Abstract
Introduction
Inflammatory cell recruitment, which is potentially mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor type 2 (CCR2) system and by C-C chemokine receptor type 5 (CCR5) activity, may play a role in the development and progression of diabetic nephropathy. PF-04634817 is a dual chemokine CCR2/5 receptor antagonist that is being developed for the treatment of diabetic nephropathy.
Methods
We evaluated the efficacy of PF-04634817 compared with matching placebo for reduction of albuminuria after 12 weeks of treatment in subjects with type 2 diabetes who received standard of care (SOC; angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy), in a randomized, double-blind, placebo-controlled, parallel-group phase 2 study.
Results
A total of 226 subjects who received SOC with baseline estimated glomerular filtration rates between 20 and 75 ml/min per 1.73 m2 and a baseline urinary albumin-to-creatinine ratio (UACR) of ≥300 mg/g were randomly assigned 3:1 to receive PF-04634817 (150 or 200 mg orally, once daily) or placebo. The primary analysis was Bayesian, with an informative prior for placebo response (equivalent to including an additional 80 subjects in the placebo arm). We observed a placebo-adjusted reduction in UACR of 8.2% (ratio 0.918; 95% credible interval: 0.75–1.09) at week 12 in the PF-04634817 arm. PF-04634817 appeared to be safe and well-tolerated.
Conclusion
Despite the good safety profile shown by PF-04634817, clinical development for this indication was discontinued in light of the modest efficacy observed.
Keywords: albuminuria, CCR2, CCR5, diabetes, diabetic nephropathy
Diabetes mellitus affects approximately 422 million adults worldwide.1 Chronic diabetes is linked with several serious complications, including kidney failure.1 In the United States, the prevalence of diabetes associated with albuminuria (UACR ≥30 mg/g) and an impaired estimated glomerular filtration rate (eGFR; <60 ml/min per 1.73 m2), or both, has increased over 2 decades in proportion to the prevalence of diabetes.2 The risk of progression to end-stage kidney disease from diabetic nephropathy is higher in patients with macroalbuminuria (UACR >300 mg/g) and doubles for each doubling of baseline proteinuria.3
In patients with diabetic nephropathy, inhibition of the renin–angiotensin–aldosterone system (RAAS) with a single-agent angiotensin-converting enzyme inhibitor (ACEi), angiotensin receptor blockers (ARBs), or direct renin inhibitors, is the SOC therapy. RAAS blockade reduces albuminuria, but does not halt the progression of disease.4, 5 When a more complete blockade of the RAAS was tested, it was associated with increased morbidity and poor outcomes.6, 7, 8 Non-RAAS approaches to the treatment of persistent albuminuria in diabetic subjects also had limited success.9, 10
Inflammatory cell recruitment, infiltration, and activation play a key role in the development and progression of diabetic nephropathy. CCR2 activation by monocyte chemoattractant protein 1 (MCP-1) stimulates the release of monocytes into blood from the bone marrow and contributes to the recruitment of monocytes into inflamed tissue.11 The major receptors expressed by monocytes for MCP-1 and RANTES (Regulated upon Activation, Normal T cell Expressed and presumably Secreted) are CCR2 and CCR5, respectively.12 In patients with diabetic nephropathy, urinary MCP-1 levels are significantly increased and are positively correlated with both the number of macrophages in the renal interstitium and with the degree of tubulointerstitial lesions, which suggests that locally produced MCP-1 may be involved in the development of diabetic nephropathy.13, 14, 15 Furthermore, CCR2 receptors are over-expressed by glomerular podocytes in patients with diabetic nephropathy, which suggests potential roles for MCP-1 and/or CCR2 beyond the recruitment of monocytes.16
The role of CCR5 in diabetic nephropathy is largely unexplored. However, it is a receptor for various proinflammatory cytokines and underwrites the chemotaxis of several inflammatory cell types and the ligands that have been shown to be increased in diabetic nephropathy.17, 18 The presence of a CCR5 polymorphism (loss of function) is associated with better survival in subjects with type 2 diabetes, which suggests that blockade of CCR5 receptors may be worth exploring as a potential treatment for diabetic nephropathy.19
With CCR2- and CCR5-mediated recruitment of monocytes and other inflammatory cells implicated in the etiology of diabetic nephropathy, combined inhibition of CCR2 and CCR5 receptors may decrease albuminuria and prevent kidney function decline in patients with diabetic nephropathy. CCR2 blockade with an orally available small-molecule antagonist (RO5234444) has been shown to alleviate proteinuria, glomerulosclerosis, and kidney failure in diabetic db/db mice.20 PF-04634817 is a small-molecule chemokine CCR2 and CCR5 receptor dual antagonist. In this phase 2 study, we evaluated the efficacy and safety of once-daily oral PF-04634817 (150 or 200 mg) versus matching placebo in adult subjects with type 2 diabetes who were on SOC ARB and/or ACEi therapy.
Methods
Study Design
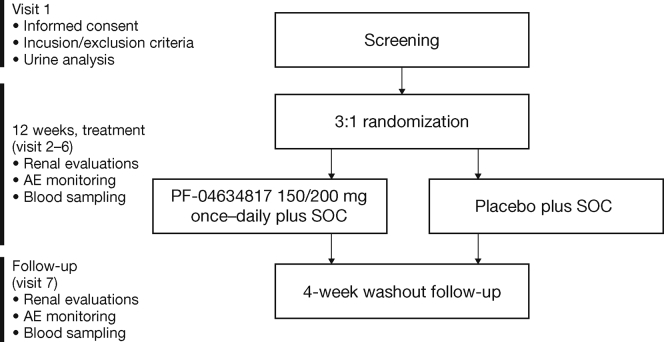
This was a 12-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group phase 2 study in subjects with type 2 diabetes. Eligible subjects were randomly assigned in a 3:1 ratio to receive oral PF-04634817 (150 or 200 mg once daily depending on kidney function status [eGFR]) or matching placebo (Supplementary Figure S1). Subjects were randomized via an automated interactive voice response system; assigned numbers were retained throughout the study and corresponded to a treatment schedule determined by a sponsor-generated randomization code. All study investigators, the sponsor, and participants were blinded to treatment allocation.
PF-04634817 was administered orally at 200 mg once daily for subjects with eGFR values of ≥30 to 75 ml/min per 1.73 m2 and at 150 mg once daily for subjects with eGFR values of 20 to 30 ml/min per 1.73 m2. The 200 mg once daily dose was selected based on previous clinical experience in phase 1 studies in healthy volunteers (NCT0109887; NCT01140672; NCT01247883; NCT01791855), in which doses up to 300 mg were shown to be safe and well tolerated. In addition, target coverage was assessed using pharmacokinetic (PK) predictions from the SimCYP simulator (www.certara.com) and an assessment of CCR2 receptor inhibition (ex vivo p-ERK assay), CCR5 receptor inhibition (ex vivo receptor internalization assay), and reduction in the total monocyte population seen in the phase 1 studies. Simulated mean coverage at the proposed doses was >99%, >97%, and >99% against CCR2, CCR5 pharmacology, and monocyte reduction, respectively. The daily dose was reduced to 150 mg in subjects with eGFR between 20 and <30 ml/min per 1.73 m2, a decision that was informed by simulation using SimCYP, which predicted a potential effect of kidney impairment on the PK of PF-04634817. The appropriateness of this dose adjustment was confirmed using data from a phase 1 PK study in subjects with varying degrees of kidney impairment (NCT01791855), which was conducted in parallel with the present study.
Objectives
The primary objective was to evaluate the efficacy of PF-04634817 150 and/or 200 mg plus SOC compared with placebo plus SOC in the reduction of albuminuria after 12 weeks of treatment. Secondary objectives included evaluation of the safety and tolerability of PF-04634817, its effect on kidney function, and PKs in this patient population. An exploratory objective characterized biomarkers that demonstrated the pharmacological effect of PF-04634817.
Efficacy and Safety Endpoints
The primary efficacy measure of reduction in albuminuria was assessed by change from baseline in UACR after 12 weeks of treatment with PF-04634817 150 and/or 200 mg plus SOC or placebo plus SOC. UACR, determined by a central laboratory, was calculated as the geometric mean from first-morning void specimens collected at home on the 3 days before each study visit.
Secondary endpoints included UACR at weeks 4, 8, and 16. The urinary protein-to-creatinine ratio was assessed at weeks 4, 8, 12, and 16, and compared with the baseline value obtained from 3 consecutive first-morning void urine samples at the time of screening.
eGFR was calculated using the 4 variable formula of the Modification of Diet in Renal Disease group21 and evaluated at weeks 1, 4, 8, 12, and 16. In addition, eGFR was calculated at weeks 12 and 16 using the eGFR cystatin formula.22
Serum creatinine and plasma glycosylated hemoglobin (HbA1c) at weeks 1 (serum creatinine only), 4, 8, 12, and 16, and serum cystatin C at weeks 12 and 16, were determined by a central laboratory.
Exploratory endpoints included serum C-reactive protein at weeks 12 and 16, and serum and urinary MCP-1 at weeks 4, 8, 12, and 16, as determined by a central laboratory using a validated enzyme-linked immunosorbent assay. Circulating total monocytes and the frequency and absolute numbers of the CD14+CD16+ subpopulation were measured at a central laboratory in samples of whole blood at weeks 1, 4, 8, 12, and 16.
Plasma samples were analyzed for PF-04634817 concentrations at baseline, and at weeks 1, 4, 8, and 12 at a central laboratory using a validated high-performance liquid chromatography tandem mass spectrometry method.
Safety and tolerability of PF-04634817 after 12 weeks of treatment were assessed by adverse event (AE) profiles and clinical laboratory evaluations, as well as other safety measures, including physical examination, body height and weight, triplicate supine 12-lead electrocardiogram, supine blood pressure (BP) and/or pulse, and fasting glucose values.
Eligibility Criteria and Recruitment
The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines, and was approved by the institutional review boards and/or independent ethics committees at each of the investigational centers participating in the study or a central institutional review board. Subjects had to sign and date an informed consent document.
Subjects were recruited from the following countries: Argentina, Australia, Canada, Germany, Hong Kong, Italy, Korea, Malaysia, Peru, Poland, Romania, Spain, and the United States. Eligible men or women aged 18 years or older or non-childbearing potential included those who had type 2 diabetes and overt nephropathy (eGFR 20–75 ml/min per 1.73 m2), as well as persistent albuminuria (UACR ≥300 mg/g for at least 3 months). Subjects had to have stable background SOC therapy with an ARB and/or an ACEi for at least 3 months before screening; these therapies were maintained throughout the study. Subjects were ineligible for the study if they had a history of albuminuria (>6.5 g/day), serum albumin (<2.0 g/dl), or poorly controlled diabetes mellitus (HbA1c >10.5%). Subjects could not have any active or latent infection of Mycobacterium tuberculosis (TB). To be eligible to participate, subjects had to have negative tests for TB at screening, confirmed by a blood test (QuantiFERON Gold; Quest Diagnostics, Springfield, NJ) or a negative tuberculin skin test defined as <5 mm induration. In countries with a high rate of TB or high rate of multidrug resistant TB (Argentina, Hong Kong, India, Korea, Malaysia, Peru, and Romania), a chest radiograph with no changes that suggested active TB infection was required or no history of TB infection, unless determined otherwise by the investigator.
Statistical Analyses
The full analysis set (FAS) included all subjects who received at least 1 dose of randomized treatment and who had at least 1 post-baseline efficacy measurement. The safety analysis population was defined as all subjects who received at least 1 dose of study drug. The primary analysis was the ratio of week 12-to-baseline UACR with no imputation. The primary Bayesian analysis of covariance model included terms for treatment, the standardized log of the baseline UACR, and standardized baseline systolic BP.
The Bayesian analysis was similar to a frequentist analysis of covariance but allowed for the incorporation of historical placebo information directly, in the form of summary data available from literature, without the need to obtain historical patient-level data. The informative prior for the placebo response (defined on the log scale) at the final analysis was a normal distribution, with a mean ± SD of −0.037 ± 0.092 (or variance of 0.086, which was derived from a meta-analysis of 632 placebo subjects from 2 historical diabetic nephropathy studies).9, 23 This implied an expected decrease in UACR of approximately 3.6% in the placebo group but allowed for uncertainty in the prior specification. Under this prior, a 95% credible interval (CI) ranged from a 20% decrease to a 15% increase of UACR. The prior could also be characterized by its equivalence to historical data records using standard calculations.24 At study design, the ratio of the expected variance of the data to the variance of the prior indicates that the information in the prior distribution is equivalent to approximately 80 placebo-treated subjects. This number was chosen to ensure that the prior would not completely overwhelm possible conflicting data obtained at the time of the study.
The sample size was based on the primary endpoint of UACR at week 12, assuming a 3:1 allocation of PF-04634817 150 and/or 200 mg once daily to placebo, and that data were analyzed on the log scale. Assuming an attrition rate of approximately 10%, 176 subjects needed to be randomized to ensure that 160 subjects completed the study.
A 2-part decision criterion for efficacy and futility was used at the end of this study, based on a Bayesian interpretation of the results, assuming an informative prior for placebo response and noninformative priors for the treatment difference and variation.25 A mixed-effects model repeated measure (MMRM) was performed for continuous data from FAS secondary endpoints collected longitudinally during the study.
An interim Bayesian analysis for futility was performed in July 2014 by an unblinded independent review committee, which used efficacy and safety data from the first 90 subjects to complete 12 weeks of randomized treatment. The committee recommended that the study be continued as planned.
The Full Methods section can be found in the Supplementary Material.
Results
Subjects
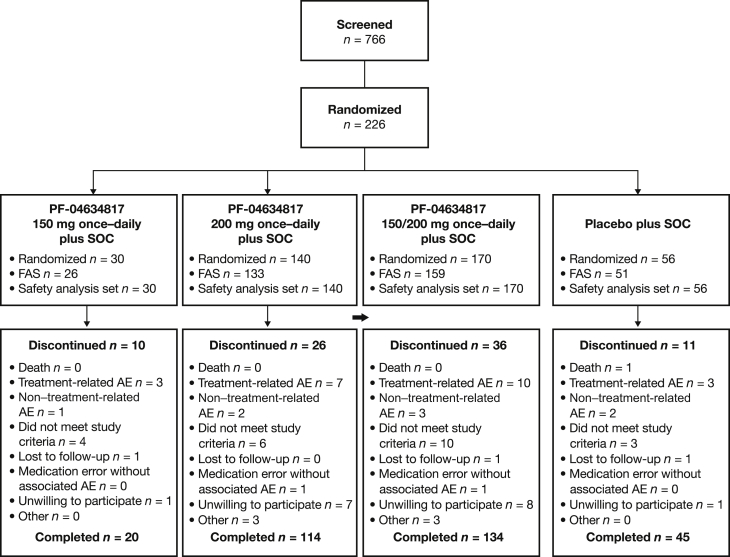
Between December 13, 2012 and September 22, 2014, 766 subjects were screened; 226 were randomized in a 3:1 ratio to the PF-04634817 150 and/or 200 mg plus SOC arm or placebo plus SOC arm (Figure 1). The number of subjects who entered screening in the last weeks of the screening period was unexpectedly high; 1 of the most common reasons for screen failure was a positive test for TB (data not shown). Baseline subject demographics, chronic kidney disease severity, and other characteristics (Table 1) were generally balanced between the study arms.
Figure 1.
Subject disposition. Full analysis set (FAS): all subjects who received at least 1 dose of randomized treatment and had at least 1 postdose efficacy measurement. Safety analysis set: all subjects who received at least 1 dose of study medication (reported data for those analyzed for adverse events [AEs]); 3 subjects in the PF-04634817 150 and/or 200 mg plus standard of care (SOC) arm and 3 subjects in the placebo plus SOC arm were not analyzed for laboratory data. (SOC: angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy.)
Table 1.
Subject demographics and baseline disease characteristics
| PF-04634817 150/200 mg once daily plus SOC n = 170 |
Placebo plus SOC n = 56 |
|
|---|---|---|
| Male, n (%) | 134 (78.8) | 49 (87.5) |
| Age, yr | 64.6 ± 8.2 | 62.7 ± 9.4 |
| Race, white | 125 (73.5) | 36 (64.3) |
| BMI, kg/m2 | 32.6 ± 6.9 | 31.6 ± 7.6 |
| UACR, mg/mmol | 157 | 50 |
| Geometric mean, % CV | 127.4 (96) | 121.7 (88) |
| Geometric 95% CI | 112.1–144.7 | 98.2–151.0 |
| UPCR, mg/mmol | 155 | 49 |
| Geometric mean, % CV | 185.4 (97) | 176.3 (82) |
| Geometric 95% CI | 162.9–211.0 | 143.4–216.6 |
| eGFR using cystatin formula, ml/min per SAB | 159 (45.2 ± 14.2) | 50 (45.3 ± 14.9) |
| eGFR using abbreviated MDRD formula, ml/min per SAB | 159 (41.8 ± 12.1) | 51 (41.6 ± 13.4) |
| Serum creatinine, mg/dl | 159 (1.7 ± 0.5) | 51 (1.7 ± 0.5) |
| Serum cystatin C, mg/l | 159 (1.5 ± 0.4) | 51 (1.5 ± 0.4) |
| HbA1c, % | 159 (7.5 ± 1.2) | 51 (7.9 ± 1.4) |
| Systolic BP, mm Hg | 168 (140.4 ± 14.0) | 53 (139.9 ± 13.6) |
| Diastolic BP, mm Hg | 168 (75.9 ± 9.0) | 53 (77.1 ± 6.8) |
Values are n, n (%), and n (mean ± SD).
BMI, body mass index; BP, blood pressure; CI, confidence interval; CV, coefficient of variance; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin A1c; MDRD, Modification of Diet in Renal Disease; SAB, standard body surface area; SOC, standard of care (angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy); UACR, urine albumin-to-creatinine ratio; UPCR, urinary protein-to-creatinine ratio.
For mg/mmol to mg/g conversion: divide by 0.113.
Efficacy
Primary Endpoint
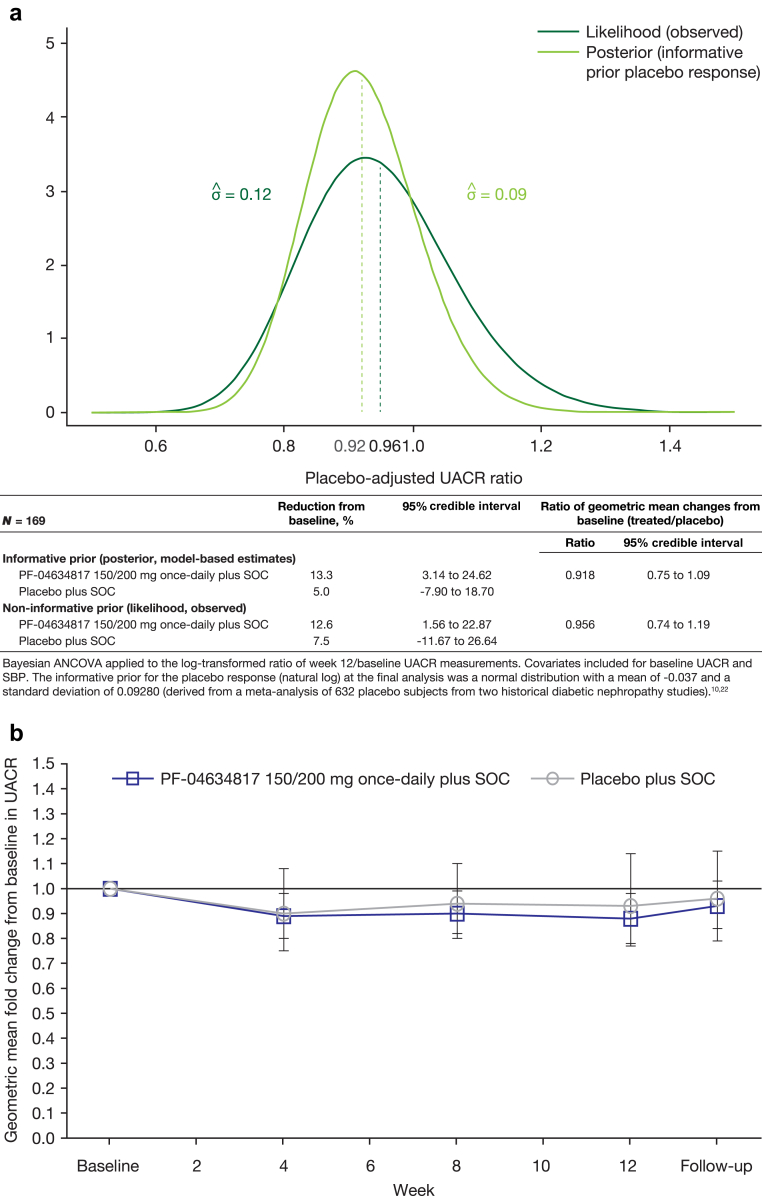
In the PF-04634817 arm, an 8.2% placebo-adjusted reduction from baseline in UACR (ratio: 0.918; 95% Bayesian CI: 0.75–1.09) (Figure 2a) was calculated at week 12 for the primary analysis (using an informative prior distribution for the placebo response equivalent to including an additional 80 placebo arm subjects).9, 23
Figure 2.
(a) Placebo-adjusted urinary albumin to creatinine ratio (UACR)–Bayesian analysis of covariance (ANCOVA) (full analysis set [FAS]). (b) Geometric mean fold change (95% confidence interval [CI]) from baseline in UACR–mixed-effects model repeated measures (MMRM) analysis (FAS). The posterior distribution is shown in gray, and the observed likelihood (assuming no prior information) is shown in black. Baseline was defined as the last pre-dose measurement. SBP, systolic blood pressure; SOC, standard of care (angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy).
A sensitivity analysis was performed to further investigate the effect of the informative prior. The sensitivity analysis, which used a noninformative prior that did not incorporate historical placebo information, showed a 4.4% placebo-adjusted reduction from baseline in UACR at week 12 (ratio 0.956; 95% Bayesian CI: 0.74–1.19). The placebo arm had an estimated 7.5% reduction from baseline in UACR calculated instead of 5.0% (Figure 2a). The observed placebo reduction of UACR of 7.5% was well within the range of plausible placebo responses as specified by the informative prior distribution used in the primary analysis, and showed no difference in the overall study conclusions. The comparison of the 2 analyses can be visually assessed in Figure 2a, which shows the posterior distribution in gray and the observed likelihood (assuming no prior information) in black. The bell-shaped curves with a small skew to the right are slightly shifted versions of each other, with substantial overlap. This was evidenced by similar 95% credible regions, which indicated similar results. As expected, the posterior distribution from the informative analysis was narrower than the distribution from the noninformative analysis, as seen in the SDs of the 2 posterior distributions (0.09 and 0.12).
An MMRM sensitivity analysis of adjusted geometric mean fold change from baseline in UACR on the FAS was consistent with the primary Bayesian analysis at week 12 using a noninformative prior (treated/placebo ratio: 0.94; 95% CI: 0.75–1.18; P = 0.5844) (Figure 2b), which supported the primary analysis. The observed mean ratios for UACR over time were similar to these modeled results (data not shown).
When the impact of baseline and change from baseline at week 12 systolic BP on log change in UACR was analyzed by including it in the analysis of covariance (as prespecified in the statistical analysis plan), the primary results were almost identical (data not shown). This indicated that the observed treatment effect was not mediated by either baseline systolic BP or the change from baseline in systolic BP.
Secondary Endpoints
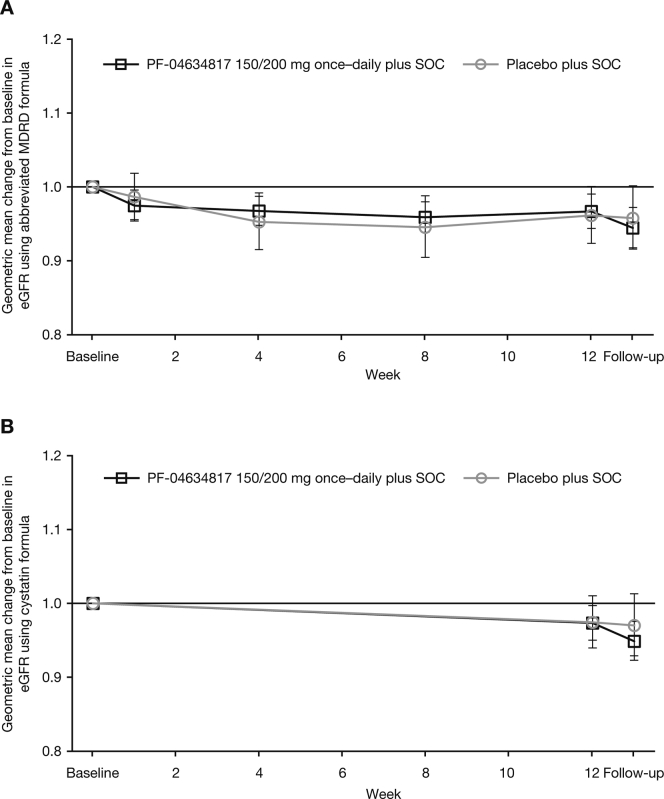
Analysis of least-squares means of the log-transformed UACR and the urinary protein-to-creatinine ratio showed that there were no significant differences at weeks 4, 8, and 12 between the PF-04634817 arm and the placebo arm (Supplementary Table S1). Corresponding analyses of eGFR calculated by both the abbreviated Modification of Diet in Renal Disease or cystatin formulas demonstrated no significant differences between treatment arms at weeks 4, 8, and 12 (Supplementary Table S1; Supplementary Figure S2A and B). A similar pattern was noted with serum creatinine, cystatin C, and HbA1c, with no significant differences between treatment arms. A supplementary analysis of the influence of baseline eGFR on the primary endpoint was conducted, but no robust correlation was identified.
Proportion of UACR Responders at Week 12
Logistic regression analyses showed that the proportion of responders who achieved UACR <300 mg/g at the end of treatment was 10.2% (13 of 128) and 6.8% (3 of 44) in the PF-04634817 and placebo arms, respectively (odds ratio [OR]: 1.228; 95% CI: 0.293–5.149). The same proportion of subjects achieved ≥50% reduction from baseline in UACR at the end of treatment; the PF-04634817 group achieved 10.2% (13 of 128 subjects) and the placebo group achieved 6.8% (3 of 44 subjects) (OR: 1.472; 95% CI: 0.395–5.481). The OR for the subjects who received PF-04634817 and who achieved a normal UACR (<30 mg/g) at week 12 was not analyzed because there were no responders in the placebo arm.
Exploratory Endpoints
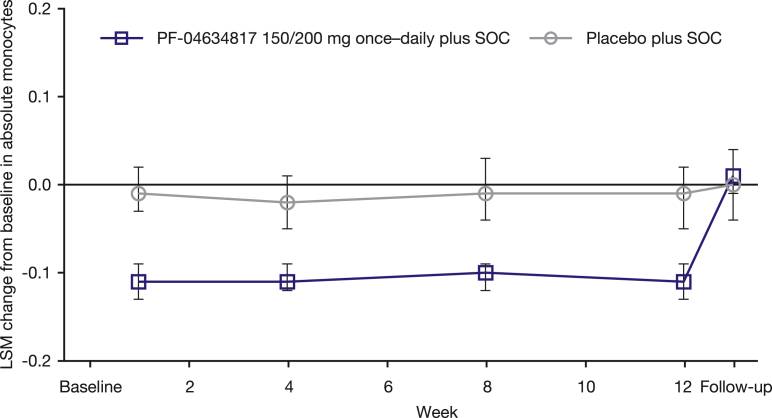
In MMRM analyses of absolute monocyte count and percentage change from baseline in absolute monocyte count, there was a statistically significant, sustained decrease in both parameters in the PF-04634817 arm compared with no change in the placebo arm (P < 0.0001 at weeks 1, 4, 8, and 12) (Figure 3); this returned to baseline levels at the post-treatment follow-up visit.
Figure 3.
Least-squares mean (LSM) (95% confidence interval) change from baseline in absolute monocytes–mixed-effects model repeated measures analysis (full analysis set). Baseline was defined as the last pre-dose measurement. SOC, standard of care (angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy).
There was no demonstrable impact on log-transformed baseline monocyte CD14+16+ subpopulations, baseline C-reactive protein, or baseline MCP-1 on log-change UACR at week 12, due to the lack of a strong treatment effect of PF-04634817 at that time point.
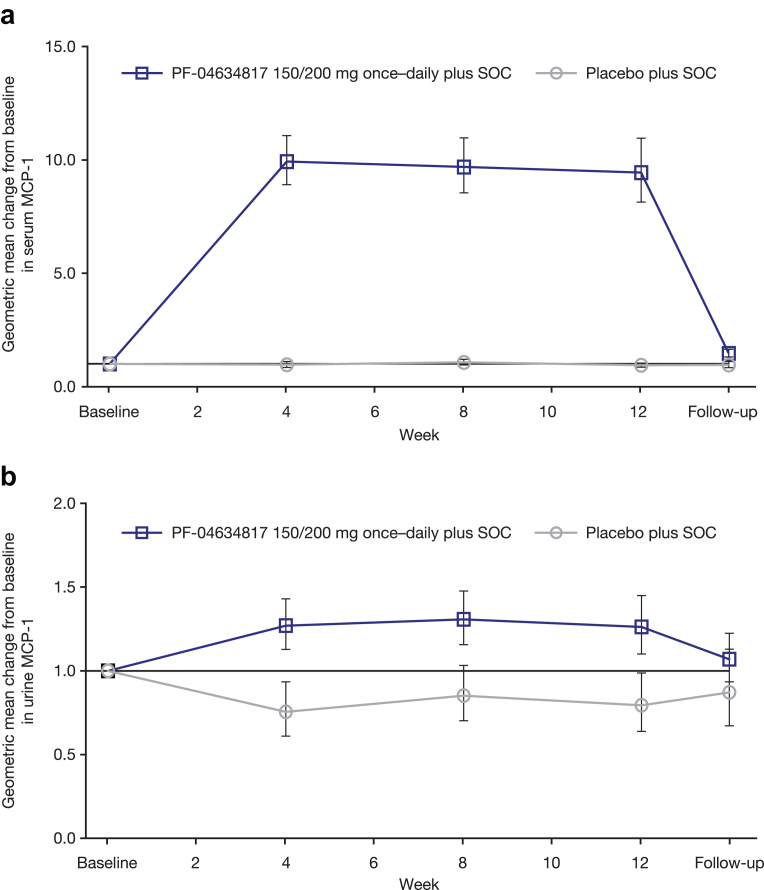
A marked and sustained increase in serum MCP-1 was observed with PF-04634817 compared with placebo, which returned to baseline levels at the follow-up visit after treatment stopped (Figure 4a). A small increase in urinary MCP-1 was demonstrated with PF-04634817 compared with placebo (Figure 4b).
Figure 4.
(a) Geometric mean change (95% confidence interval [CI]) from baseline in serum monocyte chemoattractant protein 1 (MCP-1) (fast analysis set [FAS]). (b) Geometric mean change (95% CI) from baseline in urinary MCP-1 (FAS). Baseline was defined as the last pre-dose measurement. SOC, standard of care (angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy).
PK Endpoints
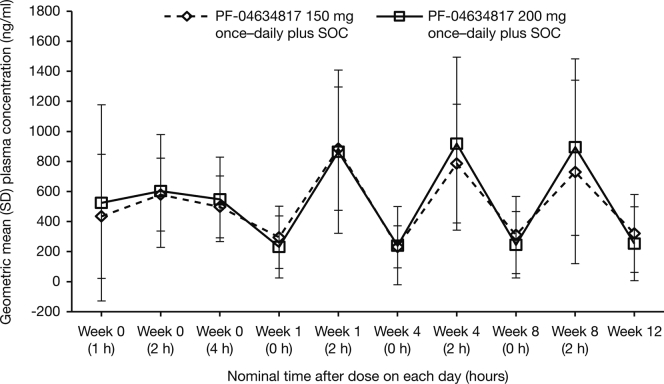
Observed plasma PF-04634817 concentrations over time indicated that steady state was achieved after approximately 1 week of treatment, with no apparent difference in exposure between the 150- and 200-mg doses (Supplementary Figure S3).
Safety
A summary of treatment-emergent AEs is presented in Table 2. There appeared to be a numerically higher frequency of all-cause (data not reported) and treatment-related serious AEs (SAEs) in subjects who received PF-04634817 150 mg once daily versus 200 mg once daily. The most frequently reported treatment-emergent, treatment-related AEs by system organ class were “investigations” (abnormal blood chemistries), followed by gastrointestinal disorders, and skin and subcutaneous tissue disorders.
Table 2.
Summary of treatment-emergent, treatment-related adverse events
| PF-04634817 150/200 mg once daily plus SOC |
Placebo plus SOC | |
|---|---|---|
| Evaluable for AEs | 170 | 56 |
| AEs | 95 | 16 |
| Subjects with AEs | 44 (25.9) | 10 (17.9) |
| Subjects with SAEs | 6 (3.5) | 0 |
| Subjects with SAEs | 6 (3.5) | 1 (1.8) |
| Subjects discontinued due to AEs | 10 (5.9) | 3 (5.4) |
| Subjects with dose reduced/temporary discontinuation due to AEs | 4 (2.4) | 0 |
| Incidence of treatment-emergent, treatment-related AEs in ≥1% of subjects across treatment arms | ||
| Gastrointestinal disorders | 11 (6.5) | 5 (8.9) |
| Diarrhea | 3 (1.8) | 1 (1.8) |
| Nausea | 5 (2.9) | 1 (1.8) |
| General disorders and administration-site conditions | 7 (4.1) | 0 |
| Fatigue | 3 (1.8) | 0 |
| Investigations | 13 (7.6) | 4 (7.1) |
| Gamma-glutamyltransferase increased | 3 (1.8) | 0 |
| GFR decreased | 2 (1.2) | 1 (1.8) |
| Lipase increased | 3 (1.8) | 1 (1.8) |
| Nervous system disorders | 6 (3.5) | 0 |
| Dizziness | 3 (1.8) | 0 |
| Skin and subcutaneous tissue disorders | 14 (8.2) | 2 (3.6) |
| Acne | 5 (2.9) | 0 |
| Rash | 3 (1.8) | 0 |
Values are n or n (%).
AE, adverse event; GFR, glomerular filtration rate; SAE, serious adverse event; SOC, standard of care (angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy).
Most treatment-emergent AEs (all-cause or treatment-related) were mild (PF-04634817 group: 191 of 299 subjects [63.9%]; placebo group: 47 of 79 subjects [59.5%]) or moderate (PF-04634817 group: 88 of 299 subjects [29.4%]; placebo group: 25 of 79 subjects [31.6%]) in severity.
Two treatment-emergent AEs of abnormal white blood cells were reported: 1 case (0.6%) of leukopenia that was considered to be related to PF-04634817 treatment, and 1 case (1.8%) of leukocytosis in the placebo arm. Three treatment-emergent AEs of cardiac disorders were reported, all in the PF-04634817 arm, including 1 case each of cardiac failure and palpitations (deemed unrelated), and 1 of bradycardia that was judged to be treatment-related. Treatment-emergent rash (not previously associated with PF-04634817) was reported by 5 subjects in the PF-04634817 arm, 2 cases of which were considered to be SAEs (treatment-related allergic dermatitis and psoriasis). None were considered severe in intensity.
A total of 13 (7.6%) and 6 (10.7%) subjects in the PF-04634817 and placebo arms, respectively, permanently discontinued treatment (including 1 subject in the placebo arm who died as a victim of homicide). Of these, 10 (5.9%) and 3 (5.4%) subjects, respectively, discontinued due to treatment-related AEs. In the PF-04634817 arm, these AEs included cerebral infarction, dermatitis (n = 2), PR prolongation on the electrocardiogram, QRS complex prolonged on the electrocardiogram, a decrease in GFR, pancreatitis, pyrexia, rash, and stomatitis. Most of the treatment-emergent AEs that led to permanent discontinuation spontaneously resolved (8 of 13 subjects in the PF-04634817 arm and 5 of 6 subjects in the placebo arm).
Thirty-two SAEs occurred in 22 subjects during the study; 26 occurred in 17 of 170 (10%) subjects treated with PF-04634817 and 6 occurred in 5 of 56 (9%) subjects treated with placebo. One death (homicide, not treatment-related) occurred in the placebo arm.
In subjects who received PF-04634817, 7 SAEs were considered to be related to treatment in 6 of 170 (4%) subjects; 3 of 30 (10%) subjects received 150 mg and 3 of 140 (2%) subjects received 200 mg of PF-04634817. SAEs included acute renal failure and dermatitis, psoriasis, pneumonia, acute pancreatitis, pyrexia, and cerebral infarction, and all resolved without sequelae, except for psoriasis and cerebral infarction.
No clinically meaningful treatment effects on BP, heart rate, or electrocardiography intervals were observed, and there were no PF-04634817 treatment-related effects on laboratory parameters.
Discussion
Analysis of the primary endpoint, UACR, indicated a modest effect of PF-04634817 in reducing albuminuria in subjects with type 2 diabetes who received SOC treatment after 12 weeks of treatment. The likely limited clinical meaningfulness of this modest reduction in albuminuria, and the failure of the effect to meet with prespecified criteria for success, resulted in the clinical development of PF-04634817 for this indication being halted.
Results of Bayesian analysis can be dependent on choice of prior distributions, and in this study, only the prior distribution for the placebo effect (a normal distribution with a mean ± SD of −0.037 ± 0.092) was informative and required consideration. This distribution was chosen to incorporate knowledge of previous control subjects, taking into account our uncertain knowledge of UACR changes based on current literature. Therefore, the prior distribution represents a mean change of 3.6%, but is consistent with a range of values from a 20% decrease to a 15% increase. The estimated placebo reduction was 7.5% (Figure 2a) in the noninformative analysis, and thus consistent with historical data.
Loss of power in the study was not a concern because 169 subjects were included in the primary analysis (i.e., 169 had both baseline and week 12 UACR measurements), with 160 being required. The 169 subjects included in the primary analysis were unbiased and representative of the study population, with similar disposition and rates of discontinuation across treatment arms.
An MMRM sensitivity analysis of adjusted geometric mean fold change from baseline in UACR was consistent with the primary Bayesian analysis at week 12. With no apparent differences between treatment arms on secondary endpoints, PF-04634817 was not associated with any effect on kidney function parameters.
Once-daily treatment with PF-04634817 150 or 200 mg was generally well tolerated when administered for 12 weeks, with no clinically meaningful treatment-related laboratory anomalies or vital signs data. PK analyses showed that subjects achieved plasma concentrations of PF-04634817 that were predicted from previous studies (see Full Methods section in the Supplementary Materials), and that PF-04634817 concentrations in the 150-mg dose subgroup were similar to the 200-mg dose subgroup. Analysis of pharmacological biomarkers believed to be indicative of CCR2 receptor antagonism, including increases in serum MCP-1 and decreases in circulating monocytes during the treatment period, showed sustained elevations and reductions, respectively, as predicted from previous data; thus, these supported high levels of CCR2 receptor antagonism by PF-04634817. The absence of a biomarker for CCR5 receptor blockade limited our ability to draw a similar conclusion for this receptor population; however, PF-04634817 had an almost equal affinity for the CCR2 and CCR5 receptors, and so it was not unreasonable for us to presume that there was also a high level of CCR5 receptor blockade throughout the treatment period.
In contrast to the results of the present study, a recent publication appeared to support the potential clinical value of targeting CCR2-mediated pathways as a treatment for diabetic nephropathy. The effect of the CCR2 receptor antagonist, CCX140-B, on residual albuminuria was evaluated in patients with type 2 diabetes and nephropathy in a 52-week, phase 2, randomized, double-blind study by De Zeeuw et al.26 The results suggested that CCX140-B, in addition to current SOC, had renoprotective effects, with reductions in albuminuria of between 16% and 10% (inversely related to dose). Subjects were selected using broadly similar inclusion criteria to those used in the present study (baseline eGFR and/or UACR requirements and stable background RAAS-inhibiting therapy). However, there were differences in the recruited population between the 2 studies, with subjects in the present study generally having a higher baseline UACR and a lower eGFR. These differences between study populations might explain the apparent differences in the effects of the investigational agents. Whether the findings of these studies were materially different will require additional, larger clinical trials. Interestingly, in the study with CCX140-B, serum MCP-1 only increased significantly in the CCX140-B 10-mg group compared with the placebo group (P = 0.01), but it did not increase in the 5-mg group, which also appeared to be associated with a lesser reduction in albuminuria in the 10-mg group.26 Researchers hypothesized that increased MCP-1 concentrations compete with the antagonist at the CCR2 receptor, thus reducing the effectiveness of pharmacological blockade and lessening the reduction in albuminuria at the 10-mg dose. In the present study, we could not exclude the possibility that high circulating concentrations of MCP-1 might compete against PF-04634817 at the CCR2 receptor. However, in phase 1 studies, the maximum increase in circulating MCP-1 was achieved at doses <200 mg once daily (∼5–10-fold lower), which suggested that at 150 or 200 mg once daily in subjects with diabetic nephropathy in the present study, CCR2 antagonism was likely on the plateau of the dose−response curve. If this assumption was correct, then the high concentrations of the antagonist (PF-04634817) would reduce the likelihood of competition from the agonist ligand, MCP-1, although this possibility could not be ruled out as a potential explanation for modest efficacy. In addition, a supplementary post hoc analysis revealed no correlation between the magnitude of reduction in UACR and circulating levels of MCP-1, both at week 12 (data not shown).
Limitations of the present study included that most subjects had high levels of albuminuria and a significant loss of kidney function at baseline. Therefore, the efficacy of PF-04634817 in subjects at earlier stages of diabetic nephropathy could not be properly assessed. The possibility existed that more significant changes in albuminuria might have been observed in subjects with albuminuria but with preserved kidney function. The study by De Zeeuw et al.26 included subjects with a mean baseline UACR across the treatment groups of between 363 and 440 mg/g and higher levels of residual kidney function (mean baseline eGFR across the treated groups was between 61.1 and 64.2 ml/min per 1.73 m2), and might support this hypothesis. However, supplementary, post hoc analyses of the data from the present study did not reveal any correlation between change in UACR at week 12 and baseline UACR or baseline eGFR (data not shown).
Although we were confident that there was a high level of pharmacological inhibition of both receptor populations throughout the duration of treatment with PF-04634817, this was determined from serum biomarkers of CCR2 receptor antagonism. We had no direct measure of CCR2 or CCR5 receptor inhibition at the level of the kidney, and no evidence that the influx of inflammatory cells into the kidney (one of the proposed mechanisms of action) was inhibited. An additional important limitation of the study was the short duration (12 weeks) compared with other studies in which treatment was assessed for up to 52 weeks.26 It was possible that a greater treatment effect might have been observed after a longer duration of treatment with regard to a chronic disease such as diabetic nephropathy. However, the data from De Zeeuw et al.26 raised the possibility that the inhibitory effect on albuminuria might not be maintained with more long-term dosing, although this will require a larger study to understand more fully. The modest short-term effect of PF-04634817 on albuminuria makes a another longer study difficult to support.
Conclusions
In this phase 2 study of adult subjects with diabetic nephropathy who had persistent macroalbuminuria despite receiving SOC therapy, CCR2 and/or 5 chemokine receptor antagonism with PF-04634817 showed a further reduction in albuminuria, although the magnitude of this reduction was modest, and therefore, the clinical meaningfulness is doubtful. Despite the tolerable safety profile shown by PF-04634817, clinical development was discontinued in light of the modest efficacy observed.
Disclosures
JDG, SG, and WS are employees of Pfizer Inc and hold shares in Pfizer Inc. KG is a previous employee of Pfizer Inc and holds shares in Pfizer Inc. He is currently an employee of EMD Serono. CP-H is a previous employee of Pfizer Inc and holds shares in Pfizer Inc. She is currently an employee of X-Chem Pharmaceuticals. All the other authors declared no competing interests.
Acknowledgments
We thank the subjects and investigators for their participation in this study. This study was sponsored by Pfizer Inc. Medical writing support under the guidance of the authors was provided by Louise Brown of Complete Medical Communications and was funded by Pfizer Inc.
JDG, CP-H, and WS were involved in the conception and design of the study and/or analyses. JDG, KG, and SG performed the data and statistical analyses. WS, TE, PEP, and SB were involved in subject recruitment and data acquisition. All authors were involved in data interpretation and manuscript drafting, reviewing, and development.
The views and opinions expressed within this manuscript are those of all authors and do not necessarily represent those of the funding organization. All authors approved final submission of the manuscript.
Central laboratories were used for the primary endpoint (UACR), and certain secondary (urinary protein-to-creatinine ratio, serum creatinine, serum cystatin C, HbA1c) and exploratory (C-reactive protein, circulating monocytes) endpoints. Clinical chemistry and hematology were also performed by a central laboratory. Good Clinical Practice and Clinical Laboratory Improvement Amendments−certified bioanalytical laboratories and appropriately validated assay systems were used for PK measurements and other exploratory endpoints (serum and urinary MCP-1, soluble tumor necrosis factor receptor 1). Additional Contract Research Organizations, meeting Pfizer’s non-protocol minimum laboratory requirements, were contracted for exploration of additional markers of kidney injury, kidney fibrosis, and metabolic status. MCP-1 serum and urine samples were analyzed by Eurofins Medinet (Breda, The Netherlands). Clinical laboratory assessments were performed by 3 Covance Central Laboratories: Asia-Pacific samples at Covance Asia Pte. Ltd. (Singapore); European samples at Covance Central Laboratory Services Meyrin (Geneva, Switzerland); and US samples at Covance Central Laboratory Services Inc. (Indianapolis, IN). PK samples were analyzed at Covance Bioanalytical Services (Shanghai, China).
Data from this study were reported as an abstract and oral presentation at the American Society of Nephrology Conference, San Diego, California, USA, November 3–8, 2015.
Footnotes
Full Methods.
Table S1. Log-transformed measurements of urinary albumin-to-creatinine ratio (UACR), urinary protein-to-creatinine ratio (UPCR), estimated glomerular filtration rate (eGFR), serum creatinine, serum cystatin C, and plasma glycosylated hemoglobin (HbA1c)– mixed-effects model repeated measure (MMRM) analysis (full analysis set [FAS])
Figure S1. Study design.
Figure S2. Geometric mean (95% confidence interval [CI]) change from baseline in (A) estimated glomerular filtration rate (eGFR)–abbreviated Modification of Diet in Renal Disease (MDRD) formula (full analysis set [FAS]), and (B) eGFR–cystatin formula mixed-effects model repeated measure (MMRM) analysis (full analysis set [FAS]).
Figure S3. Geometric mean ± SD plasma concentrations of PF-04634817.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Log-transformed measurements of urinary albumin-to-creatinine ratio (UACR), urinary protein-to-creatinine ratio (UPCR), estimated glomerular filtration rate (eGFR), serum creatinine, serum cystatin C, and plasma glycosylated hemoglobin (HbA1c)– mixed-effects model repeated measure (MMRM) analysis (full analysis set [FAS])
Figure S1.
Study design.
Figure S2.
Geometric mean (95% confidence interval [CI]) change from baseline in (A) estimated glomerular filtration rate (eGFR)–abbreviated Modification of Diet in Renal Disease (MDRD) formula (full analysis set [FAS]), and (B) eGFR–cystatin formula mixed-effects model repeated measure (MMRM) analysis (full analysis set [FAS]).
Figure S3.
Geometric mean ± SD plasma concentrations of PF-04634817.
References
- 1.World Health Organization (WHO). Global report on diabetes. Available at http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. Last updated 2016. Accessed November 21, 2017.
- 2.de Boer I., Rue T.C., Hall Y.N. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins R.C., Briganti E.M., Lewis J.B. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45:281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Lizakowski S., Tylicki L., Rutkowski B. Direct renin inhibition--a promising strategy for renal protection? Med Sci Monit. 2013;19:451–457. doi: 10.12659/MSM.883949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheele W., Diamond S., Gale J. Phosphodiesterase type 5 inhibition reduces albuminuria in subjects with overt diabetic nephropathy. J Am Soc Nephrol. 2016;27:3459–3468. doi: 10.1681/ASN.2015050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteras R., Perez-Gomez M.V., Rodriguez-Osorio L. Combination use of medicines from two classes of renin-angiotensin system blocking agents: risk of hyperkalemia, hypotension, and impaired renal function. Ther Adv Drug Saf. 2015;6:166–176. doi: 10.1177/2042098615589905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parving H.H., Brenner B.M., McMurray J.J.V. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 8.Fried L.F., Emanuele N., Zhang J.H. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–1903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 9.Mann J.F., Green D., Jamerson K. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Zeeuw D., Akizawa T., Audhya P. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crane M.J., Hokeness-Antonelli K.L., Salazar-Mather T.P. Regulation of inflammatory monocyte/macrophage recruitment from the bone marrow during murine cytomegalovirus infection: role for type I interferons in localized induction of CCR2 ligands. J Immunol. 2009;183:2810–2817. doi: 10.4049/jimmunol.0900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad P., Tiwari A.K., Kumar K.M. Association of TGFbeta1, TNFalpha, CCR2 and CCR5 gene polymorphisms in type-2 diabetes and renal insufficiency among Asian Indians. BMC Med Genet. 2007;8:20. doi: 10.1186/1471-2350-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banba N., Nakamura T., Matsumura M. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000;58:684–690. doi: 10.1046/j.1523-1755.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 14.Tesch G.H. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 15.Wada T., Furuichi K., Sakai N. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492–1499. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- 16.Tarabra E., Giunti S., Barutta F. Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes. 2009;58:2109–2118. doi: 10.2337/db08-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C.C., Chen J.S., Lu K.C. Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin Chim Acta. 2010;411:700–704. doi: 10.1016/j.cca.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Segerer S., MacK M., Regele H. Expression of the C-C chemokine receptor 5 in human kidney diseases. Kidney Int. 1999;56:52–64. doi: 10.1046/j.1523-1755.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 19.Muntinghe F.L., Gross S., Bakker S.J. CCR5Delta32 genotype is associated with outcome in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;86:140–145. doi: 10.1016/j.diabres.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Sayyed S.G., Ryu M., Kulkarni O.P. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 2011;80:68–78. doi: 10.1038/ki.2011.102. [DOI] [PubMed] [Google Scholar]
- 21.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens L.A., Coresh J., Schmid C.H. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parving H.H., Persson F., Lewis J.B. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelhalter D.J., Abrams K.R., Myles J.P. John Wiley & Sons; New York: 2004. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. [Google Scholar]
- 25.Walley R.J., Smith C.L., Gale J.D. Advantages of a wholly Bayesian approach to assessing efficacy in early drug development: a case study. Pharm Stat. 2015;14:205–215. doi: 10.1002/pst.1675. [DOI] [PubMed] [Google Scholar]
- 26.de Zeeuw D., Bekker P., Henkel E. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3:687–696. doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Log-transformed measurements of urinary albumin-to-creatinine ratio (UACR), urinary protein-to-creatinine ratio (UPCR), estimated glomerular filtration rate (eGFR), serum creatinine, serum cystatin C, and plasma glycosylated hemoglobin (HbA1c)– mixed-effects model repeated measure (MMRM) analysis (full analysis set [FAS])