Abstract
Purpose
To investigate the long-term results of a modified technique for parafoveal multiple curvilinear internal limiting membrane (ILM) peeling to preserve the epi-foveal ILM in myopic foveoschisis surgery.
Methods
Thirty-two consecutive patients (36 eyes) were retrospectively reviewed. Patients were divided into two groups according to the extent of ILM peeled: the fovea-sparing ILM peeling group (FS) (18 eyes) and total ILM peeling group (TP) (18 eyes). Patients were followed up for at least 12 months. The main outcome measures were best-corrected visual acuity changes, evolution of macular schisis and the factors associated with the development of a full-thickness macular hole (FTMH).
Results
FTMH developed in 1 of 18 eyes (5.6%) in the FS group and 3 of 18 eyes (16.7%) in the TP group (P = 0.28). Long-term follow-up showed visual improvement was better in the FS group than in the TP group (0.94 vs. 0.58 logMAR). Macular schisis disappeared in 13 of 18 eyes (72.2%) in the FS group, but disappeared in 7 of 18 eyes (38.9%) in the TP group (P = 0.04). Logistic regression analysis showed that only the preoperative outer lamellar macular hole (P = 0.016) was a significant risk factor for development of postoperative FTMH.
Conclusions
Fovea-sparing ILM peeling achieves a higher rate of macular schisis resolution over total peeling. A preoperative outer lamellar macular hole can be a risk factor for the development of a macular hole.
Introduction
Myopic foveoschisis is a unique complication of high myopic eyes and a major cause of visual impairment. The pathogenesis of myopic foveoschisis is varied; tangential vitreous traction of the disease is believed to be the primary cause [1–3]. Presently, vitrectomy and internal limiting membrane (ILM) peeling are considered to be effective in the management of high myopic foveoschisis [4–6]. However, it has been reported that the risk of developing a full-thickness macular hole (FTMH) ranges from 16.7 to 20.8% in myopic foveoschisis eyes undergoing ILM peeling [7, 8]. The mechanisms of why and how FTMHs develop postoperatively have not been fully determined. Hirakata and associates reported that the risk of postoperative FTMH was higher in eyes with foveal retinal detachment (RD). Recently, Shimada and associates hypothesized that ILM peeling on such a thinned central foveola could induce a break in the central foveolar tissue, leading to the formation of FTMH.
The development of FTMH is a serious complication in highly myopic eyes [2, 3, 7–14]. To address this, Ho et al. [15] and Shimada [7] proposed preserving the epi-foveal ILM during ILM peeling to prevent the formation of FTMH after vitrectomy. They reported that this new technique achieved better anatomical and visual results over total ILM peeling, consequently preventing long-term foveal retinal thinning and the development of FTMH [7, 9, 15]. Nevertheless, the surgical manoeuver to preserve the epi-foveal ILM is not a simple manipulation, especially for highly myopic eyes with foveal RD. Moreover, although the fovea-sparing ILM peeling technique has been evaluated, further studies in multiple centres are necessary to fully determine the effectiveness and safety of this procedure.
In the current study, we described the long-term surgical outcomes of this modified fovea-sparing ILM peeling technique for a large number of patients with a long follow-up.
Subjects and methods
Subjects
This study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of the Xinhua hospital-affiliated medical college, Shanghai Jiao Tong University. All participants provided written informed consent of the possible benefits and risks. This study was a retrospective, interventional and consecutive case series of myopic foveoschisis patients who underwent vitrectomy with fovea-sparing or total ILM peeling from January 2014 to June 2016. The inclusion criteria were a preoperative spherical equivalent higher than −8.00 dioptres (D) or axial length (AL) longer than 26 mm and progressive visual loss presumably caused by foveoschisis associated with high myopia. Eyes with dense opacities of the media such as corneal opacities or dense cataracts were excluded. The exclusion criteria also included a preoperative FTMH observed by spectral-domain optical coherence tomography (OCT), poor visual acuity (VA) due to diffuse macular chorioretinal atrophy or a large Fuchs spot, high myopic choroidal neovascularization, a history of ocular trauma, and other systemic and retinal diseases that may affect VA.
Patients were divided into two groups according to the ILM peeling procedure: Group 1: fovea-sparing ILM peeling group (FS) (18 eyes) and Group 2: total ILM peeling group (TP) (18 eyes). All patients underwent a complete ocular examination, including a slit-lamp examination, best-corrected VA (BCVA) measurement, fundus examination and OCT (RTVue-100, Optovue, Inc., Fremont, CA, USA) scanning at every visit. The AL was measured using an optical biometer (Ver 5.4.Carl Zeiss Meditec AG, Jena, Germany). All patients were followed up for at least 12 months.
Surgical procedure
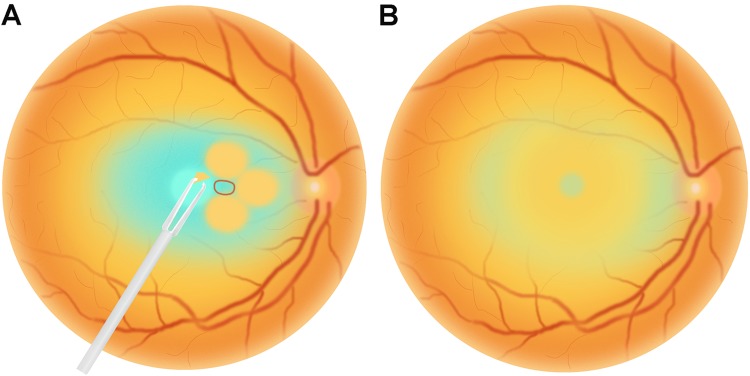
Phacoemulsification with simultaneous intraocular lens implantation followed by a standard 23-gauge three-port transconjunctival pars plana vitrectomy was performed on all eyes in the two groups under local anaesthesia. First, the central vitreous core was removed, and then the posterior hyaloid was removed by active suction using a vitreous cutter. Then, the ILM was stained with brilliant blue G for 30 s in all patients. In the FS group, the macular area was divided into four quadrants: the nasal, temporal, superior and inferior regions. The initial ILM tear was performed to make a flap away from the central fovea in one quadrant using a microforceps (Grieshaber, Alcon, Fort Worth, TX, USA). Then, the ILM was peeled in a curvilinear manner centred around the site away from the central fovea in each quadrant. Following parafoveal curvilinear ILM peeling, small areas of residual ILM between the four circular areas of ILM were removed. The edge of the residual ILM at the central fovea was trimmed by a vitreous cutter. After multiple parafoveal curvilinear ILM peeling, an epi-foveal ILM of approximately 500 μm in diameter was preserved [16]. (Fig. 1) In the TP group, the ILM was peeled over the complete macula with an approximately two-disc diameter area. Finally, fluid–air exchange was performed, followed by an injection of 16% perfluoropropane.
Fig. 1.
Schematic illustrations of the parafoveal multiple curvilinear internal limiting membrane (ILM) peeling technique. a ILM peeling was initiated and centred away from the central fovea in a continuous curvilinear manner in each quadrant, nasal, temporal, inferior and superior. b An epi-foveal ILM of about 500 μm in diameter was preserved after removing the residual ILM between the four circular areas
Statistical analysis
Statistical analysis was performed using the Sigma Stat software (SPSS, Inc., Chicago, IL, USA) for Windows software version 19.0. The age, AL, preoperative and postoperative BCVA (logarithm of the minimal angle of resolution, logMAR); visual improvement in logMAR; and preoperative and postoperative central foveolar thickness (CFT) were compared using the Mann–Whitney U test. Differences in the incidence rates of the outer lamella macular hole, foveal detachment and evolution of macular schisis between two groups were analysed using Fisher’s exact test. Logistic regression models were used to determine the influence of the potential factor on the development of postoperative macular holes. All P values were two-sided, with P < 0.05 being considered statistically significant.
Results
Baseline characteristics of patients
Thirty-two consecutive patients (36 eyes) who underwent high myopic foveoschisis surgery by one surgeon (PQZ) were enroled and retrospectively reviewed in this study. The baseline characteristics of the 36 eyes and 32 patients are shown in Table 1. The mean age was 52.8 ± 10.8 years in the FS group and 58.0 ± 13.2 years in the TP group (P = 0.10). The mean AL was 30 ± 1.7 mm in the FS group and 28.9 ± 2.6 mm in the TP group (P = 0.32). The mean refractive error was 13.5 ± 1.6 dioptric spherical equivalents in the FS group and 12.8 ± 2.3 dioptric spherical equivalents in the TP group (P = 0.31). The outer lamellar macular hole (OLMH) was observed in 2 of 18 patients (11.1%) in the fovea-sparing group and 1 of 18 (5.6%) patients in the total peeling group (P = 1.00). The foveal detachment was observed in 10 of 18 (55.6%) patients in the fovea-sparing group and 9 of 18 (50%) patients in the total peeling group (P = 1.00). The mean follow-up time was 20.9 ± 9.6 months in the FS group and 19.6 ± 4.1 in the TP group (P = 0.94). The differences in these demographic and baseline characteristics were comparable and not significant.
Table 1.
Baseline characteristics of the patients who underwent fovea-sparing or total internal limiting membrane peeling
| FS group (18 eyes) | TP group (18 eyes) | P value | |
|---|---|---|---|
| Age (years), mean ± SD | 52.8 ± 10.8 | 58.0 ± 13.2 | 0.10 |
| Sex (female/male) | 11/7 | 10/8 | 0.73 |
| AL (mm), mean ± SD | 30 ± 1.7 | 28.9 ± 2.6 | 0.32 |
| RE (D), mean ± SD | 13.5 ± 1.6 | 12.8 ± 2.3 | 0.31 |
| OLMH, no. (%) | 2 (11.1%) | 1 (5.6%) | 1.00 |
| FD, no. (%) | 10 (55.6%) | 9 (50%) | 1.00 |
| Follow-up (months), mean ± SD | 20.9 ± 9.6 | 19.6 ± 4.1 | 0.94 |
FS fovea-sparing, TP total peeling, AL axial length, RE refractive error, D dioptres, SD standard deviation, OLMH outer lamellar macular hole, FD foveal retinal detachment
Visual outcomes
There were no significant differences in the preoperative and postoperative BCVA between the two groups (Table 2). Compared to the preoperative BCVA, the postoperative BCVA improved significantly both in the FS group and the TP group (P < 0.001 vs. 0.04). The visual improvement, however, was larger in the FS group (0.94 ± 0.8 logMAR) than that in the TP group (0.58 ± 0.5 logMAR), although this was not statistically significant (P = 0.17).
Table 2.
Visual and anatomic outcomes of before and after surgery in fovea-sparing and total peeling group
| FS group (18 eyes) | TP group (18 eyes) | P value | |
|---|---|---|---|
| Preoperative BCVA in logMAR, mean ± SD | 1.46 ± 0.8a | 1.11 ± 0.8b | 0.18c |
| Postoperative BCVA in logMAR, mean ± SD | 0.56 ± 0.3a | 0.67 ± 0.5b | 0.33c |
| Visual improvement in logMAR, mean ± SD | 0.94 ± 0.8 | 0.58 ± 0.5 | 0.17c |
| Preoperative CFT (mm), mean ± SD | 615.17 ± 169.7d | 631.6 ± 146.3e | 0.28c |
| Postoperative CFT (mm), mean ± SD | 143.36 ± 52.4d | 141.6 ± 93.4e | 0.92c |
| Evolution of MS, eyes no. (%) | 0.04f | ||
| Disappeared | 13 (72.2 %) | 7 (38.9 %) | |
| Improved | 5 (27.8 %) | 11 (61.1 %) | |
| Postoperative FTMH | 1 (5.6 %) | 3 (16.7%) | 0.28f |
BCVA best-corrected visual acuity, FS fovea-sparing, TP total peeling, FTMH full-thickness macular hole, CFT central foveal thickness, SD standard deviation
aP < 0.001(Mann–Whitney U test)
bP = 0.04 (Mann–Whitney U test)
cMann–Whitney U test.
dP < 0.001 (Mann–Whitney U test)
eP < 0.001 (Mann–Whitney U test)
fP: Fisher's exact test.
In the FS group, VA improved by more than two lines in all eyes except one eye that developed FTMH, in which case, VA decreased from 0.4 logMAR to 1.0 logMAR. In the TP group, nine eyes (50%) had a more than two lines of VA improvement. However, BCVA was reduced in four eyes (22.2%) in the TP group, and three of the four eyes developed FTMH postoperatively.
Anatomic outcomes
Postoperative CFT decreased significantly both in two groups (P < 0.001). At the final follow-up, macular schisis disappeared in 13 eyes (72.2%) in the FS group and 7 eyes (38.9%) in the TP group (P = 0.04). In the FS group, the preserved ILM was observed in 2 of 18 eyes (Figs. 2 and 3).
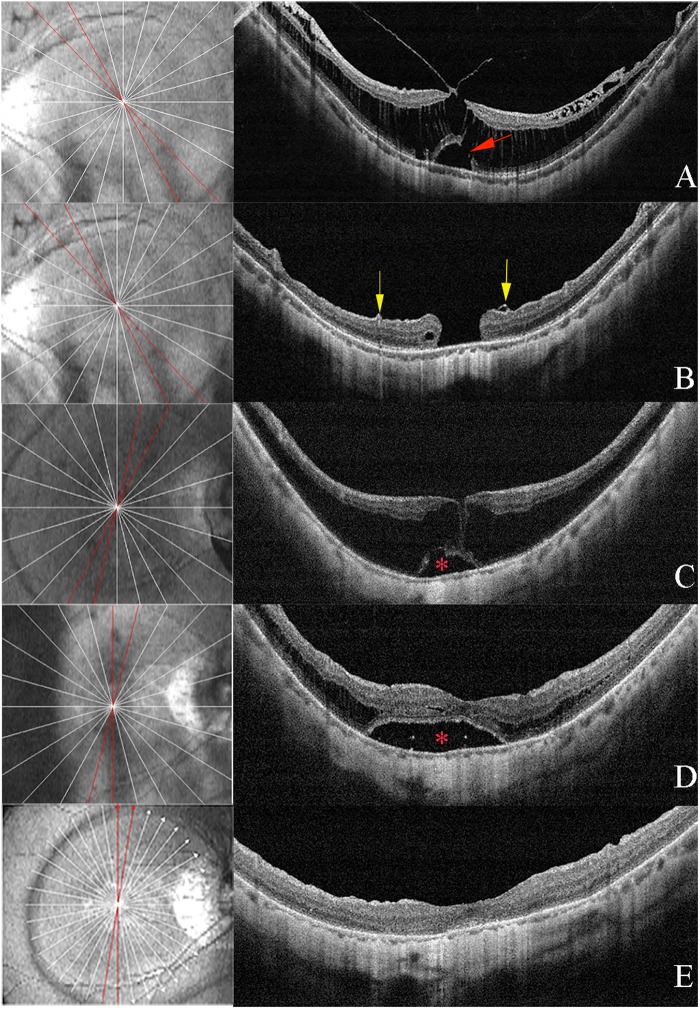
Fig. 2.
Two patients who underwent fovea-sparing internal limiting membrane (ILM) peeling. a Preoperative optical coherence tomography (OCT) image of a 30-year-old male with an axial length of 30.84 mm. Vitreous macular traction and the outer lamella hole (OLMH) (red arrow) with macular retinoschisis were observed in OCT image. The preoperative best-corrected visual acuity (BCVA) was 0.4 logMAR. b At 1 month after surgery, a full-thickness macular hole (FTMH) developed and the BCVA decreased to 1.0 logMAR. The preserved ILM was torn and the rolled margin was detected (yellow arrow). c Preoperative OCT image of a 65-year-old female with an axial length of 28.62 mm. The preoperative BCVA was 1.2 logMAR. The macular schisis with foveal retinal detachment (RD) (asterisk) was observed. d At 3 months after surgery, the retinoschisis was decreased with an enlarged foveal RD (asterisk). e At 12 months after surgery, the macular retinoschisis was resolved with no contraction of the preserved ILM
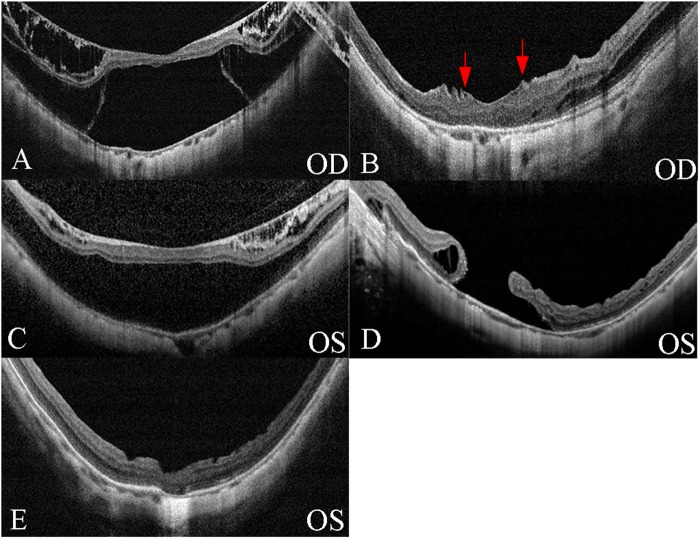
Fig. 3.
Optical coherence tomography (OCT) images of one patient who underwent fovea-sparing internal limiting membrane (ILM) and total ILM peeling for two eyes, respectively. a Preoperative OCT image of the right eye with axial length of 28.32 mm. The inner and outer layer of retinoschisis with foveal retinal detachment (RD) can be observed. The preoperative best-corrected visual acuity (BCVA) was 1.5 logMAR. b Three months after fovea-sparing ILM peeling, macular retinoschisis was resolved and the edge of the preserved ILM was detected (red arrow). The postopertative BCVA was 0.9 logMAR. c Preoperative OCT image of the left eye with an axial length of 29.27 mm. OCT showed macular retinoschisis with foveolar RD. The preoperative BCVA was 0.83 logMAR. d At 1 month after total ILM peeling, a full-thickness macular hole (MH) with RD has developed. e Autologous lens capsular transplantation was performed and MH with RD was resolved. The BCVA improved to 0.5 logMAR
Long-term follow-up showed that FTMH developed in 1 of 18 eyes (5.6%) in the FS group and in 3 of 18 eyes (16.7%) in the TP group postoperatively (P = 0.28). The details of the patients who developed FTMH postoperatively are shown in Supplementary Table 1. For the one eye that developed FTMH in the FS group, OLMH was detected by OCT scanning preoperatively (Fig. 2). For the three eyes that developed FTMH in the TP group, OLMH was observed in one eye and foveal RD was observed in the remaining two eyes. These three patients in the TP group received a secondary operation; the MHs were closed and BCVA improved in all three eyes (Fig. 3).
No significant differences in AL (P = 0.680), extent of schisis (P = 0.213), presence of posterior staphyloma (P = 0.566), presence of foveal detachment (P = 1.000), or ILM peeling type (P = 0.566) were detected between patients with and without postoperative FTMH. Among the characteristics examined in the OCT images, the height of schisis (P = 0.028) and the percentage of eyes with OLMH (P = 0.027) were statistically significantly different between patients with and without postoperative FTMH (Supplementary Table 2). Logistic regression analysis with a stepwise method showed that among all of the relevant factors, only the percentage of OLMH was significantly correlated (P = 0.016) with the development of postoperative macular holes.
Discussion
By comparing the long-term surgical outcomes of fovea-sparing and total ILM peeling for high myopic foveal schisis, this study showed that the rate of thorough resolution of foveolar schisis was higher in the FS group than in the TP group. BCVA improved significantly both in the FS group and the TP group. The visual recovery, however, was better in the FS group than in the TP group. At the last follow-up, there was no significant difference in CFT between the two groups. Preoperative OLMH can be a risk factor for the development of postoperative FTMH.
Recently, Shimada et al. [7] and Ho et al. [14] proposed the epi-foveal ILM preservation during ILM peeling. The technique used in the Shimada’s study [7] was named 'fovea-sparing ILM peeling', which started away from the central fovea and restarted from a new site when the peeled ILM flap came close to the central fovea. In the Ho’s study [15], the co-authors used microscissors to make multiple tangential cuts around the fovea during the procedure (named as 'foveola nonpeeling technique'). By contrast, Zhao’s technique [16], named 'fovea-sparing ILM peeling using multiple parafoveolar curvilinear peels', utilized peeling that was initiated and centred away from the region of the ILM, where it can hopefully remain undisturbed, instead of centring around the fovea. Since ILM peeling was initiated and centred away from the central fovea, it reduced the lift of the ILM overlying the foveal centre and left it undisturbed. The preserved epi-foveal ILM can be trimmed by a vitreous cutter and is left with a sharp ILM margin.
Until now, there has been no consensus on the size of the preserved ILM. In our study, the size of the preserved ILM was approximately 500 to 1000 µm. In the Ho’s study [15], the size of the preserved ILM was 300 to 500 µm. However, in Shimada’s study [7], the size of the spared-fovea ILM was approximately equal to the size of the optic disc. As tangential vitreous traction is believed to be a main factor in the development of myopic schisis, the preserved ILM should be small enough to release all of the traction around the fovea. Additionally, if the size is not small enough or the margin is not sharp, the preserved ILM itself may postoperatively cause contraction. On the other hand, the inner retina will follow a larger arc along with the reattachment of the retina, resulting in the preserved ILM enlargement especially in cases with staphyloma. This factor should also be considered during the ILM preserving procedure. In Shimada’s study [7], remaining ILM contraction was observed in 10 of 15 eyes 3 months after the fovea-sparing ILM peeling. The ILM contraction was not found in any cases in Ho’s study [9] at the last follow-up. In this study, a preserved ILM was observed in 2 of 18 eyes in the FS group, but no parafoveolar elevation was caused by the contraction of the preserved ILM. Regarding the foveal structure, the fovea itself is a 1500 µm depression in the centre of the macula, and the foveola is approximately 350 µm in diameter. Thus, 500 µm may be the ideal size of the preserved ILM size, as it can release tangential traction as well as offer enough area to cover the foveola to prevent the formation of postoperative FTMH.
In Shimada’s [7] and Ho’s [9] studies, no postoperative development of FTMH was found in the fovea-sparing ILM peeling group. The rate of postoperative FTMH in the TP was 16.7% in Shimada’s study [7], 28.9% in Ho’s study [9] and 16.7% in our study. In our study, however, 1 of 18 eyes (5.6%) in the FS group developed FTMH within 1 month after operation. In this case, the OLMH was detected during baseline OCT scanning. Analogously, OLMH was observed in 1 of 3 eyes that developed postoperative FTMH in the TP group. The remaining two eyes were accompanied by foveal RD. It has been reported that foveal RD and preoperative inner segment/outer segment junction defects are risk factors for the development of postoperative FTMH in myopic macular schisis [17, 18]. In the current study, logistic regression analysis showed that the presence of OLMH was correlated significantly correlated with the development of postoperative FTMH. The mechanism of why and how FTMH developed in these cases was not fully determined. We hypothesized that the foveal tissue with OLMH is extremely weak and can be more easily damaged, regardless of whether it is managed with fovea-sparing or total ILM peeling. In addition, OLMH might be enlarged and the thin foveal retina was stretched over a larger arc created by the surgical retinal reattachment, resulting in the development of postoperative FTMH. Thus, we suggested that OLMH might be another risk factor of postoperative FTMH, no matter whether in the FS group or the TP group. The rate of complete resolution of macular schisis was higher in the FS group than in the TP group (P = 0.04); however, the mechanisms remain unclear. Based on a review of the ultrastructure, Gass [19] believed that Müller cell cones supply structural support to the fovea. Thus, we hypothesized that the Müller cells endfeet within the preserved ILM may contribute to maintaining the foveolar cone skeleton. In the current study, no contraction of the retained ILM was observed in OCT scanning. We considered that the retained ILM is so thin and small that it could not generate centrifugal tangential traction after vitrectomy. Theoretically, total ILM peeling relieves all tangential traction. However, total ILM peeling can generate potential trauma to the underlying Müller cells and weaken the macular glial structure [20].
In Shimada’s study [7], patients in the fovea-sparing group had a better visual improvement than complete peeling group, which was consistent with both Ho’s study [9] and the present study’s results. However, the BCVA change was not significant or even progressively decreased in the total peeling group in their studies. In the current study, the BCVA improved significantly both in the FS group and the TP group at the last follow-up (20.9 months vs. 19.6 months). We believe that the visual improvement might be attributed to the restoration of the foveal schisis and retinal reattachment, no matter whether in the FS group or TP group.
To date, there is no consensus on the criteria for the selection of the surgical procedure to treat macular schisis with high myopia. Additionally, it is extremely challenging to preserve the epi-foveal ILM, especially for highly myopic eyes with long ALs, which suggests that further studies to clarify the criteria for appropriate surgery and more practical techniques are needed.
In conclusion, modified fovea-sparing ILM peeling has achieved better resolution of macular retinoschisis and visual improvement. Preoperative OLMH can be a risk factor for the development of postoperative FTMH. The optimal size of the preserved ILM and safety of fovea-sparing ILM peeling requires further study.
Summary
What was known before
The risk of FTMH is high in myopic foveoschisis eyes undergoing total ILM peeling.
Fovea-sparing ILM peeling contributes to better anatomical and visual results than total peeling.
What this study adds
FTMH could occur in eyes undergoing fovea-sparing ILM peeling.
In addition to foveal retinal detachment, OLMH may be another new risk factor for developing postoperative FTMH in myopic foveoschisis.
Electronic supplementary material
Acknowledgements
We thank the participating patients and the medical staff at Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
Funding
The study was supported in part by grants from the National Natural Science Foundation of China (81470642) and the Science and Technology Commission of Shanghai Municipality, China (15XD1502800).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41433-018-0178-0) contains supplementary material, which is available to authorized users.
References
- 1.Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128(4):472–6. doi: 10.1016/S0002-9394(99)00186-5. [DOI] [PubMed] [Google Scholar]
- 2.Baba T, Ohno-Matsui K, Futagami S, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol. 2003;135(3):338–42. doi: 10.1016/S0002-9394(02)01937-2. [DOI] [PubMed] [Google Scholar]
- 3.Gaucher D, Haouchine B, Tadayoni R, Massin P, Erginay R, Benhamou N, et al. Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. Am J Ophthalmol. 2007;143(3):455–62. doi: 10.1016/j.ajo.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 4.Kanda S, Uemura A, Sakamoto Y, Kita H. Vitrectomy with internal limiting membrane peeling for macular retinoschisis and retinal detachment without macular hole in highly myopic eyes. Am J Ophthalmol. 2003;136(1):177–80. doi: 10.1016/S0002-9394(03)00243-5. [DOI] [PubMed] [Google Scholar]
- 5.Ikuno Y, Sayanagi K, Ohji M, et al. Vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Am J Ophthalmol. 2004;137(4):719–24. doi: 10.1016/j.ajo.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MW. Myopic traction maculopathy: pathogenic mechanisms and surgical treatment. Retina. 2012;32( Suppl 2):S205–10. doi: 10.1097/IAE.0b013e31825bc0de. [DOI] [PubMed] [Google Scholar]
- 7.Shimada N, Sugamoto Y, Ogawa M, Takase H, Ohno-Matsui K. Fovea-sparing internal limiting membrane peeling for myopic traction maculopathy. Am J Ophthalmol. 2012;154(4):693–701. doi: 10.1016/j.ajo.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Panozzo G, Mercanti A. Vitrectomy for myopic traction maculopathy. Arch Ophthalmol. 2007;125(6):767–72. doi: 10.1001/archopht.125.6.767. [DOI] [PubMed] [Google Scholar]
- 9.Ho TC, Yang CM, Huang JS, et al. Long-term outcome of foveolar internal limiting membrane nonpeeling for myopic traction maculopathy. Retina. 2014;34(9):1833–40. doi: 10.1097/IAE.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 10.Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol. 2002;134(5):645–60. doi: 10.1016/S0002-9394(02)01883-4. [DOI] [PubMed] [Google Scholar]
- 11.Benhamou N, Massin P, Haouchine B, Erginay A, Gaudric A. Macular retinoschisis in highly myopic eyes. Am J Ophthalmol. 2002;133(6):794–800. doi: 10.1016/S0002-9394(02)01394-6. [DOI] [PubMed] [Google Scholar]
- 12.Shimada N, Ohno-Matsui K, Baba T, Futagami S, Tokoro T, Mochizuki M. Natural course of macular retinoschisis in highly myopic eyes without macular hole or retinal detachment. Am J Ophthalmol. 2006;142(3):497–500. doi: 10.1016/j.ajo.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 13.Todorich B, Scott IU, Flynn HW, Jr., Chang S. Macular retinoschisis associated with pathologic myopia. Retina. 2013;33(4):678–83. doi: 10.1097/IAE.0b013e318285d0a3. [DOI] [PubMed] [Google Scholar]
- 14.Ho TC, Yang CM, Huang JS, Yang CH, Chen MS. Foveola nonpeeling internal limiting membrane surgery to prevent inner retinal damages in early stage 2 idiopathic macula hole. Graefes Arch Clin Exp Ophthalmol. 2014;252(10):1553–60. doi: 10.1007/s00417-014-2613-7. [DOI] [PubMed] [Google Scholar]
- 15.Ho TC, Chen MS, Huang JS, Shih YF, Ho H, Huang YH. Foveola nonpeeling technique in internal limiting membrane peeling of myopic foveoschisis surgery. Retina. 2012;32(3):631–4. doi: 10.1097/IAE.0B013E31824D0A4B. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Zhang Q, Zhao P. Fovea sparing internal limiting membrane peeling using multiple parafoveal curvilinear peels for myopic foveoschisis: technique and outcome. BMC Ophthalmol. 2016;16(1):180. doi: 10.1186/s12886-016-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirakata A, Hida T. Vitrectomy for myopic posterior retinoschisis or foveal detachment. Jpn J Ophthalmol. 2006;50(1):53–61. doi: 10.1007/s10384-005-0270-4. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Ikuno Y, Fujimoto S, Nishida K. Risk factors for development of full-thickness macular holes after pars plana vitrectomy for myopic foveoschisis. Am J Ophthalmol. 2013;155(6):1021–7 e1. doi: 10.1016/j.ajo.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Gass JD. Muller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch Ophthalmol. 1999;117(6):821–3. doi: 10.1001/archopht.117.6.821. [DOI] [PubMed] [Google Scholar]
- 20.Wolf S, Schnurbusch U, Wiedemann P, Grosche J, Reichenbach A, Wolburg H. Peeling of the basal membrane in the human retina: ultrastructural effects. Ophthalmology. 2004;111(2):238–43. doi: 10.1016/j.ophtha.2003.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.