Abstract
Introduction
Little is known of the clinical outcomes of secondary oxalate nephropathy. To inform clinical practice, we performed a systematic review of case reports and case series to examine the clinical characteristics and outcomes of patients with secondary oxalate nephropathy.
Methods
Electronic databases were searched for case reports and case series of individual cases or cohorts of patients with biopsy-proven oxalate nephropathy in native or transplanted kidneys from 1950 until January 2018.
Results
Fifty-seven case reports and 10 case series met the inclusion criteria, totaling 108 patients. The case series were meta-analyzed. Mean age was 56.4 years old, 59% were men, and 15% were kidney transplant recipients. Fat malabsorption (88%) was the most commonly attributed cause of oxalate nephropathy, followed by excessive dietary oxalate consumption (20%). The mean baseline serum creatinine was 1.3 mg/dl and peaked at 4.6 mg/dl. Proteinuria, hematuria, and urinary crystals was reported in 69%, 32%, and 26% of patients, respectively. Mean 24-hour urinary oxalate excretion was 85.4 mg/d. In addition to universal oxalate crystal deposition in tubules and/or interstitium, kidney biopsy findings included acute tubular injury (71%), tubular damage and atrophy (69%), and interstitial mononuclear cell infiltration (72%); 55% of patients required dialysis. None had complete recovery, 42% had partial recovery, and 58% remained dialysis-dependent. Thirty-three percent of patients died.
Conclusion
Secondary oxalate nephropathy is a rare but potentially devastating condition. Renal replacement therapy is required in >50% of patients, and most patients remain dialysis-dependent. Studies are needed for effective preventive and treatment strategies in high-risk patients with hyperoxaluria-enabling conditions.
Keywords: acute kidney injury, chronic kidney disease, nephrolithiasis, oxalate nephropathy, oxalosis, secondary oxalosis
Primary hyperoxaluria is a rare inborn error of glyoxylate metabolism characterized by the overproduction of oxalate, which deposits as calcium oxalate in various organs as kidney function declines.1, 2 The kidney is the main target for oxalate deposition, which leads to oxalate nephropathy and kidney failure.3, 4, 5, 6, 7, 8, 9, 10 Secondary hyperoxaluria is more common and is usually the result of increased dietary oxalate intake, increased intestinal oxalate availability, decreased intestinal oxalate degradation, or increased colonic permeability to oxalate. The diagnosis of secondary forms of oxalate nephropathy is often challenging due to physician unfamiliarity with the disorder and lack of proven kidney biopsy findings. Most of the evidence is derived from individual case reports and case series.
The clinical manifestations, natural history, and prognosis of secondary forms of oxalate nephropathy have not been systematically examined. To address this knowledge gap, inform clinical practice, and provide future research directions, we performed a systematic review of patients who presented with secondary forms of oxalate nephropathy, with a focus on elucidating enabling factors, clinical presentation, kidney biopsy findings, and both kidney and patient outcomes.
Methods
Data Sources and Searches
The systematic review was conducted in accordance with the CAse REport (CARE) guidelines and Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data (The PRISMA-IPD Statement).11, 12 The following electronic databases were searched for relevant citations: PubMed, Scopus, Ovid, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) (inception from 1950 to January 2018). Eligible reports were identified using the following Medical Subject Headings search terms: “oxalate” OR “oxalic acid” OR “calcium oxalate” OR “oxaloacetate” OR “oxaloacetic acid” OR “oxaluria” OR “hyperoxaluria” AND “renal insufficiency” OR “renal replacement therapy” OR “kidney disease” OR “kidney failure” OR “CKD or CKF or CRD or CRF or ESKD or ESRD or ESKF or ESRF” OR “predialysis or dialysis” OR “hemodialysis” OR “dialysis” OR “CAPD or CCPD or APD” OR “kidney transplant” OR “renal transplant” OR “kidney graft”OR “renal graft” OR “acute renal failure” OR “acute kidney failure” OR “acute renal insufficiency” OR “acute kidney insufficiency” OR “acute tubular necrosis” OR “acute kidney injury” OR “acute renal injury” OR “nephropathy.”
Bibliographies of retrieved articles were inspected, and abstracts from the annual scientific meetings of the American Society of Nephrology (up to 2017) were searched. The search strategy was limited to human studies with no restrictions on language, sample size, duration of study, or year of publication.
Study Selection
Due to the absence of retrospective and prospective cohort studies, we focused primarily on case reports and case series (as defined by a report of ≥2 patients) that provided clinical description of patients presenting with kidney disease and biopsy-proven oxalate nephropathy in the setting of native kidneys or kidney allografts.
Criteria for excluding articles from further review were insufficient clinical data to evaluate the relation between the exposure variable and oxalate nephropathy; lack of kidney biopsy results; unknown cause of oxalate nephropathy; short duration of exposure (<30 days) to hyperoxaluria-enabling conditions such as the acute ingestion of Averrhoa bilimbi, Averrhoa carambola, fruit juice, and high-dose vitamin C; exposure to ethylene glycol and derivatives; and primary hyperoxaluria.
The criteria listed in Table 1 were used to establish the level of evidence for a causal role of oxalate in the development of kidney disease. In brief, 2 of the authors (NL and MS) independently reviewed each report to establish the level of evidence for a causal role of oxalate in the development of the nephropathy. Disagreement between the 2 reviewers was resolved through adjudication by a third author (PS).
Table 1.
Criteria for assessing reports of oxalate nephropathy and level of evidence for a causal relation between oxalate exposure and development of kidney disease
| Criterion | |
| 1 | Hyperoxaluria-enabling condition as the only identifiable trigger for the kidney disease |
| 2 | Abnormal kidney function |
| 3 | Presence of oxalate crystals, tubulitis, and interstitial inflammation on the kidney biopsy |
| 4 | Other causes of tubulointerstitial disease excluded |
| Level of evidence | |
| I | Definite: criteria 1, 2, 3, and 4 met |
| II | Probable: criteria 1, 2, and 3 met |
| III | Possible: criterion 1 and 2 met |
| IV | Unlikely: criterion 1 not met |
Data Extraction and Quality Assessment
The following variables were extracted in duplicate using a data extraction spreadsheet: study-level variables (country of origin, year of publication, study design [case report, case series, case-control, and retrospective or prospective cohort], population setting [native kidney or kidney allograft], and duration of follow-up); demographic and clinical variables (age, sex, and ethnicity); hyperoxaluria-enabling conditions; kidney-related variables (clinical presentation, baseline serum creatinine and estimated glomerular filtration rate [eGFR], serum creatinine and eGFR at initial presentation, peak serum creatinine, and serum creatinine and eGFR at the end of the follow-up period, pre-existing chronic kidney disease (CKD), urinalysis and sediment findings, 24-hour urine oxalate excretion, and serum oxalate level); kidney biopsy findings; treatment-related variables (e.g., dietary modification, use of medications, and need for renal replacement therapy); and clinical outcomes (complete, partial vs. nonrecovery of kidney function, transplantation, and death).
Hyperoxaluria-enabling conditions were recorded and divided into the following mechanisms or categories: (i) increased dietary oxalate intake through excessive consumption; (ii) increased oxalate availability in the colon due to decreased intestinal calcium availability from fat malabsorption (e.g., Crohn’s disease, celiac sprue, jejunoileal bypass, ileal resection, Roux-en-Y gastric bypass surgery, short bowel syndrome, chronic pancreatitis, pancreatic insufficiency, cystic fibrosis, and use of orlistat) and a low calcium diet; (iii) decreased intestinal oxalate degradation due to decreased intestinal colonization with oxalate-degrading bacteria (i.e., Oxalobacter formigenes); and (iv) increased colonic permeability to oxalate (e.g., injury to colonic mucosa in Clostridium difficile colitis, leading to nonselective increase in oxalate absorption).
Data Synthesis and Analysis
The results of the case reports and case series were tabulated and synthesized qualitatively. The results of the case series were synthesized quantitatively by performing random-effects model meta-analyses to compute pooled means and rates with corresponding 95% confidence intervals (CIs) to describe patient characteristics and clinical outcomes. The analyses were performed using the Comprehensive Meta-Analysis, software version 2.0 (www.meta-analysis.com; Biostat, Englewood, NJ).
Results
Study Characteristics
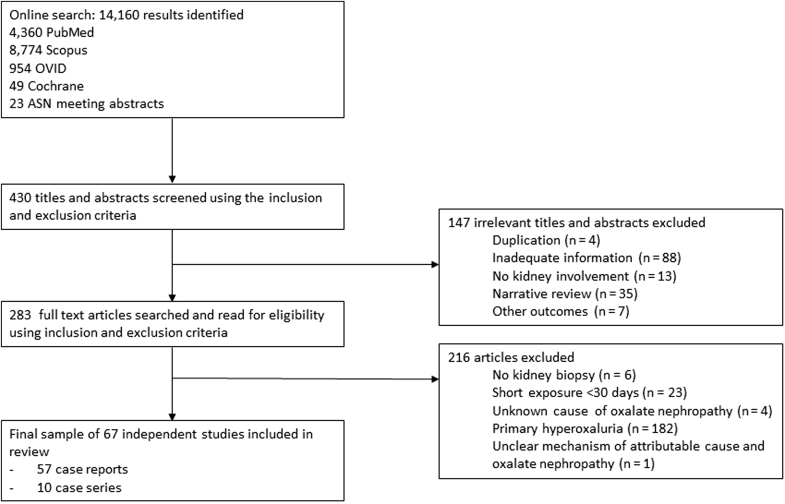
A total of 14,160 potentially relevant citations were identified and screened; 430 titles and abstracts were screened using the inclusion and exclusion criteria; 147 were excluded on the basis of the title and abstract review; and 283 articles were retrieved for detailed evaluation, of which 67 fulfilled the eligibility criteria, which involved 108 patients (Figure 1).3, 4, 5, 6, 7, 8, 9, 10, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72 There were 57 (85%) individual case reports and 10 case series that detailed 51 cases (range: 2−12 patients per case series). The case reports originated from North America (46%), Europe (38%), Asia (9%), Australia and New Zealand (5%), and Africa (2%), whereas the case series originated from North America (80%) and Europe (20%). The level of evidence was definite or level 1 for 57 articles (86%) and probable or level 2 for 9 articles (14%). A detailed description of each case is summarized in Supplementary Table S1. The 51 patients described in the 10 case series were analyzed quantitatively. Details of the case reports and case series that had short-term exposure to hyperoxularia-enabling conditions, and as result, were not included in the systematic review, are summarized in Supplementary Table S2.73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95
Figure 1.
Study selection flow diagram. ASN, American Society of Nephrology.
Characteristics of Patients Presenting With Secondary Oxalate Nephropathy
Table 2 displays the clinical characteristics, attributed causes, laboratory data, kidney biopsy findings, and outcomes of patients who presented with secondary oxalate nephropathy in the case series. In brief, mean age was 56.4 years old (95% CI: 48.6−64.3). Fifty-nine percent (95% CI: 42−75) were men, 81% (95% CI: 66−90) were white, and 15% (95% CI: 7−31) were kidney transplant recipients.
Table 2.
Summary measures of clinical characteristics and outcomes of patients with secondary oxalate nephropathy (derived from 10 case series entailing 51 patients)
| Variable | No. of studies | No. patients | Weighted mean | 95% CI | I2 index, % |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age, yr | 10 | 51 | 56.4 | 48.6−64.3 | 94 |
| Men | 10 | 51 | 59.0 | 42.0−75.0 | 16 |
| White | 10 | 51 | 81.0 | 66.0−90.0 | 0 |
| Native kidney | 9 | 48 | 85.0 | 70.0−93.0 | 0 |
| Kidney allograft | 2 | 3 | 15.0 | 7.0−31.0 | 0 |
| Attributed cause | |||||
| Increased intake of oxalate precursors | 10 | 51 | 20.2 | 9.9−36.6 | 4 |
| Increased oxalate availability | 10 | 51 | 88.3 | 75.1−95.0 | 0 |
| Serum creatinine, mg/dl | |||||
| Baseline | 10 | 51 | 1.3 | 1.1−1.5 | 92 |
| At presentation | 8 | 37 | 4.9 | 3.4−6.4 | 98 |
| Peak | 10 | 51 | 4.6 | 3.0−6.2 | 97 |
| End of follow-up | 6 | 32 | 2.7 | 2.2−3.1 | 22 |
| Urinary findings | |||||
| Proteinuria | 6 | 33 | 69.0 | 34.0−91.0 | 51 |
| Hematuria | 3 | 16 | 32.0 | 14.0−58.0 | 0 |
| Oxalate crystals | 3 | 16 | 26.0 | 10.0−53.0 | 0 |
| Urinary oxalate, mg/d | 6 | 25 | 85.4 | 68.7−102.1 | 92 |
| Kidney biopsy findings | |||||
| Tubular injury | 10 | 51 | 71.0 | 44.0−89.0 | 41 |
| Tubular atrophy | 10 | 51 | 69.0 | 43.0−87.0 | 43 |
| Interstitial cellular infiltrate | 10 | 51 | 72.0 | 45.0−89.0 | 43 |
| Glomerular findings | 10 | 51 | 59.0 | 40.0−76.0 | 23 |
| Kidney and patient outcomes | |||||
| Dialysis requirement | 9 | 40 | 55.0 | 38.0−70.0 | 0 |
| Partial recovery | 9 | 40 | 42.0 | 27.0−58.0 | 0 |
| Dialysis dependence | 9 | 40 | 58.0 | 42.0−73.0 | 0 |
| Mortality rate | 9 | 40 | 33.0 | 17.0−55.0 | 15 |
| Duration of follow-up, mo | 8 | 38 | 12.9 | 5.9−19.9 | 75 |
CI, confidence interval.
Values are %, unless otherwise noted.
Attributed Causes of Secondary Oxalate Nephropathy
Table 3 displays an overview of the attributed causes of hyperoxaluria-enabling conditions (divided into 4 etiologies) in both the individual case reports and the case series. In brief, fat malabsorption (75.0%) was the most commonly attributed cause of secondary oxalate nephropathy, followed by excessive dietary oxalate consumption (30.6%), and decreased intestinal oxalate degradation (0.9%). There was 1 case report of presumed increased colonic permeability to oxalate secondary due to Clostridium difficile colitis (0.9%).
Table 3.
Attributed causes of secondary oxalate nephropathya
| Hyperoxaluria-enabling factors | No. of studies | No. of patients | Percentage distribution | Listed causes |
|---|---|---|---|---|
| Increased intake of oxalate precursors | 30 | 33 | 30.6 |
Averrhoa carambola Vitamin C Peanuts Tea Rhubarb Chaga mushroom Piridoxylate |
| Increased oxalate availability in the colon due to decreased intestinal calcium availability from fat malabsorption | 42 | 81 | 75.0 | Crohn’s disease Celiac disease Chronic pancreatitis Small bowel resection Diabetic gastroenteropathy Pancreatic adenocarcinoma Systemic sclerosis Roux-en-Y bypass Hemicolectomy Gastric bypass Jejunoileal bypass Bariatric surgery Cystic fibrosis Orlistat Octreotide Mycophenolate mofetil |
| Decreased intestinal oxalate degradation due to decreased intestinal colonization with oxalate-degrading bacteria | 1 | 1 | 0.9 | Absence of Oxalobacter formigenes colonization |
| Increased colonic permeability to oxalate | 1 | 1 | 0.9 | Clostridium difficile colitis |
Derived from individual case reports and case series: summary of the 57 case reports and 10 case series (entailing 51 cases), totaling 108 patients. Eight patients had >1 attributed cause of secondary oxalate nephropathy, with all 8 patients having 2 contributing causes.
In the case series that were meta-analyzed, fat malabsorption (88.3%; 95% CI: 75.1−95) was the most commonly attributed contributing cause of secondary oxalate nephropathy, followed by excessive dietary oxalate consumption (20.2%; 95% CI: 9.9−36.6). There were 6 cases with >1 attributed cause (11.8%).
Laboratory Investigations and Kidney Biopsy Findings
The mean baseline serum creatinine was 1.3 mg/dl (95% CI: 1.1−1.5), with a mean peak value of 4.6 mg/dl (95% CI: 3.0−6.2). At the end of the follow-up period, the mean serum creatinine was 2.7 mg/dl (95% CI: 2.2−3.1). Proteinuria was reported in 6 studies with a prevalence rate of 69% (95% CI: 34−91). Hematuria was reported in only 3 studies with a prevalence rate of 32% (95% CI: 14−58). Urinary crystals were reported in 26% (95% CI: 10− 53) of cases. The mean 24-hour urinary oxalate excretion was 85.4 mg/d (95% CI: 68.7−102.1).
On kidney biopsy, all patients had oxalate crystal deposition in the tubules and/or interstitium. Other kidney biopsy findings included acute tubular injury (71%; 95% CI: 44−89), tubular damage and atrophy (69%; 95% CI: 43−87), interstitial mononuclear cell infiltration (72%; 95% CI: 45.0−89.0), and glomerular changes (59%; 95% CI: 40−76). There were no cases of oxalate crystal deposition in the glomeruli.
Treatment Strategies
All patients received hydration and dietary interventions, including the adoption of a normal calcium, low-oxalate diet. In addition, the use of diuretics for fluid overload,15, 46, 70 alkali therapy to alkalinize the urine,10, 19, 46, 53 and pyridoxine to convert glyoxalate (the main precursor of oxalate) to glycine15, 19, 44, 48, 65, 70 was variably reported and had inconsistent impact on recovery of kidney function (Supplemental Table S1).
Kidney and Patient Outcomes
The mean duration of follow-up was 12.9 months (95% CI: 5.9−19.9). Fifty-five percent (95% CI: 38−70) of patients required dialysis. No patient experienced complete kidney recovery, with 42% (95% CI: 27−58) experiencing partial kidney recovery, and 58% (95% CI: 42−73) remaining dialysis-dependent. The overall mortality rate was 33% (95% CI: 17−55).
Among the 48 patients with oxalate nephropathy that involved their native kidneys, 41% had partial kidney recovery, 49% remained dialysis-dependent, and 51% died. Among the 3 kidney transplant recipients with oxalate nephropathy, 2 patients remained dialysis-dependent, and 1 had partial kidney recovery. One patient eventually died.
Discussion
The aim of our systematic review was to describe the clinical manifestations, natural history, and prognosis of secondary forms of oxalate nephropathy qualitatively and quantitatively. We identified a total of 57 case reports and 10 case series, consisting of 108 patients with biopsy-proven oxalate nephropathy. Fifty-one patients described in 10 case series were quantitatively analyzed.
The true prevalence of secondary oxalate nephropathy is unknown due to a lack of prospective studies in populations at risk for hyperoxaluria-enabling conditions. In our synthesis, the mean age of onset was 57.6 years old, which contrasts with a mean age of 9.5 years at symptom onset in primary hyperoxaluria.96
Increased intestinal oxalate availability, followed by increased oxalate consumption, were the most commonly attributed causes of oxalate nephropathy. Enteric hyperoxaluria is usually a consequence of malabsorptive states, including inflammatory bowel disease,4, 53 small bowel or gastric surgery,5, 6, 9, 10 chronic pancreatitis,7, 8, 9 systemic sclerosis with bowel involvement,3 and use of orlistat.64, 97 Excessive intraluminal free fatty acids bind to and saponify calcium within the intestine, thereby inhibiting the formation of calcium oxalate. As a result, soluble free oxalate is absorbed by the colonic mucosa.98 In addition, free fatty acids and bile salts enhance the permeability of the colonic mucosa to oxalate.99 Calcium oxalate can precipitate in proximal tubules and induce tubular injury and interstitial inflammation. High-oxalate diets, such as those high in fruit juice (i.e., juicing), leafy greens, or tea, also contribute to oxalate nephropathy. Oxalobacter formigenes is a gram-negative anaerobic bacterium frequently found in the normal intestinal flora that metabolizes dietary oxalate.100 Reduced intestinal colonization with Oxalobacter formigenes is directly correlated with increased intestinal oxalate absorption, hyperoxaluria, and stone formation.101, 102 In addition to causing exocrine pancreatic insufficiency and malabsorption, cystic fibrosis also contributes to oxaluria through a defect in the solute carrier family 26 member-6, which is an apical membrane anion-exchanger located in the small intestinal brush border membrane that mediates oxalate intestinal secretion, thereby preventing hyperoxaluria.103
In our systematic review, the most common presentation of oxalate nephropathy was acute kidney injury (35%), followed by acute on CKD (29%). Twenty-six percent of patients presented with kidney disease and stones, and 10% with CKD. In contrast, 20%–50% of patients with primary hyperoxaluria present with recurrent nephrolithiasis, and CKD or kidney failure.96, 104, 105, 106
In our review, proteinuria was the most common urinary finding (69%), followed by hematuria (32%). Urinary oxalate crystals were identified in only 26% of cases. The serum oxalate level was reported in only 3 cases and was elevated, ranging from 40 to 61 μmol/l (normal value: 9 μmol/l). The 24-hour urine mean oxalate excretion was 85 mg/d (normal value: <45 mg/d).106 In a study of 48 patients with chronic pancreatitis, the prevalence of hyperoxaluria was 23%.107 Monitoring the 24-hour urinary oxalate excretion rate might be a useful tool for prevention of oxalate nephropathy in high-risk patients.
In our study, oxalate crystal deposition was universally found in tubules and/or the interstitium. Kidney biopsy findings of acute tubular injury and interstitial infiltration were reported in 71% and 72% of patients, respectively, which suggested a cause role for the oxalate crystals. Approximately 69% of patients had evidence of chronicity, which might reflect under recognition and delayed diagnosis of oxalate nephropathy. Interestingly, glomerular changes were found in 59% of the biopsy specimens, which were mostly mesangial cellular proliferation; this might explain the high prevalence of proteinuria. There were no cases of crystal deposition in the glomeruli. We can only speculate as to whether preexisting CKD predisposes patients to oxalate nephropathy in the setting of hyperoxaluric-enabling conditions.
The prognosis of secondary forms of oxalate nephropathy is somewhat guarded, with 55% of patients described in this report requiring renal replacement therapy. None of the patients had complete recovery, and 58% progressed to end-stage kidney disease. During a mean follow-up of 13 months, 33% of patients died. According to the European Primary Hyperoxaluria Registry, the 5-year survival rate of patients with primary hyperoxaluria is 76%, possibly due to a younger age at onset.104 Hydration,3, 8, 13, 19, 28, 46, 47, 60, 62, 63, 68 alkali therapy,19, 46 and pyridoxine19, 44, 48, 70 for treatment of oxalate nephropathy has yielded unsatisfactory results. Treatment of oxalate nephropathy in patients with native kidneys and kidney allografts likely depends on the underlying etiology, but low-oxalate and low-fat diets are generally recommended. Renal transplantation was proposed in a few patients with ESRD due to enteric hyperoxaluria, but recurrent oxalate deposition after transplantation could occur.24, 37, 53, 108, 109 Oral calcium supplements,110 cholestyramine,111 oxalate-binding agents,112, 113 microbiome manipulation,114 oxalate decarboxylase enzyme therapy,115 reversal of bariatric surgery,116 and kidney and intestinal transplantation117 have all been explored to reduce enteric hyperoxaluria, although none have been studied to reverse oxalate nephropathy.
To our knowledge, this is the first and largest systematic review that examines the clinical presentation, natural history, and prognosis of patients with secondary forms of oxalate nephropathy. The main strength is the strong causal association due to the selective inclusion of patients with biopsy-proven oxalate deposition (level 1 or level 2 evidence). However, there are several important limitations that should be noted. First, the data synthesis was derived from case reports and case series, and the lack of cohort studies limited the interpretation of the results. There was a potential for misclassification of the attributed causes of oxalate nephropathy, because some of the pathophysiological mechanisms were purely speculative and could be disputed. Accordingly, this systematic review is likely biased and focused on severe forms of oxalate nephropathy that warranted a kidney biopsy. Second, we excluded hyperoxaluria-enabling conditions associated with short duration of exposure and short-term follow-up, such as the ingestion of Averrhoa bilimbi fruit juice, high-dose vitamin C, or ethylene glycol; these ingestions were deemed not suitable for incorporating into the systematic review as they are associated with a different natural history of disease and prognosis. Third, the laboratory data were inconsistently reported or were lacking in most of the case reports, which might be due to physicians’ unfamiliarity with this condition at the time of the clinical presentation. Nevertheless, our systematic review has implications for raising public health awareness and making physicians more alert about this potential diagnosis, and paves the way for future studies for the prompt diagnosis and treatment of oxalate nephropathy.
In conclusion, secondary oxalate nephropathy is a rare but potentially devastating condition. Renal replacement therapy is required in >50% of patients, and most patients remain dialysis-dependent. Physicians should be more alert about this condition, especially in the setting of hyperoxaluria-enabling conditions, and increase monitoring. Well-designed prospective cohort studies or registries should be conducted. Finally, these findings call for the design and implementation of clinical trials of new drugs to prevent or treat oxalate nephropathy in high-risk patient populations.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors would like to thank the Medical Library staff at the King Chulalongkorn Memorial Hospital, Chulalongkorn University for assistance in the retrieval of articles.
Acknowledgments
Author Contributions
NL performed data extraction and took part in manuscript preparation. MS performed data extraction. BLJ conceived the research idea and study design, and took part in manuscript preparation. PS performed the data analysis and took part in manuscript preparation.
Footnotes
Table S1. Detailed description of the individual studies included in the systematic review.
Table S2. Detailed description of the individual studies excluded from the systematic review.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Detailed description of the individual studies included in the systematic review.
Detailed description of the individual studies excluded from the systematic review.
References
- 1.Bhasin B., Urekli H.M., Atta M.G. Primary and secondary hyperoxaluria: understanding the enigma. World J Nephrol. 2015;4:235–244. doi: 10.5527/wjn.v4.i2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perinpam M., Enders F.T., Mara K.C. Plasma oxalate in relation to eGFR in patients with primary hyperoxaluria, enteric hyperoxaluria and urinary stone disease. Clin Biochem. 2017;50:1014–1019. doi: 10.1016/j.clinbiochem.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ligon C.B., Hummers L.K., McMahan Z.H. Oxalate nephropathy in systemic sclerosis: case series and review of the literature. Semin Arthritis Rheum. 2015;45:315–320. doi: 10.1016/j.semarthrit.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evan A.P., Lingeman J.E., Worcester E.M. Renal histopathology and crystal deposits in patients with small bowel resection and calcium oxalate stone disease. Kidney Int. 2010;78:310–317. doi: 10.1038/ki.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troxell M.L., Houghton D.C., Hawkey M. Enteric oxalate nephropathy in the renal allograft: an underrecognized complication of bariatric surgery. Am J Transplant. 2013;13:501–509. doi: 10.1111/ajt.12029. [DOI] [PubMed] [Google Scholar]
- 6.Nasr S.H., D'Agati V.D., Said S.M. Oxalate nephropathy complicating Roux-en-Y gastric bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3:1676–1683. doi: 10.2215/CJN.02940608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefaucheur C., Hill G.S., Amrein C. Acute oxalate nephropathy: a new etiology for acute renal failure following nonrenal solid organ transplantation. Am J Transplant. 2006;6:2516–2521. doi: 10.1111/j.1600-6143.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- 8.Cartery C., Faguer S., Karras A. Oxalate nephropathy associated with chronic pancreatitis. Clin J Am Soc Nephrol. 2011;6:1895–1902. doi: 10.2215/CJN.00010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wharton R., D'Agati V., Magun A.M. Acute deterioration of renal function associated with enteric hyperoxaluria. Clin Nephrol. 1990;34:116–121. [PubMed] [Google Scholar]
- 10.Canos H.J., Hogg G.A., Jeffery J.R. Oxalate nephropathy due to gastrointestinal disorders. Can Med Assoc J. 1981;124:729–733. [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnier J.J., Kienle G., Altman D.G. The CARE guidelines: consensus-based clinical case reporting guideline development. Global Adv Health Med. 2013;2:38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart L.A., Clarke M., Rovers M. Preferred reporting items for a systematic review and meta-analysis of individual participant data: The PRISMA-IPD statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 13.Albersmeyer M., Hilge R., Schrottle A. Acute kidney injury after ingestion of rhubarb: secondary oxalate nephropathy in a patient with type 1 diabetes. BMC Nephrol. 2012;13:141. doi: 10.1186/1471-2369-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alkhunaizi A.M., Chan L. Secondary oxalosis: a cause of delayed recovery of renal function in the setting of acute renal failure. J Am Soc Nephrol. 1996;7:2320–2326. doi: 10.1681/ASN.V7112320. [DOI] [PubMed] [Google Scholar]
- 15.Beloncle F., Sayegh J., Duveau A. An unexpected cause of progressive renal failure in a 66-year-old male after liver transplantation: secondary hyperoxaluria. Int Urol Nephrol. 2013;45:1209–1213. doi: 10.1007/s11255-012-0140-1. [DOI] [PubMed] [Google Scholar]
- 16.Bernardino M., Parmar M.S. Oxalate nephropathy from cashew nut intake. CMAJ. 2017;189 doi: 10.1503/cmaj.151327. E405−e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capolongo G., Abul-Ezz S., Moe O.W., Sakhaee K. Subclinical celiac disease and crystal-induced kidney disease following kidney transplant. Am J Kidney Dis. 2012;60:662–667. doi: 10.1053/j.ajkd.2012.02.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhari D., Crisostomo C., Ganote C., Youngberg G. Acute oxalate nephropathy associated with orlistat: a case report with a review of the literature. Case Rep Nephrol. 2013;2013:124604. doi: 10.1155/2013/124604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen-Bucay A., Garimella P., Ezeokonkwo C. Acute oxalate nephropathy associated with Clostridium difficile colitis. Am J Kidney Dis. 2014;63:113–118. doi: 10.1053/j.ajkd.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Colliou E., Mari A., Delas A. Oxalate nephropathy following vitamin C intake within intensive care unit. Clin Nephrol. 2017;88:354–358. doi: 10.5414/CN109118. [DOI] [PubMed] [Google Scholar]
- 21.Cossey L.N., Rahim F., Larsen C.P. Oxalate nephropathy and intravenous vitamin C. Am J Kidney Dis. 2013;61:1032–1035. doi: 10.1053/j.ajkd.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Courtney A.E., O'Rourke D.M., Maxwell A.P. Rapidly progressive renal failure associated with successful pharmacotherapy for obesity. Nephrol Dial Transplant. 2007;22:621–623. doi: 10.1093/ndt/gfl684. [DOI] [PubMed] [Google Scholar]
- 23.Crook E.D., Cook W.J., Bergman S.M. Rapid renal deterioration secondary to oxalate in a patient with diabetic gastroenteropathy. Am J Kidney Dis. 1995;26:68–71. doi: 10.1016/0272-6386(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 24.Cuvelier C., Goffin E., Cosyns J.P. Enteric hyperoxaluria: a hidden cause of early renal graft failure in two successive transplants: spontaneous late graft recovery. Am J Kidney Dis. 2002;40:E3. doi: 10.1053/ajkd.2002.33934. [DOI] [PubMed] [Google Scholar]
- 25.Dheda S., Swaminathan R., Musk M. Acute irreversible oxalate nephropathy in a lung transplant recipient treated successfully with a renal transplant. Nephrology (Carlton) 2012;17(Suppl 1):12–15. doi: 10.1111/j.1440-1797.2012.01585.x. [DOI] [PubMed] [Google Scholar]
- 26.Escudero-Sanchez R., Villacorta-Perez J., Fernandez-Juarez G.M. Acute oxalate nephropathy and chronic pancreatitis. Rev Clin Esp. 2015;215:352–354. doi: 10.1016/j.rce.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Fakhouri F., Chauveau D., Touam M. Crystals from fat. Acute oxalate nephropathy. Nephrol Dial Transplant. 2002;17:1348–1350. doi: 10.1093/ndt/17.7.1348. [DOI] [PubMed] [Google Scholar]
- 28.Gariani K., De Seigneux S., Courbebaisse M. Oxalate nephropathy induced by octreotide treatment for acromegaly: a case report. J Med Case Rep. 2012;6:215. doi: 10.1186/1752-1947-6-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelbart D.R., Brewer L.L., Fajardo L.F., Weinstein A.B. Oxalosis and chronic renal failure after intestinal bypass. Arch Intern Med. 1977;137:239–243. [PubMed] [Google Scholar]
- 30.Getting J.E., Gregoire J.R., Phul A., Kasten M.J. Oxalate nephropathy due to 'juicing': case report and review. Am J Med. 2013;126:768–772. doi: 10.1016/j.amjmed.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Gurm H., Sheta M.A., Nivera N., Tunkel A. Vitamin C-induced oxalate nephropathy: a case report. J Commun Hosp Intern Med Perspect. 2012;2(2) doi: 10.3402/jchimp.v2i2.17718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haaskjold Y.L., Drotningsvik A., Leh S. Renal failure due to excessive intake of almonds in the absence of Oxalobacter formigenes. Am J Med. 2015;128:e29–e30. doi: 10.1016/j.amjmed.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Hicks K., Evans G.B., Rogerson M.E., Bass P. Jejuno-ileal bypass, enteric hyperoxaluria, and oxalate nephrosis: a role for polarised light in the renal biopsy. J Clin Pathol. 1998;51:700–702. doi: 10.1136/jcp.51.9.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humayun Y., Ball K.C., Lewin J.R. Acute oxalate nephropathy associated with orlistat. J Nephropathol. 2016;5:79–83. doi: 10.15171/jnp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karamadoukis L., Shivashankar G.H., Ludeman L., Williams A.J. An unusual complication of treatment with orlistat. Clin Nephrol. 2009;71:430–432. doi: 10.5414/cnp71430. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi Y., Seta K., Ogawa Y. Chaga mushroom-induced oxalate nephropathy. Clin Nephrol. 2014;81:440–444. doi: 10.5414/CN107655. [DOI] [PubMed] [Google Scholar]
- 37.Kistler H., Peter J., Thiel G., Brunner F.P. Seven-year survival of renal transplant for oxalate nephropathy due to short-bowel syndrome. Nephrol Dial Transplant. 1995;10:1466–1469. [PubMed] [Google Scholar]
- 38.Kohler G.D., Gaut J.P., Morrison A.R. The Case Diarrhea, weight loss, electrolyte abnormalities, and renal failure. Kidney Int. 2015;88:421–422. doi: 10.1038/ki.2014.344. [DOI] [PubMed] [Google Scholar]
- 39.Kwan T.K., Chadban S.J., McKenzie P.R., Saunders J.R. Acute oxalate nephropathy secondary to orlistat-induced enteric hyperoxaluria. Nephrology (Carlton) 2013;18:241–242. doi: 10.1111/j.1440-1797.2012.01649.x. [DOI] [PubMed] [Google Scholar]
- 40.Lamarche J., Nair R., Peguero A., Courville C. Vitamin C-induced oxalate nephropathy. Int J Nephrol. 2011;2011:146927. doi: 10.4061/2011/146927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawton J.M., Conway L.T., Crosson J.T. Acute oxalate nephropathy after massive ascorbic acid administration. Arch Intern Med. 1985;145:950–951. [PubMed] [Google Scholar]
- 42.Mandell I., Krauss E., Millan J.C. Oxalate-induced acute renal failure in Crohn's disease. Am J Med. 1980;69:628–632. doi: 10.1016/0002-9343(80)90479-9. [DOI] [PubMed] [Google Scholar]
- 43.Mascio H.M., Joya C.A., Plasse R.A. An unusual cause of acute kidney injury due to oxalate nephropathy in systemic scleroderma. Clin Nephrol. 2015;84:111–115. doi: 10.5414/CN108406. [DOI] [PubMed] [Google Scholar]
- 44.Mashour S., Turner J.F., Jr., Merrell R. Acute renal failure, oxalosis, and vitamin C supplementation: a case report and review of the literature. Chest. 2000;118:561–563. doi: 10.1378/chest.118.2.561. [DOI] [PubMed] [Google Scholar]
- 45.Mazzoleni L., Aydin S., De Meyer M. Acute oxalate nephropathy after renal transplantation. Acta Clin Belgica. 2013;68:389. doi: 10.2143/ACB.3331. [DOI] [PubMed] [Google Scholar]
- 46.McHugh G.J., Graber M.L., Freebairn R.C. Fatal vitamin C-associated acute renal failure. Anaesth Intensive Care. 2008;36:585–588. doi: 10.1177/0310057X0803600413. [DOI] [PubMed] [Google Scholar]
- 47.Moinuddin I., Bala A., Ali B. Acute oxalate nephropathy due to pancreatic atrophy in newly diagnosed pancreatic carcinoma. Human Pathology. 2016;48:163–166. doi: 10.1016/j.humpath.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 48.Montagnac R., Schendel A., Vuiblet V. Bariatric surgery, calcium oxalate urinary stones and oxalate nephropathy [in French] Nephrol Ther. 2011;7:38–45. doi: 10.1016/j.nephro.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Moutzouris D.A., Skaneli G., Margellos V. Oxalate nephropathy in a diabetic patient after gastric by-pass. Clin Nephrol. 2011;75(Suppl 1):16–19. [PubMed] [Google Scholar]
- 50.Mpofu S., Rhodes J.M., Mpofu C.M.A., Moots R.J. An unusual cause of acute renal failure in systemic sclerosis. Ann Rheum Dis. 2003;62:1133–1134. doi: 10.1136/ard.2002.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muñoz C.G., Martín M.V.M. Acute oxalate nephropathy in a patient with malabsorption syndrome for chronic pancreatitis. Farm Hospital. 2015;39:407–408. doi: 10.7399/fh.2015.39.6.9830. [DOI] [PubMed] [Google Scholar]
- 52.Nasr S.H., Kashtanova Y., Levchuk V., Markowitz G.S. Secondary oxalosis due to excess vitamin C intake. Kidney Int. 2006;70:1672. doi: 10.1038/sj.ki.5001724. [DOI] [PubMed] [Google Scholar]
- 53.Nazzal L., Puri S., Goldfarb D.S. Enteric hyperoxaluria: an important cause of end-stage kidney disease. Nephrol Dial Transplant. 2016;31:375–382. doi: 10.1093/ndt/gfv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niticharoenpong K., Chalermsanyakorn P., Panvichian R., Kitiyakara C. Acute deterioration of renal function induced by star fruit ingestion in a patient with chronic kidney disease. J Nephrol. 2006;19:682–686. [PubMed] [Google Scholar]
- 55.Parasuraman R., Venkat K.K. Crystal-induced kidney disease in 2 kidney transplant recipients. Am J Kidney Dis. 2010;55:192–197. doi: 10.1053/j.ajkd.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Park H., Eom M., Won Yang J. Peanut-induced acute oxalate nephropathy with acute kidney injury. Kidney Res Clin Pract. 2014;33:109–111. doi: 10.1016/j.krcp.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pipeleers L., Wissing K.M., Pirson Y. Pre-terminal renal insufficiency in a patient with enteric hyperoxaluria: effect of medical management on renal function. Acta Clin Belgica. 2012;67:39–41. doi: 10.2143/ACB.67.1.2062625. [DOI] [PubMed] [Google Scholar]
- 58.Poulin L.D., Riopel J., Castonguay V., Mac-Way F. Acute oxalate nephropathy induced by oral high-dose vitamin C alternative treatment. Clin Kidney J. 2014;7:218. doi: 10.1093/ckj/sfu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramaswamy C.R., Williams J.D., Griffiths D.F. Reversible acute renal failure with calcium oxalate cast nephropathy--possible role of ascorbic acid. Nephrol Dial Transplant. 1993;8:1387–1389. [PubMed] [Google Scholar]
- 60.Rankin A.C., Walsh S.B., Summers S.A. Acute oxalate nephropathy causing late renal transplant dysfunction due to enteric hyperoxaluria. Am J Transplant. 2008;8:1755–1758. doi: 10.1111/j.1600-6143.2008.02288.x. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki M., Murakami M., Matsuo K. Oxalate nephropathy with a granulomatous lesion due to excessive intake of peanuts. Clin Exp Nephrol. 2008;12:305–308. doi: 10.1007/s10157-008-0046-5. [DOI] [PubMed] [Google Scholar]
- 62.Sentis A., Quintana L.F., Masso E. Acute renal failure due to oxalate crystal deposition and enteric hyperoxaluria. Nefrologia. 2011;31:121–123. doi: 10.3265/Nefrologia.pre2010.Oct.10644. [DOI] [PubMed] [Google Scholar]
- 63.Singh A., Sarkar S.R., Gaber L.W., Perazella M.A. Acute oxalate nephropathy associated with orlistat, a gastrointestinal lipase inhibitor. Am J Kidney Dis. 2007;49:153–157. doi: 10.1053/j.ajkd.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Solomon L.R., Nixon A.C., Ogden L., Nair B. Orlistat-induced oxalate nephropathy: an under-recognised cause of chronic kidney disease. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-218623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Y., Horowitz B.L., Servilla K.S. Chronic nephropathy from dietary hyperoxaluria: sustained improvement of renal function after dietary intervention. Cureus. 2017;9:e1105. doi: 10.7759/cureus.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sunkara V., Pelkowski T.D., Dreyfus D., Satoskar A. Acute kidney disease due to excessive vitamin C ingestion and remote roux-en-y gastric bypass surgery superimposed on CKD. Am J Kidney Dis. 2015;66:721–724. doi: 10.1053/j.ajkd.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 67.Syed F., Mena-Gutierrez A., Ghaffar U. A case of iced-tea nephropathy. N Eng J Med. 2015;372:1377–1378. doi: 10.1056/NEJMc1414481. [DOI] [PubMed] [Google Scholar]
- 68.Van der Niepen P., Janssen van Doorn K., Van den Houte K., Verbeelen D. Nimesulide and acute renal failure caused by oxalate precipitation. Nephrol Dial Transplant. 2002;17:315–316. doi: 10.1093/ndt/17.2.315. [DOI] [PubMed] [Google Scholar]
- 69.Vigeral P., Kenouch S., Chauveau D. Piridoxilate-associated nephrocalcinosis: a new form of chronic oxalate nephropathy. Nephrol Dial Transplant. 1987;2:275–278. [PubMed] [Google Scholar]
- 70.Yaich S., Chaabouni Y., Charfeddine K. Secondary oxalosis due to excess vitamin C intake: a cause of graft loss in a renal transplant recipient. Saudi J Kidney Dis Transplant. 2014;25:113–116. doi: 10.4103/1319-2442.124518. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto R., Morita S., Aoki H. Acute renal failure and metabolic acidosis due to oxalic acid intoxication: a case report. Tokai J Exp Clin Med. 2011;36:116–119. [PubMed] [Google Scholar]
- 72.Hamidian Jahromi A., A HJ, Roberts I.S.D., Winearls C.G., Vaidya A. Acute renal failure secondary to oxalosis in a recipient of a simultaneous kidney-pancreas transplant: was mycophenolate the cause? Nephrol Dial Transplant. 2008;23:2409–2411. doi: 10.1093/ndt/gfn194. [DOI] [PubMed] [Google Scholar]
- 73.Abeysekera R.A., Wijetunge S., Nanayakkara N. Star fruit toxicity: a cause of both acute kidney injury and chronic kidney disease: a report of two cases. BMC Res Notes. 2015;8:796. doi: 10.1186/s13104-015-1640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alhamad T., Blandon J., Meza A.T. Acute kidney injury with oxalate deposition in a patient with a high anion gap metabolic acidosis and a normal osmolal gap. J Nephropathol. 2013;2:139–143. doi: 10.12860/JNP.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bakul G., Unni V.N., Seethaleksmy N.V. Acute oxalate nephropathy due to 'Averrhoa bilimbi' fruit juice ingestion. Indian J Nephrol. 2013;23:297–300. doi: 10.4103/0971-4065.114481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barman A.K., Goel R., Sharma M., Mahanta P.J. Acute kidney injury associated with ingestion of star fruit: acute oxalate nephropathy. Indian J Nephrol. 2016;26:446–448. doi: 10.4103/0971-4065.175978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergstrand A., Collste L.G., Franksson C. Oxalosis in renal transplants following methoxyflurane anaesthesia. Br J Anaesth. 1972;44:569–574. doi: 10.1093/bja/44.6.569. [DOI] [PubMed] [Google Scholar]
- 78.Bouattar T., Madani N., Hamzaoui H. Severe ethylene glycol intoxication by skin absorption [in French] Nephrol Ther. 2009;5:205–209. doi: 10.1016/j.nephro.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Buehner M., Pamplin J., Studer L. Oxalate nephropathy after continuous infusion of high-dose vitamin C as an adjunct to burn resuscitation. J Burn Care Res. 2016;37:e374–e379. doi: 10.1097/BCR.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bullock J.D., Albert D.M. Generalized oxalosis with retinal involvement following methoxyflurane anesthesia. Anesthesiology. 1974;41:296–302. doi: 10.1097/00000542-197409000-00018. [DOI] [PubMed] [Google Scholar]
- 81.Chen C.L., Fang H.C., Chou K.J. Acute oxalate nephropathy after ingestion of star fruit. Am J Kidney Dis. 2001;37:418–422. doi: 10.1053/ajkd.2001.21333. [DOI] [PubMed] [Google Scholar]
- 82.Cuvelier C., Goffin E., Cosyns J.P. Acute renal failure due to naftidrofuryl oxalate praxilene overdose in a kidney transplant recipient. Nephrol Dial Transplant. 1995;10:1756–1758. [PubMed] [Google Scholar]
- 83.Dassanayake U., Gnanathasan C.A. Acute renal failure following oxalic acid poisoning: case report. J Occup Med Toxicol. 2012;7(1) doi: 10.1186/1745-6673-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frascino J.A., Vanamee P., Rosen P.P. Renal oxalosis and azotemia after methoxyflurane anesthesia. N Engl J Med. 1970;283:676–679. doi: 10.1056/NEJM197009242831304. [DOI] [PubMed] [Google Scholar]
- 85.Le Meur Y., Moesch C., Rince M. Potential nephrotoxicity of intravenous infusions of naftidrofuryl oxalate. Nephrol Dial Transplant. 1995;10:1751–1755. [PubMed] [Google Scholar]
- 86.Moesch C., Rince M., Daudon M. Renal intratubular crystallisation of calcium oxalate and naftidrofuryl oxalate. Lancet. 1991;338:1219–1220. doi: 10.1016/0140-6736(91)92093-h. [DOI] [PubMed] [Google Scholar]
- 87.Nair S.A.-O., George J., Kumar S., Gracious N. Acute oxalate nephropathy following ingestion of averrhoa bilimbi juice. Case Rep Nephrol. 2014;2014:240936. doi: 10.1155/2014/240936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neto M.M., Silva G.E., Costa R.S. Star fruit: simultaneous neurotoxic and nephrotoxic effects in people with previously normal renal function. NDT Plus. 2009;2:485–488. doi: 10.1093/ndtplus/sfp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim M.J., Lee J.S., Kim S.W. Acute kidney injury associated with nafronyl oxalate overdose. Clin Exp Nephrol. 2013;17:437–438. doi: 10.1007/s10157-012-0752-x. [DOI] [PubMed] [Google Scholar]
- 90.Kim S.E., Kim S.J., Chu S.T. A rare case of hyperoxaluria presenting with acute liver injury and stone-free kidney injury. Kidney Res Clin Pract. 2015;34:113–116. doi: 10.1016/j.krcp.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Konta T., Yamaoka M., Tanida H. Acute renal failure due to oxalate ingestion. Intern Med (Tokyo, Japan) 1998;37:762–765. doi: 10.2169/internalmedicine.37.762. [DOI] [PubMed] [Google Scholar]
- 92.Krausz T., Sellyei M., Abranyi I. Renocerebral oxalosis after intravenous glycerol infusion. Lancet. 1977;2:89–90. doi: 10.1016/s0140-6736(77)90094-0. [DOI] [PubMed] [Google Scholar]
- 93.Pfeiffer H., Weiss F.U., Karger B. Fatal cerebro-renal oxalosis after appendectomy. Int J Legal Med. 2004;118:98–100. doi: 10.1007/s00414-003-0414-3. [DOI] [PubMed] [Google Scholar]
- 94.Pomara C., Fiore C., D'Errico S. Calcium oxalate crystals in acute ethylene glycol poisoning: a confocal laser scanning microscope study in a fatal case. Clin Toxicol (Phila) 2008;46:322–324. doi: 10.1080/15563650701419011. [DOI] [PubMed] [Google Scholar]
- 95.Seo J.W., Lee J.H., Son I.S. Acute oxalate nephropathy caused by ethylene glycol poisoning. Kidney Res Clin Pract. 2012;31:249–252. doi: 10.1016/j.krcp.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lieske J.C., Monico C.G., Holmes W.S. International registry for primary hyperoxaluria. Am J Nephrol. 2005;25:290–296. doi: 10.1159/000086360. [DOI] [PubMed] [Google Scholar]
- 97.Solomons C.C., Goodman S.I., Riley C.M. Calcium carbimide in the treatment of primary hyperoxaluria. N Engl J Med. 1967;276:207–210. doi: 10.1056/NEJM196701262760404. [DOI] [PubMed] [Google Scholar]
- 98.Dobbins J.W., Binder H.J. Importance of the colon in enteric hyperoxaluria. N Engl J Med. 1977;296:298–301. doi: 10.1056/NEJM197702102960602. [DOI] [PubMed] [Google Scholar]
- 99.Dobbins J.W., Binder H.J. Effect of bile salts and fatty acids on the colonic absorption of oxalate. Gastroenterology. 1976;70:1096–1100. [PubMed] [Google Scholar]
- 100.Arvans D., Jung Y.C., Antonopoulos D. Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J Am Soc Nephrol. 2017;28:876–887. doi: 10.1681/ASN.2016020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sidhu H., Hoppe B., Hesse A. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet. 1998;352:1026–1029. doi: 10.1016/S0140-6736(98)03038-4. [DOI] [PubMed] [Google Scholar]
- 102.Sidhu H., Schmidt M.E., Cornelius J.G. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol. 1999;10(Suppl 14):S334–S340. [PubMed] [Google Scholar]
- 103.Knauf F., Thomson R.B., Heneghan J.F. Loss of cystic fibrosis transmembrane regulator impairs intestinal oxalate secretion. J Am Soc Nephrol. 2017;28:242–249. doi: 10.1681/ASN.2016030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harambat J., van Stralen K.J., Espinosa L. Characteristics and outcomes of children with primary oxalosis requiring renal replacement therapy. Clin J Am Soc Nephrol. 2012;7:458–465. doi: 10.2215/CJN.07430711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Hoeven S.M., van Woerden C.S., Groothoff J.W. Primary hyperoxaluria type 1, a too often missed diagnosis and potentially treatable cause of end-stage renal disease in adults: results of the Dutch cohort. Nephrol Dial Transplant. 2012;27:3855–3862. doi: 10.1093/ndt/gfs320. [DOI] [PubMed] [Google Scholar]
- 106.Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012;8:467–475. doi: 10.1038/nrneph.2012.113. [DOI] [PubMed] [Google Scholar]
- 107.Demoulin N., Issa Z., Crott R. Enteric hyperoxaluria in chronic pancreatitis. Medicine (Baltimore) 2017;96:e6758. doi: 10.1097/MD.0000000000006758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bernhardt W.M., Schefold J.C., Weichert W. Amelioration of anemia after kidney transplantation in severe secondary oxalosis. Clin Nephrol. 2006;65:216–221. doi: 10.5414/cnp65216. [DOI] [PubMed] [Google Scholar]
- 109.Rifkin S.I. Transplantation for renal failure secondary to enteric hyperoxaluria: a case report. J Med Case Rep. 2007;1:31. doi: 10.1186/1752-1947-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hylander E., Jarnum S., Nielsen K. Calcium treatment of enteric hyperoxaluria after jejunoileal bypass for morbid obesity. Scand J Gastroenterol. 1980;15:349–352. doi: 10.3109/00365528009181482. [DOI] [PubMed] [Google Scholar]
- 111.Nordenvall B., Backman L., Larsson L., Tiselius H.G. Effects of calcium, aluminium, magnesium and cholestyramine on hyperoxaluria in patients with jejunoileal bypass. Acta Chir Scand. 1983;149:93–98. [PubMed] [Google Scholar]
- 112.Lindsjo M., Fellstrom B., Ljunghall S. Treatment of enteric hyperoxaluria with calcium-containing organic marine hydrocolloid. Lancet. 1989;2:701–704. doi: 10.1016/s0140-6736(89)90769-1. [DOI] [PubMed] [Google Scholar]
- 113.Lieske J.C., Regnier C., Dillon J.J. Use of sevelamer hydrochloride as an oxalate binder. J Urol. 2008;179:1407–1410. doi: 10.1016/j.juro.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lieske J.C., Goldfarb D.S., De Simone C., Regnier C. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int. 2005;68:1244–1249. doi: 10.1111/j.1523-1755.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 115.Langman C.B., Grujic D., Pease R.M. A double-blind, placebo controlled, randomized phase 1 cross-over study with ALLN-177, an orally administered oxalate degrading enzyme. Am J Nephrol. 2016;44:150–158. doi: 10.1159/000448766. [DOI] [PubMed] [Google Scholar]
- 116.Agrawal V., Wilfong J.B., Rich C.E., Gibson P.C. Reversal of gastric bypass resolves hyperoxaluria and improves oxalate nephropathy secondary to Roux-en-Y gastric bypass. Case Rep Nephrol Dial. 2016;6:114–119. doi: 10.1159/000449128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ceulemans L.J., Nijs Y., Nuytens F. Combined kidney and intestinal transplantation in patients with enteric hyperoxaluria secondary to short bowel syndrome. Am J Transplant. 2013;13:1910–1914. doi: 10.1111/ajt.12305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed description of the individual studies included in the systematic review.
Detailed description of the individual studies excluded from the systematic review.