fig. 2E). Finally, there was no effect of inulin on body weight (Fig. 2F) or food intake (Fig. 2G) in these mice. These data show that inulin did not beneficially lower plasma lipid levels and did not affect atherosclerosis development in hypercholesterolemic E3L.CETP mice.
Figure 2.
Inulin neither decreased hyperlipidemia nor ameliorated atherosclerosis development. Mice were fed a WTD containing 0.5% cholesterol ± inulin for 11 weeks. (A) Plasma TG levels, (B) plasma TC levels, and (C) cumulative TC exposure were determined in 4 hour fasted mice. (D) Representative cross-sections of the valve area of the aortic root of the heart stained with HPS are shown, and (E) the mean atherosclerotic lesion area from the four consecutive cross-sections. Finally, (F) body weight, and (G) food intake were determined. Values are presented as means ± SEM (n = 10 mice per group). P < 0.05 was considered as statistically significant.
Inulin adversely affected the liver by manifestations of hepatic inflammation
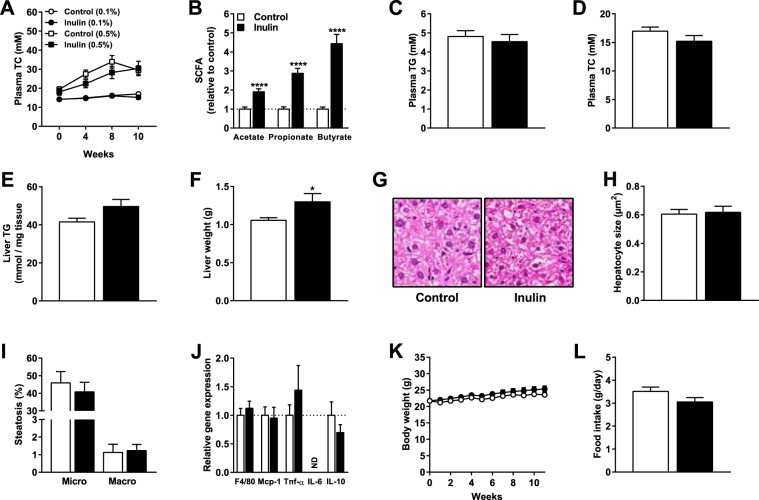
Although inulin did not affect hyperlipidemia and atherosclerosis, we found that inulin increased liver weight in mice fed a WTD containing 0.5% cholesterol (+34% vs. control; P = 0.003; Fig. 3A). We observed notable hepatic morphological derangements in the inulin group (Fig. 3B). Inulin significantly increased hepatic hypertrophy as indicated by increased hepatocyte size (+20% vs. control; P = 0.008; Fig. 3C). Inulin increased microvesicular steatosis (+37% vs. control; P = 0.006) and macrovesicular steatosis (+167% vs. control; P = 0.03)(Fig. 3D), but did not affect hepatic TG content (Fig. 3E). Hepatic gene expression of F4/80 (+65% vs. control; P = 0.002), Mcp-1 (+186% vs. control; P = 0.0009), Tnf-α (+139% vs. control; P = 0.008), and IL-6 (+67% vs. control; P = 0.04) were higher in the inulin group without any effect on IL-10 expression, indicating a pro-inflammatory phenotype in the mice fed inulin (Fig. 3F). However, inulin did not lead to changes in the macrophage marker F4/80 as quantified by immunohistochemical staining (Fig. 3G). These data indicate that inulin in combination with 0.5% dietary cholesterol adversely affected liver morphology and resulted in manifestations of hepatic inflammation in hypercholesterolemic E3L.CETP mice.
Figure 3.
Inulin adversely affected the liver by manifestations of hepatic inflammation Mice were fed a WTD containing 0.5% cholesterol ± inulin for 11 weeks. (A) Liver weight, (B) liver morphology, (C) hepatocyte size as a marker for hepatic hypertrophy, (D) microvesicular and macrovesicular steatosis, (E) liver TG content, (F) qRT-PCR analysis of F4/80, Mcp-1, Tnf-α, IL-6, IL-10, and (G) immunohistochemical analysis of hepatic F4/80 were determined. Values are presented as means ± SEM (n = 10 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
Inulin in combination with 0.1% dietary cholesterol increased SCFAs in cecum content, but did not affect hyperlipidemia or hepatic inflammation
We subsequently determined whether inulin in combination with a more moderate percentage of dietary cholesterol (0.1%) would lead to different outcomes. Using this lower percentage of dietary cholesterol, hypercholesterolemia is induced to a lower extent compared with 0.5% dietary cholesterol. Indeed, 0.5% dietary cholesterol resulted in final plasma TC levels of 27.6 ± 1.5 mM, while 0.1% dietary cholesterol resulted in plasma TC levels of 15.6 ± 0.3 mM (Fig. 4A). Similar to mice fed 0.5% cholesterol, inulin in combination with 0.1% dietary cholesterol significantly elevated levels of propionate (+188% vs. control; P < 0.0001) and butyrate (+344% vs. control; P < 0.0001) in cecum content (Fig. 4B). However, 0.1% dietary cholesterol with inulin also increased levels of acetate in cecum content (+90% vs. control; P < 0.0001; Fig. 4B). Inulin in combination with 0.1% cholesterol did not affect plasma TG levels (Fig. 4C), plasma TC levels (Fig. 4D), or liver TG content (Fig. 4E), but increased liver weight (Fig. 4F) which was similar to mice fed 0.5% cholesterol. However, in contrast to mice fed inulin with 0.5% cholesterol, inulin combined with 0.1% cholesterol did not affect hepatic morphology (Fig. 4G), hepatocyte size (Fig. 4H), microvesicular or macrovesicular steatosis (Fig. 4I), or hepatic gene expression of inflammatory markers (Fig. 4J). Finally, there was neither an effect of inulin on body weight (Fig. 4K) nor on food intake (Fig. 4L) in these mice. These results show that inulin in combination with 0.1% cholesterol also modulated gut microbiota function, did also not affect hypercholesterolemia, but in contrast to inulin with 0.5% cholesterol did not affect liver inflammation.
Figure 4.
Inulin in combination with 0.1% dietary cholesterol increased SCFAs in cecum content, but did not affect hyperlipidemia or hepatic inflammation. Mice were fed a WTD containing 0.1% cholesterol ± inulin for 11 weeks (n = 17 mice per group). (A) Plasma TC levels for control and inulin fed mice on both 0.5% dietary cholesterol (n = 10 mice per group) and 0.1% dietary cholesterol for 0, 4, 8, and 10 weeks of dietary intervention. (B) The cecal SCFAs acetate, propionate, and butyrate, (C) plasma TG levels, (D) plasma TC levels, (E) liver TG content, (F) liver weight, (G) liver morphology, (H) hepatocyte size, (I) microvesicular and macrovesicular steatosis, (J) qRT-PCR analysis of F4/80, Mcp-1, Tnf-α, IL-6, and IL-10, (K) body weight, and (L) food intake were determined. Values are presented as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ***P < 0.0001 vs. control. ND = non-detectable.
Discussion
Our data show that dietary inulin did not reduce atherosclerosis in E3L.CETP mice fed a WTD with 0.5% cholesterol. This may not have been surprising, since plasma lipid levels were not decreased by inulin supplementation. In contrast, inulin in combination with 0.5% cholesterol was associated with manifestations of hepatic inflammation. To further investigate the interaction between dietary cholesterol and inulin on plasma lipid levels and inflammation, we analysed the effect of inulin in combination with 0.1% cholesterol. In this experiment, we found no clear signs of hepatic inflammation but again no effects of inulin on plasma lipid levels. Thus, inulin appears not to have a cholesterol lowering or anti-atherogenic effect in hypercholesterolemic E3L.CETP mice.
Rault-Nania et al.17, found a 35% reduction in atherosclerotic plaque formation in hypercholesterolemic APOE-deficient mice. APOE-deficient mice are characterized by severe hypercholesterolemia due to a virtually completely blocked LDL-receptor mediated lipoprotein remnant clearance18. In contrast, E3L.CETP mice express a dominant variant of human APOE, which results in a moderately disrupted LDL-receptor mediated lipoprotein remnant clearance19. E3L.CETP mice are highly responsive to diet-induced hyperlipidemia and atherosclerosis development and have been widely used as preclinical model to study the effects and underlying mechanisms of various human drugs16. Furthermore, the APOE-deficient mice in the study of Rault-Nania et al. were fed a semi-purified diet based on sucrose, whereas the E3L.CETP mice in our study were fed a WTD. Both mouse models are based on C57BL/6 genetic background. However, whether the differential effects of inulin on atherosclerosis development in APOE-deficient mice versus E3L.CETP mice are mouse model-specific and/or a result of differences in dietary composition and/or housing conditions remain to be determined.
Nevertheless, inulin led to elevations of the SCFAs propionate and butyrate on both 0.1% and 0.5% cholesterol diets in E3L.CETP mice. Both propionate and butyrate have been suggested to have beneficial effects on cholesterol metabolism. Propionate is absorbed in the colon and transported via the portal vein to the liver where it can inhibit cholesterol synthesis20. Butyrate is mainly used as energy substrate for colonocytes, and studies in rats have suggested that ingestion of butyrate may lead to reduced hepatic cholesterol synthesis21. Furthermore, Wang et al.22, recently have shown that butyrate improves energy metabolism by reducing energy intake and enhancing fat oxidation by activating brown adipose tissue in E3L.CETP mice. Despite elevations of both propionate and butyrate in our study, no lipid lowering effects of inulin were observed. However, we cannot exclude that prior administration of an inulin containing diet may affect the subsequent response to a cholesterol WTD. This further implies that the effects of administration of inulin prior to cholesterol WTD feeding on SCFAs and atherosclerosis development remains to be further investigated.
Interestingly, inulin in combination with 0.5% dietary cholesterol led to manifestations of hepatic inflammation. This effect seemed to be dependent on the amount of cholesterol in the diet as inulin did not adversely affect the liver when combined with 0.1% cholesterol. We found similar detrimental effects of inulin and cholesterol in an inflammation-driven cuff-induced atherosclerosis model, were inulin in combination with 1% dietary cholesterol even resulted in increased plasma cholesterol levels and aggravated atherosclerosis development23. We are not the first ones to show adverse effects of dietary fiber on liver inflammation mediated via gut microbiota interactions. In a previous study by Janssen et al.24, specific modulation of the gut microbiota, by feeding mice the prebiotic indigestible carbohydrate guar gum, promoted liver inflammation and led to worsening of non-alcoholic fatty liver disease (NAFLD) in mice. In this study, the effects of guar gum on liver inflammation could be linked to altered circulating and hepatic levels of bile acids. Whether inulin in our study adversely affected the liver via changes in bile acid metabolism remains to be investigated.
Inulin has been shown to induce inflammation in previous studies, although in these studies inulin mainly exacerbated pre-existing inflammation. For instance Miles et al.25, reported that diets enriched with inulin further exacerbated the severity of dextran sulfate sodium (DSS)-induced colitis in mice. It is possible that in our study the high-cholesterol diet induced borderline liver inflammation which was exacerbated by inulin.
Inulin is well established to exert bifidogenic effects and the increased abundance of Bifidobacteria in the gut microbiota presumably explains the beneficial effects of inulin on health26. Notably, inulin in combination with 0.5% dietary cholesterol led to major shifts in microbial composition but there was no significant increase in the relative abundance of Bifidobacteria. Instead, according to both LEfSe and ANCOM analysis, inulin stimulated the relative abundance of the genera Allobacullum and Coprococcus. Catry et al.27 have previously reported that dietary intervention with inulin-type fructans (ITF) improved endothelial dysfunction in which they found increased abundance of Allobaculum in APOE−/− mice. Furthermore, Lee et al.28, have reported that feeding rats a diet rich in cholesterol specifically increased the abundance of Allobaculum and Coprococcus compared to a control AIN76A diet. Whether the relative increase in Allobaculum in these and our studies was due to inulin supplementation, hypercholesterolemia, or both is not clear. In the study of Lee et al.28, it was found that Allobaculum exhibited a negative correlation with anti-inflammatory genes in the gut, indicating that Allobaculum plays a role in inflammation. We found a relative decrease in the abundance of Bacteroides, Parabacteroides, Prevotella, Mucispirillum, Clostridium, and Coprobacillus after inulin supplementation. Whether the absence of Bifidobacteria, relative increase of Allobaculum and Coprococcus, and/or the decrease in relative abundance of certain other genera played a role in the underlying mechanism of the manifestations of liver inflammation after inulin supplementation on a high cholesterol diet, remains to be investigated.
In conclusion, in this study inulin did neither affect hypercholesterolemia nor atherosclerosis development in hypercholesterolemic E3L.CETP mice, but rather resulted in manifestations of hepatic inflammation when combined with high percentages of dietary cholesterol. Although inulin is widely acknowledged as a prebiotic with favorable effects on lipid metabolism and CVD, inulin clearly not always exerts beneficial effects. It will be important for future research to decipher potential adverse pathways and mechanisms that are induced by the interaction of inulin with high dietary cholesterol and the gut microbiota. Although the gut microbiota of mice differ significantly from humans, E3L.CETP mice respond similarly as patients to a variety of anti-atherosclerotic interventions23. Therefore, we interpret our data to indicate caution with the application of inulin to reduce lipid levels in humans.
Methods
Mice and diet
In two experiments, female E3L.CETP mice were fed a WTD containing 0.1% or 0.5% cholesterol (Diet T 0.1 (Diet T 4022.16) or Diet T 0.5 (Diet T 4022.17); AB Diets, The Netherlands) (Table 2). This WTD diet was supplemented with or without 10% inulin (FrutaFit HD, Sensus, The Netherlands) for a total period of 11 weeks in which 10% of cellulose was replaced with 10% inulin. At baseline, after a run-in of 3 weeks with WTDs, randomization of the mice was performed based on plasma TC levels, plasma TG levels, and body weight. During the intervention period, body weight and food intake were measured weekly. After 11 weeks, non-fasted mice were sacrificed using CO2 inhalation. Orbital bleeding was performed to collect blood and mice were subsequently perfused with ice-cold PBS through the heart. Cecum content, heart, and liver were collected for further analysis. Mice were housed under temperature- and humidity-controlled conditions with a 12:12 h light-dark cycle and free access to food and water. A schematic of the experimental study protocol is provided in Fig. 5. Mouse experiments were performed in compliance with Dutch government guidelines and the Directive 2010/63/EU of the European Parliament and had received approval from the University Ethical Review Board (Leiden University Medical Center, The Netherlands).
Table 2.
Diet composition (% of total weight).
| Dietary substitute (%) | Control/Inulin 0.1% | Control/Inulin 0.5% |
|---|---|---|
| Inulin | 0/10 | 0/10 |
| Cholesterol | 0.1 | 0.5 |
| Magnesium oxide | 0.2 | 0.2 |
| Methionine | 0.2 | 0.2 |
| Standard trace elements premix | 0.25 | 0.25 |
| Standard vitamin premix | 0.25 | 0.25 |
| Salt | 0.3 | 0.3 |
| Magnesium sulphate heptahydate | 0.4 | 0.4 |
| Potassium hydrogen phosphate | 0.7 | 0.7 |
| Potassium chloride | 0.7 | 0.7 |
| Calcium carbonate | 1 | 1 |
| Corn oil | 1 | 1 |
| Calcium hydrogen phosphate | 1.3 | 1.3 |
| Choline chloride | 2 | 2 |
| Corn starch | 10 | 10 |
| Cacoa butter | 15 | 15 |
| Cellulose | 16.1/6.1 | 16/6 |
| Sour casein | 20 | 20 |
| Sugar/sucrose | 30.5 | 30.2 |
| Total | 100 | 100 |
Figure 5.
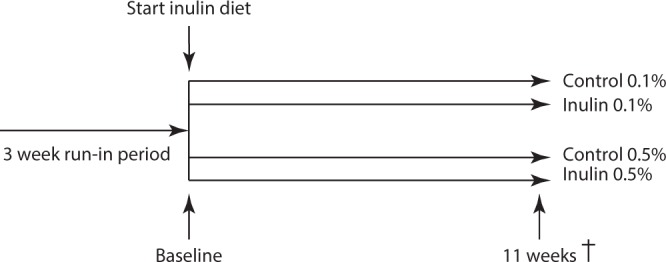
Experimental study protocol. Experiments were performed independently.
16S rRNA gene sequencing and profiling
16S rRNA sequencing in cecum samples of E3L.CETP mice fed with a WTD (0.5% cholesterol) ± inulin was performed as described previously29. Relative bacterial abundance was determined. For statistical significance, biological relevance and visualisation linear discriminant analysis (LDA) effect size (LEfSe) method (https://bitbucket.org/biobakery/biobakery/wiki/lefse)30 and ANCOM analysis were used as described previously (https://www.niehs.nih.gov/research/resources/software/biostatistics/ancom/index.cfm)31. Prior to LEfse analysis low abundant taxa were filtered out, applying a two-step filtering. First, taxa that were present in less than half of the group size were filtered out. In a second step, very rare taxa that were abundant less than 0.5% of total group taxa were filtered out. At baseline and after 11 weeks (wks) of intervention, jack-knifed β-diversity of unweighted UniFrac distances, with 10 jack-knifed replicates was measured at rarefaction depth of 22000 reads per sample, based on the unfiltered OTU table.
Cecal short-chain fatty acid analysis
Cecum SCFA content was analysed using gas chromatography mass spectrometry (GC-MS) as described previously32.
Plasma parameters
Plasma TG and TC were measured in 4 hour fasted mice as described previously29. Plasma cholesterol exposure was calculated as the cumulative exposure over the number of weeks where mice were fed a WTD ± inulin.
Atherosclerosis quantification
Atherosclerosis quantification using histological staining with hematoxylin-phloxyin-saffron (HPS) was performed in hearts of E3L.CETP mice fed a WTD (0.5% cholesterol) ± inulin as described previously29. Image J Software (NIH, USA) was used for the quantification of atherosclerotic lesion areas.
Liver (immuno)histochemistry
Livers were removed, fixed in 4% paraformaldehyde, dehydrated in 70% ethanol, embedded in paraffin and sectioned (5 µm). Paraffin-embedded liver sections were stained with haematoxylin and eosin (H&E) using standard protocols. From H&E-stained sections, hepatocyte size (hepatic hypertrophy) was quantified as the average number of hepatocytes per total microscopic field (mm2) per section. H&E-stained liver sections were scored for hepatic steatosis on the level of microvesicular and macrovesicular steatosis33, expressed as the percentage of the total liver section area affected. Immunohistochemical detection of the macrophage marker F4/80 was done on paraffin-embedded sections that were treated with proteinase K, using a primary Rat Anti-Mouse F4/80 monoclonal Ab (MCA497; 1:600, Serotec, UK) and a secondary Goat Anti-Rat immunoglobulin peroxidase (MP-7444, Vector Laboratories Inc., USA). All histological and histochemical analysis were analysed using Image J software (NIH, USA).
Liver lipids
Lipids were extracted from the liver according to a protocol from Bligh and Dyer34 and modified as described previously29. TG content was assayed as described above.
RNA isolation and qRT-PCR
Snap-frozen liver samples were used for the extraction of RNA using a NucleoSpin RNA kit (Machery-Nagel, Germany). NanoDrop ND-1000 spectrophotometer (Isogen, The Netherlands) was used to determine concentrations and purity of RNA. Reverse transcription of RNA was done using Moloney Murine Leukemia Virus Reverse Transcriptase (Promega, The Netherlands). Gene expression levels were determined using qRT-PCR, SYBR green supermix (Biorad, The Netherlands), and gene-specific primers (Table 3). Expression of mRNA was normalized to cyclophilin (CypA) and ribosomal protein large P0 (Rplp0) RNA, and expressed as fold change versus control using the ΔΔ CT method.
Table 3.
Primer sequences of forward and reverse primers (5′ → 3′).
| Gene | Sense | Antisense |
|---|---|---|
| CypA | ACTGAATGGCTGGATGGCAA | TGTCCACAGTCGGAAATGGT |
| Rplp0 | GGACCCGAGAAGACCTCCTT | GCACATCACTCAGAATTTCAATGG |
| IL-10 | GACAACATACTGCTAACCGACTC | ATCACTCTTCACCTGCTCCACT |
| IL-6 | AAGAAATGATGGATGCTACCAAACTG | GTACTCCAGAAGACCAGAGGAAATT |
| Mcp-1 | CACTCACCTGCTGCTACTCA | GCTTGGTGACAAAAACTACAGC |
| Tnf-α | GATCGGTCCCCAAAGGGATG | CACTTGGTGGTTTGCTACGAC |
| F4/80 | CTTTGGCTATGGGCTTCCAGTC | GCAAGGAGGACAGAGTTTATCGTG |
Statistical analysis
Data are presented as means ± SEM. Normal distribution of the data was tested using D’Agostino-Pearson omnibus normality test, and data were analysed with the unpaired Student’s t-test in case of normal distribution or with the nonparametric Mann–Whitney U test in case of not normally distributed data. Differences in body weight over time were evaluated for statistical significance by two-way ANOVA followed by Sidak’s post hoc multiple comparison test. P < 0.05 was considered as statistically significant. Analyses were performed using Graph Pad Prism version 7.0 (GraphPad Software, USA).
Acknowledgements
This research was supported by The Netherlands Cardiovascular Research Initiative: An initiative with support of the Dutch Heart Foundation, CVON2012-03 (IN-CONTROL). The authors would like to thank Trea Streefland, Lianne van der Wee-Pals, and Thomas Idzinga for their excellent technical assistance. The authors gratefully acknowledge Sensus The Netherlands for generously providing Frutafit HD (inulin).
Author Contributions
L.R.H., V.v.H., and K.W.v.D. conceived and designed the experiments; L.R.H., A.P., and K.K.D.V. performed the experiments; L.R.H., S.K., M.H., F.A.B., R.V., and L.D. analyzed the data; M.G. contributed reagents/materials/analysis tools; L.R.H., V.v.H., and K.W.v.D. wrote the paper. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lisa R. Hoving, Email: L.R.Hoving@lumc.nl
Ko Willems van Dijk, Email: K.Willems_van_Dijk@lumc.nl.
References
- 1.World Health Organization. WHO | Cardiovascular diseases (CVDs):Fact sheet. WHO at, http://www.who.int/mediacentre/factsheets/fs317/en/ (2017).
- 2.Chan DC, Barrett PHR, Watts GF. The metabolic and pharmacologic bases for treating atherogenic dyslipidaemia. Best Pract. Res. Clin. Endocrinol. Metab. 2014;28:369–85. doi: 10.1016/j.beem.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Jukema JW, Cannon CP, De Craen AJM, Westendorp RGJ, Trompet S. The Controversies of Statin Therapy: Weighing the Evidence. J. Am. Coll. Cardiol. 2012;60:875–881. doi: 10.1016/j.jacc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson FH, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 6.Ley RRE, Turnbaugh PPJ, Klein S, Gordon JIJ. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 7.Wilson B, Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017;32:64–68. doi: 10.1111/jgh.13700. [DOI] [PubMed] [Google Scholar]
- 8.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 9.Peng Mengfei, Biswas Debabrata. Short chain and polyunsaturated fatty acids in host gut health and foodborne bacterial pathogen inhibition. Critical Reviews in Food Science and Nutrition. 2016;57(18):3987–4002. doi: 10.1080/10408398.2016.1203286. [DOI] [PubMed] [Google Scholar]
- 10.Vogt L, et al. Immunological Properties of Inulin-Type Fructans. Crit. Rev. Food Sci. Nutr. 2015;55:414–436. doi: 10.1080/10408398.2012.656772. [DOI] [PubMed] [Google Scholar]
- 11.Dehghan P, Gargari BP, Jafar-Abadi MA, Aliasgharzadeh A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2014;65:117–23. doi: 10.3109/09637486.2013.836738. [DOI] [PubMed] [Google Scholar]
- 12.Brighenti F. Dietary fructans and serum triacylglycerols: a meta-analysis of randomized controlled trials. J. Nutr. 2007;137:2552S–2556S. doi: 10.1093/jn/137.11.2552S. [DOI] [PubMed] [Google Scholar]
- 13.Brighenti F, Casiraghi MC, Canzi E, Ferrari A. Effect of consumption of a ready-to-eat breakfast cereal containing inulin on the intestinal milieu and blood lipids in healthy male volunteers. Eur. J. Clin. Nutr. 1999;53:726–33. doi: 10.1038/sj.ejcn.1600841. [DOI] [PubMed] [Google Scholar]
- 14.Davidson MH, Maki KC, Synecki C, Torri SA, Drennan KB. Effects of dietary inulin on serum lipids in men and women with hypercholesterolemia. Nutr. Res. 1998;18:503–517. doi: 10.1016/S0271-5317(98)00038-4. [DOI] [Google Scholar]
- 15.Aliasgharzadeh A, et al. A combination of prebiotic inulin and oligofructose improve some of cardiovascular disease risk factors in women with type 2diabetes: A randomized controlled clinical trial. Adv. Pharm. Bull. 2015;5:507–514. doi: 10.15171/apb.2015.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zadelaar S, et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 2007;27:1706–21. doi: 10.1161/ATVBAHA.107.142570. [DOI] [PubMed] [Google Scholar]
- 17.Rault-Nania M-H, et al. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br. J. Nutr. 2007;96:840–844. doi: 10.1017/bjn20061913. [DOI] [PubMed] [Google Scholar]
- 18.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–71. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 19.Van den Maagdenberg AM, et al. Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J. Biol. Chem. 1993;268:10540–5. [PubMed] [Google Scholar]
- 20.Wolever TM, Spadafora P, Eshuis H. Interaction between colonic acetate and propionate in humans. Am. J. Clin. Nutr. 1991;53:681–7. doi: 10.1093/ajcn/53.3.681. [DOI] [PubMed] [Google Scholar]
- 21.Hara H, Haga S, Aoyama Y, Kiriyama S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 1999;129:942–8. doi: 10.1093/jn/129.5.942. [DOI] [PubMed] [Google Scholar]
- 22.Li Zhuang, Yi Chun-Xia, Katiraei Saeed, Kooijman Sander, Zhou Enchen, Chung Chih Kit, Gao Yuanqing, van den Heuvel José K, Meijer Onno C, Berbée Jimmy F P, Heijink Marieke, Giera Martin, Willems van Dijk Ko, Groen Albert K, Rensen Patrick C N, Wang Yanan. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2017;67(7):1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- 23.Hoving L, et al. The Prebiotic Inulin Aggravates Accelerated Atherosclerosis in Hypercholesterolemic APOE*3-Leiden Mice. Nutrients. 2018;10:172. doi: 10.3390/nu10020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen Aafke W. F., Houben Tom, Katiraei Saeed, Dijk Wieneke, Boutens Lily, van der Bolt Nieke, Wang Zeneng, Brown J. Mark, Hazen Stanley L., Mandard Stéphane, Shiri-Sverdlov Ronit, Kuipers Folkert, Willems van Dijk Ko, Vervoort Jacques, Stienstra Rinke, Hooiveld Guido J. E. J., Kersten Sander. Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids. Journal of Lipid Research. 2017;58(7):1399–1416. doi: 10.1194/jlr.M075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miles JP, et al. Supplementation of Low- and High-fat Diets with Fermentable Fiber Exacerbates Severity of DSS-induced Acute Colitis. Inflamm. Bowel Dis. 2017;23:1133–1143. doi: 10.1097/MIB.0000000000001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. European Journal of Clinical Nutrition. 2009;63:1277–1289. doi: 10.1038/ejcn.2009.64. [DOI] [PubMed] [Google Scholar]
- 27.Catry Emilie, Bindels Laure B, Tailleux Anne, Lestavel Sophie, Neyrinck Audrey M, Goossens Jean-François, Lobysheva Irina, Plovier Hubert, Essaghir Ahmed, Demoulin Jean-Baptiste, Bouzin Caroline, Pachikian Barbara D, Cani Patrice D, Staels Bart, Dessy Chantal, Delzenne Nathalie M. Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut. 2017;67(2):271–283. doi: 10.1136/gutjnl-2016-313316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S-M, Han HW, Yim SY. Beneficial effects of soy milk and fiber on high cholesterol diet-induced alteration of gut microbiota and inflammatory gene expression in rats. Food Funct. 2015;6:492–500. doi: 10.1039/C4FO00731J. [DOI] [PubMed] [Google Scholar]
- 29.R. Hoving L, et al. Dietary Mannan Oligosaccharides Modulate Gut Microbiota, Increase Fecal Bile Acid Excretion, and Decrease Plasma Cholesterol and Atherosclerosis Development. Mol. Nutr. Food Res. 2018;62:1700942. doi: 10.1002/mnfr.201700942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandal S, et al. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015;26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoving, L. R., Heijink, M., van Harmelen, V., Willems van Dijk, K. & Giera, M. In Clinical Metabolomics (ed. Giera, M.) (Springer, 2018).
- 33.Liang W, et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One. 2014;9:e115922. doi: 10.1371/journal.pone.0115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bligh E, Dyer Wa. Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]