See Clinical Research on Page 1304
The success of renal transplantation has led physicians to waitlist increasingly older and obese recipients, populations at higher risk for cardiovascular complications and patient death. Growing importance is therefore attached to the management of the adverse cardiovascular risk profile of immunosuppressive therapy while maintaining adequate immunosuppressive efficacy. The prospective and randomized multicenter study from Spain by Torres et al.1 in the present issue of KI Reports is a significant contribution in this respect, and provides important information on the potential pitfalls.
The trial selectively recruited candidates for renal transplantation at high risk to develop posttransplant diabetes mellitus (PTDM) either because of age ≥60 years or younger age (45–59 years) in combination with increased triglyceride levels and/or body mass index. Patients were treated with the gold-standard immunosuppression of calcineurin inhibitors in combination with basiliximab induction, mycophenolate mofetil (MMF), and steroids. Participants were randomly allocated to tacrolimus (Tac) with steroid withdrawal after 1 week (Tac-SW) or either Tac or cyclosporine (CsA) with progressive steroid tapering and cessation after 6 months (Tac-SM and CsA-SM arms). The primary efficacy endpoint was the presence of PTDM based on American Diabetes Association criteria at 3 and 12 months after inclusion, making this study only the second randomized head-to-head comparison of tacrolimus and cyclosporine in renal transplant recipients with PTDM as primary efficacy endpoint.2
The study first confirmed the Symphony trial in the lower efficacy of cyclosporine in preventing acute rejection during the first months after transplantation.3 The incidence of acute rejection in the CsA-SM arm (21.4%) was significantly higher than in the Tac-SM arm (4.8%) and still numerically higher than in patients receiving Tac in combination with early steroid withdrawal (11.4%). Patients receiving CsA had also a numerically higher incidence of severe and steroid-resistant acute rejection episodes, which led to the premature discontinuation of the study. It can be argued that the trough levels of cyclosporine were probably insufficient for optimal prevention of acute rejection, and that reduced bioavailability of MMF in combination with CsA probably resulted in suboptimal dosing of the drug in some patients. This, as well as systematic steroid tapering and cessation, resulted in an incidence of acute rejection that can be considered as unacceptable by current standards.
In terms of the primary efficacy endpoint, the study confirmed the higher diabetogenicity of tacrolimus by a markedly increased cumulative incidence of PTDM at 1 year in spite of the lower number of treated rejection episodes, the lower cumulative steroid dose, and the higher proportion of patients without steroids in the 2 tacrolimus arms. Interestingly, early steroid withdrawal did not provide any benefit in terms of fasting glucose levels or PTDM as compared to a low maintenance dose of steroids in the 3-month data, but was associated with a higher risk of acute rejection. This is in line with a recent double-blind intervention trial that showed no significant beneficial effect of early steroid avoidance on glucose metabolism as compared to a low maintenance dose.4
The combination of basiliximab, Tac, and MMF in combination with a low maintenance dose of steroids, which can eventually be withdrawn several months after transplantation, is thus confirmed by the present trial as the best compromise in terms of prevention of acute rejection and adverse impact on glucose metabolism. Similar efficacy in the prevention of acute rejection is possible with cyclosporine, but requires increased drug exposure with C2 levels >1000 ng/ml, C0 levels >250 ng/ml during the first months after transplantation, and the use of maintenance steroids.2, 5
The present study, the Symphony study, and the current evidence on the limited beneficial effect of steroid avoidance therefore question whether adjustment of immunosuppression to improve glucose metabolism, either by replacing tacrolimus with cyclosporine or by the avoidance of steroids, justifies the increased risk of rejection and consequent adverse graft outcomes.3, 4
In this sense, an expert panel has recommended to choose the most efficient therapy to prevent rejection irrespective of the associated metabolic risk profile, because graft dysfunction and loss are much more potent risk factors for cardiovascular disease and patient death than PTDM.6 However, this recommendation was general and did not differentiate between the early and late posttransplant period and between patients at high and low immunological risk. We completely agree with this statement in the early posttransplant period when the risk of acute rejection is high. However, after 3 to 6 months, acute rejection becomes a rare event in the large majority of renal transplant recipients. In addition, delaying changes in immunosuppression for several months enables physicians to select patients who have remained free of acute rejection, donor-specific antibodies, altered graft function, proteinuria, or histological abnormalities at protocol biopsies and can therefore be considered at low immunological risk. This delay also permits to identify patients with suboptimal control of PTDM in spite of optimal care, whose glucose metabolism is likely to benefit most from a change in the immunosuppressive regimen.
Most registry reports and randomized controlled trials concur that tacrolimus and cyclosporine are associated with equivalent long-term patient and graft survival after renal transplantation when used in combination with MMF. We therefore developed the concept that conversion from tacrolimus to cyclosporine in the maintenance setting and in patients at low immunological risk would be associated with low risk of graft injury but significant benefits in terms of glycemic control, thereby providing a favorable risk−benefit ratio for this intervention.7
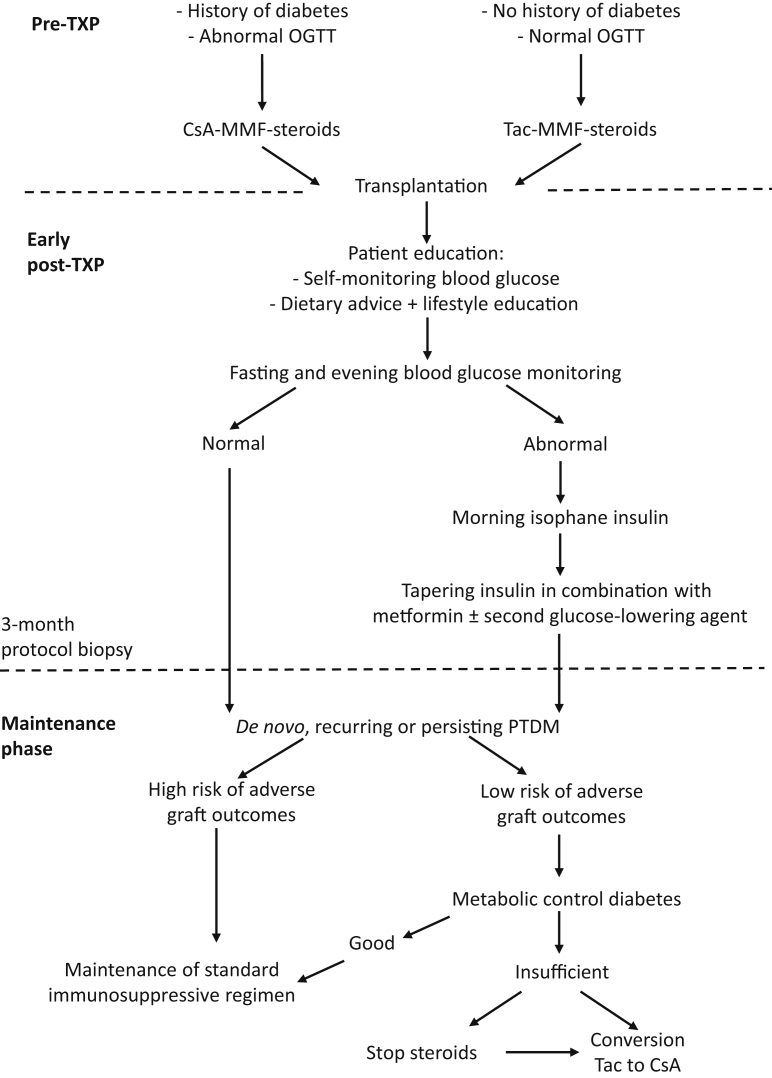
The eventual adjustment of immunosuppression has to be considered as part of a comprehensive arsenal of multiple interventions to prevent and manage PTDM that are not mutually exclusive6 (Figure 1). During the early postoperative period, a single-center randomized trial has shown that intermediate length action isophane insulin, administered as a single daily dose in the morning, can protect beta cells with a long-term preservation of functional reserve and reduced incidence or PTDM.8 This interesting hypothesis is currently validated in a large multicenter prospective and randomized study. Weight increase and a sedentary lifestyle are common in transplant recipients. Lifestyle modifications in the form of dietary advice, exercise programs, and weight loss have been validated as interventions to improve glucose metabolism in the general population and in renal transplant recipients, and should be implemented in all recipients of renal transplants.9 After discharge and when the maintenance steroid dose is progressively decreased, insulin can be stopped in the majority of patients with increased glycemia during the early posttransplant period. Oral agents then become the main glucose-lowering therapy and achieve adequate metabolic control in the majority of patients. In case adequate metabolic control cannot be achieved by optimal therapy and patients keep HbA1c levels that put them at increased risk for diabetes-related renal and vascular damage in the long term, tailoring the immunosuppressive regimen to drugs with less diabetogenic side effects has, in our opinion, to be considered.
Figure 1.
Algorithm for the prevention and management of posttransplant diabetes mellitus (PTDM) after renal transplantation. Structured follow-up for the diagnosis and management of abnormalities in glucose metabolism after renal transplantation, such as those implemented in the Universitair Ziekenhuis Brussel renal transplant program. All candidates for renal transplantation have an oral glucose tolerance test (OGTT) during the pretransplantation workup. Patients with a history of diabetes or abnormal OGTT are treated with cyclosporine except when otherwise indicated. After transplantation, all patients are followed by a diabetes nurse and receive training in self-monitoring of blood glucose levels, dietary advice, and information on physical activity. Patients with abnormal fasting and/or evening glucose receive a single dose of isophane insulin according to the protocol by Hecking et al.8 Metformin is started after the recovery of renal function and insulin is tapered. Three months after transplantation, all patients have a protocol kidney biopsy. Patients who continue to receive glucose-lowering therapy after 3 months are followed regularly at a multidisciplinary consultation with a transplant nephrologist and a dedicated diabetologist, who follow all diabetic renal transplant recipients. Patients classified as low risk for adverse graft outcome (no acute rejection after transplantation, good graft function, no significant proteinuria, no donor-specific anti−human leukocyte antigen antibodies, and absence of subclinical rejection on protocol biopsy) are eligible for conversion from tacrolimus to cyclosporine and/or steroid withdrawal in the case of insufficient metabolic control of diabetes. CsA-MMF-steroids, cyclosporine−mycophenolate mofetil−steroids group; Tac-MMF-steroids, tacrolimus−mycophenolate mofetil−steroids group; TXP, transplant.
The metabolic benefit of conversion from Tac to CsA in patients with established PTDM in the maintenance phase was first investigated in a retrospective cohort study and subsequently the multi-center prospective and randomized REVERSE study.7, 10 In this trial, conversion was associated with a significant reduction in the proportion of patients with diabetes and glucose-lowering therapy 1 year after randomization. In addition, HbA1c levels were on average at 6.0% in the CsA as compared to 7.1% in the Tac arm, translating into a clinically significant improvement in metabolic control.7 Conversion was not associated with an increase in acute rejection or significant differences in graft function between the 2 arms. In contrast to intervention trials in the early posttransplant period, improvement in metabolic control was thus obtained without a significant detrimental effect on graft and patient outcomes, although the study was not powered for key safety endpoints.
The place of steroid withdrawal during the maintenance phase in patients with PTDM is currently uncertain. Although steroid withdrawal is safe in patients receiving Tac and MMF, there is at present no evidence from controlled intervention trials that stopping low-dose steroids equivalent to ≤5 mg prednisolone per day during the maintenance phase will reduce the risk to subsequently develop PTDM or will improve glycemic control in patients with PTDM. Stopping steroids has therefore to be evaluated in the context of the coexistence of other steroid-related adverse effects, such as osteoporosis or wound healing problems. Similar to the study by Torres et al.,1 older patients are frequently transplanted with organs from older donors with age-related chronic damage that can be further worsened by ischemia-reperfusion injury and delayed graft function. In this context, calcineurin inhibitor minimization is probably the best intervention to avoid the added detrimental effect of chronic calcineurin inhibitor nephrotoxicity on an already damaged graft. Reducing calcineurin inhibitor exposure in patients without steroids has not been validated and clearly implies a risk of insufficient immunosuppression, even in low-risk patients. In the quest to balance the risk of adverse graft outcomes and metabolic side effects, systematic steroid withdrawal like in the trial by Torres et al.1 is probably not the best choice in the majority of patients at risk for PTDM.
In conclusion, the study by Torres et al.1 provided additional evidence that cyclosporine is considerably less diabetogenic than tacrolimus but is associated with a higher incidence of acute rejection, particularly when used at a relatively low dose in association with systematic steroid withdrawal by 6 months. In this setting, tacrolimus-based immunosuppression with steroid withdrawal after the early posttransplant phase provides the optimal immunosuppressive regimen for the large majority of patients. Conversion to cyclosporine remains an efficient option in the maintenance phase for patients at low immunological risk and with suboptimal metabolic control by available glucose-lowering therapies.
Disclosure
All the authors declared no competing interests.
References
- 1.Torres A., Hernández D., Moreso F. Randomized controlled trial assessing the impact of tacrolimus versus cyclosporine on the incidence of posttransplant diabetes mellitus. Kidney Int Rep. 2018;3:1304–1315. doi: 10.1016/j.ekir.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincenti F., Friman S., Scheuermann E. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–1514. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 3.Ekberg H., Tedesco-Silva H., Demirbas A. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 4.Pirsch J.D., Henning A.K., First M.R. New-onset diabetes after transplantation: results from a double-blind early corticosteroid withdrawal trial. Am J Transplant. 2015;15:1982–1990. doi: 10.1111/ajt.13247. [DOI] [PubMed] [Google Scholar]
- 5.Hardinger K.L., Bohl D.L., Schnitzler M.A. A randomized, prospective, pharmacoeconomic trial of tacrolimus versus cyclosporine in combination with thymoglobulin in renal transplant recipients. Transplantation. 2005;80:41–46. doi: 10.1097/01.tp.0000162980.68628.5a. [DOI] [PubMed] [Google Scholar]
- 6.Sharif A., Hecking M., de Vries A.P. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992–2000. doi: 10.1111/ajt.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wissing K.M., Abramowicz D., Weekers L. Prospective randomized study of conversion from tacrolimus to cyclosporine A to improve glucose metabolism in patients with posttransplant diabetes mellitus after renal transplantation. Am J Transplant. 2018;18:1726–1734. doi: 10.1111/ajt.14665. [DOI] [PubMed] [Google Scholar]
- 8.Hecking M., Haidinger M., Doller D. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol. 2012;23:739–749. doi: 10.1681/ASN.2011080835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharif A., Moore R., Baboolal K. Influence of lifestyle modification in renal transplant recipients with postprandial hyperglycemia. Transplantation. 2008;85:353–358. doi: 10.1097/TP.0b013e3181605ebf. [DOI] [PubMed] [Google Scholar]
- 10.Ghisdal L., Bouchta N.B., Broeders N. Conversion from tacrolimus to cyclosporine A for new-onset diabetes after transplantation: a single-centre experience in renal transplanted patients and review of the literature. Transpl Int. 2008;21:146–151. doi: 10.1111/j.1432-2277.2007.00589.x. [DOI] [PubMed] [Google Scholar]