Abstract
Introduction
Medial arterial calcification is common in chronic kidney disease (CKD) and portends poor clinical outcomes, but its progression relative to the severity of CKD and the role of other risk factors is unknown because of the lack of reliable quantification.
Methods
Calcification of breast arteries detected by mammography, which is exclusively medial and correlates with medial calcification in peripheral arteries and with cardiovascular outcomes, was used to measure the progression of medial arterial calcification in women with CKD and end-stage renal disease (ESRD). Measurements showed intra- and interobserver correlations of 0.98, an interstudy variability of 8% to 11%, and a correlation with computed tomographic measurements of 0.92.
Results
Progression of calcification was measured in 60 control subjects (estimated glomerular filtration rate (eGFR) ≥ 90 ml/min per 1.73 m2) and 137 subjects with CKD (eGFR < 90 ml/min per 1.73 m2). Progression in control subjects was linear over time and independent of age. The rate of progression was increased in CKD but only at eGFR < 40 ml/min per 1.73 m2 (median, 8.1 vs. 3.9 mm/breast/yr in controls; P = 0.006). Progression accelerated markedly in subjects with ESRD (median, 20 mm/breast/yr; n = 36), but did not differ from controls after kidney transplantation (n = 25). Diabetes significantly augmented progression in subjects with CKD and ESRD but not in controls.
Conclusion
Mammography is a convenient and reliable method to measure the progression of medial arterial calcification. Progression does not increase until advanced stages of CKD, accelerates markedly in ESRD, and returns to control rates after kidney transplantation. Diabetes significantly increases progression in CKD and ESRD.
Keywords: diabetes, ESRD, mammography, vascular calcification
Vascular calcification is common in subjects with advanced stages of CKD and predicts poor outcomes. This calcification comprises 2 distinct forms: atherosclerotic calcification within neointimal plaques and medial calcification within the smooth muscle layer that can occur in the absence of atherosclerosis and is linked to altered bone and mineral metabolism. Although atherosclerotic calcification is increased in advanced kidney disease,1 it is the medial form that is particularly prevalent2, 3 and is a greater risk factor for cardiovascular events or death.4, 5 However, it is not clear when the risk of medial calcification begins in CKD and how this is affected by other risk factors. This information could help clarify the relationship between medial arterial calcification and altered bone and mineral metabolism in CKD and guide potential therapies.
The study of medial calcification in humans has been hampered by the lack of specific imaging and precise quantification. Because clinical studies have focused almost exclusively on coronary arteries, the aorta, or its branches, in which both medial and atherosclerotic calcification occur, specific information on medial arterial calcification is not provided. Attempts to distinguish and quantify medial calcification through radiologic patterns or by examining peripheral arteries are problematic because of poor specificity, the existence of atherosclerosis in some peripheral arteries, the lack of validation, and the semiquantitative nature of the measurements.6, 7
Precise information on medial calcification can only come from arterial beds devoid of atherosclerosis in which calcification can be imaged with high sensitivity. Arterial calcification is easily detected on mammograms and is exclusively medial8 because atherosclerosis does not occur in breast arteries.8, 9 This calcification correlates with calcification in peripheral arteries in ESRD10 and with cardiovascular disease both in the general population11, 12, 13 and in subjects with ESRD,14 indicating that it is a marker of medial arterial calcification systemically. We have previously shown that the prevalence of breast arterial calcification (BAC) is increased at least by stage 4 CKD15 but prevalence data lack sensitivity and could be influenced by the duration of CKD, which is difficult to quantify. The effect of CKD and ESRD on medial calcification is best determined by following its progression, and to this end, we have developed and validated a measurement of medial arterial calcification on routine mammograms that can be used to quantify its progression in CKD and ESRD.
Methods
Mammography
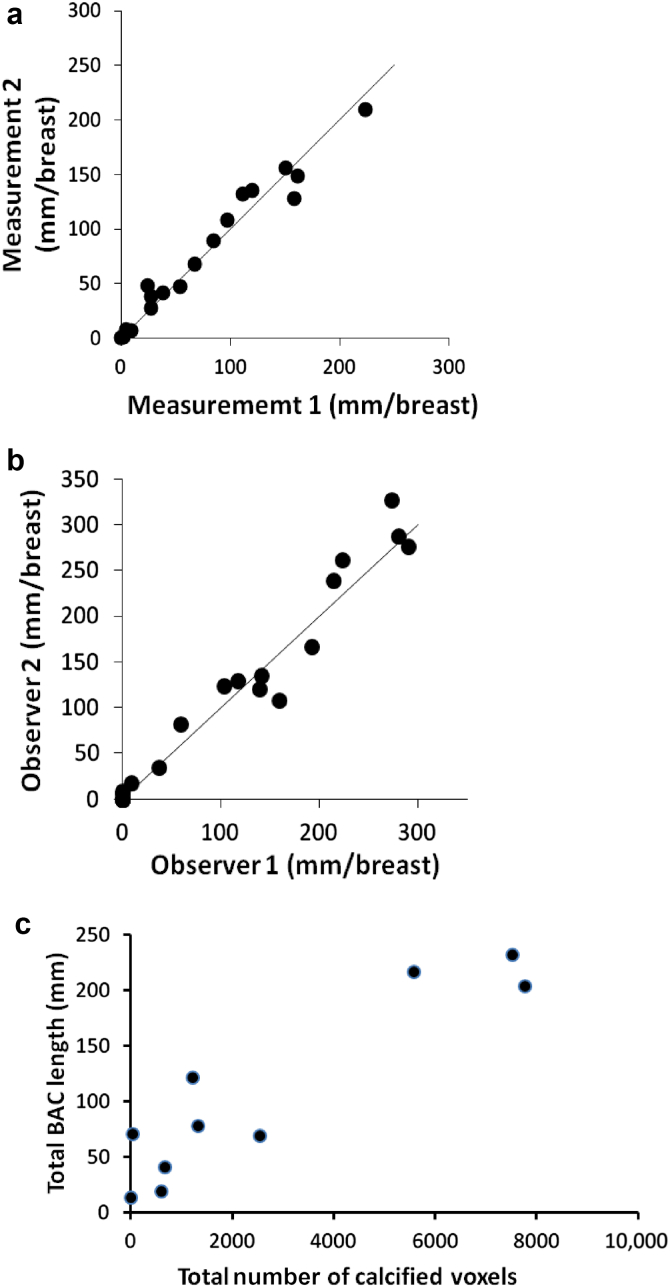
Arterial calcification was identified on standard digital mammograms as linear densities along the walls of arteries and is easily distinguished from other calcifications. Only arteries with involvement of both walls were considered calcified, and when there was any uncertainty, the region was considered not to be calcified. The lengths of calcified segments were measured using standard clinical PACS software (Centricity PACS Radiology RA1000 Workstation; GE Healthcare, Barrington, IL) and then summed and expressed in mm/breast (Figure 1). There was no correlation between breast size, modeled as a hemi-ellipsoid (π/6 × height × the square of the width at the base), and BAC score (r = 0.09; n = 43). Measurements were performed by 3 investigators, all blinded to the clinical information. The correlation between repeat measurements on 18 images by the same individual was 0.98, with a mean difference of 9.9 mm or 13%, and the correlation between measurements on 23 images by 2 individuals was 0.98, with a mean difference of 14 mm or 14% (Figure 2a and b). To minimize variability in measuring progression, all mammograms from the same patient were measured by a single individual.
Figure 1.
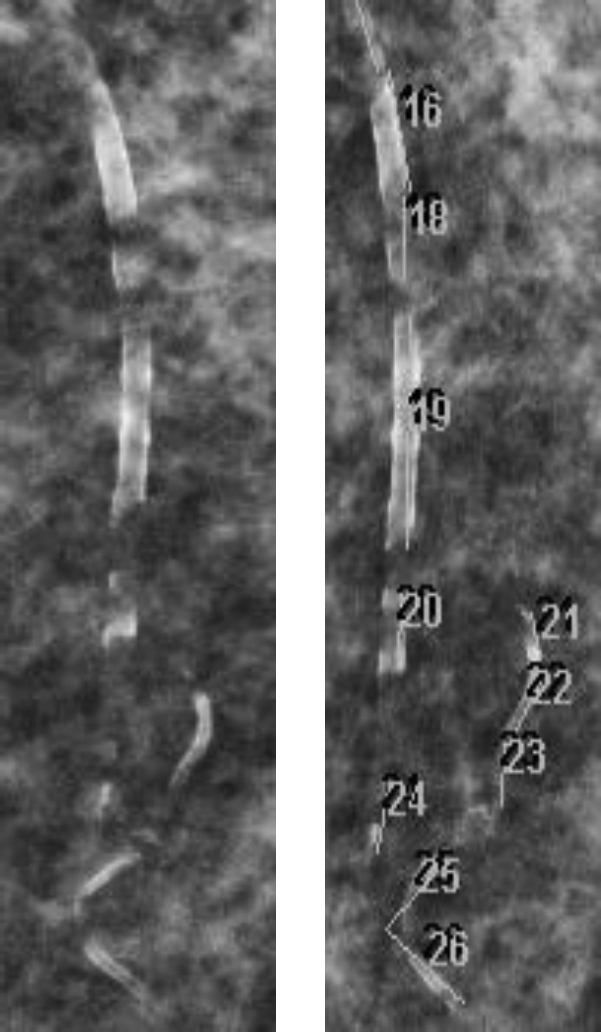
Arterial calcification and its measurement on a portion of a mammogram.
Figure 2.
Reproducibility and accuracy of the measurement of breast arterial calcification (BAC) on mammograms. (a) Two blinded measurements on the same image by the same individual. (b) Measurements on the same image by 2 individuals. (c) Correlation between BAC measured by mammography and the volume of calcified arteries measured by computed tomography.
Breast Computed Tomography
Validation of the mammography measurements was performed by computed tomography (CT) of single breasts in 10 subjects with arterial calcification, 33 to 71 days after the mammogram. This was performed using a dedicated Koning Breast CT system (Koning Corporation, West Henrietta, NY). Breast CT is a recently developed technique that results in high-resolution (273-μm) 3-dimensional images with high contrast. The images were subjected to an algorithm to correct for cupping artifacts and then analyzed using ImageJ software (National Institutes of Health, Bethesda, MD) via simultaneous inspection of coronal and sagittal planes. Regions of vascular calcification were selected, and the density of each voxel was measured. The number of voxels with a density greater than that of noncalcified arteries was determined for each region and these were then summed to give the total number of calcified voxels in the breast (volume score). Validation of the CT quantification of calcium was performed by scanning a breast phantom consisting of a cylindrical plastic container of shortening (adipose tissue equivalent) embedded with small tubes containing suspensions of varying concentrations of hydroxyapatite in shortening. The mean Hounsfield units in each standard and the background surrounding each standard were measured, and the difference yielded a linear relationship between Hounsfield units and calcium density (r2 = 0.994; Supplementary Figure S1). The slope was applied to the density of each calcified voxel (above baseline) and multiplied by voxel volume (0.02 μl) to determine the total amount of arterial calcium. The correlation between the CT volume and BAC measured on craniocaudal views was 0.92 (Figure 2c) as compared with 0.85 for BAC measured on mediolateral oblique views (not shown). Correlation with the total amount of calcium was not as strong (0.86), which is consistent with the fact that the length rather than the density of calcifications was measured on mammograms. Applying a semiquantitative weighting of severity to the mammography measurements did not improve this correlation, indicating that assessment of the density of calcification on mammograms is unreliable. Because of the stronger correlation with CT scans, craniocaudal views were used for all subsequent measurements.
Subjects
Subjects with and without CKD were identified from a computerized search of medical records at Emory Healthcare, for all mammograms scheduled between January 3, 2011, and December 17, 2013. Additional information that was obtained included date of birth, sex, race, history of diabetes or diabetes medications, history of warfarin use, serum creatinine levels, and dates of measurement. Men and subjects with current warfarin use, which is associated with medial arterial calcification,16 were excluded. The glomerular filtration rate was estimated (eGFR) in ml/min per 1.73 m2 as determined by the 4-variable equation from the Modification of Diet in Renal Disease Study.17 All available subjects with eGFR < 30 ml/min per 1.73 m2 were screened, whereas subjects with eGFR ≥ 30 ml/min per 1.73 m2 were randomly selected for screening by birth month. The cohort was supplemented with diabetic women with eGFR ≥ 60 ml/min per 1.73 m2, again selected at random, to account for the higher prevalence of diabetes in subjects with lower eGFR. Women with BAC and at least 1 subsequent mammogram underwent medical record review to confirm the CKD stage during the mammogram interval and to exclude subjects who were receiving warfarin or who had ESRD or underwent kidney transplantation. The eGFR assigned to each subject was calculated from the estimated serum creatinine value at the midpoint between the 2 mammograms, which was determined by interpolation of values obtained before and after mammograms on the basis of a linear increase in serum creatinine level over time. Women who had ESRD or underwent kidney transplantation and mammograms were identified by comparing the aforementioned mammogram search with searches for all subjects with either diagnosis. ESRD was identified by chronic outpatient hemodialysis during the mammogram interval, and only subjects with serum creatinine levels < 1.8 mg/dl (159 umol/l) after kidney transplantation during the mammogram interval were included. Confirmation of diabetes, warfarin use, onset of ESRD, dialysis modality, and transplantation were all obtained through physician review of medical records. Diabetes was identified as a diagnosis of diabetes in the medical record and the use of hypoglycemic medications during the mammogram interval. All protocols were approved by the Institutional Review Board of Emory University.
Statistics
The distribution of data was assessed for normality using the Shapiro-Wilk test. Normally distributed continuous variables are presented as mean ± SE and analyzed using the Student t test. Nonparametric data are presented as median and interquartile range and analyzed using the Mann-Whitney U test or Kruskal-Wallis test. Binary variables were analyzed using the Fisher exact test. Progression of BAC is presented as Tukey box plots, in which the median is shown within boxes representing the interquartile range and error bars indicating maximum and minimum data points within 1.5 times the interquartile range above and below the quartiles. Data points outside this range are depicted individually.
Results
The time course of BAC progression was examined in 11 subjects with at least 5 sequential mammograms. To minimize variability due to biological factors, only subjects without diabetes, warfarin use, or renal failure (eGFR > 90 ml/min per 1.73 m2) were included. Progression was analyzed by linear regression in each subject (examples shown in Supplementary Figure S2). The mean correlation coefficient for the group was 0.79, and the mean of the residuals of this regression did not differ significantly from zero, which is consistent with a linear progression. These data were also used to estimate interstudy variability, which includes variability in the imaging and variability in the measurement. The absolute values of the residuals of this regression, representing the differences between measured and expected BAC, averaged over the entire cohort, yielded a mean error of 7.24 ± 1.01 mm/breast or 11.1% ± 1.4%. The percent error is magnified by minimal BAC in some subjects. When calculated instead by dividing the error by the mean BAC score for the entire group (94.5 mm/breast), the error was 7.7%. These data were also used to determine the error associated with measuring the rate of BAC on just 2 mammograms instead of 5. This was dependent on the interval between mammograms, with an error of ∼2 mm/breast/yr for intervals of ≥3 years (Supplementary Figure S3). Therefore, an interval of at least 3 years was used to measure progression of BAC, with intervals of <3 years used only when other mammograms were not available.
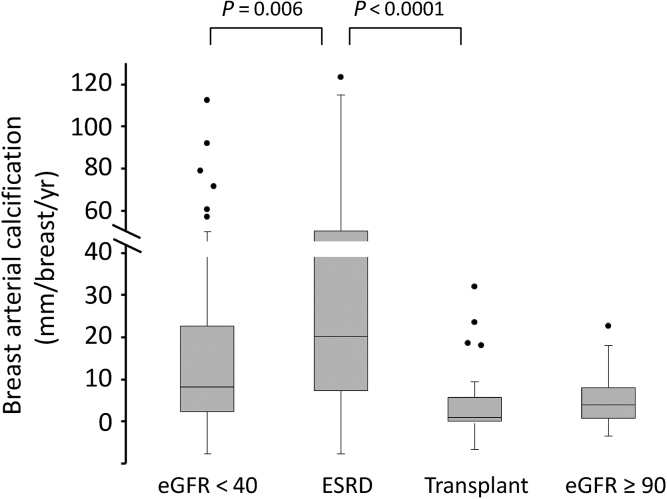
Progression of BAC was measured in 60 women without CKD (eGFR ≥ 90 ml/min per 1.73 m2) and 137 women with CKD (eGFR < 90 ml/min per 1.73 m2). The latter cohort was divided into tertiles on the basis of eGFR, and the characteristics of subjects in these tertiles and control subjects are listed in Table 1. Neither the values for BAC nor the progression rate was normally distributed, and these values are presented as medians and interquartile ranges. There were no differences in age, race, prevalence of diabetes, or baseline BAC between the groups. The mammogram interval was greater in women without CKD, but was similar in each of the CKD tertiles. The progression rate was the same in the first 2 tertiles as in controls but significantly higher in the lowest tertile of eGFR. The individual data points for subjects with CKD are shown in Figure 3 along with the interquartile range in the control cohort.
Table 1.
Characteristics of subjects with CKD and controls
| Characteristic | No CKD | CKD tertile 1 | CKD tertile 2 | CKD tertile 3 |
|---|---|---|---|---|
| n | 60 | 45 | 46 | 46 |
| Age (yr) | 77.0 ± 1.0 | 74.4 ± 1.2 | 78.3 ± 1.0 | 73.4 ± 1.5 |
| Diabetes (%) | 28 | 33 | 26 | 33 |
| Race: African American (%) | 30 | 38 | 20 | 46 |
| eGFR (ml/min per 1.73 m2)a | 90–170 | 53–90 | 40–52 | 6–39 |
| BAC0 (mm/breast) | 26 (12–71) | 22 (12–54) | 33 (13–79) | 31 (14–95) |
| MG interval (yr) | 4.1 ± 0.3 | 2.5 ± 0.1 | 2.7 ± 0.2 | 2.5 ± 0.2 |
| BAC rate (mm/breast/yr) | 3.9 (0.7–8.0) | 3.3 (0.3–6.2) | 4.2 (0.3–10) | 8.1 (2.4–23)b |
Data are expressed as mean ± SE, or median (interquartile range).
BAC, breast arterial calcification; BAC0, baseline breast arterial calcification; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MG, mammogram.
eGFR is presented as a range.
P = 0.006 by the Kruskal-Wallis test.
Figure 3.
Progression of breast arterial calcification in subjects with chronic kidney disease. The dashed lines indicate the first and third quartiles of progression in control subjects. eGFR, estimated glomerular filtration rate.
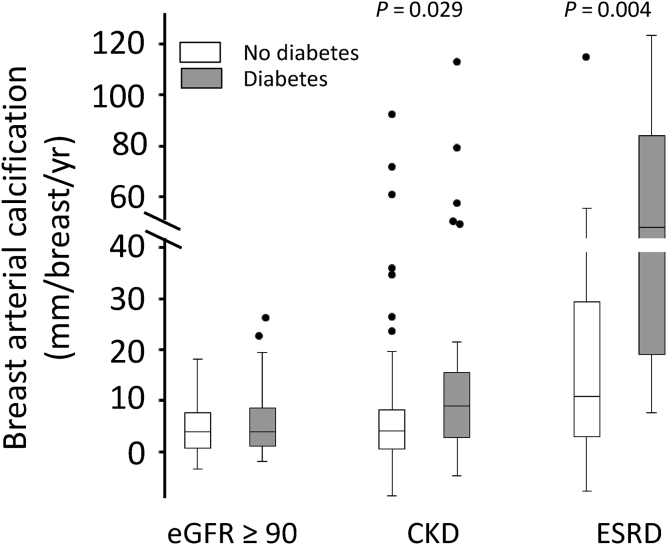
An additional 36 subjects with ESRD and 25 subjects with kidney transplants were studied, and their characteristics are listed in Table 2. Age, race, prevalence of diabetes, and baseline BAC did not differ significantly between the groups. The proportion of African Americans was higher in both these groups compared with controls and subjects with CKD, but race did not significantly affect BAC progression in a multivariable analysis of controls and subjects with CKD. The mammogram interval was shorter in subjects with ESRD. Although this could increase the error in determining the rate of progression, this is compensated by the much higher rate of progression. The progression of BAC is shown graphically and compared with that in controls and the third CKD tertile in Figure 4. Subjects with ESRD had a significantly higher rate of progression than did subjects in the third CKD tertile, whereas subjects with kidney transplants had a significantly lower progression rate than did subjects with ESRD, which was not different from the rate in subjects without CKD. The rate was negative in 40% of the subjects with kidney transplants. The effect of diabetes on progression of BAC was examined in controls, subjects with CKD, and subjects with ESRD (Figure 5). Among control subjects, progression was identical in diabetics and nondiabetics, but diabetes was associated with a significantly higher rate of progression in subjects with CKD (2-fold in median rate) and subjects with ESRD (4.4-fold in median rate).
Table 2.
Characteristics of subjects with ESRD and those who underwent kidney transplantation
| Characteristic | ESRD | Transplantation |
|---|---|---|
| n | 36 | 25 |
| Age (yr) | 60.1 ± 1.7 | 57.7 ± 1.9 |
| Diabetes (%) | 44 | 28 |
| Race: African American (%) | 86 | 64 |
| ESRD duration (yr)a | 5.2 ± 0.6 | 5.5 ± 0.7 |
| Serum creatinine level (mg/dl) | 1.09 ± 0.1 | |
| BAC0 (mm/breast) | 43 (10 to 97) | 49 (6 to 106) |
| MG interval (yr) | 2.1 ± 0.2 | 2.8 ± 0.3 |
| BAC rate (mm/breast/yr) | 20 (7.4 to 51) | 0.5 (−0.5 to 5.2) |
Data are expressed as mean ± SE, or median (interquartile range).
BAC, breast arterial calcification; BAC0, baseline breast arterial calcification; ESRD, end-stage renal disease; MG, mammogram.
ESRD duration denotes years of renal replacement therapy before the most recent mammogram or before kidney transplantation.
Figure 4.
Progression of breast arterial calcification in subjects with end-stage renal disease (ESRD) or subjects who underwent kidney transplantation. The boxes represent the interquartile range (IQR), with the intervening line indicating the median. The error bars indicate the maximum and minimum data points within 1.5× the IQR of the first and third quartiles, with symbols indicating outlying points. Data for control subjects and subjects in the lowest chronic kidney disease tertile from Figure 2 are shown for comparison. Significances are determined using the Mann-Whitney U test. eGFR, estimated glomerular filtration rate.
Figure 5.
Progression of breast arterial calcification in subjects with and without diabetes. The boxes represent the interquartile range (IQR), with the intervening line indicating the median. The error bars indicate the maximum and minimum data points within 1.5× the IQR of the first and third quartiles, with symbols indicating outlying points. Significances are determined using the Mann-Whitney U test. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease.
Discussion
This is the first quantitative measurement of medial arterial calcification and its progression in humans. The measurements correlated well with measurements obtained by CT, and the reproducibility and sensitivity were sufficient to detect the normal progression of calcification. Although limited to breast arteries, abundant data demonstrate that this calcification is indicative of medial calcification elsewhere. There is a strong correlation with cardiovascular disease both in the general population11, 12, 13 and in subjects with ESRD,14 as well as with medial calcification in peripheral arteries in subjects with ESRD10 and other subjects (K. H. Han and W. C. O’Neill, unpublished data). Furthermore, warfarin increases calcification in both breast arteries16 and peripheral arteries.18 Although measurements have been performed on radiographs of distal extremities,7 where the calcification is primarily medial,19, 20 these are only semiquantitative and are limited by their insensitivity, lack of validation, and the fact that atherosclerotic calcification can occur at these sites.20
Although CKD is known to be a risk factor for medial arterial calcification,4, 5 the stage at which this risk begins has remained unknown. This is best examined by measuring progression rather than quantity, which can be influenced by the duration of CKD and other risk factors. A significant 2-fold greater progression was seen in subjects with eGFR < 40 ml/min per 1.73 m2 than in controls but not in CKD subjects with higher eGFR, indicating that the risk for medial arterial calcification begins in advanced CKD. These results are consistent with our previous data showing an increase in the prevalence of BAC in women with stage 4 or 5 CKD, but not CKD 3,15 as well as with a recent study5 showing increased calcification of hand arteries in stages 4 and 5 compared to stage 3. However, the latter study did not examine earlier stages. A histologic study of coronary arteries showed medial calcification only in subjects with stage 4 CKD or higher1 but the numbers were small and other risk factors were not controlled.
Progression of BAC accelerated markedly in subjects undergoing hemodialysis. This could be due to poorer residual renal function or some aspect of hemodialysis or ESRD care, but it is clear that hemodialysis does not improve the risk. The progression rate in subjects with transplanted kidneys did not differ from that in control subjects. Although this could represent bias in selecting healthier subjects for transplantation, there was no difference in age, diabetes prevalence, ESRD duration, or baseline calcification between subjects with ESRD and subjects with transplanted kidneys, suggesting that progression returns to control levels after kidney transplantation and that the risk of calcification is eliminated by restoring renal function. The fact that the rate was negative in 40% of the subjects with transplanted kidneys compared with 17% of controls raises the possibility of reversal of calcification after transplantation.
Progression of calcification was strongly influenced by the presence of diabetes, which significantly increased the rate in subjects with CKD progression or ESRD. Interestingly, diabetes did not increase in control subjects, indicating an important interaction between diabetes and CKD. Although diabetes has been associated with increased BAC21, 22 and medial arterial calcification elsewhere,23 renal function was rarely evaluated and it is possible that some of these subjects had advanced CKD. Interestingly, age, which is a potent determinant of the prevalence of BAC,24 had no effect on progression in controls or subjects with CKD. This suggests that the effect of age on prevalence is due to a steady rate of accumulation over time.
The progression of medial arterial calcification in relation to eGFR may provide some insight into its linkage with altered bone and mineral metabolism, which has been ascribed to a number of factors including hyperphosphatemia, hyperparathyroidism, and elevated fibroblast growth factor 23 levels. The fact that progression of medial arterial calcification increases only in advanced CKD is not consistent with a major role for parathyroid hormone or fibroblast growth factor 23, levels of which increase at earlier stages.25, 26 However, the data are consistent with a role for hyperphosphatemia, which begins to develop at eGFR < 40 ml/min per 1.73 m2.25 Reliable data on mineral metabolism were not available in this retrospective study, and a prospective study will be required to address this. Calcification accelerated markedly in subjects undergoing hemodialysis. Although this could be due to further loss of renal function, other factors such as postdialysis alkalemia or the more frequent use of active vitamin D compounds may play a role. It is also possible that subjects with more rapid calcification may be more likely to progress to ESRD.
A major limitation of this study is that the measurement of medial arterial calcification is restricted to females. The breast is uniquely suited for the detection and measurement of medial arterial calcification because of the sensitivity of mammography and the periarterial fat, and a vascular bed of comparable ease and sensitivity of imaging does not exist in men. However, insights obtained in women are likely to be applicable to men as well. For instance, the increased prevalence of BAC in warfarin users16 was subsequently noted in the lower limb radiographs in men.18 Another limitation is the reduced frequency of mammography in women with advanced CKD, which is likely explained by current data and recommendations on the benefits versus limited life expectancy in this population.27, 28, 29 Because screening mammography is recommended only in women with a life expectancy of >5 years, the cohorts in this study likely represent the healthiest of these subjects and the results may therefore underestimate the true rates of calcification in advanced CKD. Given the correlation between clinical outcomes and medial arterial calcification in the breast and other locations in CKD and ESRD,4, 5, 14 mammography may have a useful clinical role beyond screening for cancer.
In summary, BAC can be easily and reliably quantified and followed by routine mammography and can define the risk of medial arterial calcification in CKD and ESRD. Because screening mammography within an age range applicable to cardiovascular disease has been routine for many years, a large body of retrospective data is available for analysis. The availability of serial mammograms also allows longitudinal studies to be performed in retrospective cohorts. In contrast to other protocols for quantifying vascular calcification, mammography is clinically indicated in most women within the relevant age range, and prospective studies can be performed with little or no additional imaging cost or radiation exposure.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported in part by the Data Analytics and Biostatistics Core within the Department of Medicine, Emory University. The assistance of Ms. Debra Carter in the Emory Breast Imaging Center is gratefully acknowledged.
Footnotes
Figure S1. Validation of the measurement of calcium by breast computed tomography. Relationship between Hounsfield units (HU) and the density of hydroyapatite (HA) in a breast phantom.
Figure S2. Time course of breast arterial calcification in 5 subjects. Data are the total for both breasts. Each graph represents a different subject, and the linear regression line is shown for each.
Figure S3. Error in the measurement of progression of breast arterial calcification obtained from 2 mammograms as a function of the interval between mammograms. The ordinate is the absolute difference between the rate calculated from 2 mammograms and the rate calculated by linear regression of measurements from 5 mammograms. The numbers above the bars indicate the number of subjects.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Validation of the measurement of calcium by breast computed tomography. Relationship between Hounsfield units (HU) and the density of hydroyapatite (HA) in a breast phantom.
Time course of breast arterial calcification in 5 subjects. Data are the total for both breasts. Each graph represents a different subject, and the linear regression line is shown for each.
Error in the measurement of progression of breast arterial calcification obtained from 2 mammograms as a function of the interval between mammograms. The ordinate is the absolute difference between the rate calculated from 2 mammograms and the rate calculated by linear regression of measurements from 5 mammograms. The numbers above the bars indicate the number of subjects.
References
- 1.Nakamura S., Ishibashi-Ueda H., Niizuma S. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.London G.M., Guerin A.P., Marchais S.J. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 3.Gross M.L., Meyer H.P., Ziebart H. Calcification of coronary intima and media: immunohistochemistry, backscatter imaging, and x-ray analysis in renal and nonrenal patients. Clin J Am Soc Nephrol. 2007;2:121–134. doi: 10.2215/CJN.01760506. [DOI] [PubMed] [Google Scholar]
- 4.Blacher J., Guerin A.P., Pannier B. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 5.Górriz J.L., Molina P., Cerverón M.J. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 2015;10:654–666. doi: 10.2215/CJN.07450714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kauppila L.I., Polak J.F., Cupples L.A. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 7.Adragao T., Pires A., Lucas C. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephol Dial Transplant. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill W.C., Adams A.L. Breast arterial calcification in chronic kidney disease: absence of smooth muscle apoptosis and osteogenic transdifferentiation. Kidney Int. 2014;85:668–676. doi: 10.1038/ki.2013.351. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen B.B., Holm N.V. Calcification in breast arteries: the frequency and severity of arterial calcification in female breast tissue without malignant changes. Acta Path Microbiol Immunol Scand A. 1985;93:13–16. [PubMed] [Google Scholar]
- 10.Duhn V., D’Orsi E.M., Johnson S. Breast arterial calcification: a marker of medial vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:377–382. doi: 10.2215/CJN.07190810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kataoka M., Warren R., Luben R. How predictive is breast arterial calcification of cardiovascular disease and risk factors when found at screening mammography. AJR Am J Roentgenol. 2006;187:73–80. doi: 10.2214/AJR.05.0365. [DOI] [PubMed] [Google Scholar]
- 12.Crystal P., Crystal E., Leor J. Breast arterial calcification on routine mammography as a potential marker for increased cardiovascular disease. Am J Cardiol. 2000;86:216–217. doi: 10.1016/s0002-9149(00)00860-2. [DOI] [PubMed] [Google Scholar]
- 13.Kemmeren J.M., Beijerinck D., van Noord P.A. Breast arterial calcifications: associations with diabetes mellitus and cardiovascular mortality. Radiology. 1996;201:75–78. doi: 10.1148/radiology.201.1.8816524. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Hassan N., Tantisattamo E., D’Orsi E.T., O’Neill W.C. The clinical significance of medial arterial calcification in end-stage renal disease. Kidney Int. 2011;87:195–199. doi: 10.1038/ki.2014.187. [DOI] [PubMed] [Google Scholar]
- 15.Abou-Hassan N., D’Orsi E.T., D’Orsi C.J., O’Neill W.C. The risk for medial arterial calcification in CKD. Clin J Am Soc Nephrol. 2012;7:275–279. doi: 10.2215/CJN.06490711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tantisattamo E., Han K.H., O’Neill W.C. Increased vascular calcification in patients receiving warfarin. Arterioscler Thromb Vasc Biol. 2015;35:237–242. doi: 10.1161/ATVBAHA.114.304392. [DOI] [PubMed] [Google Scholar]
- 17.Levey A.S., Coresh J., Greene T., Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Han H.K., O’Neill W.C. Increased peripheral arterial calcification in patients receiving warfarin. J Am Heart Assoc. 2016;5:e002665. doi: 10.1161/JAHA.115.002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhury U.K., Airan B., Mishra P.K. Histopathology and morphometry of radial artery conduits: basic study and clinical application. Ann Thorac Surg. 2004;78:1614–1622. doi: 10.1016/j.athoracsur.2004.03.105. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill W.C., Han K.H., Schneider T.M., Hennigar R.A. Prevalence of nonatheromatous lesions in peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2015;35:439–447. doi: 10.1161/ATVBAHA.114.304764. [DOI] [PubMed] [Google Scholar]
- 21.Kemmeren J.M., van Noord P.A., Beijerinck D. Arterial calcification found on breast cancer screening mammograms and cardiovascular mortality in women: the DOM Project. Doorlopend Onderzoek Morbiditeit en Mortaliteit. Am J Epidemiol. 1998;147:333–341. doi: 10.1093/oxfordjournals.aje.a009455. [DOI] [PubMed] [Google Scholar]
- 22.Taskin F., Akdilli A., Karaman C. Mammographically detected breast arterial calcifications: indicators for arteriosclerotic diseases? Eur J Radiol. 2006;60:250–255. doi: 10.1016/j.ejrad.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Everhart J.E., Pettitt D.J., Knowler W.C. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 24.Hendriks E.J., de Jong P.A., van der Graaf Y. Breast arterial calcifications: a systematic review and meta-analysis of their determinants and their association with cardiovascular events. Atherosclerosis. 2015;239:11–20. doi: 10.1016/j.atherosclerosis.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Levin A., Bakris G.L., Molitch M. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 26.Gutierrez O., Isakova T., Rhee E. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 27.Walter L.C., Lindquist K., O’Hare A.M., Johansen K.L. Targeting screening mammography according to life expectancy among women undergoing dialysis. Arch Int Med. 2006;166:1203–1208. doi: 10.1001/archinte.166.11.1203. [DOI] [PubMed] [Google Scholar]
- 28.Kajbaf S., Nichol G., Zimmerman D. Cancer screening and life expectancy of Canadian patients with kidney failure. Nephol Dial Transplant. 2002;17:1786–1789. doi: 10.1093/ndt/17.10.1786. [DOI] [PubMed] [Google Scholar]
- 29.Holley J.L., Von Roenn J. Screening for breast cancer in women with CKD stages 4 to 5. Am J Kid Dis. 2010;56:820–822. doi: 10.1053/j.ajkd.2010.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of the measurement of calcium by breast computed tomography. Relationship between Hounsfield units (HU) and the density of hydroyapatite (HA) in a breast phantom.
Time course of breast arterial calcification in 5 subjects. Data are the total for both breasts. Each graph represents a different subject, and the linear regression line is shown for each.
Error in the measurement of progression of breast arterial calcification obtained from 2 mammograms as a function of the interval between mammograms. The ordinate is the absolute difference between the rate calculated from 2 mammograms and the rate calculated by linear regression of measurements from 5 mammograms. The numbers above the bars indicate the number of subjects.