Abstract
Introduction
The Cure Glomerulonephropathy Network (CureGN) is a 66-center longitudinal observational study of patients with biopsy-confirmed minimal change disease, focal segmental glomerulosclerosis, membranous nephropathy, or IgA nephropathy (IgAN), including IgA vasculitis (IgAV). This study describes the clinical characteristics and treatment patterns in the IgA cohort, including comparisons between IgAN versus IgAV and adult versus pediatric patients.
Methods
Patients with a diagnostic kidney biopsy within 5 years of screening were eligible to join CureGN. This is a descriptive analysis of clinical and treatment data collected at the time of enrollment.
Results
A total of 667 patients (506 IgAN, 161 IgAV) constitute the IgAN/IgAV cohort (382 adults, 285 children). At biopsy, those with IgAV were younger (13.0 years vs. 29.6 years, P < 0.001), more frequently white (89.7% vs. 78.9%, P = 0.003), had a higher estimated glomerular filtration rate (103.5 vs. 70.6 ml/min per 1.73 m2, P < 0.001), and lower serum albumin (3.4 vs. 3.8 g/dl, P < 0.001) than those with IgAN. Adult and pediatric individuals with IgAV were more likely than those with IgAN to have been treated with immunosuppressive therapy at or prior to enrollment (79.5% vs. 54.0%, P < 0.001).
Conclusion
This report highlights clinical differences between IgAV and IgAN and between children and adults with these diagnoses. We identified differences in treatment with immunosuppressive therapies by disease type. This description of baseline characteristics will serve as a foundation for future CureGN studies.
Keywords: glomerulonephritis, Henoch-Schönlein purpura (HSP), IgA nephropathy (IgAN), IgA vasculitis (IgAV)
IgAN and IgAV (also known as Henoch-Schönlein purpura nephritis) are commonly encountered glomerular diseases across the age spectrum. IgAN is the most prevalent primary glomerular disorder in the world, affecting approximately 2.5 per 100,000 persons worldwide.1, 2 Similarly, IgAV represents 1 of the most common vasculitides in childhood with a small, but significant, incidence in adults. Although IgAN and IgAV have common kidney pathology, including the hallmark finding of dominant mesangial deposition of aberrantly glycosylated IgA1, they are different in presentation and course.3, 4 Over the past decade, much has been learned about the pathogenesis of IgAN, but progress in the treatment of this disease has been comparatively stagnant,5, 6, 7, 8, 9 and our knowledge about IgAV as a discrete entity remains limited.4 Similar to other glomerular diseases, clinical trials have been sparse and, when present, often have divergent results.10, 11, 12, 13, 14 As a critical step to improve patient treatments and outcomes, we need to understand the pathophysiology, genetics, disease trajectories, long-term outcomes, and biomarkers for patient stratification and prognosis.5, 15
Recognizing this critical knowledge gap, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) funded the CureGN study to establish a primary glomerular disease consortium with a focus on IgAN/IgAV, minimal change disease, focal segmental glomerulosclerosis, and idiopathic membranous nephropathy. Central to the mission of CureGN is the creation of a longitudinal observational cohort of adults and children with a biopsy-proven primary glomerular disease. A unique feature of this cohort is the use of broad inclusion criteria, seeking to capture the breadth of disease by all patients from childhood into adulthood who underwent biopsy within 5 years of enrollment. The study includes baseline and longitudinal follow-up data, digital pathology images, and biospecimens. The creation of a digital pathology repository is a key feature of the CureGN cohort that will allow for standardization of pathology data, grading, and diagnosis across the 66 institutions. The development of such a centralized resource is currently underway; as such, the digital pathology image reporting and analysis will not be presented here.
In May 2017, 30 months after enrollment commenced, IgAN/IgAV was the first CureGN disease cohort to reach its recruitment target of 650 patients. This report represents the initial description of this cohort. The current study has the following aims: (i) to describe the baseline characteristics of patients enrolled in the IgAN/IgAV CureGN cohort; (ii) to compare clinical characteristics of those with IgAN and IgAV; (iii) to compare clinical characteristics of adults and children with IgAN/IgAV; and (iv) to describe treatment patterns in this cohort.
Methods
Study Sample
CureGN (https://curegn.org/) is a 66-center, NIDDK-funded, longitudinal, prospective, observational study. Children (<18 years of age at biopsy) and adults with a diagnostic biopsy within the past 5 years with either IgAN or IgAV were eligible for enrollment. Each enrolling investigator assigned the clinical diagnosis of IgAV based on the presence of both renal and extrarenal manifestations (e.g., palpable purpuric rash, gastrointestinal involvement, arthralgias/arthritis). A CureGN pathologist validated the pathologic diagnosis via review of the pathology report and of slides if indicated. The pathology biopsy criteria included ≥5 glomeruli available for light microscopic evaluation and dominant or co-dominant mesangial IgA staining by immunofluorescence. Electron microscopy was not required for the diagnosis. Biopsy exclusion criteria were findings indicative of another glomerular disease (IgA-dominant post-infectious glomerulonephritis, IgA-dominant or co-dominant lupus glomerulonephritis and IgA-dominant anti−glomerular basement membrane [GBM] antibody nephritis). Cases of IgAV lacking renal involvement were not captured in this cohort.
Exclusion criteria included end-stage kidney disease or any of the following present prior to the first kidney biopsy: solid organ or bone marrow transplant, active HIV infection, hepatitis B or C infection, diabetes mellitus, systemic lupus erythematosus, or active malignancy. Additional pathology exclusion criteria were tubulo-interstitial disease, monoclonal gammopathy-related injury, nonparaprotein amyloidosis, granulomatous interstitial nephritis, infectious interstitial nephritis, IgG4-related disease, or sarcoid-related renal lesions. All enrolled patients provided either informed consent or assent, as appropriate.
The current report presents enrollment data from IgAN/IgAV patients in CureGN. The in-person enrollment visit included demographics, disease history, laboratory data, and clinical characteristics. The latter comprised duration of disease, family history of kidney disease, medication history and current use, as well as comorbidities. Blood and urine samples were also collected at the enrollment visit and processed centrally by the CureGN laboratory to measure serum creatinine and proteinuria in 24-hour, first morning void, or spot urine sample. For adults (aged ≥18 years), the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula and for children the bedside Chronic Kidney Disease in Children Study or CKiD equation were used to calculate the eGFR.16, 17
Statistical Analyses
Continuous variables are expressed as medians with interquartile ranges (IQRs), whereas categorical values are expressed as frequencies and percentages. We report demographic and clinical data at biopsy and at enrollment for each diagnosis (IgAN/IgAV), and for each age group.
We compared demographic, clinical, and medication data between the 2 disease states overall and by age. In addition, we compared the pediatric and adult cohorts separately for the IgAN and IgAV groups. The Mann−Whitney U test was used for continuous variables; a χ2 test was used for categorical variables with at least 5 patients in each group; and the Fisher exact test was used for categorical variables with fewer than 5 patients in at least 1 group. Improvement in variables such as eGFR and proteinuria were defined as absolute change (increase in eGFR and decrease in proteinuria) between biopsy and enrollment. Multivariable linear models were also used to assess the effect of age and disease type on eGFR and log-transformed proteinuria at biopsy and enrollment. All analyses used SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Demographics
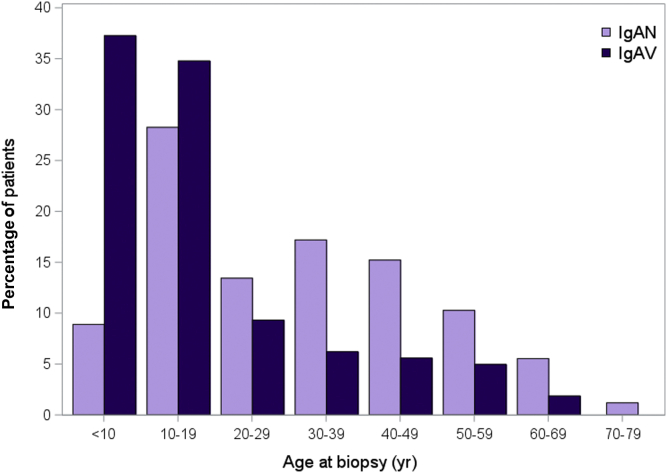
A total of 667 patients are enrolled in the CureGN IgA cohort, including 506 (75.9%) with IgAN and 161 (24.1%) with IgAV. The median disease duration at enrollment was 1 year (IQR = 0.3−2.9). Of the patients, 285 (42.7%) were children at the time of biopsy. In all, 513 of the cohort (81.6%) self-reported as white and 100 (15.1%) as Hispanic/Latino. At the time of biopsy, 64.3% of the CureGN IgA cohort with a urine protein-to-creatinine ratio (UPCR) measurement had a UPCR >1 g/g. Demographic characteristics and laboratory values at biopsy and enrollment are presented in Table 1. Figure 1 shows the age distribution of the cohort by disease.
Table 1.
Patient characteristics in the CureGN IgA nephropathy (IgAN)/IgA vasculitis (IgAV) cohort, by diagnosis
| All N = 667 |
IgAN n = 506 |
IgAV n = 161 |
P valuea | |
|---|---|---|---|---|
| Median (IQR) or n (%) | Median (IQR) or n(%) | Median (IQR) or n (%) | ||
| Demographicsb | ||||
| Age at diagnosis, yr | 23.9 (12.1–40.9) | 28.8 (14.7–43.5) | 12.7 (7.6–22.4) | <0.001 |
| Age at biopsy, yr | 24.3 (12.6–41.8) | 29.6 (15.0–43.9) | 13.0 (8.3–22.4) | <0.001 |
| Time from diagnosis to enrollment, yr | 1.0 (0.3–2.9) | 1.2 (0.3–3.1) | 0.6 (0.2–1.5) | <0.001 |
| Sex, male | 403 (60.4%) | 303 (59.9%) | 100 (62.1%) | 0.61 |
| Race, white | 513 (81.6%) | 374 (78.9%) | 139 (89.7%) | 0.003 |
| Hispanic/Latino | 100 (15.1%) | 81 (16.1%) | 19 (11.8%) | 0.18 |
| Family history of kidney disease | 188 (29.3%) | 149 (30.5%) | 39 (25.3%) | 0.22 |
| At biopsyc | ||||
| UPCR | 1.5 (0.7–3.3) | 1.4 (0.7–3.0) | 1.8 (0.7–4.6) | 0.04 |
| 3 ≤ UPCR | 141 (28.5%) | 89 (24.9%) | 52 (38.0%) | 0.01 |
| 1 ≤ UPCR < 3 | 177 (35.8%) | 140 (39.2%) | 37 (27.0%) | |
| 0.3 ≤ UPCR < 1 | 121 (24.5%) | 86 (24.1%) | 35 (25.5%) | |
| UPCR < 0.3 | 55 (11.1%) | 42 (11.8%) | 13 (9.5%) | |
| Hematuria | ||||
| Negative | 27 (5.5%) | 24 (6.8%) | 3 (2.2%) | 0.03 |
| Trace | 17 (3.5%) | 15 (4.3%) | 2 (1.4%) | |
| 1+ Small, 11–25 | 41 (8.4%) | 33 (9.4%) | 8 (5.8%) | |
| 2+ Moderate, 26–50 | 105 (21.5%) | 78 (22.2%) | 27 (19.6%) | |
| 3+ Large, 51–250 | 299 (61.1%) | 201 (57.3%) | 98 (71.0%) | |
| Serum albumin, g/dl | 3.7 (3.2–4.1) | 3.8 (3.4–4.1) | 3.4 (2.9–3.8) | <0.001 |
| Serum albumin <3 g/dl | 91 (18.2%) | 53 (14.5%) | 38 (27.9%) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 78.7 (46.1–110.9) | 70.6 (41.8–101.6) | 103.5 (70.3–122.5) | <0.001 |
| 90 ≤ eGFR | 239 (41.6%) | 150 (35.4%) | 89 (59.3%) | <0.001 |
| 60 ≤ eGFR < 90 | 131 (22.8%) | 97 (22.9%) | 34 (22.7%) | |
| 30 ≤ eGFR < 60 | 142 (24.7%) | 127 (30.0%) | 15 (10.0%) | |
| eGFR <30 | 62 (10.8%) | 50 (11.8%) | 12 (8.0%) | |
| At enrollmentd | ||||
| UPCR | 0.6 (0.2–1.7) | 0.7 (0.2–1.8) | 0.5 (0.2–1.6) | 0.37 |
| 3 ≤ UPCR | 81 (14.9%) | 58 (14.4%) | 23 (16.2%) | 0.33 |
| 1 ≤ UPCR < 3 | 138 (25.3%) | 110 (27.3%) | 28 (19.7%) | |
| 0.3 ≤ UPCR < 1 | 147 (27.0%) | 104 (25.8%) | 43 (30.3%) | |
| UPCR <0.3 | 179 (32.8%) | 131 (32.5%) | 48 (33.8%) | |
| Hematuria | ||||
| Negative | 84 (16.1%) | 68 (17.6%) | 16 (11.9%) | 0.02 |
| Trace | 48 (9.2%) | 37 (9.6%) | 11 (8.1%) | |
| 1+ Small, 11–25 | 72 (13.8%) | 60 (15.5%) | 12 (8.9%) | |
| 2+ Moderate, 26–50 | 126 (24.1%) | 95 (24.5%) | 31 (23.0%) | |
| 3+ Large, 51–250 | 192 (36.8%) | 127 (32.8%) | 65 (48.1%) | |
| Serum albumin, g/dl | 4.0 (3.6–4.3) | 4.0 (3.7–4.3) | 3.9 (3.4–4.3) | 0.03 |
| Serum albumin <3 g/dl | 48 (9.7%) | 27 (7.3%) | 21 (17.1%) | 0.001 |
| eGFR, ml/min per 1.73 m2 | 82.9 (48.8–105.2) | 75.8 (43.5–100.1) | 100.1 (82.4–118.7) | <0.001 |
| 90 ≤ eGFR | 261 (43.0%) | 167 (36.1%) | 94 (64.8%) | <0.001 |
| 60 ≤ eGFR < 90 | 150 (24.7%) | 116 (25.1%) | 34 (23.4%) | |
| 30 ≤ eGFR < 60 | 133 (21.9%) | 122 (26.4%) | 11 (7.6%) | |
| eGFR <30 | 63 (10.4%) | 57 (12.3%) | 6 (4.1%) | |
| Hypertensione | 121 (19.1%) | 89 (18.5%) | 32 (20.6%) | 0.56 |
| Trajectoryf | ||||
| eGFR higher at enrollment than at biopsy | 215 (40.6%) | 156 (39.6%) | 59 (43.7%) | 0.40 |
| UPCR lower at enrollment than at biopsy | 267 (62.8%) | 186 (62.0%) | 81 (64.8%) | 0.59 |
| UPCR ever <0.3 prior to or at enrollment | 247 (39.3%) | 184 (38.9%) | 63 (40.6%) | 0.70 |
eGFR, estimated glomerular filtration rate; IQR, interquartile range; UPCR, urine protein-to-creatinine ratio.
P value from Mann−Whitney U test for continuous variables, χ2 test, or Fisher exact test for categorical variables.
Less than 6% missing for demographic variables.
Total of 26% of UPCR, 27% of hematuria, 25% of serum albumin, and 14% of eGFR values unavailable at biopsy.
Total of 18% of UPCR, 22% of hematuria, 26% of serum albumin, 9% of eGFR, and 5% of hypertension values missing at enrollment.
Systolic blood pressure >140 or diastolic blood pressure >90 for adults; systolic or diastolic blood pressure >95th percentile for pediatric patients.
Total of 21% of eGFR trajectories and 36% of UPCR trajectories unavailable (trajectories require nonmissing values at biopsy and enrollment), and 6% of patients had no UPCR measurements recorded prior to or at enrollment.
Figure 1.
Distribution of patient age at biopsy, by IgA nephropathy (IgAN) and IgA vasculitis (IgAV).
Overall Comparison of IgAN and IgAV
Comparisons between IgAN and IgAV patients are provided in Table 1. In comparison to patients with IgAN, those with IgAV were younger at diagnosis (median 12.7 years [IQR = 7.6−22.4] vs. 28.8 years [IQR = 14.7−43.5], P < 0.001) and were more likely to be of white race (89.7% vs. 78.9%, P = 0.003). At the time of biopsy, those with IgAV had higher UPCR, more hematuria, and lower serum albumin, but a significantly higher median eGFR (103.5 ml/min per 1.73 m2 [IQR = 70.3−122.5] vs. 70.6 ml/min per 1.73 m2 [IQR = 41.8−101.6], P < 0.001). At the time of enrollment, the IgAV cohort continued to show higher levels of hematuria and lower serum albumin levels, but no significant difference in proteinuria. From biopsy to enrollment, more IgAV patients had an increase in their eGFR and decrease in UPCR compared to IgAN patients (eGFR: 43.7% vs. 39.6%, UPCR: 64.8% vs. 62.0%); however, this difference was not statistically significant. The disease duration at enrollment was shorter for IgAV compared to IgAN.
Comparison of IgAN to IgAV Within Pediatric and Adult Cohorts
Table 2 presents the demographics and laboratory data for adult and pediatric patients, comparing diseases within each age group. Of the 285 pediatric patients, 112 (39.3%) had IgAV. These 112 patients were younger, had shorter disease duration, and at biopsy had a lower serum albumin as well as a higher degree of proteinuria, compared to the 173 children with IgAN. These laboratory differences persisted at enrollment. Although there was a significant difference in eGFR at enrollment, both pediatric cohorts had a median eGFR >95 ml/min per 1.73 m2 at biopsy and enrollment. At the time of biopsy, 50.8% of pediatric IgAN and 64.4% of pediatric IgAV patients with an available UPCR measure had UPCR >1 g/g. There was substantial reduction in proteinuria in both pediatric IgA cohorts from biopsy to enrollment, resulting in normalization of the UPCR to <0.3 g/g in 49.7% and 35.9% with IgAN and IgAV, respectively.
Table 2.
Patient characteristics in the CureGN IgA nephropathy (IgAN)/IgA vasculitis (IgAV) cohort, by diagnosis and age
| IgAN |
IgAV |
P valuea |
||||||
|---|---|---|---|---|---|---|---|---|
| Pediatric n = 173 |
Adult n = 333 |
Pediatric n = 112 |
Adult n = 49 |
IgAN Ped. versus Adult | IgAV Ped. versus Adult | Ped. IgAN versus IgAV | Adult IgAN versus IgAV | |
| Median (IQR) or n (%) | Median (IQR) or n (%) | Median (IQR) or n (%) | Median (IQR) or n (%) | |||||
| Demographicsb | ||||||||
| Age at diagnosis, yr | 12.1 (9.0–14.9) | 38.3 (29.1–50.0) | 9.1 (6.8–13.3) | 34.6 (24.0–48.5) | <0.001 | <0.001 | <0.001 | 0.13 |
| Age at biopsy, yr | 12.5 (9.9–15.2) | 39.6 (30.1–50.7) | 9.5 (6.9–13.6) | 35.4 (25.8–48.5) | <0.001 | <0.001 | <0.001 | 0.13 |
| Time from diagnosis to enrollment, yr | 1.3 (0.4–3.0) | 1.1 (0.3–3.3) | 0.6 (0.2–1.5) | 0.8 (0.2–1.9) | 0.66 | 0.57 | <0.001 | 0.10 |
| Sex, male | 107 (61.8%) | 196 (58.9%) | 71 (63.4%) | 29 (59.2%) | 0.51 | 0.61 | 0.79 | 0.97 |
| Race, white | 136 (82.4%) | 238 (77.0%) | 99 (91.7%) | 40 (85.1%) | 0.17 | 0.22 | 0.03 | 0.21 |
| Hispanic/Latino | 21 (12.2%) | 60 (18.1%) | 12 (10.7%) | 7 (14.3%) | 0.09 | 0.52 | 0.70 | 0.51 |
| Family history of kidney disease | 51 (30.5%) | 98 (30.5%) | 25 (23.6%) | 14 (29.2%) | 1.00 | 0.46 | 0.21 | 0.85 |
| At biopsyc | ||||||||
| UPCR | 1.1 (0.3–2.3) | 1.6 (0.9–3.3) | 2.1 (0.7–5.0) | 1.5 (0.7–3.7) | <0.001 | 0.36 | <0.001 | 0.76 |
| 3 ≤ UPCR | 22 (18.0%) | 67 (28.5%) | 41 (40.6%) | 11 (30.6%) | <0.001 | 0.18 | 0.001 | 0.68 |
| 1 ≤ UPCR < 3 | 40 (32.8%) | 100 (42.6%) | 24 (23.8%) | 13 (36.1%) | ||||
| 0.3 ≤ UPCR < 1 | 32 (26.2%) | 54 (23.0%) | 24 (23.8%) | 11 (30.6%) | ||||
| UPCR <0.3 | 28 (23.0%) | 14 (6.0%) | 12 (11.9%) | 1 (2.8%) | ||||
| Hematuria | ||||||||
| Negative | 4 (3.1%) | 20 (9.0%) | 3 (2.9%) | 0 (0.0%) | <0.001 | 0.84 | 0.49 | 0.01 |
| Trace | 4 (3.1%) | 11 (5.0%) | 2 (2.0%) | 0 (0.0%) | ||||
| 1+ Small, 11–25 | 6 (4.6%) | 27 (12.2%) | 7 (6.9%) | 1 (2.8%) | ||||
| 2+ Moderate, 26–50 | 16 (12.3%) | 62 (28.1%) | 20 (19.6%) | 7 (19.4%) | ||||
| 3+ Large, 51–250 | 100 (76.9%) | 101 (45.7%) | 70 (68.6%) | 28 (77.8%) | ||||
| Serum albumin, g/dl | 3.7 (3.2–4.1) | 3.8 (3.4–4.2) | 3.4 (2.9–3.8) | 3.5 (2.9–3.8) | 0.07 | 0.63 | 0.001 | 0.002 |
| Serum albumin <3 g/dl | 23 (18.9%) | 30 (12.3%) | 28 (27.7%) | 10 (28.6%) | 0.10 | 0.92 | 0.12 | 0.01 |
| eGFR, ml/min per 1.73 m2 | 98.6 (75.9–122.0) | 51.8 (36.0–87.2) | 109.5 (82.4–127.7) | 67.3 (42.4–105.4) | <0.001 | <0.001 | 0.07 | 0.11 |
| 90 ≤ eGFR | 83 (59.7%) | 67 (23.5%) | 73 (67.0%) | 16 (39.0%) | <0.001 | <0.001 | 0.24 | 0.13 |
| 60 ≤ eGFR < 90 | 40 (28.8%) | 57 (20.0%) | 28 (25.7%) | 6 (14.6%) | ||||
| 30 ≤ eGFR < 60 | 13 (9.4%) | 114 (40.0%) | 4 (3.7%) | 11 (26.8%) | ||||
| eGFR <30 | 3 (2.2%) | 47 (16.5%) | 4 (3.7%) | 8 (19.5%) | ||||
| At enrollmentd | ||||||||
| UPCR | 0.3 (0.1–1.0) | 1.0 (0.3–2.2) | 0.5 (0.2–1.3) | 0.8 (0.2–2.4) | <0.001 | 0.22 | 0.07 | 0.54 |
| 3 ≤ UPCR | 14 (9.8%) | 44 (16.9%) | 17 (16.5%) | 6 (15.4%) | <0.001 | 0.24 | 0.13 | 0.91 |
| 1 ≤ UPCR < 3 | 22 (15.4%) | 88 (33.8%) | 16 (15.5%) | 12 (30.8%) | ||||
| 0.3 ≤ UPCR < 1 | 36 (25.2%) | 68 (26.2%) | 33 (32.0%) | 10 (25.6%) | ||||
| UPCR <0.3 | 71 (49.7%) | 60 (23.1%) | 37 (35.9%) | 11 (28.2%) | ||||
| Hematuria | ||||||||
| Negative | 24 (17.4%) | 44 (17.7%) | 15 (15.3%) | 1 (2.7%) | 0.06 | 0.11 | 0.55 | 0.02 |
| Trace | 10 (7.2%) | 27 (10.8%) | 7 (7.1%) | 4 (10.8%) | ||||
| 1+ Small, 11–25 | 17 (12.3%) | 43 (17.3%) | 6 (6.1%) | 6 (16.2%) | ||||
| 2+ Moderate, 26–50 | 29 (21.0%) | 66 (26.5%) | 24 (24.5%) | 7 (18.9%) | ||||
| 3+ Large, 51–250 | 58 (42.0%) | 69 (27.7%) | 46 (46.9%) | 19 (51.4%) | ||||
| Serum albumin, g/dl | 4.0 (3.7–4.3) | 4.0 (3.6–4.3) | 3.9 (3.4–4.2) | 3.9 (3.4–4.3) | 0.78 | 0.56 | 0.05 | 0.51 |
| Serum albumin <3 g/dl | 11 (9.2%) | 16 (6.4%) | 15 (16.1%) | 6 (20.0%) | 0.33 | 0.62 | 0.12 | 0.01 |
| eGFR, ml/min per 1.73 m2 | 96.5 (83.9–117.6) | 53.3 (36.5–84.4) | 104.6 (89.7–121.5) | 80.1 (49.2–100.2) | <0.001 | <0.001 | 0.05 | 0.001 |
| 90 ≤ eGFR | 98 (64.5%) | 69 (22.3%) | 77 (74.0%) | 17 (41.5%) | <0.001 | <0.001 | 0.42 | 0.02 |
| 60 ≤ eGFR < 90 | 48 (31.6%) | 68 (21.9%) | 23 (22.1%) | 11 (26.8%) | ||||
| 30 ≤ eGFR < 60 | 3 (2.0%) | 119 (38.4%) | 2 (1.9%) | 9 (22.0%) | ||||
| eGFR <30 | 3 (2.0%) | 54 (17.4%) | 2 (1.9%) | 4 (9.8%) | ||||
| Hypertensione | 20 (12.1%) | 69 (21.9%) | 24 (22.2%) | 8 (17.0%) | 0.01 | 0.46 | 0.03 | 0.45 |
| Trajectoryf | ||||||||
| eGFR higher at enrollment than at biopsy | 55 (42.3%) | 101 (38.3%) | 40 (39.6%) | 19 (55.9%) | 0.44 | 0.10 | 0.68 | 0.05 |
| UPCR lower at enrollment than at biopsy | 72 (64.9%) | 114 (60.3%) | 66 (68.8%) | 15 (51.7%) | 0.43 | 0.09 | 0.55 | 0.38 |
| UPCR ever <0.3 prior to or at enrollment | 94 (59.1%) | 90 (28.7%) | 46 (42.2%) | 17 (37.0%) | <0.001 | 0.54 | 0.01 | 0.25 |
eGFR, estimated glomerular filtration rate; IQR, interquartile range; UPCR, urine protein-to-creatinine ratio.
P value from Mann−Whitney U test for continuous variables, χ2 test, or Fisher exact test for categorical variables.
Less than 6% missing for demographic variables.
Total of 26% of UPCR, 27% of hematuria, 25% of serum albumin, and 14% of eGFR values unavailable at biopsy.
Total of 18% of UPCR, 22% of hematuria, 26% of serum albumin, 9% of eGFR, and 5% of hypertension values missing at enrollment.
Systolic blood pressure >140 or diastolic blood pressure >90 for adults; systolic or diastolic blood pressure >95th percentile for pediatric patients.
Total of 21% of eGFR trajectories and 36% of UPCR trajectories unavailable (trajectories require nonmissing values at biopsy and enrollment), and 6% of patients had no UPCR measurements recorded prior to or at enrollment.
Among the 382 adult patients (333 [87.2%] with IgAN and 49 [12.8%] with IgAV), demographics were similar across diseases (Table 2). However, those patients with IgAV had significantly more hematuria and lower serum albumin at biopsy. The median eGFR did not differ significantly between adults with IgAN and IgAV at the time of biopsy (51.8 ml/min per 1.73m2 [IQR = 36.0−87.2] vs. 67.3 ml/min per 1.73 m2 [IQR = 42.4−105.4]). However, only 38.3% of adult IgAN patients saw an improvement in eGFR from biopsy to enrollment, compared to 55.9% of IgAV patients (P = 0.05). This resulted in significant differences in eGFR at the time of enrollment: 53.3 ml/min per 1.73 m2 (IQR = 36.5−84.4) for adult IgAN patients versus 80.1 ml/min per 1.73 m2 (IQR = 49.2−100.2) for adult IgAV patients (P = 0.001). At the time of biopsy, 71.1% of adult IgAN and 66.7% of adult IgAV patients with an available UPCR measure had UPCR >1 g/g. Although 59.2% of adult patients saw an improvement in proteinuria from biopsy to enrollment, only 23.1% and 28.2% of IgAN and IgAV patients, respectively, had a UPCR <0.3 g/g at enrollment.
Comparison of Pediatric to Adult Patients by Disease
Demographics (except age) did not differ significantly between adults and children with IgAN (Table 2). However, significant differences in laboratory values were observed. Similarly, when compared to adult patients, children with IgAN had significantly higher median eGFR at the time of biopsy (98.6 ml/min per 1.73 m2 [IQR = 75.9−122.0] vs. 51.8 ml/min per 1.73 m2 [IQR = 36.0−87.2], P < 0.001) and at the time of enrollment (96.5 ml/min per 1.73 m2 [IQR = 83.9−117.6] vs. 53.3 ml/min per 1.73 m2 [IQR = 36.5−84.4], P < 0.001). In contrast, there was little difference in the percentage of pediatric and adult patients with an improvement in eGFR.
For IgAV, proteinuria, hematuria, and serum albumin were similar between pediatric and adult IgAV patients at biopsy and enrollment. Pediatric patients had significantly higher median eGFR at the time of biopsy (109.5 ml/min per 1.73 m2 [IQR = 82.4−127.7] vs. 67.3 ml/min per 1.73 m2 [IQR = 42.4−105.4], P < 0.001) and at the time of enrollment (104.6 ml/min per 1.73 m2 [IQR = 89.7−121.5] vs. 80.1 ml/min per 1.73 m2 [IQR = 49.2−100.2], P < 0.001). From biopsy to enrollment, the percentage of pediatric IgAV patients with improved eGFR was not statistically significantly different compared to that for adults, or for improvement in UPCR.
To begin to investigate the role of disease type and age on the degree of proteinuria and eGFR, preliminary analyses were performed evaluating the impact of each variable on these outcomes. In a multivariable model, age group (pediatric vs. adult, P < 0.001) and disease classification (IgAN vs. IgAV, P <0 .001) predicted eGFR at enrollment. Results were similar for eGFR at biopsy. In a similar model evaluating the degree of proteinuria at enrollment, only age group was significant (P < 0.001) and disease type was not. At biopsy, age group and disease type were both significant (P = 0.002 and P = 0.01, respectively). Although the impact of age was expected, the results also suggest an impact of disease type after accounting for age. It will be important to follow these patients longitudinally to better understand the impact of age and to assess whether there are fundamental disease differences as well.
Treatment
Table 3 presents a description of the immunosuppression treatment at or prior to enrollment, comparing patients with IgAN and IgAV. Individuals with IgAV were more likely to receive immunosuppressive therapy (79.5% vs. 54.0%, P < 0.001). Of the patients with IgAV, 35% had been treated with ≥2 immunosuppressive medications at the time of enrollment, compared to 20.5% of those with IgAN. Individuals with IgAV were significantly more likely to receive cyclophosphamide and corticosteroids at or prior to enrollment. The difference in number of immunosuppressive drug exposures persisted when the pediatric and adult cohorts were analyzed separately (Table 4). Among pediatric patients, those with IgAV were more likely to receive corticosteroids compared to those with IgAN (73.2% vs. 46.2%, P < 0.001). In the adult cohort, those with IgAV were more likely to receive cyclophosphamide and corticosteroids compared with IgAN (16.3% vs. 4.5%, P = 0.001 and 85.7% vs. 53.2%, P < 0.001, respectively). Although there was not a statistically significant difference in use of renin−angiotensin−aldosterone system (RAAS) blockade between diseases, adult patients were more likely to receive RAAS blockade irrespective of diagnosis (88.5% vs. 65.7%, P < 0.001).
Table 3.
Immunosuppression use, by diagnosisa
| IgAN n = 506 |
IgAV n = 161 |
P value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Number of medication classes usedb | |||
| 0 | 232 (46.0%) | 33 (20.5%) | <0.001 |
| 1 | 169 (33.5%) | 71 (44.1%) | |
| 2 | 77 (15.3%) | 49 (30.4%) | |
| 3 | 23 (4.6%) | 5 (3.1%) | |
| 4 | 3 (0.6%) | 3 (1.9%) | |
| Medication class | |||
| Cyclophosphamide | 22 (4.3%) | 17 (10.6%) | 0.004 |
| Azathioprine | 22 (4.3%) | 13 (8.1%) | 0.07 |
| Mycophenolate mofetil | 61 (12.1%) | 28 (17.4%) | 0.09 |
| Corticosteroids | 257 (50.8%) | 124 (77.0%) | <0.001 |
| RAAS blockade | 411 (81.2%) | 113 (70.2%) | 0.002 |
Immunosuppression use at or before enrollment visit.
Missing medication data for 2 patients.
Table 4.
Immunosuppression use, by diagnosis and agea
| Pediatric |
P value | Adult |
P value | |||
|---|---|---|---|---|---|---|
| IgAN n = 173 |
IgAV n = 112 |
IgAN n = 333 |
IgAV n = 49 |
|||
| n (%) | n (%) | n (%) | n (%) | |||
| Number of medications usedb | ||||||
| 0 | 85 (49.7%) | 27 (24.1%) | <0.001 | 147 (44.1%) | 6 (12.2%) | <0.001 |
| 1 | 44 (25.7%) | 44 (39.3%) | 125 (37.5%) | 27 (55.1%) | ||
| 2 | 34 (19.9%) | 37 (33.0%) | 43 (12.9%) | 12 (24.5%) | ||
| 3 | 8 (4.7%) | 2 (1.8%) | 15 (4.5%) | 3 (6.1%) | ||
| 4 | 0 (0.0%) | 0 (0.0%) | 3 (0.9%) | 1 (2.0%) | ||
| Medication class | ||||||
| Cyclophosphamide | 7 (4.0%) | 9 (8.0%) | 0.16 | 15 (4.5%) | 8 (16.3%) | 0.001 |
| Azathioprine | 9 (5.2%) | 10 (8.9%) | 0.23 | 13 (3.9%) | 3 (6.1%) | 0.47 |
| Mycophenolate mofetil | 25 (14.5%) | 23 (20.5%) | 0.19 | 36 (10.8%) | 5 (10.2%) | 0.90 |
| Corticosteroids | 80 (46.2%) | 82 (73.2%) | <0.001 | 177 (53.2%) | 42 (85.7%) | <0.001 |
| RAAS blockade | 116 (67.1%) | 70 (62.5%) | 0.36 | 295 (88.6%) | 43 (87.8%) | 0.86 |
Immunosuppression use at or before enrollment visit.
Missing medication data for 2 patients.
Discussion
The CureGN study represents a large, collaborative, multicenter effort to address major knowledge gaps in the field of primary glomerular diseases. We present data on the IgA cohort, the first of the 4 CureGN cohorts to reach the enrollment target. This cohort represents, to the best of our knowledge, the largest prospective cohort of patients with IgA kidney disease with full clinical data, biospecimens, and centralized digital pathology. A unique feature of this cohort is the wide range of patients enrolled, including both IgAN and IgAV, as well as children and adults. A breadth of chronic kidney disease stages are well represented in CureGN, and many participants are at moderate to high risk of progression, based on proteinuria and eGFR at biopsy and/or enrollment. Taking advantage of the diversity of this cohort, we are able to demonstrate important differences in clinical features of IgAV and IgAN between children and adults. Furthermore, we describe differences in the treatment patterns between IgAN and IgAV, showing that those with IgAV are more likely to be treated aggressively with immunosuppressive medications but are less likely to receive standard supportive care with RAAS inhibition.
Current clinical practice guidelines and observational studies suggest that patients with IgAN with UPCR >1 g/g have a moderate to high risk of progressive kidney function loss.10, 18 Using this cutpoint, we demonstrate that there are significant differences in the evolution of proteinuria in children and adults within this cohort. Based on reported proteinuria at the time of biopsy, 64.3% of the CureGN IgA cohort with an available UPCR measure had a UPCR >1 g/g, including 50.8% of pediatric IgAN, 71.1% of adult IgAN, 64.4% of pediatric IgAV, and 66.7% of adult IgAV patients. There was substantial reduction in proteinuria in both pediatric IgA disease groups between biopsy and enrollment, resulting in normalization of the UPCR to <0.3 g/g in 49.7% and 35.9% of children at the time of enrollment with IgAN and IgAV, respectively. In contrast, although more than half of the adult patients saw an improvement in proteinuria between biopsy and enrollment, only 23.1% and 28.2% of IgAN and IgAV adult patients, respectively, had a UPCR <0.3 g/g at enrollment.
Age-based differences were also observed in eGFR at the time of biopsy. Specifically, the eGFR was quite low in adults with IgAN with a median of 51.8 ml/min per 1.73 m2 and IgAV of 67.3 ml/min per 1.73 m2 compared with children (98.6 ml/min per 1.73 m2 and 109.5 ml/min per 1.73 m2, respectively). Between biopsy and enrollment, approximately 40% of all IgAN patients and pediatric IgAV patients had an improvement in eGFR, whereas 55.9% of adult IgAV patients showed an improvement. Longer observation will help to improve our understanding of differential eGFR trajectories in these patients. The long-term observation planned for the CureGN study, the anticipated entry of digitized pathology of the kidney biopsies into the CureGN database, and the availability of serial biological samples to test for biomarkers will be key components in the effort to better predict renal prognosis and to identify which patients with IgA disease may benefit the most from available therapies.
Antiproteinuric therapy using angiotensin-converting enzyme inhibitors or angiotensin receptor blockers is a hallmark of treating IgAN. Not surprisingly, use of RAAS blockers was common in CureGN participants with IgAN and IgAV, with approximately 90% of adult and 65% of pediatric participants using these therapies at some point in their disease course at or prior to enrollment. However, pediatric IgAV patients were more likely to receive immunosuppressant medications than RAAS blockade. Outside of the crescentic forms of IgAN and IgAV, the use of immunosuppression in these diseases remains controversial. A number of small randomized studies have shown efficacy in adding a course of corticosteroids to RAAS blockade for patients with IgAN.19, 20, 21 The 2012 KDIGO Clinical Practice Guideline for Glomerulonephritis suggests that IgAN patients with persistent proteinuria >1 g/d, despite optimal supportive care, receive a 6-month course of corticosteroids. However, the more recent STOP-IgAN (Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy) trial10 and TESTING (The Therapeutic Evaluation of Steroids in IgA Nephropathy Global) study12 have questioned the benefit of such a treatment strategy. In the CureGN cohort, half of the IgAN patients received immunosuppression with corticosteroids (257 of 506, 50.8%), clearly the most commonly used agent. Approximately 75% of the IgAV patients were treated with a course of corticosteroids, although it is unclear whether therapy was targeted primarily at kidney involvement, as these percentages do not correlate with the prevalence of proteinuria >1 g/d at enrollment. Also of interest, many pediatric and adult patients with IgAN or IgAV received other immunosuppressive agents—including cyclophosphamide, azathioprine, and mycophenolate mofetil—for which the evidence base is even less conclusive than that for corticosteroids. Recently, there has been a call to tailor treatment with corticosteroids (and other immunomodulatory agents) in IgAN and IgAV to those patients who will receive the most benefit and the least harm.22 The CureGN cohort will couple the clinical data presented here with detailed histopathology, biomarker, genetic, and longitudinal follow-up data on all its participants. This cohort, therefore, is well positioned to begin to inform these important questions.
Although our study has a number of strengths, there are several limitations that should be acknowledged. The main limitation is that the centralized digital pathology repository is currently under development, via which standardized biopsy scoring will be performed for this cohort. These data will surely complement the clinical data presented in this analysis, but will not be complete for several years. Nevertheless, the patients included in our cohort meet strict biopsy diagnostic criteria based on local pathology evaluations. Another limitation is that for prevalent patients, some of the clinical data were collected retrospectively and as such are subject to inherent issues such as unavailability, and a lack of granularity, which can occur in circumstances such as transfer of care following diagnosis. To this end, some particular limitations include data about the timing and duration of RAAS blockade prior to enrollment and the degree of missing data (including 25% with unavailable urine data at biopsy). Finally, the time for analysis in the cohort is still relatively short, encompassing the time from biopsy diagnosis to study enrollment. This precludes detailed analysis of kidney disease outcomes and limits our analyses to assessment of baseline data at the time of biopsy and enrollment. Furthermore, the time from biopsy to enrollment differed significantly between diseases, so caution should be paid when interpreting changes in eGFR and proteinuria between disease cohorts. Despite these limitations, our results already provide several novel insights about clinical features of these disorders, and highlight important differences in the existing treatment strategies.
In summary, the CureGN cohort represents the largest multicenter, prospectively followed cohort of IgAN and IgAV patients. The prospective design of the CureGN study, along with its stringent biopsy-based enrollment criteria, make this cohort less susceptible to the confounding factors inherent to prior retrospective and cross-sectional analyses.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 Important features of the cohort include the following: (i) enrollment of participants within 5 years of kidney biopsy; (ii) inclusion of both IgAN and IgAV; (iii) inclusion of all chronic kidney disease stages except dialysis and transplantation; (iv) inclusion of pediatric and adult patients; (v) rigorous and standardized prospective data collection across multiple sites; (vi) comprehensive longitudinal biobank for all recruited participants; and (vii) the emerging standardized central digital pathology repository. Our baseline description of this unique cohort lays the foundation for future clinical and translational studies of IgA-related glomerulonephritis within the CureGN study. The long-term observation planned for the CureGN study, the anticipated availability of digital kidney biopsy pathology, and the collection of serial biological samples to permit biomarker analysis will be key components in better predicting renal prognosis and identifying patients most appropriate for specific therapies.
Disclosure
GBA has consulting fees or paid advisory boards for Alexion, Mallincrodt, Bristol-Meyers Squibb, Pfizer, Takeda, Genentech, Merck, and Sanofi, has received lecture fees from Takeda, Genentech, and Sanofi, and receives grant support from Bristol-Myers Squibb, Genentech, Regulus, Achillion and travel support from all consultations and grants. DCC has consulting fees or paid advisory boards with Mallincrodt and Nefigan for the known future and receives grant support from NIDDK/Neptune. VDD’A receives grant support from the National Institutes of Health (NIH)/NIDDK. CJDA-S received consulting fees/paid advisory board for Advicenne, receives grant support from CureGN, and has served as an expert witness in 2017 for a 1-day trial. JJH received consulting fees or paid advisory boards from Variant, Dimerix, GlaxoSmithKline, and Aurinia, and in the known future will receive them for Variant and Dimerix. BAJ has received consulting fees from Visterra, has equity ownership/stock options in Reliant Glycosciences, LLC, has grant support under negotiation from Retrophin and Shire, and has a US patent assigned to University of Alabama at Birmingham Research foundation. RAL receives consulting fees/paid advisory boards from Mallinckrodt, Inc., Rigel, Inc., and Genentech, Inc., and receives grant support for the MENTOR study, Protocol number NCT01180036. HL has received consulting fees or paid advisory boards for Alexion and Michael and Wells Law Firm and receives grant support from the NIH. SM receives grant support from the Childhood Arthritis and Rheumatology Research Alliance. PHN receives grant support from NIDDK/Rare Disease Clinical Research Network. CMN has consulting fees/paid advisory boards from Achillion and receives grant support from Retrophine-Site PI on Duet. JN has received consulting fees/paid advisory boards from Visterra, has equity ownership/stock options in Reliant Glycosciences LLC, has grant support under negotiation with Retrophin and Shire, and has a US patent assigned to University of Alabama at Birmingham Research Foundation. MNR receives grant support from Reata, Retrophin, Regulus, and Novartis, and is negotiating with Roche. DVR has equity ownership/stock options in Reliant Glycosciences LLC, receives grant support from Reata Pharmaceuticals, Fast Biomedical, and AbbVie Inc. and has grant support under negotiation with Retrophin, Inc. and Calliditas. TS receives grant support from the NIH, Retrophin, Bristol-Myers Squibb, and Mallinckcroft. KT receives grant support from NIDDK, Takeda/Arbor, Alexion, Novartis, and Kaneka. DJW received lecture fees from Alexion Pharmaceuticals. BWG receives grant support for CureGN and Neptune. DSG has consulting agreements between the University of Michigan and the following entities: Janssen, Dimerix, and Bristol-Myers Squibb, and receives grant support from the NIH, the Centers for Disease Control and Prevention (CDC), the Patient-Centered Outcomes Research Institute (PCORI), Bristol-Myers Squibb, NephCure Kidney International, Variant, Inc., Complexa, Inc., Retrophin, Inc., Carolinas Medical Foundation, and the American Heart Association (total funding includes full project budgets [direct, indirect, and subcontracts to external sites]), and has funding under negotiation with Goldfinch Biopharma. LAG receives grant support from Bristol-Myers Squibb. LBH receives consulting fees or paid advisory boards from Bristol-Myers Squibb and in the known future with Bristol-Myers Squibb and receives grant support from the NIH. MK receives grant support from the NIH, University of Michigan, and Goldfinch Biopharma, and is in negotiation with Boehringer-Ingelheim. BMR is Principal Investigator for the Dialysis Outcomes and Practice Patterns Study (DOPPS) Program, which is supported by Amgen, Kyowa Hakko Kirin, AbbVie, Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma. Support for specific projects and countries is provided by Keryx Biopharmaceuticals, Merck Sharp & Dohme, Proteon Therapeutics, Relypsa, F. Hoffmann-LaRoche Benevolence Health Center (BHC) Medical Janssen Takeda, the Kidney Foundation of Canada Hexal DGfN Shire, and the WiNe Institute; for Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) the Japanese Society for Peritoneal Dialysis; and NIH NIDDK funding for United States Renal Data System (USRDS) and CureGN. WES receives consulting fees/paid advisory boards from Pfizer External Pediatric Committee and receives grant support from NIDDK CureGN Ancillary R01 and the NIDDK CureGN Grant. LMG-W receives grant support from the NIH (National Center for Advancing Translational Sciences [NCATS], NIDDK, and National Institute of Child Health and Human Development [NICHD]). KK receives grant support from the NIDDK and IgA Foundation of America. All the other authors declared no competing interests.
David T. Selewski, confirms that he has had full access to the data in the study and final responsibility for the decision to submit for publication.
Acknowledgments
Funding for the CureGN consortium is provided by UM1DK100845, UM1DK100846, UM1DK100876, UM1DK100866, and UM1DK100867 from the NIDDK. Patient recruitment is supported by NephCure Kidney International. Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial assistance.
Contributor Information
David T. Selewski, Email: dselewsk@med.umich.edu.
Krzysztof Kiryluk, Email: kk473@columbia.edu.
CureGN Consortium:
Ali Gharavi, Wooin Ahn, Gerald B. Appel, Rupali S. Avasare, Revekka Babayev, Ibrahim Batal, Andrew S. Bomback, Eric Brown, Eric S. Campenot, Pietro Canetta, Brenda Chan, Vivette D. D’Agati, Hilda Fernandez, Bartosz Foroncewicz, Gian Marco Ghiggeri, William H. Hines, Namrata G. Jain, Krzysztof Kiryluk, Fangming Lin, Francesca Lugani, Maddalena Marasa, Glen Markowitz, Sumit Mohan, Krzysztof Mucha, Thomas L. Nickolas, Jai Radhakrishnan, Maya K. Rao, Renu Regunathan-Shenk, Simone Sanna-Cherchi, Dominick Santoriello, Michael B. Stokes, Natalie Yu, Anthony M. Valeri, Ronald Zviti, Larry A. Greenbaum, William E. Smoyer, Amira Al-Uzri, Isa Ashoor, Diego Aviles, Rossana Baracco, John Barcia, Sharon Bartosh, Craig Belsha, Michael C. Braun, Aftab Chishti, Donna Claes, Carl Cramer, Keefe Davis, Elif Erkan, Daniel Feig, Michael Freundlich, Melisha Hanna, Guillermo Hidalgo, Amrish Jain, Myda Khalid, Mahmoud Kallash, Jerome C. Lane, John Mahan, Nisha Mathews, Carla Nester, Cynthia Pan, Hiren Patel, Adelaide Revell, Rajasree Sreedharan, Julia Steinke, Scott E. Wenderfer, Craig S. Wong, Ronald Falk, William Cook, Vimal Derebail, Agnes Fogo, Adil Gasim, Todd Gehr, Raymond Harris, Jason Kidd, Louis-Philippe Laurin, Will Pendergraft, Vincent Pichette, Thomas Brian Powell, Matthew B. Renfrow, Virginie Royal, Lawrence B. Holzman, Sharon Adler, Charles Alpers, Raed Bou Matar, Elizabeth Brown, Daniel Cattran, Michael Choi, Katherine M. Dell, Ram Dukkipati, Fernando C. Fervenza, Alessia Fornoni, Crystal Gadegbeku, Patrick Gipson, Leah Hasely, Sangeeta Hingorani, Michelle A. Hladunewich, Jonathan Hogan, J. Ashley Jefferson, Kenar Jhaveri, Duncan B. Johnstone, Frederick Kaskel, Amy Kogan, Jeffrey Kopp, Kevin V. Lemley, Laura Malaga- Dieguez, Kevin Meyers, Alicia Neu, Michelle Marie O’Shaughnessy, John F. O’Toole, Rulan Parekh, Heather Reich, Kimberly Reidy, Helbert Rondon, Kamalanathan K. Sambandam, John R. Sedor, David T. Selewski, Christine B. Sethna, Jeffrey Schelling, C. John Sperati, Agnes Swiatecka-Urban, Howard Trachtman, Katherine R. Tuttle, Joseph Weisstuch, Olga Zhdanova, Brenda Gillespie, Debbie S. Gipson, Matthias Kretzler, Bruce M. Robinson, Laura Barisoni, Sarah Mansfield, Laura Mariani, Cynthia C. Nast, Matthew Wladkowski, Jarcy Zee, and Lisa M. Guay-Woodford
Appendix
CureGN Consortium Members
Collaborators: The CureGN Consortium members listed below (from within the 4 Participating Clinical Center networks and the Data Coordinating Center, authors above have been removed from this list) are collaborators on this manuscript and should be indexed in PubMed as collaborators on this manuscript. *CureGN Principal Investigators.
Columbia University: Ali Gharavi*, Columbia; Wooin Ahn, Columbia; Gerald B. Appel, Columbia; Rupali S. Avasare, Columbia; Revekka Babayev, Columbia; Ibrahim Batal, Columbia; Andrew S. Bomback, Columbia; Eric Brown, Columbia; Eric S. Campenot, Columbia; Pietro Canetta, Columbia; Brenda Chan, Columbia; Vivette D. D’Agati, Columbia; Hilda Fernandez, Columbia; Bartosz Foroncewicz, University of Warsaw; Gian Marco Ghiggeri, Gaslini Children’s Hospital, Italy; William H. Hines, Columbia; Namrata G. Jain, Columbia; Krzysztof Kiryluk, Columbia; Fangming Lin, Columbia; Francesca Lugani, Gaslini Children’s Hospital, Italy; Maddalena Marasa, Columbia; Glen Markowitz, Columbia; Sumit Mohan, Columbia; Krzysztof Mucha, University of Warsaw; Thomas L. Nickolas, Columbia; Jai Radhakrishnan, Columbia; Maya K. Rao, Columbia; Renu Regunathan-Shenk, Columbia; Simone Sanna-Cherchi, Columbia; Dominick Santoriello, Columbia; Michael B. Stokes, Columbia; Natalie Yu, Columbia; Anthony M. Valeri, Columbia; and Ronald Zviti, Columbia.
Midwest Pediatric Nephrology Consortium: Larry A. Greenbaum*, Emory University; William E. Smoyer*, Nationwide Children’s; Amira Al-Uzri, Oregon Health & Science University; Isa Ashoor, Louisiana State University Health Sciences Center; Diego Aviles, Louisiana State University Health Sciences Center; Rossana Baracco, Children’s Hospital of Michigan; John Barcia, University of Virginia; Sharon Bartosh, University of Wisconsin; Craig Belsha, Saint Louis University/Cardinal Glennon; Michael C. Braun, Baylor College of Medicine/Texas Children’s Hospital; Aftab Chishti, University of Kentucky; Donna Claes, Cincinnati Children’s Hospital; Carl Cramer, Mayo Clinic; Keefe Davis, Washington University in St. Louis; Elif Erkan, Cincinnati Children’s Hospital Medical Center; Daniel Feig, University of Alabama, Birmingham; Michael Freundlich, University of Miami/Holtz Children’s Hospital; Melisha Hanna, Children’s Colorado/University of Colorado; Guillermo Hidalgo, East Carolina University; Amrish Jain, Children’s Hospital of Michigan; Myda Khalid, JW Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, Indiana; Mahmoud Kallash, Nationwide Children’s Hospital; Jerome C. Lane, Feinberg School of Medicine, Northwestern University; John Mahan, Nationwide Children’s; Nisha Mathews, University of Oklahoma Health Sciences Center; Carla Nester, University of Iowa Stead Family Children’s Hospital; Cynthia Pan, Medical College of Wisconsin; Hiren Patel, Nationwide Children’s Hospital; Adelaide Revell, Nationwide Children’s Hospital; Rajasree Sreedharan, Medical College of Wisconsin; Julia Steinke, Helen DeVos Children’s Hospital; Scott E. Wenderfer, Baylor College of Medicine/Texas Children’s Hospital; and Craig S. Wong, University of New Mexico Health Sciences Center.
The University of North Carolina (UNC): Ronald Falk*, UNC; William Cook, University of Alabama at Birmingham; Vimal Derebail, UNC; Agnes Fogo, Vanderbilt; Adil Gasim, UNC; Todd Gehr, Virginia Commonwealth University; Raymond Harris, Vanderbilt; Jason Kidd, Virginia Commonwealth University; Louis-Philippe Laurin, Maisonneuve-Rosemont Hospital; Will Pendergraft, UNC; Vincent Pichette, Hôpital Maisonneuve-Rosemont Montreal; Thomas Brian Powell, Columbia Nephrology Associates; Matthew B. Renfrow, University of Alabama at Birmingham; and Virginie Royal, Hôpital Maisonneuve-Rosemont Montreal.
University of Pennsylvania: Lawrence B. Holzman*, University of Pennsylvania; Sharon Adler, Los Angeles Biomedical Research Institute at Harbor, University of California Los Angeles; Charles Alpers, University of Washington; Raed Bou Matar, Cleveland Clinic; Elizabeth Brown, University of Texas Southwestern Medical Center; Daniel Cattran, University of Toronto; Michael Choi, Johns Hopkins; Katherine M. Dell, Case Western/Cleveland Clinic; Ram Dukkipati, Los Angeles Biomedical Research Institute at Harbor University of California Los Angeles; Fernando C. Fervenza, Mayo Clinic; Alessia Fornoni, University of Miami; Crystal Gadegbeku, Temple University; Patrick Gipson, University of Michigan; Leah Hasely, University of Washington; Sangeeta Hingorani, Seattle Children’s Hospital; Michelle A. Hladunewich, University of Toronto/Sunnybrook; Jonathan Hogan, University of Pennsylvania; J. Ashley Jefferson, University of Washington; Kenar Jhaveri, North Shore University Hospital; Duncan B. Johnstone, Temple University; Frederick Kaskel, Montefiore Medical Center; Amy Kogan, Children’s Hospital of Philadelphia; Jeffrey Kopp, NIDDK Intramural Research Program; Kevin V. Lemley, Children’s Hospital of Los Angeles; Laura Malaga- Dieguez, New York University; Kevin Meyers, Children’s Hospital of Pennsylvania; Alicia Neu, Johns Hopkins; Michelle Marie O'Shaughnessy, Stanford; John F. O’Toole, Case Western/Cleveland Clinic; Rulan Parekh, University Health Network, Hospital for Sick Children; Heather Reich, University Health Network; Kimberly Reidy, Montefiore Medical Center; Helbert Rondon, University of Pittsburgh Medical Center; Kamalanathan K. Sambandam, University of Texas Southwestern; John R. Sedor, Case Western/Cleveland Clinic; David T. Selewski, University of Michigan; Christine B. Sethna, Cohen Children's Medical Center, Zucker School of Medicine at Hofstra/Northwell; Jeffrey Schelling, Case Western; C. John Sperati, Johns Hopkins; Agnes Swiatecka-Urban, Children’s Hospital of Pittsburgh; Howard Trachtman, New York University; Katherine R. Tuttle, Spokane Providence Medical Center; Joseph Weisstuch, New York University; and Olga Zhdanova, New York University.
Data Coordinating Center: Brenda Gillespie*, University of Michigan; Debbie S Gipson*, University of Michigan; Matthias Kretzler*, University of Michigan; Bruce M. Robinson*, Arbor Research Collaborative for Health; Laura Barisoni, University of Miami; Sarah Mansfield, Arbor Research Collaborative for Health; Laura Mariani, University of Michigan; Cynthia C. Nast, Cedars-Sinai Medical Center; Matthew Wladkowski, Arbor Research Collaborative for Health; and Jarcy Zee, Arbor Research Collaborative for Health.
Steering Committee Chair: Lisa M. Guay-Woodford, Children’s National Health System.
References
- 1.McGrogan A., Franssen C.F., de Vries C.S. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 2.Kiryluk K., Li Y., Sanna-Cherchi S. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8:e1002765. doi: 10.1371/journal.pgen.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 4.Davin J.C., Coppo R. Henoch-Schonlein purpura nephritis in children. Nat Rev Nephrol. 2014;10:563–573. doi: 10.1038/nrneph.2014.126. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H., Kiryluk K., Novak J. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiryluk K., Novak J. The genetics and immunobiology of IgA nephropathy. J Clin Invest. 2014;124:2325–2332. doi: 10.1172/JCI74475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novak J., Rizk D., Takahashi K. New insights into the pathogenesis of IgA nephropathy. Kidney Dis (Basel) 2015;1:8–18. doi: 10.1159/000382134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai K.N., Tang S.C., Schena F.P. IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. doi: 10.1038/nrdp.2016.1. [DOI] [PubMed] [Google Scholar]
- 9.Knoppova B., Reily C., Maillard N. The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol. 2016;7:117. doi: 10.3389/fimmu.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauen T., Eitner F., Fitzner C. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373:2225–2236. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 11.Lafayette R.A., Canetta P.A., Rovin B.H. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol. 2017;28:1306–1313. doi: 10.1681/ASN.2016060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv J., Zhang H., Wong M.G. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318:432–442. doi: 10.1001/jama.2017.9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogg R.J., Lee J., Nardelli N. Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol. 2006;1:467–474. doi: 10.2215/CJN.01020905. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa N., Honda M., Iijima K. Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clin J Am Soc Nephrol. 2006;1:511–517. doi: 10.2215/CJN.01120905. [DOI] [PubMed] [Google Scholar]
- 15.Gharavi A.G., Kiryluk K., Choi M. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43:321–327. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz G.J., Munoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 19.Lv J.C., Zhang H., Chen Y.Q. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2009;53:26–32. doi: 10.1053/j.ajkd.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Manno C., Torres D.D., Rossini M. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009;24:3694–3701. doi: 10.1093/ndt/gfp356. [DOI] [PubMed] [Google Scholar]
- 21.Pozzi C., Andrulli S., Del Vecchio L. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157–163. doi: 10.1097/01.asn.0000103869.08096.4f. [DOI] [PubMed] [Google Scholar]
- 22.O'Shaughnessy M.M., Lafayette R.A. Corticosteroids for IgA nephropathy: TESTING for benefit, discovering harm. JAMA. 2017;318:429–431. doi: 10.1001/jama.2017.9359. [DOI] [PubMed] [Google Scholar]
- 23.Calvo-Rio V., Loricera J., Martin L. Henoch-Schonlein purpura nephritis and IgA nephropathy: a comparative clinical study. Clin Exp Rheumatol. 2013;31:S45–S51. [PubMed] [Google Scholar]
- 24.Coppo R., Troyanov S., Bellur S. Validation of the Oxford Classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828–836. doi: 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong S., Ahn S.M., Lim D.H. Late-onset IgA vasculitis in adult patients exhibits distinct clinical characteristics and outcomes. Clin Exp Rheumatol. 2016;34:S77–S83. [PubMed] [Google Scholar]
- 26.Kang Y., Park J.S., Ha Y.J. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schonlein purpura. J Korean Med Sci. 2014;29:198–203. doi: 10.3346/jkms.2014.29.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu H., Fujimoto S., Yoshikawa N. Clinical manifestations of Henoch-Schonlein purpura nephritis and IgA nephropathy: comparative analysis of data from the Japan Renal Biopsy Registry (J-RBR) Clin Exp Nephrol. 2016;20:552–560. doi: 10.1007/s10157-015-1177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu S., Liu D., Xiao J. Comparison between adults and children with Henoch-Schonlein purpura nephritis. Pediatr Nephrol. 2015;30:791–796. doi: 10.1007/s00467-014-3016-z. [DOI] [PubMed] [Google Scholar]
- 29.Mao S., Xuan X., Sha Y. Clinico-pathological association of Henoch-Schoenlein purpura nephritis and IgA nephropathy in children. Int J Clin Exp Pathol. 2015;8:2334–2342. [PMC free article] [PubMed] [Google Scholar]
- 30.Mao Y., Yin L., Huang H. Henoch-Schonlein purpura in 535 Chinese children: clinical features and risk factors for renal involvement. J Int Med Res. 2014;42:1043–1049. doi: 10.1177/0300060514530879. [DOI] [PubMed] [Google Scholar]
- 31.Mohey H., Laurent B., Mariat C. Validation of the absolute renal risk of dialysis/death in adults with IgA nephropathy secondary to Henoch-Schonlein purpura: a monocentric cohort study. BMC Nephrol. 2013;14:169. doi: 10.1186/1471-2369-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moriyama T., Tanaka K., Iwasaki C. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh H.J., Ahn S.V., Yoo D.E. Clinical outcomes, when matched at presentation, do not vary between adult-onset Henoch-Schonlein purpura nephritis and IgA nephropathy. Kidney Int. 2012;82:1304–1312. doi: 10.1038/ki.2012.302. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka S., Ninomiya T., Katafuchi R. The effect of renin-angiotensin system blockade on the incidence of end-stage renal disease in IgA nephropathy. Clin Exp Nephrol. 2016;20:689–698. doi: 10.1007/s10157-015-1195-y. [DOI] [PubMed] [Google Scholar]
- 35.Trimarchi H., Barratt J., Cattran D.C. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Zeng C.H., Le W., Ni Z. A multicenter application and evaluation of the Oxford Classification of IgA nephropathy in adult Ahinese patients. Am J Kidney Dis. 2012;60:812–820. doi: 10.1053/j.ajkd.2012.06.011. [DOI] [PubMed] [Google Scholar]