Abstract
Background
Prototheca is an emerging, opportunistic, pathogenic, zoonotic achlorophyllous green alga, expanding in pathogenicity and host range, causing localized and disseminated infections. This outbreak of Prototheca wickerhamii algaemia and sepsis in a tertiary care 30-bedded chemotherapy oncology unit is the first human outbreak to the best of our knowledge.
Methods
P. wickerhamii algaemia was confirmed on consecutive isolation. Person to person transmission was hypothesized considering all patients in the unit at risk. Clinico-demographic, diagnostic and treatment profile were correlated. Both manual and automated systems were used for blood culture, isolation, identification and susceptibility of Prototheca. Liposomal amphotericin B was given. Outbreak surveillance of faeces, fingertips and environmental reservoirs, retrospective surveillance during past 15 years and prospective surveillance was continued for two years.
Results
The outbreak affected 12 neutropenic patients over 50 days. No specific clinical features were noted. The hypothesis could not be substantiated. P. wickerhamii was isolated as yeast-like colonies revealing Gram positive yeast-like cells without budding and pseudohyphae which were confirmed by automated system. Post amphotericin B blood cultures were negative for Prototheca. Surveillance studies were not contributory.
Conclusion
P. wickerhamii has no documented reservoirs or transmission. Endogenous colonization in the gut followed by translocation during chemotherapy induced immunosuppression is likely to cause algaemia and sepsis. Outbreaks are difficult to detect and control as incubation period is variable and clinical presentation is muted, emphasizing the need to strengthen hospital and laboratory based surveillance systems to ensure adequate preparedness, rapid detection and response to outbreaks.
Keywords: Prototheca wickerhamii, Outbreak, Algaemia, Chlorella, Chemotherapy
Introduction
Prototheca is an emerging, opportunistic, pathogenic, achlorophyllous green alga known to cause Protothecosis, a zoonotic disease seen in dogs, cats and cattle. Earlier interpreted as contaminants in human blood and faeces, Prototheca species are emerging with expanding pathogenicity and host range causing localized and disseminated infections. Pathogenic species are Prototheca wickerhamii and Prototheca zopfii, although most human cases are caused by P. wickerhamii.1, 2 Since, the first human case identified in Sierra Leone in 1964, approximately 125 infections have been reported mostly as individual cases. Localized cutaneous infections of extremities and face, wound infections and olecranon bursitis are commonly seen in immunocompetent hosts. Patients with history of steroid use, haematological malignancy, diabetes mellitus, HIV-AIDS, organ transplantation, stem cell transplantation and alcoholism are prone to peritonitis, algaemia and disseminated fatal infections.3, 4, 5, 6, 7 Outbreaks of protothecosis are restricted to animals in research literature. This study aims to characterize an outbreak of P. wickerhamii algaemia in a tertiary care chemotherapy oncology unit. This is the first human outbreak of P. wickerhamii algaemia to the best of our knowledge.
Materials and methods
The possibility of an outbreak was considered after isolation of three consecutive P. wickerhamii isolates from routine BD BACTEC Plus Aerobic/F blood culture bottles of three different patients from the 30-bedded chemotherapy oncology unit. All patients in the chemotherapy unit were considered at risk and person to person transmission was hypothesized. All patients detected to have P. wickerhamii algaemia on blood cultures were operationally included in the case definition as suspect cases who were considered confirmed cases after consecutive isolation of P. wickerhamii in repeat blood cultures. Active case finding was done through blood cultures every alternate day for all patients irrespective of detection of algaemia. Preliminary survey of existing infrastructure, policies and practices was done. A greater emphasis on infection control measures such as standard precautions, hand hygiene, contact precautions, sterilization of medical equipment, surface disinfection and biomedical waste disposal was instituted. Clinico-demographic patient profile, diagnosis, duration of stay in hospital, algaemia specific clinical features, treatment protocol, neutrophil count, routine haematological and biochemical parameters along the temporal period of outbreak were correlated.
After positive culture screen in aerobic and/or fungal culture bottles of BACTEC™ 9120 (BD Diagnostics, USA), blood was streaked on sheep blood agar and McConkey agar and aerobically incubated at 37 °C for 48 h. All yeast-like colonies were subjected to micromorphological examination after Gram stain. They were subcultured in biological oxygen demand incubators at 22 °C as well as 37 °C on Sabouraud's dextrose agar. All isolates obtained in pure culture were subjected to tests for urease, Germ tube formation and VITEK 2 compact automated system (bioMérieux, France). Inbuilt standards for identification were utilized and Minimal Inhibitory Concentrations (MICs) were obtained.8, 9, 10 Non-repeat positive cultures with respective susceptibility patterns were considered for profiling of isolates. Identified isolates were interpreted along with colony characteristics, micromorphology, substrate utilization reactions, disc diffusion antifungal susceptibility patterns and clinical correlates. All confirmed cases were initiated on liposomal amphotericin B 5 mg/kg body-weight/day.
Faecal cultures of affected patients were undertaken to screen for any evidence of colonization or dissemination. Surveillance studies for possible environmental reservoirs such as room air, water, surfaces, antiseptic solutions, crystalloids and medical devices were undertaken. Healthcare staff and other patients were screened through cultures of faeces and fingertips.11 Retrospective surveillance for Prototheca algaemia during past 15 years as well as prospective surveillance was continued for two years post outbreak.
Results
The outbreak affected 12 patients over a temporal extent of approximately 50 days during which the unit had an average occupancy of 26 patients (86.7%). Mean age was 37 ± 6.4 years, mean stay in the unit was 60 ± 8.4 days, mean leucocyte count was 569.2 ± 88.7/dl, mean neutrophil count was 350 ± 54.4/dl, and mean haemoglobin was 9 ± 1.5 g/dl. Most common diagnosis was Non-Hodgkins Lymphoma (33.33%) followed together by osteosarcoma, carcinoma pancreas and metastatic carcinoma colon (16.67% each). No specific clinical features were noticed during the period of algaemia. Routine haematological and biochemical parameters concurred to previous values prior to P. wickerhamii algaemia. All patients were given subcutaneous granulocyte colony stimulating factor 300 μg daily.
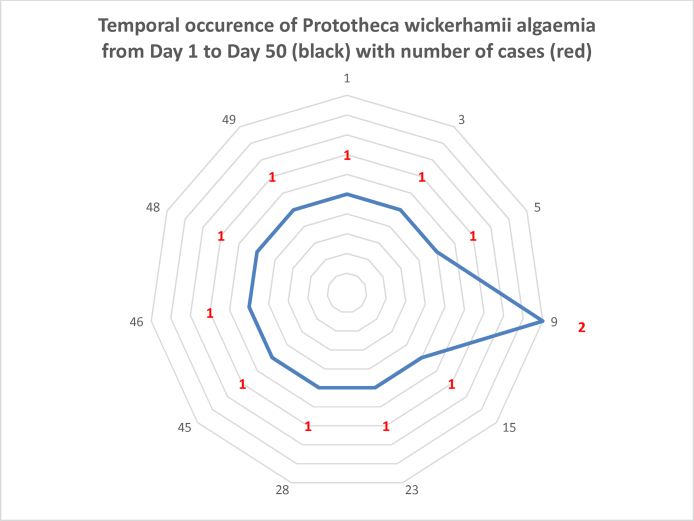
The chemotherapy unit is well spaced with private rooms for neutropenic patients. Patient care, infection control, sterilization and housekeeping protocols were as per laid out Standard Operating Procedures (SOPs). A minimum of one and a maximum of three patients having algaemia were present in the unit at any given time. The hypothesis of person to person transmission could not be substantiated. Three affected patients were on antibacterials and two on caspofungin. The clinico-demographic, diagnostic and treatment profile has been summarized in Table 1. Temporal occurrence from day 1 to day 50 plotted against number of cases is depicted in Fig. 1.
Table 1.
Clinico-demographic, diagnostic and treatment profile from outbreak of Prototheca wickerhamii algaemia in a tertiary care chemotherapy oncology unit.
| S. no. | Age (years) | Duration of stay | Leucocyte (count/dl) | Neutrophil (count/dl) | Haemoglobin (g/dl) | Diagnosis | Chemotherapy regime and antimicrobials |
|---|---|---|---|---|---|---|---|
| 1 | 31 | 65 | 650 | 390 | 12.4 | Osteosarcoma | Ifosfamide, mesna, adriamycin, cisplatin |
| 2 | 35 | 66 | 496 | 310 | 8.1 | ||
| 3 | 27 | 55 | 640 | 378 | 8.9 | Cyclophosphamide, vincristine, prednisolone, rituximab | |
| 4 | 32 | 69 | 546 | 341 | 8.7 | ||
| 5 | 36 | 63 | 475 | 280 | 8.1 | Non-Hodgkin's Lymphoma | |
| 6 | 29 | 64 | 726 | 430 | 8 | ||
| 7 | 41 | 68 | 422 | 248 | 8.4 | Metastatic carcinoma colon | 5-Fluorouracil, colistin, imipenem, caspofungin |
| 8 | 37 | 60 | 515 | 350 | 10.2 | ||
| 9 | 43 | 43 | 574 | 410 | 9 | Carcinoma pancreas | Gemcitabine, capecitabine |
| 10 | 46 | 66 | 548 | 330 | 8.4 | ||
| 11 | 43 | 47 | 672 | 400 | 11.4 | Carcinoma oesophagus | 5-Fluorouracil, cisplatin, imipenem, caspofungin |
| 12 | 44 | 54 | 566 | 333 | 7.2 | Adenocarcinoma gall bladder | Gemcitabine, cisplatin, piperacillin-tazobactam |
Fig. 1.
Temporal occurrence of Prototheca wickerhamii algaemia from day 1 to day 50 (shown in outer circumference depicted in black) with number of cases (depicted by red).
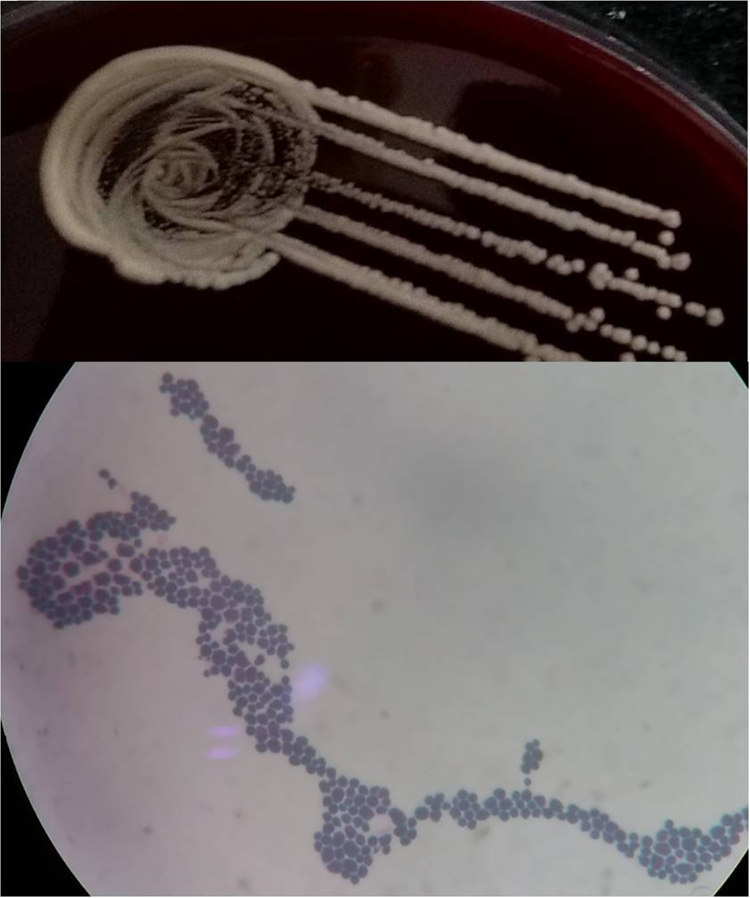
Repeat blood cultures confirmed the presence of algaemia in all affected patients. No other organisms were isolated from blood cultures. P. wickerhamii was isolated after positive culture screen from BACTEC™ 9120 on sheep blood agar as yeast-like colonies while there was no growth on McConkey agar. Preliminary micromorphological examination revealed Gram positive 3–11 μ non-capsulated yeast-like cells without budding and pseudohyphae (Fig. 2). Yeast-like colonies were isolated on subcultures at 37 °C on Sabouraud's dextrose agar. No growth was obtained in biological oxygen demand incubators at 22 °C after seven days. All isolates were negative for urease and Germ tube formation. VITEK 2 compact provided identification number 4502100000205110 with 99% identification probability through assimilation of sucrose, trehalose, glycerol, inositol, propanol and arginine. Minimal Inhibitory Concentration (MICs) in μg/ml for amphotericin B and voriconazole were 0.5 and 2 respectively. All isolates were similar for biochemical reactions and susceptibility patterns. All patients responded to liposomal amphotericin B (5 mg/kg body weight/day). Post treatment blood cultures were negative for Prototheca in all patients. One patient of metastatic carcinoma colon with neutrophil count 248/dl detected to have algaemia went into sepsis with serum procalcitonin levels between 2 and 4 ng/ml with subsequent fatal outcome under intensive care.
Fig. 2.
Prototheca wickerhamii exhibiting yeast-like colonies on sheep blood agar and Gram positive yeast-like cells without budding and pseudohyphae.
Fresh faecal cultures undertaken from affected patients to screen for any evidence of colonization or dissemination were not contributory. Prototheca was not isolated from blood cultures of any other patient in the chemotherapy oncology unit. Environmental surveillance through air, water and surface cultures as well as cultures from antiseptic solutions, crystalloids and medical devices was not corroborative. Prototheca was not isolated from faeces and fingertips of healthcare staff and other patients. There were no records of Prototheca algaemia in the past 15 years. No case of Prototheca algaemia was seen in the follow up period of two years post outbreak.
Discussion
Epidemiologically, P. wickerhamii is a rare emerging organism which has no known reservoirs, routes of transmission and a variable incubation period. Specific epidemiological correlates in this study were restricted by limited number of cases, unavailability of matched control group and disease dynamics of invasion, colonization, infection and diagnosis which may not be predictable.2, 8 However, the study was conducted with three arms viz. a prospective surveillance of the outbreak, a retrospective surveillance of historical outbreaks or case-patients, and a prospective surveillance for two years post outbreak. Retrospective surveillance may not yield as outbreaks may go unnoticed due to low index of suspicion and/or technological and diagnostic limitations. It is difficult to define risk factors and establish the incidence rates and temporal relationship between exposure and disease. P. wickerhamii could not be isolated in environmental and faecal surveillance.11 The reservoir, source, common exposure, mode of transmission and index case could not be identified. Human to human transmission was neither evident in the outbreak nor documented in protothecosis, nevertheless the possibility cannot be ruled out. The outbreak is likely due to endogenous colonization during prolonged immunosuppression in the gut followed by translocation resulting in algaemia and sepsis. The mortality following sepsis may be multifactorial, with prothecosis aggravating the condition. Variable disease dynamics may delay detection of an outbreak on epidemiological, clinical and laboratory axes. Prospective surveillance mandates a high index of suspicion for algaemia in neutropenic patients, coupled with new-age diagnostic technology for optimal detection of Prototheca. The absence of established therapeutic and immunization protocols makes control measures difficult.
This outbreak comprising 12 cases with a temporal extent of 50 days may represent only a portion of cases as special clinical units treating immunocompromised patients extend clinical suspicion only to bacteremia and fungaemia in hitherto asymptomatic patients. While algaemia has been reported in specialized cancer and transplant centres, the possibility of algaemia is often missed even if the patients are under aggressive antimicrobial cover. Prototheca algaemia may be completely missed due to administration of prophylactic systemic antifungals leading to cure, patient discharge, death or transfer to another clinical unit.8, 11 Clinically, immunocompromised states suppress clinical presentation which can lead to delay in requesting blood cultures.12, 13 The window period may be different in various patients depending upon the degree of immunosuppression, prophylactic antifungals, Prototheca infection and clinical manifestations. In the laboratory, there is delay due to prolonged incubation period through blood culture, growth on solid media, identification and susceptibility leading to delay in treatment and control of a potential outbreak.8
Prototheca has a diverse geographical distribution as a saprophyte with microhabitat in soil and wastewater, where it can achieve high concentrations of 106 cells/ml. It is also seen in cattle, dogs, fruit bats, fish, animal waste, animal buildings, agricultural sewage, tree sap and grass. Food items such as butter, potato peels, cow milk and bananas may contain Prototheca. Contamination of aquatic systems, resistance to chlorination and subsequent entry into food chain contributes to ubiquity in fresh water reservoirs such as ponds, lakes, streams as well as seawater. Growth requirements may include thiamine. It is not a part of human microflora although can rarely colonize skin, nails, respiratory tract and digestive system.2, 14, 15
Despite widespread distribution and exposure, human infection is rare and is restricted to hosts with compromised immunity. Prototheca may be transmitted by direct traumatic inoculation resulting in onychoprotothecosis and cutaneous protothecosis manifesting as papules, pustules, plaques, erosions, ulcers, crusts and nodules which need to be differentiated from other indolent conditions. Fishermen, paddy farmers, seafood handlers are at risk of exposure. Human to human transmission is not known. Arthropod borne transmission has been reported.16 Incubation period varies from 10 days to 4 months. Systemic infections can also occur in peritoneal dialysis, myasthenia gravis and polytrauma. Prototheca has been implicated in healthcare associated infections in association with central venous catheter, Hickman catheter, meningitis in HIV positive patient, endocarditis in premature neonates, ventriculoperitoneal shunts, tumour necrosis factor α inhibitors, multiple myeloma and various surgical procedures. Worldwide infections with cure rates of 59% in disseminated protothecosis and attributable mortality of 2.2% have been reported.4, 5, 6, 17, 18
Prototheca is a diagnostic and therapeutic enigma. The ability of Prototheca to grow in human blood, brain heart infusion medium in blood culture bottles and blood agar furthers colony characterization and micromorphology. Nevertheless, situations such as contamination of growth by co-infecting yeasts or bacteria, or growth of a single colony on solid media, often lead to missed diagnosis.8, 11, 19 Prototheca exhibits polymorphism in size from 2–30 μ (3–11 μ) expanding into both prokaryotic and eukaryotic cell size range, leading to confusion in characterization. Yeast-like colonies on blood and Sabouraud's agar are indistinguishable from those of Candida and Cryptococcus. Standard identification of yeasts by sporulation on nutritionally deficient media, auxanograms and chromogenic media is unyielding in case of Prototheca as it is non-sporulating, chemically inert and chromogenic media are not standardized. Prototheca species appear similar to Candida parapsilosis on CHROMagar Candida.20 The lack of further identification may lead to labelling of Prototheca as a commensal or contaminant.8, 9 Selective media such as Prototheca isolation medium supplemented with potassium hydrogen phthalate, 5-flucytosin and Rose Bengal, may not be available in clinical laboratories. Antibacterials such as gentamicin, chloramphenicol, cycloheximide and tobramycin present in selective yeast media such as Sabouraud's agar or fungal culture bottles may inhibit the growth of Prototheca. Thus, it can surpass standard laboratory identification by routine techniques. Automated systems such as Vitek 2 compact, API 20C and API 20C AUX (bioMerieux, France), MicroScan WalkAway (Siemens Healthcare Diagnostics, USA) and RapID Yeast Plus (Remel, New Mexico) can rapidly and reliably detect Prototheca and provide susceptibility, thereby reducing turnaround time, and incorporating standardization and quality control. API 32C does not identify P. wickerhamii.2, 5, 8, 9, 19 Immunological tests are not commonly available for P. wickerhamii. Fluorescent antibodies can be used to reach genus level identification and rabbit antisera against P. wickerhamii and P. zopfii have been evaluated for diagnosis.2, 20 Prototheca is reactive to Galactomannan. Indirect ELISA based on whole cell antigen of P. zopfii SAG 2021 for anti-Prototheca antibodies is restricted to veterinary labs. Prototheca cannot be diagnosed by sequencing of internal transcribed spacers as for fungi. 26S rDNA and 18S rDNA sequencing with Pw18SF 5′-TCAAAAAGTCCCGGCTAATCTCGTGC-3′ and Pw18SR 5′-CGCTTTCGTGCCTCAATGTCAGTGTT-3′ primers can be used to confirm identification.21 Molecular microbiology based applications are limited by pre-designed genus and species specific primers, laboratory infrastructure, proficiency, standardization and quality control. Flow cytometry, matrix assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry and Fourier transform infra-red (FTIR) spectroscopy FTIR are promising approaches, though have limited utility due to lack of standardization of cytometry characteristics and spectra database, and thereby generally remain beyond the purview of clinical laboratories.8, 19, 22 Disseminated infections of tissues result in chronic granulomatous inflammation with necrosis, giant cells, hyperkeratosis, focal parakeratosis, pseudoepithelialization as well as histiocytic, lymphocytic and eosinophilic infiltrates. Prototheca is subjected to polymorphonuclear cell-mediated killing in presence of specific IgG and heat-stable serum opsonins. Large oval, elliptical or spherical non-budding cells with prominent cell wall and lack of characteristic endospores may create confusion with Cryptococcus, Blastomyces, Paracoccidioides, Coccidioides, Rhinosporidium and Pneumocystis.2, 8, 9 Asexual reproduction by cytoplasmic internal septation and cleavage results in spherical or elliptical endosporulating sporangia of 7–30 μ size containing 2–8 sporangiospores in a morula-like of daisy-like form, which may be visualized on tissue biopsy after haematoxylin/eosin, Grocott's–Gomori methenamine silver, periodic acid Schiff, Gridley fungus stain and other histochemical stains. Histopathologically, it can be confused with yeasts or dimorphic fungi such as sporangia of Coccidioides immitis, however they are 10–100 times larger.2, 6, 9
Antifungal susceptibility testing methods are extended to algae. In the absence of specific guidelines and breakpoints for performance, interpretation and quality control in susceptibility testing of Prototheca species, interpreting results of disc diffusion zone diameters and MICs by automated systems or E-test remains difficult. RPMI 1640 agar with 2% glucose and Mueller Hinton agar can be used for disc diffusion and agar dilution for susceptibility testing. MICs are not always reproducible and may not relate to clinical cure. Testing is not recommended routinely and required only in cases of treatment failure. P. zopfii can grow in medium containing 16 μg of voriconazole/ml.4
Antifungals such as amphotericin B and itraconazole form the mainstay of treatment, although Prototheca is susceptible to voriconazole, miconazole, clotrimazole, tetracycline, gentamicin, amikacin and polymyxin B.23 Systemic antifungals may be required for cutaneous manifestations not responding to topical therapy. Amphotericin B and its lipid based formulations provide broad spectrum cover, however, treatment failures even with combination antifungal therapy with amphotericin B has been reported.20 Breakthrough Protothecosis under long-term administration of voriconazole, itraconazole, fluconazole and caspofungin has been reported.4, 6, 23 The limitation of newer antifungals against certain fungi such as zygomycetes warrants correct identification of isolate for optimization of therapy. Antifungal treatment needs to be reassessed in cases there is no clinical improvement. Adjunctive local thermal therapy along with systemic itraconazole has been found to be effective in cutaneous protothecosis not responding to local and systemic therapy. Newer agents such as miltefosine are being evaluated.18
Prototheca belongs to the family chlorellaceae and is considered a mutant of Chlorella, which is a photosynthetic alga. Prototheca and Chlorella can be differentiated by two layered glucosamine lacking cell wall, variations in 16s like rRNA, genetic DNA and lack of chloroplasts in the former. The parasitism of green algae such as Prototheca, Chlorella and Helicosporidium is a result of divergent evolution resulting in an ecological niche. Various species of Prototheca viz. P. wickerhamii, P. zopfii, P. stagnora, P. ulmea, P. blaschkeae have been described. P. moriformis, P. chlorelloides, P. pastoriensis, P. trispora, P. ubrizsyi, P. ciferri, P. portoricensis and P. segbwema are considered synonymous with P. zopfii of which two genotypes are known.1 P. filamenta has been reclassified under the genus Fissuricella. P. wickerhamii is likely to be reclassified under the genus Auxenochlorella.9 Canine protothecosis manifests as diarrhoea, weight loss, uveitis, retinal detachment, ataxia and seizures while bovine protothecosis manifests as enteritis and mastitis. Outbreaks of P. zopfii bovine mastitis have been widely reported.14, 16 Piscine Protothecosis has also been reported.24
The emergence of rare algae of low virulence is alarming. Epidemiological concerns are two-pronged. Firstly, Prototheca is emerging as an opportunistic human pathogen with a potential to cause outbreaks. Secondly, there is evidence of P. zopfii resistance to milk pasteurization.25 Concurrently, Chlorella pyrenoidosa is being marketed as a food supplement beneficial in boosting immunity, radioprotection, reducing cholesterol, slowing ageing, etc. with a potential probiotic status, topical treatment for post-irradiation dermatitis and fibromyalgia. Chlorella species have application in nanotechnology and biodiesel production.26, 27
Hospital outbreaks caused by bacterial (MRSA, VRE, Pseudomonas, etc.), fungi (Candida, Aspergillus) and viruses (Influenza, Hepatitis A, B, C, etc.) pathogens are frequently encountered and transmission dynamics are known which streamlines outbreak management protocols. Outbreaks of algae are likely to evade clinical, epidemiological and microbiological suspicion. De novo appearance of an outbreak should be considered in any cohort comprising immunocompromised patients affected with opportunistic pathogens such as Prototheca. Concerted laboratory based surveillance, statistical epidemic modelling, systems analysis and operations research capabilities can help early identification and investigation of such outbreaks.7, 11, 28, 29, 30
Conclusion
P. wickerhamii is a rare emerging organism which has no known reservoirs, routes of transmission and a variable incubation period. Prototheca wickerhamii outbreak of algaemia and sepsis as evidenced by repeated blood cultures in the chemotherapy oncology unit represents its expanding pathogenicity. Human to human transmission was neither evident nor documented in literature. Immunocompromised neutropenic patients having protothecosis may not manifest clinical features leaving detection to intuitive clinical acumen. Outbreaks are difficult to detect and control as incubation period is variable. Endogenous colonization in the gut followed by translocation during chemotherapy induced immunosuppression is likely to cause algaemia and sepsis. De novo appearance of outbreak should be considered in any cohort comprising immunocompromised patients affected with opportunistic pathogens. Such hospital outbreaks by emerging opportunistic pathogens in specific patient populations re-emphasize the need to strengthen hospital and laboratory based surveillance systems to ensure adequate preparedness, rapid detection and response to outbreaks.
Conflicts of interest
The authors have none to declare.
Acknowledgement
The authors acknowledge the contributions and support of various clinical superspecialists and specialists, nursing and laboratory staff involved in patient care, diagnosis and auxiliary support during the outbreak.
References
- 1.Arnold P., Ahearn D.G. The systematics of genus Prototheca with a description of new species P. filament. Mycologia. 1972;64:265–275. http://www.jstor.org/stable/37757830. [Google Scholar]
- 2.Lee W., Lagios M., Leonards R. Wound infection by Prototheca wickerhamii, a saprophytic alga pathogenic for man. J Clin Microbiol. 1975;2(1):62–66. doi: 10.1128/jcm.2.1.62-66.1975. www.ncbi.nlm.nih.gov/m/pubmed/1225929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohabeer A.J., Kaplan P.J., Southern P.M., Gander R.M. Algaemia due to Prototheca wickerhamii in a patient with myasthenia gravis. J Clin Microbiol. 1997;35(12):3305–3307. doi: 10.1128/jcm.35.12.3305-3307.1997. www.ncbi.nlm.nih.gov/m/pubmed/230169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaur S., Marrin C., Barnes R. Disseminated Protothecosis following traumatic Hickman line removal in a patient with leuakemia. Med Mycol. 2010;48(2):1–4. doi: 10.1080/13693780903188698. www.ncbi.nlm.nih.gov/m/pubmed/19688631. [DOI] [PubMed] [Google Scholar]
- 5.Lass-Flörl C., Fille M., Gunsilius E., Gastl G., Nachbaur D. Disseminated infection with Prototheca zopfii after unrelated stem cell transplantation for leukemia. J Clin Microbiol. 2004;42(10):4907–4908. doi: 10.1128/JCM.42.10.4907-4908.2004. www.ncbi.nlm.nih.gov/m/pubmed/15472379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiqueroa C.J., Camp B.J., Varghese G.I. A case of protothecosis in a patient with multiple myeloma. J Cutan Pathol. 2014;41(5):409–413. doi: 10.1111/cup.12338. www.ncbi.nlm.nih.gov/m/pubmed/24758253. [DOI] [PubMed] [Google Scholar]
- 7.Yamada N., Yoshida Y., Ohsawa T. A case of cutaneous protothecosis successfully treated with local thermal therapy as an adjunct to itraconazole therapy in an immunocompromised host. Med Mycol. 2010;48(4):643–646. doi: 10.3109/13693780903401690. www.ncbi.nlm.nih.gov/m/pubmed/20092420. [DOI] [PubMed] [Google Scholar]
- 8.Khan I.D., Sahni A.K., Bharadwaj R., Lall M., Jindal A.K., Sashindran V.K. Emerging organisms in a tertiary healthcare set up. Med J Armed Forces India. 2014;70(2):120–128. doi: 10.1016/j.mjafi.2013.09.005. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan I.D., Sahni A.K., Basu A., Haleem S. Trichosporon asahii UTI in immunocompetent patients. Med J Armed Forces India. 2014;71(4):373–376. doi: 10.1016/j.mjafi.2014.08.013. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4646908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan I.D., Lall M., Sen S., Ninawe S.M., Chandola P. Multiresistant Elizabethkingia meningoseptica infections in tertiary care. Med J Armed Forces India. 2014;71(3):66–67. doi: 10.1016/j.mjafi.2014.02.002. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4534552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan I.D., Basu A., Kiran S., Trivedi S., Pandit P., Chattoraj A. Device-associated healthcare associated infections (DA-HAI) and the caveat of multiresistance in a multidisciplinary intensive care unit. Med J Armed Forces India. 2017:1–4. doi: 10.1016/j.mjafi.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lass-Florl C., Fille M., Gunsilius E., Gastl G., Nachbaur D. Disseminated infection with Prototheca zopfii after unrelated stem cell transplantation for leukemia. J Clin Microbiol. 2004;42(10):4907–4908. doi: 10.1128/JCM.42.10.4907-4908.2004. www.ncbi.nlm.nih.gov/m/pubmed/522359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heney C., Greeff M., Davis V. Hickman catheter related protothecal algaemia in an immunocompromised child. J Infect Dis. 1991;163:930–931. doi: 10.1093/infdis/163.4.930. www.ncbi.nlm.nih.gov/m/pubmed/2010650. [DOI] [PubMed] [Google Scholar]
- 14.Mathew L.G., Pulimood S., Thomas M., Acharya M.A., Raj P.M., Mathews M.S. Disseminated Protothecosis. Indian J Pediatr. 2010;77(2):198–199. doi: 10.1007/s12098-009-0251-6. www.ncbi.nlm.nih.gov/m/pubmed/19936664. [DOI] [PubMed] [Google Scholar]
- 15.Kano R., Sobukawa H., Suzuki M. Immunohistopathology of Prototheca wickerhamii in cutaneous lesions of protothecosis. Med Mycol J. 2014;55(1):E29–E32. doi: 10.3314/mmj.55.e29. www.ncbi.nlm.nih.gov/m/pubmed/24682095. [DOI] [PubMed] [Google Scholar]
- 16.Lass-Florl C., Mayr A. Human Protothecosis. Clin Microbiol Rev. 2007;20(2):230–242. doi: 10.1128/CMR.00032-06. www.ncbi.nlm.nih.gov/m/pubmed/1865593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macesic N., Fleming S., Kidd S. Protothecosis in hematopoietic stem cell transplantation: case report and review of previous cases. Transpl Infect Dis. 2014;16(3):490–495. doi: 10.1111/tid.12223. www.ncbi.nlm.nih.gov/m/pubmed/24797402. [DOI] [PubMed] [Google Scholar]
- 18.McMullan B., Muthiah K., Stark D., Lee L., Marriot D. Prototheca wickerhamii mimicking yeast: a cautionary tale. J Clin Microbiol. 2011;49(8):3078–3081. doi: 10.1128/JCM.00487-11. www.ncbi.nlm.nih.gov/m/pubmed/3147757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan I.D., Sahni A.K. Bacterial infections and emerging resistance in renal transplant recipients. Bang J Med Sci. 2015;14(1):14–21. www.banglajol.info/index.php/BJMS/article/download/16306/14837. [Google Scholar]
- 20.Torres H.A., Bodey G.P., Tarrand J.J., Kontoyiannis D.P. Protothecosis in patients with cancer: case series and literature review. Clin Microbiol Infect. 2003;9(8):786–792. doi: 10.1046/j.1469-0691.2003.00600.x. www.ncbi.nlm.nih.gov/m/pubmed/14616698. [DOI] [PubMed] [Google Scholar]
- 21.da Silva P.C.G., da Costa e Silva S.B., Lima R.B. Cutaneous protothecosis – case report. An Bras Dermatol. 2013;88(6 suppl 1):183–185. doi: 10.1590/abd1806-4841.20132232. www.ncbi.nlm.nih.gov/m/pubmed/3876013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan I.D., Gupta N., Rangan N.M.<ET_AL>. Evaluation of pre and post analytical variables in clinical microbiology services in multidisciplinary ICU of a medical college and tertiary care hospital. J Basic Clin Med. 2016;5(1):2–4. www.sspublications.org/index.php/JBCM/article/download/63/61. [Google Scholar]
- 23.Wirth F.A., Passalacqua J.A., Kao G. Disseminated cutaneous protothecosis in an immunocompromised host: a case report and literature review. Cutis. 1999;63(3):185–188. www.ncbi.nlm.nih.gov/m/pubmed/10190075. [PubMed] [Google Scholar]
- 24.Gentles J.C., Bond P.M. Protothecosis of Atlantic salmon. Sabouraudia. 1977;15(2):133–139. doi: 10.1080/00362177785190211. www.ncbi.nlm.nih.gov/m/pubmed/905919. [DOI] [PubMed] [Google Scholar]
- 25.Melville P.A., Watanabe E.T., Benites N.R. Evaluation of the susceptibility of Prototheca zopfii to milk pasteurization. Mycopathologia. 1999;146(2):79–82. doi: 10.1023/a:1007005729711. www.ncbi.nlm.nih.gov/m/pubmed/10822507. [DOI] [PubMed] [Google Scholar]
- 26.www.amazondiscovery.com/products/chlorella-powder/, www.purellahealth.com/#!chlorellla/cegs and www.chlorella-guide.com/en/the-microalga-chlorella.htm Accessed 15.01.16.
- 27.Xu H., Miao X., Wu Q. High quality biodiesel production from a microalga Chlorealla protothecoides by heterotrophic growth in fermenters. J Biotechnol. 2006;126(4):499–507. doi: 10.1016/j.jbiotec.2006.05.002. www.ncbi.nlm.nih.gov/m/pubmed/16772097. [DOI] [PubMed] [Google Scholar]
- 28.Wolkewitz M., Dettenkofer M., Bertz H., Schumacher M., Huebner J. Statistical epidemic modeling with hospital outbreak data. Stat Med. 2008;27(30):6522–6531. doi: 10.1002/sim.3419. www.ncbi.nlm.nih.gov/m/pubmed/18759371. [DOI] [PubMed] [Google Scholar]
- 29.Ransjö U., Lytsy B., Melhus A. Hospital outbreak control requires joint efforts from hospital management, microbiology and infection control. J Hosp Infect. 2010;76(1):26–31. doi: 10.1016/j.jhin.2010.01.018. www.ncbi.nlm.nih.gov/m/pubmed/20359768. [DOI] [PubMed] [Google Scholar]
- 30.Jindal A.K., Pandya K., Khan I.D. Antimicrobial resistance: a public health challenge. Med J Armed Forces India. 2014;71(2):178–181. doi: 10.1016/j.mjafi.2014.04.011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4388962. [DOI] [PMC free article] [PubMed] [Google Scholar]