Abstract
Introduction
Sepsis is the leading cause of acute kidney injury (AKI) in critically ill patients. The Kidney in Sepsis and Septic Shock (Kid-SSS) study evaluated the value of proenkephalin A 119-159 (penkid)—a sensitive biomarker of glomerular function, drawn within 24 hours upon intensive care unit (ICU) admission and analyzed using a chemiluminescence immunoassay—for kidney events in sepsis and septic shock.
Methods
The Kid-SSS study was a substudy of Adrenomedullin and Outcome in Severe Sepsis and Septic Shock (AdrenOSS) (NCT02393781), a prospective, observational, multinational study including 583 patients admitted to the intensive care unit with sepsis or septic shock and a validation cohort of 525 patients from the French and euRopean Outcome reGistry in Intensive Care Units (FROG-ICU) study. The primary endpoint was major adverse kidney events (MAKEs) at day 7, composite of death, renal replacement therapy, and persistent renal dysfunction. The secondary endpoints included AKI, transient AKI, worsening renal function (WRF), and 28-day mortality.
Results
Median age was 66 years (interquartile range 55–75), and 28-day mortality was 22% (95% confidence interval [CI] 19%−25%). Of the patients, 293 (50.3%) were in shock upon ICU admission. Penkid was significantly elevated in patients with MAKEs, persistent AKI, and WRF (median = 65 [IQR = 45–106] vs. 179 [114–242]; 53 [39–70] vs. 133 [79–196] pmol/l; and 70 [47–121] vs. 174 [93–242] pmol/l, all P < 0.0001), also after adjustment for confounding factors (adjusted odds ratio = 3.3 [95% CI = 1.8–6.0], 3.9 [95% CI = 2.1–7.2], and 3.4 [95% CI = 1.9–6.2], all P < 0.0001). Penkid increase preceded elevation of serum creatinine with WRF and was low in renal recovery.
Conclusion
Admission penkid concentration was associated with MAKEs, AKI, and WRF in a timely manner in septic patients.
Keywords: acute kidney injury, biomarker, diagnosis, sepsis
AKI is a frequent condition in critically ill patients, with considerable impact on both short- and long-term outcome including morbidity, mortality, and long-term loss of kidney function.1, 2 However, its early detection remains challenging due to poor sensitivity or specificity of current standard biomarkers (i.e. serum creatinine and urine output).3, 4, 5, 6 Implementation of novel biomarkers that permit a reliable AKI risk stratification for ICU patients would allow developing early efficient management strategies with potential positive impact on patient outcome.7 The use of biomarkers of kidney injury or damage has shown conflicting results with respect to AKI prediction, mostly due to specificity issues and uncoupling between kidney damage and loss of renal function.4, 8
The filtration marker penkid has recently been proposed as a sensitive biomarker of glomerular function. Penkid is a 5-kDa peptide derived from the same precursor as met- and leu-enkephalins and is considered a stable surrogate marker for the unstable enkephalins.9 Although numerous functions of enkephalins have been described, including stimulation of kidney function,10 penkid might be a “connecting peptide” in the precursor only, presumably without a function in the blood circulation. There is no evidence for penkid being possibly protein bound, as linearity and recovery in the penkid assay have been found to be not impaired. Proenkephalin A is expressed in number of tissues,11 including the kidney and the heart. Plasma concentrations of penkid have been found to be strongly negatively correlated with measured glomerular filtration rate (GFR),12 and thus penkid is considered a marker of kidney function (not damage). Upon acute kidney dysfunction, penkid levels increase more quickly than creatinine.13 Increase of plasma penkid appears to be highly specific for kidney dysfunction; in contrast to other markers, it is not influenced by non−kidney-related variables such as, for instance, systemic inflammation. However, confirmation of these promising results is warranted.
In the present study, we have investigated whether penkid levels are associated with MAKEs, as well as AKI and WRF in ICU patients with severe sepsis or septic shock.
Methods
Study Design
The Kid-SSS study was an ancillary investigation of the AdrenOSS study, a European prospective, observational, multinational study in 24 centers from 5 countries (France, Belgium, the Netherlands, Italy, and Germany). Patients were recruited from June 2015 to May 2016. The study protocol was approved by the local ethical committees and conducted in accordance with Directive 2001/20/EC, as well as Good Clinical Practices (I.C.H. version 4 of May 1, 1996 and Decision of November 24, 2006) and the Declaration of Helsinki.
Patients were enrolled who were 18 years and older and who were (i) admitted to the ICU for sepsis or septic shock14 or (ii) transferred from another ICU in the state of sepsis and septic shock within less than 24 hours after primary admission. If patients were treated with vasopressors, they were considered eligible only if treatment had been started within a maximum of 24 hours after the primary admission before ICU admission. When patients were included, they were stratified by severe sepsis and septic shock based on the definitions for sepsis and organ failure from 2001.15 Exclusion criteria were pregnancy, vegetative coma, and participation in an interventional trial in the preceding month. Informed consent was obtained from all patients or their lawful representatives prior to enrollment in the study. Patients were treated according to current guidelines, and all treatments and procedures were registered. Blood for the assessment of penkid was drawn on days 0, 1, and 2.
A subset of patients (n = 536) from the FROG-ICU study with severe sepsis (n = 48) or septic shock (n = 488) was used as a validation cohort. The FROG-ICU study was a prospective, observational, multicenter cohort study designed to investigate long-term mortality of critically ill adult patients. The study was performed in accordance with Good Clinical Practice and the Declaration of Helsinki of 2002, validated by the corresponding ethical committees, and registered on ClinicalTrials.gov (NCT01367093). The protocol of the FROG-ICU study was previously published.16
The primary endpoint of the Kid-SSS investigation was MAKE at day 7. The secondary endpoints consisted of the assessment of AKI, transient AKI, WRF, and 28-day mortality.
Endpoint Definitions
Major adverse kidney events included death, need for renal replacement therapy (RRT), and persistent AKI by day 7. Acute kidney injury was defined using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria.17 Baseline serum creatinine (Screat) was assessed using the following method: If estimated glomerular filtration rate (eGFR) on admission was ≥75 ml/min per 1.73 m2, we used the actual SCreat on admission (admission SCreat). If eGFR was <75 ml/min per 1.73 m2 on admission, we used the SCreat calculated back from the Modification of Diet in Renal Disease Study (MDRD) equation set to 75 ml/min per 1.73 m2.
Persistent AKI was defined as an elevated SCreat level from baseline by >1.5-fold or ≥0.3 mg/dl (26.5 μmol/l) at day 7 (or discharge, if earlier), or RRT at day 7, or death within 7 days after ICU admission.
WRF opposes transient AKI and was defined as an increase in serum creatinine level by ≥1.5-fold or ≥0.3 mg/dl (26.5 μmol/l), or need for RRT, or death within 48 hours from admission, all representative of poor clinical evolution.
Transient AKI was defined by the highest value of admission SCreat measured during the first 48 hours after ICU admission, decreasing thereafter without need for RRT at any time.
Chronic kidney disease (CKD) was defined as known history of kidney disease.
Because prediction of kidney events may be hampered in patients with already-high Screat upon admission, we performed an analysis of 3 subgroups in patients with eGFR >75 ml/min per 1.73 m2, renal SOFA score of 0, and renal SOFA score of ≤1 on admission. The subgroup identified as “low Screat at admission” was defined as patients with renal SOFA score of 0 at admission.
Collection of Patient Data
Upon admission, demographics (age, gender), body mass index, presence of sepsis or septic shock, type of ICU admission, organ dysfunction scores (Sequential Organ Failure Assessment [SOFA], Acute Physiology And Chronic Health Evaluation II [APACHE II] score), the source of sepsis, pre-existing comorbidities treated within the past year, past medical history, laboratory values, urine output, as well as organ support were recorded.
During the first week after patient enrollment, the following data were assessed and/or collected on a daily basis: SOFA score, antimicrobial therapies, fluid balance, ventilation status, Glasgow Coma Scale score, central venous pressure, need for RRT, invasive procedures for sepsis control, and vasopressor treatment. On day 28, discharge status or mortality was recorded in all study participants.
Measurement of Penkid
Blood for the central laboratory was sampled within 24 hours after admission for penkid measurement. Samples were subsequently processed and stored at −80 °C before transfer to the central laboratory for the blinded penkid analysis organized by the study sponsor (Sphingotec GmbH, Hennigsdorf, Germany). The measurements were performed in batch within 3 months after recruitment of the last patient based on a documented real-time stability of almost 2 years. Penkid was measured in duplicate using a chemiluminescence immunoassay (Sphingotec GmbH, Hennigsdorf, Germany), as described previously.18 The lower detection limit of the immunoassay was 5.5 pmol/l. Intra-assay and interassay coefficients of variation were 6.4% and 9.5% at 50 pmol/l, and 4.0% and 6.5% at 150 pmol/l, respectively. As in previous publications, coefficients of variation are reported here only for 2 samples in concentration ranges of special interest, namely, in the low range (50 pmol/l is close to the median of a normal reference population) and for a moderately elevated concentration (150 pmol/l). Intra-assay and interassay precision profiles of the assay have been determined with many more samples covering the entire measuring range of the assay. As expected for sandwich immunoassay, the profiles are of continuous shape: Concentrations >150 pmol/l until the upper end of the calibration curve (∼2500 pmol/l) are detected with a precision similar to that reported for the 150-pmol/l sample; concentrations between 150 and 50 pmol/l are detected with a precision between those determined for the 150- and 50-pmol/l samples; and the coefficient of variation for concentrations <50 pmol/l increases at lower concentrations.
Statistical Analyses
Values are expressed as means and SDs, medians and interquartile ranges (IQRs), or counts and percentages, as appropriate. Group comparisons of continuous variables were performed using the Kruskal−Wallis test. Data for penkid, Screat, eGFR, and urine output, as well as lactate, were log transformed. Categorical data were compared using the Pearson's Chi-squared test. Logistic regression was used to evaluate penkid for the different endpoints, both for univariable and multivariable analyses. To demonstrate independence from clinical variables already known, the added value of penkid on top of these variables was evaluated based on the likelihood ratio χ2 test for nested models. The concordance index (C index or AUC) is given as an effect measure for univariable and multivariable models. For multivariable models, a bootstrap corrected version of the C index is given. For continuous variables, odds ratios (ORs) were standardized to describe the OR for a change of 1 IQR. Ninety-five percent CIs for risk factors and significance levels for χ2 are given. For illustration, receiver operating characteristic curves were constructed to assess the sensitivity and specificity of penkid to predict the kidney endpoints.
Cox proportional hazards regression was used to analyze the effect of risk factors on survival in univariable and multivariable analyses. Methods similar to those described for logistic regression were applied. Survival curves plotted by the Kaplan−Meier method were used for illustrative purposes. We also explored association between penkid and 28-day mortality. The cut-off of 84 pmol/l was used as the median value of penkid in the cohort. Of note, the cut-off was close to the upper limit of normal of penkid previously reported.
Missing data were not replaced. All statistical tests were 2-tailed, and a 2-sided P value of 0.05 was considered significant. The statistical analyses were performed using R version 2.5.1 (http://www.r-project.org, library Design, Hmisc, ROCR) and the Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL).
Results
Patient Characteristics
A total of 583 patients were included in the study. Patient characteristics on admission with respect to the primary endpoint or penkid quartiles are presented in Table 1 and Supplementary Table S1 (see also Supplementary Appendix S1). Median age was 66 years (range, 55–75 years); 62% of patients were male; the median Simplified Acute Physiology Score (SAPS II) was 50 (range, 39–63), and the 28-day mortality was 22% (95% confidence interval [CI] = 19%–25%). Of the patients, 293 (50.3%) were in shock upon ICU admission. Penkid increased with increasing renal SOFA scores (P < 0.0001). Patients with a renal SOFA score of 0 had a median penkid concentration of 53.7 pmol/l (IQR = 39.2–73.6 pmol/l), similar to that of a healthy reference population.19 Outcome data for the overall median penkid value of 84.2 pmol/l are summarized in Supplementary Table S2.
Table 1.
Patient characteristics
| All | No MAKE | MAKE | P value | |
|---|---|---|---|---|
| Characteristics | N = 579 | n = 410 (70.8%) | n = 169 (29.2%) | |
| Penkid at admission (pmol/l) | 83.4 [52.7–154.0] | 64.7 [44.9–106.0] | 178.6 [113.7–241.9] | <0.0001 |
| Age (yr) | 65.6 [55–75.4] | 64.4 [54.0–74.0] | 69.4 [59.5–77.1] | 0.0013 |
| Males, n (%) | 361 (62.3) | 258 (62.9) | 103 (60.9) | 0.7243 |
| Septic shock at admission (yes) | 292 (50.4) | 171 (58.3) | 121 (71.6) | 0.0005 |
| Medical history | ||||
| Cardiac comorbidity | 396 (68.4) | 257 (62.7) | 139 (82.2) | <0.0001 |
| Noncardiac comorbidity | 410 (70.8) | 282 (68.8) | 128 (75.7) | 0.1155 |
| CKD | 76 (13.0) | 28 (6.8) | 47 (27.8) | <0.0001 |
| Hypertension | 290 (50.1) | 187 (45.6) | 103 (60.9) | 0.0007 |
| Diabetes melitus | 158 (27.3) | 99 (24.1) | 59 (34.9) | 0.0121 |
| Physiological values at admission | ||||
| Mean blood pressure (mm Hg) | 75 [64–90] | 76 [65–93] | 73 [61–86] | 0.0271 |
| Heart rate (bpm) | 104 [90–119] | 104 [90–119] | 103 [88–117] | 0.6996 |
| Fluid balance (ml) | 1930 [600–3561] | 1655 [430–3108] | 2787 [1263–4685] | <0.0001 |
| PaO2/FiO2 | 224 [137–340] | 221 [138–343] | 234 [136–333] | 0.8092 |
| Laboratory values at admission | ||||
| Lactate | 2.4 [1.49–4] | 2.2 [1.4–3.3] | 3.65 [1.72–7.77] | <0.0001 |
| Arterial pH | 7.38 [7.3–7.44] | 7.4 [7.33–7.45] | 7.32 [7.25–7.4] | <0.0001 |
| Bilirubin (μmol/l) | 11 [6–19] | 11 [7–19] | 11 [6–21] | 0.6286 |
| Platelets (109/l) | 190 [120–275] | 196 [125–287] | 168 [103–259] | 0.0269 |
| Creatinine (mg/dl) | 1.35 [0.86–2.22] | 1.1 [0.75–1.61] | 2.64 [1.7–4.05] | <0.0001 |
| BUN or urea (mg/dl) | 61.3 [37.1–107.3] | 51.0 [33.2–81.1] | 107.5 [61.3–144.1] | <0.0001 |
| Hematocrit (%) | 34 [29–38] | 35 [30–38] | 33 [28–38] | 0.1342 |
| White blood cell count (/mm3) | 12,455 [7185–18,520] | 12,970 [7625–18,800] | 11,325 [5790–17,000] | 0.0785 |
| Organ support at admission | ||||
| Mechanical ventilation | <0.0001 | |||
| Invasive | 219 (37.8) | 132 (32.2) | 87 (51.5) | |
| Noninvasive | 128 (22.1) | 106 (25.9) | 22 (13) | |
| None | 232 (40.1) | 172 (42) | 60 (35.5) | |
| Renal replacement therapy | 49 (8.5) | 0 (0) | 49 (29) | <0.0001 |
| Vasopressor at admission | 336 (58) | 205 (50) | 131 (77.5) | <0.0001 |
| Organ dysfunction scores | ||||
| SOFA (points) | 7 [5–10] | 6 [4–9] | 10 [8–13] | <0.0001 |
| APACHE II (points) | 15 [11–20] | 14 [10–18] | 19 [15–23] | <0.0001 |
| Length of stay (d) | ||||
| ICU | 5 [2–9] | 6 [3–10] | 3 [2–7] | <0.0001 |
| Mortality (%) | ||||
| 28-Day, deaths | 126 (21.8) | 51 (12.4) | 75 (44.4) | <0.0001 |
| 90-Day, deaths | 165 (28.5) | 75 (18.3) | 90 (53.3) | <0.0001 |
APACHE II, Acute Physiology And Chronic Health Evaluation II; BUN, blood urea nitrogen; CKD, chronic kidney disease; ICU, intensive care unit; MAKE, major adverse kidney event; SOFA, Sequential Organ Failure Assessment.
Of the 536 severe sepsis and septic shock patients, penkid was measured in 532 patients on ICU admission. Of those, MAKEs and AKI were possible to determine in 525 patients, and WRF was possible to evaluate in 496 patients. The FROG-ICU cohort included 525 patients with severe sepsis or septic shock. The characteristics of the patients in the validation cohort are presented in Supplementary Table S3.
Prediction of Kidney Events with Penkid
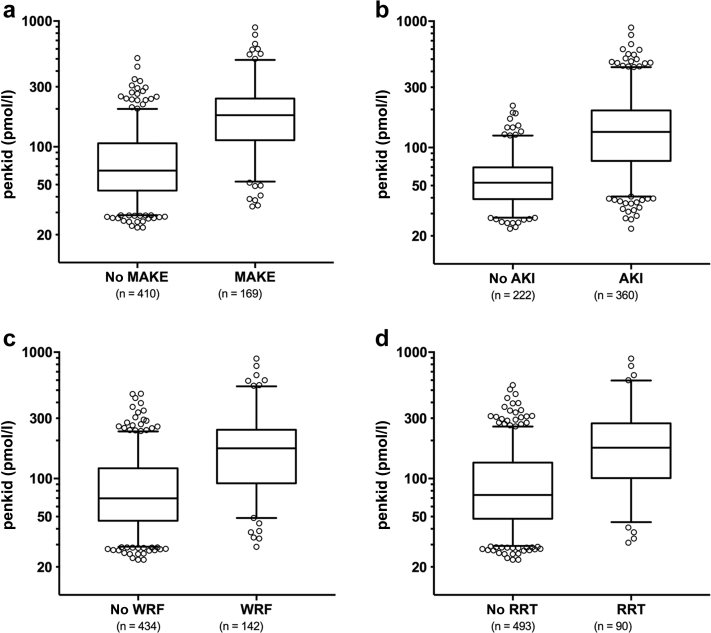
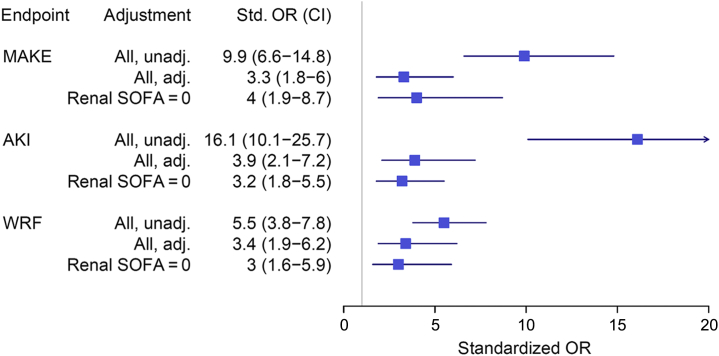
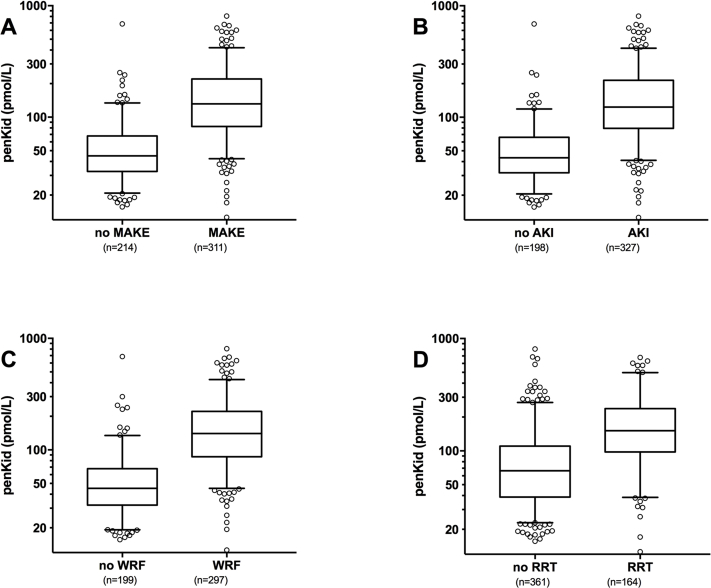
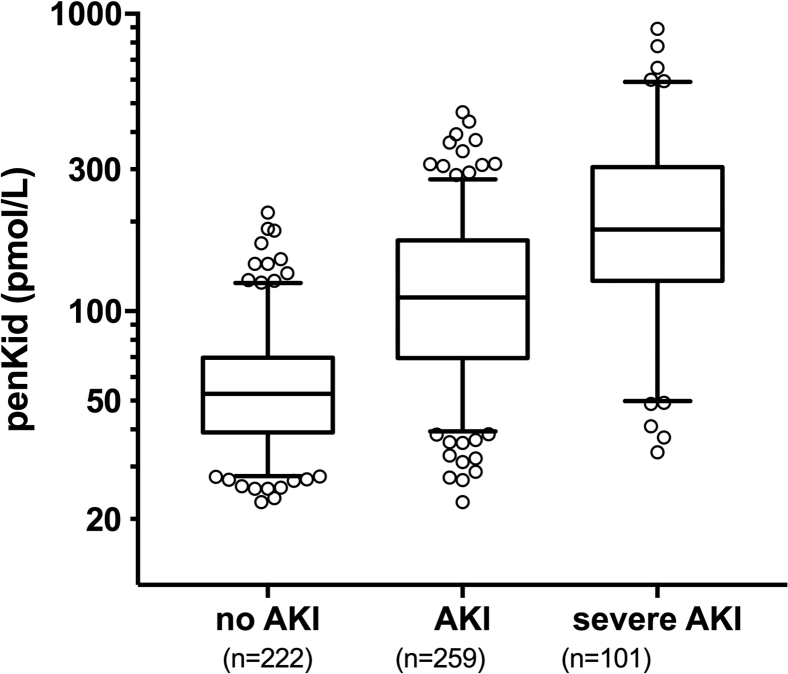
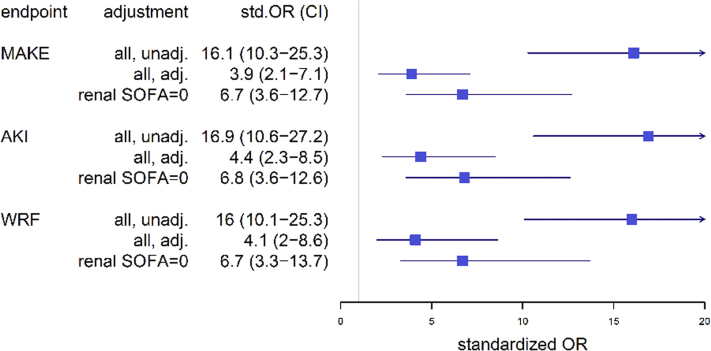
The median penkid concentration at admission in all studied patients was 84 pmol/l (IQR = 53–156 pmol/l) in the AdrenOSS cohort and 85 pmol/l (IQR = 46–156.3 pmol/l) in the FROG-ICU cohort (Table 1 and Supplementary Table S3). When investigating MAKEs as a combined endpoint after 7 days in the 579 patients with the available data in the AdrenOSS cohort, 67 patients had died, 51 patients were alive with need of RRT at some point during the first 7 days, and 51 were alive with persistent AKI without having received RRT. Accordingly, a total of 169 events were recorded in this study population. Patients who developed MAKEs had a higher penkid concentration at admission compared to patients without MAKEs (median 65, IQR = 45–106, vs. median 179, IQR = 114–242, P < 0.0001) (Figure 1). Likewise, penkid concentrations were significantly higher in patients with AKI and who developed WRF (median 53, IQR = 39–70, vs. median 133, IQR = 79–196 pmol/l, P < 0.0001, and median 70, IQR = 47–121, vs. median 174, IQR = 93–242, pmol/l, P < 0.0001 respectively). Similar results were observed in the validation cohort (Supplementary Figure S1). Also, penkid showed a stepwise increase with AKI severity (Supplementary Figure S2) and was also elevated in patients who received RRT within the first 7 days (median 74, IQR = 48–134, vs. median 176, IQR = 102–265 pmol/l, P < 0.0001) (Figure 1). Penkid concentration on admission was independently associated with MAKE in the studied population. The association remained significant after adjustment for potential confounding factors including age, gender, admission diagnosis, history of CKD, history of diabetes, history of hypertension, urine output, and eGFR on admission (adjusted standardized OR = 3.3 [95% CI = 1.8–6.0], P < 0.0001) (Figure 2). Similarly, penkid concentration on admission was significantly associated with the risk of AKI and WRF, even after adjustment for confounding variables (adjusted standardized OR = 3.9 [95% CI = 2.1–7.2] and 3.4 [1.9–6.2]). The same was true for the FROG-ICU validation cohort (Supplementary Figure S3), only here it was not possible to adjust for urine output and admission diagnosis. Likewise, penkid at admission was also significantly associated with the risk of RRT in the AdrenOSS cohort, albeit not after adjustment (adjusted standardized OR 1.9 [95% CI = 0.95–3.7], P = 0.069).
Figure 1.
Proenkephalin A 119-159 (penkid) values from the Adrenomedullin and Outcome in Severe Sepsis and Septic Shock (AdrenOSS) cohort at admission (boxplots) in (a) patients with or without major adverse kidney events (MAKEs) at day 7, (b) patients with acute kidney injury (AKI) and patients without, (c) patients with worsening renal function (WRF) and patients without, and (d) patients with or without renal replacement therapy (RRT).
Figure 2.
Standardized odds ratio (OR) for proenkephalin A 119-159 (penkid) from the Adrenomedullin and Outcome in Severe Sepsis and Septic Shock (AdrenOSS) cohort, unadjusted (unadj.) and adjusted (adj.) for age, sex, admission diagnosis, history of chronic kidney disease, history of diabetes, history of hypertension, urine output, and estimated glomerular filtration rate on admission, and in patients with low creatinine (i.e., renal Sequential Organ Failure Assessment [SOFA] ≤1 [other subgroups not reported due to low event numbers]); endpoints major adverse kidney events (MAKEs), worsening renal function (WRF), and acute kidney injury (AKI). Odds ratios are standardized to 1 interquartile range (IQR); all P < 0.05.
Predictive Value of Penkid According to Baseline Kidney Function
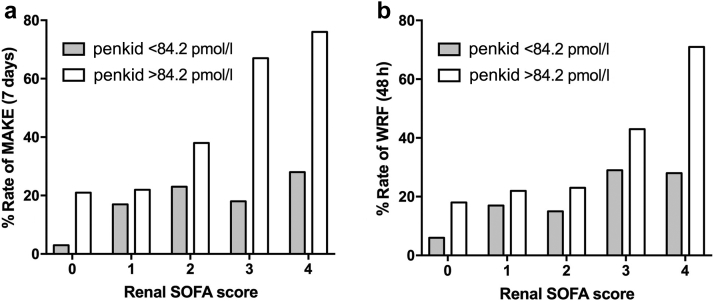
To illustrate the added value of penkid over Screat, Figure 3 shows the incidence of MAKEs and WRF according to penkid concentration <84 pmol/l or >84 pmol/l at admission across the renal component of the SOFA score. Figure 3 shows that patients in almost any renal SOFA score subgroup had a higher rate of MAKE and WRF when their penkid concentration was >84 pmol/l than when penkid was ≤84 pmol/l.
Figure 3.
Bar graphs showing incidence of (a) major adverse kidney events (MAKEs) and (b) worsening renal function (WRF) with respect to proenkephalin A 119-159 (penkid) concentration upon admission above or below the predefined cut-off value of 84.2 pmol/l (population median) across renal Sequential Organ Failure Assessment (SOFA) score stage on admission. Median serum creatinine (Screat) values are 0.8, 1.5, 2.6, 2.7, and 2.6 mg/dl for renal SOFA score categories 0, 1, 2, 3, and 4, respectively.
When restricting the analysis to patients with low Screat at admission (renal SOFA score ≤1, renal SOFA score 0 or eGFR >75) or patients without CKD, penkid was significantly associated with kidney events, and was more discriminant than Screat for their prediction (Table 2).
Table 2.
Overview of predictive performance of penkid and Screat in patients with low serum creatinine (i.e., SOFA score = 0) at admission
| Endpoint | Subgroup | n | Events | Event rate | AUC |
Added value of penkid |
|
|---|---|---|---|---|---|---|---|
| Screata | penkida | P value | |||||
| MAKE | All | 579 | 169 | 29% | 0.831 [0.795, 0.867] | 0.838 [0.802, 0.874] | <0.001 |
| No CKD | 500 | 121 | 24% | 0.815 [0.771, 0.859] | 0.818 [0.774, 0.862] | <0.001 | |
| eGFR >75 | 180 | 7 | 4% | 0.504b[0.246, 0.722] | 0.700 [0.480, 0.905] | 0.048 | |
| SOFA = 0 | 212 | 13 | 6% | 0.637b[0.459, 0.811] | 0.779 [0.639, 0.919] | <0.001 | |
| SOFA = 0/1 | 336 | 37 | 11% | 0.704 [0.617, 0.793] | 0.749 [0.665, 0.832] | <0.001 | |
| AKI | All | 582 | 360 | 62% | 0.948 [0.929, 0.967] | 0.854 [0.823, 0.884] | <0.001 |
| No CKD | 502 | 284 | 57% | 0.936 [0.912, 0.959] | 0.826 [0.791, 0.862] | <0.001 | |
| eGFR >75 | 180 | 18 | 10% | 0.627 [0.453, 0.800] | 0.624b[0.484, 0.762] | 0.052 | |
| SOFA = 0 | 212 | 30 | 14% | 0.621b[0.483, 0.755] | 0.736 [0.634, 0.839] | <0.001 | |
| SOFA = 0/1 | 337 | 138 | 41% | 0.898 [0.858, 0.939] | 0.761 [0.709, 0.813] | <0.001 | |
| WRF | All | 576 | 142 | 25% | 0.734 [0.688, 0.781] | 0.778 [0.733, 0.822] | <0.001 |
| No CKD | 497 | 106 | 21% | 0.714 [0.660, 0.768] | 0.755 [0.703, 0.808] | <0.001 | |
| eGFR >75 | 178 | 10 | 6% | 0.539b[0.341, 0.752] | 0.694 [0.506, 0.880] | 0.032 | |
| SOFA = 0 | 209 | 16 | 8% | 0.485b[0.286, 0.618] | 0.739 [0.608, 0.873] | <0.001 | |
| SOFA = 0/1 | 334 | 40 | 12% | 0.649 [0.555, 0.741] | 0.715 [0.631, 0.799] | <0.001 | |
AKI, acute kidney injury; AUC, area under the curve; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; penkid, proenkephalin A 119-159; Screat, serum creatinine; SOFA, Sequential Organ Failure Assessment; WRF, worsening renal function.
AUC with 95% confidence intervals.
P > 0.05; all other univariate P < 0.05.
A total of 76 patients had a history of CKD and showed statistically significantly higher penkid concentrations at admission (median 206, IQR = 135–318, versus median 74, IQR = 48–133, pmol/l, P < 0.0001). Penkid concentrations predicted both MAKE and WRF in both patients with and without CKD (MAKE: AUC = 0.794, 95% CI = 0.686–0.902, and AUC = 0.818, 95% CI = 0.774–0.862; WRF: AUC = 0.775, 95% CI = 0.672–0.879, and AUC = 0.755, 95% CI = 0.703–0.808).
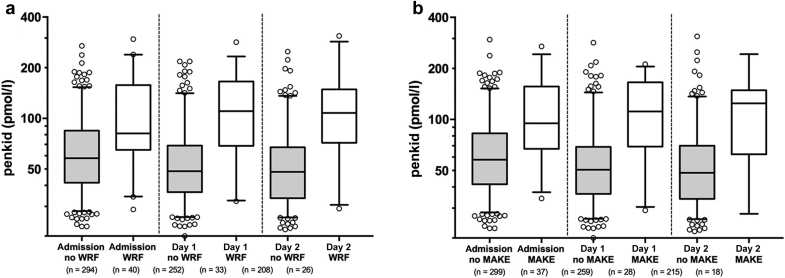
Serial measurement (i.e., at admission, day 1, and day 2 for MAKE and at admission and day 1 for WRF) (Figure 4) of penkid improved the predictive value for MAKE and WRF (added value P < 0.05 for both, AUC increase from 0.838 to 0.851 and 0.778 to 0.790 for endpoint MAKE and WRF, respectively; AUCs for multivariable models were bootstrap corrected). The gain was more pronounced for patients with renal SOFA ≤1 (added value P < 0.05 for both, AUC increase from 0.749 to 0.774 and 0.715 to 0.743 for endpoint MAKE and WRF, respectively).
Figure 4.
Proenkephalin A 119-159 (penkid) at admission, day 1, and day 2 in patients with low serum creatinine (renal Sequential Organ Failure Assessment [SOFA] score of 0 or 1) upon admission (a) according to worsening renal function (WRF) occurrence and (b) according to major adverse kidney events (MAKEs).
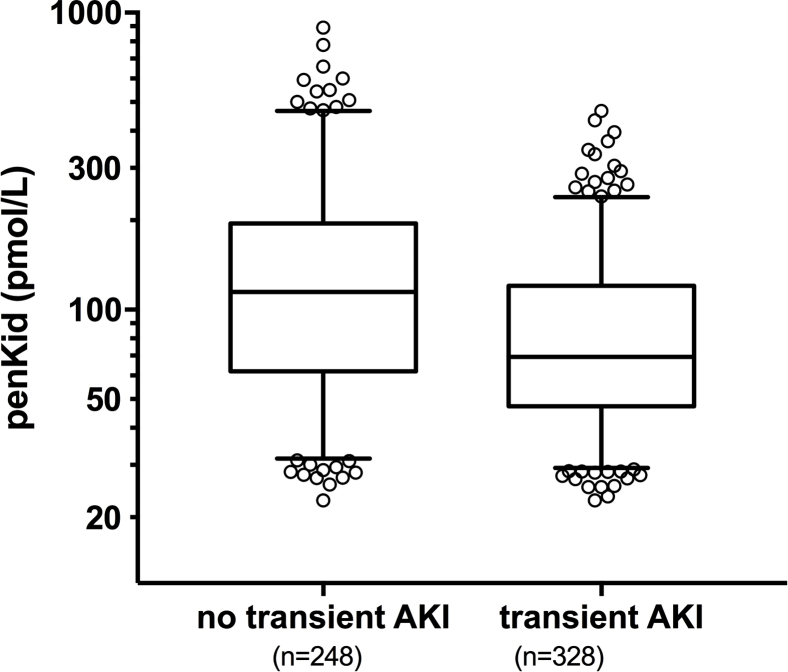
Finally, we investigated whether low penkid concentration can indicate an improvement of renal function. In patients with AKI upon admission, penkid was superior to SCreat in detecting those patients likely to recover early from AKI, hence predicting transient AKI (Supplementary Figure S4) (AUC = 0.649, 95% CI = 0.603–0.695; AUC = 0.554, 95% CI = 0.506–0.603; and AUC = 0.559, 95% CI = 0.510–0.606 for penkid, SCreat, and eGFR; penkid adjusted standardized OR = 0.36, 95% CI = 0.22–0.59).
Levels of Penkid and 28-Day Mortality
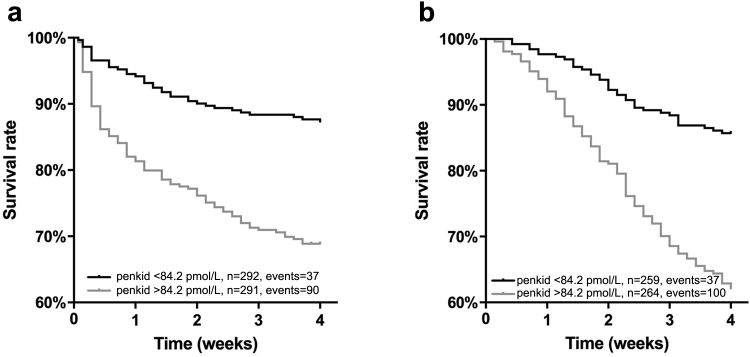
Over a 28-day follow-up period, 127 patients (22%) died. High penkid concentration on admission was associated with worse outcome in both cohorts (Figure 5a, b). In the univariate Cox regression, the C-index for prediction of 28-day mortality for penkid at admission was 0.661 (95% CI = 0.615–0.701, P < 0.0001). The C-index for creatinine, eGFR, and age was 0.589 (95% CI = 0.542–0.636, P = 0.00561), 0.589 (95% CI = 0.542–0.636, P = 0.00503), and 0.603 (95% CI = 0.554–0.651, P < 0.0001), respectively. In a Cox proportional-hazard model adjusted for age, gender, comorbidities (cardiac and noncardiac), lactate, creatinine, and diagnosis (sepsis, septic shock), penkid concentration on admission was independently associated with 28-day mortality (P < 0.002 for added value, adjusted standardized hazard ratio = 1.9 [95% CI = 1.3–2.9]). Penkid was also independent from SOFA and APACHE score (both p<0.05 for added value).
Figure 5.
Twenty-eight-day Kaplan−Meier survival curves of low versus high proenkephalin A 119-159 (penkid) concentrations at admission, (a) based upon the cut-off value of 84.2 pmol/l (population median) in all patients from the Adrenomedullin and Outcome in Severe Sepsis and Septic Shock (AdrenOSS) study cohort, and (b) based upon the cut-off value of 85 pmol/l (population median) in all patients from the French and euRopean Outcome reGistry in Intensive Care Units (FROG-ICU) study cohort.
Discussion
In the prospective, multinational, observational Kid-SSS study, we showed that elevated levels of penkid upon admission were independently associated with kidney events and death in septic and septic shock patients. Penkid was further prognostic for kidney events in patients presenting with low serum creatinine on admission and transient AKI. These findings were consistently observed in another validation cohort of 525 patients with severe sepsis or septic shock.
Previous investigations had already suggested that penkid could be a dynamic biomarker of kidney function, and that an initial measurement would allow an estimation of early patient survival.5
Current definitions of AKI have been based on serum creatinine and urine output. Both of these biomarkers, however, have limits in their sensitivity and specificity.6, 20 Although there are no doubts that oliguric patients or patients with high serum creatinine present with worse prognoses, these biomarkers may introduce delay in recognizing AKI.21 One major point is the physiological behavior of Screat, which rises after the decrease in glomerular filtration rate due to the high volume of distribution.3, 22 Septic patients are even more exposed to these limitations due to the decrease in creatinine production and the large volume of fluid that they receive. Therefore, the value of a more sensitive biomarker of AKI would be to detect early changes in GFR before Screat has risen. In this line, looking at the subcategories of the renal component of the SOFA score gives us the opportunity to explore the potential value of penkid in patients with low Screat at admission. In other words, early prediction of AKI in patients already presenting with high Screat makes little sense, and the potential additional value of the biomarker for AKI prediction lies in patients with low Screat.
Furthermore, the pathophysiology of septic AKI is complex and multifactorial. Regional immune response and inflammatory response, cellular dysfunction, and microcirculatory failure are proposed as the key contributors to septic AKI.23, 24, 25 The use of AKI biomarkers cross-reacting with the inflammatory response may therefore limit their value in predicting AKI in a septic condition. It appears that penkid is not directly affected by the inflammatory response, as penkid concentrations in patients without AKI are within the normal range. Therefore, penkid could be more specific in septic AKI than biomarkers affected by the systemic inflammatory response.20, 26
Previous findings suggest that a time-sensitive biomarker of GFR, being independent of inflammation, could therefore provide added value in septic AKI, which has led to the investigation of penkid in our study.
A substantial number of septic patients already presented to the ICU with AKI and high Screat. The clinical value of penkid may therefore be limited to predict AKI. However, we showed that penkid anticipated WRF in patients presenting with low Screat on admission and could therefore represent an early biomarker of septic AKI. Furthermore, penkid was prognostic for MAKEs and death in these patients, even after adjustment for confounding variables. On the other hand, low penkid was suggestive for rapid renal recovery (i.e., transient AKI).
Previously, it has been detected that opioid receptors are strongly expressed in the kidney,27 and that opioid agonists could stimulate kidney function4; therefore, penkid was further investigated as a biomarker for the assessment of kidney function and prediction of development of AKI. It was suggested that penkid could determine kidney function in a more accurate and timely manner when compared to Screat.28 Moreover, penkid has exhibited promising features in monitoring kidney function in acutely ill patients, including septic patients.26 In patients with acute heart failure, penkid adds value to the surveillance of kidney function in addition to the reflection of cardiorenal status after acute myocardial infarction18 and prediction of AKI in the postoperative cardiac surgery setting.13
Limitations of the present study are that the present population included patients with severe sepsis and septic shock before the release of the new Sepsis-3 definition. As such, results cannot be directly translated to the new definition, even though the definition remains close. Future studies should focus on extrapolation of our results to patients with the new definition. Second, our findings were not investigated prospectively in a validation cohort. Third, inulin clearance remains the gold standard for the assessment of kidney function, but was not investigated in this study. Also, penkid was not compared to alternative biomarkers of glomerular/tubular function (e.g., cystatin C, neutrophil gelatinase-associated lipocalin [NGAL]), nor was it compared to biomarkers of kidney injury (e.g., NGAL, kidney injury molecule-1 [KIM-1], liver-type fatty acid-binding protein [L-FABP], or TIMP2*IGFBP7, except from a subset of FROG-ICU, in which penkid has been compared with TIMP2*IGFBP727). Fourth, the uniethnicity of the studied population may impede translation of the study results to a multiethnic population. Finally, we acknowledge that impact of early recognition of AKI and prediction of AKI on treatment and patient outcomes remains elusive, even though a recent study suggested that early intervention might improve renal outcomes.29, 30
In conclusion, in a large, prospective, international cohort of critically ill patients with sepsis and septic shock (Kid-SSS), penkid was predictive of septic kidney events including MAKEs, AKI, WRF, and renal recovery, which was validated in an independent large cohort, FROG-ICU.
Disclosure
AM has received speaker’s honoraria from Novartis, Orion, and Servier, and fee as member of advisory board and/or Steering Committee from Cardiorentis, Adrenomed, Sphingotec, Sanofi, Roche, Abbott, and Bristol-Myers Squibb. EG received consulting fees from Adrenomed, Roche Diagnostics, and Magnisense, and lecture fees from Edwards LifeSciences. ABer is the managing director and holds shares in, and OH and JS are employees of, Sphingotec GmbH, the company that developed and holds patent rights in the penkid assay. BF received consulting fees from Aridis, Ferring, Arsanis, Inotrem, and Lascco. PP serves as a consultant for and has received consulting fees from Adrenomed. ML has received lecture fees from Alere, Fresenius, and Gilead and consulting fees from Adrenomed. P-FL received consulting fees from Adrenomed, Ferring, and Lascco. All the other authors declared no competing interests.
Acknowledgments
AdrenOSS (ClinicalTrials.gov Identifier identifier NCT02393781) was sponsored by Sphingotec GmbH, Neuendorfstraße 15a, 16761 Hennigsdorf, Germany. This project was partially funded by the European Union’s Horizon 2020 research and innovation program under the grant agreement No 666328. FROG-ICU was funded by the Programme Hospitalier de la RechercheClinique (AON 10-216) and by a research grant from the Société Française d’Anesthésie—Réanimation. The authors thank the Centre de Recherche Clinique (CRC) of Lariboisière University Hospital for its support. The authors are particularly grateful to Marie-Céline Fournier who coordinated organizational aspects of the study. AM and PFL had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions
Study concept and design: ML, AM; acquisition of data: EG, AH, PFL, ML, AM; analysis and interpretation of data: AH, ML; drafting of the manuscript: OH, AH, ML, JS; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: OH; obtained funding: PFL, AM; administrative, technical, or material support: ABer, PFL, AM, JS; study supervision: ML, AM; manuscript approval: all authors.
Footnotes
Figure S1. Penkid values from the FROG-ICU cohort at admission (boxplots) in (A) patients with or without major adverse kidney events (MAKEs) at day 7, (B) patients with acute kidney injury (AKI) and patients without, (C) patients with worsening renal function (WRF) and patients without, and (D) patients with or without renal replacement therapy (RRT).
Figure S2. Penkid values at admission (boxplot) according to AKI staging (P < 0.001).
Figure S3. Standardized odds ratio for penkid from the FROG-ICU cohort, unadjusted and adjusted for age, sex, history of CKD, history of diabetes, history of hypertension and eGFR on admission, and in patients with low creatinine (i.e., renal SOFA ≤1); endpoints MAKE, WRF and AKI. OR are standardized to 1 IQR; all P < 0.05.
Figure S4. Penkid values at admission (boxplot) according to rapid recovery from AKI and transient AKI (P < 0.001).
Table S1. Patient characteristics of the AdrenOSS cohort displayed in quartiles.
Table S2. Outcome data based on median penkid value of 84.2 pmol/l.
Table S3. Patient characteristics from the FROG-ICU cohort.
Appendix S1. Listing of Site Investigators for the AdrenOSS study.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Figure S1.
Penkid values from the FROG-ICU cohort at admission (boxplots) in (A) patients with or without major adverse kidney events (MAKEs) at day 7, (B) patients with acute kidney injury (AKI) and patients without, (C) patients with worsening renal function (WRF) and patients without, and (D) patients with or without renal replacement therapy (RRT).
Figure S2.
Penkid values at admission (boxplot) according to AKI staging (P < 0.001).
Figure S3.
Standardized odds ratio for penkid from the FROG-ICU cohort, unadjusted and adjusted for age, sex, history of CKD, history of diabetes, history of hypertension and eGFR on admission, and in patients with low creatinine (i.e., renal SOFA ≤1); endpoints MAKE, WRF and AKI. OR are standardized to 1 IQR; all P < 0.05.
Figure S4.
Penkid values at admission (boxplot) according to rapid recovery from AKI and transient AKI (P < 0.001).
Patient characteristics of the AdrenOSS cohort displayed in quartiles.
Outcome data based on median penkid value of 84.2 pmol/l.
Patient characteristics from the FROG-ICU cohort.
Listing of Site Investigators for the AdrenOSS study.
References
- 1.Bellomo R., Ronco C., Mehta R.L. Acute kidney injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care. 2017;7:49. doi: 10.1186/s13613-017-0260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siew E.D., Fissell W.H., Tripp C.M. Acute kidney injury as a risk factor for delirium and coma during critical illness. Am J Respir Crit Care Med. 2017;195:1597–1607. doi: 10.1164/rccm.201603-0476OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darmon M., Ostermann M., Cerda J. Diagnostic work-up and specific causes of acute kidney injury. Intensive Care Med. 2017;43:829–840. doi: 10.1007/s00134-017-4799-8. [DOI] [PubMed] [Google Scholar]
- 4.Legrand M., De Berardinis B., Gaggin H.K. Evidence of uncoupling between renal dysfunction and injury in cardiorenal syndrome: insights from the BIONICS study. PLoS One. 2014;9:e112313. doi: 10.1371/journal.pone.0112313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moledina D.G., Hall I.E., Thiessen-Philbrook H. Performance of serum creatinine and kidney injury biomarkers for diagnosing histologic acute tubular injury. Am J Kidney Dis. 2017;70:807–816. doi: 10.1053/j.ajkd.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moledina D.G., Parikh C.R. Phenotyping of acute kidney injury: beyond serum creatinine. Semin Nephrol. 2018;38:3–11. doi: 10.1016/j.semnephrol.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haase M., Kellum J.A., Ronco C. Subclinical AKI—an emerging syndrome with important consequences. Nat Rev Nephrol. 2012;8:735–739. doi: 10.1038/nrneph.2012.197. [DOI] [PubMed] [Google Scholar]
- 8.Legrand M., Januzzi J.L., Jr., Mebazaa A. Critical research on biomarkers: what's new? Intensive Care Med. 2013;39:1824–1828. doi: 10.1007/s00134-013-3008-7. [DOI] [PubMed] [Google Scholar]
- 9.Beunders R., Struck J., Wu A. Proenkephalin (PENK) as a novel biomarker for kidney function. J Appl Lab Med. 2018;3:400–412. doi: 10.1373/jalm.2017.023598. [DOI] [PubMed] [Google Scholar]
- 10.Sezen S.F., Kenigs V.A., Kapusta D.R. Renal excretory responses produced by the delta opioid agonist, BW373U86, in conscious rats. J Pharmacol Exp Ther. 1998;287:238–245. [PubMed] [Google Scholar]
- 11.Denning G.M., Ackermann L.W., Barna T.J. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides. 2008;29:83–92. doi: 10.1016/j.peptides.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Leijte G., Beunders R., van Groenendael R. Proenkephalin, the new marker for kidney function on the intensive care unit? Intensive Care Med Exp. 2017;5(Suppl 2):51–52. [Google Scholar]
- 13.Shah K.S., Taub P., Patel M. Proenkephalin predicts acute kidney injury in cardiac surgery patients. Clin Nephrol. 2015;83:29–35. doi: 10.5414/cn108387. [DOI] [PubMed] [Google Scholar]
- 14.Dellinger R.P., Levy M.M., Rhodes A. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy M.M., Fink M.P., Marshall J.C. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 16.Mebazaa A., Casadio M.C., Azoulay E. Post-ICU discharge and outcome: rationale and methods of the the French and euRopean Outcome reGistry in Intensive Care Units (FROG-ICU) observational study. BMC Anesthesiol. 2015;15:143. doi: 10.1186/s12871-015-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawla L.S., Bellomo R., Bihorac A. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 18.Ng L.L., Sandhu J.K., Narayan H. Proenkephalin and prognosis after acute myocardial infarction. J Am Coll Cardiol. 2014;63:280–289. doi: 10.1016/j.jacc.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Marino R., Struck J., Hartmann O. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J Nephrol. 2015;28:717–724. doi: 10.1007/s40620-014-0163-z. [DOI] [PubMed] [Google Scholar]
- 20.Ronco C. Acute kidney injury: from clinical to molecular diagnosis. Crit Care. 2016;20:201. doi: 10.1186/s13054-016-1373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legrand M., Payen D. Understanding urine output in critically ill patients. Ann Intensive Care. 2011;1:13. doi: 10.1186/2110-5820-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endre Z.H., Pickering J.W., Walker R.J. Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI) Am J Physiol Renal Physiol. 2011;301:F697–F707. doi: 10.1152/ajprenal.00448.2010. [DOI] [PubMed] [Google Scholar]
- 23.Legrand M., Dupuis C., Simon C. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17:R278. doi: 10.1186/cc13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez H., Ince C., De Backer D. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payen D., Lukaszewicz A.C., Legrand M. A multicentre study of acute kidney injury in severe sepsis and septic shock: association with inflammatory phenotype and HLA genotype. PLoS One. 2012;7:e35838. doi: 10.1371/journal.pone.0035838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H., Hur M., Lee S. Proenkephalin, neutrophil gelatinase-associated lipocalin, and estimated glomerular filtration rates in patients with sepsis. Ann Lab Med. 2017;37:388–397. doi: 10.3343/alm.2017.37.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gayat E., Touchard C., Hollinger A. Back-to-back comparison of penKID with NephroCheck(R) to predict acute kidney injury at admission in intensive care unit: a brief report. Crit Care. 2018;22:24. doi: 10.1186/s13054-018-1945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng L.L., Squire I.B., Jones D.J. Proenkephalin, renal dysfunction, and prognosis in patients with acute heart failure: a GREAT network study. J Am Coll Cardiol. 2017;69:56–69. doi: 10.1016/j.jacc.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 29.Meersch M., Schmidt C., Hoffmeier A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellum J.A., Sileanu F.E., Bihorac A. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195:784–791. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient characteristics of the AdrenOSS cohort displayed in quartiles.
Outcome data based on median penkid value of 84.2 pmol/l.
Patient characteristics from the FROG-ICU cohort.
Listing of Site Investigators for the AdrenOSS study.