To the Editor:
The chronic hypoxia theory states that hypoxia and interstitial fibrosis are key contributors to progression of chronic kidney disease (CKD).1 Presence of fibrosis may further enhance the hypoxia by limiting oxygen transport, resulting in a perpetual cycle of hypoxic injury and progressive loss of kidney function. Blood oxygenation level dependent (BOLD)2 and diffusion3 magnetic resonance imaging (MRI) can provide information on renal oxygenation and fibrosis, respectively. The methods rely on endogenous contrast mechanisms that do not require exogenous contrast administration and that are widely available on major vendor platforms.
We report baseline MRI data in 127 individuals with advanced CKD (mean estimated glomerular filtration rate [eGFR] = 33.4 ± 7.2 ml/min per 1.73m2) who participated in the COMBINE (CKD Optimal Management with BInders and NicotinamidE) study, a multicenter clinical trial that aimed to test whether nicotinamide and lanthanum carbonate would safely lower serum phosphate and FGF23 levels compared with placebo. Relaxation rate R2* served as the BOLD MRI index; higher values represent decreased oxygenation.2 Apparent diffusion coefficient (ADC) was the diffusion MRI index; lower values may be due to increased fibrosis.4 Similar MRI data obtained in a small group (n = 13) of healthy volunteers were used for comparison. Detailed MRI methods are provided as Supplementary Methods and specific MRI parameters are listed in Supplementary Table S1.
Results and Discussion
Table 1 summarizes the baseline characteristics of the study population.
Table 1.
Baseline characteristics of study population
| Variable | CKD, n = 127 | Control, n = 13 |
|---|---|---|
| Age, yr | 65 ± 12 | 59 ± 9 |
| Male, n (%) | 81 (64) | 6 (46) |
| Race, n (%) | ||
| White | 73 (57) | 7 (54) |
| African American | 39 (31) | 3 (23) |
| Other | 15 (12) | 3 (23) |
| Diabetes, n (%) | 64 (50) | 0 (0) |
| eGFR, ml/min per 1.73 m2 | 33.4 ± 7.2 | 90.7 ± 11.9 |
| UACR, mg/g | 161 (20–584) | n/a |
| HGB, g/dl | 12.9 ± 1.7 | n/a |
eGFR, estimated glomerular filtration rate; HGB, serum hemoglobin; n/a, not available; UACR, urine albumin to creatinine ratio shown as median (interquartile range).
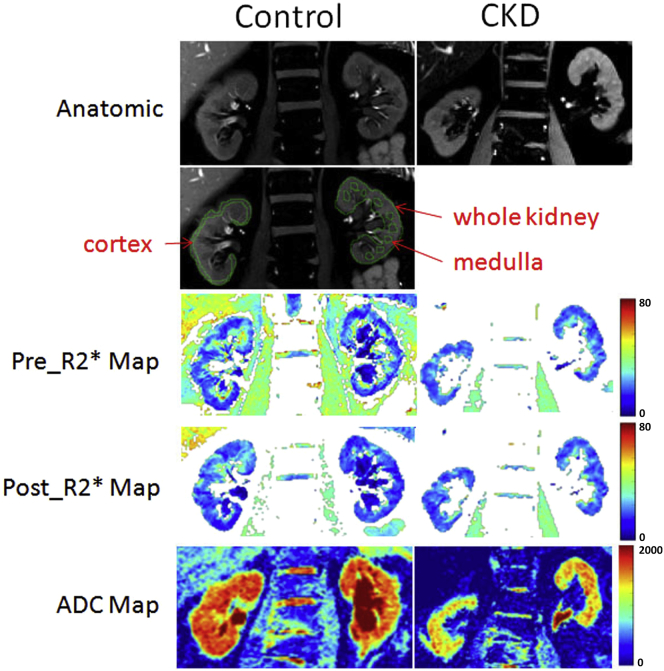
Figure 1 shows examples of MRI data in both a healthy volunteer and an individual with CKD. Table 2 summarizes the differences in MRI measurements between the trial participants with CKD and the healthy control group. Consistent with current literature on studies from single sites,5, 6, 7 cortical R2* was modestly higher in the group with CKD versus the control group, suggesting decreased cortical oxygenation. R2*_Medulla was lower in participants with CKD compared with healthy volunteers, suggesting increased medullary oxygenation. This seemingly contradictory observation has been reported before in preclinical studies8 using invasive measurements and in human studies using BOLD MRI.9, 10 In a remnant kidney model, direct oxygen levels measured using microelectrodes at 6 to 8 weeks showed increased tissue oxygen levels.8 In the same model, at an early phase (4–7 days), tissue hypoxia was observed.11 These observations suggest the possibility that with advanced kidney disease, the decrease in renal perfusion is surpassed by decreased oxygen consumption that results from reduced delivery of glomerular filtrate and reduced tubular sodium transport.12
Figure 1.
Illustration of typical magnetic resonance imaging data from a representative subject from the control and chronic kidney disease (CKD) groups. Shown are anatomical images, pre- and post-furosemide R2*, and apparent diffusion coefficient (ADC) maps. The maps are scaled similarly using the same color bar for both control and CKD. Note that changes in medullary regions in control, but not in CKD on the post-furosemide R2* map compared with the pre-furosemide R2* map. Also included is an illustration of sample regions of interest (ROIs) defined for the analysis of R2* maps. Cortical ROIs (outlined in green) are defined as thin regions parallel to the outer boundary of the kidney covering the entire length of the kidney. Also shown are whole-kidney ROI and multiple small ROIs in the medulla.
Table 2.
Comparison of magnetic resonance imaging parameters between study groups
| CKD/Control | n | Mean | SD | Pa | Mean difference (confidence interval) | |
|---|---|---|---|---|---|---|
| Oxygenation | ||||||
| R2*_Cortex (s−1) | Control | 13 | 18.74 | 2.37 | 0.022 | −1.811 (−3.322 to −0.3) |
| CKD | 123 | 20.55 | 3.10 | |||
| R2*_Medulla (s−1) | Control | 13 | 29.03 | 3.87 | <0.01 | 5.278 (2.890 to 7.665) |
| CKD | 121 | 23.75 | 3.22 | |||
| R2*_Kidney (s−1) | Control | 13 | 22.15 | 2.25 | 0.38 | 0.611 (−0.810 to 2.032) |
| CKD | 123 | 21.54 | 2.76 | |||
| R2*_(Kid-Cor)(s−1) | Control | 13 | 3.41 | 1.89 | <0.01 | 2.453 (1.303 to 3.604) |
| CKD | 123 | 0.96 | 0.89 | |||
| R2*_MC ratio | Control | 13 | 1.57 | 0.28 | <0.01 | 0.403 (0.230 to 0.577) |
| CKD | 121 | 1.17 | 0.15 | |||
| Response to furosemide | ||||||
| ΔR2*_Cortex (s−1) | Control | 13 | 0.42 | 0.86 | 0.11 | −0.461 (−1.035 to 0.112) |
| CKD | 54 | 0.88 | 1.02 | |||
| ΔR2*_Medulla (s−1) | Control | 13 | 6.28 | 3.46 | 0.002 | 3.747 (1.578 to 5.915) |
| CKD | 54 | 2.54 | 2.47 | |||
| ΔR2*_Kidney (s−1) | Control | 13 | 2.03 | 1.13 | 0.014 | 0.927 (0.217 to 1.638) |
| CKD | 54 | 1.11 | 0.87 | |||
| Fibrosis | ||||||
| ADC | Control | 13 | 1.67 | 0.08 | < 0.01 | 0.219 (0.165 to 0.273) |
| (× 10–3 mm2/s) | CKD | 126 | 1.45 | 0.17 |
ADC, apparent diffusion coefficient; CKD, chronic kidney disease; R2*_(Kid-Cor) = R2*_Kidney – R2*_Cortex; R2*_MC ratio = R2*_Medulla / R2*_Cortex.
By Student 2-tailed t test.
Because cortico-medullary contrast is reduced in CKD,13 identification of medulla may be challenging, especially when using small regions of interest (ROIs). This difficulty may result in lower interreader agreement for medullary ROIs, as has been reported previously.14 We had a high interreader agreement in medullary ROIs (Supplementary Table S2), and we included whole-kidney ROI specifically to mitigate the limitation of small ROIs for evaluating medulla. The difference between kidney and cortex ROIs could be used as an indirect estimate of medullary R2* with a higher degree of objectivity. Consistent with R2*_Medulla, changes in R2*_(Kid-Cor) show a net positive mean difference value when compared with controls (Table 2), whereas R2*_Cortex had a negative mean difference. This supports that the observed increase in R2*_Medulla is not an artifact of using small ROIs. Prior studies have reported increased medullary oxygenation using both small ROIs10 and more objective concentric ROI method.9
The response to furosemide provides an index of active tubular sodium reabsorption in the medulla.15 Consistent with prior reports, we observed a blunted response to furosemide in participants with CKD compared with controls.6, 16 Notably, for the analysis of response to furosemide, we excluded participants with CKD who were chronically treated with loop diuretics, given a prior study reporting lower response in such individuals.17
Lower ADC values are associated with the presence of fibrosis.3, 4 The cortical ADC estimates from this study in the healthy volunteers are comparable with a recent report,3 and as also previously reported, we observed lower cortical ADC in participants with CKD compared with controls (Table 2). The cortical ADC values in our participants with CKD were lower than previously reported3 (1.45 ± 0.17 vs. 1.63 ± 0.14 × 10–3mm2/s), probably due to the lower mean eGFR in our study in comparison with the mean eGFR in the prior study (33.4 vs. 71.2 ml/min per 1.73 m2). Interestingly, 50% of the group with CKD had diabetes, and participants with CKD with diabetes had significantly lower ADC compared with those without diabetes (Table 3).
Table 3.
Comparison of magnetic resonance imaging measures by diabetes status
| CKD/Control | n | Mean | SD | Pa | Mean difference (confidence interval) | |
|---|---|---|---|---|---|---|
| Oxygenation | ||||||
| R2*_Cortex (s−1) | Diabetes | 61 | 20.55 | 3.00 | 0.99 | 0.007 (−1.106 to 1.118) |
| No diabetes | 62 | 20.54 | 3.23 | |||
| R2*_Medulla (s−1) | Diabetes | 60 | 24.13 | 3.32 | 0.21 | 0.745 (−0.412 to 1.901) |
| No diabetes | 61 | 23.38 | 3.10 | |||
| R2*_Kidney (s−1) | Diabetes | 61 | 21.61 | 2.62 | 0.78 | 0.143 (−0.846 to 1.132) |
| No diabetes | 62 | 21.46 | 2.92 | |||
| R2*_MC ratio | Diabetes | 60 | 1.19 | 0.17 | 0.11 | 0.043 (−0.010 to 0.097) |
| No diabetes | 61 | 1.15 | 0.13 | |||
| Response to furosemide | ||||||
| ΔR2*_Cortex (s−1) | Diabetes | 17 | 0.78 | 1.178 | 0.634 | 0.156 (−0.510 to 0.822) |
| No diabetes | 37 | 0.93 | 0.958 | |||
| ΔR2*_Medulla (s−1) | Diabetes | 17 | 1.76 | 1.47 | 0.054 | 1.137 (−0.022 to 2.296) |
| No diabetes | 37 | 2.90 | 2.76 | |||
| ΔR2*_Kidney (s−1) | Diabetes | 17 | 0.96 | 0.93 | 0.439 | 0.208 (−0.335 to 0.751) |
| No diabetes | 37 | 1.17 | 0.84 | |||
| Fibrosis | ||||||
| ADC | Diabetes | 63 | 1.40a | 0.15 | < 0.02 | 0.097 (0.038 to 0.156) |
| (× 10–3 mm2/s) | No diabetes | 63 | 1.50 | 0.19 |
ADC, apparent diffusion coefficient; CKD, chronic kidney disease.
Bold values indicate P < 0.05.
P < 0.05 by Student 2-tailed t test.
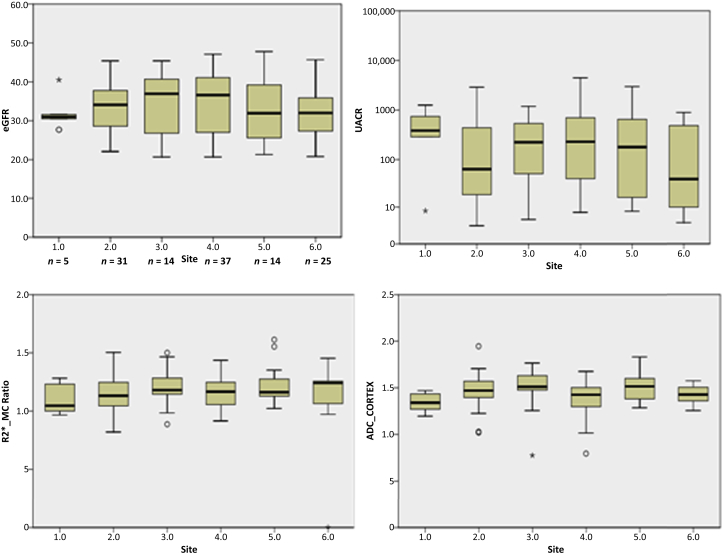
To characterize MRI measurements across all clinical sites, we examined box-whisker plots for R2*_MC Ratio, ADC_Cortex, eGFR, and urine albumin to creatinine ratio (UACR) by individual sites (Figure 2). Analysis of variance showed no significant differences in any of these parameters between sites.
Figure 2.
Box plots summarizing the measurements from each of the 6 sites. R2*_MC ratio is an objective measure to compare data from different scanners, because R2* is sensitive to field inhomogeneities and coil position, for example. It was also the parameter that showed the largest difference compared with healthy controls. For reference, we also include estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (UACR) values across the sites. Analysis of variance showed no differences in any of the measurements between sites. Circles represent outliers, and asterisks represent extreme outliers, i.e., > 1.5 × interquartile range. ADC, apparent diffusion coefficient.
Finally, we explored associations of MRI measurements with clinical parameters in participants with CKD (Table 4). R2*_Medulla and R2*_MC Ratio showed significant association with eGFR and UACR (Table 5). These relationships remained significant after adjusting for age, race, gender, and diabetes status. The association of medullary R2* with UACR may be interesting because higher UACR is considered to be a predictor of fast progression.18 R2*_Medulla was associated with ADC and remained significant even after adjusting for eGFR and UACR (Table 6). ADC was also associated with diabetes status, and remained significant even after adjusting for eGFR and UACR (Table 7). Further studies are necessary to confirm these findings. Race and gender did not show differences in any of the MRI parameters (data not shown).
Table 4.
Spearman correlations between magnetic resonance imaging parameters and renal function in the participants with CKD
| ADCdiffusion | eGFR | Log(UACR) | ||
|---|---|---|---|---|
| Oxygenation: | ||||
| ρ | 0.011 | −0.027 | −0.111 | |
| R2*_Cortex | P | 0.903 | 0.769 | 0.225 |
| n | 122 | 123 | 122 | |
| ρ | 0.184a | 0.195a | −0.311b | |
| R2*_Medulla | P | 0.044 | 0.032 | 0.001 |
| n | 120 | 121 | 120 | |
| ρ | 0.062 | 0.026 | −0.209a | |
| R2*_Kidney | P | 0.500 | 0.775 | 0.021 |
| n | 122 | 123 | 122 | |
| ρ | 0.223a | 0.150 | −0.225a | |
| R2*_MC ratio | P | 0.014 | 0.100 | 0.013 |
| n | 120 | 121 | 120 | |
| Response to furosemide: | ||||
| ρ | −0.009 | 0.045 | −0.080 | |
| ΔR2*_Cortex | P | 0.952 | 0.748 | 0.571 |
| n | 53 | 54 | 53 | |
| ρ | 0.257 | −0.018 | −0.139 | |
| ΔR2*_Medulla | P | 0.063 | 0.899 | 0.321 |
| n | 53 | 54 | 53 | |
| ρ | 0.053 | 0.086 | −0.239 | |
| ΔR2*_Kidney | P | 0.705 | 0.539 | 0.085 |
| n | 53 | 54 | 53 | |
| Fibrosis: | ||||
| ρ | 1.000 | −0.053 | −0.167 | |
| ADC | P | 0.557 | 0.063 | |
| n | 126 | 125 | 125 | |
| Conventional parameters: | ||||
| ρ | −0.053 | 1.000 | −0.105 | |
| eGFR | P | 0.557 | 0.244 | |
| n | 125 | 126 | 125 | |
| ρ | −0.167 | −0.105 | 1.000 | |
| Log(UACR) | P | 0.063 | 0.244 | |
| n | 125 | 125 | 126 |
ADC, apparent diffusion coefficient; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; UACR, urinary albumin to creatinine ratio.
Bold values indicate P < 0.05.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Table 5.
Linear regression of MRI indices with UACR and eGFR
| Dependent variable | Predictors | β | SE | P | Adjusteda |
||
|---|---|---|---|---|---|---|---|
| β | SE | P | |||||
| R2*_Medulla | UACRb | −1.125 | 0.342 | 0.001 | −0.798 | 0.364 | 0.021 |
| eGFR | 0.081 | 0.040 | 0.048 | 0.080 | 0.039 | 0.044 | |
| R2*_MC ratio | UACRb | −0.039 | 0.016 | 0.017 | −0.035 | 0.017 | 0.048 |
| eGFR | 0.004 | 0.002 | 0.049 | 0.004 | 0.002 | 0.038 | |
| R2*_Kidney | UACRb | −0.569 | 0.300 | 0.061 | −0.353 | 0.316 | 0.267 |
| eGFR | 0.016 | 0.035 | 0.649 | 0.013 | 0.034 | 0.703 | |
eGFR, estimated glomerular filtration rate; MRI, magnetic resonance imaging; UACR, urine albumin to creatinine ratio.
Bold values indicate P < 0.05.
Adjusted for age, race, gender, and diabetes status.
Log transformed.
Table 6.
Linear regression of ADC with R2* indices
| Dependent variable | Predictors | β | SE | P | Adjusteda |
||
|---|---|---|---|---|---|---|---|
| β | SE | P | |||||
| ADC | R2*_Medulla | 0.010 | 0.005 | 0.036 | 0.011 | 0.005 | 0.043 |
| R2*_MC ratio | 0.157 | 0.107 | 0.144 | 0.156 | 0.112 | 0.165 | |
ADC, apparent diffusion coefficient.
Bold values indicate P < 0.05.
Adjusted for estimated glomerular filtration rate and log-transformed urine albumin to creatinine ratio.
Table 7.
Linear regression of ADC with diabetes status (0 = no; 1 = yes)
| Dependent variable | Predictors | β | SE | P | Adjusteda |
||
|---|---|---|---|---|---|---|---|
| β | SE | P | |||||
| ADC | Diabetes status | −0.097 | 0.030 | 0.002 | −0.096 | 0.031 | 0.003 |
ADC, apparent diffusion coefficient.
Bold values indicate P < 0.05.
Adjusted for estimated glomerular filtration rate and log-transformed urine albumin to creatinine ratio.
Limitations
Our study was performed in patients with a 20 < GFR < 45 ml/min, so conclusions based on these results cannot be generalized to patients with all stages of CKD. Sodium intake was not controlled, which has been shown to affect renal medullary oxygenation.19 Use of hand-drawn ROIs for the medulla may not be objective.14 Ideally, future studies should use fully automated segmentation of the kidneys performed on high-contrast anatomic images that are coregistered to the BOLD MRIs.20 It is not clear whether stopping angiotensin-converting enzyme inhibitors/angiotensin receptor blockers for 1 day before MRI and oral loop diuretics only on the day of the MRI is sufficient in this group of participants with advanced CKD. Use of the same dose of furosemide in individuals with lower renal function may not be optimal. Participants in this study did not undergo kidney biopsy, so we cannot directly determine correlations of the MRI findings with biopsy-proven fibrosis measures. The 6 centers enrolled participants from varying clinic settings. The control group had a limited number (n = 13) and were all from a single center. All participating sites used MRI scanners from a single vendor.
In conclusion, our data support the feasibility of using renal BOLD and diffusion MRI in multicenter trials and the data are consistent with prior reports based on single-site studies. These data combined with other results from a recent report21 support planned international initiatives, such as BEAt-DKD,22 that involve longitudinal multicenter trials using multiparametric MRI. Overall, our observations in advanced CKD further confirm the reduced renal cortical oxygenation and presence of renal fibrosis consistent with the chronic hypoxia hypothesis. The medullary oxygenation was significantly increased compared with controls and is also consistent with prior reports. The significantly lower ADC in participants with diabetes is novel and may have clinical relevance. Future studies to monitor progressive changes in eGFR are needed to verify if and which of these MRI parameters are specific to progressive CKD. In participants with advanced CKD, evaluating response to furosemide may be limited by the low baseline medullary R2* and their potential chronic use of loop diuretics.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The COMBINE study is supported by grants U01DK099877, U01DK097093, U01DK099930, U01DK099933, U01DK099924, and R01DK102438 (TI) from the National Institute of Diabetes and Digestive and Kidney Diseases. The work also was supported in part by R01DK093793 (PVP) and F31HL123360 (JT).
Footnotes
Supplementary Methods.
Table S1. MRI acquisition parameters.
Table S2. Intra- and interreader agreement.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
MRI acquisition parameters.
Intra- and interreader agreement.
References
- 1.Fine L.G., Orphanides C., Norman J.T. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74–S78. [PubMed] [Google Scholar]
- 2.Prasad P.V., Edelman R.R., Epstein F.H. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 3.Mao W., Zhou J., Zeng M. Intravoxel incoherent motion diffusion-weighted imaging for the assessment of renal fibrosis of chronic kidney disease: a preliminary study. Magn Reson Imaging. 2018;47:118–124. doi: 10.1016/j.mri.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Togao O., Doi S., Kuro-o M. Assessment of renal fibrosis with diffusion-weighted MR imaging: study with murine model of unilateral ureteral obstruction. Radiology. 2010;255:772–780. doi: 10.1148/radiol.10091735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue T., Kozawa E., Okada H. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22:1429–1434. doi: 10.1681/ASN.2010111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad P.V., Thacker J., Li L.P. Multi-parametric evaluation of chronic kidney disease by MRI: a preliminary cross-sectional study. PLoS One. 2015;10:e0139661. doi: 10.1371/journal.pone.0139661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pruijm M., Milani B., Pivin E. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. 2018;93:932–940. doi: 10.1016/j.kint.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Priyadarshi A., Periyasamy S., Burke T.J. Effects of reduction of renal mass on renal oxygen tension and erythropoietin production in the rat. Kidney Int. 2002;61:542–546. doi: 10.1046/j.1523-1755.2002.00140.x. [DOI] [PubMed] [Google Scholar]
- 9.Milani B., Ansaloni A., Sousa-Guimaraes S. Reduction of cortical oxygenation in chronic kidney disease: evidence obtained with a new analysis method of blood oxygenation level-dependent magnetic resonance imaging. Nephrol Dial Transplant. 2017;32:2097–2105. doi: 10.1093/ndt/gfw362. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z.J., Kumar R., Banerjee S. Blood oxygen level-dependent (BOLD) MRI of diabetic nephropathy: preliminary experience. J Magn Reson Imaging. 2011;33:655–660. doi: 10.1002/jmri.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manotham K., Tanaka T., Matsumoto M. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277–1288. doi: 10.1097/01.asn.0000125614.35046.10. [DOI] [PubMed] [Google Scholar]
- 12.Neugarten J. Renal BOLD-MRI and assessment for renal hypoxia. Kidney Int. 2012;81:613–614. doi: 10.1038/ki.2011.462. [DOI] [PubMed] [Google Scholar]
- 13.Lee V.S., Kaur M., Bokacheva L. What causes diminished corticomedullary differentiation in renal insufficiency? J Magn Reson Imaging. 2007;25:790–795. doi: 10.1002/jmri.20878. [DOI] [PubMed] [Google Scholar]
- 14.Thacker J.M., Li L.P., Li W. Renal blood oxygenation level-dependent magnetic resonance imaging: a sensitive and objective analysis. Invest Radiol. 2015;50:821–827. doi: 10.1097/RLI.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez S.I., Warner L., Haas J.A. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009;297:F981–F986. doi: 10.1152/ajprenal.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruijm M., Hofmann L., Piskunowicz M. Determinants of renal tissue oxygenation as measured with BOLD-MRI in chronic kidney disease and hypertension in humans. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall M.E., Rocco M.V., Morgan T.M. Chronic diuretic therapy attenuates renal BOLD magnetic resonance response to an acute furosemide stimulus. J Cardiovasc Magn Reson. 2014;16:17. doi: 10.1186/1532-429X-16-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzo V., Saracho R., Zamora J. Similar renal decline in diabetic and non-diabetic patients with comparable levels of albuminuria. Nephrol Dial Transplant. 2010;25:835–841. doi: 10.1093/ndt/gfp475. [DOI] [PubMed] [Google Scholar]
- 19.Pruijm M., Hofmann L., Maillard M. Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension. 2010;55:1116–1122. doi: 10.1161/HYPERTENSIONAHA.109.149682. [DOI] [PubMed] [Google Scholar]
- 20.Cox E.F., Buchanan C.E., Bradley C.R. Multiparametric renal magnetic resonance imaging: validation, interventions, and alterations in chronic kidney disease. Front Physiol. 2017;8:696. doi: 10.3389/fphys.2017.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L.P., Thacker J., Li W. Consistency of multiple renal functional MRI measurements over 18 months. J Magn Reson Imaging. 2018;48:514–521. doi: 10.1002/jmri.26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biomarker Enterprise to Attack Diabetic Kidney Disease (BEAt-DKD). Available at: www.beat-dkd.eu. Accessed on August 6, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MRI acquisition parameters.
Intra- and interreader agreement.