Abstract
Introduction
Plasmin and its precursor, plasminogen, are detectable in urine from patients with glomerular disease. Urinary plasmin(ogen) levels correlate with blood pressure (BP) and may contribute to renal Na+ retention by activating the epithelial Na+ channel (ENaC). In a longitudinal nested-cohort study, we asked whether urinary plasmin(ogen) levels predict subsequent increase in BP, incident hypertension, or mortality in subjects with type I diabetes, who often develop proteinuria.
Methods
The Pittsburgh Epidemiology of Diabetes Complications (EDC) study followed up type I diabetic subjects for 25 years. Urine specimens from 70 subjects with a spectrum of baseline urinary albumin levels were examined. Outcomes included increased BP after 2 years (≥1 SD over baseline systolic or diastolic BP, examined via logistic regression), 25-year incident hypertension (≥140/90 mm Hg or initiating BP-lowering medications), and all-cause or cardiovascular mortality, examined using Cox regression.
Results
Subjects experiencing a 2-year increase in BP had higher baseline urinary plasmin(ogen)/creatinine levels (uPl/Cr) than other subjects (P = 0.04); the difference in baseline urinary albumin/creatinine levels (uAlb/Cr) was similar (P = 0.07). Baseline uPl/Cr was associated with increased 25-year hypertension incidence (hazard ratio = 2.05, P = 0.001), all-cause mortality (HR = 2.05, P = 0.01) and cardiovascular mortality (HR = 3.30, P = 0.005), although not independent of uAlb/Cr.
Conclusion
This is the first long-term prospective study addressing clinical outcomes associated with increased urinary plasmin(ogen). Findings are consistent with a role for plasmin(ogen) in promoting increased BP, but also demonstrate the difficulty in distinguishing effects due to plasmin(ogen) from those of albuminuria.
Keywords: albumin, ENaC, diabetes, hypertension, plasmin
See Commentary on Page 1242
Diabetes is a leading contributor to chronic and end-stage kidney disease and is often associated with hypertension.1 Urinary elaboration of protein represents 1 of the earliest clinical manifestations of diabetic kidney disease.2 Proteinuria is associated with increased BP and predicts onset of hypertension.3, 4, 5, 6, 7 Although urinary albumin is commonly monitored clinically as a marker of proteinuria, additional bloodstream proteins traverse damaged glomeruli and appear in the urine. Among these is the thrombolytic protease plasmin and its precursor, plasminogen.8, 9, 10, 11, 12, 13, 14, 15
In experimental systems, plasmin, in conjunction with the trans-Golgi protease furin, proteolytically removes an imbedded inhibitory tract from the γ-subunit of the ENaC, thereby increasing open probability.16, 17 Activation of ENaC via this mechanism has been suggested to promote renal Na+ retention and increased BP in the context of proteinuria.12, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 Evidence implicating ENaC in proteinuria-associated renal Na+-retention includes findings that nephrotic laboratory animals experience enhanced Na+ reabsorption that is prevented by the ENaC blocker amiloride.31, 32 Furthermore, in diabetic humans, amiloride’s ability to induce natriuresis is enhanced in people with proteinuria.33
A role for plasmin in Na+ retention is suggested by observations that urinary levels of plasmin and its precursor, plasminogen, are associated with increased extracellular fluid volume and BP.9, 10, 30 Moreover, serine protease inhibitors attenuate urinary Na+ retention in animal models of nephrotic syndrome and hypertension.34, 35, 36 Thus, enhanced renal Na+ retention in individuals with increased urinary plasmin(ogen) may promote subsequent increases in BP. We hypothesized that in subjects with type I diabetes (T1D), who often experience proteinuria, increased baseline urinary plasmin(ogen) would be associated with a subsequent increase in BP, risk of incident hypertension, and, by extension, an increase in cardiovascular mortality. We thus examined plasmin(ogen) levels in urine specimens collected prospectively through the EDC, a cohort of subjects with T1D enrolled from 1986 to 1988 and followed up for >25 years.37, 38 We examined the association between baseline urinary plasmin(ogen) levels normalized to creatinine (uPl/Cr) with BP and with baseline urinary albumin levels normalized to creatinine (uAlb/Cr). We further examined whether uPl/Cr was associated with a subsequent increase in BP, incident hypertension, or mortality in this cohort.
Methods
The present investigation represents an observational pilot substudy performed using longitudinally collected data and specimens.
Study Population
Recruitment of participants occurred as part of the EDC Study, previously described in detail.39, 40 Briefly, study subjects (n = 658 examined at baseline) carried a diagnosis of childhood-onset (<17 years of age) T1D and had been initially diagnosed (or seen within 1 year of diagnosis) at the Children’s Hospital of Pittsburgh between 1950 and 1980. Following enrollment in 1986 to 1988, subjects were followed up with biennial clinical examinations for the first 10 years, biennial questionnaires thereafter, and additional clinical evaluations at 18 and 25 years. For the current study, a nested subset of subjects was selected to provide a spectrum of baseline urinary protein levels: one-third with no clinical albuminuria (<30 mg/g albumin/creatinine), one-third with microalbuminuria (30−300 mg/g), and one-third with macroalbuminuria (>300 mg/g). Choice of subject was blinded to clinical outcomes. Specimens from study baseline (1986−1988) and those at 25 years (2011−2013) were examined. All participants provided written informed consent for the study, which was approved by the University of Pittsburgh institutional review board.
Clinical Variables
At each clinical examination, 3 BP readings were taken from seated participants initially using a sphygmomanometer that was random-zero, updated to aneroid at 18- and 25-year follow-up visits. The mean of the second and third readings was used in analyses, according to the Hypertension Detection and Follow-up Program protocol.41 Blood pressure medication use was ascertained through self-report.
For determination of kidney function, blood samples were collected from study subjects at each clinical visit. Serum creatinine was measured using an Ektachem 400 Analyzer (Eastman Kodak Co., Rochester, NY). Estimated glomerular filtration rate (eGFR) was calculated from demographic variables and serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.42
Study subjects provided urine specimens at each clinic visit. Urine albumin levels were measured using immunonephelemetry and normalized to urine creatinine measured via Ektachem 400 Analyzer, as previously reported.43 Units for uAlb/Cr are mg/g or log(mg/g). For urinary plasmin(ogen) measurement in the present study, archived specimens stored at −80°C were thawed on ice and centrifuged at 10,000 g for 15 minutes at 4°C to remove particulate matter. Specimens were then diluted 10- to 100-fold before plasmin(ogen) measurement via enzyme-linked immunosorbent assay (Innovative Research, Novi, MI). Although this method does not distinguish active plasmin from plasminogen, results using this assay correlate well with urine plasmin as measured by immunoblot.44 Urinary plasmin(ogen) was normalized to urine creatinine measured by enzymatic creatinine assay (Pointe Scientific, Canton, MI). Final units are in μg/g creatinine or log(μg/g). Urine Na+ and K+ concentrations were measured using flame photometry (Jenway, Staffordshire, UK) after 500-fold dilution and passage through a 0.2-μm filter.
Outcomes
Primary study outcomes included increase in BP over a 2-year period and 25-year incident hypertension; secondary outcomes included 25-year all-cause and cardiovascular mortality. Because only 4 subjects developed incident hypertension in the 2-year period between the first and second study visits, increase in BP was used as a study outcome. To avoid mis-stratification of subjects resulting from variability in BP measurement, an increase in BP was defined as an increase in systolic BP (SBP) or diastolic BP (DBP) of at least 1 SD over baseline (2.7 mm Hg for SBP or 2.4 mm Hg for DBP). Individuals started on a new antihypertensive agent were also regarded as having higher BP. Analysis of BP change as a continuous variable was not examined, as this would not account for the significant impact of initiation of new antihypertensive agents. Long-term outcomes examined hypertension incidence, defined as the first instance of BP ≥140/90 mm Hg or prescription of a medication explicitly for BP control during the 25-year follow-up. Mortality was ascertained over 25 years using death certificates, autopsy reports, medical records, and/or interviews with next of kin. Causes of death were classified using all available information according to the Diabetes Epidemiology Research International system by a committee of physicians.45 Cardiovascular mortality was defined as fatal coronary artery disease, myocardial infarction, or stroke as either the primary or a contributing cause of death.
Statistical Analyses
A preliminary pilot study and power analysis were performed to determine the number of subjects required to provide adequate power to examine the association between uPl/Cr and the primary outcome of hypertension incidence. The preliminary study included 7 subjects in each of 3 groups: no clinical albuminuria (<30 mg/g albumin/creatinine), microalbuminuria (30−300 mg/g), or macroalbuminuria (>300 mg/g). A sample size of 70 provided 90% power to detect a HR of 1.90 for hypertension incidence associated with a 1-unit increase in log(uPl/Cr), given our observed event rate of 66% and SD of 0.74 for log(uPl/Cr)
Study subjects were stratified on the basis of uPl/Cr tertile, and those from the highest tertile (≥34.9 μg/g) were compared to subjects from the combined 2 lower tertiles. In a previous, cross-sectional study examining subjects with diabetic nephropathy, uPl/Cr in excess of this level was observed in roughly two-thirds of diabetic nephropathy subjects but in no control subjects.8 Baseline characteristics were compared between uPl/Cr categories (highest tertile vs. all others) using 2-sample t tests (or Wilcoxon rank-sum test, if data were not normally distributed).
Regression analyses were performed using continuous uPl/Cr, which was log transformed to reduce skew. Logistic regression models were used to estimate the association between uPl/Cr and the odds of increased BP in the first 2 study years. Receiver operating characteristic (ROC) curves were fitted for both uPl/Cr and uAlb/Cr to evaluate evidence for a threshold effect on the odds of increased BP. Nine subjects did not attend the 2-year study visit and were excluded from the complete-cases analysis of BP change. Areas under the curves (AUCs) were compared using the DeLong test. Kaplan−Meier curves were fitted separately for survival free of hypertension, cardiovascular death, or death from any cause. Survival curves by uPl/Cr categories were compared using the log-rank test. Cox proportional hazards models were used to estimate the association between uPl/Cr and the risk of incident hypertension, cardiovascular death, or death from any cause over the entire 25 years of follow-up. Subjects not experiencing an event were censored at their most recent follow-up time. The mean follow-up time until an event or censoring was 22 years (SD = 6.5). Models were also fit adjusting individually for T1D duration, sex, eGFR, body mass index (BMI), SBP, and DBP. Age was not included in the fully adjusted multivariable models because of its high correlation with duration of T1D (r = 0.82). Because increased BP may promote transudation of plasma proteins across the glomerular barrier, and because albumin may serve as a surrogate for nonspecific proteinuria, separate modeling offering the above-mentioned variables plus uAlb/Cr was also performed. Fully adjusted multivariable models estimating the association between uPl/Cr and the outcomes were selected by first including uPl/Cr and then using backward elimination for the remaining covariates with a removal criterion of P = 0.05. Quality of model fit was assessed using the Akaike Information Criterion. The proportional hazards assumption was assessed graphically and by including a time-dependent form of uPl/Cr in the Cox models. No violations of the proportional hazards assumption were observed.
Results
At baseline, subjects were normotensive on average with mean eGFR 104.0 ± 24.6 ml/min per 1.73 m2. Of the subjects, 20% had prevalent hypertension, and only 14% were prescribed antihypertensive medications (Table 1). Participants with uPl/Cr in the highest tertile had significantly higher SBP and DBP and significantly lower eGFR. There were no differences in age, T1D duration, or BMI. Although not statistically significant, those in the highest tertile were more likely to be female, to use antihypertensives, and to have prevalent HTN.
Table 1.
Baseline characteristics
| Characteristic | All subjects | uPl/Cr tertiles |
||
|---|---|---|---|---|
| Lower 2 tertiles | Highest tertile | P | ||
| Subjects, n | 70 | 46 | 24 | |
| Age, yr | 27.8 ± 6.9 | 27.8 ±7.5 | 27.9 ±5.5 | 0.9 |
| Sex, % female | 41.4 | 34.8 | 54.2 | 0.1 |
| T1D duration, yr | 19.2 ± 7.1 | 19.0 ±7.2 | 19.4 ±7.0 | 0.9 |
| HbA1c, % | 8.9 ±1.4 | 8.8 ±1.4 | 9.2 ±1.4 | 0.2 |
| BMI, kg/m2 | 23.9 ±3.0 | 23.9 ±3.0 | 23.9 ±3.2 | 0.9 |
| SBP, mm Hg | 117.6 ± 13.8 | 114.1 ±10.7 | 124.3 ±16.4 | 0.009 |
| DBP, mm Hg | 76.8 ± 10.3 | 74.5 ±8.4 | 81.1 ±12.3 | 0.02 |
| Antihypertensive use, % subjects | 14.3 | 11.9 | 19.1 | 0.5 |
| HTN, % subjects | 20.0 | 15.2 | 29.2 | 0.2 |
| eGFR, ml/min per 1.73 m2 | 104.0 ± 24.6 | 105.9 ±19.9 | 100 ±31.9 | 0.02 |
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HTN, hypertension; SBP, systolic blood pressure; T1D, type I diabetes mellitus; uPl/Cr, urinary plasmin(ogen)/creatinine.
uPl/Cr and Blood Pressure
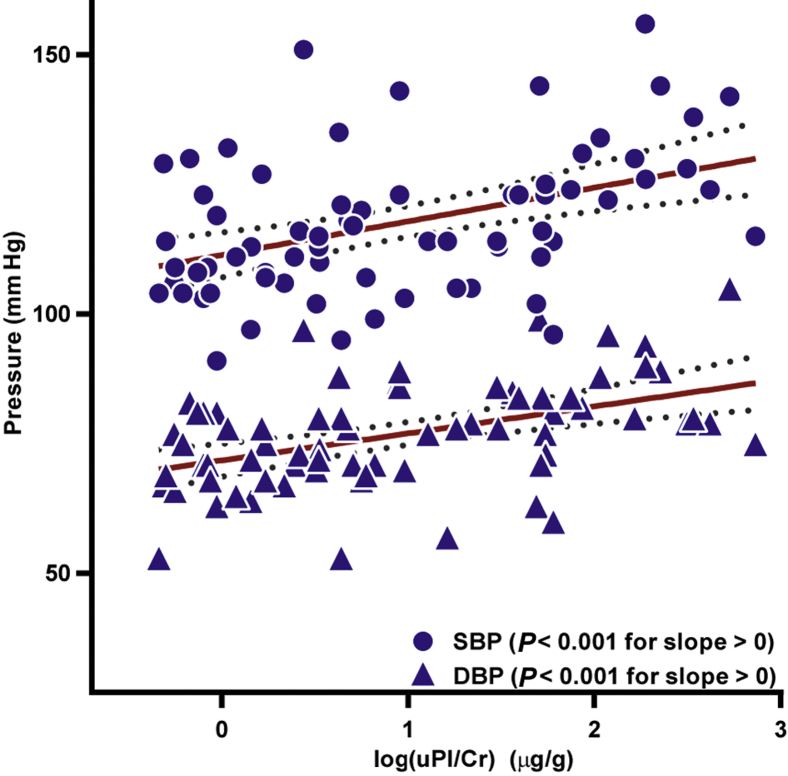
Baseline uPl/Cr correlated positively with both SBP and DBP (Figure 1). In addition, uPl/Cr correlated with uAlb/Cr (see Supplementary Figure S1) and inversely correlated with eGFR (r = −0.35, P = 0.003). Baseline uPl/Cr was not correlated with age, T1D duration, or BMI. In the subset of subjects with uPl/Cr measured at baseline and after 25 years (n = 21), there was no significant change in uPl/Cr level between measurements. There was no correlation of uPl/Cr with urinary Na+/K+ (see Supplementary Figure S2).
Figure 1.
Log of the urinary plasmin(ogen)/creatinine (log uPl/Cr) correlated with systolic blood pressure (SBP) and diastolic blood pressure (DBP) at enrollment. Both SBP and DBP correlated positively with log(uPl/Cr). Dotted lines show 95% confidence intervals. The log(uPl/Cr) slope versus SBP is 6.5 ± 1.7; the slope versus DBP is 5.2 ± 1.2. r = 0.43 and r = 0.46, respectively.
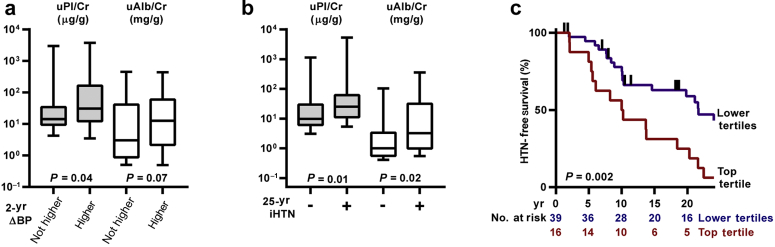
To examine whether uPl/Cr was associated with short-term worsening of BP (increased BP or addition of a BP medication), we compared baseline uPl/Cr with prospectively collected BP data (Figure 2). Baseline uPl/Cr was higher (P = 0.04) in subjects who went on to experience a worsening of BP between the first and second study visits (∼2 years). The difference in baseline urinary albumin in these subjects also approached statistical significance (P = 0.07). Frequency of 2-year increase in BP was compared in each uPl/Cr and uAlb/Cr tertile (Table 2).
Figure 2.
Longitudinal blood pressure analysis. (a) Urinary plasmin(ogen)/creatinine (uPl/Cr, gray bars) and urinary albumin/creatinine (uAlb/Cr, white bars) were compared to blood pressure (BP) change over 2 years. Subjects were stratified on the basis of change in BP. In all, 40 subjects experienced higher BP, 23 had stable BP, and 7 were excluded due to missing data. The ordinate represents log units, but the units for uPl/Cr (μg/g) differ from those of uAlb/Cr (mg/g). Whiskers represent 5th and 95th percentiles. (b) Baseline uPl/Cr levels and uAlb/Cr levels from subjects stratified for incident hypertension (iHTN) over the course of approximately 25 years. Of the subjects, 37 experienced iHTN and 19 did not. Prevalent cases of hypertension (HTN) were excluded. Statistical comparisons were performed as in panel (a). (c) Kaplan−Meier survival curve showing the association between uPl/Cr and iHTN over 25 years. Subjects in the top tertile of uPl/Cr (≥34.9 μg/g) are compared to those in the lower tertiles. Vertical ticks represent censoring events.
Table 2.
Percentage (number) of subjects with 2-year increase in blood pressure, by uPl/Cr or uAlb/Cr tertile
| Variable | Tertile |
|||
|---|---|---|---|---|
| First | Second | Third | P | |
| uPl/Cr | 42.1% (8) | 54.6% (12) | 68.2% (15) | 0.09 |
| uAlb/Cr | 45.5% (10) | 52.4% (11) | 70.0% (14) | 0.11 |
uAlb/Cr, urinary albumin/creatinine; uPl/Cr, urinary plasmin(ogen)/creatinine.
The odds of increased BP increased with higher uPl/Cr with borderline statistical significance (P = 0.06) (Table 3). Adjustment for additional covariates did not improve the fit of the model. No evidence for a threshold effect of either uPl/Cr or uAlb/Cr was observed using receiver operating characteristic curves. The areas under the curve did not differ (uPl/Cr area under the curve = 0.65 vs. uAlb/Cr area under the curve = 0.62, P = 0.45). Baseline uPl/Cr was not higher in individuals who experienced increased BP at 4 and 6 years (P = 0.96 and P = 0.62, respectively).
Table 3.
Odds ratios associated with a 1-unit increment in log(uPl/Cr)
| Odds ratio (95% CI) | P | |
|---|---|---|
| 2-yr BP increasea,b,c | ||
| Univariate | 2.30 (0.97−5.43) | 0.06 |
| Hazard ratio (95% CI) | P | |
| 25-yr Incident hypertension | ||
| Univariate | 2.05 (1.33−3.17) | 0.001 |
| Model 1 (adjusted for T1D duration, DBP)a | 2.18 (1.37−3.48) | 0.001 |
| Model 2 (adjusted for T1D duration, uAlb/Cr, DBP)b | 1.15 (0.59−2.24) | 0.7 |
| All-cause mortality | ||
| Univariate | 1.97 (1.17−3.39) | 0.01 |
| Model 1 (adjusted for T1D duration)a | 1.93 (1.15−3.24) | 0.01 |
| Model 2 (adjusted for uAlb/Cr)b | 0.93 (0.43−2.00) | 0.9 |
| Cardiovascular mortalitya,b,c | ||
| Univariate | 3.30 (1.43−7.63) | 0.005 |
BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; log(uPl/Cr), log of the urinary plasmin(ogen)/creatinine; SBP, systolic blood pressure; T1D, type I diabetes mellitus; uAlb/Cr, urinary albumin/creatinine.
Variables offered for adjustment were duration of diabetes, sex, body mass index, SBP and DBP, and estimated glomerular filtration rate. Only those listed in parentheses were retained after backward selection.
Same variables offered as in model 1 with the addition of uAlb/Cr. Only those listed in parentheses were retained after backward selection.
Only log(uPl/Cr) was retained after backward selection.
uPl/Cr and Incident Hypertension
Analyses of hypertension incidence during the 25-year follow-up excluded 14 subjects with prevalent hypertension at baseline. In subjects experiencing incident hypertension, baseline uPl/Cr and uAlb/Cr were both higher than in individuals not experiencing incident hypertension (Figure 2). Survival analysis revealed that the group of individuals with uPl/Cr in the top tertile experienced a significantly greater rate of incidence of hypertension than the group with lower uPl/Cr. In univariate analysis, uPl/Cr was associated with an increased risk of incident hypertension (Table 3). In models where T1D duration, sex, body mass index, SBP, DBP, and eGFR were offered for adjustment, only T1D duration, uAlb/Cr, and DBP were retained after backward elimination. The association between uPl/Cr and hazard of incident hypertension was lost after adjusting for uAlb/Cr.
uPl/Cr and Mortality
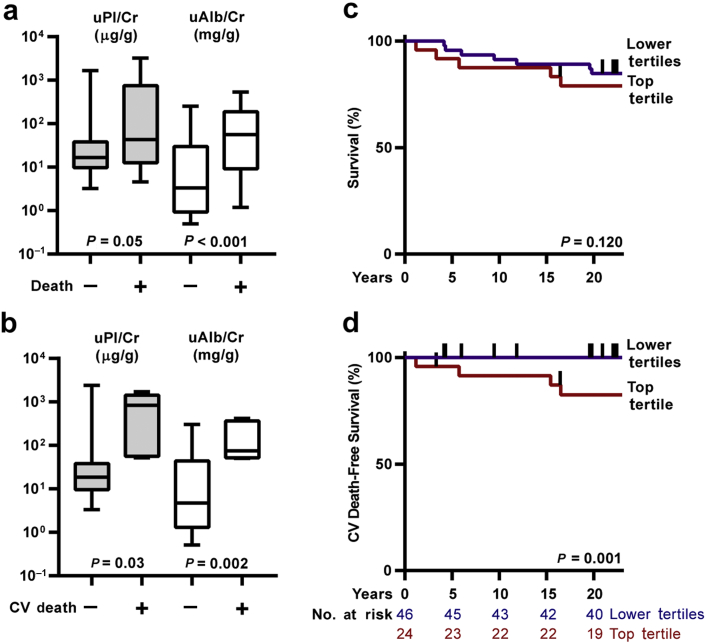
In subjects experiencing death from any cause (n = 16), baseline uPl/Cr was marginally higher than in those who survived (n = 54); uAlb/Cr was significantly higher (Figure 3). In univariate analysis, continuous uPl/Cr was significantly associated with an increased risk of all-cause mortality (Table 3). In multivariable models, T1D duration, sex, BMI, SBP, DBP, and eGFR were offered for adjustment. After backward elimination, only T1D duration was retained, but it did not affect the strength of the association between uPl/Cr and mortality. Adjusting for uAlb/Cr attenuated the correlation between uPl/Cr and all-cause mortality. The all-cause mortality risk in subjects in the top uPl/Cr tertile was similar to individuals in the lower tertiles.
Figure 3.
Study participant survival. (a,b) Baseline urinary plasmin(ogen)/creatinine (uPl/Cr, gray bars) and urinary albumin/creatinine (uAlb/Cr, white bars) from subjects stratified on the basis of all-cause mortality or cardiovascular (CV) mortality, respectively, during the ∼25-year study. Whiskers represent 5th and 95th percentiles. (c,d) Kaplan−Meier survival curves show associations between uPl/Cr tertile and survival, or survival free of CV death, respectively. Vertical hatches represent censoring events.
In subjects experiencing mortality due to cardiovascular causes (n = 5), baseline uPl/Cr and uAlb/Cr were higher than in those who did not die of cardiovascular causes (n = 65). Likewise, subjects in the top uPl/Cr tertile experienced significantly greater risk of cardiovascular death compared to individuals with lower uPl/Cr. Hazard of death due to cardiovascular causes also rose significantly with increasing uPl/Cr (Table 3). After offering the same covariates described above for multivariable adjustment, only uPl/Cr remained after backward elimination of other covariates. Adjusting for uAlb/Cr did not improve the fit of the model. However, if inclusion of uPl/Cr is not required in the model, it is eliminated after backward selection, and only uAlb/Cr remains (uAlb/Cr HR = 5.7, 95% confidence interval = 1.5−21.8). Although the model including uAlb/Cr has a slightly better fit (Akaike Information Criterion = 33.1 vs. uPl/Cr model Akaike Information Criterion = 34.5), the current data do not definitively establish which is the better model.
Similar to incident hypertension, results for progression of kidney disease (≥50% reduction in eGFR) were similar for uPl/Cr and uAlb/Cr (Supplementary Figure S3).
Discussion
We observe that uPl/Cr correlates well with SBP and DBP in this relatively young T1D cohort on few antihypertensive agents. Baseline urinary plasmin(ogen) is higher in subjects who go on to experience increased BP over 2 years than in those who do not. Baseline uPl/Cr is also associated with greater hazard of incident hypertension over 25 years and greater risk of cardiovascular, but not all-cause, mortality.
Our cross-sectional findings are consistent with previous reports demonstrating an association of urinary plasmin(ogen) and BP.9, 10 Although the strength of the association in previous studies was modest, the correlation was likely attenuated by the use of multiple antihypertensive agents in study subjects. The EDC provided a unique opportunity to examine the correlation between BP and urinary plasmin(ogen) from a group of young patients with little baseline hypertension and using data collected at a time prior to the common use of antihypertensive agents for proteinuria (1986−1988). Consequently, findings presented here demonstrate a stronger correlation between urinary plasmin(ogen) and BP than has previously been reported.
To evaluate for evidence of increased tubular cation exchange related to activation of ENaC, we examined the association between uPl/Cr and urinary Na+/K+. We did not detect a significant correlation between these variables, perhaps because dietary intake was not standardized. Oxlund et al. were similarly unable to identify a significant change in urinary Na+/K+ when subjects on nonstandardized diets were given amiloride, although an increase in blood K+ was observed.9
Although cross-sectional analyses confirm an association between BP and urinary plasmin(ogen), a cause-and-effect conundrum complicates interpretation of these findings. Increased plasmin may promote hypertension by stimulating Na+ retention, but increased BP also likely promotes urinary leakage of proteins, including plasminogen. Longitudinal analysis provides additional insight. Persistent stimulation of Na+ retention by plasmin may promote increased BP over time, but the resulting increase in BP cannot be implicated in increasing previous urinary plasmin(ogen) levels. This represents the first long-term study examining the value of urinary plasmin(ogen) in predicting clinical outcomes. For every log-unit increase in uPl/Cr, we observe 2.3-fold higher odds of experiencing increased BP over 2 years. The frequency with which subjects experienced increased BP over this period trended upward as a function of uPl/Cr tertile, but did not achieve statistical significance. This trend was similar to the trend observed with increasing uAlb/Cr tertiles. Individuals experiencing incident hypertension over roughly 25 years demonstrated elevated baseline urinary plasmin(ogen) as well as albumin. Although risk of incident hypertension increases with increasing urinary plasmin(ogen), inclusion of urinary albumin improved the regression model and rendered insignificant the association of urinary plasmin(ogen) with hazard of incident hypertension.
The relationship between baseline plasmin(ogen) and mortality are provocative. We have previously shown in the EDC cohort that excess mortality is statistically explained by increased albuminuria,43 consistent with earlier data from the FinnDiane study.46 It is intriguing that, in the present study, individuals experiencing cardiovascular mortality had elevated baseline plasmin(ogen). Consistent with a role for plasmin in increasing the risk of mortality by promoting hypertension, the association of plasmin(ogen) with cardiovascular mortality was stronger than the association with all-cause mortality. Modeling risk of cardiovascular mortality was not improved by adjustment for uAlb/Cr, if uPl/Cr was already included in the model. When examined separately, uPl/Cr and uAlb/Cr resulted a similar model fit with respect to cardiovascular mortality risk. A small number of events underlies these findings, and a larger cohort would be necessary to examine whether plasmin(ogen) improves prediction of cardiovascular mortality over albumin alone.
The results of this study should be viewed in light of important limitations. The observational design of the study limits the strength of inferences. Also, with the possible exception of increase in BP over 2 years, plasmin(ogen) generally did not predict outcomes better than albumin. A recent study in women with T1D similarly found that increased urinary plasmin(ogen) predicted increased risk of preeclampsia, although not better than urinary albumin.47 Distinguishing effects due to urinary plasmin(ogen) from albumin is challenged by a size disparity between the 2 proteins. Glomerular damage sufficient to allow transudation of plasminogen (∼91 kDa) would also be expected to allow passage of albumin (∼69 kDa). Thus, our findings do not exclude the possibility that other factors associated with albuminuria promote hypertension. Few reported studies examine the relationship between urinary concentration of filtered proteins and cardiovascular outcomes, and review of the literature revealed no examples demonstrating that urinary concentration of plasma proteins can be separated from cardiovascular outcomes.
In conclusion, this study represents the first long-term, longitudinal study examining clinical outcomes associated with increased urinary plasmin(ogen) and provides evidence consistent with a role for plasmin(ogen) in promoting increased BP, but demonstrates the challenge of differentiating the effects of urinary plasmin(ogen) from those of albuminuria on hypertension.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by grants from the Rossi Memorial Fund (TC); the University of New Mexico Clinical Sciences Center, UL1TR00449 (MLU); and the National Institutes of Health: R01 DK051391 (TRK), P30 DK079307 (TRK), T32 DK061296 (ECR), K08 DK110332 (ECR), T35 DK065521 (JAD), and R01 DK034818 (RGM, TC, and TJO). The authors appreciate Rebecca Hughey’s assistance with biochemical measurements and Linda Fried’s contribution to study design and critical review of the manuscript. These findings were presented, in abstract form, at the American Society for Nephrology—Kidney Week, November 4, 2017, New Orleans, Louisiana. The plasmin(ogen) data and analyses are unpublished; the albumin findings represent reanalysis of a subset of previously reported clinical data.43
Footnotes
Figure S1. uPl/Cr versus albumin and eGFR, and longitudinal change in uPl/Cr.
Figure S2. uPl/Cr versus urinary Na/K.
Figure S3. uPl/Cr, uAlb/Cr, and eGFR decline over 25 years.
STROBE Statement checklist.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
uPl/Cr versus albumin and eGFR, and longitudinal change in uPl/Cr.
uPl/Cr versus urinary Na/K.
uPl/Cr, uAlb/Cr, and eGFR decline over 25 years.
References
- 1.Saran R., Robinson B., Abbott K.C. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69:A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulou-Marketou N., Chrousos G.P., Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab Res Rev. 2017;33(2):1–9. doi: 10.1002/dmrr.2841. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R., Andersen M.J. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension. 2005;46:514–520. doi: 10.1161/01.HYP.0000178102.85718.66. [DOI] [PubMed] [Google Scholar]
- 4.Kim B.J., Lee H.J., Sung K.C. Comparison of microalbuminuria in 2 blood pressure categories of prehypertensive subjects. Circ J. 2007;71:1283–1287. doi: 10.1253/circj.71.1283. [DOI] [PubMed] [Google Scholar]
- 5.Forman J.P., Fisher N.D., Schopick E.L., Curhan G.C. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol. 2008;19:1983–1988. doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue T., Iseki K., Higashiuesato Y. Proteinuria as a significant determinant of hypertension in a normotensive screened cohort in Okinawa, Japan. Hypertens Res. 2006;29:687–693. doi: 10.1291/hypres.29.687. [DOI] [PubMed] [Google Scholar]
- 7.Wang T.J., Gona P., Larson M.G. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49:432–438. doi: 10.1161/01.HYP.0000256956.61872.aa. [DOI] [PubMed] [Google Scholar]
- 8.Andersen H., Friis U.G., Hansen P.B. Diabetic nephropathy is associated with increased urine excretion of proteases plasmin, prostasin and urokinase and activation of amiloride-sensitive current in collecting duct cells. Nephrol Dial Transplant. 2015;30:781–789. doi: 10.1093/ndt/gfu402. [DOI] [PubMed] [Google Scholar]
- 9.Oxlund C.S., Buhl K.B., Jacobsen I.A. Amiloride lowers blood pressure and attenuates urine plasminogen activation in patients with treatment-resistant hypertension. J Am Soc Hypertens. 2014;8:872–881. doi: 10.1016/j.jash.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Buhl K.B., Oxlund C.S., Friis U.G. Plasmin in urine from patients with type 2 diabetes and treatment-resistant hypertension activates ENaC in vitro. J Hypertens. 2014;32:1672–1677. doi: 10.1097/HJH.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 11.Andersen R.F., Buhl K.B., Jensen B.L. Remission of nephrotic syndrome diminishes urinary plasmin content and abolishes activation of ENaC. Pediatr Nephrol. 2013;28:1227–1234. doi: 10.1007/s00467-013-2439-2. [DOI] [PubMed] [Google Scholar]
- 12.Svenningsen P., Bistrup C., Friis U.G. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol. 2009;20:299–310. doi: 10.1681/ASN.2008040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaziri N.D., Gonzales E.C., Shayestehfar B., Barton C.H. Plasma levels and urinary excretion of fibrinolytic and protease inhibitory proteins in nephrotic syndrome. J Lab Clin Med. 1994;124:118–124. [PubMed] [Google Scholar]
- 14.Lau S.O., Tkachuck J.Y., Hasegawa D.K., Edson J.R. Plasminogen and antithrombin III deficiencies in the childhood nephrotic syndrome associated with plasminogenuria and antithrombinuria. J Pediatr. 1980;96:390–392. doi: 10.1016/s0022-3476(80)80678-0. [DOI] [PubMed] [Google Scholar]
- 15.Wu K.K., Hoak J.C. Urinary plasminogen and chronic glomerulonephritis. Am J Clin Pathol. 1973;60:915–919. doi: 10.1093/ajcp/60.6.915. [DOI] [PubMed] [Google Scholar]
- 16.Bruns J.B., Carattino M.D., Sheng S. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- 17.Carattino M.D., Hughey R.P., Kleyman T.R. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem. 2008;283:25290–25295. doi: 10.1074/jbc.M803931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passero C.J., Mueller G.M., Rondon-Berrios H. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586–36591. doi: 10.1074/jbc.M805676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svenningsen P., Uhrenholt T.R., Palarasah Y. Prostasin-dependent activation of epithelial Na+ channels by low plasmin concentrations. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1733–R1741. doi: 10.1152/ajpregu.00321.2009. [DOI] [PubMed] [Google Scholar]
- 20.Hamm L.L., Feng Z., Hering-Smith K.S. Regulation of sodium transport by ENaC in the kidney. Curr Opin Nephrol Hypertens. 2010;19:98–105. doi: 10.1097/MNH.0b013e328332bda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passero C.J., Hughey R.P., Kleyman T.R. New role for plasmin in sodium homeostasis. Curr Opin Nephrol Hypertens. 2010;19:13–19. doi: 10.1097/MNH.0b013e3283330fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svenningsen P., Friis U.G., Bistrup C. Physiological regulation of epithelial sodium channel by proteolysis. Curr Opin Nephrol Hypertens. 2011;20:529–533. doi: 10.1097/MNH.0b013e328348bcc7. [DOI] [PubMed] [Google Scholar]
- 23.Palmer L.G., Patel A., Frindt G. Regulation and dysregulation of epithelial Na+ channels. Clin Exp Nephrol. 2012;16:35–43. doi: 10.1007/s10157-011-0496-z. [DOI] [PubMed] [Google Scholar]
- 24.Svenningsen P., Skott O., Jensen B.L. Proteinuric diseases with sodium retention: is plasmin the link? Clin Exp Pharmacol Physiol. 2012;39:117–124. doi: 10.1111/j.1440-1681.2011.05524.x. [DOI] [PubMed] [Google Scholar]
- 25.Bockenhauer D. Over- or underfill: not all nephrotic states are created equal. Pediatr Nephrol. 2013;28:1153–1156. doi: 10.1007/s00467-013-2435-6. [DOI] [PubMed] [Google Scholar]
- 26.Svenningsen P., Friis U.G., Versland J.B. Mechanisms of renal NaCl retention in proteinuric disease. Acta Physiol [Oxf] 2013;207:536–545. doi: 10.1111/apha.12047. [DOI] [PubMed] [Google Scholar]
- 27.Ray E.C., Kleyman T.R. Cutting it out: ENaC processing in the human nephron. J Am Soc Nephrol. 2015;26:1–3. doi: 10.1681/ASN.2014060618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svenningsen P., Andersen H., Nielsen L.H., Jensen B.L. Urinary serine proteases and activation of ENaC in kidney—implications for physiological renal salt handling and hypertensive disorders with albuminuria. Pflugers Arch. 2015;467:531–542. doi: 10.1007/s00424-014-1661-5. [DOI] [PubMed] [Google Scholar]
- 29.Hoorn E.J., Ellison D.H. Diuretic resistance. Am J Kidney Dis. 2017;69:136–142. doi: 10.1053/j.ajkd.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schork A., Woern M., Kalbacher H. Association of plasminuria with overhydration in patients with CKD. Clin J Am Soc Nephrol. 2016;11:761–769. doi: 10.2215/CJN.12261115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichikawa I., Rennke H.G., Hoyer J.R. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983;71:91–103. doi: 10.1172/JCI110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deschenes G., Wittner M., Stefano A. Collecting duct is a site of sodium retention in PAN nephrosis: a rationale for amiloride therapy. J Am Soc Nephrol. 2001;12:598–601. doi: 10.1681/ASN.V123598. [DOI] [PubMed] [Google Scholar]
- 33.Andersen H., Hansen P.B., Bistrup C. Significant natriuretic and antihypertensive action of the epithelial sodium channel blocker amiloride in diabetic patients with and without nephropathy. J Hypertens. 2016;34:1621–1629. doi: 10.1097/HJH.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 34.Maekawa A., Kakizoe Y., Miyoshi T. Camostat mesilate inhibits prostasin activity and reduces blood pressure and renal injury in salt-sensitive hypertension. J Hypertens. 2009;27:181–189. doi: 10.1097/hjh.0b013e328317a762. [DOI] [PubMed] [Google Scholar]
- 35.Kitamura K., Tomita K. Proteolytic activation of the epithelial sodium channel and therapeutic application of a serine protease inhibitor for the treatment of salt-sensitive hypertension. Clin Exp Nephrol. 2012;16:44–48. doi: 10.1007/s10157-011-0506-1. [DOI] [PubMed] [Google Scholar]
- 36.Bohnert B.N., Menacher M., Janessa A. Aprotinin prevents proteolytic epithelial sodium channel (ENaC) activation and volume retention in nephrotic syndrome. Kidney Int. 2018;93:159–172. doi: 10.1016/j.kint.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Orchard T.J., Dorman J.S., Maser R.E. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 38.Orchard T.J., Dorman J.S., Maser R.E. Factors associated with avoidance of severe complications after 25 yr of IDDM. Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes Care. 1990;13:741–747. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 39.Wagener D.K., Sacks J.M., LaPorte R.E., Macgregor J.M. The Pittsburgh Study of Insulin-Dependent Diabetes Mellitus. Risk for diabetes among relatives of IDDM. Diabetes. 1982;31:136–144. doi: 10.2337/diab.31.2.136. [DOI] [PubMed] [Google Scholar]
- 40.Pambianco G., Costacou T., Ellis D. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 41.Hypertension Detection and Follow-up Program Cooperative Group The Hypertension Detection and Follow-up Program. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 42.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orchard T.J., Secrest A.M., Miller R.G., Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53:2312–2319. doi: 10.1007/s00125-010-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unruh M.L., Pankratz V.S., Demko J.E. Trial of amiloride in type 2 diabetes with proteinuria. Kidney Int Rep. 2017;2:893–904. doi: 10.1016/j.ekir.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diabetes Epidemiology Research International Mortality Study Group International evaluation of cause-specific mortality and IDDM. Diabetes Care. 1991;14:55–60. doi: 10.2337/diacare.14.1.55. [DOI] [PubMed] [Google Scholar]
- 46.Groop P.H., Thomas M.C., Moran J.L. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651–1658. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen L.H., Jensen B.L., Fuglsang J. Urine albumin is a superior predictor of preeclampsia compared to urine plasminogen in type I diabetes patients. J Am Soc Hypertens. 2018;12:97–107. doi: 10.1016/j.jash.2017.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
uPl/Cr versus albumin and eGFR, and longitudinal change in uPl/Cr.
uPl/Cr versus urinary Na/K.
uPl/Cr, uAlb/Cr, and eGFR decline over 25 years.