Introduction
Acute renal cortical necrosis (ACN) is an uncommon but often catastrophic cause of acute kidney injury (AKI). Although the exact incidence of ACN is unknown, it is reported to account for 1% to 2% of all cases of AKI in developed countries.1 Commonly, ACN occurs as a result of catastrophic obstetric complications. We describe an unusual case of AKI due to renal cortical necrosis attributed to N-methylamphetamine (“crystal meth”) use.
Case Presentation
A 27-year-old Caucasian homeless woman presented to the emergency department with symptoms of vomiting, poor oral intake, lower back pain, and decrease in urine output of 2 days’ duration. The patient reported frequent use of nonsteroidal anti-inflammatory drugs for the past 4 years. Her past medical history was significant for chronic hepatitis C infection and a spontaneous second-trimester miscarriage. She denied any history of clotting disorders or autoimmune disease. The patient had a history of i.v. drug use for the past 10 years, and her previous toxicology screens had been positive for cocaine, marijuana, and opiates. She reported frequent use of both oral and i.v. crystal meth (methamphetamines) for the past 2 months. On examination, she was conscious and oriented. She was afebrile with a heart rate of 63 bpm, respiratory rate of 16 breaths/min, and blood pressure of 119/70 mm Hg. Her remaining physical examination findings were unremarkable except for anasarca. Laboratory evaluation revealed serum creatinine of 6.4 mg/dl and blood urea nitrogen of 34 mg/dl. No recent baseline creatinine value was available, and she denied any past history of kidney disease. The rest of the laboratory data were as follows: leukocyte count 13,700/μl, hemoglobin 11.9 g/dl, platelets 96,000K/UL, bicarbonate 23 mEq/dl, and creatinine phosphokinase 108 IU/l. Aspartate aminotransferase and alanine aminotransferase were mildly elevated at 219 IU/l and 82 IU/l, respectively. A pregnancy test result was negative. Urine examination showed large blood, protein 100 mg/dl, 24 red blood cells per high-power field, and no leukocytes. Renal ultrasound revealed normal sized kidneys without any hydronephrosis. Blood culture results were negative. With differential diagnosis of acute glomerulonephritis and acute interstitial nephritis, further a workup was performed. A component of prerenal AKI was suspected, and treatment was initiated with isotonic i.v. fluids. However, on day 2, the patient remained anuric with worsening renal function and refractory hyperkalemia, following which emergent dialysis had to be initiated.
Serological workup showed mildly decreased complement C3 at 82 mg/dl (normal range, 88−206 mg/dl), normal complement C4, and positive rheumatoid factor at 23.9 IU/ml. Antinuclear antibody and antineutrophil cytoplasmic antibody serology results were negative. Both hepatitis B core antibody and hepatitis B surface antibody results were positive, whereas hepatitis B surface antigen and hepatitis C RNA result were negative. Serum cryoglobulin results were also negative. Anticardiolipin IgM was mildly elevated at 21.1 IgM phospholipid units and lupus anticoagulant results were negative.
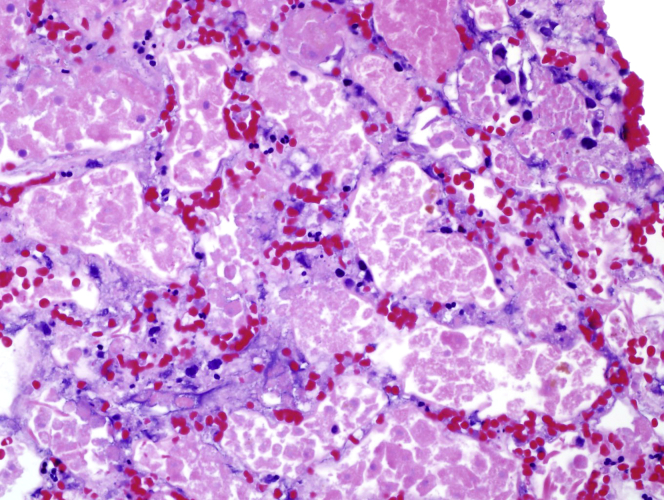
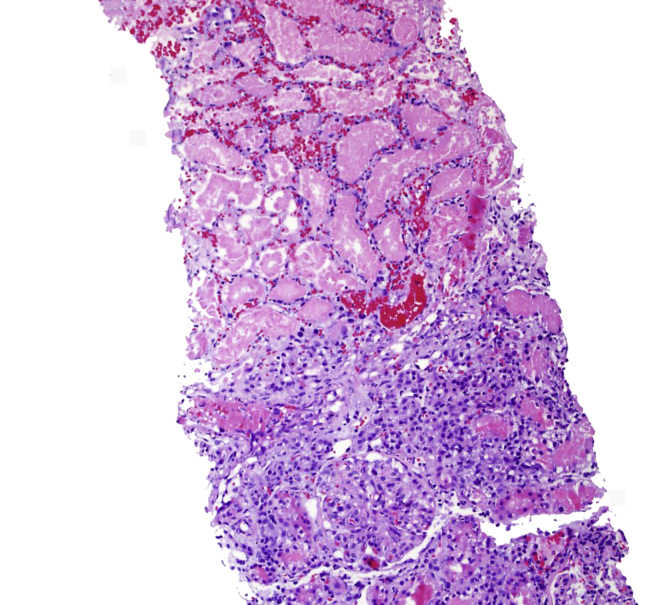
Kidney biopsy was performed on day 4. Sections for light microscopy had 19 glomeruli, 2 of which were globally sclerotic. Viable glomeruli were of normal overall size and cellularity. There was widespread coagulative necrosis of the renal cortex (Figure 1). There was also a sharp demarcation between areas of coagulative necrosis and the surrounding inflamed but nonnecrotic tissue (Figure 2). No vasculitis or evidence of thrombotic microangiopathy was seen in the viable kidney. Immunofluorescence staining was negative. Toluidine blue−stained sections revealed extensive coagulative necrosis.
Figure 1.
Necrotic tissue with ghost outlines of glomeruli and tubules with loss of cellular detail (hematoxylin and eosin stain; original magnification ×200).
Figure 2.
Sharp demarcation between eosinophilic necrotic parenchyma and inflamed but viable tissue (hematoxylin and eosin stain; original magnification ×100).
Transesophageal echocardiography showed that the patient’s ejection fraction was 55% with no evidence of emboli, vegetation, or endocarditis. The patient’s further hospital course was complicated by line-associated bacteremia, for which she was successfully treated with i.v. antibiotics. She has remained dialysis dependent during 2 months of follow-up.
Discussion
Acute renal cortical necrosis is a rare cause of AKI, with a reported incidence of about 2.0% in developed countries. In contrast, in developing countries, the incidence is higher, and about 6% to 7% of AKI cases are attributed to ACN. Pregnancy related complications remain the most common cause of ACN. About 60% to 70% of ACN cases are sequelae of obstetric complications such as septic abortion, puerperal sepsis, abruptio placentae, postpartum hemorrhage, and eclampsia. The remaining 30% to 40% are attributed to nonobstetric causes, with fulminant sepsis and hemolytic uremic syndrome being the most common ones.1 Other causes of nonobstetric ACN include snake bites, malaria, renal trauma, acute pancreatitis, diabetic ketoacidosis, and hyperacute renal transplant rejection.2, 3 With improvement in obstetric health care, and a marked decline in septic abortion, the incidence of obstetric ACN has decreased significantly. However, novel causes of non-obstetric ACN have emerged in the literature, which include prescription drugs (bisphosphonates, tranexamic acid)4, 5, 6 and drugs of abuse (synthetic cannabinoids).7 Table 1 lists the known causes of ACN. To the best of our knowledge, this is the first case report of N-methylamphetamine (crystal meth)−induced ACN as a non-obstetric cause of AKI in a young white woman.
Table 1.
Causes of acute renal cortical necrosis
| Obstetric | Non-obstetric |
|---|---|
| Septic abortion Puerperal sepsis Abruptio placentae Postpartum hemorrhage Eclampsia |
Drugs
Trauma/hemorrhagic shock Burns Pancreatitis Diabetic ketoacidosis Glucose-6-phosphate dehydrogenase deficiency with intravascular hemolysis Hyperacute kidney transplant rejection SLE-associated antiphospholipid syndrome |
SLE, systemic lupus erythematosus.
The typical histological feature of ACN is total ischemic necrosis of the affected area of the renal cortex (glomerului, blood vessels, and tubules). Two types of cortical necrosis are recognized on the basis of renal histology: (i) diffuse cortical necrosis, and (ii) patchy cortical necrosis. Diffuse cortical necrosis is characterized by confluent cortical destruction extending into columns of Bertin. There is preservation of a thin rim of subcapsular and juxtamedullary tissue. Clinically, diffuse ACN results in irreversible renal failure, leading to end-stage renal disease. The incomplete or patchy variety involves 30% to 50% of the entire cortical tissue. The latter variety can present with initial oliguria or even anuria, followed by a variable return of renal function and a stable period of moderate renal insufficiency.8 Renal function may improve until after the third year of onset.9 Even though the pathophysiology of ACN is not clearly understood, the final common pathway involves significantly diminished renal arterial perfusion. The initiating event is the vasospasm of small vessels and endothelial injury due to liberation of vasoactive substances such as endothelin-1. There is unique involvement of a part of the renal vasculature, mainly the interlobular cortical arteries and afferent arterioles, with sparing of vessels from the main renal arteries, arcuate arteries, and collateral subcapsular vessels.10 Intravascular coagulation and microvascular injury have also been implicated in the genesis of ACN.1 In our patient, we attributed ACN to methamphetamine-induced vasospasm affecting the renal vasculature. However, given the known effect of nonsteroidal anti-inflammatory drugs in blocking the vasodilatory effects of prostaglandins in the afferent arteriole (and thus reducing renal blood flow), nonsteroidal anti-inflammatory drug use may have additionally contributed to the genesis of ACN. Alternative etiologies such as thrombotic microangiopathy were ruled out.
Methamphetamine and related substances have become the second most frequently used drugs of abuse in the world after cannabis, with approximately 33,900,000 users reported worldwide in 2013.11 Although the exact prevalence of adverse effects among methamphetamine users is unknown, myriad cardiovascular and cerebrovascular complications have been described, including malignant hypertension, arrhythmias, aortic dissection, myocardial infarction, stroke (both ischemic and hemorrhagic), and cardiomyopathy.12 Methamphetamine-induced ischemic colitis13 and retinal vasculitis14 have also been described.
These effects appear to be related to the stimulated release of neurotransmitters including dopamine, serotonin, and/or noradrenaline. Noradrenaline acts via α1 receptors in arterial vasculature to stimulate vasoconstriction,15 and increases cardiac contractility and heart rate via β1 receptors.16 Catecholamine excess with associated coronary vasospasm has been postulated to be a cause of methamphetamine-associated cardiomyopathy. Other proposed mechanisms include increases in reactive oxygen species, mitochondrial injury, and changes in myocardial metabolism.17 More recently, a study has demonstrated methamphetamine-induced release of endothelin in mouse brain endothelial cells, suggesting an additional mechanism in which methamphetamine can cause arterial vasoconstriction.18 Endothelin, which is released from vascular endothelial cells, as a result of both renal hypoperfusion and endothelial injury, may act as final common factor leading to renal damage and subsequent ACN. In conclusion, it can be postulated that N-methylamphetamine causes acute cortical renal necrosis via noradrenergic and endothelin-mediated renal arterial vasoconstriction.
Renal biopsy is considered the gold standard in the diagnosis of ACN, but may be difficult to perform due to clinical instability and underlying coagulopathy in patients. In addition, biopsy may miss the diagnosis if ACN is patchy.19 Noninvasive diagnostic modalities such as ultrasound, radionuclide scintigraphy, computed tomography, magnetic resonance imaging, and renal arteriography may support the clinical diagnosis of ACN by specific findings. Contrast-enhanced computed tomography demonstrates the characteristic finding of a lack of renal cortical enhancement, and, in later stages of ACN, cortical calcifications may be seen.19 A double-line pattern of calcification resembling a tram-line has also been described.20
Table 2 shows the teaching and interesting points of our case. Our case highlights that renal cortical necrosis remains an important differential in the diagnosis of acute kidney injury, especially with emergence of an epidemic of drug abuse. As in this case, N-methylamphetamine may not be used in isolation, so the use of other drugs may contribute to its effects. Although the patchy variety may be partially reversible, the more common outcome is long-term dialysis dependence.
Table 2.
Teaching points
| • Acute renal cortical necrosis is a rare cause of acute kidney injury. |
| • Methamphetamines and other related drugs of abuse are emerging as an important cause of non-obstetric acute renal cortical necrosis. |
| • The most common presentation of acute cortical necrosis is anuric acute kidney injury that often requires initiation of renal replacement therapy. |
| • Renal biopsy is the gold standard for diagnosis of acute renal cortical necrosis. |
| • Although acute renal cortical necrosis is partially reversible in 20% to 40% of cases, patients frequently require long-term renal replacement therapy. |
| • To the best of our knowledge, this is the first case illustrating N-methylamphetamine as a cause of acute renal cortical necrosis, and clinicians should be aware of its associated potential complications. |
Disclosure
All the authors declared no competing interests.
References
- 1.Prakash J., Singh V.P. Changing picture of renal cortical necrosis in acute kidney injury in developing country. World J Nephrol. 2015;4:480–486. doi: 10.5527/wjn.v4.i5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chugh K.S., Jha V., Sakhuja V., Joshi K. Acute renal cortical necrosis—a study of 113 patients. Ren Fail. 1994;16:37–47. doi: 10.3109/08860229409044846. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R., Bansal N., Jhorawat R. Renal cortical necrosis: a rare complication of Plasmodium vivax malaria. Indian J Nephrol. 2014;24:390–393. doi: 10.4103/0971-4065.133789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko D.H., Kim T.H., Kim J.W. Tranexamic acid-induced acute renal cortical necrosis in post-endoscopic papillectomy bleeding. Clin Endosc. 2017;50:609–613. doi: 10.5946/ce.2017.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odabas A.R., Cetinkaya R., Selcuk Y. Tranexamic-acid-induced acute renal cortical necrosis in a patient with haemophilia A. Nephrol Dial Transplant. 2001;16:189–190. doi: 10.1093/ndt/16.1.189. [DOI] [PubMed] [Google Scholar]
- 6.Aksoy S., Hocaoglu E., Karahasanoglu A. Bisphosphonate-induced bilateral acute renal cortical necrosis. Radiol Case Rep. 2015;10:992. doi: 10.2484/rcr.v10i2.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansoor K., Zawodniak A., Nadasdy T., Khitan Z.J. Bilateral renal cortical necrosis associated with smoking synthetic cannabinoids. World J Clin Cases. 2017;5:234–237. doi: 10.12998/wjcc.v5.i6.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prakash J., Vohra R., Wani I.A. Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: a single-centre experience of 22 years from eastern India. Nephrol Dial Transplant. 2007;22:1213–1217. doi: 10.1093/ndt/gfl761. [DOI] [PubMed] [Google Scholar]
- 9.Beji S., Hajji M., Rais L. Acute renal cortical necrosis in pregnancy: clinical course and changing prognosis. Nephrol Ther. 2017;13:550–552. doi: 10.1016/j.nephro.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Deutsch V., Frankl O., Drory Y. Bilateral renal cortical necrosis with survival through the acute phase with a note on the value of selective nephroangiography. Am J Med. 1971;50:828–834. doi: 10.1016/0002-9343(71)90192-6. [DOI] [PubMed] [Google Scholar]
- 11.Gowing L.R., Ali R.L., Allsop S. Global statistics on addictive behaviours: 2014 status report. Addiction [Abingdon, England] 2015;110:904–919. doi: 10.1111/add.12899. [DOI] [PubMed] [Google Scholar]
- 12.Schurer S., Klingel K., Sandri M. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine-associated cardiomyopathy. JACC Heart Fail. 2017;5:435–445. doi: 10.1016/j.jchf.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Johnson T.D., Berenson M.M. Methamphetamine-induced ischemic colitis. J Clin Gastroenterol. 1991;13:687–689. doi: 10.1097/00004836-199112000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Shaw H.E., Jr., Lawson J.G., Stulting R.D. Amaurosis fugax and retinal vasculitis associated with methamphetamine inhalation. J Clin Neuro-ophthalmol. 1985;5:169–176. doi: 10.3109/01658108509079659. [DOI] [PubMed] [Google Scholar]
- 15.Kish S.J. Pharmacologic mechanisms of crystal meth. CMAJ. 2008;178:1679–1682. doi: 10.1503/cmaj.071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunser M.W., Hasibeder W.R. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intens Care Med. 2009;24:293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 17.Lord K.C., Shenouda S.K., McIlwain E. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovasc Res. 2010;87:111–118. doi: 10.1093/cvr/cvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo J.W., Jones S.M., Hostetter T.A. Methamphetamine induces the release of endothelin. J Neurosci Res. 2016;94:170–178. doi: 10.1002/jnr.23697. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A., Ferguson J., Rahman M. Acute oliguric renal failure in HELLP syndrome: case report and review of literature. Ren Fail. 2012;34:653–656. doi: 10.3109/0886022X.2012.660856. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Thomas H.G., Balme R.H., Key J.J. Tram-line calcification in renal cortical necrosis. Br Med J. 1962;1:909–911. doi: 10.1136/bmj.1.5282.909. [DOI] [PMC free article] [PubMed] [Google Scholar]